Abstract
We tested the hypotheses that the global incidence of bladder cancer was increasing but its mortality was reducing and its incidence was positively correlated with country-specific socioeconomic development. We retrieved data on age-standardized incidence and mortality rates/100,000 from the GLOBOCAN database in 2012. Temporal patterns were examined for 39 countries from the Cancer Incidence in Five Continents volumes I-X and other national registries. We evaluated the correlation between the incidence/mortality rates and Human Development Index (HDI)/ logarithmic values of Gross Domestic Product per capita (GDP). The average annual percent change of the incidence and mortality rates in the most recent 10 years was examined by joinpoint regression analysis. The highest incidence rates were observed in Southern Europe, Western Europe and North America. The mortality rates were the highest in Western Asia and Northern Africa. The incidence was positively correlated with HDI (r = 0.66 [men]; r = 0.50 [women]) and to a lesser extent logarithmic values of GDP per capita (r = 0.60 [men]; r = 0.50 [women], all p < 0.01). Many European countries experienced incidence rise. A substantial mortality reduction was observed in most countries, yet increases in mortality rates were observed in the Philippines and Iceland. These findings identified countries where more preventive actions are required.
Introduction
Bladder cancer was the ninth most common malignancy worldwide, with 430,000 newly diagnosed cases in 20121. In Europe, a total of 118,000 new cases and 52,000 deaths were estimated in the same year2. Its high prevalence, in conjunction with its vulnerability to multiple recurrences and progression despite local therapy3, leads to a substantial health service burden4. Leal and colleagues4 recently estimated that bladder cancer cost €4.9 billion in the European Union in 2012. The majority (90%) of bladder cancer consists of urothelial carcinoma as the predominant histologic type in Western Europe and the United States, although squamous cell bladder cancer is more common in Africa where schistosomiasis infections were more prevalent5. Recent studies showed that North America and Western Europe reported particularly high incidence rates6, whilst Eastern Europe and Asian countries had the lowest rates.
The major risk factors for bladder cancer include tobacco smoking; industrial exposure to potential carcinogens such as aromatic amines and carbon black dust; long-term drinking of arsenic-contaminated or chlorinated water; and family history of concordant cancers7,8. Many of these risk factors can be modified by lifestyle measures and environmental protective initiatives, implying a strong prospect for intervention. Previous studies that analyzed its global trends were based on figures in the 1990s to early 2000s, did not make direct comparisons between countries, or performed in selected regions8–11. The Global Burden of Disease Study12 does provide comparison of bladder cancer incidence and mortality over time, but the temporal incidence and mortality trends of this cancer at the global level should be examined by recognized databases. Examining the patterns and temporal trends of bladder cancer could quantify geographical variations, shed light on health planning and priority setting, and explore modifiable factors that might have brought about trend changes8–11.
At least two important knowledge gaps exist in research on bladder cancer. Firstly, previous analyses showed that the highest incidence was found in more developed countries1, and the past few decades had seen wealth and technological advancement, particularly in more developed countries. One study showed that the age-specific incidence of papillary non-invasive bladder cancer increased from 5.52 to 9.09 per 100,000 from 1998 to 2006, but this is constrained to the US population9. Temporal changes in its trends of incidence and mortality for a significant number of countries in the past decade remain unknown. Also, there is a scarcity of studies on the role of socioeconomic development on incidence and mortality rate of bladder cancer when their associations were examined on a global scale. There was some evidence that environmental and socioeconomic factors affect bladder cancer mortality, and the effects appear to vary by gender and race13. A recent study has also examined the relationship between bladder cancer incidence/mortality and the world development index, but the influence of gender was not taken into account14. These findings highlight the need for a worldwide, across-country analysis of the epidemiological data.
This study tested the a priori hypothesis that the global temporal trends in the incidence of bladder cancer increased and that in its mortality decreased with time. Also, we sought to test the hypothesis that its global incidence was positively correlated with country-specific socioeconomic development.
Methods
Source of Data
To standardize the methodology across published literature, we adopted the same analysis plan as reported in our previous study on prostate cancer15, liver cancer16, pancreatic cancer17 and that on colorectal cancer18. We retrieved the incidence and mortality figures for bladder cancer in 2012 from the GLOBOCAN database1. For all countries, data were matched with their Human Development Index (HDI) and Gross Domestic Product (GPD) per capita in the same year based on the United Nations Human Development Report19, which highlighted the progress on human development over the past quarter century by reporting different statistical indexes. HDI is a summary index of life expectancy, education period, and income per capita19. For incidence trends, we extracted data from the Cancer Incidence in Five Continents series Volumes I-X20, which provided high-quality incidence statistics of cancer documented by local registries worldwide. This study has been approved by the Survey and Behavioural Research Ethics Committee of the Chinese University of Hong Kong. As this study used routinely collected anonymised electronic data consent was not required. All methods were performed in accordance with the relevant guidelines and regulations, and there were no publication of identifying information.
To acquire incidence data for more recent years, we also utilized publicly available information from the European Union Registration (EUREG)21, National Cancer Institute of the United States22, Nordic Cancer Registries23, Australian Cancer Incidence and Mortality Books24 and the Ministry of Health of New Zealand25. We used the GLOBOCAN data to analyze the incidence and mortality patterns in 2012, which were plotted against the HDI and logarithmic values of GDP per capita of each country in the same year. For analysis of temporal trends of incidence/mortality across time, we used the data from CI5 supplemented by the national databases21–25 (Table 1). The incidence data were retrieved according to the International Classification of Diseases (ICD-10, C67, 67.9; ICD-9-CM 188).
Table 1.
Data source for the age-standardized incidence and mortality rates of bladder cancer.
| Incidence | Mortality | |
|---|---|---|
| Austria | EUREG (1990–2009) | WHO (1980–2014) |
| Croatia | CI5 (1988–2007) | WHO (1985–2013) |
| Czech Republic | CI5 (1983–2007) | WHO (1986–2013) |
| Denmark | NORDCAN (1960–2013) | NORDCAN (1960–2013) |
| Estonia | CI5 (1968–2007) | WHO (1994–2012) |
| Finland | NORDCAN (1960–2013) | NORDCAN (1960–2013) |
| France | CI5 (1988–2007) | WHO (1979–2011) |
| Germany | CI5 (1970–2007) | WHO (1990–2013) |
| Iceland | NORDCAN (1960–2013) | NORDCAN (1960–2012) |
| Italy | CI5 (1993–2007) | WHO (1979–2003, 2006–2012) |
| Latvia | CI5 (1988–2007) | WHO (1996–2012) |
| Lithuania | CI5 (1978–2007) | WHO (1993–2013) |
| Netherlands | CI5 (1989–2007) | WHO (1979–2013) |
| Norway | NORDCAN (1960–2013) | NORDCAN (1960–2013) |
| Poland | CI5 (1978–2006) | WHO (1980–1996, 1999–2013) |
| Slovakia | CI5 (1968–2007) | WHO (1992–2010, 2012–2014) |
| Slovenia | CI5 (1963–2007) | WHO (1985–2010) |
| Spain | CI5 (1993–2007) | WHO (1980–2013) |
| Sweden | NORDCAN (1960–2013) | NORDCAN (1960–2013) |
| Switzerland | CI5 (1993–2007) | WHO (1995–2013) |
| United Kingdom | CI5 (1993–2007) | WHO (1979–2013) |
| Australia | AIHW (1982–2012) | AIHW (1968–2013) |
| New Zealand | New Zealand (1960–2012) | New Zealand (1960–2012) |
| Bulgaria | EUREG (1993–2007) | WHO (1980–2012) |
| Russia | CI5 (1994–2007) | WHO (1999–2011) |
| Malta | EUREG (1994–2009) | EUREG (1995–2010) |
| Ireland | EUREG (1994–2009) | WHO (1979–2012) |
| Brazil | CI5 (1988–2007) | WHO (1979–2013) |
| Colombia | CI5 (1983–2007) | WHO (1984–2012) |
| Costa Rica | CI5 (1980–2007) | WHO(1980–2013) |
| Ecuador | CI5 (1985–2007) | WHO (1979–2013) |
| Canada | CI5 (1978–2007) | WHO (1979–2011) |
| USA | NIH (1975–2013) | NIH (1975–2013) |
| USA White | NIH (1975–2013) | NIH (1975–2013) |
| USA Black | NIH (1975–2013) | NIH (1975–2013) |
| Israel | CI5 (1963–2007) | WHO (1979–2013) |
| Japan | CI5 (1988–2007) | WHO (1979–2013) |
| Philipines | CI5 (1983–2007) | WHO (1992–2003, 2008) |
| Singapore | CI5 (1968–2007) | WHO (1979–2014) |
| Thailand | CI5 (1993–2007) | WHO (1979–1987, 1990–1992, 1994–2000, 2002–2006) |
| China | CI5 (1993–2007) | Hospital Authority (1983–2013) |
AIHW: Australian Cancer Incidence and Mortality Books24; CI5: Cancer Incidence in Five Continents V20; EUREG: European Union Registration21; Hospital Authority, Hong Kong. http://www3.ha.org.hk/cancereg/e_a1b.asp; New Zealand: the Ministry of Health of New Zealand25; NIH: National Institute of Health, United States22; NORDCAN: Nordic Cancer Registries23; WHO: World Health Organization26.
For mortality data, we used the WHO mortality database26 and the various national databases21–23, where the primary data source originated from death certificates. These data were categorized based on the ICD 9th 188 up to 2014. Table 1 showed a more detailed description of the data sources and calendar years for the present analysis. We used age-standardized rate per 100,000 (ASR) using the world standard population27. More developed regions include Europe, Northern America, Australia/New Zealand and Japan, whilst less developed regions include Africa, Asia (excluding Japan), Latin America and the Caribbean, Melanesia, Micronesia and Polynesia1.
Statistical Analysis
We used joinpoint regression analysis to study the incidence and mortality trends28. A series of joined straight lines was fit to the ASR trend28. We performed logarithmic transformation of the ASRs and computed the standard errors adopting binomial approximation. A maximum number of three joinpoints were used as analysis options, and we evaluated the average annual percent change (AAPC) and the respective 95% confidence intervals (C.I.) for data available in the most recent 10 years. The AAPC was computerized as a geometrically weighted average of the generated APCs by the joinpoint trend analysis software. Their weights were equivalent to the length of each segment within the specified time interval29. We extracted data for the incidence and mortality trends from the above sources. We selected the most recent 10 years as the timeframe for examining temporal trend changes, as this was commonly adopted in previous studies on global epidemiology of other cancers15,18,30. All AAPCs with their 95% C.I. lying above and below zero, respectively, were regarded as increasing and decreasing trends. AAPCs with 95% C.Is overlapping with zero were considered as stable trends15,18,30. We plotted the ASRs of incidence and mortality against the HDI and logarithmic values of GDP per capita, respectively, for each country. The HDI was defined as low ( ≤ 0.534), medium (0.534–0.710), high (0.710–0.796) and very high (>0.796)19. Linear regression analysis was applied and correlation coefficients were evaluated, as linear associations had the best goodness-of-fit. Also, we estimated the percent change in incidence and mortality by 2020 and 2030 when compared to the latest published figures based on the AAPC – a statistical technique employed by Bailey and colleagues in a recent article published in JAMA Surgery31. The predicted incidence/mortality rates were assumed to be a constant percentage of the rate of the previous joinpoint. All p values <0.05 were considered statistically significant.
Results
Incidence and mortality rates of bladder cancer in 2012
A total of 429,793 new cases of bladder cancer and 165,084 related deaths were reported in 2012 (Tables 2 and 3). The age-standardized rate of its incidence showed approximately ten-fold variation worldwide1. In men, the highest rates were found in Southern Europe (ASR = 21.8), Western Europe (ASR = 19.7), North America (ASR = 19.5) and Western Asia (ASR = 19.0), and the lowest were reported in Western (ASR = 2.1), Middle (ASR = 2.2) and Eastern Africa (ASR = 3.3). This geographical difference is similar for incidence rates in women. Overall, countries that were more developed had higher incidence than less developed regions in both genders. The incidence in men was substantially higher in countries with very high HDI (ASR = 16.7) than those with high (ASR = 10.8), medium (ASR = 4.7) and low HDI (ASR = 3.1), and similarly this trend was also observed for women (Supplementary Figure 1).
Table 2.
The estimated incidence and mortality of bladder cancer according to world area, 2012, males.
| World regions | Population size Male (thousands) | New cases | Mortality | Mortality to incidence ratio | ||
|---|---|---|---|---|---|---|
| n | ASR | n | ASR | |||
| Africa | 549,445 | 17685 | 6.3 | 9362 | 3.5 | 0.56 |
| Eastern Africa | 180,243 | 2824 | 3.3 | 1819 | 2.2 | 0.67 |
| Middle Africa | 69,179 | 610 | 2.2 | 420 | 1.6 | 0.73 |
| Northern Africa | 106,147 | 11225 | 15.1 | 5489 | 7.6 | 0.50 |
| Southern Africa | 29,735 | 1285 | 7.5 | 494 | 3.0 | 0.40 |
| Western Africa | 164,141 | 1741 | 2.1 | 1140 | 1.5 | 0.71 |
| Asia | 2,179,003 | 115646 | 5.5 | 52816 | 2.5 | 0.45 |
| Eastern Asia | 813,296 | 64662 | 5.8 | 27271 | 2.3 | 0.40 |
| South-Eastern Asia | 305,225 | 10784 | 4.3 | 5352 | 2.2 | 0.51 |
| South-Central Asia | 933,786 | 24415 | 3.6 | 13413 | 2.0 | 0.56 |
| Western Asia | 126,697 | 15785 | 19.0 | 6780 | 8.4 | 0.44 |
| America | 303,514 | 17610 | 6.1 | 7078 | 2.4 | 0.39 |
| Caribbean | 20,951 | 1839 | 7.6 | 773 | 3.0 | 0.39 |
| Central America | 82,227 | 2327 | 3.4 | 867 | 1.2 | 0.35 |
| South America | 200,336 | 13444 | 6.9 | 5438 | 2.7 | 0.39 |
| North America | 173,209 | 58089 | 19.5 | 13285 | 4.0 | 0.21 |
| Europe | 355,275 | 118365 | 17.7 | 39522 | 5.2 | 0.29 |
| Central and Eastern Europe | 138,249 | 30871 | 15.1 | 13231 | 6.1 | 0.40 |
| Northern Europe | 49,574 | 12722 | 12.4 | 5174 | 4.4 | 0.35 |
| Southern Europe | 74,900 | 34786 | 21.8 | 11653 | 6.0 | 0.28 |
| Western Europe | 92,553 | 39986 | 19.7 | 9464 | 4.0 | 0.20 |
| Oceania | 18,859 | 2985 | 10.6 | 988 | 3.2 | 0.30 |
| Australia/New Zealand | 13,632 | 2868 | 11.3 | 939 | 3.3 | 0.29 |
| Melanesia | 4,628 | 84 | 3.5 | 43 | 2.0 | 0.57 |
| Micronesia/Polynesia | 258 | 33 | 6.5 | 6 | 1.2 | 0.18 |
| More developed regions | 604,008 | 196077 | 16.9 | 58914 | 4.5 | 0.27 |
| Less developed regions | 2,975,297 | 134303 | 5.3 | 64137 | 2.6 | 0.49 |
| World | 3,579,305 | 330380 | 9.0 | 123051 | 3.2 | 0.36 |
ASR = Age standardized rate per 100,000. Source: GLOBOCAN 20121. Numbers are rounded to the nearest 10 or 100, and may not add up to the total. The population size of the world regions were retrieved from the Population Reference Bureau, Washington, DC. Available at: http://www.prb.org/Publications/Datasheets/2012/world-population-data-sheet/world-map.aspx#/table/population.
Table 3.
The estimated incidence and mortality of bladder cancer according to world area, 2012, Females.
| World regions | Population size Female (thousands) | New cases | Mortality | Mortality to incidence ratio | ||
|---|---|---|---|---|---|---|
| n | ASR | n | ASR | |||
| Africa | 549 608 | 6752 | 2.1 | 3906 | 1.2 | 0.57 |
| Eastern Africa | 182 469 | 1961 | 2.0 | 1290 | 1.3 | 0.65 |
| Middle Africa | 69 644 | 441 | 1.3 | 300 | 0.9 | 0.69 |
| Northern Africa | 105 353 | 2708 | 3.2 | 1337 | 1.6 | 0.50 |
| Southern Africa | 30 816 | 483 | 1.9 | 225 | 0.9 | 0.47 |
| Western Africa | 161 327 | 1159 | 1.3 | 754 | 0.9 | 0.69 |
| Asia | 2 081 150 | 32922 | 1.4 | 16478 | 0.6 | 0.43 |
| Eastern Asia | 777 374 | 20789 | 1.6 | 10220 | 0.7 | 0.44 |
| South-Eastern Asia | 306 008 | 3034 | 1.0 | 1517 | 0.5 | 0.50 |
| South-Central Asia | 881 514 | 6159 | 0.8 | 3441 | 0.5 | 0.63 |
| Western Asia | 116 253 | 2940 | 3.1 | 1300 | 1.3 | 0.42 |
| America | 310 360 | 7234 | 2.0 | 3069 | 0.8 | 0.40 |
| Caribbean | 21 313 | 542 | 1.8 | 284 | 0.9 | 0.50 |
| Central America | 83 632 | 1430 | 1.8 | 535 | 0.6 | 0.33 |
| South America | 205 415 | 5262 | 2.1 | 2250 | 0.9 | 0.43 |
| North America | 176 585 | 18660 | 5.1 | 5307 | 1.2 | 0.24 |
| Europe | 381 747 | 32932 | 3.5 | 12889 | 1.1 | 0.31 |
| Central and Eastern Europe | 155 701 | 8904 | 2.7 | 3543 | 0.9 | 0.33 |
| Northern Europe | 51 252 | 4645 | 3.6 | 2391 | 1.5 | 0.42 |
| Southern Europe | 78 393 | 8049 | 3.8 | 3001 | 1.0 | 0.26 |
| Western Europe | 96 400 | 11334 | 4.3 | 3954 | 1.1 | 0.26 |
| Oceania | 18 746 | 913 | 2.7 | 384 | 1.0 | 0.37 |
| Australia/New Zealand | 13 715 | 887 | 2.9 | 366 | 1.0 | 0.34 |
| Melanesia | 4 451 | 21 | 0.7 | 13 | 0.4 | 0.57 |
| Micronesia/Polynesia | 580 | 5 | 0.9 | 5 | 0.9 | 1.00 |
| More developed regions | 637 294 | 57766 | 3.7 | 21024 | 1.1 | 0.30 |
| Less developed regions | 2 880 901 | 41647 | 1.5 | 21009 | 0.7 | 0.47 |
| World | 3 518 195 | 99413 | 2.2 | 42033 | 0.9 | 0.41 |
ASR = Age standardized rate per 100,000. Source: GLOBOCAN 20121. Numbers are rounded to the nearest 10 or 100, and may not add up to the total. The population size of the world regions were retrieved from the Population Reference Bureau, Washington, DC. Available at: http://www.prb.org/Publications/Datasheets/2012/world-population-data-sheet/world-map.aspx#/table/population.
The mortality rates varied by seven-fold in 2012, and were higher in more developed than less developed regions (ASR = 4.5 vs. 2.6 in men; 1.1 vs. 0.7 in women). The highest mortality rates in men were reported in Western Asia (ASR = 8.4), Northern Africa (ASR = 7.6), and Central and Eastern Europe (ASR = 6.1). The lowest estimated death rates were found in Central America (ASR = 1.2), Micronesia/Polynesia (ASR = 1.2), Western Africa (ASR = 1.5), and Middle Africa (ASR = 1.6). In women, Northern Europe was amongst one of the world regions with the highest mortality (ASR = 1.5), whilst South-Eastern and South-Central Asia (ASR = 0.5) were regions that reported the lowest mortality rates.
Correlation between incidence/mortality and socioeconomic development
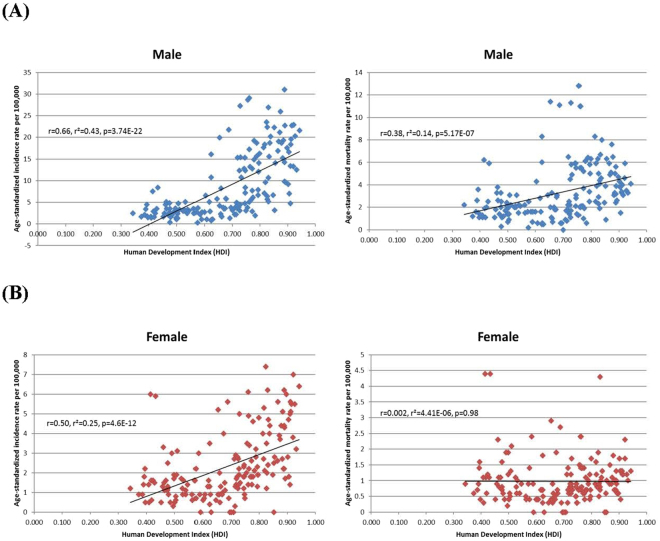
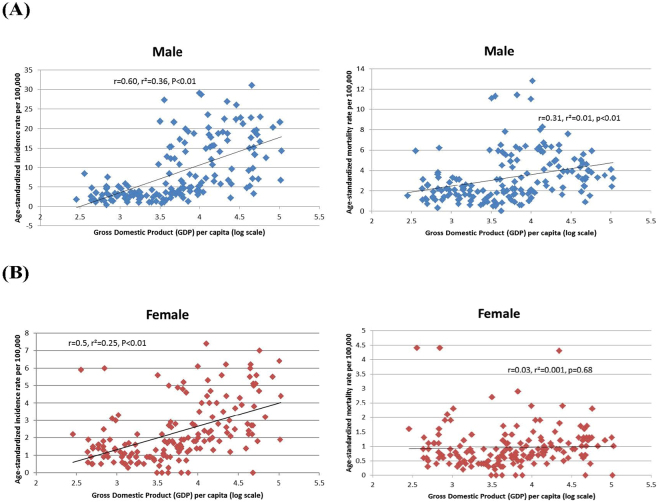
Figures 1A,B and 2A,B showed the correlation between the incidence/mortality and the two socioeconomic indicators, evaluated by simple linear regression analysis. The ASR of incidence in men (r = 0.66, r2 = 0.43, p < 0.001) and women (r = 0.50, r2 = 0.25, p < 0.001) increased with higher levels of HDI, and to a lesser extent logarithmic values of GDP per capita (r = 0.60, r2 = 0.36, p < 0.01 [men] and r = 0.50, r2 = 0.25, p < 0.01 [women]). The ASR of mortality in women was not significantly correlated with HDI and logarithmic values of GDP per capita, whereas that in men was significantly correlated with HDI (r = 0.38, r2 = 0.14, p < 0.001) and logarithmic values of GDP per capita (r = 0.31, r2 = 0.01, p < 0.01).
Figure 1.
(A) Correlation between age-standardised bladder cancer incidence (left panel) and mortality (right panel) and Human Development Index (HDI) (Male) (B) Correlation between age-standardised bladder cancer incidence (left panel) and mortality (right panel) and Human Development Index (HDI) (Female).
Figure 2.
(A) Correlation between age-standardised bladder cancer incidence (left panel) and mortality (right panel) and logarithmic values of Gross Domestic Product (GDP) (Male) (B) Correlation between age-standardised bladder cancer incidence (left panel) and mortality (right panel) and logarithmic values of Gross Domestic Product (GDP) (Female).
Temporal trends of bladder cancer
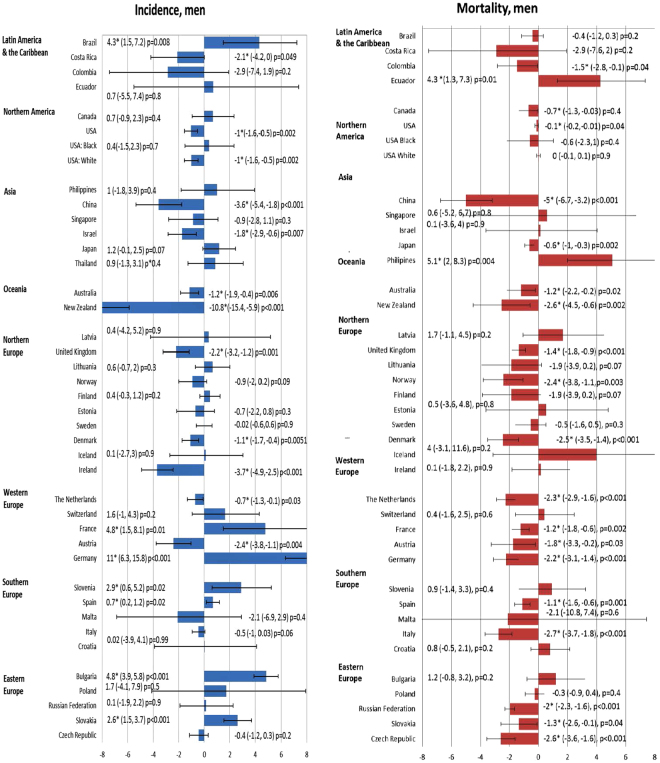
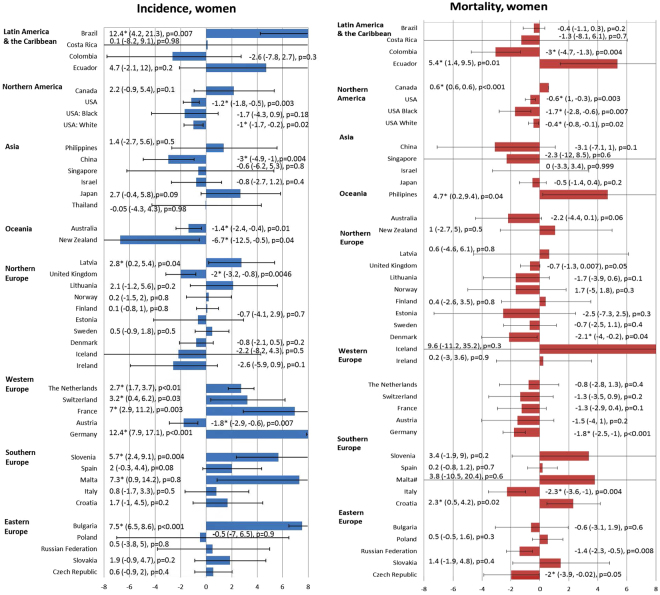
The incidence and mortality trends of each country were shown in Supplementary Figure 1, and the corresponding findings from the joinpoint regression analysis were presented in Supplementary Figures 2 and 3. The changes in incidence and mortality trends were plotted in Figs 3 and 4.
Figure 3.
The Average Annual Percent Change (AAPC) in the incidence of bladder cancer in male (left) and female (right) in the most recent 10 years.
Figure 4.
The Average Annual Percent Change (AAPC) in the incidence of bladder cancer in male (left) and female (right) in the most recent 10 years.
Incidence trend
Among men, seven countries had increases in incidence, 11 countries showed declining trends, and 21 countries reported stable trends. Out of all seven countries with rise in incidence, six were reported in Europe, including Germany (AAPC = 11.0, 95% C.I. 6.3, 15.8, p < 0.001), Bulgaria (AAPC = 4.8, 95% C.I. 3.9, 5.8, p < 0.001), France (AAPC = 4.8, 95% C.I. 1.5, 8.1, p = 0.01), Slovenia (AAPC = 2.9, 95% C.I. 0.6, 5.2, p = 0.02) and Slovakia (AAPC = 2.6, 95% C.I. 1.5, 3.7, p < 0.001). Countries with substantial incidence reduction include New Zealand (AAPC = −10.8, 95% C.I. −15.4, −5.9, p < 0.001), Ireland (AAPC = −3.7, 95% C.I. −4.9, −2.5, p < 0.001) China (AAPC = −3.6, 95% C.I. −5.4, −1.8, p < 0.001) and Austria (AAPC = −2.4, 95% C.I. −3.8, −1.1, p = 0.004). Among women, seven countries had increases in incidence, 8 countries showed declining trends, and trends in 24 countries remained stable. The majority of incidence rise occurred in Europe, and New Zealand also reported a significant incidence decline (AAPC = −6.7, 95% C.I. −12.5, −0.5, p = 0.04) (Fig. 4).
Mortality trend
In men, the only countries that showed increasing mortality trends include Ecuador (AAPC = 4.3, 95% C.I. 1.3, 7.3, p = 0.01) and the Philippines (AAPC = 5.1, 95% C.I. 2.0, 8.3, p = 0.004). A total of 19 out of 38 countries reported declining trends, and 12 occurred in European countries. Among them, Italy (AAPC = −2.7, 95% C.I. −3.7, −1.8, p < 0.001), Czech Republic (AAPC = −2.6, 95% C.I. −3.6, −1.6, p < 0.001), Denmark (AAPC = −2.5, 95% C.I. −3.5, −1.4, p < 0.001) and Norway (AAPC = −2.4, 95% C.I. −3.8, −1.1, p = 0.003) showed the most marked reduction in mortality rates.
In women, Ecuador (AAPC = 5.4, 95% C.I. 1.4, 9.5, p = 0.01), the Philippines (AAPC = 4.7, 95% C.I. 0.2, 9.4, p = 0.04), Croatia (AAPC = 2.3, 95% C.I. 0.5, 4.2, p = 0.02) and Canada (AAPC = 0.6, 95% C.I. 0.6, 0.6, p < 0.001) were the only countries where increase in mortality rates were observed. Colombia had the greatest mortality decline (AAPC = −3.0, 95% C.I. −4.7, −1.3, p = 0.004). Five European countries reported decrease in mortality trends, including Italy (AAPC = −2.3, 95% C.I. −3.6, −1.0, p = 0.004), Denmark (AAPC = −2.1, 95% C.I. −4.0, −0.2, p = 0.04), Czech Republic (AAPC = −2.0, 95% C.I. −3.9, −0.02, p = 0.05), Germany (AAPC = −1.8, 95% C.I. −2.5, −1.0, p < 0.001) and Russian Federation (AAPC = −1.4, 95% C.I. −2.3, −0.5, p = 0.008).
Projection of incidence and mortality rates in 2020 and 2030
Tables 4 and 5 summarized the projected incidence and mortality of bladder cancer by all future years up to 2030. The estimated increase in incidence by 2030 was most marked in Germany (998%), France (191%), Bulgaria (129%), and Brazil (164%) among men. In women, Brazil (1,380%), Germany (1,375%), Bulgaria (372%) and France (371%) were projected to have the highest incidence growth. Most countries included in this study showed declining mortality rates, except the Philippines (283%) and Ecuador (104%) that showed increase rates in men. Among women, Iceland (374%), the Philippines (245%), Ecuador (143%) and Malta (111%) showed prominent rise in mortality rates.
Table 4.
Projected growth rate of bladder cancer incidence rates by 2020 and 2030.
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AAPC | P-value (3 sig. fig.) | Latest available year* | Growth rate by 2020 (%) | Growth rate by 2030 (%) | AAPC | P-value | Latest available year* | Growth rate by 2020 (%) | Growth rate by 2030 (%) | |
| Latin America, the Caribbean and Northern America | ||||||||||
| Brazil | 4.31 | 0.008 | 2007 | 73 | 164 | 12.43 | 0.007 | 2007 | 359 | 1,380 |
| Costa Rica | −2.13 | 0.050 | 2007 | −24 | −39 | 0.11 | 0.979 | 2007 | 1 | 3 |
| Colombia | −2.87 | 0.200 | 2007 | −32 | −49 | −2.65 | 0.284 | 2007 | −29 | −46 |
| Ecuador | 0.71 | 0.804 | 2007 | 10 | 18 | 4.73 | 0.152 | 2007 | 82 | 189 |
| Canada | 0.68 | 0.366 | 2007 | 9 | 17 | 2.16 | 0.149 | 2007 | 32 | 63 |
| Asia | ||||||||||
| The Philippines | 1.01 | 0.437 | 2007 | 14 | 26 | 1.36 | 0.465 | 2007 | 19 | 37 |
| China | −3.59 | 0.000 | 2007 | −38 | −57 | −2.96 | 0.004 | 2007 | −32 | −50 |
| Singapore | −0.89 | 0.323 | 2007 | −11 | −19 | −0.61 | 0.837 | 2007 | −8 | −13 |
| Israel | −1.75 | 0.007 | 2007 | −21 | −33 | −0.78 | 0.386 | 2007 | −10 | −17 |
| Japan | 1.16 | 0.069 | 2007 | 16 | 30 | 2.67 | 0.090 | 2007 | 41 | 83 |
| Thailand | 0.87 | 0.381 | 2007 | 12 | 22 | −0.049 | 0.979 | 2007 | −1 | −1 |
| Oceania | ||||||||||
| Australia | −1.11 | 0.008 | 2012 | −9 | −18 | −1.23 | 0.032 | 2012 | −9 | −20 |
| New Zealand | −10.80 | 0.000 | 2012 | −60 | −87 | −6.74 | 0.037 | 2012 | −43 | −71 |
| Northern Europe | ||||||||||
| Latvia | 0.36 | 0.879 | 2007 | 5 | 9 | 2.76 | 0.038 | 2007 | 42 | 87 |
| United Kingdom | −2.22 | 0.001 | 2007 | −25 | −40 | −1.99 | 0.005 | 2007 | −23 | −37 |
| Lithuania | 0.65 | 0.344 | 2007 | 9 | 16 | 2.11 | 0.187 | 2007 | 31 | 62 |
| Norway | −0.92 | 0.091 | 2013 | −6 | −15 | 0.20 | 0.801 | 2013 | 1 | 3 |
| Finland | 0.44 | 0.237 | 2013 | 3 | 8 | 0.094 | 0.805 | 2013 | 1 | 2 |
| Estonia | −0.70 | 0.306 | 2007 | −9 | −15 | −0.66 | 0.678 | 2007 | −8 | −14 |
| Sweden | −0.019 | 0.944 | 2013 | 0 | 0 | 0.46 | 0.445 | 2013 | 3 | 8 |
| Denmark | −1.09 | 0.005 | 2013 | −7 | −17 | −0.78 | 0.248 | 2013 | −5 | −13 |
| Iceland | 0.12 | 0.926 | 2013 | 1 | 2 | −2.17 | 0.451 | 2013 | −14 | −31 |
| Ireland | −3.70 | 0.000 | 2009 | −34 | −55 | −2.58 | 0.123 | 2009 | −25 | −42 |
| Western Europe | ||||||||||
| The Netherlands | −0.72 | 0.028 | 2007 | −9 | −15 | 2.74 | 0.000 | 2007 | 42 | 86 |
| Switzerland | 1.64 | 0.220 | 2007 | 24 | 45 | 3.24 | 0.031 | 2007 | 51 | 108 |
| France | 4.76 | 0.010 | 2007 | 83 | 191 | 6.97 | 0.004 | 2007 | 140 | 371 |
| Austria | −2.42 | 0.004 | 2009 | −24 | −40 | −1.77 | 0.007 | 2009 | −18 | −31 |
| Germany | 10.98 | 0.001 | 2007 | 287 | 998 | 12.41 | 0.000 | 2007 | 358 | 1375 |
| Southern Europe | ||||||||||
| Slovenia | 2.89 | 0.019 | 2007 | 45 | 92 | 5.67 | 0.004 | 2007 | 105 | 256 |
| Spain | 0.68 | 0.015 | 2007 | 9 | 17 | 2.02 | 0.076 | 2007 | 30 | 59 |
| Malta | −2.10 | 0.356 | 2009 | −21 | −36 | 7.32 | 0.031 | 2009 | 118 | 341 |
| Italy | −0.46 | 0.065 | 2007 | −6 | −10 | 0.80 | 0.479 | 2007 | 11 | 20 |
| Croatia | 0.016 | 0.993 | 2007 | 0 | 0 | 1.68 | 0.194 | 2007 | 24 | 47 |
| Eastern Europe | ||||||||||
| Bulgaria | 3.67 | 0.000 | 2007 | 60 | 129 | 6.98 | 0.000 | 2007 | 140 | 372 |
| Poland | 1.72 | 0.524 | 2006 | 27 | 51 | −0.48 | 0.875 | 2006 | −6 | −11 |
| Russian Federation | 0.15 | 0.872 | 2007 | 2 | 4 | 0.50 | 0.801 | 2007 | 7 | 12 |
| Slovakia | 2.61 | 0.000 | 2007 | 40 | 81 | 1.87 | 0.157 | 2007 | 27 | 53 |
| Czech Republic | −0.43 | 0.213 | 2007 | −5 | −9 | 0.56 | 0.398 | 2007 | 8 | 14 |
AAPC: Average Annual Percent Change; *represents the calendar year where the latest figure is available from the respective national database.
Table 5.
Projected growth rate of bladder cancer mortality rates by 2020 and 2030.
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AAPC | P-value (3 sig. fig.) | Latest available year* | Growth rate by 2020 (%) | Growth rate by 2030 (%) | AAPC | P-value | Latest available year* | Growth rate by 2020 (%) | Growth rate by 2030 (%) | |
| Latin America, the Caribbean and Northern America | ||||||||||
| Brazil | −0.42 | 0.228 | 2013 | −3 | −7 | −0.40 | 0.244 | 2013 | −3 | −7 |
| Costa Rica | −2.92 | 0.201 | 2013 | −19 | −40 | −1.27 | 0.692 | 2013 | −9 | −19 |
| Colombia | −1.46 | 0.043 | 2012 | −11 | −23 | −3.05 | 0.004 | 2012 | −22 | −43 |
| Ecuador | 4.28 | 0.010 | 2013 | 34 | 104 | 5.37 | 0.014 | 2013 | 44 | 143 |
| Canada | −0.69 | 0.042 | 2011 | −6 | −12 | 0.60 | 0.000 | 2011 | 6 | 12 |
| Asia | ||||||||||
| China | −4.98 | 0.000 | 2013 | −30 | −58 | −3.10 | 0.121 | 2013 | −20 | −41 |
| Singapore | 0.58 | 0.826 | 2014 | 4 | 10 | −2.29 | 0.622 | 2014 | −13 | −31 |
| Israel | 0.14 | 0.937 | 2013 | 1 | 2 | −0.0017 | 0.999 | 2013 | 0 | 0 |
| Japan | −0.63 | 0.002 | 2013 | −4 | −10 | −0.50 | 0.244 | 2013 | −3 | −8 |
| The Philippines | 5.10 | 0.005 | 2003 | 133 | 283 | 4.69 | 0.043 | 2003 | 118 | 245 |
| Oceania | ||||||||||
| Australia | −1.21 | 0.025 | 2013 | −8 | −19 | −2.11 | 0.069 | 2013 | −14 | −30 |
| New Zealand | −2.56 | 0.017 | 2012 | −19 | −37 | 1.05 | 0.547 | 2012 | 9 | 21 |
| Northern Europe | ||||||||||
| Latvia | 1.67 | 0.201 | 2012 | 14 | 35 | 0.63 | 0.792 | 2012 | 5 | 12 |
| United Kingdom | −1.36 | 0.000 | 2013 | −9 | −21 | −0.67 | 0.052 | 2013 | −5 | −11 |
| Lithuania | −1.89 | 0.071 | 2013 | −12 | −28 | −1.66 | 0.133 | 2013 | −11 | −25 |
| Norway | −2.44 | 0.003 | 2013 | −16 | −34 | −1.65 | 0.298 | 2013 | −11 | −25 |
| Finland | −1.87 | 0.068 | 2013 | −12 | −27 | 0.39 | 0.777 | 2013 | 3 | 7 |
| Estonia | 0.50 | 0.791 | 2012 | 4 | 9 | −2.53 | 0.270 | 2012 | −19 | −37 |
| Sweden | −0.55 | 0.263 | 2013 | −4 | −9 | −0.70 | 0.399 | 2013 | −5 | −11 |
| Denmark | −2.46 | 0.001 | 2013 | −16 | −34 | −2.11 | 0.036 | 2013 | −14 | −30 |
| Iceland | 3.98 | 0.240 | 2013 | 31 | 94 | 9.59 | 0.344 | 2013 | 90 | 374 |
| Ireland | −0.06 | 0.960 | 2012 | −1 | −1 | 0.22 | 0.879 | 2012 | 2 | 4 |
| Western Europe | ||||||||||
| The Netherlands | −2.26 | 0.000 | 2013 | −15 | −32 | −0.77 | 0.410 | 2013 | −5 | −12 |
| Switzerland | 0.42 | 0.639 | 2013 | 3 | 7 | −1.34 | 0.203 | 2013 | −9 | −20 |
| France | −1.24 | 0.002 | 2011 | −11 | −21 | −1.26 | 0.145 | 2011 | −11 | −21 |
| Austria | −1.76 | 0.029 | 2014 | −10 | −25 | −1.55 | 0.191 | 2014 | −9 | −22 |
| Germany | −2.23 | 0.000 | 2013 | −15 | −32 | −1.77 | 0.001 | 2013 | −12 | −26 |
| Southern Europe | ||||||||||
| Slovenia | 0.92 | 0.379 | 2010 | 10 | 20 | 3.39 | 0.182 | 2010 | 40 | 95 |
| Spain | −1.12 | 0.001 | 2013 | −8 | −17 | 0.18 | 0.695 | 2013 | 1 | 3 |
| Malta | −2.13 | 0.609 | 2010 | −19 | −35 | 3.80 | 0.571 | 2010 | 45 | 111 |
| Italy | −2.74 | 0.000 | 2003 | −38 | −53 | −2.27 | 0.004 | 2003 | −32 | −46 |
| Croatia | 0.80 | 0.202 | 2013 | 6 | 15 | 2.30 | 0.020 | 2013 | 17 | 47 |
| Eastern Europe | ||||||||||
| Bulgaria | 2.09 | 0.061 | 2012 | 18 | 45 | −0.60 | 0.599 | 2012 | −5 | −10 |
| Poland | −0.26 | 0.393 | 2013 | −2 | −4 | 0.53 | 0.274 | 2013 | 4 | 9 |
| Russian Federation | −1.98 | 0.000 | 2011 | −16 | −32 | −1.39 | 0.008 | 2011 | −12 | −23 |
| Slovakia | −1.34 | 0.038 | 2010 | −13 | −24 | 1.42 | 0.353 | 2010 | 15 | 32 |
| Czech Republic | −2.60 | 0.000 | 2013 | −17 | −36 | −1.97 | 0.048 | 2013 | −13 | −29 |
AAPC: Average Annual Percent Change; *represents the calendar year where the latest figure is available from the respective national database.
Discussion
This study presented the most updated global epidemiological profiles of bladder cancer, and we described the incidence and mortality patterns and trends based on high quality data. Southern Europe, Western Europe, Northern America and Western Asia reported the highest incidence rates, whilst Western, Middle and Eastern Africa had the lowest rates. In men, the highest mortality rates were observed in Western Asia, Northern Africa, and Central and Eastern Europe. with lowest death rates found in Central America, Micronesia/Polynesia, Western Africa, and Middle Africa. In women, high mortality rates were observed in Northern Europe, whilst South-Eastern and South-Central Asia reported the lowest mortality rates. Incidence rates were positively correlated with human development levels and logarithmic values of GDP per capita with high coefficients of correlation between incidence/mortality and HDI was moderately strong (0.50–0.66). Incidence figures from 39 countries in the most recent 10 years reported that a total of 7 countries experienced increases in incidence rates in either gender - and six of them were European countries. Up to 19 and 6 out of 38 countries reported reduction in mortality trends in men and women, respectively, and the majority of them were in Europe. Bulgaria is the only country where males had significant increase in both incidence and mortality. On the contrary, male populations in Oceania (i.e. Australia and New Zealand) went in declines in both incidence and mortality. German men and women reported increases in incidence but decreases in mortality. A reduction in both incidence and mortality in both genders could only be observed in USA.
A recent study by Antoni et al.32 has examined the most recent global incidence and mortality patterns and trends of bladder cancer. They also reported the highest incidence rates in Southern and Western Europe, North America as well as in some nations in Northern Africa and Western Asia. It was found that the incidence rates were diverging, with stabilizing or decreasing rates for men but increasing rates for women in many countries. Our study utilized an approach similar to their design by using similar data sources, but the timeframe used to compute the AAPC and the registries used to extract the data were different.
Several reasons could explain the higher incidence of bladder cancer in more developed countries, and their positive correlation with HDI. Firstly, in developed nations with more rapid development and higher productivity, the prevalence of risk factors for bladder cancer including smoking, obesity, alcohol drinking and red-meat consumption was higher7,8 – and these risk factors have been reported by the World Health Organization as alarmingly high across Europe33. Tobacco use in developing nations is growing and has now surpassed that of developed nations where prevalence of tobacco use has begun to decrease34. To control it, the WHO FCTC (Framework Convention on Tobacco Control) were adopted by 174 countries and currently covering around 90% of the population globally35. There has been substantial interest in measuring the impact of tobacco use on health outcomes at the population level since the 1950s36. However, there are substantial differences between various surveys and a lag in the impact of tobacco control on smoking-related incidence and mortality37. While limited data was available to evaluate how the tobacco control has influenced the incidence or mortality of bladder cancer. In 2012, the WHO published the “WHO global report: mortality attributable to tobacco”, showing the relative risk (males: 3.0, females: 2.4) for bladder cancer death due to tobacco use was just second to lung cancer38. Therefore, we can predict that the policies of tobacco control will have a substantial impact on the incidence and mortality of bladder cancer. Another explanation for the higher incidence could be attributed to the more widespread practice of diagnostic tests in more resource-privileged countries, such as urine cytology, cystoscopy and CT scan for haematuria and other non-specific urinary symptoms presented by patients39. Yet another possible contributor includes higher rates of occupational and environmental exposures to carcinogenic agents, including the aromatic amines in the dye industry in the European Union40. Our findings that some countries outside Europe and North America reported markedly increased incidence trends warrant further studies to elucidate the underlying etiological mechanisms. The reduction in mortality trends in the recent decade could be explained by decreasing incidence or earlier diagnosis leading to stage migration to earlier stage disease, which could be treated by curative intervention. Another driver for the mortality decline could be due to improved endoscopic system for cystoscopic surveillance41, use of re-staging (second look) transurethral bladder resection42 and also better intravesical therapy for non-muscle invasive cancer43.
This study presented and analyzed the most up-to-date epidemiological data on bladder cancer, and quantified the geographical variations as well as trends in its incidence and mortality using data of high validity, completeness and comparability. We also used national mortality data that were based on WHO criteria of at least medium level quality in terms of coverage and completeness. The IARCs estimation methods have been further refined in more recent years to take into account the increasing availability and quality of the source data1. However, some limitations of this study should be discussed. Firstly, cancer registration in relatively less-developed nations could suffer from higher chance of under-reporting. Population based cancer registries that only cover parts of a country can have limited representativeness for the whole population. This is especially true for many population based cancer registries in low- and middle income countries that are mostly located in urban areas. On the contrary, in countries where estimates were based on a single cancer registry in more urbanized, resource privileged areas, the presented figures could be an overestimation if the countries consist of extensive rural populations. In addition, analysis of bladder cancer incidence is affected by the underlying data – some might include only invasive bladder cancer and others might include both invasive and non-invasive bladder cancer. Different cancer registries have different policies on how the cancer is reported, and this could change over time. Also, information regarding the tissue type of bladder cancer, such as urothelial cancer and squamous cell carcinoma, was lacking. Morover, despite our most inclusive approach to analyze the most recent data, the figures used are from 2012 at the latest and the temporal trends will need continuous updates. Last but not least, the change in coding practice of bladder cancer might influence the comparison of incidence/mortality trend of this disease across years. For instance, since 2000, the definition of bladder carcinoma was coded as invasive disease (ICD-10, C67), and it led to an apparent decrease in the incidence of bladder carcinoma as well as the survival rate due to the exclusion of carcinoma in situ that bears better prognosis. Although after years of efforts, the definition has become more universal across the cancer registries of different countries in recent years, there was a period around year 2000 with a mixed definition, which made the comparison of incidence/mortality rates among different registry systems difficult. Future analysis should be performed before and after this time period to capture the changes in trends more accurately.
Conclusion
The incidence rates of bladder cancer increased in many European countries analyzed in this study, and the mortality rates declined in a large number of nations, particularly in more developed regions. With population ageing and population growth, the absolute incidence of bladder cancer might be further escalating in European nations. Appropriate healthcare resources should be allocated to cope with the increasing need for patient treatment. Future studies are needed to explore the underlying mechanisms for these epidemiological trends with potential risk factors incorporated in further analysis.
Electronic supplementary material
Acknowledgements
We thank the expert advice from the Division of Biostatistics of the School of Public Health and Primary Care on statistical analysis. We are grateful for the International Agency for Research on Cancer and the World Health Organization for provision of data in the website http://www.iarc.fr/. We also thank Ms Sze Yeung, Ms Yan Liang and Ms. Shannon TS Li for their preparation of the graphics. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
M.C.S.W. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. M.C.S.W. and B.W.G. conceived the study. All authors contributed to the study design. F.D.H.F., W.W.L.C. and C.L. retrieved the data and composed the graphs. F.D.H.F. conducted the statistical analysis. M.C.S.W. wrote the first draft of the report. B.W.G. critically revised the manuscript. All authors contributed to the interpretation of the data and the writing and editing of the report.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19199-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay, J. et al. GLOBOCAN 2012v1.0, Cancer Incidence and Mortality Worldwide. IARC Cancer Base No. 11. Lyon, France: International Agency for Research on Cancerhttp://globocan.iarc.fr (Date of access: 06/07/2017) (2013).
- 2.Marcos-Gragera R, et al. Urinary tract cancer survival in Europe 1999-2007: Results of the population-based study EUROCARE-5. Eur J Cancer. 2015;51:2217–2230. doi: 10.1016/j.ejca.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 3.Stenzl A, et al. Treatment of muscle invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol. 2011;59:1009–1018. doi: 10.1016/j.eururo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Leal J, Luengo-Fernandez R, Sullivan R, Witjes JA. Economic Burden of Bladder Cancer Across the European Union. Eur Urol. 2016;69:438–447. doi: 10.1016/j.eururo.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Mostafa MH, Sheweita SA, O’Connor PJ. Relationship between schistosomiasis and bladder cancer. Clinical Microbiology Review. 1999;12:97–111. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelucchi C, Bosetti C, Negri E, Malvezzi M, La Vecchia C. Mechanisms of disease: The epidemiology of bladder cancer. Nat Clin Pract Urol. 2006;3:327. doi: 10.1038/ncpuro0510. [DOI] [PubMed] [Google Scholar]
- 7.Daneshmand, S. Epidemiology and risk factors of urothelial (transitional cell) carcinoma of the bladder. UpToDate http://www.uptodate.com/contents/epidemiology-and-risk-factors-of-urothelial-transitional-cell-carcinoma-of-the-bladder (Date of access: 06/07/2017) (2016).
- 8.Burger M, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen ME, et al. Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006. Cancer. 2014;120:86–95. doi: 10.1002/cncr.28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David, K. A. et al. Surveillance of urothelial carcinoma: stage and grade migration, 1993–2005 and survival trends, 1993–2000. Cancer. 115, 1435-1447 (2009). [DOI] [PubMed]
- 11.Kohler BA, et al. Annual report to the Nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith ND, et al. Bladder cancer mortality in the United States: a geographic and temporal analysis of socioeconomic and environmental factors. J Urol. 2016;195:290–296. doi: 10.1016/j.juro.2015.07.091. [DOI] [PubMed] [Google Scholar]
- 14.Mahdavifar N, et al. Epidemiology, incidence and mortality of bladder cancer and their relationship with the development index in the world. Asia Pac J Cancer Prev. 2016;17:381–386. doi: 10.7314/APJCP.2016.17.1.381. [DOI] [PubMed] [Google Scholar]
- 15.Wong MC, et al. Global incidence and mortality of prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. 2016;70:862–874. doi: 10.1016/j.eururo.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 16.Wong MC, et al. International incidence and mortality trends of liver cancer: a global profile. Sci Rep. 2017;7:45846. doi: 10.1038/srep45846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong MC, et al. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep. 2017;7:3165. doi: 10.1038/s41598-017-02997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 19.Human Development Report 2013. The rise of the south: human progress in a diverse world. New York: United Nations Development Programme (UNDP)http://hdr.undp.org/sites/default/files/reports/14/hdr2013_en_complete.pdf (Date of access: 06/07/2017) (2013).
- 20.Forman, D. et al. Cancer Incidence in Five Continents, Vol. X (electronic version). Lyon: IARC http://ci5.iarc.fr (Date of access: 06/07/2017) (2013).
- 21.Steliarova-Foucher E, et al. The European Cancer Observatory: A new data resource. Eur J Cancer. 2015;51:1131–1143. doi: 10.1016/j.ejca.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute. Surveillance, Epidemiology and End Results Program. http://seer.cancer.gov/faststats/selections.php?series=data (Date of access: 06/07/2017).
- 23.Engholm, G. et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.1. Association of the Nordic Cancer Registries. Danish Cancer Society http://www.ancr.nu (Date of access: 06/07/2017) (2015).
- 24.Australian Institute of Health and Welfare, Australian Government. Australian Cancer Incidence and Mortality (ACIM) books. http://www.aihw.gov.au/acim-books/ (Date of access: 06/07/2017) (2016).
- 25.New Zealand National Ministry of Health. Cancer: Historical summary 1948–2012. http://www.health.govt.nz/publication/cancer-historical-summary-1948-2012 (Date of access: 06/07/2017) (2016).
- 26.Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull. World Health Organ. 2005;83:171–177. [PMC free article] [PubMed] [Google Scholar]
- 27.Segi M, Fujisaku S, Kurihara M. Geographical observation on cancer mortality by selected sites on the basis of standardised death rate. Gan. 1957;48:219–225. [PubMed] [Google Scholar]
- 28.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 29.Clegg LX, et al. Estimating average annual percent change in trend analysis. Stat Med. 2009;28:3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeSantis CE, et al. International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer Epidemiol Biomarkers Prev. 2015;24:1495–1506. doi: 10.1158/1055-9965.EPI-15-0535. [DOI] [PubMed] [Google Scholar]
- 31.Bailey CE, et al. Increasing Disparities in the Age-Related Incidences of Colon and Rectal Cancers in the United States, 1975–2010. JAMA Surgery. 2015;150:17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antoni S, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz-Drager BJ, et al. Molecular Markers for Bladder Cancer Screening, Early Diagnosis, and Surveillance: The WHO/ICUD Consensus. Urol Int. 2015;94:1–24. doi: 10.1159/000369357. [DOI] [PubMed] [Google Scholar]
- 34.Ng M, Michael, Freeman K, et al. Smoking Prevalence and Cigarette Consumption in 187 Countries, 1980-2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. WHO Framework Convention on Tobacco Control. http://www.who.int/fctc/text_download/en/index.html. (Date of access: 06/07/2017)
- 36.Doll R, Hill AB. Lung Cancer and Other Causes of Death in Relation to Smoking. British Medical Journal. 1956;2:1071–1081. doi: 10.1136/bmj.2.5001.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Tobacco Questions for Surveys: A Subset of Key Questions from the Global Adult Tobacco Survey. http://www.who.int/tobacco/publications/surveillance/en_tfi_tqs.pdf. (Date of access: 06/07/2017)
- 38.World Health Organization.WHO global report: mortality attributable to tobacco. http://apps.who.int/iris/bitstream/10665/44815/1/9789241564434_eng.pdf (Date of access: 06/07/2017).
- 39.Reuters. Europe has ‘alarming’ rates of smoking, drinking and obesity: WHOhttp://www.reuters.com/article/us-health-europe-who-idUSKCN0RM2U620150922 (Date of access: 06/06/2016) (2015).
- 40.Kauppinen T, et al. Occupational exposure to carcinogens in the European Union. Occup Environ Med. 2000;57:10–18. doi: 10.1136/oem.57.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zlatev DV, Altobelli E, Liao JC. Advances in imaging technologies in the evaluation of high-grade bladder cancer. Urol Clin North Am. 2015;42:147–157. doi: 10.1016/j.ucl.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramírez-Backhaus M, Domínguez-Escrig J, Collado A, Rubio-Briones J, Solsona E. Restaging transurethral resection of bladder tumor for high-risk stage Ta and T1 bladder cancer. Curr Urol Rep. 2012;13:109–114. doi: 10.1007/s11934-012-0234-4. [DOI] [PubMed] [Google Scholar]
- 43.Malmström PU, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56:247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.