Abstract
Anemia is common in colorectal cancer (CRC) but its relationships with tumor characteristics, systemic inflammation, and survival have not been well characterized. In this study, blood hemoglobin levels and erythrocyte mean corpuscular volume (MCV) levels were measured in two independent cohorts of 148 CRC patients and 208 CRC patients, and their correlation with patient and tumor characteristics, systemic inflammatory markers (modified Glasgow Prognostic Score: mGPS; serum levels of thirteen cytokines, C-reactive protein, albumin), and survival were analyzed. We found that anemia, most frequently normocytic, followed by microcytic, was present in 43% of the patients. Microcytic anemia was most commonly associated with proximal colon tumor location. Average MCV and blood hemoglobin levels were lower in tumors with high T-class. Low blood hemoglobin associated with systemic inflammation, including high mGPS and high serum levels of C-reactive protein and IL-8. Particularly, normocytic anemia associated with higher mGPS. Normocytic anemia associated with a tendency towards worse overall survival (multivariate hazard ratio 1.61, 95% confidence interval 1.07–2.42, p = 0.023; borderline statistical significance considering multiple hypothesis testing). In conclusion, anemia in CRC patients is most frequently normocytic. Proximal tumor location is associated with predominantly microcytic anemia and systemic inflammation is associated with normocytic anemia.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies and causes of cancer deaths in the Western world1. CRC patients frequently have anemia at the time of the diagnosis, and anemia is one of the reasons why CRC patients enter the primary care2. Previously, anemia has been reported to be more common in CRC patients with tumors in proximal colon and of advanced stage3–6. Several studies have assessed the prognostic or predictive value of anemia in various CRC subgroups7–20, and reported an association between anemia and adverse outcome8–11,13,16,17,19. However, more data is needed, especially, of the prognostic significance of anemia relative to several important potentially confounding prognostic parameters, including tumor, node, metastasis (TNM) classification, lymphatic and venous invasion, and systemic inflammation, as well as of the prognostic value of different anemia subgroups.
Based on the erythrocyte mean corpuscular volume (MCV), anemia can be categorized as microcytic (MCV < 80 fL), normocytic (MCV 80–100 fL) or macrocytic (MCV > 100 fL)21. Microcytic anemia is most commonly due to iron deficiency (other, less common causes include thalassemia and anemia of chronic diseases), while the differential diagnosis of normocytic and macrocytic anemia is more diverse21. Of the factors associated with low MCV, thalassemias are rare in the Finnish population22. One of the main causes of anemia in CRC patients is blood loss to the bowel leading to iron deficiency23. Indeed, anemia in CRC has been reported to frequently show microcytic phenotype, especially in higher stages3. However, there are few studies that have assessed the relationships between clinical and histological findings of CRC and different anemia subgroups3.
In addition to CRC, anemia is also prevalent in other malignancies, in which no iron is lost into feces5,7. Anemia of inflammation, also known as anemia of chronic disease, is associated with increased circulating cytokine levels, commonly observed in infections, rheumatic and other inflammatory diseases, and cancer24. A proportion of CRC patients present with a systemic inflammatory response, as evidenced by increased serum levels of C-reactive protein (CRP) and decreased serum levels of albumin25,26. The combination of these measurements (modified Glasgow Prognostic Score, mGPS), is associated with adverse prognosis in CRC25. Cytokines, especially IL-6, lead to increased synthesis of CRP and decreased synthesis of albumin in the liver27,28. We have previously shown that CRC patients have increased serum levels of IL-6, IL-7, and IL-8 and decreased serum CCL2 levels26,29. Patients with advanced disease have increased serum levels of IL-1RA, IL-4, IL-6, IL-7, IL-8, CCL2, and PDGF-BB29. A few studies have reported that anemia is more common in CRC patients with increased mGPS14,30. However, to our knowledge, more detailed analyses of the relationships between anemia subgroups, serum cytokines, and other markers of systemic inflammation in CRC have not been conducted.
The objective of this study was to evaluate the determinants and clinical significance of blood hemoglobin (Hb) levels, erythrocyte MCV levels, and different anemia subgroups in two independent prospectively recruited successive cohorts of 148 CRC patients (Cohort 1) and 208 CRC patients (Cohort 2), with special emphasis on the relationships between blood Hb and the systemic inflammatory response.
Results
General characteristics
The average Hb level was 126.7 g/L (SD 17.3 g/L) in Cohort 1 and 126.5 g/L (SD 17.4 g/L) in Cohort 2 (Table 1), and average MCV was 88.6 fL (SD 7.0 fL) in Cohort 1 and 86.6 fL (SD 6.3 fL) in Cohort 2 (Table 1). A total of 57 (38.5%) patients in Cohort 1 and 97 (46.6%) patients in Cohort 2 had anemia (Tables S1 and S2), and it was most frequently normocytic (Cohort 1: 42 patients, 28.3%; Cohort 2: 67 patients, 32.2%), followed by microcytic (Cohort 1: 14 patients, 9.5%; Cohort 2: 29 patients, 13.9%). Only one patient in both cohorts had macrocytic anemia. Since the basic characteristics, as well as the main findings of the associations between blood Hb levels and clinicopathological variables (Tables S3 and S4), were similar in both cohorts, we combined the two cohorts for subsequent analyses, to increase the statistical power of the analyses.
Table 1.
Characteristics of the colorectal cancer patients.
| Cohort 1 (n = 148) | Cohort 2 (n = 208) | |
|---|---|---|
| Age, mean (SD) | 66.7 (11.1) | 69.2 (12.2) |
| Sex | ||
| Male | 80 (54.1%) | 110 (52.9%) |
| Female | 68 (45.9%) | 98 (47.1%) |
| Tumor location | ||
| Proximal colon | 48 (32.4%) | 75 (36.1%) |
| Distal colon | 28 (18.9%) | 45 (21.6%) |
| Rectum | 72 (48.6%) | 88 (42.3%) |
| Preoperative radiotherapy or chemoradiotherapy | ||
| No | 116 (78.4%) | 170 (81.7%) |
| Yes | 32 (21.6%) | 38 (18.3%) |
| WHO grade | ||
| Grade 1 | 21 (14.3%) | 58 (28.0%) |
| Grade 2 | 108 (73.5%) | 121 (58.5%) |
| Grade 3 | 18 (12.2%) | 28 (13.5%) |
| TNM Stage | ||
| Stage I | 27 (18.4%) | 54 (26.0%) |
| Stage II | 54 (36.7%) | 59 (28.4%) |
| Stage III | 44 (29.9%) | 71 (34.1%) |
| Stage IV | 22 (15.0%) | 24 (11.5%) |
| Blood hemoglobin, g/L, mean (SD) | 126.7 (17.3) | 126.5 (17.4) |
| Erythrocyte mean corpuscular volume (MCV), fL, mean (SD) | 88.6 (7.0) | 86.6 (6.3) |
Relationships between anemia and clinicopathological features
In the combined cohort, decreased Hb levels associated with female gender (Cohort 1: p < 0.001; Table 2). However, anemia was approximately as prevalent in male patients as in female patients (Table 3). Older patients had a tendency towards lower blood Hb levels (p = 0.0017; borderline statistical significance considering multiple hypothesis testing). Lower blood Hb associated with proximal tumor location (p < 0.001; Table 2). Particularly, microcytic anemia was common in patients with proximal colon tumors (Table 3) and average MCV was lower in subjects with proximal colon carcinomas (p < 0.001; Table 2). Preoperative RT/CRT was considered potential confounding factor. However, blood Hb levels (p = 0.986) or erythrocyte MCV levels (p = 0.636) of the rectal cancer patients who received preoperative RT/CRT did not differ from other rectal cancer patients (Table 2).
Table 2.
Relationships between blood hemoglobin (Hb) levels (g/L), erythrocyte mean corpuscular volume (MCV) levels (fL) and clinicopathological characteristics in the combined cohort.
| Variable (n) | Blood Hb, Mean (SD) | P value | Erythrocyte MCV, Mean (SD) | P value |
|---|---|---|---|---|
| All Patients (n = 356) | 126.6 (17.3) | 87.5 (6.7) | ||
| Age | ||||
| <65 (n = 130) | 129.5 (17.4) | 0.017 | 87.4 (7.1) | 0.908 |
| ≥65 (n = 226) | 124.9 (17.1) | 87.5 (6.4) | ||
| Sex | ||||
| Male (n = 190) | 130.8 (18.4) | <0.001 | 88.1 (7.0) | 0.063 |
| Female (n = 166) | 121.8 (14.7) | 86.8 (6.2) | ||
| Location of tumor | ||||
| Proximal colon (n = 123) | 116.4 (15.5) | <0.001 | 84.5 (7.0) | <0.001 |
| Distal colon (n = 73) | 125.1 (15.9) | 86.8 (6.4) | ||
| Rectum (n = 160) | 135.1 (14.8) | 90.1 (5.5) | ||
| Preoperative radiotherapy or chemoradiotherapy in rectal cancer patients | ||||
| No (n = 91) | 135.0 (16.0) | 0.986 | 89.9 (5.1) | 0.636 |
| Yes (n = 69) | 135.1 (13.3) | 90.3 (5.9) | ||
| WHO grade | ||||
| Grade 1 (n = 79) | 126.3 (17.6) | 0.023 | 87.4 (7.5) | 0.582 |
| Grade 2 (n = 229) | 128.0 (17.4) | 87.7 (6.3) | ||
| Grade 3 (n = 46) | 120.4 (15.7) | 85.6 (6.9) | ||
| TNM Stage | ||||
| Stage I (n = 81) | 132.4 (17.4) | <0.001 | 89.7 (6.2) | <0.001 |
| Stage II (n = 113) | 122.9 (17.1) | 85.9 (6.9) | ||
| Stage III (n = 115) | 128.0 (17.3) | 87.8 (6.6) | ||
| Stage IV (n = 46) | 122.3 (14.8) | 86.4 (6.2) | ||
| Primary tumor | ||||
| T1 (n = 16) | 136.4 (15.8) | <0.001 | 91.7 (5.7) | <0.001 |
| T2 (n = 89) | 131.5 (16.6) | 89.6 (6.1) | ||
| T3 (n = 218) | 125.1 (16.9) | 86.6 (6.8) | ||
| T4 (n = 32) | 119.0 (18.3) | 85.8 (5.8) | ||
| Lymph node metastasis | ||||
| N0 (n = 200) | 126.6 (17.7) | 0.135 | 87.5 (6.9) | 0.588 |
| N1 (n = 97) | 129.0 (16.8) | 87.9 (6.1) | ||
| N2 (n = 57) | 123.2 (16.7) | 86.7 (6.9) | ||
| Distant Metastasis | ||||
| M0 (n = 310) | 127.2 (17.6) | 0.072 | 87.6 (6.7) | 0.271 |
| M1 (n = 46) | 122.3 (14.8) | 86.4 (6.2) | ||
| Infiltrative growth pattern | ||||
| No (n = 278) | 127.1 (17.3) | 0.382 | 87.6 (6.5) | 0.475 |
| Yes (n = 77) | 125.1 (17.6) | 87.0 (7.4) | ||
| Lymphatic invasion | ||||
| No (n = 192) | 127.0 (17.4) | 0.698 | 87.8 (6.7) | 0.303 |
| Yes (n = 160) | 126.3 (17.4) | 87.1 (6.6) | ||
| Blood vessel invasion | ||||
| No (n = 293) | 126.9 (17.4) | 0.567 | 87.5 (6.8) | 0.867 |
| Yes (n = 59) | 125.5 (17.5) | 87.4 (6.0) | ||
| Mismatch repair (MMR) enzyme status | ||||
| MMR Proficient (n = 315) | 127.8 (17.0) | <0.001 | 85.1 (6.2) | 0.016 |
| MMR Deficient (n = 40) | 117.2 (17.2) | 87.8 (6.7) | ||
| BRAF VE1 immunohistochemistry | ||||
| Negative (n = 322) | 127.3 (17.5) | 0.010 | 87.5 (6.7) | 0.234 |
| Positive (n = 33) | 119.2 (14.8) | 86.1 (6.4) | ||
| Modified Glasgow Prognostic Score (mGPS) | ||||
| 0 (n = 269) | 128.8 (17.2) | <0.001 | 88.1 (6.8) | 0.018 |
| 1 (n = 63) | 120.0 (15.8) | 85.5 (6.2) | ||
| 2 (n = 8) | 105.5 (5.8) | 86.2 (2.7) | ||
| Mean corpuscular volume (MCV) | ||||
| <80 (n = 45) | 107.3 (11.0) | <0.001 | ||
| 80–100 (n = 306) | 129.4 (16.4) | |||
| >100 (n = 5) | 130.4 (14.0) | |||
Table 3.
Relationships between different categories of anemia and clinicopathological characteristics in the combined cohort.
| Variable | No anemia (n = 202) | Microcytic anemia (n = 43) | Normocytic anemia (n = 109) | P value |
|---|---|---|---|---|
| Age | ||||
| <65 | 82 (63.1%) | 14 (10.8%) | 34 (26.2%) | 0.223 |
| ≥65 | 120 (53.6%) | 29 (12.9%) | 75 (33.5%) | |
| Sex | ||||
| Male | 107 (56.9%) | 27 (14.4%) | 54 (28.7%) | 0.335 |
| Female | 95 (57.2%) | 16 (9.6%) | 55 (33.1%) | |
| Location of tumor | ||||
| Proximal colon | 39 (31.7%) | 28 (22.8%) | 56 (45.5%) | <0.001 |
| Distal colon | 40 (54.8%) | 10 (13.7%) | 23 (31.5%) | |
| Rectum | 123 (77.8%) | 5 (3.2%) | 30 (19.0%) | |
| Preoperative radiotherapy or chemoradiotherapy in rectal cancer patients | ||||
| No | 69 (76.7%) | 3 (3.3%) | 18 (20.0%) | 0.944 |
| Yes | 54 (79.4%) | 2 (2.9%) | 12 (17.6%) | |
| WHO grade | ||||
| Grade 1 | 42 (54.5%) | 14 (18.2%) | 21 (27.3%) | 0.139 |
| Grade 2 | 139 (60.7%) | 23 (10.0%) | 67 (29.3%) | |
| Grade 3 | 21 (45.7%) | 6 (13.0%) | 19 (41.3%) | |
| TNM Stage | ||||
| Stage I | 53 (65.4%) | 5 (6.2%) | 23 (28.4%) | 0.004 |
| Stage II | 50 (44.2%) | 22 (19.5%) | 41 (36.3%) | |
| Stage III | 76 (66.7%) | 12 (10.5%) | 26 (22.8%) | |
| Stage IV | 23 (51.1%) | 4 (8.9%) | 18 (40.0%) | |
| Primary tumor | ||||
| T1 | 12 (80.0%) | 0 (0%) | 3 (20.0%) | 0.007 |
| T2 | 59 (66.3%) | 5 (5.6%) | 25 (28.1%) | |
| T3 | 120 (55.3%) | 33 (15.2%) | 64 (29.5%) | |
| T4 | 11 (34.4%) | 5 (15.6%) | 16 (50.0%) | |
| Lymph node metastasis | ||||
| N0 | 105 (52.8%) | 28 (14.1%) | 66 (33.2%) | 0.159 |
| N1 | 65 (67.7%) | 7 (7.3%) | 24 (25.0%) | |
| N2 | 32 (56.1%) | 8 (14.0%) | 17 (29.8%) | |
| Distant Metastasis | ||||
| M0 | 179 (57.9%) | 39 (12.6%) | 91 (29.4%) | 0.322 |
| M1 | 23 (51.1%) | 4 (8.9%) | 18 (40.0%) | |
| Infiltrative growth pattern | ||||
| No | 158 (57.2%) | 32 (11.6%) | 86 (31.2%) | 0.792 |
| Yes | 44 (57.1%) | 11 (14.3%) | 22 (28.6%) | |
| Lymphatic invasion | ||||
| No | 107 (56.0%) | 24 (12.6%) | 60 (31.4%) | 0.894 |
| Yes | 93 (58.5%) | 18 (11.3%) | 48 (30.23%) | |
| Blood vessel invasion | ||||
| No | 167 (57.4%) | 36 (12.4%) | 88 (30.2%) | 0.821 |
| Yes | 33 (55.9%) | 6 (10.2%) | 20 (33.9%) | |
| Mismatch repair (MMR) enzyme status | ||||
| MMR Proficient | 191 (61.0%) | 34 (10.9%) | 88 (28.1%) | <0.001 |
| MMR Deficient | 11 (27.5%) | 9 (22.5%) | 20 (50.0%) | |
| BRAF VE1 immunohistochemistry | ||||
| Negative | 188 (58.8%) | 38 (11.9%) | 94 (29.4%) | 0.095 |
| Positive | 13 (39.4%) | 5 (15.2%) | 15 (45.5%) | |
| Modified Glasgow Prognostic Score (mGPS) | ||||
| 0 | 168 (62.9%) | 30 (11.2%) | 69 (25.8%) | <0.001 |
| 1 | 25 (39.7%) | 9 (14.3%) | 29 (46.0%) | |
| 2 | 0 (0%) | 0 (0%) | 8 (100%) | |
Due to the small number of macrocytic anemia cases (n = 2), macrocytic anemia category was not included in the analysis.
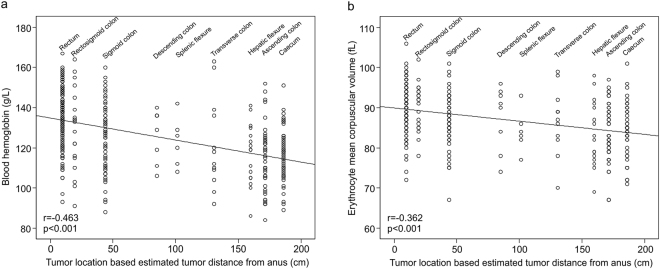
To study the association between tumor location and blood Hb levels in more detail, we recoded the tumor location into a continuous variable, based on average distance of each subsite to anus, utilizing recent computed tomography colonography data31. This approach has been successfully applied previously32. This estimate (tumor location based estimated tumor distance from anus) had moderate negative correlation with blood hemoglobin levels (p < 0.001) and erythrocyte MCV levels (p < 0.001; Fig. 1).
Figure 1.
Correlation between tumor location and blood hemoglobin levels (a) and erythrocyte mean corpuscular volume (MCV) levels (b) in the combined colorectal cancer cohort (n = 356). Utilizing CT colonography data31, tumor location was coded into an estimation of tumor distance from anus. This estimation had linear negative correlation with blood hemoglobin levels and erythrocyte MCV levels.
Of the other clinicopathological variables, decreased Hb levels associated with advanced TNM stage (p < 0.001), especially higher T-class (p < 0.001; Table 2). Average MCV was lower in tumors with higher T-class (p < 0.001; Table 2), suggesting higher prevalence of iron deficiency in these patients.
Both BRAF mutation and MMR deficiency are molecular features commonly associated with serrated pathway of CRC and have been reported to be common in tumors in proximal colon33. We therefore hypothesized that these molecular features could associate with anemia in CRC. Indeed both microcytic and normocytic anemia were more common in the patients with MMR deficient tumors relative to the patients with MMR proficient tumors (p < 0.001; Table 3). BRAF mutation (p = 0.010; borderline statistical significance considering multiple hypothesis testing) associated with a tendency towards lower Hb levels, while MMR deficiency associated with significantly lower blood Hb (p < 0.001; Table 2). However, when the analysis was restricted to the tumors in proximal colon (Table S5), there were no significant associations between anemia and MMR enzyme status (p = 0.577) or BRAF mutation (p = 0.885), suggesting that the association between proximal tumor location and MMR deficiency (and BRAF mutation) could mainly account for the observed association between MMR deficiency and anemia.
There were no significant associations between blood Hb levels and infiltrative tumor growth, lymphatic invasion or blood vessel invasion.
Relationships between anemia, serum cytokine levels, and systemic inflammation
A major hypothesis of the study was that anemia would be associated with systemic inflammation in CRC. Supporting the hypothesis, blood Hb negatively correlated with mGPS (p < 0.001; Table 2) and serum C-reactive protein (univariate p < 0.001; tumor stage and location and patient gender adjusted p = 0.012; borderline statistical significance considering multiple hypothesis testing; Table S6) and positively correlated with serum albumin (univariate p < 0.001; tumor stage and location and patient gender adjusted p < 0.001). Higher mGPS associated with predominantly normocytic anemia (p < 0.001; Table 3).
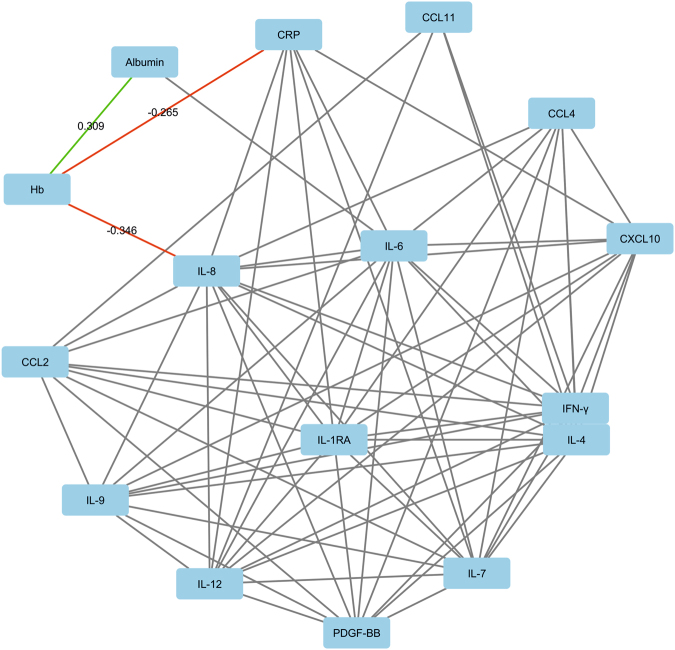
Serum analysis of thirteen cytokines was conducted in Cohort 1, and blood Hb negatively correlated with serum IL-8 (univariate p < 0.001; tumor stage and location and patient gender adjusted p = 0.009; borderline statistical significance considering multiple hypothesis testing; Table S7; Fig. 2). Normocytic anemia was associated with increased serum levels of CRP (p = 0.003; borderline statistical significance considering multiple hypothesis testing; Table S8), and IL-8 (p = 0.001), and decreased serum levels of albumin (p < 0.001), while microcytic anemia did not show significant associations with serum CRP, albumin, or cytokines (Table S8).
Figure 2.
2D visualization of the relationships between blood hemoglobin (Hb), serum C-reactive protein (CRP) levels, serum albumin levels, and serum cytokine levels in Cohort 1. The edges (connecting lines) depict the associations between the variables (only those with p < 0.0015 shown). The edge length illustrates the significance of the association. The correlations between Hb and other variables are represented by green (positive correlation) and red (negative correlation) edges, with the label indicating corresponding Pearson r for the correlation. The other associations are indicated by the grey edges. The 2D visualization was created with Cytoscape software platform55, utilizing the Prefuse force directed algorithm weighted by the statistical significances of the correlations between individual variables. Abbreviations: CCL: Chemokine (C-C motif) ligand; CRP: C-reactive protein; CXCL: Chemokine (C-X-C motif) ligand; Hb: Hemoglobin; IFN: interferon IL: interleukin; PDGF: Platelet-derived growth factor.
Multiple linear regression model for blood hemoglobin levels
A multiple linear regression model was constructed to evaluate the individual contribution of different explanatory variables, assessed in univariate analyses in Table 2 and S6, to blood Hb levels (Table 4). The model indicated that female gender, distal tumor location, higher T-class, and lower serum albumin levels independently associated with decreased blood Hb levels in CRC.
Table 4.
Multiple linear regression model for blood hemoglobin levels in the combined cohort.
| Variable | Beta | p value |
|---|---|---|
| Patient age | −0.107 | 0.023 |
| Patient gender (male vs. female) | −0.163 | <0.001 |
| Tumor location based estimated tumor distance from anus | −0.384 | <0.001 |
| T classification (ordinal categorical; T1, T2, T3, T4) | −0.165 | <0.001 |
| N classification (N0 vs. N1–2) | 0.073 | 0.142 |
| M classification (M0 vs. M1) | −0.033 | 0.507 |
| Mismatch repair (MMR) enzyme status (deficient vs. proficient) | −0.021 | 0.688 |
| Serum C-reactive protein | −0.083 | 0.080 |
| Serum albumin | 0.229 | <0.001 |
Serum C-reactive protein was logarithmically transformed because of positive skewness. R2 = 0.359.
Survival analyses
Finally, survival analyses were carried out to evaluate the prognostic value of blood Hb levels in CRC. In the univariate analyses (Table 5), high blood Hb (≥140 g/L) associated with a tendency towards improved CSS (HR = 0.37, 95% CI = 0.19–0.75; p = 0.005; borderline statistical significance considering multiple hypothesis testing) and OS (HR = 0.46, 95% CI = 0.27–0.78; p = 0.004; borderline statistical significance considering multiple hypothesis testing) and normocytic anemia associated with a tendency towards poor OS (HR = 1.69, 95% CI = 1.15–2.48; p = 0.007; borderline statistical significance considering multiple hypothesis testing). Of these, the association between normocytic anemia and poor OS was highest in multivariate Cox regression models, but it was borderline statistical significance due to multiple hypothesis testing (HR = 1.61, 95% CI = 1.07–2.42, p = 0.023; Table 6).
Table 5.
Univariate analysis of time to recurrence (TTR), cancer-specific survival (CSS), and overall survival (OS) according to blood hemoglobin levels with different cut-off points, erythrocyte mean corpuscular volume (MCV), and different anemia categories.
| Variable | TTRA | CSSB | OSC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Cohort 1 | |||||||||
| Blood hemoglobin | |||||||||
| <110 g/L vs. ≥ 110 g/L | 0.79 | 0.43–1.45 | 0.444 | 0.66 | 0.39–1.12 | 0.119 | 0.64 | 0.42–0.99 | 0.045 |
| <120 g/L vs. ≥ 120 g/L | 1.39 | 0.79–2.45 | 0.257 | 0.70 | 0.44–1.11 | 0.132 | 0.65 | 0.44–0.95 | 0.026 |
| <130 g/L vs. ≥ 130 g/L | 1.30 | 0.79–2.13 | 0.301 | 0.61 | 0.38–0.97 | 0.037 | 0.59 | 0.40–0.87 | 0.008 |
| <140 g/L vs. ≥ 140 g/L | 1.06 | 0.62–1.84 | 0.829 | 0.37 | 0.19–0.75 | 0.005 | 0.46 | 0.27–0.78 | 0.004 |
| Erythrocyte mean corpuscular volume (MCV) | |||||||||
| <80 fL vs. ≥ 80 fL | 1.14 | 0.54–2.40 | 0.726 | 1.16 | 0.58–2.32 | 0.682 | 1.28 | 0.70–2.33 | 0.422 |
| <90 fL vs. ≥ 90 fL | 1.09 | 0.67–1.80 | 0.722 | 0.91 | 0.57–1.44 | 0.683 | 1.12 | 0.77–1.64 | 0.542 |
| Anemia | |||||||||
| No vs. Yes | 0.74 | 0.44–1.24 | 0.252 | 1.26 | 0.80–1.98 | 0.320 | 1.44 | 0.99–2.10 | 0.055 |
| Microcytic anemia | |||||||||
| No vs. Yes | 0.91 | 0.43–1.91 | 0.798 | 0.80 | 0.38–1.66 | 0.546 | 0.75 | 0.40–1.40 | 0.363 |
| Normocytic anemia | |||||||||
| No vs. Yes | 0.67 | 0.36–1.23 | 0.194 | 1.39 | 0.87–2.24 | 0.171 | 1.69 | 1.15–2.48 | 0.007 |
An = 299; median follow-up time 51.2 months (IQR 24.7–71.4); 63 (21.0%) events; 57 (16.0%) cases excluded from the analysis because the operation was not radical or no follow-up data available.
Bn = 354; median follow-up time 56.0 months (IQR 32.9–78.6); 75 (21.2%) events; 2 (0.6%) cases excluded from the analysis because no follow-up data available.
Cn = 356; median follow-up time 56.0 months (IQR 32.9–78.6); 110 (30.9%) events.
Abbreviations: CI: confidence interval; CSS: cancer specific survival; HR: hazard ratio; OS: overall survival; TTR: time to recurrence.
Table 6.
Cox proportional hazard regression models for time to recurrence (TTR), cancer-specific survival (CSS), and overall survival (OS) according to normocytic anemia and clinicopathological characteristics.
| TTR | CSS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Tumor invasion (T1-T2 vs. T3-T4) | 1.92 | 0.94–3.91 | 0.073 | 1.26 | 0.65–2.44 | 0.495 | 1.10 | 0.67–1.80 | 0.720 |
| Nodal metastases (N0 vs. N1-N2) | 5.25 | 2.89–9.56 | <0.001 | 4.08 | 2.15–7.75 | <0.001 | 2.34 | 1.49–3.68 | <0.001 |
| Distant metastases (M0 vs. M1) | — | — | — | 6.77 | 3.94–11.6 | <0.001 | 4.30 | 2.68–6.89 | <0.001 |
| Tumor location (Colon vs. Rectum) | 1.46 | 0.78–2.74 | 0.236 | 0.93 | 0.54–1.60 | 0.791 | 0.91 | 0.57–1.44 | 0.679 |
| Preoperative radiotherapy or chemoradiotherapy (No vs. Yes) | 0.96 | 0.48–1.89 | 0.896 | 1.03 | 0.51–2.09 | 0.930 | 0.97 | 0.54–1.76 | 0.928 |
| Normocytic anemia (No vs. Yes) | 0.89 | 0.47–1.72 | 0.733 | 1.36 | 0.82–2.27 | 0.239 | 1.61 | 1.07–2.42 | 0.023 |
| mGPS (0 vs. 1–2) | 0.63 | 0.27–1.49 | 0.292 | 1.31 | 0.76–2.26 | 0.331 | 1.40 | 0.89–2.19 | 0.145 |
The models aimed to enlighten the prognostic value of normocytic anemia in CRC, relative to TNM variables and systemic inflammation (mGPS). Abbreviations: CI: confidence interval; CSS: cancer specific survival; HR: hazard ratio; mGPS: modified Glasgow Prognostic Score; OS: overall survival; TTR: time to recurrence.
Discussion
To our knowledge, this is so far the most extensive study on the relationships between blood Hb and erythrocyte MCV, CRC patient characteristics, tumor histopathological and molecular features, and survival in CRC. The main findings indicate that decreased blood Hb, and especially normocytic anemia, in CRC is associated with systemic inflammation, while low MCV and microcytic anemia are associated with advanced T-class and proximal tumor location. These findings indicate that in CRC, systemic inflammatory effects are important determinants of blood Hb. However, also the tumor burden itself manifests in anemia and in decrease of red blood cell size.
We found significantly lower blood Hb in patients with tumors in proximal colon relative to distal colon and rectum. This confirms the results of several previous studies3–5. Fecal occult blood screening has been reported to have high sensitivity for the detection of both colon and rectal tumors34, indicating that both colon and rectal cancers frequently bleed into the lumen. The differences between proximal and distal CRC can be mechanistically related to the bleeding, but also other effects – e.g. immunological mechanisms – are needed to be taken into account.
The tumors in the proximal colon often have distinct characteristic genetic properties (particularly, BRAF V600E mutation and MMR deficiency), resulting from the development of serrated precursor lesions through the serrated route of colorectal carcinogenesis33. Interestingly, the patients with MMR deficient tumors had a very high prevalence of anemia (72.5%), and both microcytic and normocytic anemia were overrepresented in MMR deficient subgroup. However, MMR deficient tumors are mostly located in proximal colon, and our subsequent analyses indicated that there was no significant difference in the prevalence of different anemia subtypes between patients with MMR deficient and MMR proficient tumors in proximal colon. Moreover, MMR deficiency was not a significant predictor of blood hemoglobin levels in multiple linear regression. Nevertheless, the number of MMR deficient cases in this study was rather small (n = 40), and further research is required to reliably analyze blood Hb levels in MMR deficient cases of different tumor locations.
Our results indicate that blood Hb levels in CRC inversely associate with systemic inflammation. High mGPS, high serum IL-8, and low serum albumin, particularly, associated with normocytic anemia. IL-8 is a proinflammatory chemokine associated with the promotion of neutrophil chemotaxis and degranulation35. Serum IL-8 levels are increased in many malignancies, including CRC29, and IL-8 is considered an important contributor of cancer-associated inflammation35. Serum albumin levels depict systemic inflammation, since the synthesis of albumin decreases as a response to IL-625,28. Overall, these findings support the idea that, especially, normocytic anemia in CRC is associated with the systemic inflammation. This association might also have therapeutic significance, since the modulation of the inflammatory response has shown some promise in the treatment of the anemia of inflammation36.
The reported mechanisms linking inflammation and anemia are diverse24. First, pro-inflammatory cytokines including IL-6 stimulate the hepatic expression of hepcidin, which inhibits the absorption of iron in the duodenum37. Second, in inflammatory conditions, there is limited availability of iron for erythroid cells, due to the alterations in macrophage functions24. Cytokine stimulus leads to the activation of macrophages, which phagocytose and degrade erythrocytes. IFN-γ stimulates the uptake of iron by macrophages by increasing the expression of divalent metal transporter 1, and IL-10 stimulates the uptake of transferrin-bound iron by upregulating transferrin receptor expression24. Several cytokines, including IL-6 and IL-10 induce ferritin expression and stimulate the storage and retention of iron within macrophages24. Third, several cytokines, including TNF-α and IFNγ, inhibit the synthesis of erythropoietin in the kidney, leading to diminished erythropoiesis38. Fourth, pro-inflammatory cytokines, such as TNF-α and IFNγ directly inhibit the proliferation of erythroid progenitor cells24. Against our hypotheses, our results did not indicate significant correlations between blood Hb levels and these cytokines, including IL-6 and IFNγ.
The earlier studies on the prognostic significance of blood Hb levels in CRC have been controversial, with reports of the association of anemia with lower survival in advanced colorectal cancer9, lower OS in stage II-III CRC treated with FOLFOX chemotherapy10, lower OS in stage I-III CRC13, no independent prognostic value in stage I-III CRC14, adverse OS in metastasized CRC15,19, poor OS in stage I-III rectal cancer, and no prognostic value in unselected colon cancer material20. Moreover, iron deficiency anemia has been associated with diminished disease-free survival in T3N0M0 colon cancer8. In the univariate analyses of our study, anemia did not significantly associate with disease outcome. Instead, normocytic anemia associated with a trend towards adverse OS in both univariate and multivariate survival analyses (multivariate HR = 1.61, 95% CI = 1.07–2.42, p = 0.023; borderline statistical significance due to multiple hypothesis testing). In the multivariate Cox regression model, the significance of normocytic anemia was superior to mGPS. Earlier studies have established mGPS as an independent additional prognostic parameter in CRC25, and the relatively small number of cases in the analyses could have affected the results of this study. Relying solely on Bonferroni corrected p values could increase the risk of type 2 statistical error39. Therefore, this result encourages further studies to assess the prognostic significance of normocytic anemia, especially in relation to mGPS and other systemic inflammatory biomarkers, in larger cohorts. Moreover, the limited sample size in our study does not allow sensible subgroup analysis in, e.g., stage II patients, which would be required for firm conclusions on the prognostic value of blood Hb in these different patient subgroups.
In addition to the relatively low numbers of specific patient groups, such as MMR deficient cases, additional limitations need to be considered in the interpretation of the results. First, MCV was used in the categorization of anemia. While reduced MCV is relatively specific for iron deficiency, its sensitivity is lower than, e.g., that of serum transferrin receptor, especially in the presence of chronic diseases such as CRC40. For this study, no additional markers for iron deficiency were available, and further characterization of anemia using additional parameters would be beneficial in subsequent studies. Second, no data was available on the preoperative iron supplementation of the patients. However, current available data do not provide conclusive evidence on preoperative iron supplements significantly affecting blood Hb in patients undergoing surgery for CRC41. Preoperative RT/CRT was considered another potential confounding factor but the Hb levels of the patients who received preoperative RT/CRT did not significantly differ from those of the other rectal cancer patients. Multiple hypotheses were tested in this observational study. However, we adjusted the level of statistical significance to p = 0.0015 (≈0.05/34) by Bonferroni correction and interpreted the results with p = 0.05–0.0015 (considered borderline statistical significance) cautiously. This approach could result in some increase in type 2 statistical error but reduce the risk of type 1 error. The strengths in the study was the inclusion of two independent well-characterized, prospectively recruited study cohorts. The broad array of analyzed tumor characteristics and systemic inflammatory markers enabled us to investigate their relative significance for blood Hb levels. In addition to extensive characterization of the associations between blood Hb and systemic inflammation, this is, to our knowledge, the first study to analyze the correlations between different anemia subgroups in CRC and MMR enzyme status and BRAF mutation.
In conclusion, anemia is common in CRC patients and it is most frequently normocytic followed by microcytic. Proximal tumor location is preferentially associated with microcytic anemia, while systemic inflammation is associated with normocytic anemia. Further data is needed on the prognostic value of anemia in different patient subgroups.
Methods
Patients
This study was introduced to all newly diagnosed CRC patients operated in Oulu University Hospital in 2006–2014, of which the patients who signed an informed consent to participate were included. The patients with earlier or simultaneously diagnosed other malignant diseases were excluded. The study includes two independent, consecutive, prospectively recruited cohorts of CRC patients. Cohort 1 is an earlier described cohort of 148 CRC patients operated in Oulu University Hospital in 2006–2010 (Table 1)29,42,43, with up to 120-month follow-up data (Time to recurrence, TTR; cancer specific survival, CSS; overall survival, OS) collected from the clinical records and from Statistics Finland44–46. Cohort 2 consists of 208 CRC patients operated in Oulu University Hospital in 2010–2014, with up to 60-month follow-up data (TTR, CSS, OS) collected from the clinical records and from Statistics Finland (Table 1). TTR was defined as time from the operation to the recurrence of the same cancer, CSS was defined as time from the operation to death from the same cancer, and OS was defined as time from the operation to death, irrespective of cause. Tumor location data, acquired from the clinical records was recoded into a continuous variable, based on average distance of each subsite to anus, utilizing recent computed tomography colonography data31: rectum 9.75 cm, rectosigmoid colon 19.5 cm, sigmoid colon 44 cm, descending colon 85 cm, splenic flexure 101,5 cm, transverse colon 130,65 cm, hepatic flexure 159,8 cm, ascending colon 171,35 cm, and caecum 187,25 cm. This approach has been successfully applied previously32. In both cohorts, the preoperative staging of rectal cancer was performed with magnetic resonance imaging, and the patients with cT3 or cT4 rectal tumors (Cohort 1: n = 32, 21.6%; Cohort 2: n = 38, 18.3%) received preoperative radiotherapy or chemoradiotherapy (RT/CRT). The study was performed with the approval of the Ethics Committee of Oulu University Hospital (58/2005, 184/2009) and in accordance with the Declaration of Helsinki. All the patients and the controls had signed an informed consent to participate. The REporting recommendations for tumor MARKer prognostic studies (REMARK) were taken into account in the study design and reporting47.
Blood analyses
Preoperative blood and serum samples from the patients were collected29. In both cohorts, blood Hb levels, erythrocyte MCV levels, serum CRP levels and serum albumin levels were measured in the laboratory of Oulu University Hospital29,42. Anemia was defined according to WHO criteria as blood Hb levels < 120 g/L in women or < 130 g/L in men48. It was classified according to erythrocyte MCV levels as microcytic (MCV < 80 fL), normocytic (MCV 80–100 fL), and macrocytic (MCV > 100 fL). mGPS was determined according to the established criteria (mGPS0: serum CRP ≤ 10 mg/L and serum albumin ≥ 35 g/L or < 35 g/L; mGPS1: serum CRP > 10 mg/L and serum albumin ≥ 35 g/L; mGPS2: serum CRP > 10 mg/L and serum albumin < 35 g/L)25,49. In Cohort 1, the serum analysis of 27 cytokines was performed with Bio-Plex Pro Human pre-manufactured 27-Plex Cytokine Panel (Bio-Rad, Hercules, CA, USA), as described earlier29. As described earlier in more detail, 14 cytokines had many values below or above the assay detection limits, and therefore, 13 cytokines (IL-1ra, IL-4, IL-6, IL-7, IL-8, IL-9, IL-12, IFN-γ, CXL10, CCL2, CCL4, CCL11, and PDGF-BB) with less than four values outside the assay working range were included in this study29.
Histopathological analysis
The staging of the tumors was conducted according to TNM6 (Cohort 1) or TNM7 (Cohort 2) and the grading according to the World Health Organization (WHO) criteria (both cohorts). Lymphatic invasion was defined as tumor cells present in vessels with an endothelial lining but lacking a muscular wall, and blood vessel invasion was evaluated positive if there were tumor cells in vessels with a thick muscular wall or in vessels containing red blood cells50. Tumor growth pattern at the tumor border was classified using the earlier described criteria as infiltrative or expanding50,51. All the histological analyses were performed blinded to the clinical data.
Immunohistochemistry
For both cohorts, tissue microarrays were utilized in immunohistochemical analyses. For both cohorts, the arrays included 1–4 cores of 3.0 mm diameter (Cohort 1, median 3; Cohort 2, median 4), depending on the size of the tumor, from the invasive margin and the tumor center52. Immunohistochemistry for mismatch repair (MMR) enzymes MLH1, MSH2, MSH6, and PMS2 was conducted, as described earlier, to evaluate MMR enzyme status42,53. BRAF V600E specific VE1 immunohistochemistry was conducted with Ventana Bench-Mark XT immunostainer (Ventana Medical Systems, Tucson, AZ)54, to evaluate BRAF mutation status of both cohorts. Our earlier study has indicated that the method had a sensitivity of 100% and a specificity of 99.3% in detecting BRAF V600E mutation54.
Statistical analyses
The statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY). Pearson correlation coefficients (r) were used to determine the correlation between two continuous variables. To normalize their distribution, logarithmic transformation was applied to variables with positive skewness. The statistical significances of the associations between categorical and continuous variables were analyzed by independent samples t-test or Mann-Whitney test (comparing two classes), or one-way analysis of variances (ANOVA) or Kruskal-Wallis test (comparing three or more classes), while the statistical significances of the associations between two categorical variables were analyzed with χ2 test of Fisher exact test, as appropriate. A multiple linear regression analysis of the correlation of blood Hb levels with selected clinicopathological factors was conducted. Cytoscape, an open source software platform for visualizing complex networks, was used in creating a 2D visualization of the relationships between blood Hb levels and serum levels of systemic inflammatory markers with the Prefuse force directed algorithm weighted by the statistical significances of the correlations between individual variables55. The survival outcomes of the patient subgroups were analyzed with Kaplan-Meier method, log-rank tests, and Cox regression analysis. All p values are two-tailed. We assessed the associations between blood Hb levels and 34 parameters. Due to multiple hypothesis testing, we adjusted the level of statistical significance to p = 0.0015 (≈0.05/34) by Bonferroni correction, regarded the results with p = 0.05–0.0015 as of borderline statistical significance, and interpreted the results cautiously.
Data availability statement
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The authors are grateful for Ms. Riitta Vuento for her expert assistance.
Author Contributions
Study conception and design: J.P. Väyrynen, A. Tuomisto, K. Klintrup, J. Mäkelä, K.-H. Herzig, T.J. Karttunen, M.J. Mäkinen. Data collection: J.P. Väyrynen, A. Tuomisto, S.A. Väyrynen, K. Klintrup, T. Karhu, J. Mäkelä, K.-H. Herzig, T.J. Karttunen, M.J. Mäkinen. Statistical analysis: J.P. Väyrynen. Manuscript draft: J.P. Väyrynen. Manuscript review and editing: J.P. Väyrynen, A. Tuomisto, S.A. Väyrynen, K. Klintrup, T. Karhu, J. Mäkelä, K.-H. Herzig, T.J. Karttunen, M.J. Mäkinen.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19572-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA. Cancer J. Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Jellema P, et al. Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis. BMJ. 2010;340:c1269. doi: 10.1136/bmj.c1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadahiro S, et al. Anemia in patients with colorectal cancer. J. Gastroenterol. 1998;33:488–494. doi: 10.1007/s005350050120. [DOI] [PubMed] [Google Scholar]
- 4.Dunne JR, et al. Preoperative anemia in colon cancer: assessment of risk factors. Am. Surg. 2002;68:582–7. [PubMed] [Google Scholar]
- 5.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am. J. Med. 2004;116(Suppl):11S–26S. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Edna T, Karlsen V, Jullumstrø E, Lydersen S. Prevalence of anaemia at diagnosis of colorectal cancer: assessment of associated risk factors. Hepatogastroenterology. 2012;59:713–6. doi: 10.5754/hge11479. [DOI] [PubMed] [Google Scholar]
- 7.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–21. doi: 10.1002/1097-0142(20010615)91:12<2214::AID-CNCR1251>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 8.Zhen L, et al. Iron-deficiency anemia: a predictor of diminished disease-free survival of T3N0M0 stage colon cancer. J. Surg. Oncol. 2012;105:371–5. doi: 10.1002/jso.22032. [DOI] [PubMed] [Google Scholar]
- 9.Graf W, Glimelius B, Pahlman L, Bergstrom R. Determinants of prognosis in advanced colorectal cancer. Eur. J. Cancer. 1991;27:1119–1123. doi: 10.1016/0277-5379(91)90307-Y. [DOI] [PubMed] [Google Scholar]
- 10.An MS, et al. T4 stage and preoperative anemia as prognostic factors for the patients with colon cancer treated with adjuvant FOLFOX chemotherapy. World J. Surg. Oncol. 2015;13:64. doi: 10.1186/s12957-015-0488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berardi R, et al. Anemia may influence the outcome of patients undergoing neo-adjuvant treatment of rectal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2006;17:1661–4. doi: 10.1093/annonc/mdl285. [DOI] [PubMed] [Google Scholar]
- 12.Dreyer, S. B. et al. The Pretreatment Systemic Inflammatory Response is an Important Determinant of Poor Pathologic Response for Patients Undergoing Neoadjuvant Therapy for Rectal Cancer. Ann. Surg. Oncol. 10.1245/s10434-016-5684-3 (2016). [DOI] [PMC free article] [PubMed]
- 13.Mörner MEM, Edgren G, Martling A, Gunnarsson U, Egenvall M. Preoperative anaemia and perioperative red blood cell transfusion as prognostic factors for recurrence and mortality in colorectal cancer-a Swedish cohort study. Int. J. Colorectal Dis. 2017;32:223–232. doi: 10.1007/s00384-016-2678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roxburgh CSD, Wallace AM, Guthrie GK, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative surgery for colon cancer. Colorectal Dis. 2010;12:987–94. doi: 10.1111/j.1463-1318.2009.01961.x. [DOI] [PubMed] [Google Scholar]
- 15.Sanoff HK, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J. Clin. Oncol. 2008;26:5721–7. doi: 10.1200/JCO.2008.17.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tampellini M, et al. The role of haemoglobin level in predicting the response to first-line chemotherapy in advanced colorectal cancer patients. Br. J. Cancer. 2006;95:13–20. doi: 10.1038/sj.bjc.6603204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Halteren HK, Houterman S, Verheij CDGW, Lemmens VEPP, Coebergh JWW. Anaemia prior to operation is related with poorer long-term survival in patients with operable rectal cancer. Eur. J. Surg. Oncol. 2004;30:628–32. doi: 10.1016/j.ejso.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Weissenberger C, et al. Anemia and long-term outcome in adjuvant and neoadjuvant radiochemotherapy of stage II and III rectal adenocarcinoma: the Freiburg experience (1989–2002) World J. Gastroenterol. 2006;12:1849–58. doi: 10.3748/wjg.v12.i12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zacharakis M, et al. Predictors of survival in stage IV metastatic colorectal cancer. Anticancer Res. 2010;30:653–60. [PubMed] [Google Scholar]
- 20.Fjørtoft I, et al. Pre-operative anaemia in colon cancer patients became normal after more than a year post-operatively but did not influence oncological outcome in the final analysis. Scand. J. Gastroenterol. 2013;48:663–71. doi: 10.3109/00365521.2013.781216. [DOI] [PubMed] [Google Scholar]
- 21.Moreno Chulilla JA, Romero Colás MS, Gutiérrez Martín M. Classification of anemia for gastroenterologists. World J. Gastroenterol. 2009;15:4627–37. doi: 10.3748/wjg.15.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajantie J. Thalassemias–what should Finnish physicians know? Duodecim. 2010;126:1137–44. [PubMed] [Google Scholar]
- 23.Mandel JS, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N. Engl. J. Med. 2000;343:1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 24.Weiss G, Goodnough LT. Anemia of chronic disease. N. Engl. J. Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 25.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat. Rev. 2013;39:534–40. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Kantola T, et al. Reply: Comment on ‘Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma’. Br. J. Cancer. 2013;108:1917–1918. doi: 10.1038/bjc.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 28.Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC, Yap SH. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J. Clin. Invest. 1987;79:1635–41. doi: 10.1172/JCI113000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantola T, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br. J. Cancer. 2012;107:1729–36. doi: 10.1038/bjc.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyes LH, et al. Preoperative systemic inflammation predicts postoperative infectious complications in patients undergoing curative resection for colorectal cancer. Br. J. Cancer. 2009;100:1236–9. doi: 10.1038/sj.bjc.6604997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khashab MA, Pickhardt PJ, Kim DH, Rex DK. Colorectal anatomy in adults at computed tomography colonography: normal distribution and the effect of age, sex, and body mass index. Endoscopy. 2009;41:674–8. doi: 10.1055/s-0029-1214899. [DOI] [PubMed] [Google Scholar]
- 32.Yamauchi M, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–54. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mäkinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007;50:131–150. doi: 10.1111/j.1365-2559.2006.02548.x. [DOI] [PubMed] [Google Scholar]
- 34.Hirai HW, et al. Systematic review with meta-analysis: faecal occult blood tests show lower colorectal cancer detection rates in the proximal colon in colonoscopy-verified diagnostic studies. Aliment. Pharmacol. Ther. 2016;43:755–764. doi: 10.1111/apt.13556. [DOI] [PubMed] [Google Scholar]
- 35.Waugh DJJ, Wilson C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 36.Sun CC, Vaja V, Babitt JL, Lin HY. Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am. J. Hematol. 2012;87:392–400. doi: 10.1002/ajh.23110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr. Opin. Hematol. 2005;12:107–111. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Spivak JL. The anaemia of cancer: death by a thousand cuts. Nat. Rev. Cancer. 2005;5:543–55. doi: 10.1038/nrc1648. [DOI] [PubMed] [Google Scholar]
- 39.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. doi: 10.1097/00001648-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Goddard AF, James MW, McIntyre AS, Scott BB, British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309–16. doi: 10.1136/gut.2010.228874. [DOI] [PubMed] [Google Scholar]
- 41.Borstlap WAA, et al. Iron therapy for the treatment of preoperative anaemia in patients with colorectal carcinoma: a systematic review. Colorectal Dis. 2015;17:1044–54. doi: 10.1111/codi.13110. [DOI] [PubMed] [Google Scholar]
- 42.Väyrynen JP, et al. Serum MMP-8 levels increase in colorectal cancer and correlate with disease course and inflammatory properties of primary tumors. Int. J. Cancer. 2012;131:E463–74. doi: 10.1002/ijc.26435. [DOI] [PubMed] [Google Scholar]
- 43.Väyrynen JP, et al. Decreased preoperative serum 25-Hydroxyvitamin D levels in colorectal cancer are associated with systemic inflammation and serrated morphology. Sci. Rep. 2016;6:36519. doi: 10.1038/srep36519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kantola T, et al. Serum endostatin levels are elevated in colorectal cancer and correlate with invasion and systemic inflammatory markers. Br. J. Cancer. 2014;111:1605–1613. doi: 10.1038/bjc.2014.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moilanen JM, et al. Collagen XVII expression correlates with the invasion and metastasis of colorectal cancer. Hum. Pathol. 2015;46:434–442. doi: 10.1016/j.humpath.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 46.Sirniö P, et al. Decreased serum apolipoprotein A1 levels are associated with poor survival and systemic inflammatory response in colorectal cancer. Sci. Rep. 2017;7:5374. doi: 10.1038/s41598-017-05415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McShane LM, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK) Eur. J. Cancer. 2005;41:1690–6. doi: 10.1016/j.ejca.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 48.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System (2011). Available at: http://www.who.int/vmnis/indicators/haemoglobin.pdf. (Accessed: 10th March 2017).
- 49.Väyrynen JP, et al. The relationships between serum cytokine levels and tumor infiltrating immune cells and their clinical significance in colorectal cancer. Int. J. Cancer. 2016;139:112–21. doi: 10.1002/ijc.30040. [DOI] [PubMed] [Google Scholar]
- 50.Väyrynen SA, et al. Clinical impact and network of determinants of tumour necrosis in colorectal cancer. Br. J. Cancer. 2016;114:1334–42. doi: 10.1038/bjc.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jass JR, et al. Assessment of invasive growth pattern and lymphocytic infiltration in colorectal cancer. Histopathology. 1996;28:543–548. doi: 10.1046/j.1365-2559.1996.d01-467.x. [DOI] [PubMed] [Google Scholar]
- 52.Väyrynen JP, et al. Characteristics and significance of colorectal cancer associated lymphoid reaction. Int. J. Cancer. 2014;134:2126–35. doi: 10.1002/ijc.28533. [DOI] [PubMed] [Google Scholar]
- 53.Sajanti SA, et al. Annexin A10 is a marker for the serrated pathway of colorectal carcinoma. Virchows Arch. 2015;466:5–12. doi: 10.1007/s00428-014-1683-6. [DOI] [PubMed] [Google Scholar]
- 54.Sajanti SA, et al. VE1 immunohistochemistry accurately detects BRAF V600E mutations in colorectal carcinoma and can be utilized in the detection of poorly differentiated colorectal serrated adenocarcinoma. Virchows Arch. 2014;464:637–43. doi: 10.1007/s00428-014-1555-0. [DOI] [PubMed] [Google Scholar]
- 55.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are available from the corresponding author on reasonable request.