Abstract
Automatic motor mimicry is essential to the normal processing of perceived emotion, and disrupted automatic imitation might underpin socio-emotional deficits in neurodegenerative diseases, particularly the frontotemporal dementias. However, the pathophysiology of emotional reactivity in these diseases has not been elucidated. We studied facial electromyographic responses during emotion identification on viewing videos of dynamic facial expressions in 37 patients representing canonical frontotemporal dementia syndromes versus 21 healthy older individuals. Neuroanatomical associations of emotional expression identification accuracy and facial muscle reactivity were assessed using voxel-based morphometry. Controls showed characteristic profiles of automatic imitation, and this response predicted correct emotion identification. Automatic imitation was reduced in the behavioural and right temporal variant groups, while the normal coupling between imitation and correct identification was lost in the right temporal and semantic variant groups. Grey matter correlates of emotion identification and imitation were delineated within a distributed network including primary visual and motor, prefrontal, insular, anterior temporal and temporo-occipital junctional areas, with common involvement of supplementary motor cortex across syndromes. Impaired emotional mimesis may be a core mechanism of disordered emotional signal understanding and reactivity in frontotemporal dementia, with implications for the development of novel physiological biomarkers of socio-emotional dysfunction in these diseases.
Introduction
Motor mimicry supports the decoding of perceived emotions by the healthy brain1,2. Viewing emotional facial expressions rapidly and involuntarily engages the facial muscles of neurologically normal observers3,4. Emotional mimesis may have evolved as a specialized ‘exaptation’ of action observation, and by promoting emotional contagion and affective valuation may have facilitated the development of advanced human social behaviour and theory of mind2,5,6. In line with this interpretation, motor recoding of observed emotion correlates with empathy and emotion identification ability7 and predicts authenticity judgments on facial expressions8; while conversely, facial paralysis induced by botulinum toxin attenuates emotional reactivity9. The linkage between emotion observation, recognition and mimesis is precise: viewing of universal facial emotional expressions10 produces signature profiles of electromyographic (EMG) activity in the facial muscles conveying each expression3,11. This phenomenon is mediated by distributed, cortico-subcortical brain regions that may together instantiate a hierarchically organised neural substrate for inferring the intentions and subjective states of others12–15: primary visual representations of emotions would comprise the lowest level of the hierarchy, ascending through sensorimotor representations of emotional movement kinematics, prediction of movement goals and affective states, and encoding of intentions, including affective mentalising.
On clinical, pathophysiological and neuroanatomical grounds, altered motor recoding might be anticipated to underlie impaired emotional and social signal processing in the frontotemporal dementias (FTD). This diverse group of neurodegenerative diseases manifests as three canonical clinico-anatomical syndromes16; behavioural variant (bvFTD), semantic variant primary progressive aphasia (svPPA) and nonfluent variant primary progressive aphasia (nfvPPA). These broad syndromic groupings encompass various sub-syndromes: in particular, within the heterogeneous bvFTD syndrome at least two major variants can be defined, based on the relative selectivity of right temporal lobe atrophy17,18. Deficits in emotion recognition, empathy and social understanding and behaviour are defining features of bvFTD but integral to all FTD syndromes19–24 and collectively engender substantial distress and care burden25. Impaired facial emotion recognition in bvFTD, svPPA and nfvPPA has been linked to atrophy of an overlapping network of cerebral regions including orbitofrontal cortex, posterior insula and antero-medial temporal lobe23,26, implicated in evaluation of facial emotional expressions and integration with bodily signals27–29. Moreover, various abnormalities of physiological reactivity have been documented in FTD, including changes in resting skin conductance and heart rate variability in bvFTD and altered homeostatic and affective autonomic responses in bvFTD, svPPA and nfvPPA30–36. Patients with bvFTD have been noted to have reduced facial expressivity37 and indeed, deficient volitional imitation of emotional faces38. However, whereas impaired facial EMG reactivity to facial expressions has been linked to emotion processing deficits in Parkinson’s disease39,40, Huntington’s disease41 and schizophrenia42, the motor physiology of emotional reactivity has not been addressed in the FTD spectrum.
In this study, we investigated facial motor responses to viewing facial emotional expressions in a cohort of patients representing all major phenotypes of FTD (bvFTD, svPPA and nfvPPA) relative to healthy older individuals. In addition to the canonical syndromic FTD variants, we identified a subset of patients presenting with behavioural decline and selective right temporal lobe atrophy (right temporal variant, rtvFTD): this entity has been proposed previously to account for much of the heterogeneity of the broader bvFTD syndromic spectrum and is associated with particularly severe disturbances of facial empathy18,38,43,44. We compared facial EMG response profiles with emotion identification accuracy on a stimulus set comprising video recordings of dynamic, natural facial expressions: such expressions are more faithful exemplars of the emotions actually encountered in daily life and are anticipated to engage mechanisms of motor imitation more potently than the static images conventionally used in neuropsychological studies45,46. Neuroanatomical associations of facial expression identification and EMG reactivity in the patient cohort were assessed using voxel-based morphometry (VBM). Based on previous clinical and physiological evidence3,4,30,31,33,34,36,37,43,47, we hypothesised that healthy older individuals would show rapid and characteristic patterns of facial muscle responses to perceived emotional expressions coupled with efficient emotion identification. In contrast, we hypothesised that all FTD syndromes would be associated with impaired emotion identification but would exhibit separable profiles of facial muscle reactivity. In particular, we predicted that bvFTD and rtvFTD would be associated with reduced EMG responses while svPPA would be associated with aberrant coupling of muscle reactivity to emotion identification and nfvPPA with a more selective, emotion-specific reactivity profile. Based on previous neuroimaging studies both in the healthy brain and in FTD14,23,26,45,48–50, we further hypothesised that facial emotion identification and EMG reactivity would have partly overlapping neuroanatomical correlates within the extensive cortical circuitry previously implicated in the decoding of visual emotional signals, supplementary motor and insular cortices mediating the integration of somatic representations and antero-medial temporal and prefrontal circuitry involved in the evaluation of emotion. Within these distributed networks (given the known neuroanatomical heterogeneity of the target syndromes) we predicted a differential emphasis of grey matter correlates, with more marked involvement of inferior frontal, anterior cingulate and insular cortices in bvFTD and nfvPPA and more extensive, lateralised temporal lobe involvement in svPPA and rtvFTD16–18.
Materials and Methods
Participants
Thirty-seven consecutive patients fulfilling consensus criteria for a syndrome of FTD51,52 (19 with bvFTD, nine with svPPA, nine with nfvPPA) and 21 healthy older individuals with no history of neurological or psychiatric illness participated. General characteristics of the participant groups are summarised in Table 1. No participant had a history of facial palsy or clinically significant visual loss after appropriate correction. There was clinical evidence of orofacial apraxia in seven patients in the nfvPPA group, but none in any of the other participant groups. General neuropsychological assessment (see Table 1) and brain MRI corroborated the syndromic diagnosis in all patients; no participant had radiological evidence of significant cerebrovascular damage. Based on visual inspection of individual brain MR images, six patients with a behavioural syndrome and relatively selective right temporal lobe atrophy were re-categorised as a rtvFTD subgroup (throughout this paper, we use ‘bvFTD’ to refer to those patients with a behavioural presentation not re-classified as rtvFTD). Between group differences in demographic and neuropsychological variables were analysed using ANOVAs with post hoc T-tests when main effects were found, except for categorical variables, for which a chi-squared test was used.
Table 1.
Demographic, clinical and neuropsychological characteristics of participant groups.
| Characteristic | Controls | bvFTD | rtvFTD | svPPA | nfvPPA |
|---|---|---|---|---|---|
| Demographic/clinical | |||||
| No. (male:female) | 9:12 | 10:3 | 6:0a | 7:2 | 4:5 |
| Age (years) | 69.1 (5.3) | 66.2 (6.3) | 63.8 (6.4) | 66.1 (6.5) | 69.6 (6.5) |
| Handedness (R:L) | 20:1 | 12:1 | 6:0 | 8:1 | 7:2 |
| Education (years) | 15.7 (3.5) | 13.2c (2.5) | 18.0 (3.1) | 14.9 (2.8) | 15.0 (2.1) |
| MMSE (/30) | 29.6 (0.6) | 24.5a (4.6) | 25.3a (4.3) | 21.8a (6.9) | 23.7a (6.0) |
| Symptom duration (years) | N/A | 7.7 (6.0) | 6.5 (3.5) | 5.6 (3.0) | 4.7 (2.2) |
| Neuropsychological | |||||
| General intellect | |||||
| WASI Verbal IQ | 125 (6.7) | 89a (21.9) | 87a (22.2) | 77a (19.7) | 80a (17.3) |
| WASI Performance IQ | 125 (10.2) | 104a (20.3) | 107 (24.6) | 108 (23.5) | 99a (21.5) |
| Episodic memory | |||||
| RMT words (/50) | 49.0 (1.4) | 37.4a (7.9) | 37.2a (9.3) | 30.0a,c (6.3) | 41.4a (9.5) |
| RMT faces (/50) | 44.7 (3.5) | 33.5a (6.9) | 34.8a (7.9) | 32.8a (6.9) | 39.5 (6.6) |
| Camden PAL (/24) | 20.4 (3.3) | 10.8a (8.1) | 12.5a (6.2) | 2.2a,b,c,e (3.7) | 16.3 (7.8) |
| Executive skills | |||||
| WASI Block Design (/71) | 44.8 (10.5) | 32.5 (16.7) | 37.2 (22.1) | 39.1 (21.7) | 25.1a (19.7) |
| WASI Matrices (/32) | 26.6 (3.9) | 19.3a (9.4) | 19.0a (9.8) | 19.8a (10.6) | 17.4a (9.0) |
| WMS-R DS forward (max) | 7.1 (1.1) | 6.6 (1.2) | 6.8 (1.2) | 6.7 (1.2) | 4.8a,b,c,d (0.8) |
| WMS-R DS reverse (max) | 5.6 (1.2) | 4.0a (1.5) | 4.7 (1.4) | 5.3 (1.8) | 3.0a, d (0.7) |
| D-KEFS Stroop: | |||||
| color (s) | 33.4 (7.2) | 48.0 (20.5) | 48.8 (21.4) | 53.2a (28.2) | 87.0a,b,c,d (6.7) |
| word (s) | 23.9 (5.6) | 32.5 (19.0) | 38.7 (26.1) | 36.0 (24.0) | 85.4a,b,c,d (10.3) |
| interference (s) | 57.6 (16.7) | 99.6a (47.5) | 98.3 (45.1) | 90.1 (56.1) | 165a,b,c,d (30.8) |
| Fluency: | |||||
| letter (F total) | 18.1 (5.6) | 7.8a (4.6) | 9.0a (4.7) | 8.9a (7.1) | 3.5a (1.7) |
| category (animals total) | 24.4 (6.0) | 13.8a (7.5) | 10.3a (2.3) | 5.7a,b (5.1) | 8.8a (3.5) |
| Trails A (s) | 33.7 (7.3) | 56.5 (32.3) | 59.8 (32.9) | 49.7 (20.1) | 81.7a (48.4) |
| Trails B (s) | 67.3 (21.5) | 171.7a (88.2) | 186.7a (100.4) | 134.9 (101.7) | 211.1a (94.6) |
| Language skills | |||||
| WASI Vocabulary | 72.3 (3.2) | 42.4a (21.5) | 47.0a (19.1) | 33.6a (22.0) | 31.7a (13.9) |
| BPVS | 148.6 (1.1) | 120.8 (38.7) | 141.8 (7.2) | 85.8a,b,c,e (53.8) | 142.6 (10.1) |
| GNT | 26.1a (2.7) | 12.2a (10.2) | 12.5 (10.1) | 1.6a,b,c,e (4.7) | 15.5a (6.6) |
| Other skills | |||||
| GDA (/24) | 15.8 (5.3) | 7.8a (6.6) | 7.5a (6.3) | 11.9 (8.6) | 5.4a (1.9) |
| VOSP (/20) | 19.0 (1.5) | 15.9a (3.4) | 16.7 (2.3) | 15.8 (4.5) | 15.3a (4.7) |
Mean (standard deviation) scores are shown unless otherwise indicated; maximum scores are shown after tests (in parentheses). asignificantly different from healthy controls, bsignificantly different from bvFTD, csignificantly different from rtvFTD, dsignificantly different from svPPA, esignificantly different from nfvPPA (all p < 0.05). BPVS, British Picture Vocabulary Scale (Dunn LM et al., 1982); bvFTD, patient group with behavioural variant frontotemporal dementia (excluding right temporal cases); Category fluency totals for animal category and letter fluency for the letter F in one minute (Gladsjo et al., 1999); Controls, healthy control group; D-KEFS, Delis Kaplan Executive System (Delis et al., 2001); DS, digit span; GDA, Graded Difficulty Arithmetic (Jackson M and Warrington, 1986); GNT, Graded Naming Test (McKenna and Warrington, 1983); MMSE, Mini-Mental State Examination score (Folstein et al., 1975); N/A, not assessed; NART, National Adult Reading Test (Nelson, 1982); nfvPPA, patient group with nonfluent variant primary progressive aphasia; PAL, Paired Associate Learning test (Warrington, 1996); RMT, Recognition Memory Test (Warrington, 1984); rtvFTD, patient subgroup with right temporal variant frontotemporal dementia; svPPA, patient group with semantic variant primary progressive aphasia; Synonyms, Single Word Comprehension: A Concrete and Abstract Word Synonyms Test (Warrington et al., 1998); Trails-making task based on maximum time achievable 2.5 minutes on task A, 5 minutes on task B (Lezak et al., 2004); VOSP, Visual Object and Spatial Perception Battery – Object Decision test (Warrington and James, 1991); WAIS-R, Wechsler Adult Intelligence Scale‐-Revised (Wechsler, 1981); WASI, Wechsler Abbreviated Scale of Intelligence (Wechsler, 1997); WMS, Wechsler Memory Scale (Wechsler, 1987).
This study was approved by the University College London institutional ethics committee and all methods were performed in accordance with the relevant guidelines and regulations. All participants gave informed consent in accordance with the Declaration of Helsinki.
Facial expression stimuli
Videos of emotional facial expressions were obtained from the Face and Gesture Recognition Research Network (FG-NET) database53. This database comprises silent recordings of healthy young adults viewing emotional scenarios: the scenarios were designed to elicit spontaneous, canonical facial expressions, but were presented without any instruction to pose or inhibit particular expressions (further details in Supplementary Material). In order to sample the spectrum of facial expressions, for each of the canonical emotions of anger, fear, happiness, surprise and disgust10 we selected 10 videos (50 stimuli in total; see Table S1) that clearly conveyed the relevant expression (the canonical emotion of sadness was omitted because its more diffuse time course sets it apart from other emotional expressions). Each video stimulus lasted between four and eight seconds (mean 4.9 seconds), commencing as a neutral facial expression and evolving into an emotional expression (further information in Supplementary Material). The video frame in which an emotional expression first began to develop unambiguously from the neutral baseline (previously determined by independent normal raters and provided with the FG-NET database) was used to align data traces across trials.
Stimuli were presented in randomised order via the monitor of a notebook computer running the Cogent toolbox of Matlab R2012b. The participant’s task on each trial was to identify from among the five alternatives (verbally or by pointing to the appropriate written name) which emotion was displayed; participant responses were recorded for offline analysis. Participants were first familiarised with the stimuli and task to ensure they understood and were able to comply with the protocol. During the test, no feedback was given and no time limits were imposed on responses. Emotion identification scores were compared among groups using ANOVAs, with Bonferroni-corrected post hoc T-tests when main effects were found.
EMG acquisition and analysis
While participants viewed the video stimuli, facial EMG was recorded continuously from left corrugator supercilii, levator labii and zygomaticus major muscles with bipolar surface electrodes, according to published guidelines for the use of EMG in research54. These facial muscles were selected as the key drivers of the canonical expressions represented by the video stimuli3,11. Expressions of anger and fear engage corrugator supercilii (which knits the brow) and inhibit zygomaticus major (which raises the corner of the mouth); expressions of happiness and surprise are associated with the reverse muscle activity profile, while disgust engages both corrugator supercilii and levator labii (which curls the top lip). EMG data were sampled at 2048Hz with a 0.16–100Hz band-pass filter and the EMG signal was rectified, high-pass filtered to correct for baseline shifts and smoothed with a 100 data point sliding filter using MATLAB R2012b; trials with signal amplitude >3 standard deviations from the mean (attributable to large artifacts, e.g., blinks) were removed prior to analysis. For each trial, the mean change in EMG activity from baseline (mean activity during a 500 ms period prior to trial onset) was analysed for each muscle in 500 ms epochs, starting 1s before the onset of expression change in the video stimuli; the EMG response for each muscle was calculated as the area under the curve of EMG signal change from baseline.
We first assessed the presence of automatic imitation (any EMG change from baseline) and emotion-specific muscle activation (any interaction of muscle EMG response with emotion) for the healthy control group, using a repeated measures ANOVA (mean EMG activity for five emotions in eight 500 ms time bins for the three muscles). To determine if there was an overall effect of participant group on the degree of emotion-specific muscle activation, EMG responses were compared across all participants using a restricted maximum likelihood mixed effects model incorporating interactions between emotion, muscle and participant group, with participant identity as a level variable and time bin as a covariate of no interest. After assessing the overall effect of participant group in the omnibus test, we proceeded to establish the basis for any group differences by examining particular emotion-specific muscle contrasts. Emotion-specific EMG response profiles were quantified for each trial by combining individual muscle responses pairwise as follows: for anger and fear, (corrugator response minus zygomaticus response); for happiness and surprise, (zygomaticus response minus corrugator response); for disgust, (corrugator response plus levator response). These pairwise muscle contrasts have been shown to improve reliability and internal consistency of facial EMG analysis55. Muscle contrast EMG reactivity for each trial was then analysed as a dependent variable in an ANOVA incorporating participant group and emotion as fixed factors. Significant main effects in the ANOVA were explored with post hoc T-tests, using Bonferroni correction for multiple comparisons.
To test the hypothesis that emotional imitation supports identification, we assessed any relationship between overall EMG reactivity and emotion identification score using Spearman’s rank correlation across the participant cohort. In addition, we compared EMG responses on trials with correct versus incorrect emotion identification and assessed any interaction with participant group membership using an ANOVA.
To generate an overall measure of reactivity for each participant for use in the voxel based morphometry analysis, EMG reactivity was averaged over all trials for that participant and then normalised as the square root of the absolute value of the change in muscle activity from baseline (subzero values corresponding to muscle activity changes in the reverse direction to that expected were restored).
For both emotion recognition and EMG reactivity, we assessed correlations with neuropsychological instruments indexing general nonverbal intellectual ability (nonverbal executive performance on the WASI Matrices task) and semantic knowledge (performance on the British Picture Vocabulary Scale), to examine the extent to which the experimental parameters of interest were influenced by disease severity and background semantic deficits.
For all tests, the criterion for statistical significance was thresholded at p < 0.05.
Brain image acquisition and analysis
Each patient had a sagittal 3-D magnetization-prepared rapid-gradient-echo T1-weighted volumetric brain MR sequence (echo time/repetition time/inversion time 2.9/2200/900 msec, dimensions 256 256 208, voxel size 1.1 1.1 1.1 mm), acquired on a Siemens Trio 3T MRI scanner using a 32-channel phased-array head-coil. Pre-processing of brain images was performed using the New Segment56 and DARTEL57 toolboxes of SPM8 (www.fil.ion.ucl.ac.uk/spm), following an optimised protocol58. Normalisation, segmentation and modulation of grey and white matter images were performed using default parameter settings and grey matter images were smoothed using a 6 mm full width-at-half-maximum Gaussian kernel. A study-specific template mean brain image was created by warping all bias-corrected native space brain images to the final DARTEL template and calculating the average of the warped brain images. Total intracranial volume was calculated for each patient by summing grey matter, white matter and cerebrospinal fluid volumes after segmentation of tissue classes.
Processed brain MR images were entered into a VBM analysis of the patient cohort. Separate regression models were used to assess associations of regional grey matter volume (indexed as voxel intensity) with mean overall emotion identification score and EMG reactivity, for each syndromic group. Age, total intracranial volume and WASI Matrices score (a measure of nonverbal executive function and index of disease severity) were incorporated as covariates of no interest in all models. Statistical parametric maps of regional grey matter associations were assessed at threshold p < 0.05 after family-wise error (FWE) correction for multiple voxel-wise comparisons within pre-specified regional volumes of interest. For the emotion identification contrast, these regions were informed by previous studies of emotion processing in FTD and in the healthy brain, comprising insula, anteromedial temporal lobe (including amygdala, fusiform gyrus and temporal pole), inferior frontal cortex, anterior cingulate and supplementary motor cortices23,26,48. For the EMG reactivity contrast, regions of interest were based on previous functional imaging studies of facial mimicry and dynamic facial stimuli14,45,49,50, comprising visual (V1, MT/V5, parahippocampal and fusiform gyri) and primary and supplementary motor cortices.
Results
General characteristics of participant groups
General clinical characteristics of the participant groups are presented in Table 1. There was a significant gender difference between participant groups (chi24 = 10.31, p = 0.036), but no significant age difference. The patient groups did not differ in mean symptom duration or level of overall cognitive impairment (as indexed using WASI Matrices score; ANOVAs and post hoc T-tests all p > 0.4).
Emotion identification
Group data for facial emotion identification are summarised in Table 2.
Table 2.
Summary of emotion identification and EMG reactivity findings for participant groups
| Response parameter | Controls | bvFTD | rtvFTD | svPPA | nfvPPA |
|---|---|---|---|---|---|
| Emotion identification | |||||
| Anger | 4.6 (2.2) | 1.8 (1.4)a | 2.5 (1.6) | 1.1 (0.9)a | 3.4 (1.7) |
| Disgust | 8.1 (1.0) | 5.3 (3.3)a | 3.5 (3.9)a | 3.8 (3.3)a | 5.4 (3.3) |
| Fear | 5.4 (2.1) | 2.6 (2.0)a | 2.0 (1.7)a | 3.9 (2.0) | 4.4 (2.4) |
| Happiness | 9.2 (0.8) | 8.0 (3.2) | 8.3 (1.9) | 7.0 (3.2) | 7.8 (1.6) |
| Surprise | 8.4 (1.0) | 4.9 (2.8)a | 3.7 (2.8)a | 4.1 (3.2)a | 5.8 (3.0) |
| Overall (/50) | 35.7 (4.6) | 22.7 (9.5)a | 20.0 (9.7)a | 20.2 (7.9)a | 26.9 (9.3)a |
| Facial EMG reactivity | |||||
| Anger | 1.3 (3.3) | 0.5 (1.5) | 0.2 (1.0) | 1.2 (5.1) | 0.3 (4.0) |
| Disgust | 2.6 (8.9) | −0.9 (9.0)a | 0.5 (1.7) | 1.4 (6.2) | 0.9 (3.7) |
| Fear | 0.7 (2.9) | 0.3 (1.3) | −0.1 (1.9) | 0.8 (4.4) | −0.9 (3.5)a,b,c |
| Happiness | 1.3 (2.3) | 0.5 (1.3)d | 0.2 (1.6)d | 1.8 (8.2) | 2.3 (4.9) |
| Surprise | 1.0 (2.5) | 0.01 (3.1)c,d | 0.3 (1.8) | 1.7 (5.3) | 1.7 (3.8) |
| Overall | 1.4 (4.7) | 0.09 (4.4)a,c,d | 0.2 (1.6)a,c | 1.4 (6.0) | 0.9 (4.2) |
Mean (standard deviation) scores on the emotion identification task and mean facial EMG reactivity (as defined in Fig. 1) to viewed emotional expressions are shown for each emotion, in each participant group. asignificantly less than healthy controls, bsignificantly less than bvFTD, csignificantly less than svPPA, dsignificantly less than nfvPPA (all pbonf < 0.05). bvFTD, patient group with behavioural variant frontotemporal dementia (excluding right temporal cases); Controls, healthy control group; nfvPPA, patient group with nonfluent variant primary progressive aphasia; rtvFTD, patient subgroup with right temporal variant frontotemporal dementia; svPPA, patient group with semantic variant primary progressive aphasia.
Overall accuracy of facial emotion identification showed a main effect of participant group (F4 = 10.89, p < 0.001), and was reduced in all syndromic groups relative to controls (all pbonf < 0.012) (Table 2). There was no significant relationship between emotion identification accuracy and age but a significant effect of gender (p = 0.04), with higher identification scores overall in female participants. The main effect of participant group persisted after covarying for gender (F4 = 13.852, p < 0.001). Emotion identification accuracy in the patient cohort correlated with standard measures of nonverbal executive function (WASI Matrices score, an index of disease severity; rho = 0.547, p < 0.001) and semantic competence (British Picture Vocabulary Scale; rho = 0.676, p < 0.001).
Facial EMG reactivity
Mean time courses of EMG responses for each facial muscle and emotion are shown for all participant groups in Fig. 1. Group data for EMG reactivity are summarised in Table 2 and Fig. 2.
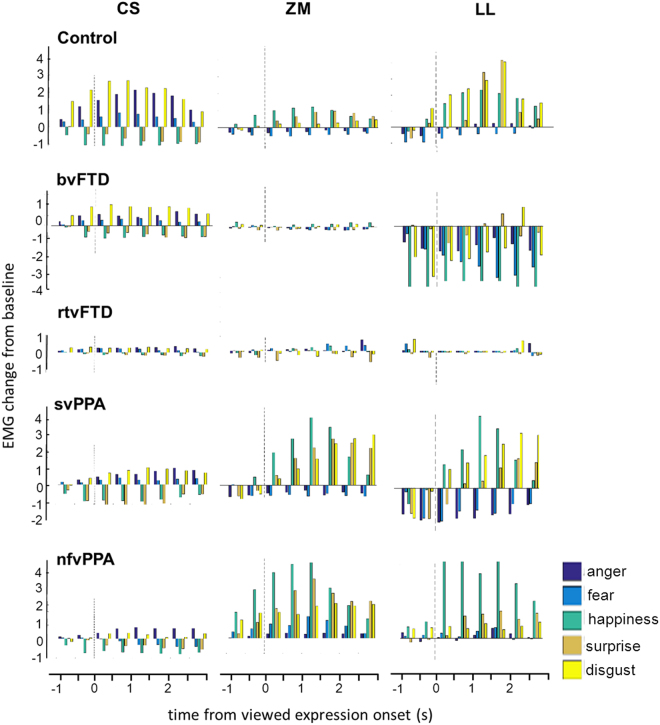
Figure 1.
Patterns of EMG reactivity for each muscle in each participant group. For each participant group, the plots show the time course of average EMG reactivity (in microvolts) for key facial muscles while participants watched videos of emotional facial expressions. EMG reactivity, here indexed in arbitrary units as mean EMG change from baseline, is shown on the y-axis (after rectifying, high-pass filtering and removing artefacts as described in Methods). Onset of the viewed facial expression (as determined in a prior independent analysis of the video stimuli) is at time 0 (dotted line) in each panel. In healthy controls, corrugator supercilii (CS) was activated during viewing of anger, fear and disgust, but inhibited during viewing of happiness and surprise; zygomaticus major (ZM) was activated during viewing of happiness and surprise, but inhibited during viewing of anger and fear; and levator labii (LL) was inhibited during viewing of anger and fear, and maximally activated during viewing of disgust. Note that in healthy controls muscle responses consistently preceded the unambiguous onset of viewed emotional expressions. bvFTD, patient group with behavioural variant frontotemporal dementia (excluding right temporal cases); Control, healthy control group; nfvPPA, patient group with nonfluent variant primary progressive aphasia; rtvFTD, patient subgroup with right temporal variant frontotemporal dementia; svPPA, patient group with semantic variant primary progressive aphasia.
Figure 2.
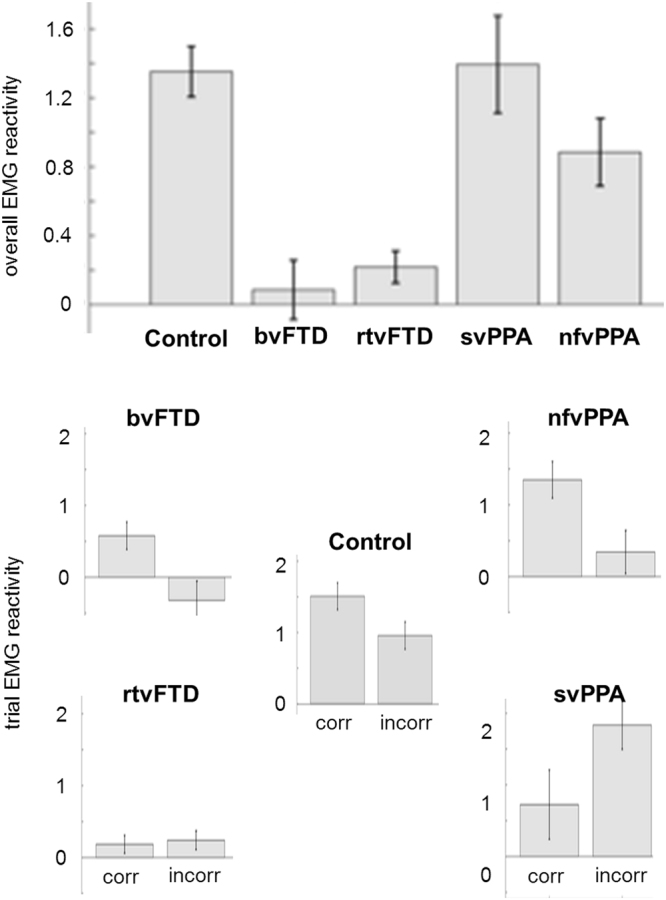
EMG reactivity in each participant group, and the relationship with identification accuracy. For each participant group, the histograms show mean overall facial muscle EMG reactivity (top) and EMG reactivity separately (below) for those trials on which viewed emotional expressions were identified correctly (corr) versus incorrectly (incorr); error bars indicate standard error of the mean (see also Table 2). bvFTD, patient group with behavioural variant frontotemporal dementia; Control, healthy control group; nfvPPA, patient group with nonfluent variant primary progressive aphasia; rtvFTD, patient subgroup with right temporal variant frontotemporal dementia; svPPA, patient group with semantic variant primary progressive aphasia.
Healthy older participants showed the anticipated profiles of facial muscle activity in response to viewing facial expressions (Fig. 1): corrugator supercilii was activated by anger, fear and disgust, and inhibited by happiness and surprise; zygomaticus major was activated by happiness and surprise, and inhibited by anger and fear; and levator labii activity was maximal for disgust. Due to the proximity of levator labii and zygomaticus major, and the limited spatial specificity of surface electrodes54, there was substantial electrical leakage between these two muscles. However, zygomaticus major was maximally activated by happiness and surprise, and levator labii by disgust; moreover, these muscles were not combined in any of the pairwise muscle contrasts.
EMG reactivity to viewed facial expressions was modulated in an emotion- and muscle-specific manner in healthy controls (F(2.20,43.94) = 5.03, p = 0.009) and the participant cohort as a whole (chi2(8) = 80.05, p < 0.001). There was further evidence that this interaction between emotion and muscle reactivity varied between participant groups (interaction of group, emotion and muscle: (chi2(32) = 143.91, p < 0.001). After the generation of a muscle contrast reactivity measure for each trial, ANOVA revealed significant main effects of participant group (F(4) = 10.84, p < 0.001), emotion (F(4) = 3.40, p = 0.009) and the interaction of group and emotion (F(16) = 2.79, p < 0.001; Table 2). In post hoc T-tests comparing participant groups (with Bonferroni correction), overall EMG reactivity across the five emotions was significantly reduced in the bvFTD group relative to the healthy control group (pbonf < 0.001), the svPPA group (pbonf < 0.001) and the nfvPPA group (pbonf = 0.042); and significantly reduced in the rtvFTD group relative to the healthy control group (pbonf = 0.001) and the svPPA group (pbonf = 0.005).
There was no significant relationship between EMG reactivity and age (p = 0.1), gender (p = 0.42), or WASI Matrices score (used here as a measure of disease severity; p = 0.63) in the patient cohort, nor with a standard measure of semantic knowledge (British Picture Vocabulary Scale; p = 0.5).
Relationship between emotion identification and facial EMG reactivity
Across the participant cohort, overall EMG reactivity was significantly correlated with emotion identification accuracy (rho = 0.331, p = 0.011) and mean trial EMG reactivity was significantly higher for trials on which the emotion was correctly identified (n = 1586) than on error trials (n = 1314; p = 0.002). This differential effect of correct versus incorrect trials showed a significant interaction with participant group (F(4) = 4.18, p = 0.002; see Fig. 2). Among healthy controls, there was a strong trend towards greater reactivity predicting correct identification (p = 0.087). Comparing trial types within patient groups, EMG reactivity was significantly higher on correct identification trials than error trials in the bvFTD group (p = 0.009) and the nfvPPA group (p = 0.01) but not the rtvFTD group (p = 0.76) or the svPPA group (p = 0.06, here signifying a trend towards greater EMG reactivity on incorrect trials).
Neuroanatomical associations
Significant grey matter associations of emotion identification and EMG reactivity for the patient cohort are summarised in Table 3 (all thresholded at pFWE < 0.05 within pre-specified anatomical regions of interest); statistical parametric maps are presented in Fig. 3.
Table 3.
Neuroanatomical correlates of emotion identification and reactivity in patient groups.
| Group | Region | Side | Cluster | Peak (mm) | T score | PFWE | ||
|---|---|---|---|---|---|---|---|---|
| (voxels) | x | y | z | |||||
| Emotion identification | ||||||||
| bvFTD | Anterior cingulate | L | 196 | −8 | 44 | 12 | 5.59 | 0.003 |
| Anterior insula | L | 123 | −30 | 27 | 0 | 4.07 | 0.047 | |
| Supplementary motor area | L | 5 | −10 | 4 | 50 | 3.81 | 0.044 | |
| Opercular IFG | L | 32 | −57 | 12 | 18 | 5.14 | 0.003 | |
| Anteromedial temporal: | ||||||||
| Temporal pole | L | 2133 | −32 | 8 | −38 | 5.11 | 0.010 | |
| Amygdala | −24 | 2 | −38 | 4.94 | 0.015 | |||
| Fusiform gyrus | −30 | −9 | −38 | 4.82 | 0.019 | |||
| rtvFTD | Supplementary motor area | L | 34 | −3 | −10 | 57 | 4.15 | 0.022 |
| Temporo-occipital junction | R | 18 | 66 | −50 | −8 | 4.06 | 0.038 | |
| svPPA | STG/STS | L | 536 | −58 | −30 | 14 | 7.21 | 0.005 |
| Supplementary motor area | L | 19 | −4 | −2 | 50 | 4.23 | 0.019 | |
| Opercular IFG | L | 25 | −57 | 12 | 18 | 5.05 | 0.003 | |
| Anterior cingulate | L | 24 | −2 | 44 | 3 | 4.11 | 0.042 | |
| Fusiform gyrus | R | 44 | 22 | −4 | −44 | 4.43 | 0.042 | |
| nfvPPA | Supplementary motor area | L | 37 | −4 | −2 | 50 | 4.14 | 0.023 |
| Opercular IFG | L | 9 | −52 | 8 | 18 | 3.96 | 0.033 | |
| Facial EMG reactivity | ||||||||
| bvFTD | Supplementary motor area | L | 12 | −8 | −9 | 56 | 3.99 | 0.030 |
| Temporo-occipital junction | L | 25 | −54 | −45 | −4 | 4.29 | 0.064 | |
| rtvFTD | Temporo-occipital junction | R | 8 | 62 | −62 | 2 | 3.96 | 0.046 |
| svPPA | Parahippocampal gyrus | L | 59 | −20 | −28 | −24 | 4.25 | 0.028 |
| Parahippocampal gyrus | R | 72 | 18 | −33 | −18 | 5.25 | 0.003 | |
| nfvPPA | Primary visual cortex | R | 291 | 12 | −80 | 3 | 5.92 | 0.001 |
| Primary motor cortex | R | 521 | 56 | 8 | 27 | 5.43 | 0.007 | |
| Supplementary motor area | R | 18 | 8 | 8 | 68 | 4.42 | 0.012 | |
The table presents regional grey matter correlates of mean overall emotion identification score and facial EMG reactivity (as defined in Fig. 1) during viewing of facial expressions in the four patient groups, based on voxel-based morphometry. Coordinates of local maxima are in standard MNI space. P values are all significant after family-wise error (FWE) correction for multiple voxel-wise comparisons within pre-specified anatomical regions of interest (see text). bvFTD, patient group with behavioural variant frontotemporal dementia (excluding right temporal cases); IFG, inferior frontal gyrus; nfvPPA, patient group with nonfluent variant primary progressive aphasia; rtvFTD, patient subgroup with right temporal variant frontotemporal dementia; STG/S, superior temporal gyrus/sulcus; svPPA, patient group with semantic variant primary progressive aphasia.
Figure 3.
Neuroanatomical correlates of emotion identification and EMG reactivity for each syndromic group. Statistical parametric maps (SPMs) show regional grey matter volume positively associated with overall emotion identification accuracy and facial EMG reactivity during viewing of emotional facial expressions, based on voxel-based morphometry of patients’ brain MR images (see also Table 3); T-scores are coded on the colour bar. SPMs are overlaid on sections of the normalised study-specific T1-weighted mean brain MR image; the MNI coordinate (mm) of the plane of each section is indicated (coronal and axial sections show the left hemisphere on the left). Panels code syndromic profiles of emotion identification (ID) or EMG reactivity (EMG). Note that the correlates of emotion identification and EMG reactivity in different syndromes overlapped in particular brain regions, including supplementary motor cortex and temporo-occipital junction (see Table 3). SPMs are thresholded for display purposes at p < 0.001 uncorrected over the whole brain, however local maxima of areas shown were each significant at p < 0.05 after family-wise error correction for multiple voxel-wise comparisons within pre-specified anatomical regions of interest (see Table 3). bvFTD, patient group with behavioural variant FTD; nfvPPA, patient group with nonfluent variant primary progressive aphasia; rtvFTD, patient subgroup with right temporal variant frontotemporal dementia; svPPA, patient group with semantic variant primary progressive aphasia.
Accuracy identifying dynamic emotional expressions was correlated with regional grey matter volume in left supplementary motor cortex in all syndromic groups. Additional regional grey matter correlates of emotion identification were delineated for particular syndromic groups. The bvFTD, svPPA and nfvPPA groups showed syndromic grey matter correlates within a bi-hemispheric (predominantly left-lateralised) frontotemporal network including opercular inferior frontal gyrus, anterior cingulate, anterior insula and antero-inferior temporal lobe; while the svPPA group showed a further correlate in left posterior superior temporal cortex and the rtvFTD group showed a correlate in right temporo-occipital junctional cortex in the vicinity of MT/V5 complex59.
Across the patient cohort, overall mean EMG reactivity was correlated with regional grey matter in an overlapping but more posteriorly directed and right-lateralised network, with variable emphasis in particular syndromic groups. The bvFTD and nfvPPA groups showed grey matter correlates of EMG reactivity in supplementary and primary motor cortices, while all syndromic groups showed grey matter associations in cortical areas implicated in the analysis of visual signals, comprising primary visual cortex in the nfvPPA group; temporo-occipital. junction (MT/V5 complex) in the bvFTD and rtvFTD groups; and parahippocampal gyrus in the svPPA group.
Discussion
Here we have demonstrated facial motor signatures of emotional reactivity in the FTD spectrum. As anticipated, healthy older individuals showed characteristic profiles of facial muscle engagement by observed facial emotions; moreover, facial muscle reactivity predicted correct trial-by-trial identification of facial emotions. These findings provide further evidence that (in the healthy brain) facial mimesis is an automatic, involuntary mechanism supporting stimulus decoding and evaluation, rather than simply an accompaniment of conscious emotion recognition. In contrast, overall facial muscle reactivity and the normal coupling of muscle reactivity to facial emotion identification were altered differentially in the patient groups representing major FTD syndromes. As predicted, identification of facial expressions was impaired across the patient cohort: however, whereas the bvFTD group showed globally reduced facial muscle reactivity to observed emotional expressions, the svPPA group had preserved overall muscle reactivity but loss of the linkage between muscle response and correct expression identification. Among those patients with syndromes dominated by behavioural decline, the profile of facial muscle reactivity stratified cases with rtvFTD from other cases of bvFTD: the subgroup with rtvFTD had a particularly severe phenotype, exhibiting both globally reduced facial reactivity and also aberrant coupling of muscle reactivity to facial expression identification.
Considered collectively, the motor signatures of emotional reactivity identified in our patient cohort amplify previous clinical, neuropsychological and physiological evidence in particular FTD syndromes. The generalised impairment of emotional mimesis in our bvFTD and rtvFTD groups is consistent with the clinical impression of facial impassivity37,60, impaired intentional imitation38 and blunting of autonomic responsiveness30,31,33,35,36 in these patients. Abnormal coupling of facial mimesis to facial expression identification in our svPPA group is in line with the disordered autonomic signalling of affective valuation previously documented in this syndrome33,35, and suggests a method of dissociating emotional reactivity from the declarative, semantic categorisation of emotions. The present findings suggest that aberrant motor recoding of perceived expressions may constitute a core physiological mechanism for impaired emotion processing in FTD.
This mimetic mechanism may be particularly pertinent to the dynamically shifting and subtle emotions of everyday interpersonal encounters. Our own emotional expressions are normally subject to continual modulation by the expressed emotions of others, including tracking of transient ‘micro-expressions’61; this modulation occurs over short timescales (a few hundred milliseconds) and contributes importantly to the regulation of social interactions, prosociality and empathy28,62–64. If facial mimesis plays a key role in tuning such responses, loss of this modulatory mechanism (most notably in bvFTD and rtvFTD) might underpin not only impaired socio-emotional awareness in FTD but also the ‘poker-faced’ sense of unease these patients commonly provoke in others37.
The neuroanatomical correlates we have identified speak to the coherent nature of dynamic emotion mimesis and identification. In line with previous evidence38, these processes mapped onto a distributed cerebral network within which FTD syndromes showed separable profiles of grey matter atrophy. Involvement of supplementary motor cortex was a feature across syndromes and associated both with emotion identification and motor reactivity, though joint correlation was observed in the bvFTD and nfvPPA groups but not the rtvFTD and svPPA groups (see Table 3). Supplementary motor cortex is a candidate hub for the computation of sensorimotor representations unfolding over time, an integral function of the mirror neuron system: this region generates both facial sensory-evoked potentials and complex facial movements65 and it is activated during facial imitation and empathy66 as well as by dynamic auditory emotional signals48. Furthermore, transcranial magnetic stimulation of the supplementary motor region disrupts facial emotion recognition67. The uncoupling of motor reactivity from emotion identification in the rtvFTD and svPPA groups may reflect disconnection of this key hub from linked mechanisms for affective semantic appraisal12, perhaps accounting for lack of an EMG reactivity correlate in supplementary motor cortex in these syndromic groups. Two further cortical hubs correlating both with emotion identification and mimesis were delineated in our patient cohort. In the svPPA and rtvFTD groups, a joint correlate was identified in the temporo-occipital junction zone, overlapping posterior superior temporal sulcus and MT/V5 visual motion cortices59,68: this region has been implicated in the imitation and decoding of dynamic facial expressions15,49,69,70, integration of dynamic social percepts, action observation and theory of mind71,72. In the svPPA group, infero-medial temporal cortex was linked both to emotion identification and mimesis: this region has previously been shown to respond to dynamic facial stimuli45.
Additional grey matter associations of facial expression identification accuracy were delineated in cingulo-insular, antero-medial temporal and inferior frontal areas previously implicated both in the detection and evaluation of salient affective stimuli and in canonical FTD syndromes15,20,21,23,26,73,74. Additional grey matter associations of facial motor reactivity were identified (for the nfvPPA group) in primary visual and motor cortices: enhanced responses to emotional facial expressions have previously been demonstrated in visual cortex75, while motoric responses to social stimuli have been located in precentral gyrus14. However, it is noteworthy that certain grey matter associations emerging from this analysis - in particular, the ‘hub regions’ of supplementary motor cortex and temporo-occipital junction and (in the nfvPPA group) primary visual and motor cortices - lie beyond the brain regions canonically targeted in particular FTD syndromes or indeed, in previous studies of emotion processing in FTD21. It is likely that the dynamic expression stimuli employed here allowed a more complete picture of the cerebral mechanisms engaged in processing naturalistic emotions. Moreover, involvement of brain regions remote from zones of maximal atrophy may reflect distributed functional network effects (for example, visual cortical activity has been shown to be modulated by amygdala75) in conjunction with disease-related network connectivity changes, which are known to extend beyond the atrophy maps that conventionally define particular FTD syndromes76. Taken together, the present neuroanatomical findings are compatible with the previously proposed, hierarchical organisation of embodied representations supporting emotional decoding and empathy13,48,77,78: whereas early visual and motor areas may support automatic imitation via low-level visual and kinematic representations, higher levels of the processing hierarchy engage the human ‘mirror’ system and substrates for semantic, evaluative and mentalising processes that drive explicit emotion identification.
From a clinical perspective, this work suggests a pathophysiological framework for deconstructing the complex social and emotional symptoms that characterise FTD syndromes. Such symptoms are difficult to measure using conventional neuropsychological tests, and may only be elicited by naturalistic social interactions: dynamic motor physiological surrogates might index both the affective dysfunction of patients’ daily lives and the underlying disintegration of culprit neural networks38. These physiological metrics might facilitate early disease detection and tracking over a wider spectrum of severity than is currently possible and enable socio-emotional assessment in challenging clinical settings (such as aphasia), especially since our results suggest that (in contrast to explicit emotion recognition) automatic motor reactivity may be relatively insensitive to semantic deficits. Our findings further suggest that such metrics are not simply ciphers of reduced cognitive capacity but may help stratify broad disease groupings (such as the heterogeneous bvFTD syndrome) and at the same time, may capture mechanisms that transcend traditional syndromic boundaries. We therefore propose that the paradigm of emotional sensorimotor reactivity may yield a fresh perspective on FTD nosology and candidate novel biomarkers of FTD syndromes. Looking forward, this paradigm suggests a potential strategy for biofeedback-based retraining of emotional responsiveness, perhaps in conjunction with disease-modifying therapies79.
This study establishes a preliminary proof of principle but the findings require further corroboration. There are several clear limitations that suggest caution in interpreting our findings and directions for future work. We have studied a small, intensively phenotyped patient cohort: the most pressing issue will be to replicate the findings in larger clinical populations. Future studies should encompass a wider range of pathologies, in order to determine the general applicability of the paradigm and the specificity of syndromic motor profiles; it would be of interest, for example, to assess the heightened emotional contagion previously documented in Alzheimer’s disease80 in this context. Longitudinal cohorts including presymptomatic mutation carriers will be required in order to assess the diagnostic sensitivity of mimetic indices and their utility as biomarkers; ultimately, histopathological correlation will be necessary to establish any molecular correlates of the syndromic stratification suggested here. It will be relevant to explore the cognitive milieu of emotional motor responses in greater detail: for example, the effects of other sensory modalities (in particular, audition48, micro-expressions61, sincere versus social emotions81 and emotional ‘caricatures’ in FTD82) and the correlation of mimetic markers with measures of social cognition and daily life empathy38. Emotional reciprocity might be modeled using virtual reality techniques to generate model social interactions62. Beyond mimesis, integration of somatic and cognitive mechanisms during social emotional exchanges demands the joint processing of autonomic and neuroendocrine signals under executive control29,83,84: future work should assess other physiological modalities alongside EMG. Functional MRI and magnetoencephalography would amplify the present structural neuroanatomical correlates by capturing disease-related changes in underlying brain network connectivity and dynamics. Multimodal studies of this kind may set motor mimicry in the context of a comprehensive physiology of socio-emotional reactivity in neurodegenerative diseases. The ultimate goal will be to identify practical physiological markers that can be widely translated for the diagnosis and dynamic tracking of these diseases and the evaluation of new therapies.
Electronic supplementary material
Acknowledgements
We are grateful to all participants for their involvement. The Dementia Research Centre is supported by Alzheimer’s Research UK, the Brain Research Trust and the Wolfson Foundation. This work was funded by the Alzheimer’s Society, Leonard Wolfson Experimental Neurology Centre, Wellcome Trust, Medical Research Council UK and NIHR UCLH Biomedical Research Centre.
Author Contributions
Study conception and design: C.R.M., J.M.K., J.D.W. Data acquisition and analysis: C.R.M., C.J.D.H., L.L.R., C.N.C., R.L.B., K.M.D., E.V.B., C.J.M., J.M.S., J.D.R., J.M.K., J.D.W. Drafting of the manuscript: C.R.M., J.D.W.
Competing Interests
Dr. Marshall is supported by the Leonard Wolfson Experimental Neurology Centre. Mr Hardy holds a MRC UK PhD studentship. Ms Russell reports no biomedical financial interests or potential conflicts of interest. Dr. Clark was supported by The National Brain Appeal – Frontotemporal Dementia Research Fund. Ms Bond holds a MRC UK PhD studentship. Ms Dick is funded by the Alzheimer’s Society. Ms Brotherhood reports no biomedical financial interests or potential conflicts of interest. Dr. Mummery reports no biomedical financial interests or potential conflicts of interest. Dr. Schott reports no biomedical financial interests or potential conflicts of interest. Dr. Rohrer is a MRC UK Clinical Scientist. Dr. Kilner reports no biomedical financial interests or potential conflicts of interest. Prof Warren was supported by a Wellcome Trust Senior Clinical Fellowship and receives funding from the Alzheimer’s Society.
Footnotes
James M. Kilner and Jason D. Warren jointly supervised this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19528-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Niedenthal PM. Embodying emotion. Science. 2007;316:1002–1005. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- 2.Wood A, Rychlowska M, Korb S, Niedenthal P. Fashioning the Face: Sensorimotor Simulation Contributes to Facial Expression Recognition. Trends in cognitive sciences. 2016;20:227–240. doi: 10.1016/j.tics.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Dimberg U, Thunberg M. Rapid facial reactions to emotional facial expressions. Scand J Psychol. 1998;39:39–45. doi: 10.1111/1467-9450.00054. [DOI] [PubMed] [Google Scholar]
- 4.Dimberg U, Thunberg M, Grunedal S. Facial reactions to emotional stimuli: Automatically controlled emotional responses. Cognition Emotion. 2002;16:449–471. doi: 10.1080/02699930143000356. [DOI] [Google Scholar]
- 5.Neumann R, Schulz SM, Lozo L, Alpers GW. Automatic facial responses to near-threshold presented facial displays of emotion: imitation or evaluation? Biological psychology. 2014;96:144–149. doi: 10.1016/j.biopsycho.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Tramacere, A. & Ferrari, P. F. Faces in the mirror, from the neuroscience of mimicry to the emergence of mentalizing. Journal of anthropological sciences = Rivista di antropologia: JASS/Istituto italiano di antropologia, 10.4436/jass.94037 (2016). [DOI] [PubMed]
- 7.Kunecke J, Hildebrandt A, Recio G, Sommer W, Wilhelm O. Facial EMG responses to emotional expressions are related to emotion perception ability. PLoS One. 2014;9:e84053. doi: 10.1371/journal.pone.0084053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korb S, With S, Niedenthal P, Kaiser S, Grandjean D. The perception and mimicry of facial movements predict judgments of smile authenticity. PLoS One. 2014;9:e99194. doi: 10.1371/journal.pone.0099194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MJ, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: preliminary findings from an A-B-A design. Biology of mood & anxiety disorders. 2014;4:11. doi: 10.1186/2045-5380-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekman P, Sorenson ER, Friesen WV. Pan-Cultural Elements in Facial Displays of Emotion. Science. 1969;164:86–88. doi: 10.1126/science.164.3875.86. [DOI] [PubMed] [Google Scholar]
- 11.Vrana SR. The psychophysiology of disgust: differentiating negative emotional contexts with facial EMG. Psychophysiology. 1993;30:279–286. doi: 10.1111/j.1469-8986.1993.tb03354.x. [DOI] [PubMed] [Google Scholar]
- 12.Leslie KR, Johnson-Frey SH, Grafton ST. Functional imaging of face and hand imitation: towards a motor theory of empathy. NeuroImage. 2004;21:601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Kilner J, Friston K, Frith C. Predictive coding: an account of the mirror neuron system. Cogn Process. 2007;8:159–166. doi: 10.1007/s10339-007-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schilbach L, Eickhoff SB, Mojzisch A, Vogeley K. What’s in a smile? Neural correlates of facial embodiment during social interaction. Soc Neurosci. 2008;3:37–50. doi: 10.1080/17470910701563228. [DOI] [PubMed] [Google Scholar]
- 15.Foley E, Rippon G, Thai NJ, Longe O, Senior C. Dynamic facial expressions evoke distinct activation in the face perception network: a connectivity analysis study. Journal of cognitive neuroscience. 2012;24:507–520. doi: 10.1162/jocn_a_00120. [DOI] [PubMed] [Google Scholar]
- 16.Warren JD, Rohrer JD, Rossor MN. Clinical review. Frontotemporal dementia. BMJ (Clinical research ed.) 2013;347:f4827. doi: 10.1136/bmj.f4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamminga J, et al. Differentiating between right-lateralised semantic dementia and behavioural-variant frontotemporal dementia: an examination of clinical characteristics and emotion processing. Journal of Neurology, Neurosurgery & Psychiatry. 2015;86:1082–1088. doi: 10.1136/jnnp-2014-309120. [DOI] [PubMed] [Google Scholar]
- 18.Ranasinghe KG, et al. Distinct Subtypes of Behavioral Variant Frontotemporal Dementia Based on Patterns of Network Degeneration. JAMA neurology. 2016;73:1078–1088. doi: 10.1001/jamaneurol.2016.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen HJ, et al. Recognition of emotion in the frontal and temporal variants of frontotemporal dementia. Dementia and geriatric cognitive disorders. 2004;17:277–281. doi: 10.1159/000077154. [DOI] [PubMed] [Google Scholar]
- 20.Omar R, et al. The structural neuroanatomy of music emotion recognition: evidence from frontotemporal lobar degeneration. NeuroImage. 2011;56:1814–1821. doi: 10.1016/j.neuroimage.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumfor F, Piguet O. Disturbance of emotion processing in frontotemporal dementia: a synthesis of cognitive and neuroimaging findings. Neuropsychology review. 2012;22:280–297. doi: 10.1007/s11065-012-9201-6. [DOI] [PubMed] [Google Scholar]
- 22.Rohrer JD, Sauter D, Scott S, Rossor MN, Warren JD. Receptive prosody in nonfluent primary progressive aphasias. Cortex. 2012;48:308–316. doi: 10.1016/j.cortex.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couto, B. et al. Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Frontiers in human neuroscience7, 10.3389/fnhum.2013.00467 (2013). [DOI] [PMC free article] [PubMed]
- 24.Hazelton, J. L., Irish, M., Hodges, J. R., Piguet, O. & Kumfor, F. Cognitive and Affective Empathy Disruption in Non-Fluent Primary Progressive Aphasia Syndromes. Brain Impairment, 1–13, 10.1017/BrImp.2016.21 (2016).
- 25.Hsieh S, Irish M, Daveson N, Hodges JR, Piguet O. When one loses empathy: its effect on carers of patients with dementia. J Geriatr Psychiatry Neurol. 2013;26:174–184. doi: 10.1177/0891988713495448. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh S, Hornberger M, Piguet O, Hodges JR. Brain correlates of musical and facial emotion recognition: evidence from the dementias. Neuropsychologia. 2012;50:1814–1822. doi: 10.1016/j.neuropsychologia.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Hale J, Hamilton AF. Cognitive mechanisms for responding to mimicry from others. Neurosci Biobehav Rev. 2016;63:106–123. doi: 10.1016/j.neubiorev.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Kraaijenvanger EJ, Hofman D, Bos PA. A neuroendocrine account of facial mimicry and its dynamic modulation. Neurosci Biobehav Rev. 2017;77:98–106. doi: 10.1016/j.neubiorev.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Eckart JA, Sturm VE, Miller BL, Levenson RW. Diminished disgust reactivity in behavioral variant frontotemporal dementia. Neuropsychologia. 2012;50:786–790. doi: 10.1016/j.neuropsychologia.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi A, et al. Skin conductance levels may reflect emotional blunting in behavioral variant frontotemporal dementia. The Journal of neuropsychiatry and clinical neurosciences. 2014;26:227–232. doi: 10.1176/appi.neuropsych.12110332. [DOI] [PubMed] [Google Scholar]
- 32.Downey LE, et al. Altered body schema processing in frontotemporal dementia with C9ORF72 mutations. Journal of Neurology, Neurosurgery & Psychiatry. 2014 doi: 10.1136/jnnp-2013-306995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fletcher PD, et al. Dementias show differential physiological responses to salient sounds. Frontiers in behavioral neuroscience. 2015;9:73. doi: 10.3389/fnbeh.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fletcher, P. D. et al. Pain and temperature processing in dementia: a clinical and neuroanatomical analysis. Brain: a journal of neurology (2015). [DOI] [PMC free article] [PubMed]
- 35.Fletcher PD, et al. Physiological phenotyping of dementias using emotional sounds. Alzheimer’s & dementia (Amsterdam, Netherlands) 2015;1:170–178. doi: 10.1016/j.dadm.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo, C. C. et al. Dominant hemisphere lateralization of cortical parasympathetic control as revealed by frontotemporal dementia. Proceedings of the National Academy of Sciences, 10.1073/pnas.1509184113 (2016). [DOI] [PMC free article] [PubMed]
- 37.Edwards-Lee T, et al. The temporal variant of frontotemporal dementia. Brain: a journal of neurology. 1997;120:1027. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- 38.Gola KA, et al. A neural network underlying intentional emotional facial expression in neurodegenerative disease. NeuroImage. Clinical. 2017;14:672–678. doi: 10.1016/j.nicl.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Argaud S, et al. Does Facial Amimia Impact the Recognition of Facial Emotions? An EMG Study in Parkinson’s Disease. PLoS One. 2016;11:e0160329. doi: 10.1371/journal.pone.0160329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balconi M, et al. Facial feedback and autonomic responsiveness reflect impaired emotional processing in Parkinson’s Disease. Scientific Reports. 2016;6:31453. doi: 10.1038/srep31453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trinkler I, et al. Embodied emotion impairment in Huntington’s Disease. Cortex. 2017;92:44–56. doi: 10.1016/j.cortex.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Peterman JS, Bekele E, Bian D, Sarkar N, Park S. Complexities of emotional responses to social and non-social affective stimuli in schizophrenia. Frontiers in psychology. 2015;6:320. doi: 10.3389/fpsyg.2015.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendez MF, Perryman KM. Disrupted facial empathy in drawings from artists with frontotemporal dementia. Neurocase. 2003;9:44–50. doi: 10.1076/neur.9.1.44.14375. [DOI] [PubMed] [Google Scholar]
- 44.Whitwell JL, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain: a journal of neurology. 2009;132:2932–2946. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trautmann SA, Fehr T, Herrmann M. Emotions in motion: dynamic compared to static facial expressions of disgust and happiness reveal more widespread emotion-specific activations. Brain research. 2009;1284:100–115. doi: 10.1016/j.brainres.2009.05.075. [DOI] [PubMed] [Google Scholar]
- 46.Rymarczyk K, Zurawski L, Jankowiak-Siuda K, Szatkowska I. Do Dynamic Compared to Static Facial Expressions of Happiness and Anger Reveal Enhanced Facial Mimicry? PLoS One. 2016;11:e0158534. doi: 10.1371/journal.pone.0158534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rohrer JD, Sauter D, Scott S, Rossor MN, Warren JD. Receptive prosody in nonfluent primary progressive aphasias. Cortex. 2012;48:308–316. doi: 10.1016/j.cortex.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warren JE, et al. Positive Emotions Preferentially Engage an Auditory–Motor “Mirror” System. The Journal of Neuroscience. 2006;26:13067–13075. doi: 10.1523/JNEUROSCI.3907-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Likowski KU, et al. Facial mimicry and the mirror neuron system: simultaneous acquisition of facial electromyography and functional magnetic resonance imaging. Frontiers in human neuroscience. 2012;6:214. doi: 10.3389/fnhum.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vrticka P, et al. Neural substrates of social emotion regulation: a FMRI study on imitation and expressive suppression to dynamic facial signals. Frontiers in psychology. 2013;4:95. doi: 10.3389/fpsyg.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rascovsky K, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain: a journal of neurology. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gorno-Tempini ML, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallhoff, F. (Technische Universität München, 2006–2015).
- 54.Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 55.Hess U, et al. Reliability of surface facial electromyography. Psychophysiology. 2017;54:12–23. doi: 10.1111/psyp.12676. [DOI] [PubMed] [Google Scholar]
- 56.Weiskopf N, et al. Unified segmentation based correction of R1 brain maps for RF transmit field inhomogeneities (UNICORT) NeuroImage. 2011;54:2116–2124. doi: 10.1016/j.neuroimage.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Ridgway GR, et al. Ten simple rules for reporting voxel-based morphometry studies. NeuroImage. 2008;40:1429–1435. doi: 10.1016/j.neuroimage.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Dumoulin SO, et al. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex. 2000;10:454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- 60.Lee GJ, et al. Neuroanatomical correlates of emotional blunting in behavioral variant frontotemporal dementia and early-onset Alzheimer’s disease. Journal of Alzheimer’s disease JAD. 2014;41:793–800. doi: 10.3233/JAD-132219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen X, Wu Q, Zhao K, Fu X. Electrophysiological Evidence Reveals Differences between the Recognition of Microexpressions and Macroexpressions. Frontiers in psychology. 2016;7:1346. doi: 10.3389/fpsyg.2016.01346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Baaren R, Janssen L, Chartrand TL, Dijksterhuis A. Where is the love? The social aspects of mimicry. Philos Trans R Soc Lond B Biol Sci. 2009;364:2381–2389. doi: 10.1098/rstb.2009.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuhn S, et al. Neural correlates of emotional synchrony. Social cognitive and affective neuroscience. 2011;6:368–374. doi: 10.1093/scan/nsq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chartrand TL, Lakin JL. The antecedents and consequences of human behavioral mimicry. Annual review of psychology. 2013;64:285–308. doi: 10.1146/annurev-psych-113011-143754. [DOI] [PubMed] [Google Scholar]
- 65.Allison T, McCarthy G, Luby M, Puce A, Spencer DD. Localization of functional regions of human mesial cortex by somatosensory evoked potential recording and by cortical stimulation. Electroencephalography and Clinical Neurophysiology - Evoked Potentials. 1996;100:126–140. doi: 10.1016/0013-4694(95)00226-X. [DOI] [PubMed] [Google Scholar]
- 66.Braadbaart L, de Grauw H, Perrett DI, Waiter GD, Williams JH. The shared neural basis of empathy and facial imitation accuracy. NeuroImage. 2014;84:367–375. doi: 10.1016/j.neuroimage.2013.08.061. [DOI] [PubMed] [Google Scholar]
- 67.Rochas V, et al. Disrupting pre-SMA activity impairs facial happiness recognition: an event-related TMS study. Cereb Cortex. 2013;23:1517–1525. doi: 10.1093/cercor/bhs133. [DOI] [PubMed] [Google Scholar]
- 68.Tootell R, et al. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. The Journal of Neuroscience. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kilts CD, Egan G, Gideon DA, Ely TD, Hoffman JM. Dissociable neural pathways are involved in the recognition of emotion in static and dynamic facial expressions. NeuroImage. 2003;18:156–168. doi: 10.1006/nimg.2002.1323. [DOI] [PubMed] [Google Scholar]
- 70.Pelphrey KA, Morris JP, McCarthy G, LaBar KS. Perception of dynamic changes in facial affect and identity in autism. Social cognitive and affective neuroscience. 2007;2:140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in cognitive sciences. 2000;4:267–278. doi: 10.1016/S1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 72.Yang DYJ, Rosenblau G, Keifer C, Pelphrey KA. An integrative neural model of social perception, action observation, and theory of mind. Neuroscience & Biobehavioral Reviews. 2015;51:263–275. doi: 10.1016/j.neubiorev.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 74.De Winter F-L, et al. Amygdala atrophy affects emotion-related activity in face-responsive regions in frontotemporal degeneration. Cortex. 2016;82:179–191. doi: 10.1016/j.cortex.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 76.Goll JC, Ridgway GR, Crutch SJ, Theunissen FE, Warren JD. Nonverbal sound processing in semantic dementia: a functional MRI study. NeuroImage. 2012;61:170–180. doi: 10.1016/j.neuroimage.2012.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ondobaka S, Kilner J, Friston K. The role of interoceptive inference in theory of mind. Brain and cognition. 2017;112:64–68. doi: 10.1016/j.bandc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simon, S. & Mukamel, R. Sensitivity to perception level differentiates two subnetworks within the mirror neuron system. Social cognitive and affective neuroscience, 10.1093/scan/nsx015 (2017). [DOI] [PMC free article] [PubMed]
- 79.Kempnich CL, Wong D, Georgiou-Karistianis N, Stout JC. Feasibility and Efficacy of Brief Computerized Training to Improve Emotion Recognition in Premanifest and Early-Symptomatic Huntington’s Disease. Journal of the International Neuropsychological Society: JINS. 2017;23:314–321. doi: 10.1017/S1355617717000145. [DOI] [PubMed] [Google Scholar]
- 80.Sturm VE, et al. Heightened emotional contagion in mild cognitive impairment and Alzheimer’s disease is associated with temporal lobe degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9944–9949. doi: 10.1073/pnas.1301119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Slessor G, et al. Examining the time course of young and older adults’ mimicry of enjoyment and nonenjoyment smiles. Emotion. 2014;14:532–544. doi: 10.1037/a0035825. [DOI] [PubMed] [Google Scholar]
- 82.Clark CN, Warren JD. Emotional caricatures in frontotemporal dementia. Cortex. 2016;76:134–136. doi: 10.1016/j.cortex.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 83.Hess U, Fischer A. Emotional mimicry as social regulation. Personality and social psychology review: an official journal of the Society for Personality and Social Psychology, Inc. 2013;17:142–57. doi: 10.1177/1088868312472607. [DOI] [PubMed] [Google Scholar]
- 84.Kret ME. Emotional expressions beyond facial muscle actions. A call for studying autonomic signals and their impact on social perception. Frontiers in psychology. 2015;6:711. doi: 10.3389/fpsyg.2015.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.