Abstract
Cadmium (Cd), as an extremely toxic metal could accumulate in kidney and induce renal injury. Previous studies have proved that Cd impact on renal cell proliferation, autophagy and apoptosis, but the detoxification drugs and the functional mechanism are still in study. In this study, we used mouse renal tubular epithelial cells (mRTECs) to clarify Cd-induced toxicity and signaling pathways. Moreover, we proposed to elucidate the prevent effect of activation of Ca2+ sensing receptor (CaSR) by Calcimimetic (R-467) on Cd-induced cytotoxicity and underlying mechanisms. Cd induced intracellular Ca2+ elevation through phospholipase C-inositol 1, 4, 5-trisphosphate (PLC) followed stimulating p38 mitogen-activated protein kinases (MAPK) activation and suppressing extracellular signal-regulated kinase (ERK) activation, which leaded to increase apoptotic cell death and inhibit cell proliferation. Cd induced p38 activation also contribute to autophagic flux inhibition that aggravated Cd induced apoptosis. R-467 reinstated Cd-induced elevation of intracellular Ca2+ and apoptosis, and it also increased cell proliferation and restored autophagic flux by switching p38 to ERK pathway. The identification of the activation of CaSR-mediated protective pathway in renal cells sheds light on a possible cellular protective mechanism against Cd-induced kidney injury.
Introduction
Occupational and environmental pollutant of Cadmium (Cd) caused various organs damage, especially the kidney, which is the major site of Cd accumulation1–3. In kidney, the renal proximal tubule is the first opportunistic site of Cd reabsorption following plasma filtration in the glomerulus4,5. Therefore, the renal proximal tubular cells are excellent model to study Cd-induced cytotoxicity and renoprotective strategies6,7. Exposure to Cd could induce various cellular responses such as carcinogenesis, necrosis, apoptosis, proliferation and autophagy8–10. Previous studies had reported that Cd induced apoptotic cell death in the renal proximal tubule cells, i.e. porcine (LLC-PK1)11 and human (HK-2) proximal tubular epithelial cell12. More importantly, the molecular mechanisms underlying Cd-induced proximal tubular damage and renoprotective strategies are still in study.
Intracellular calcium homeostasis is very important in the control of many cellular processes13–15. Previous studies suggested that Cd disrupted intracellular Ca2+ homeostasis, resulting in cell apoptosis in a variety of cells9,16–20, including renal tubular cells21,22. Cd disrupted intracellular Ca2+ homeostasis through reducing the influx of extracellular Ca2+23,24, or increasing Ca2+ release from intracellular Ca2+ store22,25. Endoplasmic reticulum (ER) is a major intracellular store of Ca2+26 and Cd induces Ca2+ release from ER store, associated with ER stress through cation-sensing receptor (CSR) mediated phospholipase C (PLC)-inositol 1, 4, 5-trisphosphate (IP3) signaling pathway18,27. Cd induced elevation of intracellular Ca2+ level also triggers mitochondrial damage18, evoking reactive oxygen species (ROS) generation from mitochondria19,22,28–30. Both ER stress and mitochondrial damage lead to up-regulation of expression of caspase-3, resulting cell apoptotic death16–18. Additionally, intracellular Ca2+ signaling pathway also mediated Cd-induced autophagy17, which played a renoprotective role in both acute kidney injury and chronic kidney diseases31, and was indicated as a protective way against Cd-induced apoptosis in lung epithelial fibroblast cells WI3832, pheochromocytoma cell line PC-1233, and rat renal tubular cells34. However, initial autophagic protection would switch to disruption of autophagic flux and result in cell death during Cd stress accrual in renal NRK-52E cells6. Therefore, it is important to understand the roles of intracellular Ca2+ signaling pathways in Cd-induced apoptosis and autophagy, and their relationship in renal tubular cells.
In addition, a great number of studies have show that Cd regulates the functions of many Ca2+-dependent regulatory proteins such as protein kinase C (PKC), mitogen-activated protein kinase (MAPK), calmodulin (CaM), and calcium/calmodulin-dependent protein kinase II (CaMKII), inducing dysregulation of intracellular Ca2+ homeostasis16,35–41. Moreover, these intracellular signals can be induced by the extracellular calcium-sensing receptor (CaSR), a G-protein-coupled receptor (GPCR), which is responsible for the control of calcium homeostasis in body fluids42–46. Faurskov and Bjerregaard’s study showed the CaSR agonist, neomycin diminished Cd-evoked increase of intracellular Ca2+ in renal distal epithelial A6 cells27. However, the underlying mechanism and function of activation of CaSR on Cd-induced disruption of intracellular Ca2+ homeostasis and Cd-regulated pathways were still undeclared. In addition, although due to CaSR agonist neomycin and Gd3+ (Gadolinium ion) could not stimulate CSR, suggesting CaSR is different from CSR, both receptors mediate activation of PLC-IP3 pathway and intracellular Ca2+ level27. However, it is still unknown whether there is competition or crosstalk between CaSR and CSR mediated pathways.
The results of RT-PCR and immunohistochemistry staining had detected the expression of CaSR in rat renal proximal tubule47–49. Interestingly, our previous study indicated that activation of CaSR by calcimimetic R-467 could as a protective pathway to reduce Ca2+-induced cytotoxicity in gill cells of Japanese eels50. Given this observation together with previous reports on biological functions of CaSR, we attempt to decipher the protective role and its underlying mechanism in activation of CaSR against Cd induce cytotoxicity in renal proximal tubular cells. Here, we demonstrated that activation of CaSR by calcimimetic R-467 reduced Cd-evoked intracellular Ca2+ elevation, followed ROS generation and apoptosis, and R-467 also restored autophagic flux and increased cell proliferation by switching Cd-activated calcium-p38 MAPK to R-467 activated PLC-ERK pathway. Our findings provide the groundwork for future studies on renoprotective therapy of Cd-induced kidney injury.
Results
Detection of expressions of CaSR in renal cells and R-467 prevented Cd-induced renal cells death and cytotoxicity
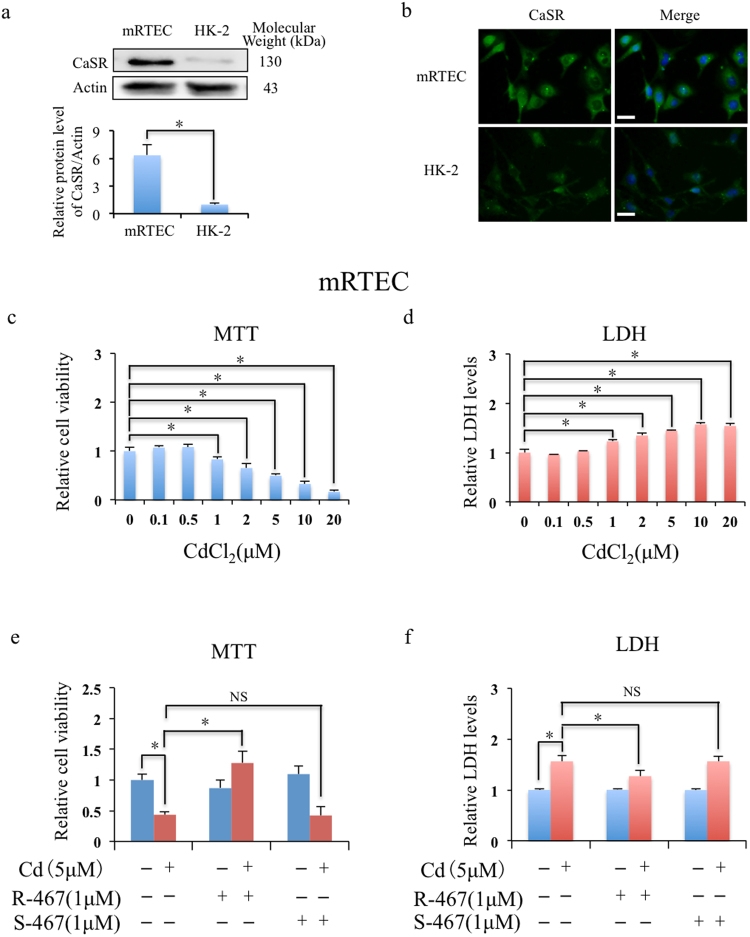
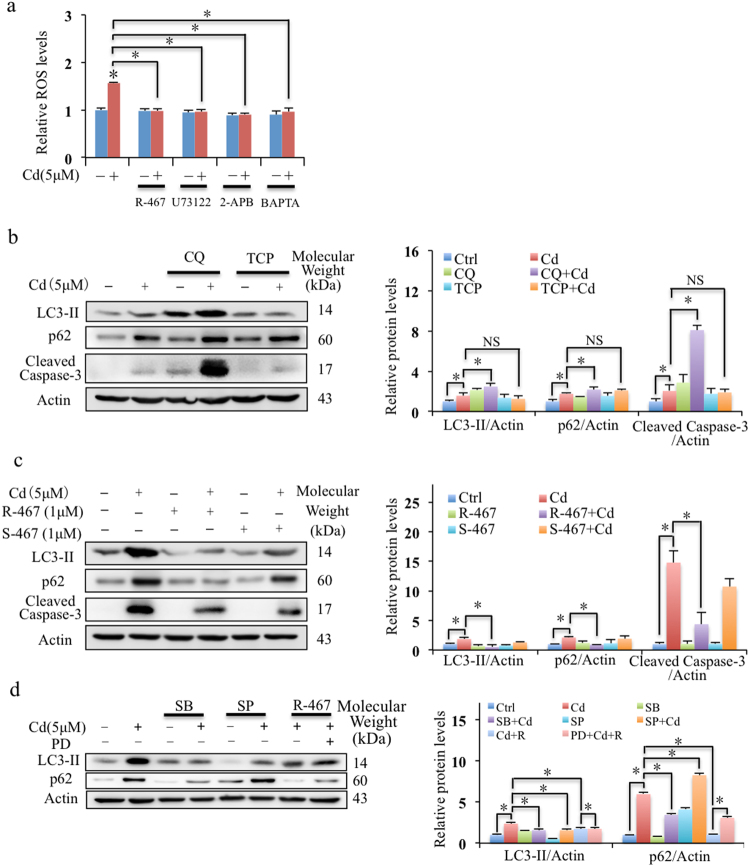
To detect the expressions of CaSR in renal cells (i.e. mRTEC and HK-2 cells), western blotting (Fig. 1a) and immunofluorescence (Fig. 1b) were performed and the results showed higher expression of CaSR in mRTECs than HK-2 cells. The results of MTT assay (Fig. 1c) and LDH cytotoxicity assay (Fig. 1d) showed Cd induced cytotoxicity of renal cell mRTEC in a dose dependent manner from 1 μM, and LD50 (lethal dose, 50%) was around 5 μM that was used in the following experiments. However, Cd induced cytotoxicity were significantly prevented when co-treated with calcimimetic R-467, but not with S-467 in mRTEC cells (Fig. 1e,f). And consistent results were also detected in HK-2 cells (Fig. S1a–d). As a positive allosteric modulator of CaSR, calcimimetic R-467 (R-enantiomer) could enhance the sensitivity of activation of CaSR, but S-467 (S-enantiomer) is less activator. In hence, co-treatment of R-467 but not S-467 prevented Cd-induced cytotoxicity (Fig. 1e,f and Fig. S1c,d) and suggested that the protective effect was specific and effectively mediated by CaSR. It is noted that Cd could not affect the expressions of CaSR in mRTEC and HK-2 cells (Fig. S1e). Considering higher expression of CaSR in mRTEC cells, we chose mRTEC as cell model in the following experiments.
Figure 1.
Expression of CaSR in renal cells and Cd induced cell death and cytotoxicity in mRTEC cells. (a) Western blotting shows expressions of CaSR in mRTEC and HK-2 cells. *P < 0.05, using Student’s t-test. (b) Immunofluorescence detection of expressions of CaSR in mRTEC and HK-2 cells. The cells were counterstained with CaSR (green) and DAPI fluorescence (blue). Scale bar: 20 μM. (c,d) Cd induced cytotoxicity in mRTEC. After treated with 0–20 μM Cd for 24 h, cell viability of mRTEC was evaluated by MTT assay. The condition mediums were collected for LDH cytotoxicity assay. Results are presented as mean ± SD (n = 4). (e,f) Effects of calcimimetics on Cd induced cytotoxicity in mRTEC. After treated with Cd (5 μM), R-467 (1 μM), S-467 (1 μM), Cd (5 μM) + R-467 (1 μM), or Cd (5 μM) + S-467 (1 μM) for 24 h, cell viability of mRTEC was evaluated by MTT assay. The condition mediums were collected for LDH cytotoxicity assay. Results are presented as mean ± SD (n = 4). *Statistical significance between control and treatments, or Cd treatment and co-treatment of Cd + R-467, *P < 0.05, using Student’s t-test.
R-467 increased PLC activity and reinstated Cd-induced intracellular Ca2+ level elevation
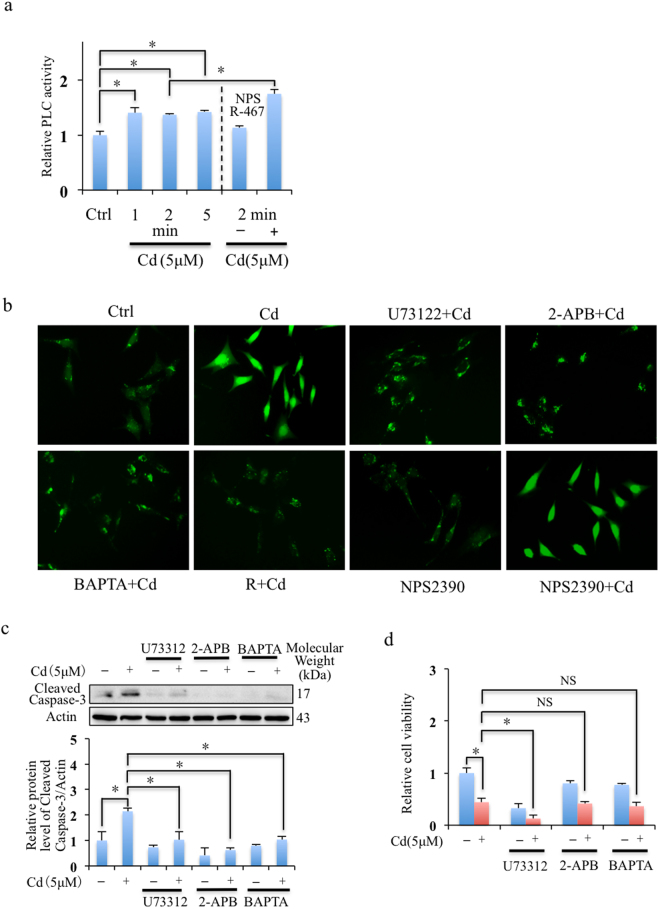
To confirm whether Cd induced elevation of intracellular Ca2+ level from Ca2+ store in ER through PLC-IP3 pathway in mRTEC cells, we measured PLC activity and intracellular Ca2+ levels with or without the inhibitors, including U73122 (inhibitor of PLC), 2-APB (inhibitor of IP3R), and BAPTA (intracellular Ca2+ chelator). Cd induced activation of PLC activity (Fig. 2a) and Cd induced intracellular Ca2+ level elevation in mRTEC cells was suppressed by the inhibitors, U73122 and 2-APB, BAPTA, and R-467 (Fig. 2b). The results were consistent with previous study that Cd induced elevation of intracellular Ca2+ level from Ca2+ store in ER through PLC-IP3 pathway. R-467 increased instead of decreased Cd-induced activation of PLC activity (Fig. 2a) but it could effectively restore intracellular Ca2+ level (Fig. 2b), the mechanism was further studied in the following sections. In addition, Cd induced intracellular Ca2+ elevation did not depend on CaSR, since it could not be blocked by CaSR antagonist NPS 2390 (Fig. 2b). Although block of PLC-IP3 pathway and chelation of intracellular Ca2+ inhibit Cd induced apoptosis (Fig. 2c), it did not prevent Cd to reduce cell viability (Fig. 2d), suggesting intracellular Ca2+ signaling was important for cell and might more complex mechanisms were involved.
Figure 2.
Effects of calcimimetics on Cd induced PLC activity and intracellular Ca2+ level elevation in mRTEC cells. (a) Effects of Cd and R-467 on PC-PLC activity in mRTEC cells. After post-exposed to Cd treatment (5 μM) for 1, 2, 5 min or co-treated with R-467 (1 μM) for 2 min, the PC-PLC activity of mRTEC cells were detected. Cd induced the activity of PC-PLC in mRTEC cells in a time-dependent manner. R-467 increased PC-PLC activity in presence of Cd. Result is presented as mean ± SD (n = 4). (b) Calcium imaging shows Cd induced elevation of intracellular Ca2+ level in mRTEC cells was eliminated by U73122, 2-APB, BAPTA and R-467. The mRTEC cells incubated with 1 μM Fluo-3 AM dye for 30 min, then pre-treated with U73122 (1 μM), 2-APB (50 μM), and BAPTA (10 μM) for another 30 min, then treated with Cd (5 μM) or Cd (5 μM) + R-467 (1 μM) for 1 hour and observed by Olympus IX73 microscopy. Scale bar: 20 μM. (c) Western blotting shows effects of U73122, 2-APB and BAPTA on Cd-induced apoptosis. Cells pretreated with U73122 (1 μM), 2-APB (50 μM), and BAPTA (10 μM) for 30 min, followed Cd treatment (5 μM) for 24 h, total proteins were extracted for western blotting analysis of expressions of cleaved caspase-3. (d) Effects of U73122, 2-APB and BAPTA on Cd-reduced cytotoxicity in mRTEC. Cells pretreated with U73122 (1 μM), 2-APB (50 μM), and BAPTA (10 μM) for 30 min, followed Cd treatment (5 μM) for 24 h, cell viability was evaluated by MTT assay. *Statistical significance between control and treatments, or Cd treatment and in presence of inhibitors, *P < 0.05, using Student’s t-test and one-way ANOVA followed by Duncan’s multiple range tests.
R-467 reversed Cd-altered p38 and ERK signaling pathways
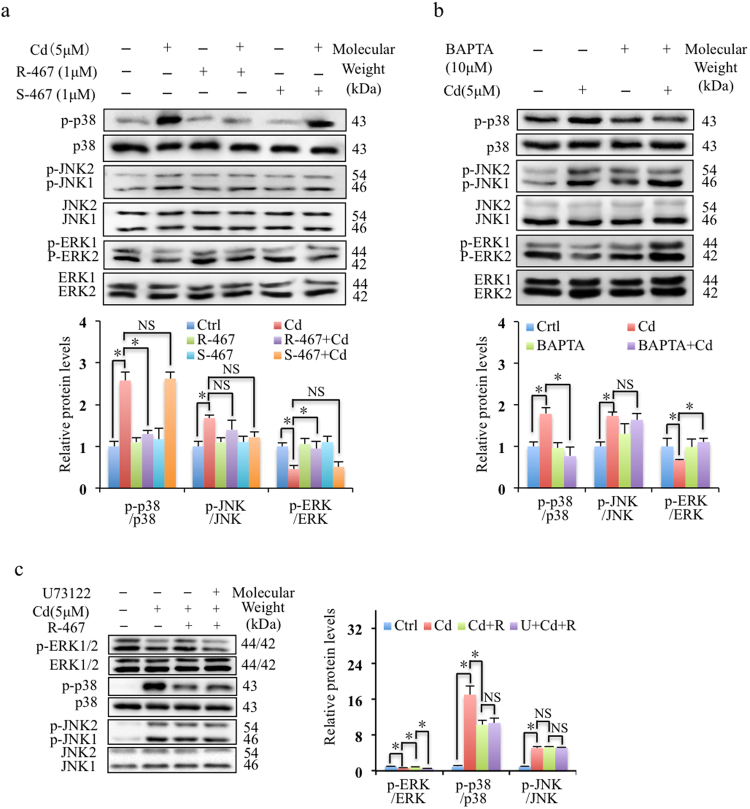
To further clarify the mechanism of R-467 reinstated Cd-induced intracellular Ca2+ level, we studied the activation of MAPK pathway. The results showed Cd induced activations of p38 and JNK while suppressed ERK1/2 MAPK signaling pathways (Fig. 3a). Effects of calcimimetics R-467 and S-467 on the activations of MAPK signaling pathway suggested that R-467 prevented Cd-induced activations of p38, but not JNK1/2 (Fig. 3a). In addition, Cd-suppressed activation of ERK1/2 was restored back by R-467 (Fig. 3a). Application of the intracellular Ca2+ chelator BAPTA indicated that Cd-induced elevation of intracellular Ca2+ level resulted in activation of p38, but not JNK1/2 (Fig. 3b). Interestingly, Cd suppressed activation of ERK1/2 was also restored back in the presence of BAPTA (Fig. 3b). It suggested that R-467 reinstated Cd-induced intracellular Ca2+ level to reverse Cd-altered p38 and ERK1/2 pathways. PLC inhibitor, U73122 eliminated the reverse effect of R-467 on Cd-suppressed activation of ERK, but did not alter R-467 reduced p38 activation (Fig. 3c). These results indicated that PLC activation was indispensable in reactivation of Cd-suppressed activation of ERK pathway.
Figure 3.
Effect of calcimimetics on Cd-regulated MAPK signaling pathway. (a,b) Effects of Cd, calcimimetics and intracellular Ca2+ chelator (BAPTA) on activation of MAPK signaling pathway. Cells treated with Cd treatment (5 μM), co-treated with calcimimetics R-467 and S-467 (1 μM), or pre-treated with BAPTA (10 μM) for 30 min, followed Cd treatment (5 μM) for 24 h, total proteins were extracted for western blotting analysis. Activations of and p38 MAPK and JNK represented by in vitro phosphorylations of p38 MAPK and JNK and showed significantly increase in response to Cd treatment. R-467 suppressed Cd-induced activation of p38 MAPK, but not JNK. Activation of ERK was decreased by Cd treatment, but it was increased when co-treated with R-467. BAPTA suppressed Cd-induced activation of p38 MAPK, but not JNK, and BAPTA increased Cd-suppressed activation of ERK. Total p38 MAPK, JNK and ERK1/2 served as loading control. (c) Inhibition of PLC on activation of MAPK pathway. Cells pretreated with U73122 (1 μM) for 30 min, followed by Cd treatment (5μM) or co-treated with R-467 (1 μM) for 24 h, total proteins were extracted for western blotting analysis of expression of phosphorylation of ERK, p38 and JNK. Total ERK1/2, p38 and JNK served as loading control. *Statistical significance between control and treatments or Cd treatment and in presence of inhibitors or calcimimetics, *P < 0.05, using Student’s t-test and one-way ANOVA followed by Duncan’s multiple range tests.
Cd-altered p38 and ERK signaling induced cytotoxicity
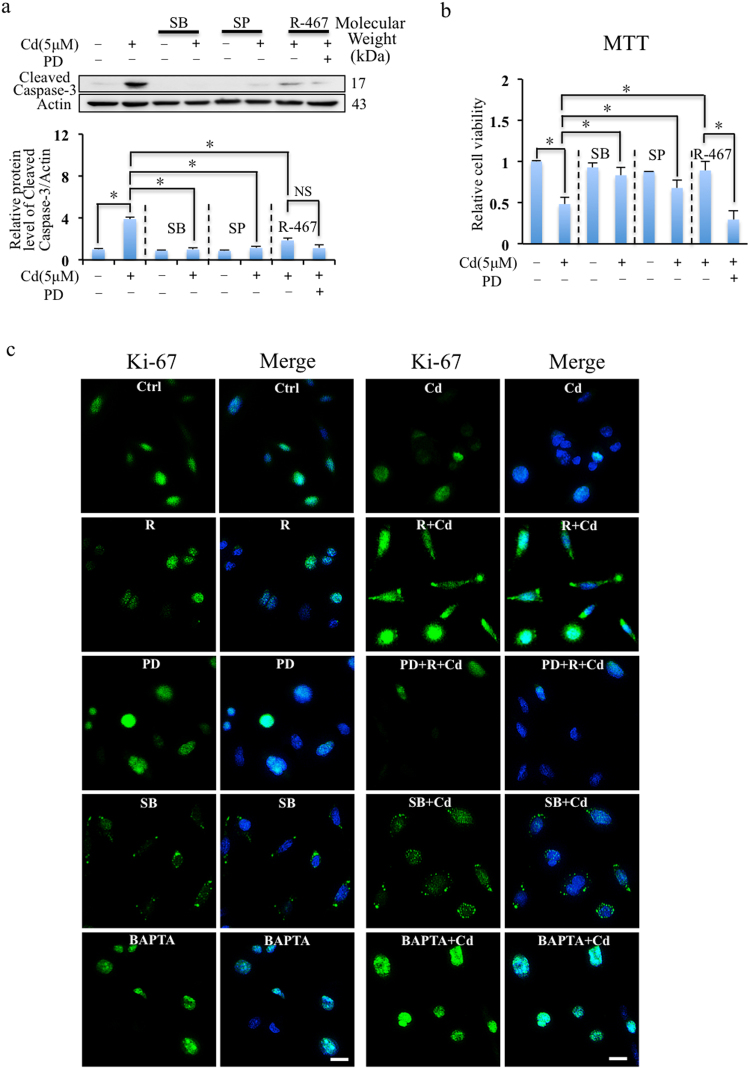
To investigate the roles of MAPK signaling pathway (i.e. p38 MAPK, JNK and ERK) in Cd-induced cell death, the specific inhibitors of these pathways, i.e. SB202190 (p38 inhibitor), SP600125 (JNK inhibitor) and PD98059 (ERK inhibitor) were applied. Inhibition of p38 and JNK reduced the expressions of cleaved caspase-3 (Fig. 4a) and decreased Cd induced cytotoxicity (Fig. 4b). It indicated that p38 and JNK pathways mediated Cd-induced apoptosis. In addition, ERK inhibitor, PD98059 did not alter R-467 reduced expression of cleaved caspase-3 in presence of Cd (Fig. 4a), which indicated R-467 could still prevent Cd-induced apoptosis when ERK activation was inhibited. However, PD98059 aggravated Cd-induced cytotoxicity in presence of Cd and R-467 (Fig. 4b), suggesting R-467-induced ERK activation played other roles in mRTEC cells.
Figure 4.
R-467 reduced apoptosis and induced cell proliferation. (a) Inhibition of MAPK signaling pathway on Cd and R-467-regulated apoptosis. Cells pretreated with SB202190 (10 μM), SP600125 (10 μM) or PD98059 (10 μM), for 30 min, followed by Cd treatment (5 μM) or co-treated with R-467 (1 μM) for 24 h, total proteins were extracted for Western blotting analysis of expression of cleaved caspase-3. (b) Inhibition of MAPK signaling pathway on Cd induced cytotoxicity. Cells pretreated with SB202190 (10 μM), SP600125 (10 μM) or PD98059 (10 μM), for 30 min, followed by Cd treatment (5 μM) or co-treated with R-467 (1 μM) for 24 h, the cell viability was evaluated by MTT assay. SB202190, SP600125 significantly inhibited Cd-induced cell death. Co-treatment of R-467 eliminated Cd-induced cytotoxicity, but suppressed by pretreatment of PD98059. Results are presented as mean ± SD (n = 4). *Statistical significance between control and treatments or Cd treatment and in presence of inhibitors or calcimimetics, *P < 0.05, using Student’s t-test and one-way ANOVA followed by Duncan’s multiple range tests. (c) Immunocytochemistry staining detection of Ki-67 expressions in mRTEC cells in Cd treatment (5 μM) or co-treated with R-467 (1 μM) for 24 h, or pretreat with SB202190 (10 μM), PD98059 (10 μM), or BAPTA (10 μM) for 30 min. The cells were counterstained with Ki-67 (green) and DAPI fluorescence (blue). Scale bar: 20 μM.
To further understand the function of R-467 altered MAPK pathway, we detected expression and localization of Ki-67, which is used as a marker to indicate cell proliferation51–53. As shown in Fig. 4c, in control, most of the Ki-67 staining was completely superimposition with nucleus, and some of them localized to the periphery of the nucleus those are in proliferation. Cd suppressed expression of Ki-67 and restricted their localization in the nuclei (Fig. 4c), suggesting Cd inhibited cell proliferation. Co-treatment of Cd and R-467 not only significantly increased expression of Ki-67, but also improved translocalization of Ki-67 out of the nucleus (Fig. 4c), suggesting more cell proliferation. However, co-treatment of Cd and R-467-increased expression and translocalization of Ki-67 were suppressed by ERK inhibitor, PD98059 (Fig. 4c). It suggested co-treatment of Cd and R-467 induced expression of Ki-67 through ERK activation, which was necessary for translocalization of Ki-67 but was suppressed by Cd. In addition, enhanced p38 activation restricted translocalization of Ki-67 and SB202190 pre-treatment (p38 inhibitor) increased Ki-67 expression and stimulated most of the Ki-67 translocated to the periphery or out of the nuclei in presence of Cd (Fig. 4c), suggesting cell proliferation. Interestingly, BAPTA treatment increased Ki-67 expression but restricted localization of Ki-67 in the nuclei (Fig. 4c), suggested intracellular Ca2+ was necessary for translocalization of Ki-67 and cell proliferation. In hence, pretreatment of BAPTA could not increase cell proliferation, although enhanced ERK activation and suppressed p38 activation in presence of Cd (Fig. 3b). Taken together, these results indicated that increased ERK activation and suppressed p38 activation but without dysregulation of intracellular Ca2+ homeostasis could stimulate cell proliferation when exposed to Cd, which were coincidently satisfied when co-treatment with R-467.
R-467 reduced Cd-evoked ROS generation, autophagic flux inhibition and apoptosis
To understand the role of Cd-increase intracellular Ca2+, ROS generation in mRTECs was determined. Cd significantly evoked generation of ROS in mRTEC cells (Fig. S2a), which could be abolished by inhibitors, U73122 and 2-APB, and intracellular Ca2+ chelator, BAPTA (Fig. 5a). This suggested Cd increased ROS generation by elevation of intracellular Ca2+ level and depended on PLC-IP3 pathway. To determine the roles of ROS generated by Cd exposure in Cd induced mRTEC cell death, TCP (ROS scavenger) and CQ (autophagy inhibitor) were applied. In Fig. S2b and Fig. S2c, it showed these two reagents reduced Cd-induced ROS generation but aggravated Cd-induced cell death. The rate of autophagic flux can be approximated by the amount of LC3-II with degradation of p62, whereas both of them are elevated when autophagic flux was impaired6. Cd increased the expressions of LC3-II and p62, which was aggravated by CQ (Fig. 5b). Addition of CQ could increase Cd-induced expression of cleaved caspase-3 (apoptosis marker) (Fig. 5b) and aggravate Cd induced cytotoxicity (Fig. S2b). These results indicated that Cd impaired autophagic flux, and increased apoptotic cell death. The pretreatment of TCP alleviated the Cd-induced accumulation of LC3-II and p62 (Fig. 5b), suggesting ROS could stimulate cell autophagy. However, scavenging of ROS by TCP aggravated instead of preventing Cd-induced cytotoxicity as show in Fig. S2b, suggesting that the cytotoxicity was not attributed to ROS generation and the induced autophagy signal under the exposure of Cd.
Figure 5.
Effects of calcimimetics on Cd-induced ROS generation, autophagic flux inhibition and apoptosis. (a) Effect of U73122, 2-APB, BAPTA and Cd increased ROS generation in mRTEC cells. Cells pretreated with U73122 (1 μM), 2-APB (50 μM), and BAPTA (10 μM) for 30 min, followed Cd treatment (5 μM) or Cd (5 μM) + R-467 (1 μM) for 6 h, relative ROS levels in mRTEC cells were determined. Results are presented as mean ± SD (n = 4). (b,c) Western blotting shows effects of TCP, CQ and co-treatments of calcimimetics R-467 or S-467 with Cd on Cd-induced autophagy flux inhibition and apoptosis. Cells pretreated with TCP (100 μM) or CQ (20 μM) for 30 min, followed Cd treatment (5 μM) or Cd (5 μM) + R-467 (1 μM) for 24 h, total proteins were extracted for western blotting analysis of expressions of LC3-II and p62, and cleaved caspase-3. (d) Inhibition of MAPK signaling pathway on Cd and R-467-regulated cell autophagy. Cells pretreated with SB202190 (10 μM), SP600125 (10 μM) or PD98059 (10 μM), for 30 min, followed by Cd treatment (5 μM) or co-treated with R-467 (1 μM) for 24 h, total proteins were extracted for Western blotting analysis of expression of LC3-II and p62. *Statistical significance between control and treatments or Cd treatment and in presence of inhibitors or calcimimetics, *P < 0.05, using Student’s t-test and one-way ANOVA followed by Duncan’s multiple range tests.
Co-treatment of R-467 eliminated Cd-induced generation of ROS (Fig. 5a), and R-467 effectively reducing Cd-increased of p62 accumulation and cleaved caspase-3 expression (Fig. 5c). This indicated that R-467 restored Cd-impaired autophagic flux and decreased Cd induced cell apoptosis, but which was not dependent on ROS generation. On the other hand, inhibition of p38 reduced the expressions of p62 but inhibition of JNK increased Cd-increased expression of p62 (Fig. 5d), indicating p38 pathway mediated Cd-impaired autophagic flux, whereas inhibition of JNK aggravated Cd-impaired autophagic flux. ERK inhibitor, PD98059 alone or in presence of Cd enhanced expression of p62 (Fig. S2d), and it also reversed co-treatment of R-467-reduced expression of p62 (Fig. 5d). Together with the effects of Cd and R-467 on activation of MAPK (Fig. 3a), the results indicated that R-467 restored autophagic flux by reversing Cd-altered p38 MAPK and ERK activations.
Discussion
The expression of CaSR in renal proximal tubules had been report in rat47–49. The renal expression of CaSR plays important roles in calciotropic signals responsible for Ca2+ homeostasis54,55. Altered CaSR expression and disruption of Ca2+ homeostasis had been shown to be associated with renal insufficiency56,57. Previous studies suggested that Cd disrupted intracellular Ca2+ homeostasis through reducing the influx of extracellular Ca2+23,24, or increasing Ca2+ release from intracellular Ca2+ store22,25 and resulting in cell apoptosis of renal tubular cells21,22. In renal distal epithelial A6 cells, Cd disrupted intracellular Ca2+ homeostasis through a divalent cation receptor (CSR) mediated PLC-IP3 pathway27. Moreover, CSR was different from CaSR and CaSR agonist neomycin could diminish the effect of Cd on intracellular Ca2+27. However, the underlying mechanism and function of activation of CaSR on Cd-induced disruption of intracellular Ca2+ homeostasis and Cd-regulated pathways were still unclear. Interestingly, our previous study in gill cells of Japanese eels indicated that activation of CaSR and it mediated PLC-ERK pathway could as a protective pathway to reduce Ca2+-induced cytotoxicity50. It naturally raised a question that whether there is crosstalk between CaSR and CSR for competition of PLC pathway. In present study, we investigated whether activation of CaSR affected Cd-induced cell death of renal proximal tubular cells. Calcimimetics as allosteric modulator of CaSR is currently being tested for the treatment of primary hyperparathyroidism, and CaSR-based therapeutics will likely be applicable to other disorders in which CaSRs are under- or overactive58. In this study, the R-enantiomer (R-467) was used, which is classified as a type II calcimimetic and functions as a positive allosteric modulator of CaSR to amplify receptor sensitivity to Ca2+ or other full agonists59. The S-enantiomer S-467 is known to be less active60. Activation of CaSR by R-467 effectively protected renal cells from Cd-induced cytotoxicity and reducing Cd-evoked disruption of intracellular Ca2+ homeostasis, followed ROS generation, autophagic flux inhibition and apoptosis. The present study demonstrated that activation of CaSR could restore of the intracellular Ca2+ homeostasis and related physiological disorders.
Previous studies had show that Cd disrupted intracellular Ca2+ homeostasis, resulting in cell apoptosis in a variety of cells9,16–19, including renal tubular cells21,22. One source of disruption of cadmium on intracellular Ca2+ homeostasis was through increasing Ca2+ release from ER store mediated by PLC-IP3 pathway18. Our data confirmed this notion by pretreatment of U73122 and 2-APB, inhibitors of PLC and IP3 receptor respectively, inhibited Cd-induced elevation of intracellular Ca2+ levels in mRTEC. However, inhibition of PLC and IP3 receptor by U73122 and 2-APB, and chelation of intracellular Ca2+ by BAPTA could not inhibited Cd-induced cytotoxicity of mRTEC. It indicated that there should be other pathways mediated Cd-induced cytotoxicity and the underlying mechanism is more complex than our speculation. On the other hand, our results suggested that activation of CaSR by R-467 reduced Cd-evoked disruption of intracellular Ca2+ homeostasis in mRTECs, which was similar with the effect of CaSR agonist neomycin on Cd-increased intracellular Ca2+ in renal distal epithelial A6 cells27. The co-treatment of Cd + R-467 increased even higher PLC activity than Cd, but they displayed reverse effects on intracellular Ca2+ level. However, what elements decide the downstream effectors and fate of PLC pathway needs further study.
Furthermore, previous study suggested Cd-induced intracellular Ca2+ elevation resulted in induction of ROS, triggering cell apoptosis19,61. Pretreatment with BAPTA attenuated Cd-induced ROS in the neuronal cells19,61. In this study, our results confirmed that Cd induced elevation of intracellular Ca2+ level resulted in ROS generation in mRTEC. Notably, previous studies also had indicated that Cd induced autophagy through ROS-activated pathways22,62. Cd recruited Ca2+ and ROS to act as 2nd messengers to control key Ca2+- and redox-sensitive molecular switches dictating cell function and fate. Severe ROS/Ca2+ signals activate cell death, whereas low localized Ca2+ and ROS levels promote cellular adaptation and survival63. In addition, ROS promoted autophagic flux serves as a cell survival mechanism to protect cell death64–66. The data in present study showed Cd induced generation of ROS and intracellular ROS in mRTEC cells stimulated autophagy, but Cd impaired autophagic flux (accumulation of p62), which contributed to apoptotic cell death. It suggested Cd induced ROS generation and autophagy signal as passive responses of mRTEC cells to promote adaptation and cell survival in Cd exposure. Autophagy is considered as an adaptive and protective mean against Cd-induced damage and dysfunction. Hence, inhibition of autophagic flux, such as by CQ, aggravated Cd-induced apoptosis. More importantly, activation of CaSR by R-467 could eliminate Cd-induced generation of ROS, but restore Cd-impaired autophagic flux (reduced expression of p62) and reduce Cd-induced apoptosis (reduced expression of cleaved caspase-3).
To further understand the molecular mechanisms of Cd-induced cytotoxicity and the protective roles of action of R-467, we detected the role of MAPK pathway. Previous studies had indicated Cd regulated activation of MAPK pathway (i.e. JNK, p38 and ERK) and induced cell apoptosis35,36,67,68. The JNK pathway usually mediates stress response and apoptosis69–71, and p38 activation was indicated to be involved in suppressing autophagy but promoting apoptosis72–75. Conversely, ERK is activated to induce cell proliferation76 or promote cellular adaptation and survival63. Our results showed Cd disrupt autophagic flux by p38 activation, which was consent with previous studies. In addition, the results indicated Cd induced apoptotic cell death through activation of p38 MAPK and JNK pathways, and Cd inhibited cell proliferation through reducing activation of ERK. It agreed with the effects of Cd on activation of MAPK in the human non-small cell lung carcinoma cell line, CL336, human osteosarcoma cell line, MG6377, and human renal proximal tubular cells, HK-212. In rat pheochromocytoma (PC12), human neuroblastoma (SH-SY5Y) cell lines and primary murine neurons, intracellular Ca2+ signaling mediated Cd-induced neuronal apoptosis via induction of activation of MAPK61. In mesangial cells, Cd-stimulated Ca2+ release from the endoplasmic reticulum induced activation of ERK, which leaded to predominantly autophagic cell death and a minor level of apoptotic cell death and resulted in nephrotoxicity78. In present study, the results suggested that in mRTEC cells, Cd-induced activation of p38 MAPK depended on intracellular Ca2+ signaling, while Cd-induced activation of JNK was intracellular Ca2+ independent manner. Pretreatment with BAPTA attenuated Cd-reduced activation of ERK. It indicated that different MAPK families mediated Cd-induced intracellular Ca2+ signaling in different cell types. Our previous study had suggested CaSR mediated PLC-ERK pathway to regulate calcium transport in gill cells50. Here, the results indicated that activation of CaSR by R-467 induced activation of PLC-ERK pathway while reduced activation of p38 MAPK, which was similar with effects of BAPTA on activation of p38 MAPK and ERK. It was notable that although both R-467 and BAPTA could restore intracellular Ca2+ level, p38 and ERK activations, R-467 instead of BAPTA prevent Cd-induced cytotoxicity and proliferation inhibition in mRTEC cells, suggesting R-467 might play important role in restoring back of intracellular Ca2+ to ER store or induce other pathways to stimulate cell proliferation that need further studies. Through reducing activation of p38 MAPK and increasing activation of ERK pathway, R-467 restored autophagic flux and reduced apoptotic cell death and increased cell proliferation. The results here indicated that activation of CaSR by R-467 could switch Cd-activated PLC-Ca2+-p38 MAPK to activate PLC-ERK pathway. However, it cannot rule out other possible mechanisms, such as R-467 might also stimulate other receptors to prevent Cd-induced cytotoxicity or alter the binding affinity of Cd onto its receptor CSR, or there might be interaction or cross-talking between the receptors (CSR and CaSR) and their other downstream effectors which were altered by R-467, and how can R-467 switch Cd-activated PLC-Ca2+-p38 MAPK to activate PLC-ERK pathway and what key elements decide the fate of PLC activation are still unclear. Therefore, the clarification of other pathways and deep mechanisms behind the protective effect of R-467 need further studies.
In summary, the results of this study demonstrated that Cd induced Ca2+ release from ER store through PLC-IP3 pathway in mRTEC cells, and elevation of intracellular Ca2+ level leaded to ROS generation. Cd-induced elevation of intracellular Ca2+ also leaded to p38 activation, which impaired autophagic flux, stimulated apoptosis and suppressed cell proliferation. In addition, Cd induced apoptosis through JNK activation, and inhibited cell proliferation through suppression of ERK activation. Activation of CaSR by R-467 reinstated intracellular Ca2+ level and ROS generation, restored Cd-impaired autophagic flux and reduced Cd-induced apoptosis, and increased cell proliferation by competing with Cd for PLC to switch Cd activated p38 MAPK to R-467 activated PLC-ERK pathway (Fig. 6). The identification of the activation of CaSR-mediated protective pathway in mRTEC cells sheds light on a possible cellular protective mechanism against Cd-induced kidney injury.
Figure 6.
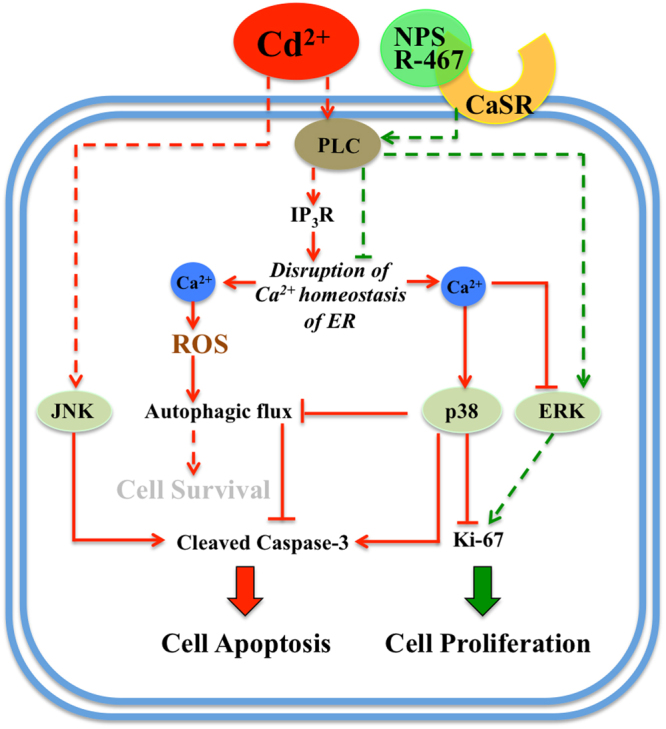
A schematic to illustrate calcimimetic R-467 reversed Cd-induced signaling pathways, followed reinstated intracellular Ca2+ level and ROS generation, restored autophagic flux, alleviated cell apoptosis and stimulated cell proliferation.
Materials and Methods
Chemicals
Cadmium chloride (Sigma, St. Louis, MO, USA) was dissolved in sterile distilled water to prepare the stock solutions (0–50 mM), aliquoted and stored at room temperature. Fluo-3/AM, and 2’7’-dichlorodihydrofluorescein diacetate (H2DCFDA) were purchased from Thermo Fisher Scientific (Waltham, MA, USA); U73122, 2-Aminoethoxydiphenylborane (2-APB), 1, 2-bis (o-aminophenoxy) ethane-N, N, N’, N’-tetraacetic acid (BAPTA/AM), PD98059, SB202190, SP600125 were purchased from Tocris Bioscience (Ellisville, MO, USA); α-tocopherol (TCP) and chloroquine (CQ) were purchased from Sigma (St. Louis, MO, USA); Calcimimetics NPS R-467 or S-467 were from NPS Pharmaceuticals (Salt Lake, UT, USA).
Cell culture and treatments
Mouse renal tubular epithelial cells (mRTECs) and human kidney proximal tubule (HK-2) cells obtained from American Tissue Culture Collection (ATCC, Manassas, VA, USA) were grown at 37 °C in RPMI 1640 medium (pH 7.4) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin (pH 7.4) in a humidified atmosphere containing 5% CO2. RPMI 1640 medium, FBS, penicillin, and streptomycin were purchased from Gibco (CA, USA). The mRTEC and HK-2 cells (at 50% confluence) were treated with CdCl2 (0–20 μM or 0–50 μM respectively) for indicated time periods. In some experiments, cells were either untreated or pre-treated with kinase inhibitors for 30 min, i.e. U73122 (1 μM), 2-APB (50 μM), BAPTA/AM (10 μM), PD98059 (10 μM), SB202190 (10 μM), SP600125 (10 μM), TCP (100 μM) and CQ (20 μM). After 24 hours, the culture mediums were collected for cytotoxicity analysis, and the cell viability was measured by MTT assay. In some experiments, cell lysates were collected for western blotting analysis.
Measurement of cell growth and cytotoxicity assay
After treated for 24 h, the culture mediums were collected to measure the activity of lactate dehydrogenase (LDH) using the LDH Cytotoxicity Assay Kit (Cayman) according to the manufacturer’s instructions. The absorbance at 490 nm was detected using a Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, USA). Cell growth was measured by the MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5- diphenyl-2-H- tetrazolium bromide) assay. In brief, cells were incubated in 100 μL MTT solution (0.5 mg/ml in RPMI 1640 medium) in 96-well plate for 4-h before the end of incubation. The supernatant was then discarded, and 100 μL DMSO was added to dissolve the colored product (formazan). The absorbance was measured at 540 nm (690 nm as reference) using a Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, USA).
PC-PLC assay
The mRTEC cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris of pH 7.4, 150 mM NaCl, 1% NP40) at 1, 2, 5 min post-treatment with CdCl2 (5 μM), or 2 min post-treatment with R-467 (1 μM), and R-467 (1 μM) + CdCl2 (5 μM). After centrifugation at 13 000 g for 15 min at 4 °C, the supernatant was collected and the total protein concentration was determined by the Bradford method (Bio-Rad). The protein lysates (15 μg/sample) were analyzed for PC-PLC activity using an Amplex Red PC-PLC assay kit according to the manufacturer’s instruction (Molecular Probes, Eugene, OR, USA). The reactions were incubated in darkness at 37 °C for 1 h. The fluorescence was measured with a Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, USA) with excitation at 530 nm and emission at 590 nm.
Measurement of intracellular calcium
To visualize the effect of Cd on intracellular Ca2+ levels in renal cells, mRTEC cells were seeded at a density of 2 × 104 cells/well in 24-well plates. Next day, the cells were loaded with 1 μM Fluo-3/AM in the RMPI 1640 medium (phenol red free) for 30 min at 37 °C in the dark. After dye loading, the cells were washed twice with the medium. Then, the cells were pre-treated with inhibitors for 30 min, i.e. U73122 (1 μM), 2-APB (50 μM), and BAPTA/AM (10 μM), then added CdCl2 (5 μM) or CdCl2 (5 μM) + R467 (1 μM) for 1 hour. Finally, calcium imaging was observed by Olympus IX73 microscopy (Japan).
ROS detection
The ROS level was measured using H2DCFDA (Thermo Fisher Scientific). Briefly, mRTEC cells were seeded at a density of 5 × 103 cells/well in a 96-well plate. The next day, cells were treated with CdCl2 (5 μM) for 0.5, 0.75, 1.5, 3 and 6 h, or incubated in the presence or absence of CdCl2 (5 μM) for 3 h with R-467 (1 μM), or pretreated with U73122 (1 μM), 2-APB (50 μM), BAPTA/AM (10 μM), TCP (100 μM) and CQ (20 μM) for 30 min, followed by incubation with H2DCFDA for 30 min. Fluorescent intensity was recorded by excitation at 485 nm and emission at 525 nm using a Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, USA).
Western blotting
The mRTEC cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris of pH 7.4, 150 mM NaCl, 1%NP40) at period time after treatments. After centrifugation at 13 000 g for 15 min at 4 °C, the supernatant was collected and the total protein concentration was determined by the Bradford method (Bio-Rad). The protein lysates containing 40 μg total cellular protein in RIPA buffer were subjected to electrophoresis on 8–12% polyacrylamide gels. The gels were then blotted onto PVDF membranes (PerkinElmer Life Sciences, Foster City, CA, USA). Western blotting was conducted using rabbit monoclonal antibodies against CaSR (D6D9V), phospho-p38 mitogen-activated protein kinases (Thr180/Tyr182) (p-p38 MAPK), phospho- extracellular signal-regulated kinase (Thr202/Tyr204) (pERK1/2), phospho-stress-activated protein kinase/c-Jun NH(2)-terminal kinase (Thr183/Tyr185) (p-SAPK/JNK) and total p38, ERK, JNK; light chain 3-II (LC3-II) and p62; cleaved caspase-3, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit antibody (1:4000). Specific bands were visualized using chemiluminescent reagent (Western-lightening Plus, PerkinElmer Life Sciences). The blots were then washed in PBST and re-probed with rabbit anti-β-actin (1:1000). All the antibodies were purchased from Cell Signaling Technology (Shanghai, China). The intensity of each band was measured by Image J software (National Institutes of Health, USA).
Immunofluorescence
To detect the expression of Ki-67, the mRTEC cells were treated with CdCl2 (5 μM) with or without R-467 (1 μM), or S-467 (1 μM) for 24 h, or pre-treated with PD98059 (10 μM), SB202190 (10 μM) for 30 min. The cells were fixed for 30 min in 4% Formaldehyde (FA, Sigma-Aldrich). Then, the cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in PBS for 20 min. After blocked with 3% normal goat serum, the cells were incubated with mouse anti-Ki-67 (1:100, Cell Signaling Technology) antibody overnight at 4 °C, followed by 1 h incubation with Alexa Fluor 488 goat anti-mouse IgG (1:200, Molecular Probes, Invitrogen). After washing twice with PBS, cell nucleus was stained by the DAPI (Invitrogen) for several minutes. The cells were washed in PBS for 10 min and the cells were mounted, then examined by Olympus IX73 microscopy (Japan). To detect the expression of CaSR (anti-CaSR, 1:40, Thermo fisher Scientific) on the membranes of HK-2 and mRTEC cells, the process was performed without the permeabilization step.
Statistical analysis
Drug treatments were performed in triplicate in each experiment and every experiment was repeated at least three times. All data are represented as means ± s.e.m. Statistical significance was assessed with Student’s t-test or one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests. Groups were considered significantly different if P < 0.05.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31600952, 31271272 and 81402100), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Foundation of Health and Family Planning Commission of Jiangsu Province (Q201408), the Social Development Foundation of Zhenjiang (SH2016031 and SH2014026), and the Start-Up Research Funding of Jiangsu University for Distinguished Scholars (5501330001).
Author Contributions
J.G., S.D., and Y.L. performed the research; J.G. and H.S. designed the research study; C.K.C.W. and H.S. contributed essential reagents or tools; J.G., Y.D., M.L., H.L., Y.Z., X.J., F.Y., Y.Z., L.C., W.K.F.T., C.K.C.W., B.C. and H.S. analyzed and interpreted the data; J.G. and H.S. wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Jie Gu and Shuya Dai contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19327-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brzoska MM, Kaminski M, Supernak-Bobko D, Zwierz K, Moniuszko-Jakoniuk J. Changes in the structure and function of the kidney of rats chronically exposed to cadmium. I. Biochemical and histopathological studies. Arch. Toxicol. 2003;77:344–352. doi: 10.1007/s00204-003-0451-1. [DOI] [PubMed] [Google Scholar]
- 2.Fujiki K, Inamura H, Matsuoka M. Phosphorylation of FOXO3a by PI3K/Akt pathway in HK-2 renal proximal tubular epithelial cells exposed to cadmium. Arch. Toxicol. 2013;87:2119–2127. doi: 10.1007/s00204-013-1077-6. [DOI] [PubMed] [Google Scholar]
- 3.Satarug S, et al. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol. Lett. 2003;137:65–83. doi: 10.1016/S0378-4274(02)00381-8. [DOI] [PubMed] [Google Scholar]
- 4.Felley-Bosco E, Diezi J. Fate of cadmium in rat renal tubules: A micropuncture study. Toxicol. Appl. Pharmacol. 1989;98:243–251. doi: 10.1016/0041-008X(89)90229-9. [DOI] [PubMed] [Google Scholar]
- 5.Robinson MK, Barfuss DW, Zalups RK. Cadmium transport and toxicity in isolated perfused segments of the renal proximal tubule. Toxicol. Appl. Pharmacol. 1993;121:103–111. doi: 10.1006/taap.1993.1134. [DOI] [PubMed] [Google Scholar]
- 6.Lee WK, et al. Initial autophagic protection switches to disruption of autophagic flux by lysosomal instability during cadmium stress accrual in renal NRK-52E cells. Arch. Toxicol. 2017;91:3225–3245. doi: 10.1007/s00204-017-1942-9. [DOI] [PubMed] [Google Scholar]
- 7.Ljubojević M, Breljak D, Herak-Kramberger CM, Anzai N, Sabolić I. Expression of basolateral organic anion and cation transporters in experimental cadmium nephrotoxicity in rat kidney. Arch. Toxicol. 2016;90:525–541. doi: 10.1007/s00204-015-1450-8. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, et al. 2D-DIGE and MALDI TOF/TOF MS analysis reveal that small GTPase signaling pathways may play an important role in cadmium-induced colon cell malignant transformation. Toxicol. Appl. Pharmacol. 2015;288:106. doi: 10.1016/j.taap.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Templeton DM, Liu Y. Multiple roles of cadmium in cell death and survival. Chem. Biol. Interact. 2010;188:267–275. doi: 10.1016/j.cbi.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, et al. Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic. Biol. Med. 2011;50:624–632. doi: 10.1016/j.freeradbiomed.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuoka M, Call KM. Cadmium-induced expression of immediate early genes in LLC-PK1 cells. Kidney Int. 1995;48:383–389. doi: 10.1038/ki.1995.306. [DOI] [PubMed] [Google Scholar]
- 12.Komoike Y, Inamura H, Matsuoka M. Effects of salubrinal on cadmium-induced apoptosis in HK-2 human renal proximal tubular cells. Arch. Toxicol. 2012;86:37–44. doi: 10.1007/s00204-011-0742-x. [DOI] [PubMed] [Google Scholar]
- 13.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 14.Carafoli E. Intracellular calcium homeostasis. Annu. Rev. Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 15.Felsenfeld A, Rodriguez M, Levine B. New insights in regulation of calcium homeostasis. Curr. Opin. Nephrol. Hypertens. 2013;22:371–376. doi: 10.1097/MNH.0b013e328362141e. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, et al. Calcium-calmodulin signaling elicits mitochondrial dysfunction and the release of cytochrome c during cadmium-induced apoptosis in primary osteoblasts. Toxicol. Lett. 2014;224:1–6. doi: 10.1016/j.toxlet.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Wang SH, Shih YL, Ko WC, Wei YH, Shih CM. Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell Mol. Life Sci. 2008;65:3640–3652. doi: 10.1007/s00018-008-8383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biagioli M, et al. Endoplasmic reticulum stress and alteration in calcium homeostasis are involved in cadmium-induced apoptosis. Cell Calcium. 2008;43:184–195. doi: 10.1016/j.ceca.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Y, et al. Cadmium-induced apoptosis in primary rat cerebral cortical neurons culture is mediated by a calcium signaling pathway. PLoS One. 2013;8:e64330. doi: 10.1371/journal.pone.0064330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Hao W, Shi H, Hou Y, Xu Q. Calcium homeostasis disruption - a bridge connecting cadmium-induced apoptosis, autophagy and tumorigenesis. Oncol. Res. Treat. 2015;38:311. doi: 10.1159/000431032. [DOI] [PubMed] [Google Scholar]
- 21.Yeh JH, et al. Cadmium-induced cytosolic Ca2+ elevation and subsequent apoptosis in renal tubular cells. Basic. Clin. Pharmacol. Toxicol. 2009;104:345–351. doi: 10.1111/j.1742-7843.2009.00391.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, et al. Role of oxidative stress, apoptosis, and intracellular homeostasis in primary cultures of rat proximal tubular cells exposed to cadmium. Biol. Trace Elem. Res. 2009;127:53–68. doi: 10.1007/s12011-008-8223-7. [DOI] [PubMed] [Google Scholar]
- 23.Beyersmann D, Hechtenberg S. Cadmium, gene regulation, and cellular signalling in mammalian cells. Toxicol. Appl. Pharmacol. 1997;144:247–261. doi: 10.1006/taap.1997.8125. [DOI] [PubMed] [Google Scholar]
- 24.Thevenod F, Jones SW. Cadmium block of calcium current in frog sympathetic neurons. Biophys. J. 1992;63:162–168. doi: 10.1016/S0006-3495(92)81575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Z, et al. Cd-induced apoptosis was mediated by the release of Ca 2+ from intracellular Ca storage. Toxicol. Lett. 2010;192:115–118. doi: 10.1016/j.toxlet.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Somlyo AP, Bond M, Somlyo AV. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985;314:622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- 27.Faurskov B, Bjerregaard HF. Evidence for cadmium mobilization of intracellular calcium through a divalent cation receptor in renal distal epithelial A6 cells. Pflügers Arch. 2002;445:40. doi: 10.1007/s00424-002-0912-z. [DOI] [PubMed] [Google Scholar]
- 28.Chacon E, Acosta D. Mitochondrial regulation of superoxide by Ca2+: an alternate mechanism for the cardiotoxicity of doxorubicin. Toxicol. Appl. Pharmacol. 1991;107:117–128. doi: 10.1016/0041-008X(91)90336-D. [DOI] [PubMed] [Google Scholar]
- 29.Roy SS, Hajnoczky G. Calcium, mitochondria and apoptosis studied by fluorescence measurements. Methods. 2008;46:213–223. doi: 10.1016/j.ymeth.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sureshbabu A, Ryter SW, Choi ME. Oxidative stress and autophagy: Crucial modulators of kidney injury. Redox Biol. 2015;4:208–214. doi: 10.1016/j.redox.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim SC, Hahm KS, Lee SH, Oh SH. Autophagy involvement in cadmium resistance through induction of multidrug resistance-associated protein and counterbalance of endoplasmic reticulum stress WI38 lung epithelial fibroblast cells. Toxicology. 2010;276:18–26. doi: 10.1016/j.tox.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, et al. Induction of cytoprotective autophagy in PC-12 cells by cadmium. Biochem. Biophys. Res. Commun. 2013;438:186–192. doi: 10.1016/j.bbrc.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 34.Liu G, et al. The role of autophagy during cadmium induced apoptosis in rat renal tubular epithelial cells. Toxicol. Lett. 2016;259:S174–S174. doi: 10.1016/j.toxlet.2016.07.414. [DOI] [Google Scholar]
- 35.Chen S, et al. CaMKII is involved in cadmium activation of MAPK and mTOR pathways leading to neuronal cell death. J. Neurochem. 2011;119:1108–1118. doi: 10.1111/j.1471-4159.2011.07493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuang, S.M., Wang, I.C. & Yang, J.L. Roles of JNK, p38 and ERK mitogen-activated protein kinases in the growth inhibition and apoptosis induced by cadmium. Carcinogenesis. 21, 1423-1432 (2000). [PubMed]
- 37.Ha TT, Burwell ST, Goodwin ML, Noeker JA, Heggland SJ. Pleiotropic roles of Ca + 2/calmodulin-dependent pathways in regulating cadmium-induced toxicity in human osteoblast-like cell lines. Toxicol. Lett. 2016;260:18–27. doi: 10.1016/j.toxlet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Templeton DM. Cadmium activates CaMK-II and initiates CaMK-II-dependent apoptosis in mesangial cells. FEBS Lett. 2007;581:1481–1486. doi: 10.1016/j.febslet.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Long GJ. Cadmium perturbs calcium homeostasis in rat osteosarcoma (ROS 17/2.8) cells; a possible role for protein kinase C. Toxicol. Lett. 1997;91:91–97. doi: 10.1016/S0378-4274(97)03880-0. [DOI] [PubMed] [Google Scholar]
- 40.Martin P, Pognonec P. ERK and cell death: cadmium toxicity, sustained ERK activation and cell death. FEBS J. 2010;277:39–46. doi: 10.1111/j.1742-4658.2009.07369.x. [DOI] [PubMed] [Google Scholar]
- 41.Misra UK, Gawdi G, Akabani G, Pizzo SV. Cadmium-induced DNA synthesis and cell proliferation in macrophages: the role of intracellular calcium and signal transduction mechanisms. Cell. Signal. 2002;14:327–340. doi: 10.1016/S0898-6568(01)00268-6. [DOI] [PubMed] [Google Scholar]
- 42.Brennan SC, et al. Calcium sensing receptor signalling in physiology and cancer. Biochim. Biophys. Acta. 2013;1833:1732–1744. doi: 10.1016/j.bbamcr.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Tennakoon S, Aggarwal A, Kállay E. The calcium-sensing receptor and the hallmarks of cancer. Biochim. Biophys. Acta. 2016;1863:1398. doi: 10.1016/j.bbamcr.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 44.Colella, M., Gerbino, A., Hofer, A.M. & Curci, S. Recent advances in understanding the extracellular calcium-sensing receptor. F1000Res. 5, (2016). [DOI] [PMC free article] [PubMed]
- 45.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 46.Ward DT. Calcium receptor-mediated intracellular signalling. Cell Calcium. 2004;35:217–228. doi: 10.1016/j.ceca.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 48.Riccardi D, et al. Localization of the extracellular Ca(2+)-sensing receptor and PTH/PTHrP receptor in rat kidney. Am. J. Physiol. 1996;271:951–956. doi: 10.1152/ajprenal.1996.271.4.F951. [DOI] [PubMed] [Google Scholar]
- 49.Riccardi D, et al. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am. J. Physiol. 1998;274:611–622. doi: 10.1152/ajprenal.1998.274.3.F611. [DOI] [PubMed] [Google Scholar]
- 50.Gu J, Law AY, Yeung BH, Wong CK. Activation of gill Ca2+ -sensing receptor as a protective pathway to reduce Ca2+ -induced cytotoxicity. J. Mol. Endocrinol. 2014;53:155–164. doi: 10.1530/JME-14-0060. [DOI] [PubMed] [Google Scholar]
- 51.Kill IR. Localisation of the Ki-67 antigen within the nucleolus. Evidence for a fibrillarin-deficient region of the dense fibrillar component. J. Cell Sci. 1996;109(Pt 6):1253. doi: 10.1242/jcs.109.6.1253. [DOI] [PubMed] [Google Scholar]
- 52.MacCallum DE, Hall PA. The location of pKi67 in the outer dense fibrillary compartment of the nucleolus points to a role in ribosome biogenesis during the cell division cycle. J. Pathol. 2000;190:537–544. doi: 10.1002/(SICI)1096-9896(200004)190:5<537::AID-PATH577>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 53.Zhang C, Wu JM, Liao M, Wang JL, Xu CJ. The ROCK/GGTase Pathway Are Essential to the Proliferation and Differentiation of Neural Stem Cells Mediated by Simvastatin. J. Mol. Neurosci. 2016;60:474–485. doi: 10.1007/s12031-016-0811-y. [DOI] [PubMed] [Google Scholar]
- 54.Ba J, Friedman PA. Calcium-sensing receptor regulation of renal mineral ion transport. Cell Calcium. 2004;35:229–237. doi: 10.1016/j.ceca.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Ward DT, Riccardi D. Renal physiology of the extracellular calcium-sensing receptor. Pflugers Arch. 2002;445:169–176. doi: 10.1007/s00424-002-0914-x. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki Y, et al. Gain-of-function haplotype in the epithelial calcium channel TRPV6 is a risk factor for renal calcium stone formation. Hum. Mol. Genet. 2008;17:1613–1618. doi: 10.1093/hmg/ddn048. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki Y, Landowski CP, Hediger MA. Mechanisms and regulation of epithelial Ca2+ absorption in health and disease. Annu. Rev. Physiol. 2008;70:257–271. doi: 10.1146/annurev.physiol.69.031905.161003. [DOI] [PubMed] [Google Scholar]
- 58.Brown EM, Pollak M, Hebert SC. The extracellular calcium-sensing receptor: its role in health and disease. Annu. Rev. Med. 1998;49:15–29. doi: 10.1146/annurev.med.49.1.15. [DOI] [PubMed] [Google Scholar]
- 59.Harrington PE, Fotsch C. Calcium sensing receptor activators: calcimimetics. Curr. Med. Chem. 2007;14:3027–3034. doi: 10.2174/092986707782794096. [DOI] [PubMed] [Google Scholar]
- 60.Nemeth EF, et al. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J. Pharmacol. Exp. Ther. 2004;308:627–635. doi: 10.1124/jpet.103.057273. [DOI] [PubMed] [Google Scholar]
- 61.Xu B, et al. Calcium Signaling Is Involved in Cadmium-Induced Neuronal Apoptosis via Induction of Reactive Oxygen Species and Activation of MAPK/mTOR Network. PLoS One. 2011;6:e19052. doi: 10.1371/journal.pone.0019052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Son YO, et al. Cadmium induces autophagy through ROS-dependent activation of the LKB1–AMPK signaling in skin epidermal cells. Toxicol. Appl. Pharmacol. 2011;255:287–296. doi: 10.1016/j.taap.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thévenod F, Lee WK. Cadmium and cellular signaling cascades: interactions between cell death and survival pathways. Arch. Toxicol. 2013;87:1743–1786. doi: 10.1007/s00204-013-1110-9. [DOI] [PubMed] [Google Scholar]
- 64.Poilletperez L, Despouy G, Delagemourroux R, Boyerguittaut M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015;4:184–192. doi: 10.1016/j.redox.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jian X, et al. Importance of ROS-mediated autophagy in determining apoptotic cell death induced by physapubescin B. Redox Biol. 2017;12:198–207. doi: 10.1016/j.redox.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J. Autophagy and Mitophagy in Cellular Damage Control. Redox Biol. 2013;1:19. doi: 10.1016/j.redox.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L, Liu L, Luo Y, Huang S. MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. J. Neurochem. 2008;105:251–261. doi: 10.1111/j.1471-4159.2007.05133.x. [DOI] [PubMed] [Google Scholar]
- 68.Chen L, Liu L, Huang S. Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic. Biol. Med. 2008;45:1035–1044. doi: 10.1016/j.freeradbiomed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 69.Goillot E, et al. Mitogen-activated protein kinase-mediated Fas apoptotic signaling pathway. Proc Natl Acad Sci USA. 1997;94:3302–3307. doi: 10.1073/pnas.94.7.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson NL, et al. Signal transduction pathways regulated by mitogen-activated/ extracellular response kinase kinase kinase induce cell death. J. Biol. Chem. 1996;271:3229–3237. doi: 10.1074/jbc.271.6.3229. [DOI] [PubMed] [Google Scholar]
- 71.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 72.Hwang CJ, et al. Parkin deficiency exacerbate ethanol-induced dopaminergic neurodegeneration by P38 pathway dependent inhibition of autophagy and mitochondrial function. Redox Biol. 2017;11:456–468. doi: 10.1016/j.redox.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang Q, et al. Involvement of p38 in signal switching from autophagy to apoptosis via the PERK/eIF2α/ATF4 axis in selenite-treated NB4 cells. Cell Death Dis. 2014;5:e1270. doi: 10.1038/cddis.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keil E, et al. Phosphorylation of Atg5 by the Gadd45β-MEKK4-p38 pathway inhibits autophagy. Cell Death Differ. 2013;20:321. doi: 10.1038/cdd.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu D, Cederbaum AI. Inhibition of autophagy promotes CYP2E1-dependent toxicity in HepG2 cells via elevated oxidative stress, mitochondria dysfunction and activation of p38 and JNK MAPK. Redox Biol. 2013;1:552–565. doi: 10.1016/j.redox.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 77.Hu KH, et al. Cadmium Induced Apoptosis in MG63 Cells by Increasing ROS, Activation of p38 MAPK and Inhibition of ERK 1/2 Pathways. Cell Physiol. Biochem. 2015;36:642–654. doi: 10.1159/000430127. [DOI] [PubMed] [Google Scholar]
- 78.Yang LY, Wu KH, Chiu WT, Wang SH, Shih CM. The cadmium-induced death of mesangial cells results in nephrotoxicity. Autophagy. 2009;5:571–572. doi: 10.4161/auto.5.4.8311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.