Abstract
Multiple cytokines and inflammatory markers control TB infection. We aimed to investigate the changes in multiple cytokines and inflammatory markers in active TB patients following anti-TB drug therapy. Twenty-nine patients with active TB were recruited prospectively between December 2010 and July 2017. Blood samples were collected before (T0), after 2 months (T2), and at the end of anti-TB treatment (Tend). We measured the levels of Interferon (IFN)-γ, interleukin (IL)-2, IL-12, IL-10, IL-13 and tumor necrosis factor (TNF)-α in supernatants collected from the QuantiFERON-TB Gold In-Tube assay (QFT-GIT), as well as the WBC, neutrophil, platelet count and neutrophil to lymphocyte ratio (NLR) in whole blood. Compared with baseline levels, WBC, neutrophil, and platelet counts were significantly lower following treatment. In addition, the NLR after treatment significantly decreased compared with baseline, whereas the IL-2/IFN-γ ratio increased after treatment. In conclusion, the levels of IL-2/IFN-γ ratios in the supernatant and the NLR might be useful biomarkers to evaluate the effectiveness of drug therapy in active TB patients.
Introduction
Tuberculosis remains a major cause of death and morbidity worldwide, responsible for an estimated 1.7 million deaths each year1. Prolonged treatment is required for adequate therapy, resulting in significant costs and medical resources.
The ability to monitor the response of TB to therapy and confirm adequate treatment would constitute a major advancement. There have been efforts to develop biomarkers for the diagnosis and treatment of TB. Identifying biomarkers of treatment success might provide insight into surrogate markers of protective immunity against TB. Cell-mediated immunity plays an important role in human host defenses against TB2. The essential role of T cells in protection against TB infection has been well-documented2. IFN-γ, a cytokine produced by T cells, is used to diagnose TB infection3. Other cytokines, including TNF-α4, IL-125, IL-136, and IL-27 are essential in controlling TB infection. Furthermore, IFN-γ and IL-2 profiles have been associated with antigenic load and treatment in active TB8.
Although several studies have evaluated IFN-γ to monitor the response to anti-TB treatment, these studies yielded conflicting results, with IFN-γ responses decreasing9–11, increasing12, or remaining not significantly changed13,14 in response to treatment. An in vitro IFN- γ immune diagnostic assay for active TB disease, the novelty of which consists of the use of multiepitopic region of difference (RD)1 peptides selected by computational analysis was reported15–17. In the study of Goletti et al., the response to selected RD1 peptides was associated with TB disease in HIV-infected individuals, and decreased after successful therapy9. Several exploratory studies have evaluated the diagnostic potential of cytokine biomarkers other than IFN-γ for monitoring anti-TB treatment such as TNF-α18, or IFN- γ /TNF-α19, or IFN- γ inducible protein (IP)-1020.
However, no single cytokine or combination of cytokines has shown a strong correlation with treatment success. To date, relatively few studies have evaluated the usefulness of multiple cytokines in monitoring the response to anti-TB treatment21,22. Previously, we examined multiple cytokines, including IFN-γ and IL-2, to determine the TB infection status and assess mycobacterial loads among smear-positive, smear-negative, and latent TB infection23. In the current study, we investigated the changes in multiple cytokines and inflammatory markers in active TB patients following anti-TB drug therapy.
Materials and Methods
Study population
Patients with active pulmonary TB were recruited prospectively between December 2010 and July 2017 at Severance Hospital, a tertiary referral hospital in Seoul, South Korea, after approval of the study protocol by the Ethics Review Committee. The patients who could not be followed for at least 6 months, those with HIV infection, end-stage renal disease, or leukemia/lymphoma, and those undergoing immunosuppressive therapy within 3 months of enrollment were excluded. After providing informed consent, each patient completed a set of questionnaires pertaining to demographics, history of TB, and cigarette use. The QFT-GIT was performed in each patient T0, T2, and Tend. We also measured the levels of IFN-γ, IL-2, IL-12 (p40), IL-10, IL-13, and TNF-α in the supernatant obtained from the QFT-GIT, as well as the white blood cell (WBC) count, neutrophil count, lymphocyte count, platelet count, red cell distribution width (RDW), mean platelet volume, neutrophil to lymphocyte ratio (NLR), the ratio of monocytes and lymphocytes (MLR) and platelet to lymphocyte ratio (PLR) in all patients at T0, T2, and Tend.
All participants provided a written, informed consent for the collection of samples and subsequent analysis. All the individuals participating in this study were over 18 years old. All methods were carried out in accordance with relevant guidelines and regulations. The study protocol was reviewed and approved by the Institutional Review Board of Yonsei University Health Service, Severance Hospital (4-2013-0803).
Diagnosis and TB treatment
The diagnosis of active pulmonary TB was made based on clinical, radiological, microbiological, and pathological data. Active pulmonary TB was confirmed in TB cultures derived from respiratory specimens or by the presence of caseating granulomas in lung tissue. One patient who was highly suspected of having active TB but had a negative mycobacterial culture showed good clinical and radiographic responses to TB treatment and therefore was also included. Treatment was based on the Korean Guidelines for Tuberculosis 2011. The standard regimen of rifampicin (10 mg/kg body weight [BW]/day), isoniazid (5 mg/kg BW/day), ethambutol (15–25 mg/kg BW/day), and pyrazinamide (15–30 mg/kg BW/day) was used. The duration of treatment was at least 6 months, depending on the risk of recurrence, the clinical response to treatment, and drug intolerance. Patients who had positive mycobacterial culture at the end of the intensive phase (after two months of the treatment) with cavity lesion in their chest radiograph were considered as having high risk of relapse.
QFT-GIT assay
IFN-γ release assays were performed using the QFT-GIT assay kit according to the manufacturer’s instructions. Whole blood (1 mL) was collected in each of three tubes precoated with saline (nil control, Nil), TB-specific antigen (TB Ag; ESAT-6, CFP-10, and TB7.7), or mitogen and incubated for 20 h at 37 °C within 8 h of blood sampling. The plasma supernatant was collected after centrifugation and stored at −20 °C until assayed for IFN-γ using the QFT GOLD ELISA. Results were calculated using the manufacturer’s QFT-GIT software.
Multiplex analysis of cytokine production
The levels of multiple cytokines, IFN-γ, IL-2, IL-12(p40), IL-10, IL-13, and TNF-α, in the supernatants obtained from the QFT-GIT assays were measured using the commercial MILLIPLEX® MAP human cytokine/chemokine kit (HCYTOMAG-60K-06, Millipore, Billerica, MA, USA) with fluorescently labeled microsphere beads and a Luminex reader. The cytokine levels were measured in the QFT-GIT supernatants at T0, T2, and Tend. The undiluted supernatants were used in this study. The minimum detectable concentrations were 0.8 pg/mL for IFN-γ, 1.0 pg/mL for IL-2, 7.4 pg/mL for IL-12(p40), 1.1 pg/mL for IL-10, 1.3 pg/mL for IL-13, and 0.8 pg/mL of TNF-α according to the protocol. The value under minimum detectable concentrations were considered as zero.
Statistical analysis
The quantitative data for cytokines were expressed as numbers with percentage in brackets or as medians with the interquartile range (IQR) in brackets. Cytokine levels were analyzed using repeated measures analysis of variance to compare the expression of each cytokine among three groups. The Wilcoxon signed rank test was used to compare cytokine expression between two related groups. The GraphPad Prism program version 4.0 (GraphPad software, San Diego, CA, USA) was used to create the graphics. A two-tailed p-value < 0.05 was considered significant. All statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline patient characteristics
The clinical characteristics of the study patients, including the extent of the disease in chest CT, acid-fast bacilli (AFB) smear and culture positivity, and profiles obtained from the blood tests and QFT-GIT assay, are summarized in Table 1. Twenty-two (75.9%) patients were culture-positive on sputum specimens, and all 22 achieved sputum conversion. The median (IQR) level of the IFN-γ concentration obtained in the TB Ag tube in all patients was 9.47 (4.51–10.0) IU (international units)/mL.
Table 1.
Demographics and clinical data.
| Total (N = 29) | |
|---|---|
| Age, median (IQR) | 27 (25–38) |
| Male, n (%) | 16 (55.1) |
| BMI, median (IQR) | 20.3 (19.2–21.9) |
| Symptom, n (%) | |
| cough | 13 (44.8) |
| sputum | 15 (51.7) |
| fever | 4 (13.8) |
| weight loss | 3 (10.3) |
| fatigue | 9 (31.0) |
| chest pain | 7 (24.1) |
| dyspnea | 3 (10.3) |
| hemoptysis | 3 (10.3) |
| others | 1 (3.4) |
| Chest CT finding, n (%) | |
| centrilobular | 9 (31.0) |
| consolidation | 11 (37.9) |
| cavity + centrilobular | 8 (27.6) |
| mass | 1 (3.4) |
| Extent of the disease in chest CT, the number of lobes (%) | |
| One lobe | 20 (69.0) |
| 2–3 lobes | 9 (31.0) |
| >3 lobes | 0 |
| Blood tests, median (IQR) | |
| WBC count (/µL) | 6990.0 (5750.0–8380.0) |
| Hemoglobin level (g/dL) | 13.8 (12.3–14.9) |
| Platelet count (×103/µL) | 292.0 (251.0–387.0) |
| Sputum exam, n (%) | |
| AFB smear, positive | 3 (10.3) |
| TB culture, positive | 22 (75.9) |
| sputum conversion | 22 (100.0) |
| QFT-GIT assay, median (IQR) | |
| Nil | 0.21 (0.13–0.28) |
| TB Ag | 9.47 (4.51–10.00) |
| mitogen | 10.00 (10.00–10.00) |
| TB Ag-Nil | 8.6 (4.2–10.00) |
| Extrapulmonary TB, n (%) | 1 (3.4) |
Multiple cytokine responses to TB –specific antigens before, after 2 months, and after treatment of active pulmonary TB
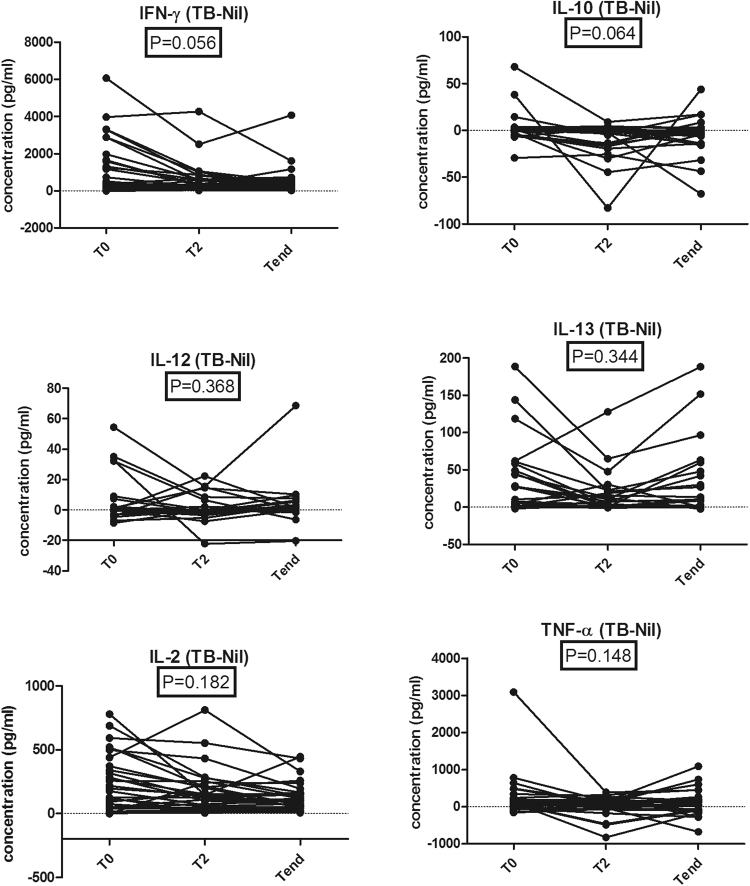
We measured the levels of multiple cytokines at the major time points of anti-TB treatment for active pulmonary TB (Fig. 1). The response was calculated by subtracting the level in the Nil sample from that in the TB Ag sample. The mean changes from T0 to Tend of IFN-γ, IL-10, IL-12, IL-13, IL-2 and TNF-α were −770.21 pg/ml (95% CI, −1150.72 to −389.69), −7.52 pg/ml (95% CI, −14.05 to −1.00), −1.80 pg/ml (95% CI, −7.17 to 3.57), −5.09 pg/ml (95% CI, −21.39 to 11.20), −118.37 pg/ml (95% CI, −180.69 to −56.05), and −176.36 pg/ml (95% CI, −389.22 to 36.48). The proportions of subjects that achieved these change for IFN-γ, IL-10, IL-12, IL-13, IL-2, and TNF-α were 65.5%, 68.9%, 55.1%, 62.0%, 58.6%, and 62.0%.
Figure 1.
The changes of levels of IFN-γ, IL-10, IL-12, IL-13, IL-2 and TNF-α in supernatants of QFT-GIT before (T0), after 2 months (T2), and at the end of anti-TB treatment (Tend).
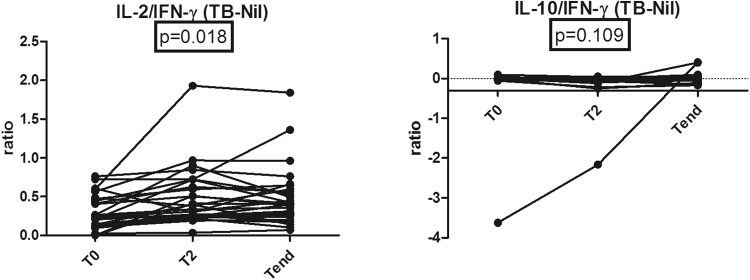
The median decreases in IFN-γ, IL-10, IL-12, IL-13, IL-2, and TNF-α levels were not significant (Fig. 1). In addition the mean changes from T0 to Tend of IL-2/IFN-γ ratio and IL-10/IFN-γ were 0.19 (95% CI, 0.07 to 0.30) and 0.12 (95% CI, −0.15 to 0.41). The proportions of subjects that achieved these change for IL-2/IFN-γ ratio and IL-10/IFN-γ were 24.1% and 27.5%. The IL-2/IFN-γ ratio measured in the supernatant after treatment was significantly increased compared with baseline (P = 0.018), whereas the median decrease in IL-10/IFN-γ ratio not significant (Fig. 2).
Figure 2.
The changes of IL-2/IFN-γ and IL-10/IFN-γ ratios in supernatant of QFT-GIT during anti-TB treatment.
Inflammatory markers in peripheral blood before, at 2 months, and after treatment of active pulmonary TB
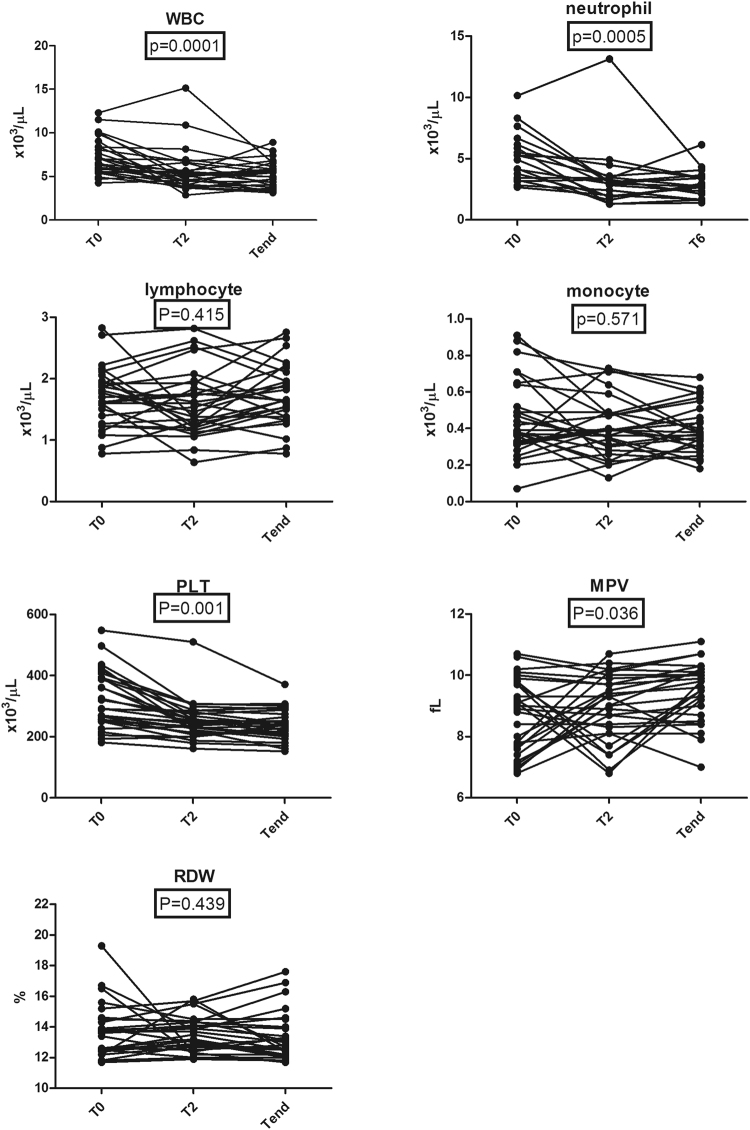
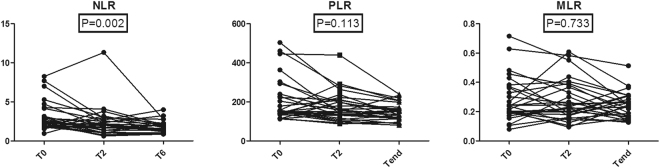
The mean changes from T0 to Tend of WBC, neutrophil, lymphocyte, monocyte, platelet, MPV, and RDW were −1.93 × 103/μL (95% CI, −2.64 to −1.22), −1.77 × 103/μL (95% CI, −2.44 to −1.09), 0.03 × 103/μL (95% CI, −0.13 to 0.19), −0.05 × 103/μL (95% CI, −0.12 to 0.01), −80.75 × 103/μL (95% CI, −110.80 to −50.71), 0.77 fL (95% CI, 0.24 to 1.30), and −0.41% (95% CI, −1.16 to 0.34). The proportions of subjects that achieved these change for WBC, neutrophil, lymphocyte, monocyte, platelet, MPV, and RDW were 55.1%, 57.1%, 42.8%, 53.5%, 58.6%, 35.7%, and 64.2%. Compared with baseline levels, the WBC, neutrophil, and platelet counts decreased in response to treatment (P = 0.0001, P = 0.0005, and P = 0.001, respectively) (Fig. 3). The decreases in the median lymphocyte count and monocyte count were not statistically significant (Fig. 3). In addition, The mean changes from T0 to Tend of NLR, PLR, and MLR were −1.34 (95% CI, −1.99 to −0.69), −59.21 (95% CI, −95.90 to −22.52), and −0.04 (95% CI, −0.09 to 0.01). The proportions of subjects that achieved these change for NLR, PLR, and MLR were 67.8%, 60.7%, and 64.2%. The NLR after treatment was significantly decreased compared with baseline (P = 0.002) (Fig. 4). The PLR and MLR were not significantly changed (Fig. 4).
Figure 3.
The changes of serum inflammatory markers during anti-TB treatment.
Figure 4.
The changes of neutrophil to lymphocyte, platelet to lymphocyte, and monocyte to lymphocyte ratios during anti-TB treatment.
Discussion
This study investigated the changes in multiple cytokine and inflammatory markers in 29 patients with active TB following anti-TB drug therapy. We found that the WBC, neutrophil, and platelet counts were decreased following anti-TB drug therapy. The IL-2/IFN-γ ratio and NLR were significantly increased after treatment compared with baseline.
Several exploratory studies have evaluated the potential of cytokine biomarkers other than IFN-γ, particularly TNF-α, IL-2, IL-10, and IL-12, for monitoring anti-TB therapy. TNF-α has been one of the most studied cytokines in previous studies. The largest of these studies, by Eum et al., found that TNF-α levels were increased during anti-TB treatment18. However, most studies on TB-Ag-stimulated TNF-α responses to anti-TB treatment have consistently reported a reduction in TNF-α levels in whole blood or peripheral blood mononuclear cells during treatment19,24–26. Those results were not consistent with the findings of our study. However, our study is different in that these markers were investigated in supernatants collected from the QFT-GIT.
IL-10 is an important anti-inflammatory cytokine reported to affect multiple cell types, such as macrophages, monocytes, dendritic cells, CD4+ T cells, and CD8+ T cells27. It inhibits CD4+ T cell responses by inhibiting the antigen-presenting cell function of TB-infected cells28. Recently, the secretion of Mycobacterium tuberculosis (MTB)-enhanced intracellular survival protein from MTB cells was reported, which possibly increases IL-10 expression29. Of the studies that have evaluated the role of IL-10 in TB infection, the findings are not consistent18,30–32. The study by Eum et al. reported that IL-10 levels increased with treatment18. However, another large longitudinal study by Sai Priya et al. found a reduction in IL-10 levels in response to treatment30. In the current study, we found IL-10 responses to TB specific antigens showed the tendency of decrement following anti-TB therapy. This decrease may be due to modified cytokine expression in infected individuals after treatment.
Several studies have assessed the role of IL-2, which is produced by TB-specific polyfunctional T cells, in monitoring anti-TB treatment responses. Two studies reported lower IL-2 levels at the end compared with the start of treatment19,33. Furthermore, Mattos et al. reported an increase in IL-2-producing TB-specific T-cells during treatment34. In the current study, the changes observed in IL-2 levels were not significant during treatment. However, the IL-2/IFN-γ ratio was significantly increased after treatment. Thus, evaluation of a combination of cytokine biomarkers might be the alternative way to monitor the treatment responses instead of the single cytokine response. Simultaneous measurements of IFN-γ and IL-2 levels help determine T cell cytokine profiles, which reflect the memory phenotype and comprise three main functional T cell subsets: effector cells secreting primarily IFN-γ only, effector memory cells secreting primarily both IFN-γ and IL-2, and central memory cells secreting IL-2 only35. Casey et al. stated that the proportion of ESAT-6/CFP-10-specific cells secreting IFN-γ only was decreased during treatment. Conversely, the proportion of ESAT-6/CFP-10-specific IFN-γ/IL-2-secreting cells was increased36. The increased IL-2/IFN-γ ratio found in our study can be explained by the dynamics of TB-specific T cells.
Several studies have investigated the responses of IL-12 during anti-TB treatment. Eum et al. found an increase in IL-12 levels after 2 months of treatment, followed by a decrease to baseline levels after 6 months18, whereas Sai Priya et al. found a reduction in IL-12 levels over the treatment course30. In the current study, the decrease in median IL-12 levels was not statistically significant.
Inflammation plays a significant role in the pathogenesis of pulmonary TB. Recently, the relationships between inflammatory markers, such as neutrophil and platelet counts, and TB have been reported. Several studies demonstrated an important contribution of poly-morphonuclear neutrophils (PMN) to the pathogenesis of TB. Eum et al. identified PMNs as the primary immune cell population in sputum and bronchoalveolar lavage (BAL) samples from patients infected with active pulmonary TB37. Furthermore, PMN in sputum, BAL, and pulmonary cavity samples carried the greatest mycobacterial loads37.
The NLR has emerged as a biomarker of inflammation38,39. There are several studies regarding the relationship between chronic obstructive pulmonary disease, TB, or pneumonia and the NLR38,40–43. Iliaz et al. found a higher NLR in patients with advanced pulmonary TB as opposed to patients with mild to moderate pulmonary TB38. Additionally, Abakay et al. found reported a significantly higher WBC count, neutrophil count, RDW, and NLR in patients with advanced pulmonary TB compared with mild to moderate pulmonary TB44. However, the change in the NLR in TB patients following anti-TB therapy has not been fully evaluated. In the current study, we found that the NLR was significantly decreased after treatment compared with baseline. The NLR is easily determined from routine laboratory tests and is easier and less expensive to measure compared with other inflammatory biomarkers. Therefore, the NLR might be used as a biomarker to evaluate the response to anti-TB treatment.
There are several limitations to this study. First, the number of participants was relatively small, and cases of relapse or treatment failure were not analyzed in this study. Further prospective cohort studies involving larger study samples with variable treatment outcomes are needed. More validation studies are needed in the future which are conducted in other settings. Second, several potential biomarkers such as IP-10, monocyte chemotactic protein 2, and epidermal growth factor were not measured in this study. The studies for other candidate markers or sample types other than Quantiferon supernatants are needed in the future. Third, because of the small numbers of subjects and the absence of a drug resistant cohort, subjects with non-TB pneumonia were not studied as a control group. Further prospective studies involving subjects with non-TB pneumonia are needed.
In conclusion, the current study showed that WBC, neutrophil, and platelet counts were decreased following anti-TB therapy. The IL-2/IFN-γ ratio and the NLR might be potential biomarkers to evaluate the effectiveness of drug therapy in active TB patients.
Electronic supplementary material
Acknowledgements
This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1324). The funding source had no role in the study process, including the design, sample collection, analysis, and interpretation of the results.
Author Contributions
Ah Young Leem, Joo Han Song, and Young Ae Kang conceived and designed the experiments; Hyejon Lee, Bora Sim, Eun Hye Lee, Song Yee Kim, Kyung Soo Chung, and Eun Young Kim performed the experiments; Ji Ye Jung, Moo Suk Park and Young Sam Kim analyzed the data; Joon Chang contributed reagents/materials/analysis tools; Ah Young Leem, and Young Ae Kang wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Ah Young Leem and Joo Han Song contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19523-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 2.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldsack L, Kirman JR. Half-truths and selective memory: Interferon gamma, CD4(+) T cells and protective memory against tuberculosis. Tuberculosis (Edinb) 2007;87:465–473. doi: 10.1016/j.tube.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Keane J, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 5.Verreck FA, et al. Human host defense and cytokines in mycobacterial infectious diseases: interleukin-18 cannot compensate for genetic defects in the interleukin-12 system. Clin Infect Dis. 2002;35:210–212. doi: 10.1086/341314. [DOI] [PubMed] [Google Scholar]
- 6.Wei M, et al. Regulation network of serum cytokines induced by tuberculosis-specific antigens reveals biomarkers for tuberculosis diagnosis. Genet Mol Res. 2015;14:17182–17192. doi: 10.4238/2015.December.16.18. [DOI] [PubMed] [Google Scholar]
- 7.Lindenstrom T, Knudsen NP, Agger EM, Andersen P. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. J Immunol. 2013;190:6311–6319. doi: 10.4049/jimmunol.1300248. [DOI] [PubMed] [Google Scholar]
- 8.Suter-Riniker F, et al. Clinical significance of interleukin-2/gamma interferon ratios in Mycobacterium tuberculosis-specific T-cell signatures. Clin Vaccine Immunol. 2011;18:1395–1396. doi: 10.1128/CVI.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goletti D, et al. Response to M. tuberculosis selected RD1 peptides in Ugandan HIV-infected patients with smear positive pulmonary tuberculosis: a pilot study. BMC Infect Dis. 2008;8:11. doi: 10.1186/1471-2334-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi K, Harada N, Mori T. Interferon-gamma responses after isoniazid chemotherapy for latent tuberculosis. Respirology. 2008;13:468–472. doi: 10.1111/j.1440-1843.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- 11.Aiken AM, et al. Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases. BMC Infect Dis. 2006;6:66. doi: 10.1186/1471-2334-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connell TG, et al. Reversion and conversion of Mycobacterium tuberculosis IFN-gamma ELISpot results during anti-tuberculous treatment in HIV-infected children. BMC Infect Dis. 2010;10:138. doi: 10.1186/1471-2334-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pai M, et al. Serial testing of health care workers for tuberculosis using interferon-gamma assay. Am J Respir Crit Care Med. 2006;174:349–355. doi: 10.1164/rccm.200604-472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosshard V, et al. Do results of the T-SPOT.TB interferon-gamma release assay change after treatment of tuberculosis? Respir Med. 2009;103:30–34. doi: 10.1016/j.rmed.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Vincenti D, et al. Identification of early secretory antigen target-6 epitopes for the immunodiagnosis of active tuberculosis. Mol Med. 2003;9:105–111. [PMC free article] [PubMed] [Google Scholar]
- 16.Goletti D, et al. Selected RD1 peptides for active tuberculosis diagnosis: comparison of a gamma interferon whole-blood enzyme-linked immunosorbent assay and an enzyme-linked immunospot assay. Clin Diagn Lab Immunol. 2005;12:1311–1316. doi: 10.1128/CDLI.12.11.1311-1316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goletti D, et al. Accuracy of immunodiagnostic tests for active tuberculosis using single and combined results: a multicenter TBNET-Study. PLoS One. 2008;3:e3417. doi: 10.1371/journal.pone.0003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eum SY, et al. Association of antigen-stimulated release of tumor necrosis factor-alpha in whole blood with response to chemotherapy in patients with pulmonary multidrug-resistant tuberculosis. Respiration. 2010;80:275–284. doi: 10.1159/000283687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petruccioli E, et al. IFNgamma/TNFalpha specific-cells and effector memory phenotype associate with active tuberculosis. J Infect. 2013;66:475–486. doi: 10.1016/j.jinf.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Kabeer BS, et al. IP-10 response to RD1 antigens might be a useful biomarker for monitoring tuberculosis therapy. BMC Infect Dis. 2011;11:135. doi: 10.1186/1471-2334-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djoba Siawaya JF, Beyers N, van Helden P, Walzl G. Differential cytokine secretion and early treatment response in patients with pulmonary tuberculosis. Clin Exp Immunol. 2009;156:69–77. doi: 10.1111/j.1365-2249.2009.03875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs R, et al. Identification of novel host biomarkers in plasma as candidates for the immunodiagnosis of tuberculosis disease and monitoring of tuberculosis treatment response. Oncotarget. 2016;7:57581–57592. doi: 10.18632/oncotarget.11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SY, et al. The responses of multiple cytokines following incubation of whole blood from TB patients, latently infected individuals and controls with the TB antigens ESAT-6, CFP-10 and TB7.7. Scand J Immunol. 2012;76:580–586. doi: 10.1111/j.1365-3083.2012.02776.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim CH, et al. Comparative analysis of whole-blood interferon-gamma and flow. cytometry assays for detecting post-treatment immune responses in patients with active tuberculosis. Cytometry B Clin Cytom. 2014;86:236–243. doi: 10.1002/cyto.b.21110. [DOI] [PubMed] [Google Scholar]
- 25.Mattos AM, et al. Increased IgG1, IFN-gamma, TNF-alpha and IL-6 responses to Mycobacterium tuberculosis antigens in patients with tuberculosis are lower after chemotherapy. Int Immunol. 2010;22:775–782. doi: 10.1093/intimm/dxq429. [DOI] [PubMed] [Google Scholar]
- 26.Young JM, Adetifa IM, Ota MO, Sutherland JS. Expanded polyfunctional T cell response to mycobacterial antigens in TB disease and contraction post-treatment. PLoS One. 2010;5:e11237. doi: 10.1371/journal.pone.0011237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore KW, de W Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 28.Rojas M, Olivier M, Gros P, Barrera LF, Garcia LF. TNF-alpha and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J Immunol. 1999;162:6122–6131. [PubMed] [Google Scholar]
- 29.Duan L, Yi M, Chen J, Li S, Chen W. Mycobacterium tuberculosis EIS gene inhibits macrophage autophagy through up-regulation of IL-10 by increasing the acetylation of histone H3. Biochem Biophys Res Commun. 2016;473:1229–1234. doi: 10.1016/j.bbrc.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 30.Sai Priya VH, et al. In vitro levels of interleukin 10 (IL-10) and IL-12 in response to a recombinant 32-kilodalton antigen of Mycobacterium bovis BCG after treatment for tuberculosis. Clin Vaccine Immunol. 2009;16:111–115. doi: 10.1128/CVI.00243-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A, Dey AB, Mohan A, Sharma PK, Mitra DK. Foxp3+ regulatory T cells among tuberculosis patients: impact on prognosis and restoration of antigen specific IFN-gamma producing T cells. PLoS One. 2012;7:e44728. doi: 10.1371/journal.pone.0044728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres M, Herrera T, Villareal H, Rich EA, Sada E. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 1998;66:176–180. doi: 10.1128/iai.66.1.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiacchio T, et al. Higher frequency of T-cell response to M. tuberculosis latency antigen Rv2628 at the site of active tuberculosis disease than in peripheral blood. PLoS One. 2011;6:e27539. doi: 10.1371/journal.pone.0027539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millington KA, et al. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J Immunol. 2007;178:5217–5226. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 36.Casey R, et al. Enumeration of functional T-cell subsets by fluorescence-immunospot defines signatures of pathogen burden in tuberculosis. PLoS One. 2010;5:e15619. doi: 10.1371/journal.pone.0015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eum SY, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–128. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iliaz S, et al. Value of neutrophil/lymphocyte ratio in the differential diagnosis of sarcoidosis and tuberculosis. Ann Thorac Med. 2014;9:232–235. doi: 10.4103/1817-1737.140135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 40.Gunay E, et al. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: a retrospective study. Inflammation. 2014;37:374–380. doi: 10.1007/s10753-013-9749-1. [DOI] [PubMed] [Google Scholar]
- 41.Yoon NB, Son C, Um SJ. Role of the neutrophil-lymphocyte count ratio in the differential diagnosis between pulmonary tuberculosis and bacterial community-acquired pneumonia. Ann Lab Med. 2013;33:105–110. doi: 10.3343/alm.2013.33.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jager CP, et al. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One. 2012;7:e46561. doi: 10.1371/journal.pone.0046561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diacon AH, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J. 2003;22:589–591. doi: 10.1183/09031936.03.00017103a. [DOI] [PubMed] [Google Scholar]
- 44.Abakay O, Abakay A, Sen HS, Tanrikulu AC. The relationship between inflammatory marker levels and pulmonary tuberculosis severity. Inflammation. 2015;38:691–696. doi: 10.1007/s10753-014-9978-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.