Highlights
-
•
Extracellular phytase from A. niger has attractive biochemical properties as an animal feed additive
-
•
A new biocatalyst was high thermostability and resistance to acidic pHvalues was produced and purified.
-
•
Phytase was as able to hydrolyze a large number of phosphate substrates, with high yields.
-
•
A great variety of metal ions showed to have a beneficent effect on the enzyme, significantly increasing its catalysis.
Keywords: Phytase, Biochemical characterization, Animal feed, Aspergillus niger, Solid-state fermentation
Abstract
In this study, an extracellular phytase produced by Aspergillus niger 7A-1, was biochemically characterized for possible industrial application. The enzyme was purified from a crude extract obtained by solid-state fermentation (SSF) of triticale waste. The extract was obtained by microfiltration, ultrafiltration (300, 100 and 30 kDa) and DEAE-Sepharose column chromatography. The molecular weight of the purified enzyme was estimated to be 89 kDa by SDS-PAGE. The purified enzyme was most active at pH 5.3 and 56 °C, and retained 50% activity over a wide pH range of 4 to 7. The enzymatic thermostability assay showed that the enzyme retained more than 70% activity at 80 °C for 60 s, 40% activity for 120 s and 9% after 300 s. The phytase showed broad substrate specificity, a Km value of 220 μM and Vmax of 25 μM/min. The purified phytase retained 50% of its activity with phosphorylated compounds such as phenyl phosphate, 1-Naphthyl phosphate, 2-Naphthyl phosphate, p-Nitrophenyl phosphate and Glycerol-2-phosphate. The inhibition of phytase activity by metal ions was observed to be drastically inhibited (50%) by Ca++ and was slightly inhibited (10%) by Ni++, K+, and Na+, at 10 and 20 mM concentrations. A positive effect was obtained with Mg++, Mn++, Cu++, Cd++ and Ba++ at 25 and 35% with stimulatory effect on the phytase activity.
1. Introduction
Phytases are hydrolytic enzymes (phosphatases), belonging to the subfamily of histidine acid phosphatases. They catalyze the hydrolysis of phytate phosphomonoester bonds (salts myo-inositol hexakisphosphate) or myo-inositol 1, 2, 3, 4, 5, 6- hexakis dihydrogenphosphate (phytic acid), and produce derivatives such as tetra, tri, di and inositol monophosphate, as well as inorganic phosphate (Pi) [[1], [2]]. The main application of this enzyme is in the animal feed industry, where it is used as feed supplement for non-ruminant animals (such as pigs, chickens, turkeys, etc). This is because phytic acid is the largest reservoir of phosphorus in plants: 60–80% of plant phosphorus is contained in phytic acid. Since their digestive system lacks phytase, monogastric animals are incapable of metabolizing phosphorus. Phytic acid is excreted in their stool, causing soil pollution and eutrophication of water by phosphates [[3], [4], [5]].
During animal digestion, phytase liberates the Pi present in phytic acid [5]. When it is used as a supplement, Pi is reduced in manure by about 33%, which ensures a decrease in environmental pollution by a third, in addition to improved animal nutrition. The main limitation to the use of this enzyme with high nutritional and environmental interest, is the high market price and in some cases, low production levels and thermostability [[6], [7]].
Phytase producing microorganisms include filamentous fungi of the genus Aspergillus. In various studies, these fungi were found to produce the most active extracellular enzyme with the most suitable characteristics of both pH and temperature stability. Hence, microorganisms of the genus Aspergillus, are the most used in the industrial production of this enzyme [[8], [6]]. Solid-state fermentation is a process commonly applied for the production of extracellular enzymes [9].
As a result of the favourable characteristics of phytase, as well as its practical application as an additive in the diets of non-ruminants, this enzyme has taken a position of great interest in biotechnological applications to reduce the phytate content of fodder and commercial foods [10]. Phytases used as additive must be effective in releasing phosphate from phytic acid and must demonstrate their effectiveness at the digestive tract level, as well as withstand the conditions of pH and temperature [[11], [12]].
This paper reports the purification, characterization and enzymatic properties of a novel phytase produced by Aspergillus niger 7A-1, by solid state fermentation of the agro-industrial waste of triticale with special interest in its application in animal feed at the industrial level.
2. Materials and methods
2.1. Microorganism production and enzyme recovery
The A. niger 7A-1 strain was provided by the Nanobioscience Group, University Autonomy of Coahuila, Saltillo, México. Prior to the commencement of the study, the strain was grown and maintained on potato dextrose agar (PDA) slants at 28 ± 1 °C, to obtain the inoculum. After seven days of fungal growth, spores were collected with 0.1% Tween 80 solution. Their concentration was adjusted to1 × 106 spores/mL.
The substrate used for phytase production by solid state fermentation (SSF), was provided by the Agrarian Autonomy Universidad “Antonio Narro”, México. It consisted of a mixture of the agroindustrial waste of triticale. The substrate was washed with distilled water to remove soil and impurities, dried at 60 °C, ground to obtain a particle size of approximately 0.3 mm, and stored in sealed bags until use.
The substrate (5 g) was placed in a Petri dish and sterilized at 121 °C for 20 min. After cooling, the substrate was moistened with 3 mL of sterile solution which contained: NH4NO3 (40 g/L), dextrose (168 g/L), lactose (4.8 g/L), Tween 80 (10 mL/L) and KCl (2 g/L) to adjust moisture at 60% approximately. The substrate was inoculated with 0.5 mL spore suspension (1 × 106 spores/mL) and the content was mixed and incubated for five days at 28 ± 1 °C under static condition.
The enzyme extract was obtained from the fermented samples with distilled water (5 mL/(g of substrate)) after stirring for 1 h (at 200 rpm and 25 ± 1 °C). The suspension was centrifuged at 10,000g for 10 min. The clear brown coloured supernatant was termed crude extract (CE) and stored at 4 °C until further use.
2.2. Phytase enzyme purification
The crude extract obtained by the SSF was concentrated using a Millipore Amicon ultrafiltration cell model 8200 (Bedford, MA, USA) with a 30, 100 and 300 kDa molecular cut-off PM30 Amicon membrane at 4 °C. The retained fraction was cleared by centrifugation (10,000g for 15 min at 4 °C). Subsequently, the pH of this concentrated extract was adjusted to 7.0 with 0.1 M NaOH solution. Thereafter, a 2 mL aliquot of the enzyme was loaded onto a 4.7 mL DEAE-Sepharose CL 6 B column (Pharmacia), pre-equilibrated with 50 mM sodium acetate at pH 5.15. The total unbound protein was removed by washing with two bed volumes of equilibration buffer. Bound protein was then eluted using a linear salt gradient (0 to 0.5 M NaCl in 50 mM sodium acetate buffer at pH 5.15 containing 0.5% (w/v) sucrose) with flow rate: 0.2 mL/min, 2.0 mL fractions were recovered. The fraction (2 mL) with the greatest specific activity was selected for subsequent analysis and characterization.
2.3. Enzyme activity assay
Phytase activity was determined by measuring the Pi released from sodium phytate solution [[13], [12]]. The reaction mixture consisted of 1 mL of 0.1 M MgSO4*7H2O, 2.4 mL of 6.82 mM phytic acid and 0.6 mL of appropriately diluted crude enzyme solution. Solutions of MgSO4*7H2O and phytic acid were prepared with 0.2 M sodium acetate buffer (pH 5.15). Subsequently, the reactants were incubated at 55 °C for 60 min, and the reaction was stopped by adding 0.5 mL of 10% trichloroacetic acid. Thereafter, 1 mL of distilled water and 2.4 mL of Taussky-Schorr reagent (10 mL of 10N H2SO4, 1 g of (NH4)Mo7O24*4H2O and 5 g of FeSO4*7H2O graduated to 100 mL distilled water) were added to generate a blue chromophore [13]. The content was mixed for 30 min and then the absorbance was determined at 660 nm. Measured values were correlated with a standard curve that was constructed using monopotassium phosphate. One unit of phytase activity was defined as the amount of enzyme that released 1 μmol of phosphate per minute under assay conditions. All the enzyme activity analyses were performed in triplicate.
2.4. Other analytical determinations
Protein concentration was determined by the Bradford method using bovine serum albumin as the standard at 0 to 20 μg/mL [14].
The Km and Vmax values were determined by means of enzyme activity assay performed using different phytic acid concentrations to plot the obtained results in Lineweaver-Burk coordinates [15].
2.5. Molecular characterization of A. niger purified phytase
One-dimensional SDS gel electrophoresis was performed using 10% (w/v) acrylamide gel in a vertical electrophoresis system, and staining was carried out using Coomassie Blue R-250 [16]. The molecular weight was determined using high molecular weight markers (Sigma-Aldrich Co.). For zymogram analysis, non-denaturing electrophoresis was carried out in the same manner, but with the omission of SDS from the gel running and loading buffers, the sample was not pre-treated under denaturing conditions. After electrophoresis, the gel was equilibrated with 0.2 M sodium acetate buffer at pH 5.15 for 30 min, and then incubated in the same buffer containing 0.04% (w/v) p-nitrophenyl phosphate (pNPP) at 55 °C for 30 min. After incubation, the gel was rinsed with distilled water, and then a 0.5 M Na2CO3 solution was added for visualization of yellow zones, indicating substrate hydrolysis [12].
2.6. Effect of temperature and pH on the purified enzyme activity
Enzyme thermostability. Phytase activity versus reaction temperature and pH were determined according to the method of Howson and Davis. [17]. A temperature range of 20–80 °C and a pH range of 2.0–8.0 were assayed. The following buffers were used: 0.2 M glycine-HCl (pH 2–3), 0.2 M sodium acetate (pH 4.0–5.5), 0.2 M 2-(N-morpholino) ethanesulfonic acid MES (pH 6.0–6.5) and 0.2 M Tris-HCl (pH 7.0–8.0). Thermostability was assayed using purified phytase preincubated at 80 °C for 300 s before enzyme activity determination by the previously described method [8].
2.7. Determination of substrate specificity
The specificity of the purified phytase for different substrates was evaluated by replacing phytic acid in the reaction mixture with other phosphate containing compounds. All substrates (listed below) were used at a concentration of 3 mM. The phytase activity was evaluated in accordance with the phytase activity assay [18].
2.8. Effect of metal ions on A. niger phytase activity
The inhibitory or stimulatory effects of metal ions on the phytase activity were determined. Before enzymatic activity assay, the enzyme was preincubated for 10 min at 39 °C in the presence of the different salts defined below, and applied at two different concentrations (10 and 20 mM). In the calculation of relative enzyme activity, the phytase activity determined without salt addition was considered as 100% [9].
2.9. Determination of substrate specificity
The specificity of purified phytase for different substrates was evaluated by replacing phytic acid in the reaction mixture with other phosphate containing compounds. All substrates (defined below) were used at concentrations of 3 mM. The evaluation of phytase activity was in accordance with the phytase activity assay [18].
3. Results
3.1. Phytase purification and concentration
The extracellular phytase produced by the A. niger strain was purified using step ultrafiltration followed by ion-exchange chromatography. The phytase purified by ion-exchange chromatography exhibited only one strong band and some shadows on SDS-PAGE gel (Fig. 1). The results of enzyme purification are shown in Table 1. Phytase was purified at 7.4-fold and 15.5% yield. [18] reported the purification yield of the phytase obtained with A. niger ATCC 9142 at 48% and 3.5-fold.
Fig. 1.
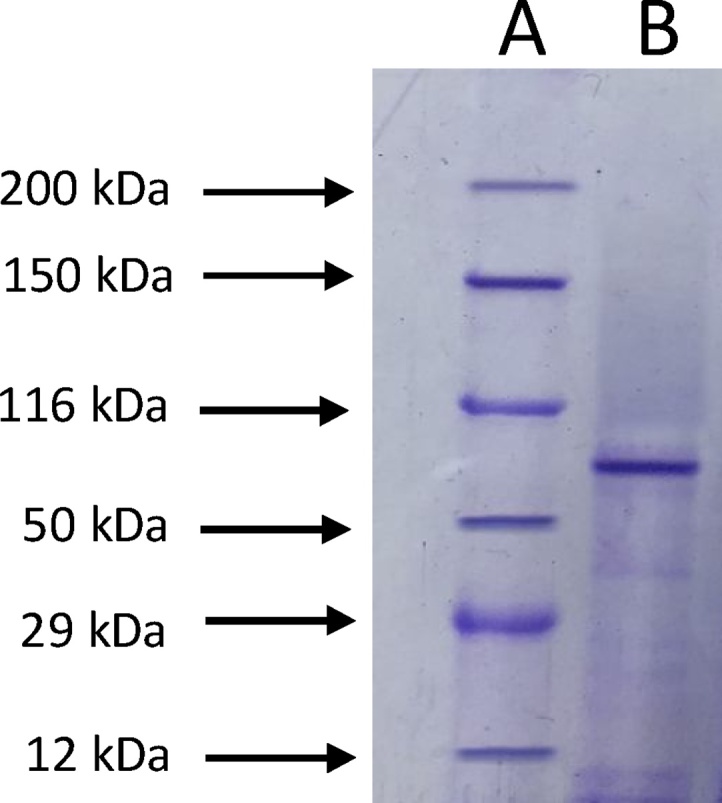
SDS-PAGE analysis of phytase from A. niger: A, – molecular weight markers bands; B, – purified phytase.
Table 1.
Purification of Aspergillus niger extracellular phytase.
| Step | Total protein mg/mL | Volumetric activity U/mL | Total activity U | Specific activity U/mg protein | Purification (Fold) | Yield% |
|---|---|---|---|---|---|---|
| Crude extract | 48.34 | 54.56 | 272.8 | 1.13 | 1.0 | 100.0 |
| Microfiltration | 44.19 | 50.69 | 243.3 | 1.15 | 1.0 | 89.2 |
| Ultrafiltration | 24.36 | 46.94 | 93.9 | 1.93 | 1.7 | 34.4 |
| Fraction 6 of DEAE-Sepharose | 2.52 | 21.13 | 42.3 | 8.38 | 7.4 | 15.5 |
3.2. Molecular weight determination of the purified phytase
According to SDS-PAGE, the molecular weight of A. niger phytase purified by ion-exchange chromatography was estimated at 89 kDa (Fig. 1). Literature data has reported the molecular weight of phytase to range between 40 and 100 kDa. Fig. 2 shows the zymogram obtained by means of non-denaturing electrophoresis. A single band represents the phytase.
Fig. 2.

Zymogram of phytase from A. niger, on a non-denaturing electrophoretic gel.
3.3. Characterization of A. niger purified phytase
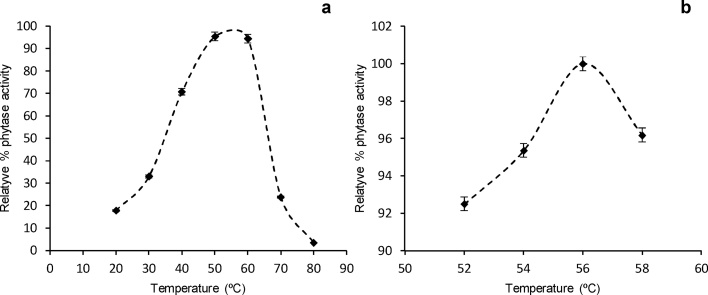
Fig. 3 shows the phytase activity at different temperatures. The optimum temperature of the purified enzyme was 56 °C (Fig. 3b) while the temperature range appropriate for catalysis was between 50 and 60 °C (Fig. 3a). An increase in temperature to 70 °C resulted in a decrease in enzyme activity to 24%, due to partial denaturation of the enzyme. Moreover, at 40 °C, the activity of the purified phytase enzyme was nearly 70% of the maximum value. Considering that the pig’s physiological temperature is about 39 °C, it may be assumed that the enzyme will be active under this temperature. The total digestion of a pig takes from 13 to 15 h, from food intake to deposition, the phytase enzyme begins its action when it is mixed with the food and increasing its activity when going from room temperature to body temperature of the animal [19]. The enzymatic stability may vary widely depending on the formulation that used to protect the enzyme. However these can be effective in a time range of one to eight hours, with an optimum range of action of between 30 and 50 min, depending largely on the food with which to mix, the animal's race and the water content in the food. Since the enzyme carries its action to the stomach level, where it releases the P that will be absorbed later at the intestinal level [[9], [10], [19]].
Fig. 3.
Effect of temperature on the activity of A. niger purified phytase: A, – screening to determine the best temperature range; B, – optimum temperature estimation.
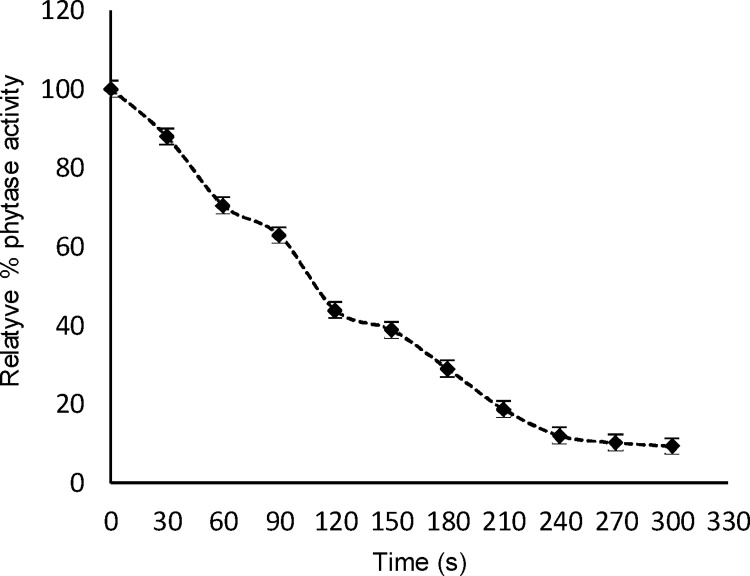
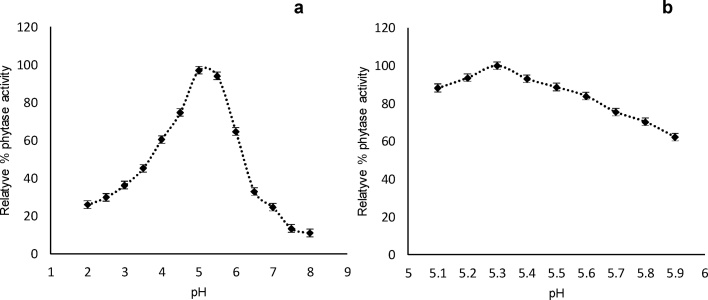
The thermostability profile of phytase at 80 °C is shown in Fig. 4. It has been proven to be a thermotolerant enzyme, which maintains 70% of its activity after 60 s of incubation at 80 °C and 9.3% after 300 s. The thermostability of the studied phytase was similar to that reported by [12]. This result is of paramount importance since the inclusion of this enzyme in industrial processes of animal feed. Has the advantage that it can withstand temperatures which are around 60 °C for two to three minutes, without being affected, besides this the enzyme used in the present work was in the pure state which poses the possibility of using some type of protection as the nanoaccounting which provides greater thermal resistance. Usually this enzyme is added to the feed prior to ingestion by the animal, but the possibility of designing complete diets that already contain the enzyme and avoid premixing at the moment of feeding the animal is not ignored. Fig. 5 shows the purified enzyme activity at different pH. The maximum activity was determined at pH 5.3, which is similar to some phytases reported in the literature: Aspergillus flavus ITCC 6720 at pH 5.5 [9]; A. niger ATCC 9142 at pH 5 [20]. By varying the pH value, the activity may be reduced to between 25 and 75% in pH values from 2.0 to 4.5. However, prolonged exposure to lower pH, could result in a loss of up to 95% of the total activity (Fig. 5a).
Fig. 4.
Thermostability profile of A. niger purified phytase at 80 °C.
Fig. 5.
Effect of pH on the A. niger phytase: A,– screening to determine the best pH range; B, – optimum pH).
3.4. Kinetic and substrate analysis
To define Km and Vmax, enzymatic reactions were carried out in the presence of various concentrations of phytate (0.5–3 mM). The parameters were calculated by using a Lineweaver-Burk plot. These values correspond to 220 and 25 μM (phosphate)/min, respectively. These values fall well within the range previously reported for microbial phytases [20].
The phytase substrate specificity is demonstrated in Table 2 and the ability of the purified enzyme to dephosphorylate various substrates, in addition to sodium phytate, can be observed. However, it was also shown that the substrate on which the enzyme had a higher activity, was sodium phytate. The activity value with this substrate was taken as 100%. The purified enzyme dephosphorylated phenyl phosphate, 1-naphthyl phosphate and 2-naphthyl phosphate, had relative activity above 70%. These results are similar to the values reported for purified phytases by different authors [[9], [21], [22]].
Table 2.
Affinity of the Aspergillus niger purified phytase to various substrates.
| Substrates | Relative activity% |
|---|---|
| Sodium phytate* | 100.00 |
| Phenyl phosphate | 87.38 |
| 1-Naphthyl phosphate | 82.61 |
| 2-Naphthyl phosphate | 74.54 |
| p-Nitrophenylphosphate | 63.65 |
| Glycerol-2-phosphate | 55.52 |
| Adenosine monophosphate | 35.95 |
| Glucose-1-phosphate | 32.28 |
| Adenosine diphosphate | 19.72 |
100% activity was obtained when sodium phytate.
The influence of various metal ions on phytase activity is an important characteristic (Table 3) which can be taken into account to predict enzyme behaviour in animal feed. It was observed that calcium exert a negative effect on the A. niger phytase activity. All other tested ions displayed a significant positive stimulatory effect. These results agree with those reported previously for phytases derived from A. niger or Rhizopus, which were inhibited by calcium ions [23]. Previous studies have reported an increase in the presence of copper, manganese and magnesium ions [[12], [18], [22], [24], [25]]. Some reports about A. flavus phytase showed that its activity was affected by barium and magnesium ions [9]. From a practical point of view, this is of great interest because both the positive and negative influences of ions on phytase activity are useful parameters in making food formulations or for combination with any supplement. On the other hand, it must be determined in order to evaluate the performance of phytase in the presence of animal feed, considering that on average, it contains 1.5% (w/w) minerals. The effect of such ions on phytase activity is also of academic interest [[21], [23], [26]].
Table 3.
Effect of metal ions on Aspergillus niger purified phytase activity.
| Metal ion | 10 mM | 20 mM |
|---|---|---|
| MgSO4*7H2O | 138.94 | 129.22 |
| MnSO4 | 128.57 | 119.57 |
| CuSO4 | 135.27 | 125.80 |
| ZnCl2 | 112.84 | 104.94 |
| CaCl2 | 52.34 | 48.68 |
| Fe2(SO4)3 | 105.11 | 97.76 |
| Fe(SO4) | 102.85 | 95.65 |
| HgCl2 | 112.48 | 104.61 |
| CdCl2 | 125.73 | 116.93 |
| BaCl2 | 131.81 | 122.58 |
| NiCl2 | 99.63 | 92.66 |
| KCl | 98.21 | 93.30 |
| NaCl | 95.91 | 89.19 |
The 100% of phytase activity corresponds to a blank test, which was performed in the absence of said ions.
4. Discussion
In the present study, the increase in specific activity was two times more than the value reported by [18]. However, a greater loss of total activity was observed (Table 1) probably due to obtaining only one fraction on ion-exchange chromatography.
In various previous reports with different microorganisms, a 7-fold purification index was obtained. [12] reported 8.55-fold purification of the phytase produced by the thermotolerant A. niger UFV-1. They reported that the active phytase is a dimer with a molecular weight of 186 kDa. Thus, the applied purification technique allows purity-fold value similar to that reported previously with less enzyme recovery yield.
The molecular weight of phytase isolated and characterized in the present study is within this range and is similar to various reports. For example, [18] reported that the molecular weight of phytase produced by A. niger ATCC 9142 was 84 kDa, while according to [8], the molecular weight of A. niger NCIM 563 phytase was estimated as 87 kDa.
Considering the SDS-PAGE result (Fig. 1), it was confirmed that the phytase obtained using A. niger isolated from Coahuila semi-desert is a monomer because in the non-denaturing electrophoresis, and SDS-PAGE a single band was exhibited with the same Rf, which precludes the possibility that it is a protein bound by more than one subunit (Fig. 2). Similar results were obtained for phytase produced by different fungi of the Aspergillus genus [[18], [27]]. However, there are reports that indicate the existence of phytases which exist as dimers and even tetramers. The molecular weight of these enzymes is generally higher than the molecular weight of A. niger phytase [[21], [25]].
Casey and Walsh. [18] demonstrated 60% residual activity of phytase from A. niger ATCC 9142 after 60 s of incubation at 80 °C and 12% of activity after 300 s. Greiner et al. (2009) observed that Aspergillus niger 11T53A9 phytase retained 8% of its activity after 300 s. On average, a fungal phytase retains 40 to 60% of its activity when exposed to high temperatures for a period of 60 s. However, thermoresistant microorganisms, for example A. niger UFV-1 [12], produce phytases which keep 80% of their activity at 80 °C for a long period of time. Enzyme thermostability is a parameter that must be considered for introduction in the industrial processes of animal feed production [[9], [19]].
In the literature, a wide range of phytase from different sources, which stand out for their catalytic and physical properties have been documented. These include those produced by A. niger, since it has been demonstrated that these enzymes act in a broader pH range.
Considering this parameter from a commercial perspective, the enzyme exhibited significant activity in the pH range found in the digestive tract. Thus, it may be helpful to promote enzymatic phytate degradation, starting from the salivary glands (pH 5.0), then the stomach (pH 2.0–4.0) and finally, the upper part of the large intestine (pH 4.0–6.0). This ensures better adsorption of nutrients along the digestive process [[5], [19]].
The A. niger phytase purified in the present study was characterized as an enzyme with a wide range of substrates that might be degraded under its activity. It is of great importance because various authors noted that the phytase usually acted best when used in animal feed, as a result of the wide range of substrate specificity [[12], [28]]. According to [20] and [12], phytases from Aspergillus were able to hydrolyze calcium phytate, phenyl phosphate, and glucose-1-phosphate. However, [9] reported phytases with a very small margin of substrate specificity, like Aspergillus flavus ITCC 6720, which displays specificity only for sodium phytate and p-nitrophenylphosphate.
5. Conclusions
The extracellular phytase produced by A. niger7A-1 in SSF using triticale waste as solid support, was successfully purified. The biochemical characterization showed interesting properties, thereby making it suitable for its potential commercial use as a feed additive. Its thermostability is highlighted because high temperatures are needed for more resistant enzymes in industrial animal feed processing. The specificity of phytase to various substrates as well as enzyme activity at a wide pH range, ensure its competitive viability for industries. The use of enzymes for animal feed production may increase the assimilation of phytate Pi, reduce the implementation of inorganic Pi in feed formulations, minimize Pi in excretion, and reduce soil pollution.
Conflict of interest
None.
Acknowledgements
Authors thank National Council for Science and Technology, CONACYT-Mexico for providing a research grant for the execution of this study. We also thank Dr. Javier Lozano-Del Rio of the breeding department of the “Universidad Autónoma Agraria Antonio Narro”, for providing the residues used in carrying out the fermentation.
References
- 1.Bilgiçli N., Elgün A., Türker S. Effects of various phytase sources on phytic acid content, mineral extractability and protein digestibility of tarhana. Food Chem. 2006;98:329–337. [Google Scholar]
- 2.Albarracín M., González R.J., Drago S.R. Effect of soaking process on nutrient bio-accessibility and phytic acid content of brown rice cultivar. LWT – Food Sci. Technol. 2013;53:76–80. [Google Scholar]
- 3.Fei B., Xu H., Zhang F., Li X., Ma S., Cao Y.Y., Xie J., Qiao D., Cao Y.Y. Relationship between Escherichia coli AppA phytase’s thermostability and salt bridges. J. Biosci. Bioeng. 2013;115:623–627. doi: 10.1016/j.jbiosc.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Ma X.-F., Tudor S., Butler T., Ge Y., Xi Y., Bouton J., Harrison M., Wang Z.-Y. Transgenic expression of phytase and acid phosphatase genes in alfalfa (Medicago sativa) leads to improved phosphate uptake in natural soils. Mol. Breed. 2011;30:377–391. doi: 10.1007/s11032-011-9628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vats P., Bhushan B., Banerjee U.C. Studies on the dephosphorylation of phytic acid in livestock feed using phytase from Aspergillus niger van Teighem. Bioresour. Technol. 2009;100:287–291. doi: 10.1016/j.biortech.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Haefner S., Knietsch A., Scholten E., Braun J., Lohscheidt M., Zelder O. Biotechnological production and applications of phytases. Appl. Microbiol. Biotechnol. 2005;68:588–597. doi: 10.1007/s00253-005-0005-y. [DOI] [PubMed] [Google Scholar]
- 7.Romero C., Salas M., García A.C., Mendoza G., Plata F., Cervantes M., Viana T., Morales A. Effect of phytase from Aspergillus niger on nutrient digestibility and activity of trypsin and chymotrypsin in weanling pigs. Arch. Zootec. 2009;58:363–369. [Google Scholar]
- 8.Bhavsar K., Ravi V., Khire J.M. Downstream processing of extracellular phytase from Aspergillus niger: chromatography process vs. aqueous two phase extraction for its simultaneous partitioning and purification. Process Biochem. 2012;47:1066–1072. [Google Scholar]
- 9.Gaind S., Singh S. Production, purification and characterization of neutral phytase from thermotolerant Aspergillus flavus ITCC 6720. Int. Biodeterior. Biodegrad. 2015;99:15–22. [Google Scholar]
- 10.Omogbenigun F.O., Nyachoti C.M., Slominski B.A. The effect of supplementing microbial phytase and organic acids to a corn-soybean based diet fed to early-weaned pigs. J. Anim. Sci. 2003;81:1806–1813. doi: 10.2527/2003.8171806x. [DOI] [PubMed] [Google Scholar]
- 11.Jondreville C., Schlegel P., Hillio S., Chagneau ‐M A.M., Nys Y. Effects of additional zinc and phytase on zinc availability in piglets and chicks fed diets containing different amounts of phytates. Livest. Sci. 2007;109:60–62. [Google Scholar]
- 12.Monteiro P.S., Guimarães V.M., de Melo R.R., de Rezende S.T. Isolation of a thermostable acid phytase from Aspergillus niger UFV-1 with strong proteolysis resistance. Br. J. Microbiol. 2015;46:251–260. doi: 10.1590/S1517-838220120037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harland B.F., Hraland J. Fermentative reduction of phytate in rye white, and whole wheat Breads. Cereal Chem. 1980;57:226–229. [Google Scholar]
- 14.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.In M.-J., Seo S.-W., Kim D.C., Oh N.-S. Purification and biochemical properties of an extracellular acid phytase produced by the Saccharomyces cerevisiae CY strain. Process Biochem. 2009;44:122–126. [Google Scholar]
- 16.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Howson S.J., Davis R.P. Production of phytate hydrolysing enzyme by some fungi. Enz. Microbiol. Technol. 1983;5:377–382. [Google Scholar]
- 18.Casey A., Walsh G. Purification and characterization of extracellular phytase from Aspergillus niger ATCC 9142. Bioresour. Technol. 2003;86:183–188. doi: 10.1016/s0960-8524(02)00145-1. [DOI] [PubMed] [Google Scholar]
- 19.Morales G.A., Moyano F.J., Marquez L. In vitro assessment of the effects of phytate and phytase on nitrogen and phosphorus bioaccessibility within fish digestive tract. Anim. Feed Sci. Technol. 2011;170:209–221. [Google Scholar]
- 20.Casey A., Walsh G. Identification and characterization of a phytase of potential commercial interest. J. Biotechnol. 2004;110:313–322. doi: 10.1016/j.jbiotec.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu M. Purification and characterization of phytase and acid phosphatase produced by aspergillus oryzae K1. Biosci. Biotechnol. Biochem. 2014;57:1364–1365. [Google Scholar]
- 22.Zou L., Wang H., Pan X., Tian G., Xie Z., Wu Q., Chen H., Xie T., Yang Z. Expression, purification and characterization of a phyA m -phyCs fusion phytase. J. Zhejiang Univ. Sci. B. 2008;9:536–545. doi: 10.1631/jzus.B0720006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selle P.H., Cowieson A.J., Ravindran V. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Livest. Sci. 2009;124:126–141. [Google Scholar]
- 24.Quan C., Tian W., Fan S.-D., Kikuchi J. Purification and properties of a low-molecular-weight phytase from cladosporium sp. FP-1. J. Biosci. Bioeng. 2004;97:260–266. doi: 10.1016/S1389-1723(04)70201-7. [DOI] [PubMed] [Google Scholar]
- 25.Soni S.K., Magdum A., Khire J.M. Purification and characterization of two distinct acidic phytases with broad pH stability from Aspergillus niger NCIM 563. World J. Microbiol. Biotechnol. 2010;26:2009–2018. doi: 10.1007/s11274-010-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H., Zhou Y.Y., Ma J., Zhou Y.Y., Jiang H. The effects of phytic acid on the Maillard reaction and the formation of acrylamide. Food Chem. 2013;141:18–22. doi: 10.1016/j.foodchem.2013.02.107. [DOI] [PubMed] [Google Scholar]
- 27.Bhavsar K., Kumar V.R., Khire J.M. High level phytase production by Aspergillus niger NCIM 563 in solid state culture: response surface optimization, up-scaling, and its partial characterization. J. Ind. Microbiol. Biotechnol. 2010;38:1407–1417. doi: 10.1007/s10295-010-0926-z. [DOI] [PubMed] [Google Scholar]
- 28.Mehdipour-Moghaddam M.J., Emtiazi G., Bouzari M., Salehi Z. Novel phytase and cellulase activities in endophytic azospirilla. World Appl. Sci. J. 2010;10:1129–1135. [Google Scholar]