Graphical abstract
Keywords: AChE, Behavioural pattern, Channa punctatus, Deltamethrin, LC50, Triazophos
Highlights
-
•
The pesticides have adverse effect on the health of aquatic biota including fishes.
-
•
Comparative acute toxicity of both pesticides was determined in the present study.
-
•
Both pesticides have affected the behavioural activities of C. punctatus.
-
•
Alteration of behavioural patterns may be due to strong inhibition of AChE activity.
-
•
Triazophos (organophosphate) is more neurotoxic than deltamethrin (pyrethroid).
Abstract
Pesticides are applied to control the pests indoor and outdoor; however, their remarkable amount reaches to the aquatic system through various routes like run-off, leaching, spray-drift, effluent from factories. These are reported to have negative metabolic impact on different non-target aquatic organisms like fishes. Thus, present study is aimed to evaluate the acute toxicity of two groups of pesticides, organophosphate and pyrethroid, namely triazophos and deltamethrin, respectively. The test was conducted for 96 h period in a freshwater teleost, Channa punctatus. The LC50 values for triazophos and deltamethrin after 96 h treatment was found to be 0.069 mg/L and 7.33 μg/L. The deltamethrin was found to be about ten times more toxic than triazophos to the fish. In treated fish, alterations in various behavioural patterns were observed with increasing concentrations of both the pesticides as compared to control. Further, tissue specific as well as dose dependent inhibition in the acetylcholinesterase (AChE, EC 3.1.1.7) activity was found in brain, muscle and gills in Channa punctatus exposed to both the insecticides. However, the effect was more pronounced in triazophos treated fishes than the deltamethrin. A futuristic approach on biochemical and molecular studies may throw light on the mechanism of action of these pesticides.
1. Introduction
The chemical pesticide formulations employed to agricultural land very often contaminate aquatic habitat which in turn causes detrimental effects to the aquatic biota particularly to the economically important non-target organisms i.e. fishes [1].
Among different classes of pesticide, organophosphates as well as pyrethroids are more frequently used because of their high insecticidal property, low mammalian toxicity, less persistence and rapid biodegradability in the environment. These compounds are used extensively in agriculture supposed to bioaccumulate in humans and exhibit relatively low level of toxicity in mammals [2]. Fishes exhibit different kinds of behaviours such as schooling where fishes form groups; surfacing i.e. frequent movement to water surface; hanging due to loss of balance, opercular movement rate and convulsions [[3], [4]]. Such behaviours are subjected to olfaction and visual stimuli [5]. Pesticide exposure affects the fish behaviour in diversified manners [[6], [7], [8]].
High toxicity of synthetic pesticides has been found to aquatic, zooplankton and mammalian species [9]. Present study reflects two group of pesticides namely an organophosphate, triazophos (O,O-Diethyl O-(1-phenyl-1H-1,2,4-triazol- 3yl – phosphorothioate; PubChem CID: 32184) which is widely used insecticide to control the pests on the paddy and on cotton fields due to its low toxicity to mammals (human) [10]. Other pesticide is pyrethroid, deltamethrin [(S)-cyano-(3-phenoxyphenyl) methyl] 3-(2, 2-dibromoethenyl)-2,2-dimethylcyclopropane-1-carboxylate; PubChem CID: 40585) which is extensively used in agriculture and forestry because of its high activity against a broad spectrum of insect pests [11]. It is also used as an alternative pesticide in malaria control programs in India and other developing countries [12]. Deltamethrin is known to be toxic to fish and various other aquatic organisms [13].
Acetylcholinesterase (AChE, EC 3.1.1.7) activity plays role as a biomarker upon the exposure to organophosphate and pyrethroid insecticides [[14], [15]]. As there is an increase in newly discovered potential pesticides day by day and also variations in their impact on various aquatic genera and species, thus it is relevant to study the impact of organophosphate and pyrethroid on fish, C. punctatus which may also act as a bioindicator of pollution caused by pesticides in water. Further, Channa punctatus is regarded as one of the most important teleost in India being the rich source of nutritive materials available in abundance and thus affordable to the low income group of people. Besides this, it also has therapeutic use. The exposure of pesticides may cause the mortality and reduction in its population.
Therefore, the aim of present study is to evaluate the comparative impact of acute toxicity of formula grade pesticides, namely triazophos (OP) and deltamethrin (pyrethroid) on AChE activity in a freshwater teleost, Channa punctatus.
2. Materials and methods
2.1. Animals and maintenance
Healthy fresh water teleosts, Channa punctatus (average length 11 ± 2 cm and weight 23 ± 2 g) were obtained from fish market and treated with potassium permanganate (PubChem CID: 516875) solution (0.5% w/v) for 1 min to remove any dermal adherent. Laboratory stock of fish was maintained in glass aquarium containing dechlorinated aerated tap water (capacity: 50 l). Quality of water was assessed before exposure and was tested according to APHA guidelines [16]. Fishes were acclimatized for one week at room temperature (26 ± 1.2 °C) and were fed with commercially available pellet fish food (Tokyu, Japan) ad libitum. During the entire experimental period the dissolved oxygen concentration (DOC) in control and experimental aquaria was between 60% and 70%.
2.2. Chemicals
Two formula grade pesticides namely Sutathion (40% EC Triazophos, Pune, Maharashtra, India) and Decis (2.8% EC Deltamethrin, Bayer crop science limited, Powai, Mumbai, India) were used for acute and sub-acute toxicity studies. All of the chemicals used in the present study were of analytical grades and were procured from Sigma-Aldrich (St. Louis, USA) and Merck (Germany).
2.3. Determination of 96 h LC50
Feeding was stopped 24 h prior to the initiation of experiment. Ten fishes were placed in each of the eight glass aquaria containing 12L water (n = 10/glass aquarium). After that, triazophos was added as per the following concentrations 0.03, 0.05, 0.065, 0.075, 0.085 and 0.10 mg/L. At an interval of 24 h, the water of aquaria was changed and different concentrations of triazophos were restored afresh as mentioned above. The stock solution of triazophos used in this study was always freshly prepared when needed. The fish kept into the pesticide free medium served as a control. Equal volume of acetone (PubChem CID: 180) was maintained in the control aquarium, as the pesticides (triazophos and deltamethrin) used were solubilised in acetone. Also, ten fishes were placed in each eight glass aquaria containing 12L water. After that, the same procedure was carried out for deltamethrin exposure with different concentrations such as 3.75, 4.58, 5.42, 6.25, 7.08, 7.92, 8.75 and 9.58 μg/L.
The test was performed for 96 h treatment period and dead fishes were removed as the test proceeded. The number of dead fish per group was recorded against the time of their death in a tabular form as specified by Sprague [17]. The 96 h LC50 value of triazophos and deltamethrin was calculated by using software US EPA (version 1.5; 1US EPA (version 1.5; 1993). The behavioural pattern of fish was monitored regularly under above treatment conditions as suggested by Kumari et al. [18].
2.3.1. Sub-acute bioassays
2.3.1.1. Biochemical assays
2.3.1.1.1. Preparation of tissue extracts
The fishes were sacrificed by using mild anaesthesia (Trichloromethane; PubChem CID: 6212) after exposure to each of the insecticides (triazophos and deltamethrin) and dissected. Brain, muscle and gills were surgically removed, thoroughly rinsed in cold saline (0.9%), at 4–6 °C and blotted dry. The tissues were weighed and homogenized (10%, w/v) in 50 mM sodium phosphate buffer (pH 8.0) containing 0.1% Triton X-100 (PubChem CID: 5590) using Potter–Elvehjam homogenizer fitted with a Teflon-coated pestle under ice cold condition. The homogenates were kept for 30 min in cold with intermittent stirring and centrifuged at 4 °C for 30 min at 10,000g in a refrigerated centrifuge (Model- 3K30 Sigma, St. Louis, USA).
2.3.1.1.2. Assay of acetylcholinesterase
AChE activity was determined according to Ellman et al. [19]. Acetylcholinesterase (AChE) efficiently catalyses the hydrolysis of acetylthiocholine (ATCh) (sulphur analog of natural substrate Acetylcholine, ACh). The surface protein AChE is required to dissolve in solution with the help of non-ionic detergent, Triton-X 100. After centrifugation, supernatant contain soluble AChE. AChE catalyses the hydrolysis of ATCh into thiocholine and acetate. The thiocholine reacts with an oxidising agent, 5,5-dithiobis-(2-nitrobenzoic acid) to form two products, one of which is 5-thio 2-nitrobenzoic acid or nitrobenzoate (PubChem CID: 123648; unstable intermediate) which absorbs the UV radiation at 412 nm. The activity of enzyme can thus be measured by following the increase in absorbance at 412 nm in a double beam UV–vis spectrophotometer. The reaction mixture (3 ml) contained 1.5 ml of 100 mM sodium phosphate buffer (pH 8.0), 0.3 ml of 5 mM DTNB [5,50-dithiobis-(nitrobenzoic acid); PubChem CID: 6254] prepared in 10 mM sodium phosphate buffer, pH 7.5 containing 15 mg sodium bicarbonate (PubChem CID: 516892) added per 10 ml of solution], 0.3 ml of 5 mM acetylthiocholine iodide (ATI; PubChem CID: 74629), 0.1 ml of 10% homogenate, and 0.8 ml of distilled water. The increase in absorbance was monitored at 412 nm for 3 min in a UV–visible double beam spectrophotometer (Model: UV1800, SL-02480, Shimadzu, Japan) with quartz cuvettes against distilled water as blank. Measurements were made in triplicate for each tissue homogenate. Simultaneously, two blanks were also used. One (enzyme blank) contained phosphate buffer, DTNB, and ATI but not the enzyme to determine the spontaneous hydrolysis of ATI, and the second (substrate blank) contained phosphate buffer, DTNB, and enzyme protein but no substrate (ATI) to correct for any non- AChE-dependent formation of thionitrobenzoic acid (TNB; PubChem CID: 123648). One unit of AChE activity was expressed as nanomoles of substrate (ATI) hydrolyzed/min/mg protein under experimental conditions.
2.3.1.1.3. Determination of total protein
Protein contents in fish tissue homogenates were determined according to the method of Lowry et al. [20], using bovine serum albumin (BSA) as standard.
2.4. Statistical analyses
LC50 and 95% confidence limits were calculated by a computer programme [21]. The data of AChE activity was represented as mean ± SEM and was analysed by One Way Analysis of Variance (One Way ANOVA) and was further analysed by Duncan’s Multiple Range Post-hoc Test. The results were considered to be significant at P ≤ 0.05 and P ≤ 0.01 levels. All of the statistical analyses were done in accordance to Bruning and Knitz [22] and were carried out in three replicates.
3. Results and discussion
3.1. Determination of LC50 values for triazophos and deltamethrin
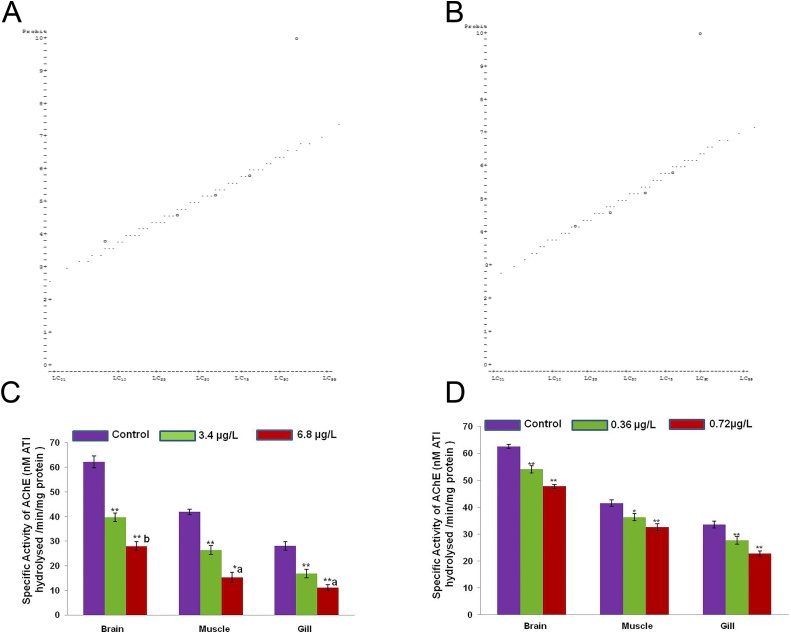
The fish in the control aquarium were observed to be healthy and normal and no mortality was recorded in it. In triazophos treated group, there was no mortality recorded at 0.03 mg/L concentration after 96 h exposure. However, at 0.05, 0.065, 0.075, 0.085 and 0.1 mg/L concentrations, the% mortality recorded were 20%, 40%, 60%, 80% and 100%, respectively. The LC50 of triazophos, after 96 h treatment was found to be 0.069 mg/L for Channa punctatus following probit analysis (Table 1, Fig. 1A). Similarly, fishes were exposed to different concentrations of deltamethrin for 96 h to observe mortality rate. After 96 h treatment, deltamethrin caused 10%, 20%, 30%, 40%, 60%, 80% and 100% mortality at concentrations of 4.58, 5.42, 6.25, 7.08, 7.92, 8.75 and 9.58 μg/L respectively. The LC50 for deltamethrin was calculated and found to be 7.33 μg/L (Table 2, Fig. 1B). From this study, it can be inferred that under identical condition and treatment duration the deltamethrin was about ten times more toxic than triazophos to C. punctatus.
Table 1.
Lethal concentrations (LC1–99) of triazophos for freshwater fish, Channa punctatus (average length 11 ± 2 cm and weight 23 ± 2 g, n = 10).
| Lethal Concentrations | Triazophos(10-3 g/L) | 95% confidence limits |
|
|---|---|---|---|
| Lower | Upper | ||
| LC1 | 0.041 | 0.016 | 0.052 |
| LC5 | 0.048 | 0.024 | 0.058 |
| LC10 | 0.052 | 0.029 | 0.061 |
| LC15 | 0.055 | 0.033 | 0.063 |
| LC50 | 0.069 | 0.056 | 0.077 |
| LC85 | 0.088 | 0.078 | 0.116 |
| LC90 | 0.093 | 0.082 | 0.131 |
| LC95 | 0.101 | 0.088 | 0.159 |
| LC99 | 0.118 | 0.098 | 0.232 |
| Slope ± SEM | 10.07 ± 3.06 | ||
| Intercept ± SEM | 16.68 ± 3.47 | ||
| χ2 value | 1.80 | ||
| p | <0.05 | ||
Control group (theoretical spontaneous response rate) = 0.000.
Fig. 1.
A) Plot of adjusted probits and predicted regression line for Triazophos to a freshwater fish Channa punctatus. B) Plot of adjusted probits and predicted regression line for Deltamethrin to a freshwater fish Channa punctatus. C) Specific activity of AChE (nM ATI hydrolysed/min/mg protein) in brain, muscle and gills of C. punctatus exposed by triazophos. Data represents mean ± SEM. * p < 0.05; ** p < 0.01; Control vs 3.4 μg/L and 6.8 μg/L. a p < 0.05; b p < 0.01; 3. 4 μg/L vs 6.8 μg/L. D) Specific activity of AChE (nM ATI hydrolysed/min/mg protein) in brain, muscle and gills of C. punctatus exposed by deltamethrin. Data represents mean ± SEM.* p < 0.05; ** p < 0.01; Control vs 0.36 μg/L and 0.72 μg/L.
Table 2.
Lethal concentrations (LC1–99) of deltamethrin for freshwater fish, Channa punctatus (average length 11 ± 2 cm and weight 23 ± 2 g, n = 10).
| Lethal Concentrations | Deltamethrin (10-6g/L) | 95% confidence limits |
|
|---|---|---|---|
| Lower | Upper | ||
| LC1 | 4.620 | 2.354 | 5.644 |
| LC5 | 5.289 | 3.184 | 6.182 |
| LC10 | 5.684 | 3.736 | 6.498 |
| LC15 | 5.967 | 4.156 | 6.727 |
| LC50 | 7.329 | 6.345 | 8.013 |
| LC85 | 9.001 | 8.206 | 11.265 |
| LC90 | 9.450 | 8.536 | 12.474 |
| LC95 | 10.156 | 9.009 | 14.575 |
| LC99 | 11.625 | 9.902 | 19.646 |
| Slope ± SEM | 11.61 ± 3.31 | ||
| Intercept ± SEM | −5.04 ± 2.92 | ||
| χ2 value | 2.72 | ||
| p | <0.05 | ||
Control group (theoretical spontaneous response rate) = 0.000.
Acute toxicity assessment for triazophos and deltamethrin in different species of fish has been done earlier by several workers. The LC50 of triazophos for 96 h treatment period in different fish species are as follows: 0.008 mg/L for Peseudorasobora parva, 0.035 mg/L for Tilapia nilotica, 0.060 mg/L for Colossoma brachypomum [23] and1.05 mg/L for Cirrhinus mrigala [24]. The LC50 value of deltamethrin for 96 h treatment period in different fish species have been reported as follows: 2.6 μg/L for Brycon amazonicus [25], 4.7 μg/L for Salmo trutta fario (Brown trout) [26] and 15.47 μg/L for O. niloticus [27].
There are reports suggesting that the temperature of the aquatic system plays an important role in the intensity of toxicity of pyrethroids. A negative correlation has been observed between temperature and acute toxicity of synthetic pyrethroids [28]. In another report it has been indicated that there may be an increase in the toxic impact of pyrethroids on reproduction during spawning season in the cold aquatic environment [29] (Table 1, Table 2).
3.2. Behavioural response and AChE activity in C. punctatus upon triazophos and deltamethrin treatment
Organophosphates (OPs) are substrate analogues of Acetylcholine (ACh) and bind to the active site irreversibly [[30], [31]] thus preventing hydrolysis of neurotransmitter, Acetylcholine [32]. Due to the accumulation of acetylcholine, an uninterrupted signal thus generated results into various altered behavioural pattern.
Pyrethroids are axonic poisons which bind to the voltage gated sodium channel in nerve membrane that affect the nerve fiber through opening and closing of the voltage gated sodium channel [33]. Pyrethroids bind to this gate and prevent it from closing normally, which results in continuous nerve stimulation and tremors in poisoned organisms, resulting uncoordinated movement and altered behavioural pattern such as convulsion, surfacing, opercular movement [34].
The behavioural response of triazophos and deltamethrin started appearing only after 3 h of treatment. The alterations in the behaviour were observed to different degrees with varying concentrations of these pesticides as compared to control fish (Table 3). The effects of pesticides were found to be in dose and duration dependent manner and these effects may due to AChE activity which is effective both at neural and neuromotor junctions present in muscles. Upon triazophos treatment, we noted decrease in AChE activities in brain (36.11% at 5% of LC50 and 55.08% at 10% of LC50 doses; in muscles 36.98% at 5% of LC50 and 63.60% at 10% of LC50 doses and in gills 40.26% at 5% of LC50 and 60.34% at 10% of LC50 doses as compared to control; Fig. 1C). Similarly, upon deltamethrin treatment, we noted decrease in AChE activities in brain (13.59% at 5% of LC50 and 18.90% at 10% of LC50 doses as compared to control; Fig. 1D), in muscle (12.6% at 5% of LC50 and 21.65% at 10% of LC50 doses as compared to control; Fig. 1D) and in gills (17.56% at 5% of LC50 and 32.4% at 10% of LC50 doses as compared to control; Fig. 1D). Thus, it is evident that the alterations in the behavioural patterns were more pronounced with triazophos than deltamethrin at different concentrations which are in coherence with the observations of other workers [35]. Most of the organophosphates including triazophos inhibit AChE activity in particular in cholinergic neurons [36]. The behavioural changes like increased opercular movements, hyper-activity of all fins, increased rate of swimming, loss of balance etc. observed in the pesticide (triazophos and deltamethrin) exposed fishes are probably due to caudal bending which was noted during entire exposure period being higher in higher pesticide concentration exposure group. This in turn affected the normal swimming pattern of the fish. Caudal bending, a kind of paralysis might have induced imbalance in swimming pattern leading in turn to surfacing behaviour. All these behavioural alterations may be the result of inhibition of neuromuscular AChE activity and finally blocking the neural transmissions. The anomalies in fish behaviour recorded in present study may be due to the inhibitory action of deltamethrin and triazophos on AChE and subsequent amassing of ACh at the nerve endings. Further inhibition of AChE activity resulted in a progressive accumulation of ACh, especially during periods of repetitive stimulation, leading to desensitization of nAChRs (nicotinic acetylcholine receptors) and consequently muscular weakness [[37], [38]]. Similarly, Balint et al [39] reported 21.4% decrease in acetylcholinesterase activity in serum of carp after three days exposure to deltamethrin but, our results are in agreement with several other researchers [[40], [41], [42], [43]]. Additionally, the toxic impact of pesticides can also be reversed as suggested by Rezakhalatbary et al. [44] where the olive oil can ameliorate the deltamethrin induced nephrotoxicity in albino mouse.
Table 3.
Impact of triazophos and deltamethrin on the behavioural pattern of Channa punctatus (average length 11 ± 2 cm and weight 23 ± 2 g, n = 10) exposed to the pesticides up to 96 h.
| Parameters | Control | Triazophos (10-6 g/L) | Deltamethrin (10-6 g/L) | ||
|---|---|---|---|---|---|
| 3.4 | 6.8 | 0.36 | 0.72 | ||
| Colour of skin | – | + | ++ | ++ | +++ |
| Loss of balance | – | ++ | +++ | + | ++ |
| Hyperactivity | – | ++ | +++ | – | + |
| Rate of swimming | – | + | ++ | + | + |
| Surfacing activity | + | ++ | +++ | – | + |
| Rate of opercular activity | + | + | ++ | + | + |
| Convulsions | – | + | ++ | – | – |
The increase or decrease in the level of behavioural parameters is shown by numbers of (+) sign. The (−) sign indicate normal behavioural conditions.
4. Conclusion
Present study plays an important role in pesticide risk assessment. Fishes serve as a key indicator of environmental toxicity. The toxicity effect of deltamethrin to Channa punctatus is high even at very low concentration. The results may help to understand the toxicity of the pesticide in the field and may work as pre- alarming indicators of pesticide toxicity in the freshwater fish, Channa punctatus.
Acknowledgements
Authors express their gratitude to the Head of the Department of Zoology, for providing Central Instrumental facility developed with the financial assistance from Department of Science and Technology-Fund for Improvement of Science and Technology Infrastructure (DST-FIST), New Delhi, India and University Grants Commission-Special Assistance Programme (UGC-SAP), New Delhi, India Phase I & II for carrying out this work. Financial assistance to the authors (SS and RKT) from UGC is gratefully acknowledged.
References
- 1.Tripathi G., Harsh S.J. Fenvalerate–induced macromolecular changes in the catfish Clarias batrachus. J. Environ. Biol. 2002;23:143–146. [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva Switzerland: 1992. Alpha-cypermethrin. Environmental Health Criteria 142. [Google Scholar]
- 3.Moyle P.B., Cech J.J. fourth ed. Prentice Hall, Upper Saddle Ridge; NJ, USA: 2000. Fishes: An Introduction to Ichthyology. [Google Scholar]
- 4.Kasumyan A.O. Effects of chemical pollutants on foraging behavior and sensitivity of fish to food stimuli. J. Ichthyol. 2001;41:76–87. [Google Scholar]
- 5.Chivers D.P., Smith R.J.F. Chemical alarm signalling in aquatic predator–prey systems: a review and prospectus. Ecoscience. 1998;5:338–352. [Google Scholar]
- 6.Zhou T., Weis J.S. Swimming behavior and predator avoidance in three populations of Fundulus heteroclitus larvae after embryonic and/or larval exposure to methylmercury. Aquat. Toxicol. 1998;43:131–148. [Google Scholar]
- 7.Zhou T., Weis J.S. Predator avoidance in mummichog larvae from a polluted habitat. J. Fish Biol. 1999;54:44–57. [Google Scholar]
- 8.Little E.E., Finger S.E. Swimming behavior as an indicator of sublethal toxicity in fish. Environ. Toxicol. Chem. 1990;9:13–19. [Google Scholar]
- 9.Mossa A.T.H., Swelam E.S., Mohafrash S.M.M. Sub-chronic exposure to fipronil induced oxidative stress, biochemical and histopathological changes in the liver and kidney of male albino rats. Toxicol. Rep. 2015;2:775-784. doi: 10.1016/j.toxrep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naveed A., Janaiah C. Effect of triazophos on protein metabolism in the fish, Channa punctatus (Bloch) Curr. Res. J. Biol. Sci. 2011;3:124–128. [Google Scholar]
- 11.Villarini M., Moretti M., Pasquini R., Scassellati-Sforzolini G., Fatigoni C., Marcarelli M., Monarca S., Rodriguez A.V. In vitro genotoxic effects of the insecticide deltamethrin in human peripheral blood leukocytes: DNA damage (‘comet’ assay) in relation to the induction of sister-chromatid exchanges and micronuclei. Toxicology. 1998;130:129–139. doi: 10.1016/s0300-483x(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 12.Yadav R.S., Sampath R.R., Sharma V.P. Deltamethrin treated bednets for control of malaria transmitted by Anopheles culicifacies (Diptera: culicidae) in India. J. Med. Entomol. 2001;38:613–622. doi: 10.1603/0022-2585-38.5.613. [DOI] [PubMed] [Google Scholar]
- 13.Mittal P.K., Adak T., Sharma V.P. Comparative toxicity of certain mosquitocidal compounds to larvivorous fish, Poecilia reticulata. Indian J. Malariol. 1994;31:43–47. [PubMed] [Google Scholar]
- 14.Grue C.E., Gilbert P.L., Seeley M.E. Neurophysiological and behavioural changes in non-target wildlife exposed to organophosphate and carbamate pesticide: thermoregulation, food consumption and reproduction. Am. Zool. 1997;37:369–388. [Google Scholar]
- 15.Dobsikova R., Velisek J., Wlasow T., Gomulka P., Svobodova Z., Novotny L. Effects of cypermethrin on some haematological, biochemical and histopathological parameters of common carp (Cyprinus carpio) Neuroendocrinol. Lett. 2006;27:91–95. [PubMed] [Google Scholar]
- 16.American Public Health Association (APHA) 16th edn. 1985. Standard Methods for the Examination of Water and Wastewater; p. 1268. Washington, DC. [Google Scholar]
- 17.Sprague J.B. Measurement of pollutant toxicity to fish: i. Bioassay methods for acute toxicity. Water Res. 1969;3:793–821. [Google Scholar]
- 18.Kumari R., Singh R.K., Khanna Y.P., Sharma B. Carbofuran induced stress mediated syndromes in Clarias batrachus. Proceedings of International Conference on Industrial Pollution Control Technology. 1997:113–119. [Google Scholar]
- 19.Ellman G.L., Courtney K.D., Jr., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–90. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 20.Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951;193:265-275. [PubMed] [Google Scholar]
- 21.US EPA . U.S. Environmental Protection Agency; Cincinnati: 1993. LC50 Software Program, Version 1.5 Ecological Monitoring Research Division, Environmental Monitoring Systems Laboratory. EPA/600/4-90/027F. [Google Scholar]
- 22.Bruning J.L., Knitz B.L. second edition. Scott, Foresman and Company; USA; Illinois: 1977. Computational Handbook of Statistics. [Google Scholar]
- 23.Shaonan Li. Acute toxicity of isofenophos-methyl and triazophos to 3 kinds of freshwater. Environ. Pollut. Control. 1998;20:15–16. [Google Scholar]
- 24.Mahboob S., Ghazala Al-Ghanim K.A., Sultana S., Al-Balawi H.F.A., Sultana T., Al-Misned F., Ahmed Z. A study on acute toxicity of triazophos, profenofos, carbofuran and carbaryl pesticides on Cirrhinus mrigala. Pakistan J. Zool. 2015;47:461-466. [Google Scholar]
- 25.Moraes de F.D., Venturini F.P., Cortella L.R.X., Rossi P.A., Moraes G. Acute toxicity of pyrethroid-based insecticides in the Neotropical freshwater fish Brycon amazonicus. Ecotoxicol. Environ. Contam. 2013;8:59–64. [Google Scholar]
- 26.Karatas T. Effects of deltamethrin on some haematological parameters of brown trout (Salmo trutta fario) Indian J. Anim. Res. 2016;50:89–92. [Google Scholar]
- 27.Boateng J.O., Nunoo F.K.E., Dankwa H.R., Ocran M.H. Acute toxic effects of deltamethrin on tilapia, Oreochromis niloticus (Linnaeus, 1758) West Afr. J. Appl. Ecol. 2006;9:1–5. [Google Scholar]
- 28.Kumaragura A.K., Beamish F.W.H. Lethal toxicity of permethrin (NRDC-143) to rainbow trout Salmo gairdneri, in relation to body weight and temperature. Water Res. 1981;15:503–505. [Google Scholar]
- 29.Moore A., Waring C.P. The effect of a synthetic pyrethroid pesticide on some aspects of reproduction in atlantic salmon (Salmo salar L.) Aquatic Toxicol. 2001;52:1–12. doi: 10.1016/s0166-445x(00)00133-8. [DOI] [PubMed] [Google Scholar]
- 30.Aldridge W.N., Reiner E. Acylated amino acids in inhibited B-esterases. In: Neuberger A., Tatum E.L., editors. Enzyme Inhibitors as Substrates. North-Holland Publishing Company; Amsterdam: 1972. pp. 170–175. [Google Scholar]
- 31.World Health Organization . Organophosphorus Insecticides: A General Introduction. 1986. Metabolism and mode of action; pp. 39–48. Geneva. [Google Scholar]
- 32.Boublik Y., Saint-Aguet P., Lougarre A., Arnaud M., Villatte F. Acetylcholinesterase engineering for detection of insecticide residues. Protein Eng. Des. Selet. 2002;15:43–50. doi: 10.1093/protein/15.1.43. [DOI] [PubMed] [Google Scholar]
- 33.Shafer T.J., Meyer D.A. Effects of pyrethroids on voltage/sensitive calcium channels: a critical evaluation of strengths, weaknesses, data needs, an relationship to assessment of cumulative neurotoxicity. Toxicol. Appl. Pharmacol. 2004;196:303–318. doi: 10.1016/j.taap.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 34.He F. Synthetic pyrethroids. Chapter in a book. Toxicology. 1994;91:43–49. doi: 10.1016/0300-483x(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 35.Baser S., Erkoc F., Selvi M., Kocak O. Investigation of acute toxicity of permethrin on guppies Poecilia reticulate. Chemosphere. 2003;5:469–474. doi: 10.1016/S0045-6535(03)00033-X. [DOI] [PubMed] [Google Scholar]
- 36.Cavanagh J.B. Peripheral neuropathy caused by chemical agents. CRC J. Crit. Rev. Toxicol. 1973;2:365–417. doi: 10.3109/10408447309082021. [DOI] [PubMed] [Google Scholar]
- 37.Chebbi S.G., David M. Neurobehavioral responses of the freshwater teleost, Cyprinus carpio (Linnaeus) under quinalphos intoxication. Biotechnol. Anim. Husb. 2009;25:241–249. [Google Scholar]
- 38.David M., Sangeetha J., Harish E.R., Shrinivas J., Naik V.R. Alterations in the levels of ACh and associated AChE in the tissues of fresh water fish Cirrhinus mrigala exposed to deltamethrin. Int. J. Pharm. Biol. Arch. 2013;4:1237–1241. [Google Scholar]
- 39.Balint T., Szegletes T., Szegletes Z., Halasy K., Nemcsok J. Biochemical and subcellular changes in carp exposed to the organophosphorous metidation and the pyrethroid deltamethrin. Aquat. Toxicol. 1995;33:279–295. [Google Scholar]
- 40.Kabeer A.S.I., Ramanarao K.V. Correlation between subacute toxicity of malathion and AChE inhibition in the tissue of the teleost, Tilapia mossambica. Bull. Environ. Contam. Toxicol. 1980;24:711–718. doi: 10.1007/BF01608178. [DOI] [PubMed] [Google Scholar]
- 41.Giniatullin R.A., Magazanik L.G. Desensitization of the postsynaptic membrane of neuromuscular synapses induced by spontaneous quantum secretion of mediator. Neurosci. Behav. Physiol. 1998;28:438–442. doi: 10.1007/BF02464803. [DOI] [PubMed] [Google Scholar]
- 42.Reddy M.P., Philip H.G., Bashamohideen M. Regulation of AChE system of freshwater fish: Cyprinus carpio under fenvalerate toxicity. Bull. Environ. Contam. Toxicol. 1992;48:18–22. doi: 10.1007/BF00197478. [DOI] [PubMed] [Google Scholar]
- 43.Rao J.V., Rani C.H.S., Kavitha P., Rao R.N., Madhavendra S.S. Toxicity of chlorpyrifos to the fish: Oreochromis mossambicus. Bull. Environ. Contam. Toxicol. 2003;70:985–992. doi: 10.1007/s00128-003-0079-0. [DOI] [PubMed] [Google Scholar]
- 44.Rezakhalatbary A., Ahmadvand H., Ghabaee D.N.Z., Malekshah A.K., Navazesh A. Virgin olive oil ameliorates deltamethrin-induced nephrotoxicity in mice: a biochemical and immunohistochemical assessment. Toxicol. Rep. 2016;3:584–590. doi: 10.1016/j.toxrep.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]