Abstract
To further investigate the mechanism of surround inhibition (SI) and to determine whether adopting different attentional strategies might have an impact on the modulation of SI, the effects of adopting an external (EF) or internal focus of attention (IF) on SI and motor performance were investigated. While performing an index flexion with either an EF or IF, transcranial magnetic stimulation was applied at various time points in 14 healthy subjects. When adopting an EF compared to an IF, the results show an improved motor performance (+14.7% in MVC) and a reduced bEMG in the adjacent APB (−22.3%) during maximal index flexion. This was accompanied by an increased SI in the APB with an EF (+26.4%). Additionally, the decrease in bEMG correlated with the magnitude of SI in APB. The current results demonstrate an efficient way to modulate SI by changing the attentional focus in healthy subjects and might, at least in part, explain the better motor performance being associated with an EF. The present findings help to better understand the positive mechanisms of an EF on SI in the healthy motor system and may also points towards a treatment strategy in pathologies with disturbed SI such as focal hand dystonia.
Introduction
Surround inhibition (SI) is a physiological mechanism that shapes neuronal activity in both sensory1 and motor systems2,3. Thus, SI reflects the capacity of excited or active neurons to reduce the activity of the surrounding neurons. In the human motor system, SI can be demonstrated by applying supra-threshold transcranial magnetic stimulation (TMS) over the primary motor cortex (M1) and measuring the amplitude of the resulting motor evoked potential (MEP) in the adjacent muscles2. In this specific context, SI within the primary cortex is expressed as the selective reduction in the MEP amplitude in the surrounding muscles before and during a contraction of the target muscle compared to a rest condition. By shaping neural drive during voluntary movements, SI in M1 is supposed to reflect the summation of inhibitory influences on the representation of surrounding muscles2,4–6 and is therefore considered to be essential for skilled motor behaviour3. For example, it was shown that the force level has an influence on the modulation of SI7. More precisely, it was demonstrated that the level of SI increased at low-force levels but was absent at force levels higher than 40% of MVC, strengthening the assumption that SI is primarily involved in the generation of skilled motor tasks. This is further supported by a study of Beck and Hallett8, showing that SI is greater and appears earlier when task complexity is increased. Apart from the task, the phase of the movement also influences the level of SI. Surround inhibition is more pronounced during the preparation and initiation phase of a movement but is dramatically reduced or absent during the hold phase of a ramp-and-hold contraction4. Furthermore, it is widely accepted that certain pathologies can influence SI. In contrast to healthy individuals, patients suffering from focal hand dystonia (FHD)4,9 or Parkinson’s disease10 show much lower levels of SI. Disturbances of SI in the cortical motor system of those patients result in a reduced contrast of active target neurons and supposedly inactive neurons that are not directly involved and thereby lead to an overflow of muscle activation in surrounding and usually non-active muscles4.
However, little is known about how the activity of the SI-network can be modulated in healthy subjects and in patients. Previous research has demonstrated that SI can be strengthened11,12 or weakened13,14 by motor training resulting in down- or upregulated MEP amplitudes in the non-trained adjacent muscles. However, in professional musicians, SI was shown to be significantly reduced14,15. The reduction in SI was assumed to favor functional coupling in order to facilitate synergistic finger movements13,14. However, at the same time, reduced SI has previously been shown to make these experts more prone to pathological states such as dystonia16. Thus, for both healthy subjects and patients it seems important to know how to (instantly) modulate SI and how this relates to behavioural outcome measures.
It has been demonstrated that populations with low levels of intracortical inhibition demonstrate impaired motor control in general such as dexterity17 or interlimb coordination18. In this regard, the relation between intracortical inhibition and motor performance when adopting either an external (EF) or an internal focus of attention (IF) has previously been investigated19. Interestingly, when participants were verbally asked to adopt an EF, it was shown that they could prolong sustaining submaximal isometric contractions with their index finger (time to task failure) and that the activity of inhibitory intracortical circuits within M1 (expressed as SICI and subTMS-induced EMG suppression) was instantly enhanced contrasted to when using an IF strategy. Thus, manipulating the focus of attention did not only have a positive influence on motor performance but also had a direct and immediate effect on cortical inhibition within M1. However, this modulatory effect of intracortical inhibition was outlined in the prime-mover and the effects on surrounding muscles were not tested. According to previous research3,4, it seems that the local inhibitory GABAergic circuits within M1 play a crucial role in the generation of SI. This hypothesis was supported by showing that FHD patients do not only have a reduction in SI leading to abnormal motor execution, but also display a loss of inhibition on multiple levels of the central nervous system including SICI4,20,21.
Based on these previous findings, it was hypothesized that changing the attentional focus may be a powerful tool to influence SI in M1, especially as it was previously suggested that SICI and SI might share common mechanisms4,22,23. Thus, adopting an EF might inhibit surrounding muscles what would reflect a focused neural drive and therefore enhanced movement efficiency. Accordingly, the present study aimed to investigate the immediate influence of different attentional strategies (EF vs IF) on (i) motor performance when executing maximal voluntary contractions (MVC) of the first dorsal interosseous muscle (FDI) and (ii) on the amount of SI within M1 influencing the activity of the adjacent muscle APB.
Methods
Subjects
Fourteen right-handed subjects (22–35 years; 3 women) agreed to participate in this study. All subjects were free from any known orthopaedic or neurological disorders. They gave their informed written consent before the experiments, which were approved by the local ethics committee (Commission cantonale d'éthique de la recherche sur l'être humain, CER-VD, Switzerland) and were in accordance with the Declaration of Helsinki.
Recording
All tests were conducted in one laboratory session. During this session, participants were seated in a comfortable and adjustable chair in an upright position. After skin preparation, bipolar surface electrodes (BlueSensor P, Ambu A/S, Ballerup, Denmark) were placed on the right hand skin over the muscles of interest with 1 cm inter-electrode distance. The reference electrode was also placed on the right hand, on the phalanx of the digitus medius. EMG recordings were obtained from the first dorsal interosseous (FDI) and the abductor pollicis brevis (APB) muscles. EMG recordings were amplified (x 1000), sampled at 4 kHz and bandpass filtered (Butterworth 10–1000 Hz). The individual MEP amplitudes were measured at four different phases of the contraction (see motor task below and Fig. 1B). All data was recorded and stored on a computer for off-line analysis using IMAGO Record software (Pfitec Biomedical Systems, Endingen, Germany).
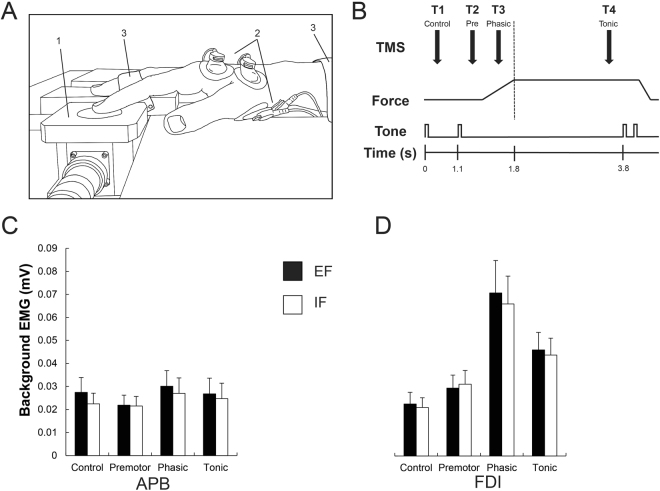
Figure 1.
(A) Experimental setup. The tip of the right index finger is resting on the force transducer (1). Two pairs of surface electrodes were placed over the (2) APB and FDI. The right forearm, middle, ring and pinky fingers were fixed by straps in order to minimize movements (3). (B) Time course of the task. Shown are the transcranial magnetic stimulation (TMS) time points, the force produced and the acoustic tones. Brain stimuli were applied during a resting or control phase (T1; 500 ms after the first tone), during the premotor phase (T2; between 100 and 200 ms after the second tone), the phasic phase (T3; between 300 and 600 ms after the second tone) and the tonic phase (T4; 2700 ms after the second tone). (C,D) Shown are the background EMG levels, obtained in a 50 ms time window before the brain stimulation, for the surrounding muscle APB (C) and the prime mover FDI (D) in all four different phases (control, premotor, phasic and tonic). There was a significant main effect of phase in the prime mover FDI, reflecting its activation during the motor task and no significant modulation of background EMG was found in the surrounding muscle APB. Black bars represent the external (EF) and the whites ones the internal focus of attention (IF).
Experiment 1
Motor task
In experiment 1, subjects were asked to perform maximum isometric contractions by pushing down on a force transducer (MC3A-500; Advanced Mechanical Technologies Inc., Watertown, MA, USA) with the tip of their right index finger in response to an acoustic signal (see Fig. 1A and B). The right forearm, the middle, ring and pinky fingers were fixed by straps in order to minimize all movements. The left arm rested in a comfortable and relaxed position on the table. The force signal was displayed on a computer screen placed 1 m in front them. At the beginning of the session, subjects were asked to perform three MVCs with their right index finger in order to determine the maximal force. Then, after a two minutes break, participants had to perform five MVCs with an IF and, in a second serie, with an EF. The order of the two series was randomized and a five minutes break was given between the two series. Between two consecutive contractions, a rest of 30 seconds was given. During all contractions, EMG and the force signals were recorded.
External vs. internal focus of attention
The instruction for the IF condition was ‘Contract your finger flexor muscles so that the moving line increases as fast as possible to the maximum after the tone’. For the EF condition, the instruction was ‘Exert pressure on the force transducer so that the moving line increases as fast as possible to the maximum after the tone’.
Experiment 2
In experiment 2, participants were asked to perform an isometric ramp-and-hold contraction by pushing down with their right index finger on the same force transducer as in experiment 1. In response to an acoustic signal, participants were asked to match a moving line, which represented the actual force level, with a horizontal target line. The target line represented 10% of the MVC and was individually adjusted based on the results obtained in experiment 1 (MVC without any specific focus). Ten percent of MVC was chosen based on previous research showing that SI is more pronounced when executing a contraction at low-force levels7. The target line and the force signal (running line) were displayed on a computer screen placed 1 m in front of the participants. A first tone indicated the start of each trial and a period of rest that lasted for 1100 ms. Participants were instructed to react to a second tone that was given 1100 ms after the first tone. Thus, they had to increase their force level from zero to 10% of MVC within 700 ms. As participants reacted to the second tone, the onset of force occurred approximately 150–250 ms later. Once the target line was matched, they had to hold the contraction for at least 2000 ms and then returned to the relaxed position (see Fig. 1B). This motor task allowed to differentiate specific phases and to deliver single-pulse TMS during each of these phases (see Fig. 1B): at rest (the control phase, T1; TMS 500 ms after the first acoustic signal), movement preparation (the premotor phase,T2; TMS between 100 and 200 ms after the second tone), the phasic phase (T3; TMS between 300 and 600 ms after the second tone) and the tonic phase (T4; TMS 2700 ms after the second tone). As a first step, participants had to perform practice trials in order to adjust the timing of the stimulation for the premotor- and phasic-phases. The time points of brain stimulation were individually adjusted in order that T2 coincide with the preparatory phase and T3 with the phasic phase. Once determined, the timing of the stimulation was identical for the IF and EF conditions. After these practice trials, participants had to repeat two series of twenty repetitions of the ramp-and-hold contraction with both an IF and an EF, resulting in eighty stimulation (2 foci (EF vs IF) * 4 time points (T1 vs T2 vs T3 vs T4) * 10 trials). Thus, 10 MEPs were recorded and used in the final analysis for each time-point and focus.
External vs. internal focus of attention
The instruction for the IF condition was like in experiment 1: ‘Contract your finger muscles so that the moving line displayed on the monitor increases from the baseline to the target value after the tone’. The instruction for the EF condition was ‘Exert pressure on the force transducer so that the moving line displayed on the monitor increases from the baseline to the target value after the tone’. After five trials, the participants were reminded to ‘concentrate on your finger muscles’ (IF) or ‘exert pressure on the force transducer’ (EF).
Brain stimulation
Transcranial magnetic stimuli were applied to the left M1 using a MagVenture Pro stimulator (MagVenture A/S, Farum, Denmark) with a 95 mm focal figure-of-eight coil (MagVenture D-B80). The initial stimulation point was set approximately 5 mm anterior to the vertex and over the midline. In order to ensure that the induced current flow is approximately perpendicular to the central sulcus, the TMS coil was oriented tangential to the scalp with the handle pointing backwards and laterally at 45° angle towards the contralateral forehead24. Induced currents were in the reverse mode (posterior to anterior) and the waveform monophasic throughout the whole experiment. The motor hotspot for eliciting MEPs in the APB with minimal intensity was determined by moving the coil anterior and left from the vertex, while MEP size was monitored. Once found, the APB motor hotspot position was recorded and constantly controlled by a neuronavigation system (Polaris Spectra, Northern Digital Inc., Waterloo, Canada and Localite TMS Navigator Version 2.0.5, LOCALITE GmbH, Sankt Augustin, Germany). Then, the resting motor threshold (rMT) was determined to the nearest of 1% of maximal stimulator output. The rMT was defined as the minimal stimulation intensity required to evoke MEPs bigger or equal to 50 μV peak-to-peak amplitude in 3 out of 5 trials. A stimulation intensity of 140% rMT was used when assessing SI. Ten stimuli at each of the four different phases (rest, premotor, phasic, tonic) were delivered in a randomized order in each condition (EF, IF). The order of conditions was randomized between participants.
Data analyses and statistics
EMG and reaction time analysis
In experiment 1, the maximal force was considered as the highest peak in the force signal (low pass filtered at 50 Hz) during the contraction. In addition, the five trials were averaged and compared between both conditions (EF vs IF). Background EMG (bEMG) was obtained in the FDI (prime mover) and APB (adjacent) muscles and compared by assessing the root mean square in a time window of 100 ms following the peak of force (in experiment 1) and 50 ms preceding the brain stimulation (in experiment 2). In addition, the power spectrum density function of the EMG signal during the MVCs was computed by a fast Fourier transformation. The mean (MNF) and median frequencies (MDF) were used as the parameter to compare the power spectrum between both focus of attention conditions (EF vs IF). The MNF was computed as the mathematical mean of the spectrum curve and the MDF as the parameter that divides the total power area into two equal parts. Finally, the reaction time (RT) was compared between both conditions as the time from the second acoustic signal – indicating the participants to press against the force transducer in order to reach the target line representing the 10% of the MVC – to the onset of force.
Motor evoked potentials analysis
In experiment 2, the peak-to-peak amplitude of the TMS evoked MEPs at each time points (control, premotor, phasic and tonic) in the APB and FDI muscles were compared between both conditions (EF vs IF). Additionally, the correlation coefficient was computed using a robust regression model to investigate association between the modulations of bEMG in the APB muscle (experiment 1) and SI (experiment 2). A robust regression model was applied as it is recommended when data might be contaminated with influential observations and/or outliers25. Differences in APB bEMG and MEPs (related to the control phase in percent) used for the regression analysis were obtained by subtracting the value found in the EF condition from the value found in the IF condition. The bEMG in the APB muscle was taken from the first experiment as participants strongly activated their FDI in order to fulfil the task and consequently, only in this experiment, it was difficult for the subjects to not co-activate with their APB muscle. In contrast, FDI activation in experiment 2 was not challenging enough to elicit any co-activation in the adjacent APB.
Statistics
Before the statistical analyses, normal distribution of the data was tested using a Shapiro-Wilk test. In experiment 1, separate paired Student t-tests were performed for each output parameter of the behavioural tests (force, bEMG and power spectrum analysis) to compare the two conditions (EF vs. IF). In experiment 2, neurophysiological outcome measures (peak-to-peak MEP amplitudes and bEMG) were compared individually using a two-way repeated measures ANOVA to compare the effect of “focus” (two levels: EF and IF) and the effect of “phase” (four levels: control, premotor, phasic and tonic). If sphericity was violated (Maulchy’s test), degrees of freedom were corrected by Greenhouse-Geisser estimates of sphericity. Effect sizes are presented as general eta-squared values26 and the Benjamini-Hochberg procedure was used to correct for multiple comparisons in case of significant F values27. Unless indicated otherwise, data are reported as mean ± standard deviation. R version 3.2.428 was used for all statistical analyses and the level of significance was set at P ≤ 0.05.
Data availability
The datasets generated during the present study are available from the corresponding author on reasonable request.
Results
Experiment 1
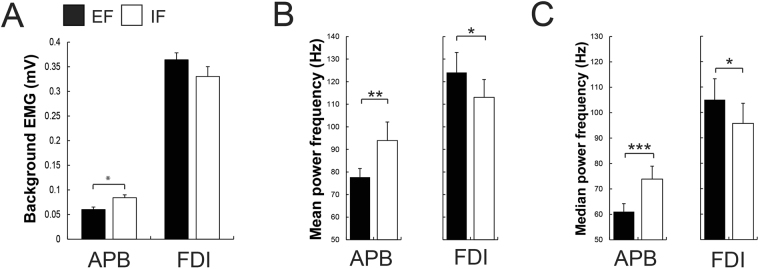
Participants could generate more force (the best of the 5 trials) when adopting an EF (43.98 ± 14.76 N) compared to an IF (38.34 ± 13.53 N; t13 = 5.46, P < 0.001, Fig. 2). In addition, they displayed more force on average (the 5 trials averaged) with an EF (38.43 ± 12.23 N) contrasted to an IF (34.75 ± 13.65 N; t13 = 3.77, P = 0.002, Fig. 2). Additionally, subjects showed less bEMG activity in the adjacent muscle APB (t13 = −2.48, P = 0.02, Fig. 3A) when executing the maximal force task with an EF (0.059 ± 0.05 mV) compared to an IF (0.076 ± 0.07 mV). Additionally, they showed smaller MNF and MDF in the adjacent APB muscle when adopting an EF contrasted to an IF (MNF: t13 = −2.99, P = 0.01, EF = 77.67 ± 13.28 Hz, IF = 93.95 ± 28.39 Hz; MDF: t13 = −3.99, P = 0.001, EF = 61.05 ± 10.92 Hz, IF = 73.87 ± 17.67 Hz, Fig. 3B and C). Regarding the bEMG of the prime mover FDI between both conditions, no significant difference was found (P = 0.14, Fig. 3A). However, participants displayed significant greater MNF and MDF when focusing externally compared to internally (MNF: t13 = 2.53, P = 0.02, EF = 124.07 ± 30.66 Hz, IF = 113.04 ± 27.30 Hz; MDF: t13 = 2.41, P = 0.03, EF = 105.01 ± 28.57 Hz, IF = 95.66 ± 27.54 Hz, Fig. 3B and C).
Figure 2.
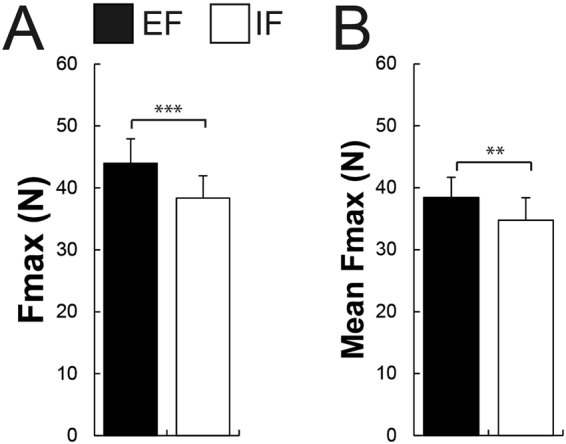
Shown are mean values and SEM (n = 14) of (A) the force during maximal voluntary contraction (MVC, the best trial) and (B) the force during the 5 MVC trials averaged under both attentional focus conditions (EF vs IF). Participants could generate more force and more force when the 5 MVC trials were averaged with an EF compared to an IF. Black bars represent the external (EF) and the whites ones the internal focus of attention (IF). **P ≤ 0.01, ***P ≤ 0.001.
Figure 3.
In all bar graphs, shown are mean values and SEM (n = 14). (A) Participants showed less background EMG during the MVC in the adjacent APB muscle when focusing externally (EF) compared to focusing internally (IF) while no significant difference was found in the prime mover FDI muscle. (B) Shown are the mean power frequency (MNF) and (C) median power frequency (MDF) of adjacent APB and prime mover FDI muscles during the MVC under both focus of attention conditions. Participants showed a smaller MNF and MDF in the APB muscle and a greater MNF and MDF in the prime mover FDI when adopting an EF contrasted to an IF. Black bars represent the external (EF) and the whites ones the internal focus of attention (IF). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Experiment 2
Brain stimulation paradigm
The same subjects as in experiment 1 took part in the second experiment. However, two participants showed a very inconsistent motor performance during this second experiment and were therefore excluded from the final data analysis.
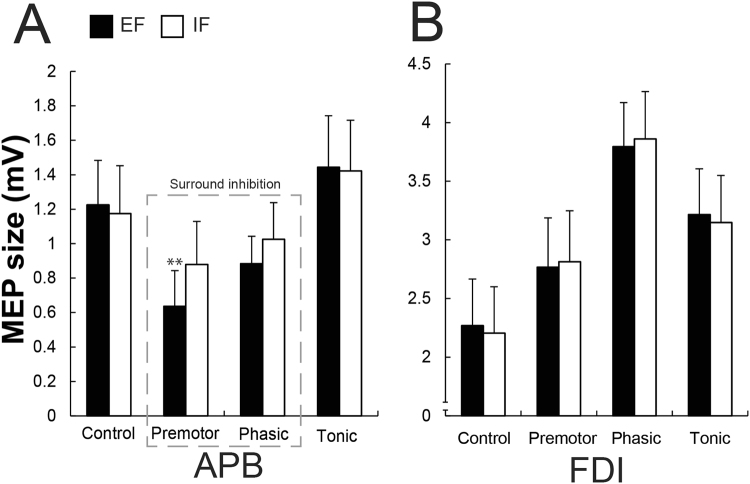
When analysing MEP peak-to-peak amplitudes in the APB muscle, there was no main effect of focus (F1,11 = 3.75, P = 0.07, η2 = 0.002) but a significant main effect of phase (F1.92,21.12 = 9.30, P = 0.001, η2 = 0.086). Additionally, a significant interaction effect of focus x phase was found (F3,33 = 7.18, P < 0.001, η2 = 0.005). Pairwise Post hoc comparisons for the main effect of phase revealed that the premotor phase (P < 0.001) and phasic phase (P = 0.04) were smaller than the control phase and that all phases differed from the tonic phase (all P < 0.001, see Table 1 for more details). Regarding the interaction effect of focus x phase, the pattern of modulation of MEP size in the APB muscle for the different phases showed a significant reduction in MEP size (reflecting the SI) before the initiation of the index finger flexion (premotor) compared to the control phase (P = 0.001 in the EF and P = 0.04 in the IF condition). Moreover, a clear enhancement in MEP size during the tonic phase when compared to all other phases (all P < 0.04, see Table 1 for details) was shown. Most importantly, MEP sizes during the premotor phase were significantly smaller when adopting an EF contrasted to an IF (P = 0.004), indicating a differential SI of the MEP in the APB regarding the type of attentional focus (Fig. 4A). No significant inhibition was found during the phasic phase compared to the control condition for both attentional foci (P = 0.10 in the EF and P = 0.38 in the IF condition, respectively).
Table 1.
Single-pulse TMS in surrounding muscle APB for all four phases of the movement and the two conditions
| EF | IF | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Premotor | Phasic | Tonic | Control | Premotor | Phasic | Tonic | |
| MEP size (mV) | 1.22 ± 0.89 | 0.63 ± 0.71a,b,c | 0.88 ± 0.55a,b,e | 1.44 ± 1.03a,c,d | 1.17 ± 0.96 | 0.87 ± 0.86a,b,d,f,g | 1.02 ± 0.73a,b,g | 1.42 ± 1.02a,f |
| MEP (% of control MEP) | 100 | 45.30 ± 17.92 | 79.69 ± 44.82 | 121.76 ± 29.73 | 100 | 71.69 ± 27.04 | 95.79 ± 47.31 | 130.76 ± 39.03 |
| bEMG (mV) | 0.024 ± 0.02 | 0.021 ± 0.01 | 0.028 ± 0.02 | 0.021 ± 0.02 | 0.019 ± 0.01 | 0.020 ± 0.01 | 0.017 ± 0.02 | 0.017 ± 0.02 |
Shown are mean values and SD (n = 12) for single-pulse MEP size, MEP size related to control MEP expressed in percent and the background EMG (bEMG) for both conditions (EF and IF) in the surrounding muscle APB. Main effect of phase: significant difference (P < 0.05) for this phase in both conditions compared with the control phase (a) and compared with the tonic phase (b). Interaction effect focus x phase: significant difference (P < 0.05) for this phase compared to control (c), premotor (d), tonic phase (e) in the EF condition and compared to control (f) and tonic phase (g) in the IF condition. EF = external focus, IF = internal focus of attention.
Figure 4.
(A,B) Shown are mean values and SEM (n = 12) of the MEP peak-to-peak amplitude in the two surrounding muscle APB (A) and the prime mover FDI (B) at four different brain stimulation time points (Control, Premotor, Phasic and Tonic) under the two attentional focus conditions (EF and IF). In the surrounding muscle APB, there was a significant interaction effect showing that when adopting a EF contrasted to an IF, surround inhibition was enhanced during the premotor phase. There was a significant main effect of phase in the prime mover FDI (B), reflecting its activation during the motor task. Black bars represent the external (EF) and the whites ones the internal focus of attention (IF). **P ≤ 0.01.
In the prime-mover FDI muscle, the MEPs were recorded with the coil over the APB hotspot. There was no main effect of focus (F1,11 = 0.01, P = 0.91, η2 < 0.001) but a significant main effect of phase (F2.03,22.37 = 12.60, P < 0.001, η2 = 0.15, Fig. 4B). Pairwise post hoc comparisons revealed that all phases differed from the control phase (premotor, P = 0.04; phasic, P < 0.001; tonic, P = 0.001, Table 2 for more details). Additionally, MEPs were significantly enhanced compared to the premotor (P < 0.001) and tonic phases (P = 0.001) during the phasic phase. Furthermore, MEPs in the tonic phase were significantly bigger contrasted to the premotor phase (P = 0.002). There was no significant interaction effect of focus x phase (F3,33 = 2.81, P = 0.054, η2 < 0.001, Fig. 4B). Due to the fact that the P value was close to significance threshold, we performed additional separate paired Student’s t-tests (uncorrected), to test whether differences between both conditions (EF vs IF) occurred for any phase. Actually, no significant difference was found between the conditions (EF vs IF) in the control phases (t11 = −1.19, P = 0.25), premotor phases (t11 = 0.92, P = 0.37), phasic phases (t11 = 0.85, P = 0.41), and tonic phases (t11 = −1.59, P = 0.13).
Table 2.
Single-pulse TMS in the prime mover (FDI) for all four phases of the movement and the two conditions.
| EF | IF | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Premotor | Phasic | Tonic | Control | Premotor | Phasic | Tonic | |
| MEP size (mV) | 2.26 ± 1.37 | 2.76 ± 1.45a | 3.79 ± 1.29a,b | 3.21 ± 1.35a,b,c | 2.20 ± 1.36 | 2.81 ± 1.50a | 3.86 ± 1.39a,b | 3.14 ± 1.38a,b,c |
| MEP (% of control) | 100 | 133.13 ± 78.48 | 206.42 ± 117.50 | 165.45 ± 90.64 | 100 | 138.13 ± 69.54 | 214.84 ± 111.80 | 164.98 ± 79.30 |
| bEMG (mV) | 0.022 ± 0.01 | 0.029 ± 0.01 | 0.07 ± 0.04 | 0.046 ± 0.02 | 0.021 ± 0.01 | 0.031 ± 0.02 | 0.066 ± 0.04 | 0.043 ± 0.02 |
Shown are mean values and SD (n = 12) for single-pulse MEP size, MEP size related to control MEP expressed in percent and the background EMG (bEMG) for both conditions (EF and IF) in the prime mover FDI muscle. Main effect of phase: significant difference (P < 0.05) for this phase in both conditions compared with control phase (a), compared with the premotor phase (b), and compared with the phasic phase (c). No interaction effect of focus x condition were found in FDI. EF = external focus, IF = internal focus of attention.
Background EMG and reaction time
Background EMG recordings in the APB muscle revealed no significant main effect of focus (F1,11 = 2.28, P = 0.15, η2 = 0.004), main effect of phase (F1.26,14.19 = 0.86, P = 0.39, η2 = 0.015) and no interaction effect of focus x phase (F1.56,17.16 = 0.65, P = 0.58, η2 = 0.001, Table 1 and Fig. 1C).
In the prime mover FDI muscle, there was no significant main effect of focus (F1,11 = 1.78, P = 0.20, η2 < 0.001). However, there was a significant main effect of phase (F1.47,16.17 = 14.36, P < 0.001, η2 = 0.28). Post hoc comparisons revealed that background EMG in all phases was bigger than the control phase (premotor, P = 0.01; phasic, P < 0.001; tonic, P < 0.001), showing that FDI was active during the movement (see Fig. 1D). Additionally, background EMG was significantly enhanced during the phasic (P < 0.001) and tonic (P = 0.003) phases compared with the premotor phase. Background EMG was significantly enhanced in the phasic phase compared to the tonic phase (P < 0.001, Table 2 for details). No significant interaction effect of focus x phase (F3,33 = 1.36, P = 0.27, η2 = 0.001) was found.
Regarding the RT (the onset in the force signal after the second tone), no significant difference was found between both conditions (t11 = −0.95, P = 0.35; EF = 198.11 ± 37.77 ms and IF = 203.32 ± 35.08 ms).
Correlation
A potential connection between the functional data (experiment 1) and the neurophysiological data (experiment 2) was tested applying a robust regression model. The difference in the bEMG of the adjacent muscle APB between EF and IF in experiment 1 was related to differences in SI between EF and IF in experiment 2. The results showed a significant coefficient of correlation between the difference in bEMG in the APB muscle during the maximal contraction and the amount of SI in the APB muscle during the premotor phase (r = 0.61, P = 0.001, Fig. 5).
Figure 5.
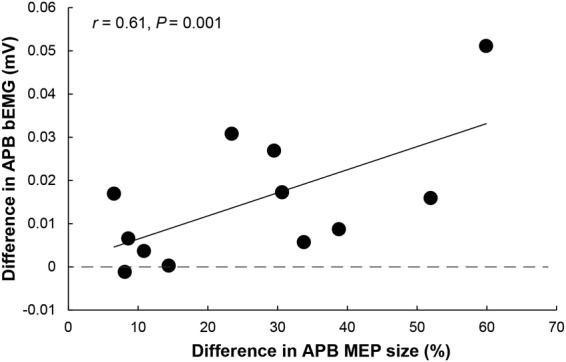
Shown are the attentional foci-related differences (IF–EF) of APB MEPs size during the premotor phase (related to control as percentage changes) on the abscissa and the differences (IF–EF) in APB bEMG during maximal contraction of the index finger on the ordinate. The robust regression model clearly demonstrates that greater decrease in bEMG found in the adjacent muscle APB during maximal contraction of the prime mover FDI (experiment 1) is accompanied by greater surround inhibition during the premotor phase in the adjacent APB (experiment 2, r = 0.61, P = 0.001).
Discussion
The current results from experiment 2 demonstrate an efficient way to instantly modulate SI by changing the focus of attention. Furthermore, as demonstrated by experiment 1, adopting an EF resulted in an improved motor performance and a reduced muscular activity in the adjacent muscle APB. As the decreased bEMG found in the APB muscle during maximal index finger contraction showed a significant correlation with the enhanced SI found during the premotor phase, the improved motor performance that is usually observed with an EF may at least partly be explained by an enhanced activation of neural circuits that shape motor SI.
Attentional foci and motor performance
In the present study, experiment 1 was designed (a) to confirm that motor performance can be enhanced when adopting an EF and (b) to assess (unwanted) muscular activity in the adjacent muscle APB.
In line with previous research (see29 for a review) and as expected, motor performance was improved when adopting an EF contrasted to an IF. More specifically, asking the participants to concentrate on the force transducer they were pushing against (EF), rather than on their index finger (IF), led to an enhanced maximal force of the index finger (+14.7%). At the same time and in line with previous research, subjects revealed less bEMG activity (−22.3%), MNF and MDF in the adjacent APB muscle with an EF30. Furthermore, despite no significant difference in bEMG between conditions in the prime-mover FDI, participants showed significantly larger power spectral density in the FDI muscle, expressed as enhanced MNF and MDF, when focusing externally.
As the force a muscle can produce depends not only on the number of motor units activated (recruitment following the size principle), but also on the rates at which motor neurones discharge action potentials (rate coding)31, this enhanced frequency found in the FDI muscle under an EF might be explained in at least two ways: first, additional motor units (MUs) might have been recruited that fire with higher frequencies and/or the already active MUs have increased their firing frequency. The first mechanism would suggest that maximal force production with an IF is not able to fully recruit all MUs and that the upward shift in the MNF and MDF with an EF in the prime mover FDI is indicative of an additional recruitment of MUs that, according to the size principle32, consist of larger MUs with faster conduction velocities33. The second mechanism does not necessarily involve recruitment of additional MUs but may be due to the fact that an increased incidence of doublet discharges occurred at the very beginning of the MVC. The presence of doublet discharges at the onset of muscle contraction known as the catch-like property of skeletal muscle34 is thought to improve force production35,36 and might, therefore, help to generate more force with an EF.
In addition, our results confirm previous findings showing reduced muscular activity with an EF30,37–42. Based on this observation, it was previously argued that an EF increases movement efficiency by reducing unnecessary muscular contributions30. Therefore, our results suggest that adopting an EF contrasted to an IF modified the pattern of muscle activation (less activation of the adjacent muscle APB coupled with an upward shift in the power spectral density in the FDI) during the motor task and led to an increased MVC. However, the underlying mechanisms for this focus-specific EMG reduction in the adjacent muscle remains generally poorly understood and, therefore, experiment 2 was designed.
Attentional foci and motor surround inhibition
In experiment 2, we investigated the influence of changing the focus of attention on the amount of SI in the APB muscle. Similar to previous studies4,7,8, a reduction in the amplitudes of APB MEPs in the premotor and phasic phases during the index finger flexion occurred, indicating the presence of SI in this adjacent muscle. In the present study, the amount of SI during the movement initiation phase could be instantly altered by changing the focus of attention: in both the premotor (+26.4%; p = 0.004) and the phasic phase (+8.1%; non-significant change), adopting an EF resulted in a greater SI than an IF. Furthermore, the advantage of the present study is that we could compare one and the same subject performing the identical task with an EF or IF (using a repeated measures design) in contrast to the first study investigating the underlying mechanism of adopting different attentional foci by means of fMRI43.
Surround inhibition and hand motor control
So far, the relationship between motor behaviour and SI is a key but unanswered part in understanding the role of SI in hand motor control and performance. As SI is more prominent with low-force levels and disappears at intensities higher than 40% of MVC, it seems that SI is predominantly involved in the generation of skilled and fine motor tasks8. Additionally, previous research showed that FHD patients demonstrate unusual co-contractions, which are thought to be associated with a reduced SI during movement initiation of the index finger4. Thus, it is argued that an enhanced SI improves the contrast on the motor cortical level and thereby facilitates dynamic manual tasks3. In addition, motor training was shown to not only improve motor performance but also to strengthen SI11,12.
The observations of the current study may suggest that the increased efficiency usually associated with an EF on a behavioral level29,44,45 may in fact be due to differential neural activation of motor areas such as M1: as shown in experiment 2, the increased SI seems to focus the motor command to the prime mover, avoiding – as shown in experiment 1 – unnecessary contractions of muscles that are not directly involved. The finding of a correlation between the decrease in bEMG and increase in SI in the APB further strengthens this notion. The current results in the adjacent APB also complement our previous study concentrating on the prime mover FDI. In this previous study, increased levels of SICI and sub-TMS induced EMG suppression with an EF were shown for the agonist FDI19. Thus, adopting an EF seems to influence motor cortical inhibitory control for both, the prime mover as well as the surrounding muscles that are not directly involved in the motor task.
It was formerly suggested that SICI might contribute to the inhibitory network shaping SI4,22,23. Indeed, previous research revealed a reduction of short-interval intracortical inhibition (SICI) in dystonic patients2,9,46 accompanied by an impaired SI in the premotor and phasic phase of the movement4. Thus, it seems that these inhibitory processes are both closely interrelated and influence the quality of motor execution in general4. Nonetheless, at this point, the link between SI and SICI remains unclear and further research is needed.
Limitations of the current study
Although the present study demonstrates an instant modulation of motor cortical SI when changing the focus of attention, it cannot clarify whether SI is created in M1 or if it is derived from input coming from other brain areas such as the basal ganglia3 and/or cerebellum47. For example, in patients with impaired SI, the basal ganglia circuits have been assumed to display imbalanced activity between direct – responsible for sculpting the desired movement –, and indirect pathways – responsible for inhibiting unwanted movements3. In addition, FHD patients show an hyper-activation of cerebellum in fMRI during voluntary movement, which might be related to the disrupted SI47. Therefore, the finding of the present study cannot render more precisely these underlying mechanisms but shed more light on the interrelation between behavioural changes and modulation in SI.
Conclusion and functional considerations
The current study provides new evidence that SI can be instantly modulated according to attentional foci in healthy individuals. Importantly, the increase in SI is associated with better motor performance. Therefore, the instant modulation of SI induced by verbal instructions leaves open the possibility of influencing SI in a therapeutic setting, when abnormal SI is thought to impair motor function in neurological conditions such as FHD3.
Author Contributions
All authors designed the study and reviewed the manuscript. Y.-A.K. analysed the data, wrote the main manuscript text and prepared all figures. All authors approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Angelucci A, Levitt JB, Lund JS. Anatomical origins of the classical receptive field and modulatory surround field of single neurons in macaque visual cortical area V1. Progress in brain research. 2002;136:373–388. doi: 10.1016/S0079-6123(02)36031-X. [DOI] [PubMed] [Google Scholar]
- 2.Sohn YH, Hallett M. Surround inhibition in human motor system. Experimental brain research. 2004;158:397–404. doi: 10.1007/s00221-004-1909-y. [DOI] [PubMed] [Google Scholar]
- 3.Beck S, Hallett M. Surround inhibition in the motor system. Experimental brain research. 2011;210:165–172. doi: 10.1007/s00221-011-2610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck S, et al. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:10363–10369. doi: 10.1523/JNEUROSCI.3564-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Progress in neurobiology. 1996;50:381–425. doi: 10.1016/S0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 6.Hallett M. Surround inhibition. Suppl Clin Neurophysiol. 2003;56:153–159. doi: 10.1016/S1567-424X(09)70216-8. [DOI] [PubMed] [Google Scholar]
- 7.Beck S, Schubert M, Richardson SP, Hallett M. Surround inhibition depends on the force exerted and is abnormal in focal hand dystonia. J Appl Physiol. 2009;107:1513–1518. doi: 10.1152/japplphysiol.91580.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck S, Hallett M. Surround inhibition is modulated by task difficulty. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2010;121:98–103. doi: 10.1016/j.clinph.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sohn YH, Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann Neurol. 2004;56:595–599. doi: 10.1002/ana.20270. [DOI] [PubMed] [Google Scholar]
- 10.Shin HW, Kang SY, Sohn YH. Disturbed surround inhibition in preclinical parkinsonism. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2007;118:2176–2179. doi: 10.1016/j.clinph.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 11.Sugawara K, et al. Functional plasticity of surround inhibition in the motor cortex during single finger contraction training. Neuroreport. 2012;23:663–667. doi: 10.1097/WNR.0b013e3283556522. [DOI] [PubMed] [Google Scholar]
- 12.Kassavetis P, et al. Adaptation of surround inhibition in the human motor system. Experimental brain research. 2012;222:211–217. doi: 10.1007/s00221-012-3207-4. [DOI] [PubMed] [Google Scholar]
- 13.Kang SY, Hallett M, Sohn YH. Exercise-induced strengthening of inter-digital connections in musicians. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2013;124:1622–1627. doi: 10.1016/j.clinph.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Shin HW, Kang SY, Hallett M, Sohn YH. Reduced surround inhibition in musicians. Experimental brain research. 2012;219:403–408. doi: 10.1007/s00221-012-3102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akkad H, et al. Motor training reduces surround inhibition in the motor cortex. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2016;127:2482–2488. doi: 10.1016/j.clinph.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Altenmuller E, Ioannou CI, Lee A. Apollo’s curse: neurological causes of motor impairments in musicians. Progress in brain research. 2015;217:89–106. doi: 10.1016/bs.pbr.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Heise K-F, et al. The Aging Motor System as a Model for Plastic Changes of GABA-Mediated Intracortical Inhibition and Their Behavioral Relevance. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:9039–9049. doi: 10.1523/JNEUROSCI.4094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiyama H, Hinder MR, Schmidt MW, Garry MI, Summers JJ. Age-related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiology of aging. 2012;33(1484):e1481–1414. doi: 10.1016/j.neurobiolaging.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn YA, Keller M, Ruffieux J, Taube W. Adopting an external focus of attention alters intracortical inhibition within the primary motor cortex. Acta physiologica (Oxford, England) 2016;220:289–299. doi: 10.1111/apha.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen LG, Hallett M. Hand cramps: clinical features and electromyographic patterns in a focal dystonia. Neurology. 1988;38:1005–1012. doi: 10.1212/WNL.38.7.1005. [DOI] [PubMed] [Google Scholar]
- 21.Chen RS, Tsai CH, Lu CS. Reciprocal inhibition in writer’s cramp. Movement disorders: official journal of the Movement Disorder Society. 1995;10:556–561. doi: 10.1002/mds.870100505. [DOI] [PubMed] [Google Scholar]
- 22.Keller A. Intrinsic synaptic organization of the motor cortex. Cereb Cortex. 1993;3:430–441. doi: 10.1093/cercor/3.5.430. [DOI] [PubMed] [Google Scholar]
- 23.Stinear CM, Byblow WD. Impaired modulation of intracortical inhibition in focal hand dystonia. Cereb Cortex. 2004;14:555–561. doi: 10.1093/cercor/bhh017. [DOI] [PubMed] [Google Scholar]
- 24.Rossini PM, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampel, F. R., Ronchetti, E. M., Rousseeuw, P. J. & Stahel, W. A. In Robust Statistics 307–341 (John Wiley & Sons, Inc., (2005).
- 26.Bakeman R. Recommended effect size statistics for repeated measures designs. Behav Res Methods. 2005;37:379–384. doi: 10.3758/BF03192707. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 28.R: A language and environment for statistical computing (R Foundation for Statistical Computing., Vienna, Austria, (2010).
- 29.Wulf G. Attentional focus and motor learning: a review of 15 years. Int Rev Sport Exerc Psychol. 2012;6:77–104. doi: 10.1080/1750984X.2012.723728. [DOI] [Google Scholar]
- 30.Lohse KR, Sherwood DE, Healy AF. Neuromuscular Effects of Shifting the Focus of Attention in a Simple Force Production Task. J Mot Behav. 2011;43:173–184. doi: 10.1080/00222895.2011.555436. [DOI] [PubMed] [Google Scholar]
- 31.Maffiuletti NA, et al. Rate of force development: physiological and methodological considerations. European journal of applied physiology. 2016;116:1091–1116. doi: 10.1007/s00421-016-3346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henneman E. Relation between size of neurons and their susceptibility to discharge. Science (New York, N.Y.) 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- 33.Desmedt JE, Godaux E. Fast motor units are not preferentially activated in rapid voluntary contractions in man. Nature. 1977;267:717–719. doi: 10.1038/267717a0. [DOI] [PubMed] [Google Scholar]
- 34.Burke D, Hagbarth KE, Löfstedt L, Wallin BG. The responses of human muscle spindle endings to vibration during isometric contraction. J. Physiol. (Lond.) 1976;261:695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binder-Macleod S, Kesar T. Catchlike property of skeletal muscle: recent findings and clinical implications. Muscle Nerve. 2005;31:681–693. doi: 10.1002/mus.20290. [DOI] [PubMed] [Google Scholar]
- 36.Cheng AJ, Place N, Bruton JD, Holmberg HC, Westerblad H. Doublet discharge stimulation increases sarcoplasmic reticulum Ca2+ release and improves performance during fatiguing contractions in mouse muscle fibres. J Physiol. 2013;591:3739–3748. doi: 10.1113/jphysiol.2013.257188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohse KR, Sherwood DE, Healy AF. How changing the focus of attention affects performance, kinematics, and electromyography in dart throwing. Hum Mov Sci. 2010;29:542–555. doi: 10.1016/j.humov.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Marchant DC, Greig M, Scott C. Attentional focusing instructions influence force production and muscular activity during isokinetic elbow flexions. Journal of strength and conditioning research/National Strength & Conditioning Association. 2009;23:2358–2366. doi: 10.1519/JSC.0b013e3181b8d1e5. [DOI] [PubMed] [Google Scholar]
- 39.Vance J, Wulf G, Töllner T, McNevin N, Mercer J. EMG activity as a function of the performer’s focus of attention. J Mot Behav. 2004;36:450–459. doi: 10.3200/JMBR.36.4.450-459. [DOI] [PubMed] [Google Scholar]
- 40.Wälchli M, Ruffieux J, Bourquin Y, Keller M, Taube W. Maximizing Performance: Augmented Feedback, Focus of Attention, and/or Reward? Medicine and science in sports and exercise. 2015;48:714–719. doi: 10.1249/MSS.0000000000000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wulf G, Dufek JS, Lozano L, Pettigrew C. Increased jump height and reduced EMG activity with an external focus. Hum Mov Sci. 2010;29:440–448. doi: 10.1016/j.humov.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Zachry T, Wulf G, Mercer J, Bezodis N. Increased movement accuracy and reduced EMG activity as the result of adopting an external focus of attention. Brain Res Bull. 2005;67:304–309. doi: 10.1016/j.brainresbull.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 43.Zentgraf K, et al. Neural correlates of attentional focusing during finger movements: A fMRI study. J Mot Behav. 2009;41:535–541. doi: 10.3200/35-08-091. [DOI] [PubMed] [Google Scholar]
- 44.Wulf G, McNevin N, Shea CH. The automaticity of complex motor skill learning as a function of attentional focus. Q J Exp Psychol A. 2001;54:1143–1154. doi: 10.1080/713756012. [DOI] [PubMed] [Google Scholar]
- 45.McNevin N, Shea CH, Wulf G. Increasing the distance of an external focus of attention enhances learning. Psychol Res. 2003;67:22–29. doi: 10.1007/s00426-002-0093-6. [DOI] [PubMed] [Google Scholar]
- 46.Hallett M. Dystonia: abnormal movements result from loss of inhibition. Advances in neurology. 2004;94:1–9. [PubMed] [Google Scholar]
- 47.Nakamura Y, Sakamoto H, Ueno S, Hirano M, Toge T. P002 Abnormal surround inhibition of finger tapping task and cerebellar hyper-activation of functional MRI in patients with focal hand dystonia. Clinical Neurophysiology. 2017;128:e11–e12. doi: 10.1016/j.clinph.2016.10.133. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the present study are available from the corresponding author on reasonable request.