Abstract
Modern broiler chickens are a major animal husbandry success story, both in terms of efficient resource utilisation and environmental sustainability. However, continuing artificial selection for both efficiency and rapid growth will be subject to both biological limits and animal welfare concerns. Using a novel analytical energy flow modelling approach, we predict how far such selection can go, given the biological limits of bird energy intake and partitioning of energy. We find that the biological potential for further improvements in efficiency, and hence environmental impact reduction, is minimal relative to past progress already made via artificial selection. An alternative breeding strategy to produce slower-growing birds to meet new welfare standards increases environmental burdens, compared to current birds. This unique analytic approach provides biologically sound guidelines for strategic planning of sustainable broiler production.
Introduction
Livestock production systems impact considerably on the environment1. However, amongst different livestock systems, chicken meat production has been found to have relatively low environmental impacts2,3. This is in part due to artificial selection over the recent decades, aiming for increased energy use efficiency and faster growth rates4–6. As a result of increased growth rate, the birds reach their slaughter weight earlier than ever before. This has reduced the resource use of the bird, mainly because during the shorter growth cycle, less energy is now needed to maintain the body functions7,8. This improved energy efficiency has considerably reduced the feed consumption of the birds and therefore improved the environmental sustainability of broiler production.
The worldwide demand for chicken meat continues to grow substantially9; this is in part due to associated health claims, lack of cultural limitations on its consumption, the efficiency at which it is produced and human population growth10. The key questions are how this predicted increase in chicken meat production will be achieved, and what the consequences of this change on the sustainability of the production system will be. The poultry industry is confident that further improvements in growth rate and resource use efficiency can be achieved via genetic selection into the foreseeable future11–15. However, these predictions are not substantiated with biological evidence in the scientific literature, with some suggesting that growth rate will soon reach a maximum biological threshold that may be insurmountable with conventional breeding16–19. Industry data suggest that the actual rate of annual improvement in daily weight gain of birds has begun to decrease in recent years4,5,20,21. This may be partly explained by the changing objectives of artificial selection. Consumer concerns about the welfare of fast-growing chickens22,23 and their meat quality24–27, for instance, may have shifted selection pressures away from increasing growth rate in favour of other traits28–30 (e.g. robustness, reproduction and adaptability31). On the other hand, selection for increased growth rate will ultimately be subject to limitations dictated by the biology of the bird and, as a matter of course, a plateau will inevitably be reached. Such biological limits have not generally been considered by the poultry industry, when making predictions on the potential and the consequences of further genetic improvements of the birds in the future.
The growth of an animal is ultimately driven by the following thermodynamic processes: (1) energy (feed) intake, (2) transfer of the energy to the metabolic system (digestion), (3) loss of energy in metabolic heat production and (4) partitioning the chemical energy within the body. We construct a modelling framework based on evidence of the apparent biological limit of each of these processes to systematically analyse the potential for breeding for increased efficiency and concomitant changes in the associated environmental burdens. The environmental burdens considered are the greenhouse gas (GHG) emissions and agricultural land use (ALU) associated with feed production, as well as the excretion of nitrogen (N) and phosphorus (P); each of these indicators has potential implications on environmental impact (e.g. global warming, eutrophication and acidification) and food security. Our analysis shows that the physical limits of the biological processes determining bird growth are likely to be reached much earlier than currently predicted by the poultry industry. As a result, the potential to improve the environmental sustainability of broiler production through further artificial selection is limited. On the other hand, an alternative breeding strategy to produce slow-growing birds to meet expectations of improved animal welfare, via reducing growth rate so that slaughter weight is not reached until 56 days, will inevitably lead to increased resource use and therefore higher environmental burdens.
Results
Limits of feed intake
In order to increase broiler growth rate, and therefore increase the energy use efficiency towards the biological limit, the daily metabolizable energy (ME) intake must be increased to facilitate growth (Equation (1)). This can be achieved by increasing the daily feed intake and this has actually been the trend in commercial broiler breeding over recent decades32–35. In practice, this means that the birds must eat increasingly higher amounts at an increasingly younger age, which is biologically challenging. Ultimately, the theoretical maximum of feed intake would be set by the capacity of the digestive system. Experiments where the energy density of the feed was reduced (so the birds are forced to increase their feed intake) provide data on the fast-growing broiler feed intake limit36–38. The highest potential feed intake shown in literature was presented by Leeson et al.36 In that study, broilers increased their gross feed intake by a total of 25% upon reaching a live weight (LW) of 2.8 kg on a low energy content feed compared to a high energy feed fed control group. The potential daily feed intake can therefore be determined from these data. As an outcome, the average daily feed intake at a LW of 1.0 kg and 2.8 kg could be increased by 10% and 1.1% respectively compared to current fast-growing birds21 (Fig. 1). This indicates that younger birds have the greatest potential to increase feed intake which reduces as they approach slaughter weight (2.2 kg39). Although much genetic progress has been achieved since the study of Leeson, et al.36, more recently Linares and Huang37 showed that the feed intake of current fast-growing broilers could be increased by a further 6% between day 10 and day 42 when fed on a low energy content feed, compared to a high energy content feed. The limit to feed intake considered here is consistent with the latter study37.
Figure 1.
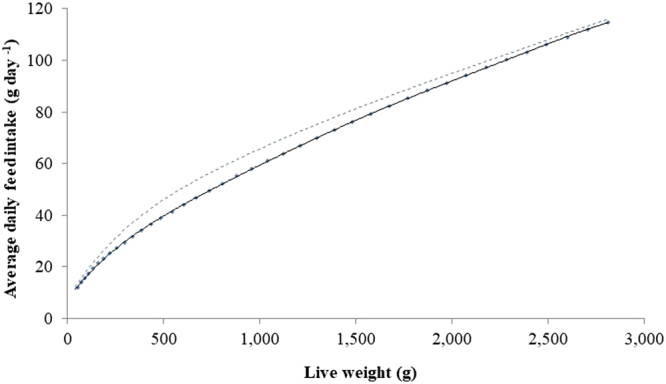
The average daily feed intake of a current fast-growing broiler ( ) and the potential average daily feed intake defined by the apparent biological limit of feed intake (broken line). Based on the data presented by Leeson et al.36.
) and the potential average daily feed intake defined by the apparent biological limit of feed intake (broken line). Based on the data presented by Leeson et al.36.
Limits of digestive efficiency
In artificial selection programmes, emphasis has been placed on the growth of certain body parts, such as the breast muscles, in order to increase carcass yield5,34,40. Consequently, the morphometries of the internal structures, in particular the organs that comprise the digestive system, have been shown to differ between high digestive efficiency genotypes and birds bred for high commercial performance41,42, i.e. increased energy use efficiency. In modern fast-growing birds, digesta throughput each day has increased to facilitate growth. Despite this, there is no evidence that breeding for increased commercial performance has led to any change in overall digestive efficiency per unit mass of digesta7; thus, selection pressure placed on increasing energy use efficiency and carcass yield at the very least must have conserved digestive efficiency whilst the size of the system has not increased at the same rate as other components of the body. Hence, the digestive efficiency as used in the energy flow model was expected to remain at its current level despite continuing selection for increasing energy efficiency. Since the digestible energy content of the feed per unit mass does not appear to be substantially compromised by augmented throughput5,7, nor does it appear to be improved genetically via selection for increased energy use efficiency42–44, it follows that the ME available to the broiler will be limited only by the capacity of feed intake.
Potential changes in energy partitioning
Broilers currently have a body protein and lipid content of around 20% and 8%, respectively, based on recent data presented by Mussini5. The abdominal fat pad constitutes about 2% of the body weight45,46. Reducing this feature to zero would result in a bird with a body lipid content of around 6%. This value places the animal firmly at the lower end of the estimated biological limit for fatness47,48. Less energy is required to grow a leaner bird than a fatter bird at the same overall growth rate (Equation (1)). Therefore, reducing the body lipid content to its minimum redirects a higher proportion of the ME into the growth of the fat-free body components, thus allowing the bird to reach slaughter weight faster. As a result, reducing the fat content from the current level to the apparent biological limit would reduce the necessary energy intake upon reaching slaughter by 1.7% (Table 1).
Table 1.
The effects of changing different processes of energy flow on the growth rate, total metabolizable energy (ME) intake and ME intake per unit mass of gain of a broiler grown to 2.2 kg.
| Scenario | Age at 2.2 kg slaughter weight (days) | Growth rate (g day−1) | Total ME intake (MJ) | ME intake per unit gain (kJ g−1) |
|---|---|---|---|---|
| Current fast-growing broiler | 34.2 | 63.1 | 45.9 | 21.3 |
| Increased feed intake only | 33.6 | 64.2 | 43.8 | 20.3 |
| Increased leanness only | 34.1 | 63.2 | 45.1 | 20.9 |
| Increased feed intake and leanness (maximum energy efficiency breeding strategy) | 33.0 | 65.3 | 42.0 | 19.4 |
| Reduced growth rate and increased leanness (Increased welfare breeding strategy) | 57.0 | 38.6 | 58.3 | 27.0 |
When changed, the feed intake and leanness are increased to their apparent biological limits.
In an earlier study, we found that the rate of metabolic heat production (MHR; MJ kg−1 d−1) of commercial broilers has either remained the same or been weakly positively correlated with the increase in growth rate7, over the recent decades, indicating that selection has not reduced the energy used for metabolic processes. Based on performance data for the current fast-growing birds21, the MHR was calculated to be 0.36 kg−1d−1. This same value was used to determine the energy distribution in the birds with maximum energy efficiency, as a conservative estimate for the further change.
Predicted future broiler performance
The average age modern fast-growing broiler lines reach a LW of 2.2 kg (slaughter weight) is currently between 34 and 35 days of growth21,49,50. The outcome of our analysis shows that even if the broiler growth rate is increased to the apparent biological limit, this will result in birds that reach their slaughter weight only 1.2 days sooner (Fig. 2). This results in an 8% reduction in the total feed energy intake of the bird upon reaching slaughter weight (Table 1).
Figure 2.

The growth rate of a current fast-growing broiler ( ) and the potential growth rate of future birds as defined by the different scenarios accessed; maximum energy efficiency (broken line) and increased welfare scenario (
) and the potential growth rate of future birds as defined by the different scenarios accessed; maximum energy efficiency (broken line) and increased welfare scenario ( ).
).
The analysis made above can be considered to represent a broiler bird with a maximum energy efficiency (and maximum growth rate). In an alternative scenario (increased welfare scenario), we calculated the energy intake of a slow-growing bird resulting from a higher welfare breeding strategy (i.e. growth rate is reduced so that birds reach a LW of 2.2 kg at 56 days of age, 23 days later than the current fast-growing bird). 5.7 MJ more energy per g of LW gain is required by birds from this slow-growing line to reach slaughter weight than is required by current fast-growing birds. This equates to 27% more total feed energy upon reaching slaughter than current fast-growing birds (Table 1).
Environmental impact assessment of future breeding scenarios
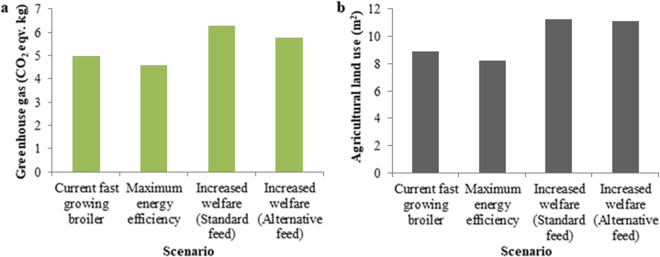
The maximum energy efficency scenario showed slightly reduced environmental burdens compared to current fast-growing birds, whereas the opposite was true for the scenario aiming to produce slow-growing “increased welfare” birds. The GHG emissions (Fig. 3(a)) and ALU (Fig. 3(b)) associated with feed production in the maximum energy efficency scenario were reduced by 8% when compared to current production. For the increased welfare scenario, both of these environmental indicators were increased by 27% when compared to current production on a standard feed. The environmental burdens were also calculated for the slow-growing line based on an alternative feeding programme (see Supplementary Table S1); this alternative feed had a lower protein content (19.6%) than the standard diet (21%), as to cater to the slow-growing line’s lower daily protein intake needed to maintain the required growth rate. When fed the alternative feed, GHG and ALU were increased by 16% and 24% respectively compared to current fast-growers reared on a standard feed (Fig. 3).
Figure 3.
The environmental impact implications associated with feed provision for one broiler of each scenario grown to 2.2 kg. (a) shows greenhouse gas emissions (CO2 eqv.) and (b) shows the agricultural land use (m2). The following scenarios are presented: current fast-growing birds, maximum energy efficiency birds and slow-growing increased welfare birds placed on a standard feed, as well as slow-growing increased welfare birds placed on an alternative feed formulated specifically for the slow-growing line.
The excretion of N and P were reduced by 23% and 15% respectively in the maximum energy efficiency scenario compared with current production, whereas these nutrients were excreted in higher quantities in the increased welfare scenario: an increase of 64% and 50% in the total N and P excretion was shown compared to current production on a standard feeding programme (Fig. 4). Applying the alternative feeding programme increased N and P excretion less than when the birds were raised on the standard feed, although this increase was still substantial (43% and 26% respectively).
Figure 4.
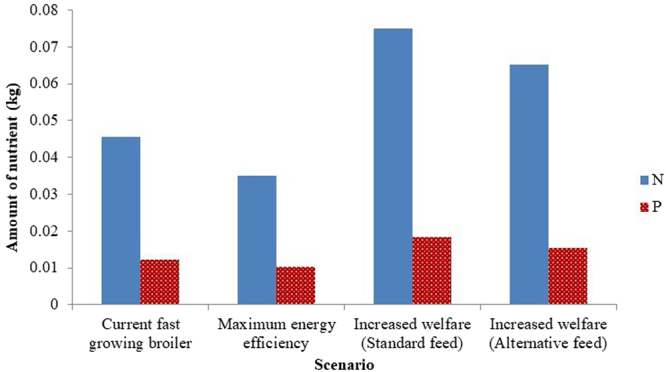
The nutrients, nitrogen (N) and phosphorus (P), that are expected to be excreted when one broiler is raised to 2.2 kg slaughter weight. The following scenarios are presented: current fast-growing birds, maximum energy efficiency birds and slow-growing increased welfare birds placed on a standard feed, as well as slow-growing increased welfare birds placed on an alternative feed formulated specifically for the slow-growing line.
Compared on a standard feeding programme with the maximum energy efficiency scenario, the slow-growing birds (increased welfare scenario) were associated with 37% more GHG and ALU, along with a 115% and 77% increase in N and P excretion respectively. When the alternative feeding programme was applied, with reduced feed protein content, the difference between the environmental burdens of the two future lines were reduced to 26%, 35%, 87% and 48% for GHG, ALU, N and P respectively.
Discussion
Our results contrast with previous predictions11–15 and indicate that the biological potential for further improvements in energy efficiency of broiler production via artificial selection is low. Of the energy flow processes determining growth, we found no evidence that either the digestive efficiency or the MHR have changed as a result of recent artificial selection in a way that could improve the energy efficiency of the bird. In contrast, an earlier analysis shows that the MHR may have slightly increased (thus allowing less energy to be allocated to growth) during the recent decades7. Therefore, the current analysis may even overestimate the energy efficiency of the future bird. Overall, it would be very difficult to improve the bird energy efficiency by changing the MHR through artificial selection. In practice, this would require producing less active birds that use less energy for physical movement. This direction in future artificial selection is not very likely, taking into account the animal welfare concerns.
Reducing the carcass fat allows more energy to be allocated to the growth of fat-free body components. As the energy density of these components (consisting mainly of water and protein), is much lower than that of fat8, this allows the bird to be grown to a certain slaughter weight with lower energy intake. Although reducing the fat content of the body to its apparent minimum can improve the energy efficiency of the bird, this effect is rather small (Table 1) because the fat content of the current broiler birds is already low. This is due to their young slaughter age, and probably also due to continued artificial selection during the recent decades6,51. As a result, the only component of energy flow that can still substantially affect the bird growth and efficiency is the intake of energy. Therefore, the potential to increase the feed intake of the future bird largely determines the potential to increase its energy efficiency. Obviously, there are physical and biological limits in this process; although increased feed intake inevitably facilitates faster growth, it should be kept in mind that faster growing birds are also getting younger at the slaughter age, which sets limits to their feed intake capacity. The faster growth is largely allocated to certain parts of the body (e.g. breast muscle), and therefore the growth of the digestive system does not follow the increased growth of the bird as a whole7. The maximum feed intake capacity applied was based on the highest value found in literature for the current broiler bird36. Whilst increasing the feed intake further could facilitate even faster growth and more efficient birds, there is no evidence in literature to indicate birds can increase feed intake beyond this level. Furthermore, it should also be kept in mind that the maximum growth rate and energy efficiency can be achieved only if the voluntary feed intake of the bird is equal to the physical capacity of the digestive system or if the energy content of the feed were to be increased52.
The results show that even if the full potential of increasing growth rate and energy efficiency of the broiler birds is utilized, there is apparently very little room for improvement in the considered environmental sustainability indicators, relative to the total improvement in recent decades5,6 and when compared to earlier predictions of further selection11,15. For instance, when raised to a LW of 2.2 kg, a commercial broiler in 1978 could be estimated to be responsible for 25% more GHG and ALU associated with feed provision than a modern commercial fast-growing line6. In contrast, according to this study, the improvements still available to be made via artificial selection equate to only a further 8% reduction in GHG and ALU (Fig. 3). However, since we only compared the environmental burdens of growing one bird from each line to 2.2 kg, we did not consider any changes in carcass quality (e.g. white striping, woody breast and green muscle disease), which can occur with increased growth rate and breast muscle yield25,27,34,53,54 and lead to rejections at the meat processing stage. The need to produce a higher number of birds to replace the rejected meat would result in increased environmental burdens.
It is widely acknowledged that many instances of bird ill-health are associated with fast growth rate (e.g. musculoskeletal disorders, myopathies and organ failures)55–60. Hence, there has been a growing market demand for slow-growing broilers, which have perceived higher welfare, as an alternative to the fast-growing, energy efficient broilers61–65. The alternative future breeding scenario presented in this study followed the recommendation of ‘welfare-friendly’ policies adopted by some businesses across Europe61. Such policies stipulate that chickens must have a reduced growth rate and live a minimum of 56 days54,62. Growing this slow-growing line would result in a substantial increase of environmental burdens in every environmental indicator considered in this study due to its increased feed consumption. The difference in the environmental burdens between the two breeding scenarios was found to be slightly reduced when the slow-growing line was predicted to be fed the alternative feed, which had a reduced protein inclusion, instead of the standard feed. The alternative feed incorporated less soya meal, which is associated with high ALU relative to other crops (e.g. wheat and rapeseed) and high GHG emissions arising from land use change66,67. However, it should be noted that such an outcome is indicative only, as the future composition of the feed and the cultivation techniques of future feed crops are very difficult to predict.
This study demonstrates the apparent biological potential for environmental impact reduction in the poultry industry that is still available via artificial selection of broiler chickens. However, it is possible that the resource inputs into the broiler growing facilities could change in the future due to technological advancements and policy changes in animal welfare. Such changes will have environmental impact implications. Furthermore, producing birds that grow more slowly will also change the amounts of other resources spent on them besides feed, e.g. energy needed for heating of the growing facility and to power ventilation, lights and feed dispensers. The scenarios described in this study may also produce differences in mortality rates34; in conventional broiler systems, the proportional environmental impact of bird mortality is currently very low67 and is intrinsically linked to factors that may change in the future in order to optimise production efficiency or meet different welfare standards, such as stocking density (combined weight of birds allowed per floor area). Furthermore, despite there being no evidence that digestive efficiency can be increased via artificial selection for current performance objectives, the digestibility of feed ingredients could be increased in the future, e.g. via advancements in the understanding and application of exogenous enzymes and prebiotics68. The increased growth rate of broilers can only be facilitated by feed with high inclusions of highly digestible protein. In Europe, imported soybean meal is incorporated as the main protein source in broiler feeds (see Supplementary Table S1). The GHG emissions associated with chicken meat may therefore be mitigated in the future by incorporating protein alternatives to imported soybean, such as European grown soybean, microalgae or insect meal69.
This study shows that the potential to increase the environmental sustainability of broiler production through artificial selection for higher energy efficiency is low compared to what has been achieved in recent decades. It is the first time that the biological limits have been analytically considered and applied to predict the potential environmental consequences of breeding strategies in this way, despite the fact that there is a substantial interest in predicting the environmental impacts of future livestock scenarios70,71. These results raise important questions as to whether the magnitude of further reductions in the environmental burdens can justify the continuation down the route of artificial selection towards maximum energy efficiency, until the biological limits of the birds are reached. Such a breeding strategy may prove unsustainable for the industry considering the market shift in Europe in favour of slow-growing broilers. On the other hand, reducing the growth rate of the birds following consumer desire for increased bird welfare will unequivocally result in a less efficient bird with higher environmental impact than current fast-growers. Balancing these social, economic and environmental aspects of the sustainability of livestock production will continue to challenge the poultry industry in the foreseeable future. It is therefore in the industry’s interest to continue to pay close attention to both consumer demands and their associated environmental impact implications.
Methods
Energy flow model
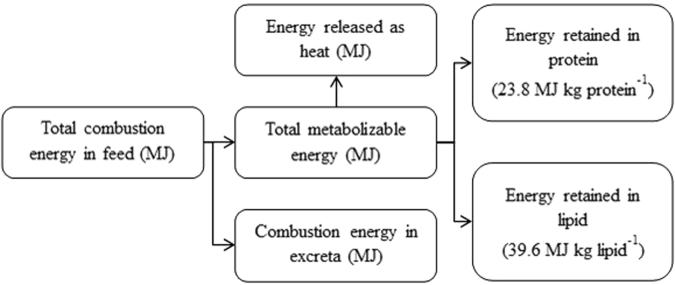
We described the energy flow through the chicken body using a simple analytical model consisting of the following thermodynamic processes: (1) energy intake in the form of feed, (2) transfer of the energy to metabolic system, i.e. making a proportion of the combustible energy of feed utilizable in metabolic process through the process of digestion, (3) loss of energy in metabolic heat production, including all life-sustaining biochemical transformations within the cells, such as those related to physical activity, protein turnover and the maintenance of energetically expensive systems and (4) partitioning the chemical energy within the body, i.e. storing the energy in the form of lipid and protein (Fig. 5).
Figure 5.
The components of energy flow through a broiler chicken.
If the energy use efficiency is to increase further, and consequently the environmental impact of broiler systems is to reduce through reduced resource use, this will be achieved through changes in the above processes. Hence, the biological limits of efficiency were assessed with an analytical energy flow model, which was used to predict possible future broiler growth trends based on the apparent biological limits of these processes incorporated into the model.
The structure of the energy flow model is as follows (Fig. 5): The “gross” energy (GE) intake equates to the total combustion energy in the feed which is consumed by the bird. Increasing feed intake towards the bird’s apparent intake capacity automatically increases the GE intake rate (GER; MJ d−1). A proportion of this energy is not utilised by the bird and is lost in the excreta. The net energy intake which is available to the bird is hence referred to as the metabolizable energy (ME) intake. The ME intake rate (MER; MJ d−1) is thus determined by the coefficient of digestive efficiency (Defficiency); increased digestive efficiency will increase the amount of the GE that can be utilised by the bird and therefore increase the energy use efficiency (Equation (1)). The ME must then be distributed between what is stored, as protein and lipids, and what is lost as heat. The chemical energy retained in the body as protein and lipid can be quantified based on their heats of combustion, i.e. 23.8 and 39.6 MJ kg−1 respectively8,72. The overall fat-free body composition (i.e. water, protein and minerals) can be approximated based on allometric relationships73, and as a result, the combustion energy content of the body for birds with a certain body weight and a given fat content can be calculated. Since less chemical energy is stored in fat-free body components compared to lipid, the energy that is taken in can be used more efficiently for weight gain when leanness is increased. Energy is lost as heat through the metabolic processes related to lean and fat body growth, as well as other metabolic pathways, such as those essential processes for maintaining normal bodily functioning. Thus the total energy lost as heat is the ME intake minus the energy stored by the body in protein and lipids, and is accounted for by the coefficient for the MHR, which can be calculated on the basis of the total energy intake and the composition of the total LW (kg).
| 1 |
where:
MER = Metabolizable energy intake rate;
GER = Gross energy intake rate;
Defficiency = Coefficient of digestive efficiency;
MHR = Rate of metabolic heat production;
LW = Live weight;
ΔProtein and ΔLipid = The daily increase of the protein and lipid mass, respectively.
To determine the coefficients Defficiency and MHR, we went through the available literature on the energy efficiency of the current broiler lines, and the trends of their genetic changes over the recent decades (summarized by Tallentire et al.7). Based on this review, we could specify the values of these constants for the current, fast-growing birds and specify any possible changes in the current values compared to the historic data. If no past trends in these coefficients were found, we could expect that they are not affected by artificial selection, and will therefore remain unchanged also in the future breeding programmes.
Daily feed intake and growth
We used literature on feeding experiments where the birds were forced to increase their feed intake, in order to determine their maximum daily feed intake capacity. This was then converted to GER, assuming that the composition of feed will remain unchanged (i.e. the production would be based on high-energy concentrate feed). The relationship between LW and the average daily feed intake for a current fast-growing bird was then quantified using a nonlinear curve21,50 (Fig. 1). This relationship is linear between 0.3 kg LW and the average slaughter weight of standard indoor broilers (2.2 kg39), therefore the potential feed intake each day beyond 0.3 kg LW was derived from the regression line equation for the maximum average daily feed intake limit each day presented in the literature36. As an outcome of this analysis, the daily live weight gain for the birds resulting from future breeding scenarios was calculated using Equation (1), with the expected values of GE, Defficiency and MHR, and ΔProtein and ΔLipid, based on the expected changes in the body composition.
Future broiler production scenarios
Two potential broiler breeding scenarios were addressed in this study. The first was based on the continuation of artificial selection for increased energy efficiency, as applied to current fast-growing lines reared commercially21,50. The performance of broilers subjected to further selection for increased energy use efficiency was calculated based on evidence of current genetic trends and apparent biological limits in the underlying biology7. In order to improve the energy use efficiency and increase the growth rate of the line further, the apparent biological limit of feed intake was applied to the current genotype, and the potential of the other energy flow processes (digestive efficiency, body composition and MHR) were changed to their apparent biological limits to facilitate this breeding strategy’s objectives. Finally, using the model shown in Equation (1), we specified the growth rate of the bird produced as an outcome of this scenario, and calculated the time and energy intake needed to reach the 2.2 kg slaughter weight.
In an alternative future breeding scenario, birds must be selected so that they reach slaughter weight no sooner than in 56 days62,74; this is also the minimum slaughter age currently required in free range chickens65. It is reasonable to assume, however, that for economic reasons the breeders will try to produce the most efficient birds possible within the limits of the welfare standard envelope, as well as the biological limits, via placing selective pressure on other traits than growth, i.e. body composition. Therefore, for the future slow-growing birds, we applied the following scenario: (1) We reduced the body fat content to its apparent biological limit. (2) We set the age when the slaughter weight is reached to be 56 days. (3) For other constants in Equation (1), we applied the same procedure as for the increased energy efficiency scenario described above. (4) Finally, we used Equation (1) to calculate the energy intake of the birds needed to reach a slaughter weight of 2.2 kg with a growth rate specified by the welfare standards. Hence, due to different selection strategy objectives, this procedure differed from that used in the scenario for the increased energy efficiency, where we specified the rate of energy intake according to biological limits of the bird only.
For the purpose of this study, the composition of the broiler feed was represented by two feeding programmes (see Supplementary Table S1). The first was based on the standard nutritional recommendations for current fast-growing birds50,75; this feed had an average energy and crude protein content of 13.2 MJ kg−1 and 21% respectively, and was here presumed to be fed to birds representing both of the future breeding scenarios; this was done in order to show the environmental implications of the artificial selection only. The second feeding programme was based on current nutritional specifications recommended for current slow-growing birds76; this “alternative feed” had a lower crude protein content compared to the standard feed and was fed to the increased welfare scenario only (see Supplementary Table S1). The composition of both feeds were formulated using a least cost formulation method, on the basis of current UK ingredient prices77. The feeds used in this study were, therefore, expected to be typical of current UK broiler production as a case in point for European systems. The primary energy ingredient of both feeds was wheat, whilst the main source of protein was provided by soybean meal, which is mainly imported to Europe from South America66.
Environmental indicators
Energy provision (in the form of feed) represents the poultry industry’s greatest environmental hotspot67,77, hence the environmental indicators considered include inputs and outputs that are related to producing the feed required by one broiler bird to achieve a slaughter LW of 2.2 kg39. All upstream processes associated with feed production, such as resource inputs to fertilizer production and the emissions that arise as a result of their application to fields, as well as the energy inputs to processing and transport of ingredients, were based on current practices.
For the purpose of this study, the differences in the most relevant feed-related environmental indicators of broiler production were quantified. As such the environmental burdens of GHG emissions, measured in carbon dioxide equivalent (CO2 eqv.) with a 100 year timescale, and the ALU (m2), both associated with the feed provision, were calculated. The main agricultural sources of GHG are nitrous oxide (N2O) together with CO2 from fossil fuel and methane (CH4), although non-ruminant species produce negligible amounts of enteric CH42. GHG emissions have impacts, such as global warming. 1 kg of CH4 and N2O emitted was considered to be equivalent to 25 and 298 kg of CO2 respectively78. The CO2 released due to land transformation was included following the PAS2050:2012-1 methodology79. The emission data for feed ingredients were based on national inventory reports, SimaPro databases and literature77.
The excretion of the environmentally important nutrients N and P were also considered as environmental indicators. Although the manure containing these nutrients can be used in the place of synthetic fertilizers (especially important in organic farming), excess of nutrients is associated with acidification and localised eutrophication, whilst N is responsible for the ammonia emissions at housing, manure storage and field spreading78. The N and P deposition in manure were calculated using the mass balance principle; the nutrients retained in the broiler’s body were subtracted from the total N and P supplied in the feed.
Data availability
All data analysed during this study are publicly available and cited within this article. Furthermore, all data generated during this study are included within this published article (and its Supplementary Information files).
Electronic supplementary material
Acknowledgements
We would like to acknowledge that this research was made possible by a Departmental Training Award from Newcastle University to C.W. Tallentire.
Author Contributions
The paper arises from the doctoral thesis of C.W.T.; I.L. and I.K. designed the research; C.W.T., I.L. and I.K. performed the research; C.W.T. run the model; C.W.T., I.L. and I.K. interpreted the outcome and wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
A correction to this article is available online at https://doi.org/10.1038/s41598-018-23133-8.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19231-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Steinfeld, H. et al. Livestocks long shadow - environmental issues and options (2006).
- 2.Williams, A. G., Audsley, E. & Sandars, D. L. D. Determining the environmental burdens and resource use in the production of agricultural and horticultural commodities., (Cranfield University and Defra, Bedford, UK, 2006).
- 3.De Vries, M. & De Boer, I. Comparing environmental impacts for livestock products: A review of life cycle assessments, Livestock Science, vol. 128 (2009).
- 4.Laughlin, K. The Evolution of Genetics, Breeding and Production. (Harper Adams University College, Newport, Shropshire 2007).
- 5.Mussini, F. J. Comparative response of different broiler genotypes to dietary levels, University of Arkansas (2012).
- 6.Zuidhof MJ, Schneider BL, Carney VL, Korver DR, Robinson FE. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tallentire CW, Leinonen I, Kyriazakis I. Breeding for efficiency in the broiler chicken: A review. Agronomy for Sustainable Development. 2016;36:66. doi: 10.1007/s13593-016-0398-2. [DOI] [Google Scholar]
- 8.Emmans GC. Effective energy: a concept of energy utilization applied across species. British Journal of Nutrition. 1994;71:801–821. doi: 10.1079/BJN19940188. [DOI] [PubMed] [Google Scholar]
- 9.Alexandratos, N. & Bruinsma, J. World agriculture towards 2030/2050: the 2012 revision. (ESA Working paper Rome, FAO, 2012).
- 10.Magdelaine P, Spiess M, Valceschini E. Poultry meat consumption trends inEurope. World’s Poultry Science Journal. 2008;64:53–64. doi: 10.1017/S0043933907001717. [DOI] [Google Scholar]
- 11.Leinonen I, Williams AG, Kyriazakis I. Potential environmental benefits of prospective genetic changes in broiler traits. Poultry Science. 2016;95:228–236. doi: 10.3382/ps/pev323. [DOI] [PubMed] [Google Scholar]
- 12.Muir WM, et al. Genome-wide assessment of worldwide chicken SNP genetic diversity indicates significant absence of rare alleles in commercial breeds. Proceedings of the National Academy of Sciences. 2008;105:17312–17317. doi: 10.1073/pnas.0806569105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill WG. Estimation, effectiveness and opportunities of long term genetic improvement in animals and maize. Lohmann information. 2008;43:3–20. [Google Scholar]
- 14.Leinonen I, Kyriazakis I. How can we improve the environmental sustainability of poultry production? Proceedings of the Nutrition Society. 2016;75:265–273. doi: 10.1017/S0029665116000094. [DOI] [PubMed] [Google Scholar]
- 15.Defra. A study of the scope of application of research in animal genomics and breeding to reduce nitrogen and methane emissions from livestock based food chains. Report No. AC0204, (Defra, 2008).
- 16.Albers, G., Rattink, A. & Vereijken, A. In XII European Poultry Conference, Verona, Italy (2006).
- 17.Pollock DL. A geneticist’s perspective from within a broiler primary breeder company. Poultry Science. 1999;78:414–418. doi: 10.1093/ps/78.3.414. [DOI] [PubMed] [Google Scholar]
- 18.MacRae V, Mahon M, Gilpin S, Sandercock D, Mitchell M. Skeletal muscle fibre growth and growth associated myopathy in the domestic chicken (Gallus domesticus) British poultry science. 2006;47:264–272. doi: 10.1080/00071660600753615. [DOI] [PubMed] [Google Scholar]
- 19.Gous RM. Nutritional limitations on growth and development in poultry. Livestock Science. 2010;130:25–32. doi: 10.1016/j.livsci.2010.02.007. [DOI] [Google Scholar]
- 20.Aviagen. Ross 308 Broiler Performance Objectives, http://www.thepoultrysite.com/downloads/vars/a/10/offset/8/ (2007).
- 21.Aviagen. Ross 308 Broiler Performance Objectives, http://en.aviagen.com/ross-308/ (2014).
- 22.Clark B, Stewart GB, Panzone LA, Kyriazakis I, Frewer LJ. A Systematic Review of Public Attitudes, Perceptions and Behaviours Towards Production Diseases Associated with Farm Animal Welfare. Journal of Agricultural and Environmental Ethics. 2016;29:455–478. doi: 10.1007/s10806-016-9615-x. [DOI] [Google Scholar]
- 23.Clark B, Stewart GB, Panzone LA, Kyriazakis I, Frewer LJ. Citizens, consumers and farm animal welfare: A meta-analysis of willingness-to-pay studies. Food Policy. 2017;68:112–127. doi: 10.1016/j.foodpol.2017.01.006. [DOI] [Google Scholar]
- 24.Petracci M, Mudalal S, Soglia F, Cavani C. Meat quality in fast-growing broiler chickens. World’s Poultry Science Journal. 2015;71:363–374. doi: 10.1017/S0043933915000367. [DOI] [Google Scholar]
- 25.Kuttappan VA, et al. Estimation of factors associated with the occurrence of white striping in broiler breast fillets. Poultry Science. 2013;92:811–819. doi: 10.3382/ps.2012-02506. [DOI] [PubMed] [Google Scholar]
- 26.Kuttappan VA, et al. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poultry Science. 2012;91:1240–1247. doi: 10.3382/ps.2011-01947. [DOI] [PubMed] [Google Scholar]
- 27.Kuttappan VA, Hargis BM, Owens CM. White striping and woody breast myopathies in the modern poultry industry: a review. Poultry Science. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- 28.RSPCA. Science Group Reviewhttps://www.rspca.org.uk/search?searchKey=broiler&x=0&y=0 (2008).
- 29.RSPCA. Most chickens we eat are dangerously heavy for their age, https://www.rspcaassured.org.uk/get-involved/most-chickens-we-eat-are-dangerously-heavy-for-their-age/ (2015).
- 30.Compassion in World Farming. Higher welfare for meat chickens, https//www.ciwf.org.uk/farm-animals/chickens/meat-chickens/higher-welfare-alternatives (2017).
- 31.Neeteson-van Nieuwenhoven A-M, Knap P, Avendaño S. The role of sustainable commercial pig and poultry breeding for food security. Animal Frontiers. 2013;3:52–57. doi: 10.2527/af.2013-0008. [DOI] [Google Scholar]
- 32.Siegel P, Wisman E. Selection for body weight at eight weeks of age 6. Changes in appetite and feed utilization. Poultry Science. 1966;45:1391–1397. [Google Scholar]
- 33.Pym R, Nicholls P. Selection for food conversion in broilers: Direct and correlated responses to selection for body‐weight gain, food consumption and food conversion ratio. British Poultry Science. 1979;20:73–86. doi: 10.1080/00071667908416551. [DOI] [Google Scholar]
- 34.Havenstein GB, Ferket PR, Qureshi MA. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult Sci. 2003;82:1500–1508. doi: 10.1093/ps/82.10.1500. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt C, Persia M, Feierstein E, Kingham B, Saylor W. Comparison of a modern broiler line and a heritage line unselected since the 1950s. Poultry science. 2009;88:2610–2619. doi: 10.3382/ps.2009-00055. [DOI] [PubMed] [Google Scholar]
- 36.Leeson S, Caston L, Summers JD. Broiler Response to Diet Energy. Poultry Science. 1996;75:529–535. doi: 10.3382/ps.0750529. [DOI] [PubMed] [Google Scholar]
- 37.Linares, L. & Huang, K. In Proceedings of the XIII European Poultry Conference (Tours, France, 2010).
- 38.Pauwels J, et al. Selection for Growth Performance in Broiler Chickens Associates with Less Diet Flexibility. PLOS ONE. 2015;10:e0127819. doi: 10.1371/journal.pone.0127819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Defra. United Kingdom Poultry and Poultry Meat Statistics – May 2014, https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/336250/poultry-statsnotice-26jun14.pdf (2014).
- 40.Carré B, Mignon-Grasteau S, Juin H. Breeding for feed efficiency and adaptation to feed in poultry. World’s Poultry Science Journal. 2008;64:377–390. doi: 10.1017/S004393390800010X. [DOI] [Google Scholar]
- 41.Mitchell MA, Smith MW. The effects of genetic selection for increased growth rate on mucosal and muscle weights in the different regions of the small intestine of the domestic fowl (Gallus domesticus) Comparative Biochemistry and Physiology Part A: Physiology. 1991;99:251–258. doi: 10.1016/0300-9629(91)90268-H. [DOI] [PubMed] [Google Scholar]
- 42.Péron A, et al. Effects of wheat quality on digestion differ between the D + and D-chicken lines selected for divergent digestion capacity. Poultry science. 2006;85:462–469. doi: 10.1093/ps/85.3.462. [DOI] [PubMed] [Google Scholar]
- 43.Carré, B. et al. In Proceedings of the 15th European Symposium on poultry nutrition. 42–44 (World’s Poultry Science Association(WPSA).
- 44.Rougière N, Carré B. Comparison of gastrointestinal transit times between chickens from D + and D− genetic lines selected for divergent digestion efficiency. Animal. 2010;4:1861–1872. doi: 10.1017/S1751731110001266. [DOI] [PubMed] [Google Scholar]
- 45.Gaya LG, et al. Heritability and genetic correlation estimates for performance and carcass and body composition traits in a male broiler line. Poultry science. 2006;85:837–843. doi: 10.1093/ps/85.5.837. [DOI] [PubMed] [Google Scholar]
- 46.Grosso J, et al. Comparison of different models to estimate genetic parameters for carcass traits in a commercial broiler line. Genetics and Molecular Research. 2010;9:908–918. doi: 10.4238/vol9-2gmr773. [DOI] [PubMed] [Google Scholar]
- 47.Emmans, G. C. In 28th British Poultry Breeders’ Round Table (Cambridge, uk, 1987).
- 48.Schiavon S, et al. Use of simple body measurements and allometry to predict the chemical growth and feed intake in pigs. Italian Journal of Animal Science. 2007;6:27–44. doi: 10.4081/ijas.2007.27. [DOI] [Google Scholar]
- 49.Aviagen. Arbor Acres Plus Broiler Performance Objectives, http://en.aviagen.com/assets/Tech_Center/AA_Broiler/AA-Broiler-PO-2014-EN.pdf (2014).
- 50.Cobb. Cobb 500. Performance and nutrition supplement, http://www.winmixsoft.com/en/blog/item/cobb500 (2014).
- 51.Fleming, E. C., Fisher, C. & McAdam, J. In Proceedings of the British Society of Animal Science. 67.
- 52.Emmans, G. & Kyriazakis, I. Issues arising from genetic selection for growth and body composition characteristics in poultry and pigs. BSAS Occasional Publication, 39–52 (2000).
- 53.Bilgili S, Hess J. Green muscle disease. Reducing the incidence in broiler flock. Ross Tech. 2008;8:3. [Google Scholar]
- 54.Efsa Panel. on Animal Health and Welfare. Scientific Opinion on the influence of genetic parameters on the welfare and the resistance to stress of commercial broilers. EFSA Journal. 2010;8:1666–n/a. doi: 10.2903/j.efsa.2010.1666. [DOI] [Google Scholar]
- 55.Bessei W. Welfare of broilers: a review. World’s Poultry Science Journal. 2006;62:455–466. doi: 10.1079/WPS2005108. [DOI] [Google Scholar]
- 56.Rodenburg T, Turner S. The role of breeding and genetics in the welfare of farm animals. Animal frontiers. 2012;2:16–21. doi: 10.2527/af.2012-0044. [DOI] [Google Scholar]
- 57.Morton, D. et al. In Efsa Scientific Colloquium Summary Report.
- 58.Fanatico AC, et al. Performance, Livability, and Carcass Yield of Slow- and Fast-Growing Chicken Genotypes Fed Low-Nutrient or Standard Diets and Raised Indoors or with Outdoor Access. Poultry Science. 2008;87:1012–1021. doi: 10.3382/ps.2006-00424. [DOI] [PubMed] [Google Scholar]
- 59.Mikulski D, Celej J, Jankowski J, Majewska T, Mikulska M. Growth performance, carcass traits and meat quality of slower-growing and fast-growing chickens raised with and without outdoor access. Asian-Aust. J. Anim. Sci. 2011;24:1407–1416. doi: 10.5713/ajas.2011.11038. [DOI] [Google Scholar]
- 60.Meseret S. A review of poultry welfare in conventional production system. Livestock Research for Rural Development. 2016;28:12. [Google Scholar]
- 61.Jansen, M. The tipping point of the perceptions of the Dutch broiler industry: the case of the ‘plofkip’, Wageningen University, (2014).
- 62.Neilson, Z. The case of the exploded Dutch chickens, http://sustainablefoodtrust.org/articles/?category = food-ethics (2016).
- 63.Clarke, P. Slow growing chicken developed for meat market, http://www.fwi.co.uk/poultry/slow-growing-chicken-developed-for-meat-market.htm (2014).
- 64.Stichting W Dier. Help de plofkip de supermarkten uit!, https://www.youtube.com/watch?v=hL-aSxy1uis (2012).
- 65.RSPCA. RSPCA welfare standards for chickens, https://www.berspcaassured.org.uk/media/1086/rspca-standards-chickens-nov2013.pdf (2013).
- 66.Kebreab, E. et al. Environmental impact of using specialty feed ingredients in swine and poultry production: A life cycle assessment. Journal of Animal Science, 10.2527/jas.2015-9036 (2016). [DOI] [PubMed]
- 67.Leinonen I, Williams AG, Wiseman J, Guy J, Kyriazakis I. Predicting the environmental impacts of chicken systems in the UK through a Life Cycle Assessment: broiler production systems. Poultry science. 2012;91:8–25. doi: 10.3382/ps.2011-01634. [DOI] [PubMed] [Google Scholar]
- 68.Pourabedin M, Zhao X. Prebiotics and gut microbiota in chickens. FEMS Microbiology Letters. 2015;362:fnv122–fnv122. doi: 10.1093/femsle/fnv122. [DOI] [PubMed] [Google Scholar]
- 69.van Krimpen, M. M., Bikker, P., van der Meer, I. M., van der Peet-Schwering, C. M. C. & Vereijken, J. M. Cultivation, processing and nutritional aspects for pigs and poultry of European protein sources as alternatives for imported soybean products. (Wageningen UR, Wageningen, The Netherlands, 2013).
- 70.van Middelaar, C., Berentsen, P., Dijkstra, J. & I, D. B. In Proceedings of the 9th International Conference on Life Cycle Assessment in the Agri-Food Sector. 1445–1454.
- 71.Besson M, et al. Environmental impacts of genetic improvement in growth rate and feed conversion in fish farming under density and nitrogen limitation. Journal of Cleaner Production. 2016;116:100–109. doi: 10.1016/j.jclepro.2015.12.084. [DOI] [Google Scholar]
- 72.Boekholt HA, et al. C. P. Effect of dietary energy restrictions on retention of protein, fat and energy in broiler chickens. British Poultry Science. 1994;35:603–614. doi: 10.1080/00071669408417725. [DOI] [PubMed] [Google Scholar]
- 73.Gous R, Moran E, Stilborn H, Bradford G, Emmans G. Evaluation of the parameters needed to describe the overall growth, the chemical growth, and the growth of feathers and breast muscles of broilers. Poultry Science. 1999;78:812–821. doi: 10.1093/ps/78.6.812. [DOI] [PubMed] [Google Scholar]
- 74.van der Aar, P., Doppenberg, J. & Kwakernaak, C. Which feedstuffs will be used in the future. Sustainable poultry production in Europe, 103–111 (2016).
- 75.Aviagen. Ross 308 Broiler Nutrition Specifications, http://en.aviagen.com/ross-308/ (2014).
- 76.Aviagen. Managing the Rowan Rangerhttp://en.aviagen.com/assets/Tech_Center/Rowan_Range/RowanRangerManagement2016EN.pdf (2016).
- 77.Tallentire CW, Mackenzie SG, Kyriazakis I. Environmental impact trade-offs in diet formulation for broiler production systems in the UK and USA. Agricultural Systems. 2017;154:145–156. doi: 10.1016/j.agsy.2017.03.018. [DOI] [Google Scholar]
- 78.IPCC. IPCC Guidelines for National Greenhouse Gas Inventories. (2006).
- 79.BSI. In Assessment of life cycle greenhouse gas emissions from horticultural products. (BSI, 2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analysed during this study are publicly available and cited within this article. Furthermore, all data generated during this study are included within this published article (and its Supplementary Information files).