Abstract
The roles of myelin in maintaining axonal integrity and action potential (AP) propagation are well established, but its role in synapse maintenance and neurotransmission remains largely understudied. Here, we investigated how Purkinje axon myelination regulates synaptic transmission in the Purkinje to deep cerebellar nuclei (DCN) synapses using the Long Evans Shaker (LES) rat, which lacks compact myelin and thus displays severe locomotion deficits. DCN neurons fired spontaneous action potentials (APs), whose frequencies were dependent on the extent of myelin. In the LES cerebellum with severe myelin deficiency, DCN neurons were hyper-excitable, exhibiting spontaneous AP firing at a much higher frequency compared to those from wild type (LE) and heterozygote (LEHet) rats. The hyper-excitability in LES DCN neurons resulted from reduced inhibitory GABAergic inputs from Purkinje cells to DCN neurons. Corresponding with functional alterations including failures of AP propagation, electron microscopic analysis revealed anatomically fewer active zones at the presynaptic terminals of Purkinje cells in both LEHet and LES rats. Taken together, these studies suggest that proper axonal myelination critically regulates presynaptic terminal structure and function and directly impacts synaptic transmission in the Purkinje cell-DCN cell synapse in the cerebellum.
Introduction
Myelin sheaths wrap around axons to create electrical insulation, increasing efficiency of AP conduction and maintenance and protection of axons from damage due to injury or disease1–3. Defects in central myelination can lead to neurodegenerative diseases, such as multiple sclerosis (MS)2,4,5. When the myelination process is impaired, axons are unable to carry APs at their highest efficiency, leading to slower conduction velocity or failures of AP propagation6 that can be rescued with remyelination7. In addition to an increase in the central conduction time, dys-/demyelination alters synaptic neurotransmission in the auditory nervous system8. The reduction in the temporal fidelity of AP firing and synaptic transmission caused by dys-/demyelination critically influences the synchrony of neuronal activities with sub-millisecond accuracy in the neurosensory and motor system6,8,9.
Central demyelination is the primary cause of symptoms in MS, including tremor and lack of motor coordination. It has been recently suggested that AP firing of Purkinje cells in the cerebellum is highly synchronous10–12 and that this temporal synchrony of APs is important for motor coordination12. In dys-/demyelination, the temporal synchrony of conduction along the highly myelinated Purkinje axon could be easily disrupted by conduction inefficiency due to myelin loss, and consequently affect the synaptic outputs of these axons to the DCN. DCN neurons have been characterized previously and have been shown to exhibit spontaneous AP firing13,14. The excitability of DCN neurons is regulated by GABA-mediated inhibitory inputs from Purkinje cells and glutamate-mediated excitable inputs from climbing and mossy fibers. In addition to axonal deficits, synaptic dysfunction and loss also plays a critical role in cerebellar pathology during progressive MS15,16. A recent study in the post-mortem cerebellum of an MS patient demonstrated a reduction in the number and density of axosomatic synapses, as well as widened intercellular clefts between pre-and post-synaptic sites in the DCN15. Glutamatergic neurotransmission alterations independent of myelination have been implicated in experimental autoimmune encephalomyelitis, a rodent model of MS17. However, the impact of demyelination without immune insult on synaptic transmission is still unknown. In particular, structural and functional consequences in GABAergic Purkinje terminal-DCN neuron synapses remain largely unexplored, although the DCN is a prominent site for lesion development in demyelinating conditions.
The present study demonstrates how lack of myelination impacts synaptic function in the cerebellum using the LES rat, which has a genetic deletion of myelin basic protein (MBP) and fails to condense the myelin sheath18,19. Axons from young LES rats before postnatal 4 weeks are loosely covered by 2–3 thin layers of myelin, but then undergo progressive demyelination until, by three months of age, most axons are demyelinated20. Previous studies in the LES rat have shown that loss of tight and condensed myelination leads to alterations in ion channel expression at nerve terminals, and a reduction in conduction velocity and synaptic transmission in the central auditory circuit8,9,21. Here, we addressed that myelin deficiency in the cerebellum specifically reduced inhibitory synaptic transmission in the Purkinje cell-DCN synapse and consequently altered cerebellar output.
Results
Loss of MBP leads to dysmyelination and disrupts axonal structures of Purkinje cells
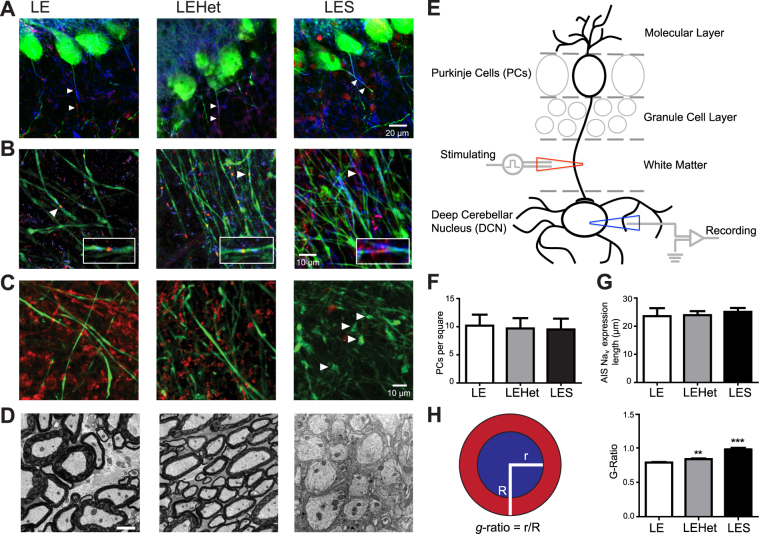
Axons of Purkinje cells, located in the cortex of the cerebellum, follow white matter tracks and form synapses on DCN neurons (Fig. 1A,E). Immunohistochemistry and fluorescent imaging demonstrated how reduced MBP and myelin affected Purkinje cell number and axonal structures in LE, LEHet, and LES rats. We counted Purkinje cells in either cerebellar hemisphere of each genotype, in the same sagittal slices used for DCN experiments (see Methods). The number of Purkinje cells was not significantly different between genotypes (Fig. 1A,F), indicating the loss of MBP does not directly induce Purkinje cell loss. The expression of contactin-associated protein (Caspr)22 and Nav channels in the proximal region near the soma of Purkinje cells, including the axon initial segment (AIS), was structurally not different among these genotypes (Fig. 1B). To quantify the AIS length, we measured the length of Nav expression from three genotypes. There was no significant difference in the length (Fig. 1A,G). In LES Purkinje axons, nodal structure was disrupted, displaying dispersed Nav channel expression at nodes (Fig. 1B). The major alteration in the integrity of axonal domains along myelinated fibers was observed in the pattern of Caspr expression, a prominent component of the paranodal axon glial junctions. LE and LEHet Purkinje axons, which were labeled with Calbindin, presented strong and well-defined labeling for Caspr at both edges of nodal Nav channels, whereas Caspr labeling was weak and disperse around nodes of Ranvier from the LES. Thus it was difficult to detect the segregation of nodal and paranodal structures along LES Purkinje axons (Fig. 1B). In the LES, abnormal spheroid swellings along Purkinje axons were found in high quantity distal to the Purkinje soma, near the DCN, whereas few could be found more proximal to the soma (Fig. 1C). These swellings have been described as indication of axon deterioration leading to cerebellar damage and degeneration23. Thus, it could be speculated that the proximal axon close to the nerve terminal is more susceptible to myelin loss. In LE and LEHet rats, Purkinje axons were covered by myelin expressing MBP, whereas LES rats had no distinct MBP expressed along Purkinje axons (Fig. 1C). EM imaging of Purkinje axons revealed that the loss of MBP impacts myelination, as evidenced by the increased g-ratio in LES, and to a lesser extent LEHet (Fig. 1D,H). The ratio of the inner axonal radius to the total radius of the axon and myelin, g-ratio, is widely utilized as a functional and structural index of optimal axonal myelination. Thus, these structural deficits in Purkinje axons seen in the LES animals indicate that MBP-mediated compact myelination is critically important to maintain axonal health of Purkinje cells in the cerebellum.
Figure 1.
Structural changes of the Purkinje axon in the LES cerebellum (A). Calbindin (CB)-expressing Purkinje cells (green) in LE, LEHet, and LES cerebella. Caspr (blue) and NavPan (red) expression along Purkinje axons (arrowheads). (B) Purkinje axons expressing CB (green) in the white matter of the cerebellum of LE, LEHet, and LES. Arrowheads mark nodal and paranodal structures, expressing Caspr (blue) and NavPan (red), enlarged in inset. Inset width is 18 μm. While the nodal structure of Purkinje axons in LE and LEHet remain intact, Nav and Caspr expression in LES Purkinje axons is diffuse. (C) CB-expressing Purkinje axons (green) and myelin basic protein (MBP) expression (red) in the white matter of the cerebellum of LE, LEHet, and LES. MBP expression is absent in the LES cerebellum, where spheroid swellings were observed in Purkinje axons (arrowheads). (D) EM images of myelination around Purkinje axons in LE, LEHet, and LES. Myelin was thinner in LEHet than LE, and nearly absent in LES. (E) Diagram of a Purkinje cell with dendrites that extend into the molecular layer and an axon projecting through the granule cell layer and white matter that synapses in the DCN. Recording electrode (blue) represents the recording site in Fig. 2 through 4. Stimulating electrode (red) indicates the stimulating site in Fig. 4. (F) Number of Purkinje cells per 304.5 μm × 304.5 μm square was not different between LE, LEHet, and LES animals. (G) There were no significant differences between the length of NavPan staining along Purkinje AIS between LE, LEHet, and LES animals. (H) Quantification of the g-ratio is calculated by dividing the radius of the axon (blue) by the total radius of the axon and myelin (red). The g-ratio quantified from electron microscopy (EM) images was significantly higher in LEHet and LES compared to LE.
Lack of compact myelin increased spontaneous firing in DCN neurons
To examine the effect on cerebellar output resulting from alterations in Purkinje axon myelination due to MBP mutation, we recorded spontaneous AP firing from DCN cells of LE, LEHet, and LES rats and filled the cells with Alexa 568 (at P14–18, Fig. 2A). In the DCN, heterogeneous cell populations show various morphological and electrophysiological properties13. Based on their soma size and intrinsic properties, we chose larger DCN neurons for whole-cell recordings, which were defined by the diameter of soma (>25 μm), capacitance (>100 pF), and input resistance (241 ± 34.4 MΩ, n = 14).
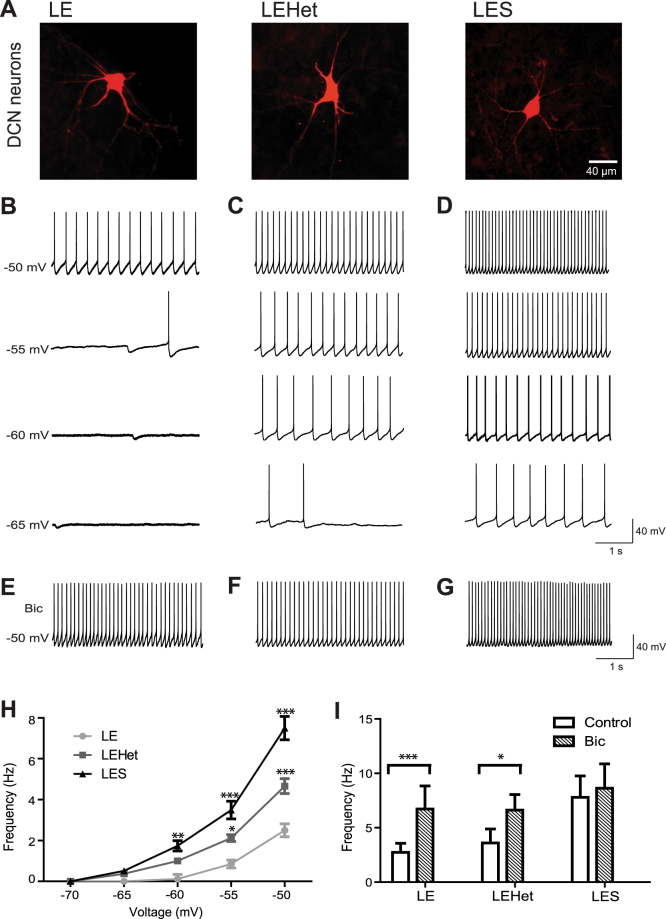
Figure 2.
LES and LEHet DCN neurons display altered tonic inhibition and spontaneous AP firing. (A) Representative deep cerebellar nuclei (DCN) cells in LE, LEHet, and LES, which were filled with Alexa 568 during whole-cell recordings. (B–D) Whole-cell current clamp recordings of spontaneous APs at membrane potentials of −50, −55, −60, and −65 mV in DCN neurons of LE (B), LEHet (C), and LES (D) rats, demonstrating increased firing frequency in LES, and to a lesser extent, LEHet DCN neurons. (E–G) Spontaneous action potential firing in DCN neurons of LE (E), LEHet (F), and LES (G) rats at −50 mV in the presence of 10 μM bicuculline. (H) Summary of spontaneous firing frequency at various membrane potentials from −70 mV to −50 mV in LE, LEHet, and LES DCN cells. (I) The effect of bicuculline on spontaneous firing frequency (at −50 mV) in LE, LEHet, and LES DCN cells. *, **, and *** indicate p < 0.05, p < 0.01, and p < 0.001, respectively.
In LE rat cerebellum, DCN cells fired spontaneous APs regularly at 2.5 ± 1.23 Hz (n = 9) at the physiological membrane potential of around −50 mV in LE rat brains (Fig. 2B). Spontaneous AP firing in LE DCN cells was not observed at membrane potentials below −60 mV in current-clamp recordings, but in 30% of recordings we detected inhibitory postsynaptic potentials (IPSPs) with amplitude of 5.2 ± 1.10 mV (n = 3). At −55 mV, spontaneous APs began to appear, and AP firing rate increased as the membrane potential depolarized to −50 mV, when no current was injected (Fig. 2B). LEHet and LES DCN cells fired spontaneous APs at membrane potentials as low as −65 mV, when hyperpolarizing current was injected, with increased firing frequency as the membrane potential depolarized (Fig. 2C,D). Average firing rate at −50 mV was 4.7 ± 1.33 Hz in LEHet DCN neurons and 7.5 ± 1.89 Hz in LES DCN cells (n = 7 LEHet cells and n = 9 LES cells, Fig. 2H). LES DCN cells displayed a higher firing rate at all membrane potentials compared with LE and LEHet (Fig. 2H). Furthermore, spontaneous IPSPs were not observable in LEHet and LES neurons. The results indicate that the extent of Purkinje axon myelination modulates spontaneous firing in DCN neurons, thus lack of myelination may impact the output of cerebellar signals from the DCN.
Spontaneous firing rate of DCN neurons is regulated by GABA-mediated tonic inhibition from presynaptic Purkinje cell terminals24,25. We tested whether increased spontaneous firing rate in LEHet and LES was due to changes in GABAA receptor activation into these DCN cells from Purkinje cell terminals. The additional application of bicuculline, a GABAA receptor antagonist, increased spontaneous firing rate from 2.7 ± 2.05 Hz to 6.7 ± 5.20 Hz (n = 6, t(5) = 2.756 p = 0.040, paired t-test, Fig. 2E,I) at membrane potential of −50 mV in LE DCN cells, indicating that tonic inhibitory input plays a role in spontaneous AP firing in normal DCN neurons. Firing rate in LEHet cells also increased from an average of 3.6 ± 3.14 Hz to an average of 6.6 ± 3.54 Hz in the presence of bicuculline (n = 6, t(5) = 3.244 p = 0.023, paired t-test, Fig. 2F,I), while firing rate in LES DCN cells did not significantly increase (n = 5, t(4) = 2.404 p = 0.074, paired t-test, Fig. 2G,I). The increased firing rate of both LE and LEHet DCN cells in the presence of bicuculline was not statistically different from the firing rate of LES DCN cells, suggesting that the GABAergic inhibitory input is the major factor underlying AP firing rate differences between genotypes. To avoid the non-specific effect of bicuculline, we tested the effect of another GABAA receptor antagonist, SR 95531, on spontaneous firing in DCN cells. The effect of increased spontaneous firing in LE and LEHet cells was also observed in the presence of SR 95531, and an increase in firing rate was similar to that in the presence of bicuculline (data not shown). Together, these results indicated that the LES cerebellum lacking compact myelination had a reduction in GABAA receptor activation in DCN cells, leading to hyper-excitability.
The extent of myelin regulates GABA-mediated inhibitory synaptic transmission in DCN cells
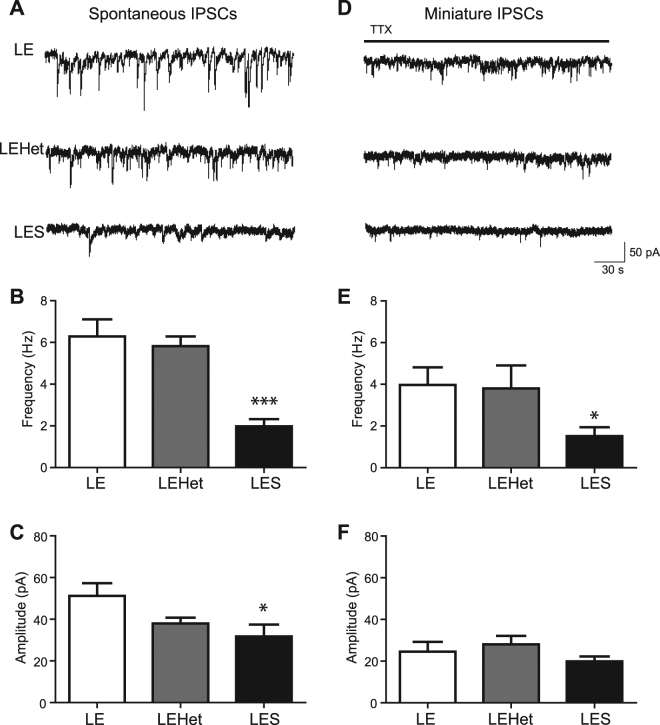
To determine whether the reduction in GABAA receptor activation seen in LES DCN cells was due to reduced inhibitory input to these cells, we evaluated GABA-mediated inhibitory inputs from Purkinje cells by recording spontaneous inhibitory postsynaptic currents (sIPSCs) in DCN cells from LE, LEHet, and LES animals in the presence of CNQX. Average sIPSC frequency was 6.3 ± 0.82 Hz (n = 7) in LE and 5.8 ± 0.47 Hz (n = 7) in LEHet DCN neurons, while in LES DCN neurons sIPSC frequency was significantly less than LE at 2.0 ± 0.35 Hz (n = 7 per group, t(12) = 4.833, p = 0.0004, two-tailed t-test, Fig. 3A,B). This indicates that the LES DCN has fewer GABA-mediated inhibitory inputs. Average sIPSC amplitude was 51.3 ± 6.04 pA (n = 7) in LE, while in LEHet the amplitude of sIPSCs (38.0 ± 2.79 pA) was lower than, but not significantly different from, LE sIPSC amplitude (n = 7 per group, t(12) = 1.998, p = 0.0688, two-tailed t-test, Fig. 3A,C). LES sIPSC amplitude was significantly lower than LE at 31.8 ± 5.62 pA (n = 7 per group, t(12) = 2.362, p = 0.0359, two-tailed t-test, Fig. 3A,C). Average decay time of sIPSCs was similar among these groups (data not shown). The reduction in the amplitude and frequency of sIPSCs observed in LES DCN neurons indicate that proper myelination of the cerebellum regulates GABA-mediated synaptic inputs from Purkinje cell terminals to DCN neurons.
Figure 3.
LES animals show reduced spontaneous inhibitory input into DCN cells. (A) Recordings of sIPSCs in DCN cells from LE, LEHet, and LES animals in the presence of CNQX (25 μM). (B,C) Summary of sIPSC frequency (B) and amplitude (C) in LE, LEHet, and LES DCN cells. (D) Recordings of mIPSCs in the presence of TTX (1 μM) in LE, LEHet, and LES DCN cells. (E,F) Summary of mIPSC frequency (E) and amplitude (F) in LE, LEHet, and LES DCN cells. *, **, and *** indicate p < 0.05, p < 0.01, and p < 0.001, respectively.
To test whether this reduction originates from reduced presynaptic release or reduced postsynaptic receptors, we recorded miniature IPSCs (mIPSCs) in the presence of TTX (1 μM) to exclude the effect of spontaneous APs. Average mIPSC frequency was 4.0 ± 0.85 Hz (n = 5) in LE and 3.8 ± 1.10 Hz (n = 4) in LEHet, while LES mIPSC frequency was significantly decreased (1.6 ± 0.43 Hz, n = 5, t(8) = 2.587, p = 0.0322, two-tailed t-test, Fig. 3D,E). The average amplitude of mIPSCs was not significantly different between LES, LEHet, and LE (n = 5 LE vs. 4 LEHet vs. 5 LES, Fig. 3D,F). This indicates that the number of inhibitory inputs or presynaptic GABA release is reduced in Purkinje terminal-DCN neuron synapses in the LES cerebellum, without alterations in the quantal size of vesicles or in postsynaptic receptor properties. Furthermore, in LES DCN cells, sIPSC and mIPSC frequency were not different. Thus, both sIPSCs and mIPSCs may be mediated by AP-independent vesicle release, indicating that few or no APs reach the LES Purkinje terminal due to failures spontaneous AP propagation.
MBP mutation-induced dysmyelination reduced Purkinje cell AP efficacy
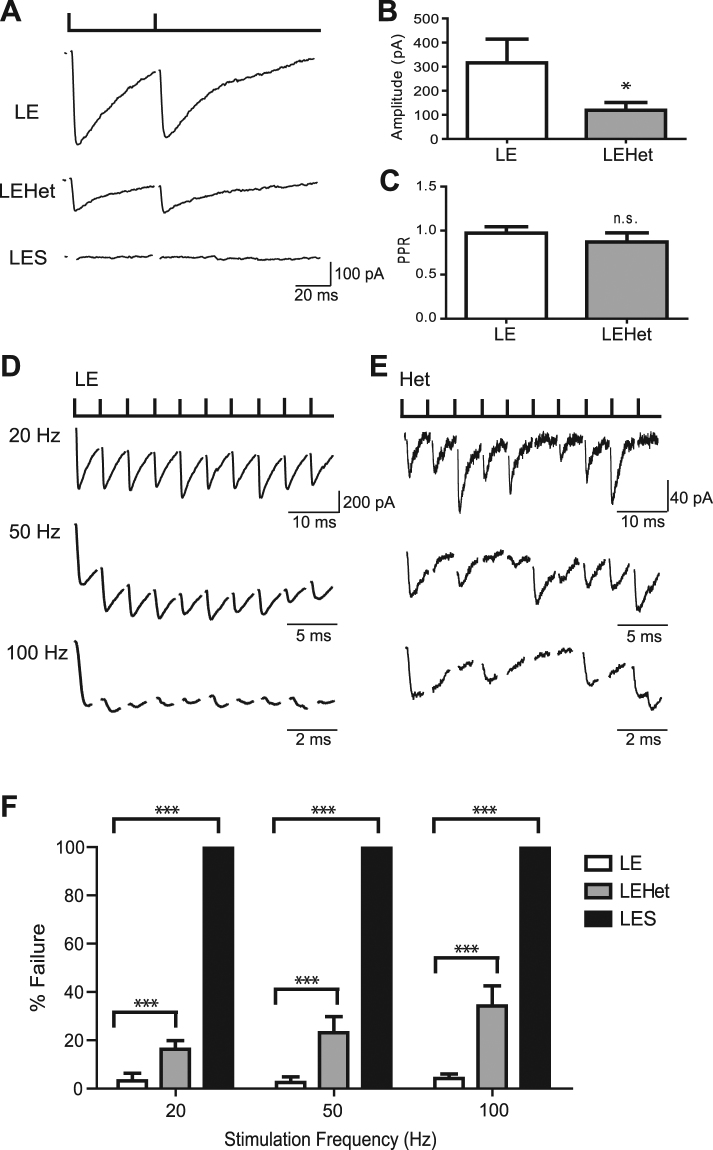
To evaluate the MBP mutation-induced deficits in AP–mediated synaptic transmission in the Purkinje cell-DCN neuron synapse, we recorded evoked inhibitory postsynaptic currents (eIPSCs) in DCN cells by stimulating Purkinje axons in the white matter surrounding the DCN using a bipolar stimulator. Recordings were performed in the presence of AMPA/Kainate antagonist CNQX in order to isolate GABA-mediated inhibitory inputs from the Purkinje cells. In LE DCN neurons, eIPSCs had an average amplitude of 316.1 ± 98.4 pA (n = 6). The amplitude of eIPSCs gradually increased as the intensity of stimulus increased, thus we stimulated at the minimum intensity that evoked the maximum response. Compared with LE, LEHet DCN neurons had reduced eIPSC amplitude (119.5 ± 31.8 pA, n = 6 LE vs. 7 LEHet, t(10) = 1.902, p = 0.0268, two-tailed t-test, Fig. 4B). There was no significant difference in paired pulse ratio (Fig. 4A–C, LEHet average = 0.9 ± 0.3, LE average = 1.0 ± 0.1, n = 6 LE vs. n = 6 LEHet, t(10) = 0.8096, p = 0.4282, two-tailed t-test, Fig. 4C). Remarkably, LES DCN cells had no observable response to Purkinje axon stimulation (n = 9, Fig. 4A). To attempt to elicit a response from LES DCN cells, we increased stimulus intensity and stimulated closer to the DCN area, as close as ~70 μm from the recorded cell, to no avail. To determine whether conduction failure along the Purkinje axon causes this failure, we tested the extent to which AP propagation was impaired in LES Purkinje axons. LES Purkinje cells fired spontaneous APs, similarly observed in LE Purkinje cells. Recording the back-propagation action currents in the Purkinje cell revealed that APs can propagate along the axon within 200 μm of the soma in the LES, but failures occur at high frequency (50 Hz), while no failures occur in LE (Supplementary Figure 1). The severe phenotype of a lack of response from LES DCN cells to Purkinje axon stimulation is likely due to a combination of AP propagation and alterations in axonal and presynaptic properties.
Figure 4.
LES and LEHet show reduced inhibitory transmission at the Purkinje cell-DCN cell synapse. (A) Recordings of eIPSCs in DCN neurons evoked by stimulating Purkinje axon with bipolar stimulator placed in the cerebellar white matter in LE, LEHet, and LES rats. Paired pulse stimulation with an interval of 50 ms. Note no identifiable current in LES DCN cells in response to Purkinje axon stimulation. (B) Summary of eIPSC amplitude in LE and LEHet DCN neurons. (C) No significant difference between the paired pulse ratio (PPR) in eIPSCs from LEHet and LE DCN neurons. (D) and (E) Whole cell voltage clamp recordings of eIPSCs in DCN neurons of LE (D) and LEHet (E) rats after stimulation at 20, 50, and 100 Hz. (F) Summary of failure rate in LE, LEHet, and LES DCN cells at 20 Hz, 50 Hz, and 100 Hz. LES cells showed 100% failure rate. *, **, and *** indicate p < 0.05, p < 0.01, and p < 0.001, respectively.
Our previous study in the auditory brainstem showed that LES mutants have reduced AP conduction efficiency, which resulted in neurotransmission failures in a frequency-dependent manner8,9. To determine whether synaptic transmission failures in response to high frequency stimulation also occurred in Purkinje cell-DCN neuron synapses of LEHet animals, we stimulated Purkinje axons at 20, 50, and 100 Hz while recording from DCN cells in LE and LEHet rats. In LE DCN cells, the failure rate of eIPSCs was 3.2 ± 3.2% at 20 Hz, 2.6 ± 2.3% at 50 Hz, and 4.2 ± 1.9% at 100 Hz (Fig. 4D,F, n = 6). LEHet DCN cells had an average of 16.3 ± 3.6% failure at 20 Hz, 23.1 ± 6.6% failure at 50 Hz, and 34.2 ± 8.4% failure at 100 Hz (Fig. 4E, n = 7). The failure rate of eIPSCs was significantly increased at 20 Hz (n = 6 LE vs. 7 LEHet, t(11) = 2.686, p = 0.0212, two-tailed t-test), 50 Hz (n = 6 LE vs. n = 7 LEHet, t(11) = 2.737, p = 0.0193, two-tailed t-test), and 100 Hz (n = 6 LE vs. 7 LEHet, t(11) = 3.242, p = 0.0078, two-tailed t-test, Fig. 4D–F). This indicates that inhibitory synaptic transmission at the Purkinje cell-DCN neuron synapse is influenced by axon myelination in a frequency-dependent manner.
Reduced inhibitory input to DCN neurons from Purkinje terminals in LES mutants
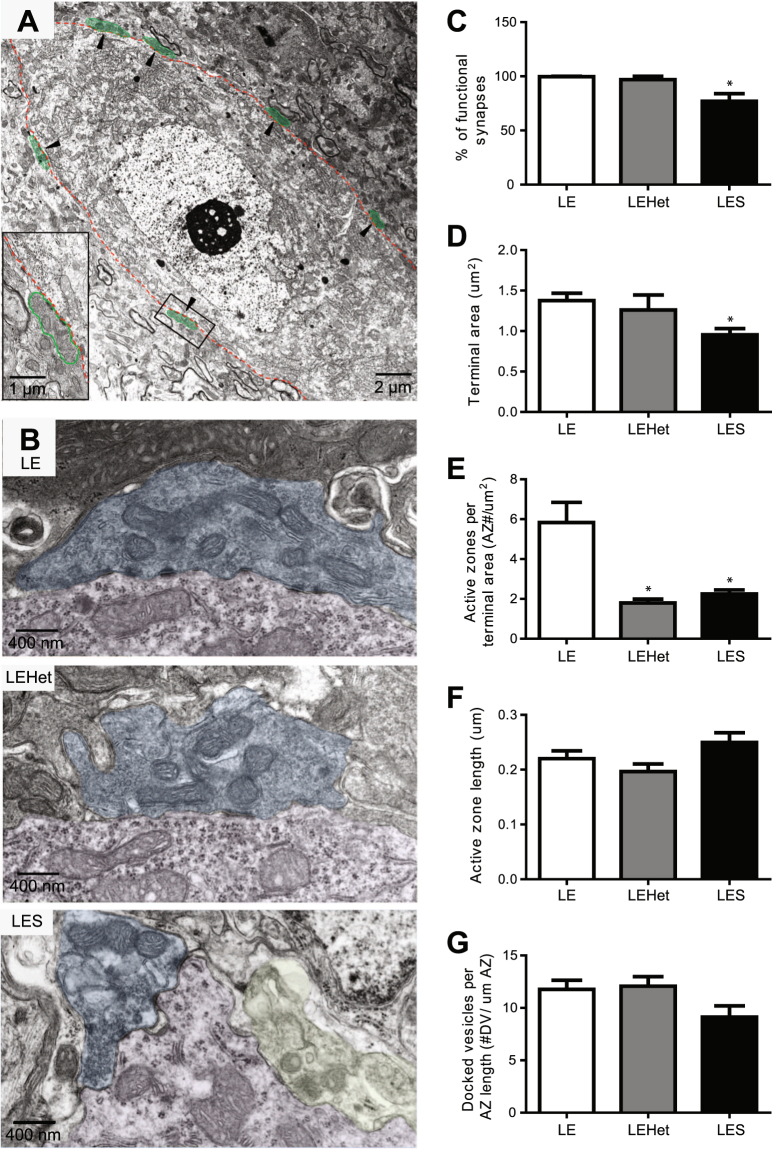
The electrophysiological measurements in LES and LEHet mutants revealed reduced tonic inhibition and hyper-excitability of DCN neurons, which is associated with failure of spontaneous AP propagation along dys- or hypo-myelinated Purkinje axons. In addition to axonal deficits and conduction failures, a decrease in the number of functional Purkinje terminals or reduced GABA release at each Purkinje terminal can sufficiently contribute to synaptic transmission deficits at the Purkinje cell-DCN neuron synapse. To address the potential role of axon myelination in proper structural properties of nerve terminals and synaptic contacts, we performed ultrastructural analysis of the DCN area in LE, LEHet, and LES rats at P18, using electron microscopy. Purkinje cell terminals that form synapses on the DCN neuronal soma were recognized by their unique morphology (Fig. 5A), as previously described26. In the LE and LEHet animals, presynaptic terminals of Purkinje cells were filled with a dark filamentous matrix containing synaptic vesicles and mitochondria (Fig. 5B). However, in the LES DCN, Purkinje terminals displayed two distinct morphologies, which we characterized as “functional” terminals, with preserved ultrastructure containing mitochondria and synaptic vesicles similar to LE, or as “degenerating” terminals, with mitochondria showing abnormal morphology and lack of synaptic vesicles (Fig. 5B). Quantitative analysis of the total Purkinje terminal numbers around DCN cell bodies revealed no changes across genotypes, however in LES rats there was a significant 22% reduction of “functional” synapses compared to LE (n = 3 animals per group, t(4) = 3.247, p = 0.0315, two-tailed t-test, Fig. 5C) compared to LE controls. In addition, we found that axonal dysmyelination could affect the presynaptic apparatus at a single nerve terminal. Quantification analysis showed a significant reduction in the area of a single terminal in LES rats (n = 3 animals per group, t(4) = 3.515, p = 0.0246, two-tailed t-test, Fig. 5D), as well as the number of active zones per terminal in the LES rat compared to LE (n = 3 animals per group, t(4) = 3.466, p = 0.257, two-tailed t-test, Fig. 5E). Interestingly, the number of active zones was also altered in LEHet Purkinje terminals compared to LE (n = 3 animals per group, t(4) = 3.912, p = 0.0174, two-tailed t-test, Fig. 5E). However, the active zone length (n = 3 animals per group, F(2) = 2.999, p = 0.1251, one-way ANOVA, Fig. 5F) and the number of docked vesicles was not statistically different across genotypes (n = 3 animals per group, F(2) = 2.881, p = 0.1327, one-way ANOVA, Fig. 5G). Taken together, the ultrastructural analysis of the DCN demonstrated anatomically reduced inhibitory inputs to LES DCN neurons from Purkinje terminals, due to significantly increased population of degenerated synapses and decreased number of active zones in functional population of terminals that can potentially reduce presynaptic GABA release. Our results indicate that axonal myelin is crucial for the structural formation, maturation, or functional maintenance of Purkinje terminals synapsing onto DCN neurons.
Figure 5.
Purkinje cell-DCN neuron synapse structure disruptions in LES and LEHet animals. (A) EM image from the DCN of an LE rat. Arrowheads indicate examples of Purkinje cell terminals (colored green). (B) Electron microscopy images of Purkinje cell-DCN neuron synapses including all active zones in LE, LEHet, and LES rats. Yellow colored terminal indicates a degenerating terminal. (C–G) Summary of the number of functional (non-degenerating) Purkinje terminals per DCN cell (C), Purkinje terminal area (D), number of active zones per terminal (E), length of active zones (F) and number of docked vesicles (DV) per active zone (G) in LE, LEHet and LES rats. *, **, and *** indicate p < 0.05, p < 0.01, and p < 0.001, respectively.
Discussion
In the LES rat, the absence of MBP prevents the multi-layered wrapping of the myelin sheath and resulted in either no myelin or a thin myelin sheath with a single layer. In addition to axonal deficits, lack of compact myelination reduced GABA release from Purkinje terminals, led to hyper-excitability of DCN neurons, and eventually induced an unstable output from the cerebellum in the LES.
The hyper-excitability of DCN neurons in LES animals, and to a lesser extent in LEHet animals, was influenced by a reduction of GABAA receptor activation due to decreased inhibitory inputs of Purkinje terminals. Reduced frequency and amplitude of sIPSCs in LES DCN cells indicated that there were fewer inhibitory inputs into these cells or that there were significant alterations in GABA release from Purkinje cell terminals. The reduction in sIPSC frequency and amplitude is likely caused by failures of AP propagation along Purkinje axon in LES, also contributing to the lack of observable eIPSCs. In addition, our EM data provide an explanation for our electrophysiological findings regarding changes in functional properties. There was no difference in the total number of Purkinje terminals contacting DCN cells in LE, LEHet, and LES rats; however, there were a significant number of Purkinje terminals on LES DCN cells that were degenerated, lessening the number of functional inputs to these cells compared to the normal LE DCN neurons. This result suggests that axon myelination is able to modulate proper synapse formation and maintenance, and control synaptic inputs into the DCN in addition to AP propagation. Correspondingly, post-mortem assessment of the DCN of MS patients with marked demyelination showed anatomically the reduction in terminals contacting DCN cells, as well as degradation of synapses in the DCN15.
Changes in the amplitude of spontaneous postsynaptic currents could be associated with alterations in presynaptic properties including release probability, the quantal size of neurotransmitters, or the readily releasable pool size at presynaptic terminals27,28. Concerning a potential alteration in release probability, the paired pulse ratio between LEHet and LE was not significantly different, indicating that release probability is not responsible for the sIPSC amplitude reduction in the Purkinje-DCN cell synapse due to dysmyelination. To address potential alterations in the quantal size of neurotransmitter, the amplitude of mIPSCs was measured. mIPSCs are a result of single vesicle fusion, and their amplitude is an indicator of quantal size of vesicles containing neurotransmitter. mIPSC amplitude was similar among the three groups (LE, LEHet, and LES), indicating that quantal size in this synapse was not impacted by myelin deficiency. Changes in the vesicle number around the active zone, including the readily releasable pool size, can lead to reduced IPSC amplitude. However, EM imaging of the active zones in Purkinje terminals revealed that there were no differences in the number of docked vesicles in the Purkinje terminals of LE, LEHet, and LES animals. Interestingly, the terminal size and the number of active zones per Purkinje terminal were significantly decreased in LES mutants compared to those in LE animals. This alteration in structural properties of terminals may contribute to reduced GABA release from LES Purkinje terminals.
The reduced amplitude of eIPSCs in LEHet and no detectable eIPSCs in LES DCN cells is explained by conduction failure in Purkinje axons of LEHet and LES animals. In addition, high frequency stimulation of 100 Hz of Purkinje axons resulted in eIPSC failure of 34.2% in LEHet DCN cells, while nearly no failure (4.2%) was seen in normal LE DCN neurons, indicating that high frequency APs fail to propagate and invade the Purkinje terminal in LEHet DCN cells. We observed failures of back-propagated action currents in LES Purkinje axons, while there was no failure in LE Purkinje axons (Supplementary Figure 1). EM imaging revealed reduced myelin thickness in LEHet and LES Purkinje axons, an important contributor to conduction velocity29,30. Myelin shapes the structure of the node of Ranvier31, and thus demyelination leads to disrupted nodal ion channel distribution32. Ion channel expression and distribution along the axon is crucial for AP propagation and conduction velocity31,33–35. Previous studies in the MBP-deficient LES rat have revealed that ion channel distribution alterations and conduction failure at the nerve terminal critically resulted in synaptic transmission failure in the auditory brainstem8,9,21.
eIPSCs were not seen in LES DCN cells, despite the evidence from electrophysiological recordings of substantially detectable mIPSCs, as well as EM images displaying that Purkinje terminals contact LES DCN cells. It is likely that a contributor to this synaptic phenotype is a disruption of AP conduction along the deteriorating Purkinje axon. Immunofluorescence images of Purkinje axons in the LES cerebellum showed that the proximal region of Purkinje axons appeared predominately normal, despite a few spheroid swellings. However, toward the nerve terminal in the DCN area, the distal axons displayed abnormal structures of nodes and paranodes with a greater number of spheroid swellings, indicating severe Purkinje axon deterioration. This suggests that very few APs invade the Purkinje terminal in LES mutants, although APs can be generated at the AIS.
How is axonal dysmyelination associated with structural alterations in axons and presynaptic terminals? MBP deletion-induced dysmyelination causes deterioration of the Purkinje presynaptic terminal in LES animals, as well as structural alterations in this terminal in both LES and LE animals, through several potential mechanisms. Synaptic remodeling has been shown to occur as a result of synapse strengthening or weakening in the form of increased or decreased utilization of the synapse during development in the neuromuscular junction and CNS36. A recent study showed that TEA-induced long-term potentiation, which can be considered an increased utilization of the synapse, resulted in an increase in synaptic contacts and the number of active zones at mossy fiber boutons in the hippocampus37. Conversely, alterations in the Purkinje cell-DCN cell synaptic structure may occur due to increased AP failures at Purkinje terminals caused by axon demyelination. Lack of APs reaching Purkinje presynaptic terminals, and thus extreme underutilization of these terminals in LES animals could even lead to deterioration of the terminal itself. Activity-dependent synapse loss such as this has been previously described as a key mechanism in normal physiology, development, and pathology36,38,39. Alternatively, myelin-producing oligodendrocytes located near the axon or terminal may be important for formation, maintenance, or stabilization of the synapse. Several neurotrophic factors, such as BDNF, have impacts on synaptic maturation, including axon arborization and presynaptic differentiation40,41. Oligodendrocytes are one of the major providers of BDNF in the CNS42–45, and therefore abnormal maturation of oligodendrocytes due to MBP deletion may have a potential side effect on their roles in synapse maturation and stabilization.
The DCN is the sole output of the cerebellum. Neurons in this area take information from the cerebellar cortex and send signals to premotor areas, which affect motor function. Recent studies suggest that Purkinje cells relay highly synchronous signals, resulting in time-locked AP firing in the DCN12. Myelination is essential for this synchrony6. Abnormally increased AP firing from DCN neurons may result in infidelity of the signal and incoherent output from the DCN in the LES. In addition, synaptic transmission is disrupted as a result of presynaptic terminal structure alterations in the Purkinje cell-DCN neuron in both LES and LEHet animals. These findings suggest that myelination deficits alter axonal and presynaptic terminal structures, disrupt AP propagation, and obstruct functional neurotransmission.
Materials and Methods
Animals
Experiments using Long-Evans Shaker rats (LES, from Dr. Kwiecien, McMaster University, Ontario, Canada) of either sex between the ages of postnatal day 14 (P14) and P18 were approved and performed in accordance with the guidelines and protocols of the University of Texas Health Science Center at San Antonio (UTHSCSA) Institutional Animal Care and Use Committee (IACUC, approved protocol #14005×). Animals were maintained as heterozygotes, and crossed to generate pups homozygous for the MBP mutation. A fraction of offspring from the heterozygous animals exhibited the LES phenotype and could be easily identified by gross movement disorders (a distinguishable tremor) starting around 2 weeks following birth19,46. The shaking phenotype of the LES rat is autosomal recessive, present only in animals that are homozygous for the MBP mutation whereas wild type (LE,+/+) and heterozygous (LEHet,+/−) rats show no symptoms18–20,47.
Slice preparation
Sagittal cerebellar slices (300 μm thick) were prepared from rat pups at P14–P18. After rapid decapitation, the cerebellum was removed from the skull and immersed in ice-cold artificial CSF (aCSF) containing the following (in mM): 125 NaCl, 2.5 KCl, 3 MgCl2, 0.1 CaCl2, 25 glucose, 25 NaHCO3, 1.25 NaH2PO4, 0.4 ascorbic acid, 3 myo-inositol, and 2 Na-pyruvate, pH 7.3–7.4, when bubbled with carbogen (95% O2, 5% CO2; osmolality of 310–320 mOsm/kg water). After cutting, the slices were transferred to an incubation chamber containing aCSF bubbled with carbogen and maintained at 35 °C for 30 min, and thereafter at room temperature.
Electrophysiology
Whole-cell patch-clamp recordings were performed in normal aCSF at room temperature (22–24 °C) using the voltage or current-clamp mode of the EPC-10 (HEKA Electronik). Normal aCSF was the same as slicing aCSF, but with 1 mM MgCl2 and 2 mM CaCl2. For current clamp recordings, K-gluconate-based pipette solution contained the following (in mM): 130 K-gluconate, 20 KCl, 5 Na2-phosphocreatine, 10 HEPES, 4 Mg-ATP, 0.2 EGTA, and 0.3 GTP, pH = 7.3. For IPSC recordings, CsCl-based pipette solution included the following (in mM): 150 CsCl, 10 TEA-Cl, 0.2 EGTA, 1 MgCl2, 10 HEPES, 2 ATP, 0.3 GTP, and 10 phosphocreatine, pH = 7.3. Occasionally, pipette solution additionally contained an Alexa 568 (50 μM) to visualize the cell through dye-filling and subsequent imaging after whole-cell recordings. Patch electrodes had resistances of 3–4 MΩ, and the initial uncompensated series resistance (Rs) was <25 MΩ. APs were elicited by a bipolar platinum/iridium electrode placed in the white matter fiber tract surrounding the DCN. Postsynaptic currents were measured under whole-cell voltage-clamp with a holding potential of −60 mV. For all experiments, Rs was <25 MΩ. Spontaneous postsynaptic currents were analyzed using Mini-analysis software (Synaptosoft Inc., Decatur, GA, USA; RRID: SCR_002184). Data were analyzed off-line and presented using Igor Pro (Wavemetrics, Lake Oswego, OR, USA; RRID: SCR_000325). Statistical significance was determined using either one-way ANOVA or Student’s t-test (GraphPad Prism; RRID: SCR_002798) and P values < 0.05 were considered significant. Data values are reported as means ± SEM. n represents the cell number.
Immunohistochemistry
Cerebellar slices (100 μm thick) were prepared as for electrophysiology, but subsequently fixed with 8% (wt/vol) paraformaldehyde in phosphate buffer solution (PBS) for 20 min. Free-floating sections were blocked in 4% goat serum and 0.3% Triton X-100 in PBS for 1 h. For double immunohistochemistry, slices were incubated with antibodies for rabbit anti-calbindin (CB; 1:200; Cell Signaling Technologies, Cat # 13176), mouse anti-sodium channel (PanNav; 1:200; Sigma-Aldrich, Cat # S8809, RRID: AB_477552), guinea pig anti-Caspr (Caspr; 1:1,000)22, and mouse anti-MBP (MBP; 1:500; BioLegend, Cat # 808401, RRID: AB_2564741) overnight at 4 °C. Antibody labeling was reported by incubation with different Alexa dye–conjugated secondary antibodies (1:500, Invitrogen) for 2 h at room temperature. Slices were mounted onto Superfrost slides in photobleaching-protective medium (Fluoroshield; Sigma-Aldrich). Stained slices were viewed with laser lines at 488 nm (for green), 555 nm (for red) and 633 nm (for blue) using a 20×, 40×, or 64× oil-immersion objective on a confocal laser-scanning microscope (LSM-510, Zeiss). Stack images were acquired at a digital size of 1,024 × 1,024 pixels with optical section separation (z interval) of 0.5 μm and were later cropped to the relevant part of the field without changing the resolution.
Purkinje Cell Count
Purkinje cell number was counted using 20×tiled images of entire sagittal cerebellar slices containing DCN from a cerebellar hemisphere. A 20 × 20 grid was constructed across the images, and 340 μm × 340 μm squares within the grid were randomly selected. CB-positive cells were counted only in the Purkinje layer, and only cells entirely in focus were counted. The number reported is the average of CB-positive cells per square in each genotype. Squares with zero CB-positive cells were not included. Blinded cell counting was performed in two to three slices (100 μm thick) from each animal (n = 3 in LE, n = 4 in LEHet, and n = 4 in LES).
Transmission Electron Microscopy
Transmission electron microscopy was carried out as described in Saifetiarova et al.48. Briefly, animals were anesthetized and intracardially perfused with normal saline, followed by 5% glutaraldehyde/4% paraformaldehyde fixative. Brains were removed and post-fixed for another 2 weeks in the same fixative. 1 mm-thick samples of DCN area were dissected out and incubated overnight in 0.1 M sodium cacodylate buffer followed by incubation in 2% OsO4 solution and gradient ethanol dehydration. Finally, samples were incubated in propylene oxide, infiltrated with PolyBed resin and embedded in flat molds. After embedding, samples were submitted to the UTHSCSA Electron Microscopy Lab for further processing. Briefly, 90 nm sections were collected on copper mesh grids and poststained with uranyl acetate and Reynold’s lead. Samples were imaged on a JEOL 1230 electron microscope using Advanced Microscopy Techniques software. Elongated Purkinje cell terminals converging on cell bodies of DCN were recognized by presence of polymorphic synaptic vesicles in relatively dense matrix and collection of several profiles of slender mitochondria26. A total of 180–200 synapses were analyzed from 3 animals per group (LE, LEHet, and LES rats). The following parameters at final magnification of 30,000× were measured in each synapse: area of presynaptic terminal, number of active zones per terminal, active zone length, thickness of postsynaptic density and number of docked vesicles. Docked vesicles were defined as those within one vesicle diameter of the presynaptic active zone49,50. Only non-degenerated synaptic terminals were included in analysis of the LES group. The number of Purkinje cell terminals per neuron was quantified at magnification of 20,000×.
Data availability
The authors declare that all data generated or analyzed in this study are available within the article. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Acknowledgements
This work was supported by a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD, R01 DC03157) to J. H. Kim, and NIGMS GM063074 and the Zachry Foundation to M.A. Bhat.
Author Contributions
Conceptualization of the study by J.H.K. Design of the study and experiments by T.B. and J.H.K. Performance of the experiments and analysis of the data by T.B. and J.S. Writing of the manuscript T. B., J.S., M.A.B., and J.H.K.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19314-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baumann N, Pham-Dinh D. Biology of Oligodendrocyte and Myelin in the Mammalian Central Nervous System. Physiol Rev. 2001;81(2):871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 2.Haines JD, Inglese M, Casaccia P. Axonal Damage in Multiple Sclerosis. Mt Sinai J Med. 2011;78(2):231–243. doi: 10.1002/msj.20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbert AL, Monk KR. Advances in myelinating glial cell development. Curr Opin Neurobiol. 2017;42:53–60. doi: 10.1016/j.conb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith KJ, McDonald WI. The pathophysiology of multiple sclerosis: the mechanisms underlying the production of symptoms and the natural history of the disease. Philos Trans R Soc Lond B Biol Sci. 1999;354:1649–1673. doi: 10.1098/rstb.1999.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolswijk G. Oligodendrocyte survival, loss and birth in lesions of chronic-stage multiple sclerosis. Brain. 2000;123(1):105–115. doi: 10.1093/brain/123.1.105. [DOI] [PubMed] [Google Scholar]
- 6.Pajevic S, Basser PJ, Fields RD. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience. 2014;276:135–147. doi: 10.1016/j.neuroscience.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utzschneider DA, Archer DR, Kocsis JD, Waxman SG, Duncan ID. Transplantation of glial cells enhances action potential conduction of amyelinated spinal cord axons in the myelin-deficient rat. Proc Natl Acad Sci. 1994;91(1):53–57. doi: 10.1073/pnas.91.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SE, Turkington K, Kushmerick C, Kim JH. Central dysmyelination reduces the temporal fidelity of synaptic transmission and the reliability of postsynaptic firing during high-frequency stimulation. J Neurophysiol. 2013;110(7):1621–1630. doi: 10.1152/jn.00117.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Renden R, von Gersdorff H. Dysmyelination of auditory afferent axons increases the jitter of action potential timing during high-frequency firing. J Neurosci. 2013;33(22):9402–9407. doi: 10.1523/JNEUROSCI.3389-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Solages C, et al. High-frequency organization and synchrony of activity in the purkinje cell layer of the cerebellum. Neuron. 2008;58(5):775–788. doi: 10.1016/j.neuron.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Wise AK, Cerminara NL, Marple-Horvat DE, Apps R. Mechanisms of synchronous activity in cerebellar Purkinje cells. J Physiol. 2010;588(13):2373–90. doi: 10.1113/jphysiol.2010.189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2011;481(7382):502–5. doi: 10.1038/nature10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uusisaari M, Obata K, Knöpfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol. 2007;97(1):901–11. doi: 10.1152/jn.00974.2006. [DOI] [PubMed] [Google Scholar]
- 14.Uusisaari M, Knöpfel T. Functional classification of neurons in the mouse lateral cerebellar nuclei. Cerebellum. 2011;10(4):637–46. doi: 10.1007/s12311-010-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albert, M. et al. Synaptic Pathology in the Cerebellar Dentate Nucleus in Chronic Multiple Sclerosis. Brain Pathol. 1015–6305 (2016). [DOI] [PMC free article] [PubMed]
- 16.Mandolesi G, et al. Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nat Rev Neurol. 2015;11(12):711–24. doi: 10.1038/nrneurol.2015.222. [DOI] [PubMed] [Google Scholar]
- 17.Centonze D, et al. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci. 2009;29(11):3442–52. doi: 10.1523/JNEUROSCI.5804-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwiecien JM, et al. Morphological and morphometric studies of the dysmyelinating mutant, the Long Evans shaker rat. J Neurocytol. 1998;27(8):581–91. doi: 10.1023/A:1006922227791. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor LT, et al. Insertion of a retrotransposon in Mbp disrupts mRNA splicing and myelination in a new mutant rat. J Neurosci. 1999;19:3404–3413. doi: 10.1523/JNEUROSCI.19-09-03404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CM, Cooksey E, Duncan ID. Myelin loss does not lead to axonal degeneration in a long-lived model of chronic demyelination. J Neurosci. 2013;33:2718–2727. doi: 10.1523/JNEUROSCI.4627-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berret E, Kim SE, Lee SY, Kushmerick C, Kim JH. Functional and structural properties of ion channels at the nerve terminal depends on compact myelin. J Physiol. 2016;594(19):5593–609. doi: 10.1113/JP272205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhat MA, et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/S0896-6273(01)00294-X. [DOI] [PubMed] [Google Scholar]
- 23.Ljungberg L, et al. Transient Developmental Purkinje Cell Axonal Torpedoes in Healthy and Ataxic Mouse Cerebellum. Front Cell Neurosci. 2016;10:248. doi: 10.3389/fncel.2016.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauck V, Jaeger D. The Control of Rate and Timing of Spikes in the Deep Cerebellar Nuclei by Inhibition. J Neurosci. 2000;20(8):3006–3016. doi: 10.1523/JNEUROSCI.20-08-03006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng N, Raman IM. Synaptic Inhibition, Excitation, and Plasticity in Neurons of the Cerebellar Nuclei. Cerebellum. 2009;9(1):56–66. doi: 10.1007/s12311-009-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palay, S. L. & Chan-Palay, V. Cerebellar Cortex: Cytology and Organization. Springer, New York (1974).
- 27.Bekkers JM, Richerson GB, Stevens CF. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc Natl Acad Sci USA. 1990;87:5359–5362. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16(6):1197–207. doi: 10.1016/S0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 29.Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3(2):141–50. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- 30.Seidl AH. Regulation of conduction time along axons. Neuroscience. 2014;276:126–34. doi: 10.1016/j.neuroscience.2013.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arancibia-Cárcamo, I. L. et al. Node of Ranvier length as a potential regulator of myelinated axon conduction speed. Elife. 6 (2017). [DOI] [PMC free article] [PubMed]
- 32.Hamada MS, Kole MH. Myelin loss and axonal ion channel adaptations associated with gray matter neuronal hyperexcitability. J Neurosci. 2015;35(18):7272–86. doi: 10.1523/JNEUROSCI.4747-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tasaki I. The electro-saltatory transmission of the nerve impulse and the effect of narcosis upon the nerve fiber. Am J Physiol. 1939;127:211–227. [Google Scholar]
- 34.Vabnick I, Shrager P. Ion channel redistribution and function during development of the myelinated axon. J Neurobiol. 1998;37(1):80–96. doi: 10.1002/(SICI)1097-4695(199810)37:1<80::AID-NEU7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 35.Khaliq ZM, Raman IM. Relative contributions of axonal and somatic Na channels to action potential initiation in cerebellar Purkinje neurons. J Neurosci. 2006;26(7):1935–1944. doi: 10.1523/JNEUROSCI.4664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purves D, Lichtman JW. Elimination of synapses in the developing nervous system. Science. 1980;210(4466):153–7. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- 37.Zhao S, et al. Structural plasticity of spines at giant mossy fiber synapses. Front Neural Circuits. 2012;6:103. doi: 10.3389/fncir.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lohof AM, Delhaye-Bouchard N, Mariani J. Synapse Elimination in the Central Nervous System: Functional Significance and Cellular Mechanisms. Rev Neurosci. 1996;7:85–101. doi: 10.1515/REVNEURO.1996.7.2.85. [DOI] [PubMed] [Google Scholar]
- 39.Nelson PG. Activity-Dependent Synapse Modulation and the Pathogenesis of Alzheimer Disease. Curr Alzheimer Res. 2005;2(5):497–506. doi: 10.2174/156720505774932232. [DOI] [PubMed] [Google Scholar]
- 40.Sallert M, et al. Brain-derived neurotrophic factor controls activity-dependent maturation of CA1 synapses by downregulating tonic activation of presynaptic kainate receptors. J Neurosci. 2009;29(36):11294–303. doi: 10.1523/JNEUROSCI.0560-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolakopoulou AM, Meynard MM, Marshak S, Cohen-Cory S. Synaptic maturation of the Xenopus retinotectal system: effects of brain-derived neurotrophic factor on synapse ultrastructure. J Comp Neurol. 2010;518(7):972–89. doi: 10.1002/cne.22258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byravan S, Foster LM, Phan T, Verity AN, Campagnoni AT. Murine oligodendroglial cells express nerve growth factor. Proc Natl Acad Sci USA. 1994;91(19):8812–8816. doi: 10.1073/pnas.91.19.8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai X, Qu P, Dreyfus CF. Neuronal signals regulate neurotrophin expression in oligodendrocytes of the basal forebrain. Glia. 2001;34(3):234–239. doi: 10.1002/glia.1057. [DOI] [PubMed] [Google Scholar]
- 44.Dai X, et al. The trophic role of oligodendrocytes in the basal forebrain. J Neurosci. 2003;23(13):5846–5853. doi: 10.1523/JNEUROSCI.23-13-05846.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagayogo IP, Dreyfus CF. Regulated release of BDNF by cortical oligodendrocytes is mediated through metabotropic glutamate receptors and the PLC pathway. ASN Neuro. 2009;1(1):e00001. doi: 10.1042/AN20090006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delaney KH, et al. Familial dysmyelination in a Long Evans rat mutant. Lab Anim Sci. 1995;45(5):547–553. [PubMed] [Google Scholar]
- 47.Kwiecien JM. Cellular compensatory mechanisms in the CNS of dysmyelinated rats. Comp Med. 2010;60(3):205–17. [PMC free article] [PubMed] [Google Scholar]
- 48.Saifetiarova J, Taylor AM, Bhat MA. Early and Late Loss of the Cytoskeletal Scaffolding Protein, Ankyrin G Reveals Its Role in Maturation and Maintenance of Nodes of Ranvier in Myelinated Axons. J Neurosci. 2017;37(10):2524–2538. doi: 10.1523/JNEUROSCI.2661-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickinson-Nelson A, Reese TS. Structural changes during transmitter release at synapses in the frog sympathetic ganglion. J Neurosci. 1983;3(1):42–52. doi: 10.1523/JNEUROSCI.03-01-00042.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21(12):4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data generated or analyzed in this study are available within the article. The data that support the findings of this study are available from the corresponding author upon reasonable request.