Abstract
The genus Ceratostomella has a long history of taxonomic confusion. While species with evanescent asci have been transferred to the Microascales and Ophiostomatales, the taxonomic status of species with persistent asci has not been completely resolved. In previous studies using DNA sequence data, cultures and morphology, several Ceratostomella spp. were allocated in 13 genera in the Eurotiomycetes and Sordariomycetes. In our study, the systematics of the remaining Ceratostomella spp. with persistent asci is revisited with new collection data, cultures and phylogeny based on novel DNA sequences from six nuclear loci. Bayesian inference and Maximum Likelihood analyses support the monophyly of several wood-inhabiting species formerly classified in Ceratostomella and other unknown morphologically similar taxa and their division into four genera, i.e. Lentomitella, Spadicoides, Torrentispora and the newly described Calyptosphaeria. This robust clade represents the order Xenospadicoidales in the Sordariomycetidae. Comparative analysis of the ITS2 secondary structure revealed a genetic variation among Lentomitella isolates; 11 species were recognised, of which five are newly introduced and two are new combinations. Other taxonomic novelties include four new species and eight new combinations in Calyptosphaeria, Spadicoides, and Torrentispora. Molecular data suggest that Spadicoides is polyphyletic. The core of the genus is positioned in the Xenospadicoidales; Spadicoides s. str. is experimentally linked with sexual morphs for the first time. Based on DNA sequence data, the monotypic genera Xenospadicoides and Pseudodiplococcium are reduced to synonymy under Spadicoides, while Fusoidispora and Pseudoannulatascus are synonymised with Torrentispora. Members of the Xenospadicoidales inhabit decaying wood in terrestrial and freshwater environments and share a few morphological characters such as the absence of stromatic tissue, ascomata with a cylindrical or rostrate neck, similar anatomies of the ascomatal walls, thin-walled unitunicate asci with a non-amyloid apical annulus, disintegrating paraphyses, usually ellipsoidal to fusiform ascospores and holoblastic-denticulate or tretic conidiogenesis. Revised Ceratostomella spp. with persistent asci are listed and the taxonomic status of each species is re-evaluated based on revision of the holotype and other representative material, published details and available phylogenetic data.
Key words: Ceratostomella, Conidiogenesis, Holoblastic-denticulate, Molecular systematics, New taxa, Phaeoisaria-like, Selenosporella-like, Tretic, Taxonomy, Xenospadicoidales
Taxonomic novelties: New genus: Calyptosphaeria Réblová & A.N. Mill
New species: Calyptosphaeria collapsa Réblová & A.N. Mill., C. tenebrosa Réblová & A.N. Mill., Lentomitella magna Réblová, L. obscura Réblová, L. striatella Réblová, L. sulcata Réblová, L. tenuirostris Réblová, Torrentispora calembola Réblová & A.N. Mill., T. novae-zelandiae Réblová & A.N. Mill
New combinations: Calyptosphaeria subdenudata (Peck) Réblová & A.N. Mill., C. tropica (Huhndorf et al.) Réblová & A.N. Mill., Lentomitella conoidea (Feltg.) Réblová, L. investita (Schw.) Réblová, Spadicoides fuscolutea (Rehm) Réblová, S. hyalostoma (Munk) Réblová, Spadicoides iberica (Hern.-Restr. et al.) Réblová & A.N. Mill., Torrentispora aquatica (Vijaykr. et al.) Réblová & A.N. Mill., T. biatriispora (K.D. Hyde) Réblová & A.N. Mill., T. dubia (Sacc.) Réblová & A.N. Mill
Introduction
The perithecial ascomycete genus Ceratostomella (Saccardo 1878a) has a long history of taxonomic debate. Although the simple generic diagnosis comprised only hyaline, aseptate ascospores, asci and perithecia, which are similar to those of Ceratostoma (Fries 1818), Ceratostomella soon became a large, heterogeneous assemblage of fungi for which Index Fungorum lists 110 epithets. Although widely distributed throughout the Northern Hemisphere, members of Ceratostomella are inconspicuous and difficult to find due to their small immersed to superficial, long-necked ascomata. The asci are persistent or evanescent containing septate or aseptate, hyaline or brown ascospores, and most of the species are difficult to culture.
The homogeneity of Ceratostomella was soon challenged by Kuntze (1898), who transferred 29 species with persistent asci and mostly hyaline ascospores to Amphitrichum (Nees & Nees 1818). Amphitrichum was emended by Corda (1837) based on A. olivaceum (= ? Cladosporium sp. fide Hughes 1958), but later it was determined to be a nomen dubium fide Hughes (1958), as no type specimen was given. Kuntze (1898) clearly misinterpreted the generic concept of Amphitrichum, which is likely a dematiaceous hyphomycete. Another step towards clarification of the concept of Ceratostomella was made by Höhnel (1906a). Lentomitella, originally described as a monotypic genus for Ceratostomella vestita, was introduced in order to segregate taxa with ellipsoidal, 1-septate, hyaline, longitudinally striate ascospores from species with similar ascospores containing more than one septum and 2–4 large drops. Höhnel (1906a) suggested that such taxa should belong to Ceratosphaeria and Lentomita. However, von Arx (1952) did not accept Höhnel's narrow concept and designated Ceratostomella as the correct generic name.
The broadly perceived Ceratostomella was redefined by Réblová (2006) based on the lectotype species, C. rostrata (Clements & Shear 1931), and three other accepted species. Using comparative morphology, Ceratostomella was confined to taxa with non-stromatic ascomata with a cylindrical neck, ascomatal wall up to 100 μm thick, persistent clavate asci arising from supporting ascogenous cells, broad-celled paraphyses and brown, aseptate, ellipsoidal to reniform ascospores. Based on DNA sequence data of two representative species, C. cuspidata and C. pyrenaica, the genus was classified as Sordariomycetidae incertae sedis. Species with evanescent asci and dark perithecia with filiform necks were recombined and placed in Ceratocystis, Huntiella and Thielaviopsis of the Microascales, or Leptographium, Ophiostoma and Pesotum of the Ophiostomatales (Von Höhnel, 1918, Elliott, 1925, Moreau, 1952, Hunt, 1956, De Beer et al., 2013a, De Beer et al., 2013b, De Beer et al., 2014). Based on multigene phylogenetic analyses, the placement of the remaining Ceratostomella spp. with persistent asci was partially resolved resulting in the recovery of three robust phylogenetic lineages centred around the Amplistromatales, Calosphaeriales and Ophiostomatales. The genus Wallrothiella (Saccardo 1882) (Amplistromatales) based on W. congregata [= Ceratostomella sphaerosperma] was redefined with the aid of DNA sequence data, recently collected material and an acrodontium-like asexual morph (Réblová and Seifert, 2004, Huhndorf et al., 2009). Several other Ceratostomella species were reclassified in Jattaea and Togniniella (Calosphaeriales) and Phaeoacremonium (Togniniales) based on the revision of type material, evidence from molecular data and phialophora- or acremonium-like asexual morphs producing phialidic conidia in vitro (Réblová et al., 2004, Réblová et al., 2015a, Réblová, 2011, Gramaje et al., 2015). The “ophiostomataceous” lineage comprised Ceratostomella s. str. and also Barbatosphaeria, Lentomitella, Natantiella, and Xylomelasma (Von Höhnel, 1906a, Réblová, 2006, Réblová, 2007, Huhndorf et al., 2008, Marincowitz et al., 2008, Réblová and Štěpánek, 2009). The asexual morphs linked with genera of this lineage are dematiaceous hyphomycetes with holoblastic conidia produced on a sympodially extending rachis or on a terminal cluster of denticles. They are part of the life cycle of Barbatosphaeria as ramichloridium- and sporothrix-like (Samuels and Candoussau, 1996, Réblová, 2007, Réblová et al., 2015b) and Lentomitella as phaeoisaria-like (Réblová 2006) asexual morphs. Other Ceratostomella spp. were dispersed to Ceratosphaeria (Magnaporthales) (Von Niessl, 1876, Huhndorf et al., 2008), Chaetosphaeria (Chaetosphaeriales) (Booth, 1957, Huhndorf and Fernández, 2005), Daruvedia (Pyrenulales) (Dennis 1988) and Pseudorhynchia (Hypocreales) (Samuels & Barr 1997).
The ongoing taxonomic revision of species of Ceratostomella s. lat. with persistent asci revealed for many of them striking morphological similarities with Lentomitella. Based on nucLSU and nucSSU rDNA sequence data, comparative morphology and cultures, Lentomitella was reinstated in the Sordariomycetidae and shown to be distantly related to Ceratostomella (Réblová 2006). The generic concept of Lentomitella was expanded to include species with 1–3-septate, longitudinally striate, hyaline ascospores, and also aseptate, smooth-walled, hyaline (Réblová 2006) and brown ascospores (Huhndorf et al. 2008). Members of Lentomitella bear a certain resemblance to Torrentispora (Hyde et al. 2000) and Pseudoannulatascus (Luo et al. 2015) characterised by ascomata with a long-neck, cylindrical asci and fusiform, hyaline, smooth- and thick-walled, usually aseptate ascospores, rarely with delayed formation of septa (Hyde et al., 2000, Fryar and Hyde, 2004, Barbosa et al., 2013). A monotypic family, the Lentomitellaceae, was introduced by Zhang et al. (2017).
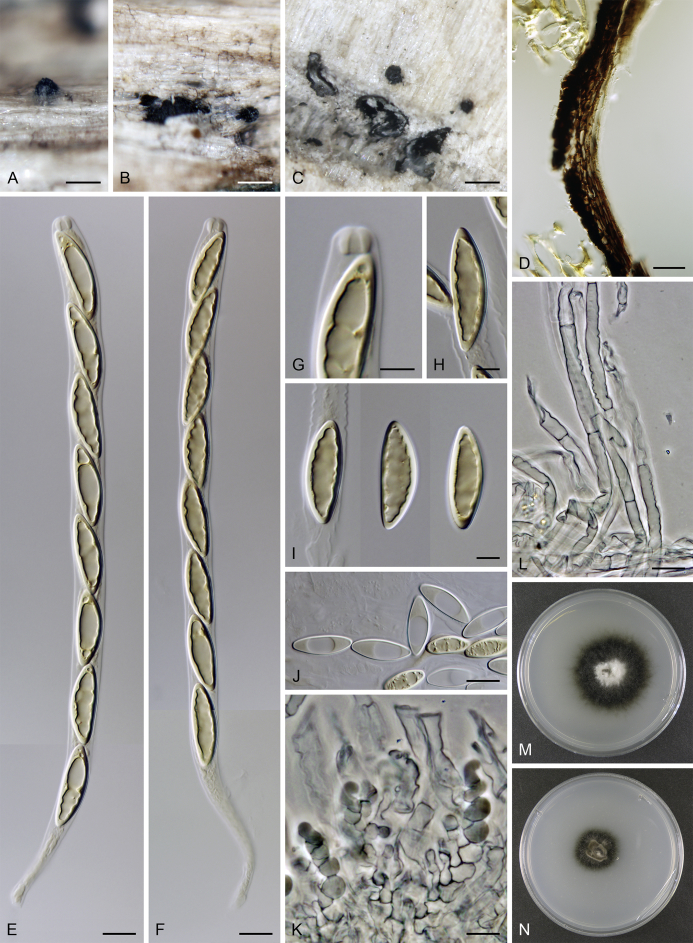
In this study, several species historically treated in Ceratostomella were recollected and isolated in axenic culture. Together with other unknown, morphologically similar taxa resembling Lentomitella and Torrentispora were subjected to phylogenetic analyses. Fungi of this assemblage occur on decaying wood or in bark in terrestrial habitats or on wood submerged in water. They share a simple inconspicuous morphology of non-stromatic ascomata with a cylindrical or rostrate neck, hyaline or brown, aseptate or septate, ornamented or smooth-walled ascospores, persistent asci with a non-amyloid apical annulus and partially disintegrating paraphyses. Little is known about their asexual morphs, which so far include only phaeoisaria-like morphs linked with Lentomitella. However, the majority of these fungi are difficult to culture or they produce only sterile mycelium in vitro. Recently, we found Ceratostomella fuscolutea (Rehm 1908), Ceratostomella hyalostoma (Untereiner 1993), and an unknown lentomitella-like species to produce Spadicoides asexual morphs in vitro. This dematiaceous hyphomycete is characterised by polytretic conidiogenous cells, unbranched conidiophores and dark brown septate or aseptate conidia borne singly or in short chains (Hughes, 1958, Ellis, 1963) and has not yet been linked with any sexually reproducing ascomycetes as a part of their life cycle. DNA sequence data suggest that Spadicoides is polyphyletic (Shenoy et al. 2010); S. atra was shown closely related to Lentomitella. Hernández-Restrepo et al. (2017) confirmed the placement of S. bina, the type species, in the Cordanales and segregated S. atra from Spadicoides into a monotypic genus Xenospadicoides in the Xenospadicoidales.
In order to unravel this part of the fungal tree of life, determine the placement of Ceratostomella spp. listed above and other similar taxa in monophyletic genera and resolve their familial and ordinal relationships, we employed a polyphasic approach in this study. We generated a multigene-based phylogeny of six nuclear ribosomal and protein-coding loci of the new isolates and intensively examined morphological characters of specimens and isolates in pure culture. We also investigated intraspecific relationships among members of Lentomitella using the Compensatory Base Change (CBC) criterion in the ITS2 secondary (2D) structure in two most conserved helices II and III (Mai and Coleman, 1997, Coleman, 2009) and also in helix I (Müller et al. 2007). The ITS2 is a fast-evolving part of the nuclear-coded rRNA operon, which has proven useful for formulating molecular taxonomic concepts, and its 2D structure has a potential to predict sexual incompatibility among closely related organisms. The CBC hypothesis is based on occurrence of compensatory base changes, i.e. co-evolution of nucleotides involved in the double-sided substitution in helices of the ITS2 molecule (Coleman, 2000, Müller et al., 2007). We performed in-depth comparative analyses of ITS2 2D structures of Lentomitella spp. and mapped all existing substitutions among co-evolving nucleotides onto the predicted 2D model of ITS2 of the type species L. vestita.
Material and methods
Herbarium material and fungal strains
Herbarium material was rehydrated with water and examined with an Olympus SZX12 dissecting microscope; hand-sectioned ascomata, centrum material (including asci, ascospores and paraphyses), conidiophores and conidia from living cultures were mounted in 90 % lactic acid, Melzer's reagent or Lugol's iodine. All measurements were made in Melzer's reagent. Means ± standard deviation (SD) based on 20–25 measurements are given for dimensions of asci, ascospores, conidiogenous cells and conidia. Microscopic structures were examined using an Olympus BX51 compound microscope (Olympus America, Inc., Melville, USA) with differential interference contrast (DIC) and phase contrast (PC) illumination. Images of microscopic structures were captured with an Olympus DP70 camera operated by Imaging Software Cell^D (Olympus). Macroscopic images of colonies were documented using an Olympus C-3030 digital camera with daylight spectrum 5600K 16W LED lights. All images were processed with Adobe Photoshop CS6 (Adobe Systems, San Jose, USA).
Cultures were maintained on Modified Leonian's agar (MLA) (Malloch 1981). For comparative purposes, strains were grown on MLA and potato-carrot agar (PCA) (Gams et al. 1998). Descriptions of colonies are based on 28-d-old cultures. Ex-type and other cultures are maintained at the Westerdijk Fungal Biodiversity Institute (CBS), Utrecht, the Netherlands and the International Collection of Microorganisms from Plants (ICMP), Auckland, New Zealand. Type and other herbarium material are deposited in the Herbarium of the Institute of Botany (PRA), Průhonice, Czech Republic, the New Zealand Fungarium (PDD), Auckland, New Zealand, and the Illinois Natural History Survey Fungarium (ILLS), Champaign, Illinois, USA.
DNA extraction, amplification and sequencing
Total genomic DNA was extracted from either mycelium removed from 14-d-old cultures grown on MLA or mature ascomata from herbarium material using the UltraClean Microbial DNA Kit (MoBio Laboratories Inc., Carlsbad, USA). For DNA extracted from herbarium material, an alternative lysis method was incorporated: the gelatinous centrum of 10–15 ascomata was saturated with distilled water, carefully removed with a needle and placed in a 1.9 mL MicroBead tube provided by the manufacturer. After the fungal material was dissolved in 300 μL of MicroBead Solution and 50 μL of Solution MD1, the preparations were heated to 65 ºC for 10 min. The remaining steps for DNA extraction from cultures and herbarium material followed the manufacturer's protocol for filamentous fungi. All amplifications were carried out in 0.5 mL thin-walled PCR tubes (Eppendorf AG, Hamburg, Germany) using a PTC-200 thermal cycler (MJ Research Inc., Watertown, USA). PCR reactions and primers used for the amplification and sequencing of the internal transcribed spacer (ITS) of the nuclear rRNA cistron, portions of the nuclear ribosomal large subunit (nucLSU) and small subunit (nucSSU) RNA gene, and segments 5–7 of the second largest subunit of RNA polymerase II (rpb2) were carried out according to the methods of Réblová et al. (2017).
Primers used for the amplification and sequencing of other genes included: 1) ACT-512F and ACT-783R (Carbone & Kohn 1999) for alpha-actin (act1) gene and 2) T1 and Bt2a in combination with Bt2b (Glass and Donaldson, 1995, O'Donnell and Cigelnik, 1997) for exons 2‒6 of beta-tubulin (tub2) gene. PCR reactions containing 4 mM MgSO4 were performed using Platinum® Taq DNA polymerase High Fidelity (Invitrogen, Carlsbad, USA) in 25 μL volume reactions. PCR conditions were (act1) 2 min at 94 °C, 45–48 cycles of 30 s at 94 °C, 30 s at 54–55 °C and 30 s at 68 °C; (tub2) 2 min at 94 °C, 40–48 cycles of 30 s at 94 °C, 30 s at 54–56 °C and 45–60 s at 68 °C, with a final extension of 10 min at 68 °C for all amplifications. Amplicons were either purified directly from PCR solution after amplification or isolated from agarose gel using the High Pure PCR Product Purification Kit (Roche Applied Science, Mannheim, Germany) following the manufacturer's directions. Automated sequencing was carried out by GATC Sequencing Service (Cologne, Germany). Raw sequence data were assembled, examined and edited using Sequencher v. 5.4.1 software (Gene Codes Corp., Ann Arbor, USA).
GenBank accession numbers for act1, ITS, nucLSU, nucSSU, rpb2 and tub2 sequences generated during this study and homologous sequences of representatives of the Sordariomycetes and Leotiomycetes retrieved from GenBank are listed in Table 1. Retrievable sequences have been published in various studies, e.g. Suh and Blackwell, 1999, Huhndorf et al., 2004, Miller and Huhndorf, 2004a, Miller and Huhndorf, 2005, Réblová and Seifert, 2004, Réblová, 2006, Réblová, 2013, Arzanlou et al., 2007, Spatafora et al., 2007, Damm et al., 2008, Schoch et al., 2009, Shenoy et al., 2010, Réblová et al., 2011, Réblová et al., 2015b, Réblová et al., 2016, Jaklitsch et al., 2013 Untereiner et al., 2013, Hernández-Restrepo et al., 2014, Su et al., 2016.
Table 1.
List of fungi, isolate information and new sequences determined for this study and those retrieved from GenBank. The asterisk (*) denotes ex-type strains of members of the Xenospadicoidales. GenBank accession numbers in bold were generated for this study.
Sequence alignment
ITS, nucLSU, nucSSU and rpb2 sequences were manually aligned in BioEdit v. 7.1.8 (Hall 1999). Alignments of act1 and tub2 sequences were generated in MAFFT v. 7 (Katoh & Standley 2013) and manually corrected where necessary. Consensus 2D structure models for the ITS1 and ITS2 were obtained for all members of the Xenospadicoidales and used to determine positions of homologous nucleotides in the ITS alignment. Introns occurring in nucLSU and nucSSU were delimited manually and excluded from the alignment; in addition, 438 nucleotides (nt) of nucLSU at the 3′-end and 127 nt of nucSSU at the 5′-end were excluded from the alignment because of the incompleteness in the majority of sequences.
The single-locus data sets were examined for topological incongruence among loci for members of the Xenospadicoidales (act1: 35 sequences/338 characters including gaps, ITS: 37/757, nucLSU: 39/1 842, nucSSU: 34/1 668, rpb2: 29/1 127, tub2: 28/966), and members of the Sordariomycetidae (nucLSU: 104/1 973, nucSSU: 71/1 787, rpb2: 65/1 189). Congruence among the loci was tested using the 70 % reciprocal bootstrap criterion (Mason-Gamer & Kellogg 1996). For each individual partition, 1 000 bootstrap replicates were generated with RAxML-HPC v. 7.0.3 (Stamatakis 2006) and PAUP v. 4.0b10 (Swofford 2002) and compared visually for topological conflict among supported clades in phylogenetic trees. The conflict-free alignments were concatenated into a multi-locus alignment that was subjected to subsequent phylogenetic analyses. The multiple sequence alignment is deposited in TreeBASE (S21034).
Phylogenetic analyses
In order to explore monophyly and infrageneric relationships within Lentomitella, Spadicoides, Torrentispora and other morphologically similar taxa, and to resolve their phylogenetic relationships in a broader context we performed analyses of combined act1, ITS, nucLSU, nucSSU, rpb2 and tub2 sequences on two datasets: a reduced dataset consisting of members of these genera and a full dataset consisting of these taxa along with homologous sequences of representatives of the subclass Sordariomycetidae. In order to resolve relationships among Pseudodiplococcium, Spadicoides and Xenospadicoides, a third analysis was based on a reduced ITS-nucLSU dataset of their representatives, mainly due to the availability of only a nucLSU sequence for Pseudodiplococcium ibericum. Two Ceratostomella species, C. cuspidata and C. pyrenaica (Sordariomycetidae, incertae sedis), and Leotia lubrica and Microglossum rufum (Helotiales, Leotiomycetes) were used to root the individual trees in the reduced and full analyses.
The combined datasets were partitioned into several subsets of nucleotide sites, i.e. ITS, nucLSU, nucSSU, rpb2 and coding and non-coding regions of act1 and tub2. Bayesian Inference (BI) and Maximum Likelihood (ML) analyses were used to estimate phylogenetic relationships. BI analyses were performed in a likelihood framework as implemented in MrBayes v. 3.2.6 (Huelsenbeck & Ronquist 2001) through the CIPRES Science Gateway v. 3.3 (http://www.phylo.org). For the BI approach, MrModeltest2 v. 2.3 (Nylander 2008) was used to infer the appropriate substitution model that would best fit the model of DNA evolution. The following models were selected according to the Akaike information criterion for partitions for which we assumed rate heterogeneity: GTR+I+G for ITS, nucLSU, rpb2 and coding region of act1, GTR+G for nucSSU and coding region of tub2 and HKY+I+G for non-coding regions of act1 and tub2. Two Bayesian searches were performed using default parameters. The B-MCMCMC analyses lasted until the average standard deviation of split frequencies was below 0.01 with trees saved every 1 000 generations. The first 25 % of saved trees, representing the burn-in phase of the analysis, were discarded. The remaining trees were used for calculating posterior probabilities (PP) of recovered branches. ML analyses were performed with RAxML-HPC v. 7.0.3 with a GTRCAT approximation. Nodal support was determined by non-parametric bootstrapping (BS) with 1 000 replicates. Maximum Parsimony (MP) analyses conducted with PAUP v. 4.0b10 (Swofford 2002) were used as supplementary to ML analyses to evaluate congruence among loci and topological variation of single-gene phylogenetic trees. A heuristic search was performed with the stepwise-addition option with 1 000 random taxon addition replicates and TBR branch swapping. All characters were unordered and given equal weight. Gaps were treated as missing data. Branch support was estimated on the recovered topologies by performing a heuristic search of 1 000 bootstrap replicates consisting of ten random-addition replicates for each bootstrap replicate.
Prediction of 2D structure models of ITS of Lentomitella
Predicting the 2D structure of the variable and rapidly evolving ITS region is essential for constructing a reliable multiple sequence alignment to compare nucleotides at homologous positions (in helices and loops) while searching for non-conserved co-evolving nucleotides which maintain base pairing. Consensus 2D structure models for the ITS1 and ITS2 were built using the PPfold program v. 3.0 (Sukosd et al. 2012) which uses an explicit evolutionary model and a probabilistic model of structures, and relies on multiple sequence alignment of related RNA sequences. The obtained 2D consensus models created for all members of Xenospadicoidales were further improved using the program Mfold (Zuker 2003) and then adjusted manually if necessary, based on comparison of homologous positions in the multiple sequence alignment. The predicted 2D RNA structures were obtained in a dot bracket notation and were visualized and drawn using the program VARNA: Visualization Applet for RNA (Darty et al. 2009). The final 2D model of ITS2, which was further utilized in formulating taxonomic hypotheses, was processed with CorelDRAW Graphics Suite X4.
We performed in-depth comparative analyses of ITS2 2D structures of Lentomitella spp. We identified three types of substitutions in the aligned ITS sequences. The compensatory base changes (CBCs) occur when both nucleotides of a paired site mutate, i.e. G=C ↔ C=G, A-U or U-A, while maintaining a canonical base pair. The hemi-compensatory base changes (hCBCs) inflict the change of a canonical base pair to a near-canonical so called “wobble” base pair, i.e. G=C → G/U. The non-compensatory base changes (non-CBC) involve the replacement of a canonical pair or a wobble pair with any non-canonical pair. While the CBCs and hCBCs are responsible for maintaining the RNA helix arrangement, non-CBCs lead to its disruption (Leontis et al. 2002). All existing substitutions among Lentomitella species identified in the ITS2 were mapped onto the predicted 2D structure of ITS2 of L. vestita, the generic type (ITS sequence: KY931794).
Results
Topological variation in single-gene, five- and six-gene phylogenetic trees
We studied molecular phylogenies of the Xenospadicoidales based on six nuclear markers in order to compare their phylogenetic utility. Three loci were shown to provide the highest number of distinct alignment patterns (RAxML) and parsimony informative characters (PAUP): rpb2 (556/459), tub2 (671/414) and ITS (496/347), which is one or more times as much as provided by nucLSU (318/168), act1 (255/151) and nucSSU (181/65) loci.
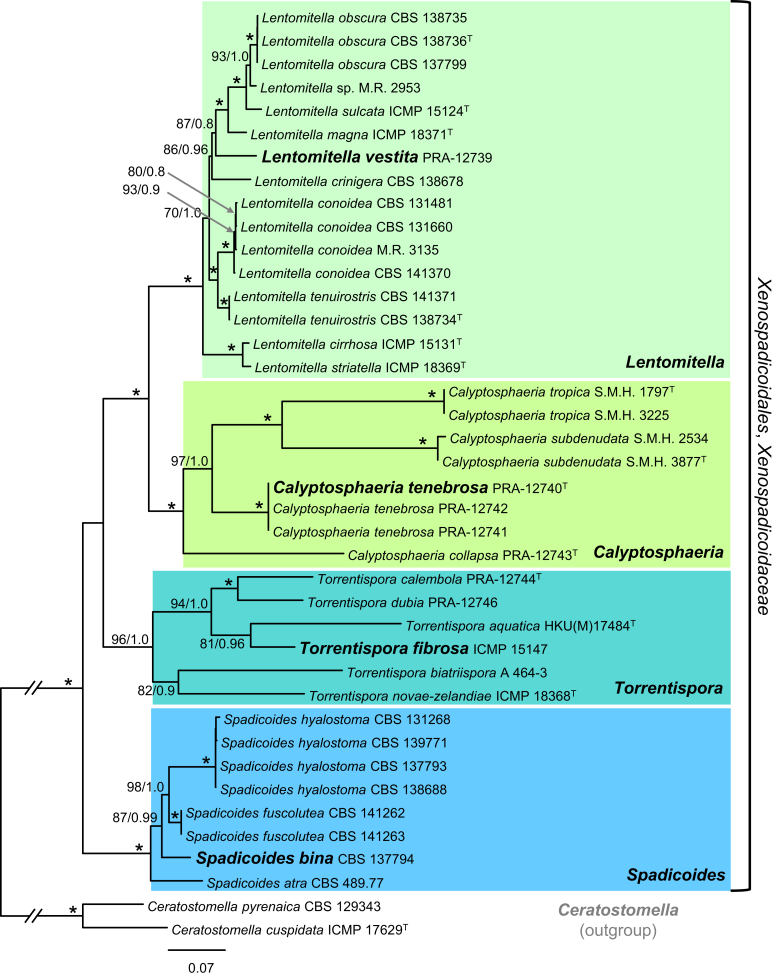
Although topologies of gene trees were generally concordant, there was some incongruence regarding the position of specimen PRA-12743. This specimen is morphologically highly similar to species for which a new genus Calyptosphaeria is introduced below. PRA-12743 was shown either nested in the strongly supported Calyptosphaeria clade in ITS (100 % ML BS) and nucSSU (99) trees or as sister to Calyptosphaeria in the tree based on tub2 (82). In addition, it was resolved on a separate branch; at the base (100) of the Xenospadicoidales clade in the act1 tree or at the base of the Lentomitella/Calyptosphaeria clade without support in the nucLSU tree or supported in the rpb2 tree (not shown). This internode received 92 % and 69 % bootstrap support in the ML and MP analyses, respectively, in the rpb2 tree. The absence of tub2 and rpb2 sequences of C. subdenudata and C. tropica may affect tree topologies based on these genes. In all single-gene phylogenies, Lentomitella, Spadicoides and Torrentispora were always resolved as strongly supported monophyletic clades except in the nucSSU where Spadicoides is paraphyletic with low statistical support.
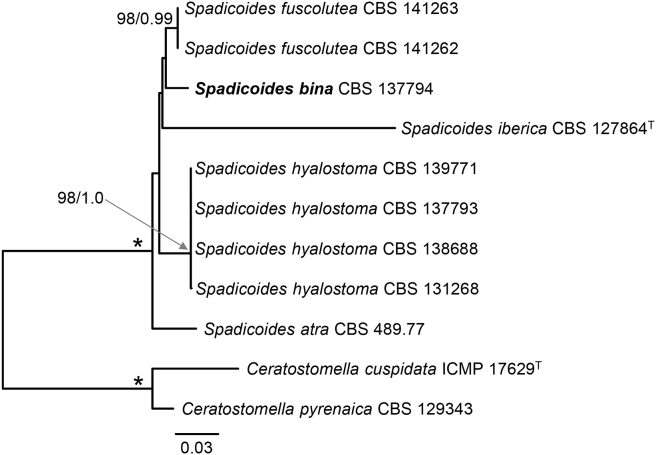
Two ML and BI phylogenetic analyses were performed for comparison based on a five-gene data set (excluding rpb2, results not shown) and a six-gene data set (Fig. 1). In the five-gene tree inferred from ML analysis, PRA-12743 is placed within the Calyptosphaeria clade (100) on a branch next to C. tenebrosa without support. In the five-gene tree inferred from BI analysis, PRA-12743 is shown at the basal position within a strongly supported clade (1.0 PP) as sister to Calyptosphaeria (0.81). In the combined six-gene analysis, the position of PRA-12743 basal in the Calyptosphaeria clade (97/1.0) is strongly supported (100/1.0), and thus this analysis supports its inclusion in Calyptosphaeria.
Fig. 1.
Phylogenetic analysis of members of the Xenospadicoidales. Phylogram inferred from the act1-ITS-nucLSU-nucSSU-tub2-rpb2 sequences with ML analysis using a GTRCAT model of evolution. An asterisk (*) indicates branches with ML BS = 100 %, PP values = 1.0. Branch support of nodes ≥ 70 % ML BS and ≥ 0.80 PP is indicated above or below branches. ‘T’ after the name indicates type strain. Taxa given in bold are type species of Calyptosphaeria, Lentomitella, Spadicoides and Torrentispora.
Because the only incongruence among data sets based on individual genes was the placement of PRA-12743 in the rpb2 tree inferred from ML analysis, the data sets were concatenated. Combination of these six nuclear loci provided robust phylogenetic support for all genera, based on a mixture of the faster evolving ITS region and non-coding regions of act1 and tub2 and generally more preserved and slower evolving regions like nucSSU.
The comparison of ITS sequences among the three species of Calyptosphaeria and PRA-12743 indicated that the latter taxon has the highest divergence among these species. While the length of the ITS sequences of Calyptosphaeria spp. varies between 553–569 nt, the ITS sequence of PRA-12743 (655 nt) is longer by ca. 100 nt with the longest insertion in the helix III of the ITS2.
Phylogeny
In the reduced analysis, 38 combined act1, ITS, nucLSU, nucSSU, rpb2 and tub2 sequences were assessed for 24 species in four genera of the Xenospadicoidales. The alignment consisted of 6 698 characters including gaps and 2 491 distinct alignment patterns (ML analysis). No topological conflicts occurred between trees generated from ML and BI analyses; the ML tree is shown in Fig. 1. The Xenospadicoidales (100 % ML BS/1.0 PP) are resolved with four strongly supported subclades that represent Lentomitella (100/1.0), Spadicoides (100/1.0), Torrentispora (96/1.0) and the newly introduced Calyptosphaeria (100/1.0). Lentomitella comprises 16 strains belonging to nine species, of which five are newly introduced to science. Ceratostomella fuscolutea and Ceratostomella hyalostoma grouped in the Spadicoides clade and therefore these two species are combined in Spadicoides. Xenospadicoides atra, the type species, formerly described as Spadicoides atra, is nested in the Spadicoides clade and therefore is accepted in the latter genus. Ceratostomella dubia, Fusoidispora aquatica, Pseudoannulatascus biatriisporus and two unknown torrentispora-like fungi grouped with T. fibrosa, the type species, in a monophyletic clade resulting in two new species and three new combinations in Torrentispora. The new genus Calyptosphaeria is introduced for Ceratostomella subdenudata, Lentomitella tropica and two morphologically similar hitherto unknown fungi. They are shown on a separate strongly supported branch unrelated to the core of Lentomitella.
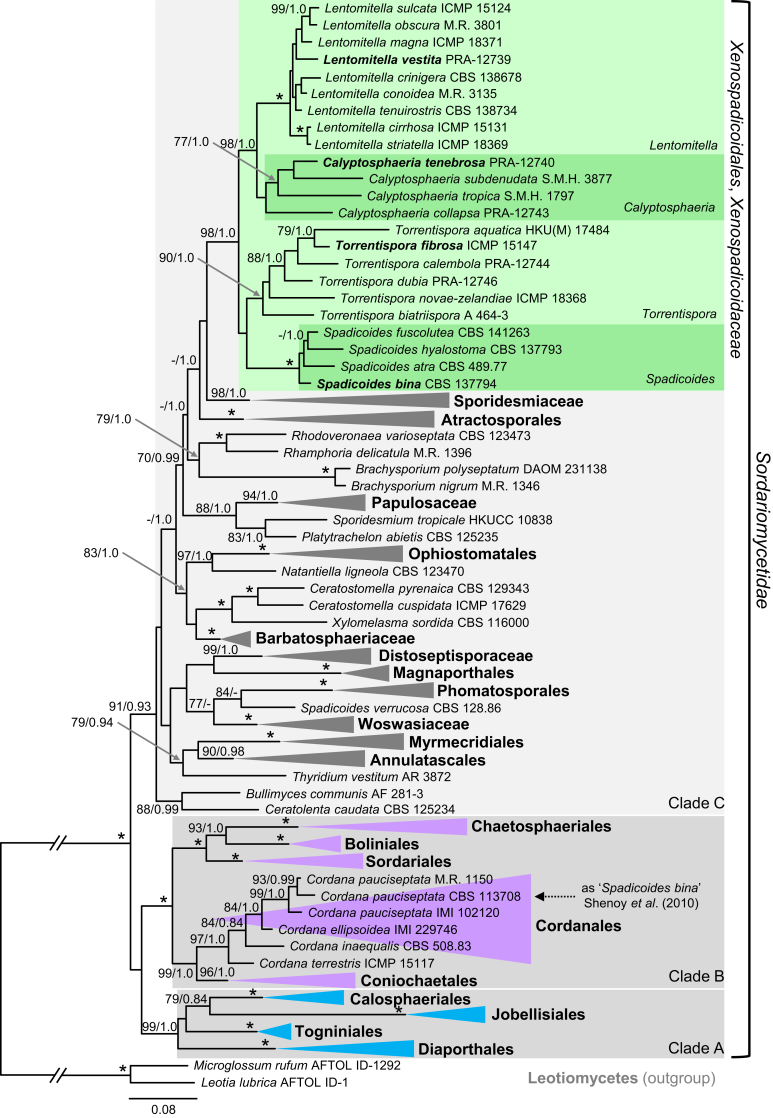
The full data set consisted of combined nucLSU, nucSSU and rpb2 sequences of 102 members of the Sordariomycetidae. This alignment consisted of 4 949 characters and 2 280 distinct alignment patterns (ML analysis). The ML tree is shown in Fig. 2. The BI and ML tree topologies differed in the position of C. collapsa. In the ML analysis, the Calyptosphaeria clade with C. collapsa at the basal position is shown to be monophyletic but statistically unsupported, while in the BI analysis C. collapsa is shown on a separate branch. Another topological difference lies in the position of Sporidesmiaceae and Atractosporales clades as sisters to Xenospadicoidales in the BI analysis. The Sordariomycetidae contain three major clades (A‒C). Clade A (99/1.0) includes orders Calosphaeriales, Diaporthales, Jobellisiales and Togniniales. Clade B (100/1.0) comprises five orders including the Boliniales, Chaetosphaeriales, Coniochaetales, Cordanales and Sordariales. Clade C (91/0.93) contains orders Annulatascales, Atractosporales, Magnaporthales, Myrmecridiales, Ophiostomatales and Phomatosporales, and five families including Barbatosphaeriaceae, Distoseptisporaceae, Papulosaceae, Sporidesmiaceae and Woswasiaceae. The Xenospadicoidales are shown as a strongly supported monophyletic group (98/1.0) embedded in clade C and distantly related to Ceratostomella represented by C. cuspidata and C. pyrenaica in our phylogeny. Its closest relatives are members of the Atractosporales, Ophiostomatales, Papulosaceae and Sporidesmiaceae and several genera of non-stromatic perithecial ascomycetes and dematiaceous hyphomycetes of uncertain positions. The strain CBS 113708 of Cordana pauciseptata, formerly misidentified as Spadicoides bina (Shenoy et al. 2010, Hernández-Restrepo et al. 2017), is grouped within the Cordanales; for details see Discussion.
Fig. 2.
Phylogenetic analysis of selected members of the Sordariomycetidae. Phylogram inferred from the nucLSU-nucSSU-rpb2 sequences with ML analysis using a GTRCAT model of evolution. Details as in Fig. 1.
The third analysis consisted of combined ITS and nucLSU sequences of representatives of Pseudodiplococcium, Spadicoides and Xenospadicoides. The ITS sequence of ex-type strain CBS 127864 of P. ibericum (KY853465, Hernández-Restrepo et al. 2017) was excluded from the analysis due to suspected contamination; in the Blast search it shows 91 % similarity with Cordyceps emeiensis (AJ309347) and 90 % similarity with Hirsutella jonesii (KJ524687) of the Hypocreales. The alignment for ML analysis consisted of 2 601 characters and 232 distinct alignment patterns. No topological conflicts occurred between trees generated from ML and BI analyses; the ML tree is shown in the Supplementary Fig. 1. Spadicoides is shown as a highly supported clade (100/1.0) including S. bina, the type species, X. atra (as S. atra) and two other Spadicoides species. Pseudodiplococcium ibericum, the type species, is nested in the Spadicoides clade, and therefore is synonymised with the latter genus.
Consensus 2D structure of ITS2 of Lentomitella
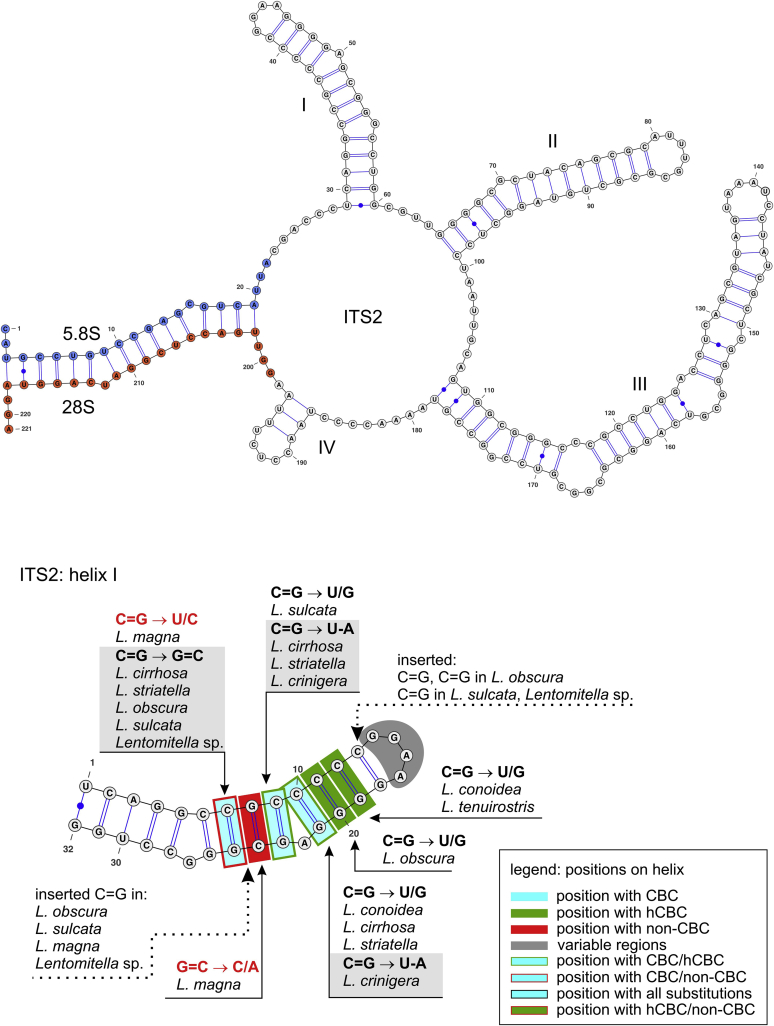
The predicted consensus 2D structure of ITS2, modelled for the type species L. vestita (Fig. 3, Fig. 4), is folded into the common core structure typical for Eukaryota, i.e. a ring structure with four main helices I–IV, of which helices II (35 nt) and III (71 nt) are highly conserved. The folding pattern of the last region corresponding to helix IV is highly variable among Lentomitella spp.; in the case of L. vestita it adopts a short helix. Therefore, only helices I–III were evaluated.
Fig. 3.
ITS2 secondary structure of Lentomitella vestita (GenBank accession no. KY931794) and 5.8S-28S rRNA gene hybridization (proximal stem region) (above); detail of helix I (below). ITS2 helices are numbered I–IV. All substitutions recorded among members of Lentomitella are mapped on the 2D model. Identified substitutions are colour-coded: CBC (blue), hCBC (green) and non-CBC (red); position with all types of substitution (black). Parts of the text highlighted with grey colour refer to CBCs. Parts of hairpin loops and a helix highlighted with grey colour represent regions with a variable number of nucleotides or sequence variation.
Fig. 4.
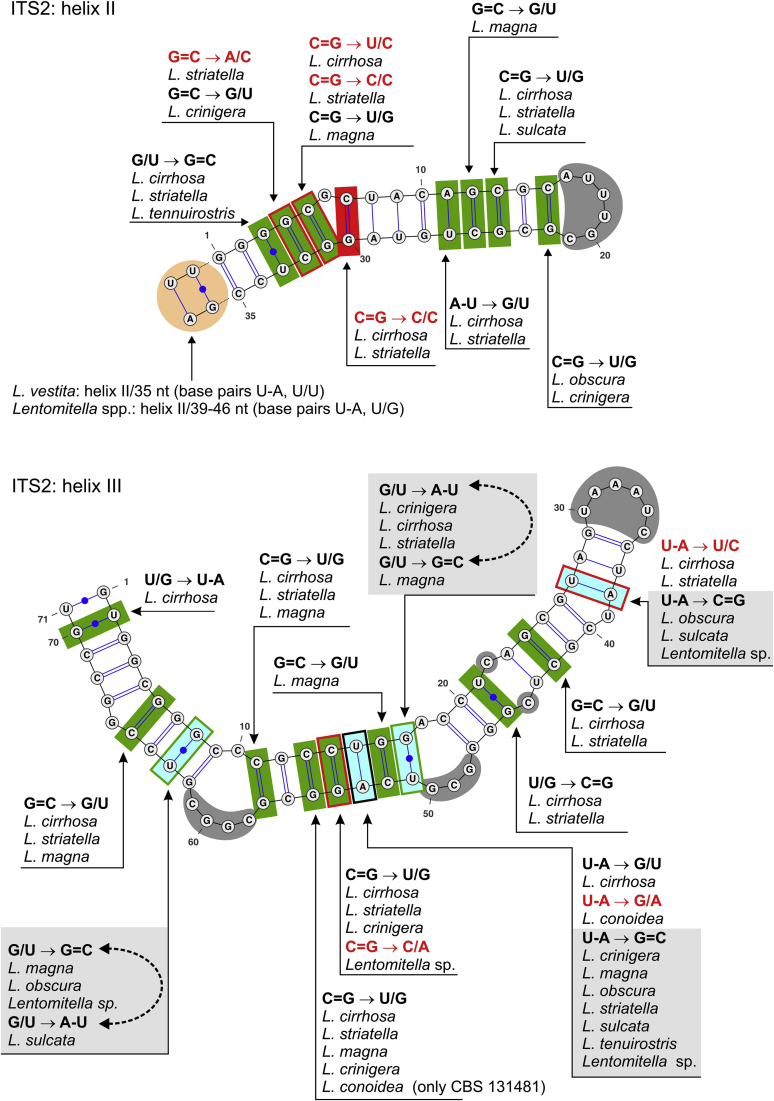
ITS2 secondary structure of Lentomitella vestita (ITS sequence KY931794), helices II and III. Details as in Fig. 3.
Three CBCs on 7, 9 and 10th base pairs were identified in helix I but only the CBC on a 10th base pair (C=G → U-A) is unique between L. crinigera and other Lentomitella species. The other two CBCs show a certain degree of homoplasy and characterise several clades or single branches corresponding to individual species. At the same position, the CBC was accompanied by either hCBC in two cases and by a non-CBC in a single case. In addition, four hCBCs and two non-CBCs were identified in helix I (Fig. 3).
In helix II no CBC was identified, only seven hCBCs and three non-CBCs occur here (Fig. 4). The length of helix II is longer by two base pairs in all Lentomitella species than in L. vestita. In L. vestita the folding of the first two nucleotides at the 5′-end and the two last nucleotides at the 3′-end has not been predicted (leading to U-A, U/U base pairs), while in all other species of the genus the folding pattern of the first two pairs is preserved and nucleotides are conserved (base pairs U-A, U/G). The pyrimidine-pyrimidine mismatch in helix II (Mai and Coleman, 1997, Schultz et al., 2005) was observed only in L. cirrhosa and L. striatella (base pairs U/C, C/C).
Helix III is the longest with two asymmetrical loops, bulges and a hairpin loop. It contains four CBCs on 8, 14, 16, and 24th base pairs, but also an additional 11 hCBCs and two non-CBCs. At the same position, CBC was accompanied by additional substitutions: by hCBC in four cases and in two events by non-CBC. Only the 14th base pair contained all types of substitutions involving CBC, hCBC, and non-CBC and is valuable for studying the evolution of CBC. The CBCs that characterise CBC clades and correspond to biological species were identified for L. magna, L. sulcata, L. vestita and one CBC delimits a clade containing L. obscura, L. sulcata, and Lentomitella sp. Two pairs of species that do not have CBCs between them but are separated by a hCBC in L. cirrhosa (G/U) and L. striatella (G=C), and by a non-CBC in L. conoidea (G/A) and L. tenuirostris (G=C) on the 14th base pair, suggesting an unfinished segregation of these species, are discussed below.
Taxonomy
Xenospadicoidales Hern.-Restr. et al., Stud. Mycol. 86: 91. 2017; emend. Réblová & A.N. Mill.
Emended description: Lignicolous. Ascomata perithecial, non-stromatic. Ostiole periphysate. Hamathecium of paraphyses. Asci unitunicate, persistent, 8-spored, with a non-amyloid apical annulus. Ascospores hyaline or pale brown prior to discharge, aseptate or septate, variable in shape. Asexual morphs dematiaceous hyphomycetes. Conidiophores macronematous, mononematous. Conidiogenous cells tretic or holoblastic-denticulate, sympodially proliferating. Conidia hyaline or brown, aseptate or septate, variable in shape.
Type family: Xenospadicoidaceae Hern.-Restr. et al.
Xenospadicoidaceae Hern.-Restr. et al., Stud. Mycol. 86: 91. 2017; emend. Réblová & A.N. Mill.
Synonym: Lentomitellaceae H. Zhang et al., Fungal Div. 85: 95. 2017.
Emended description: Lignicolous. Ascomata perithecial, non-stromatic, with venter immersed, partially erumpent becoming superficial. Neck cylindrical or rostrate with or without sulcations. Ostiole periphysate. Hamathecium consisting of septate, tapering paraphyses. Asci unitunicate, persistent, cylindrical or cylindrical-clavate, 8-spored, with a non-amyloid apical annulus. Ascospores hyaline or pale brown prior to discharge, aseptate or septate, variable in shape, smooth-walled or ornamented. Asexual morphs dematiaceous hyphomycetes producing effuse colonies. Conidiophores macronematous, mononematous, branched or unbranched. Conidiogenous cells tretic or holoblastic-denticulate, sympodially proliferating. Conidia hyaline or brown, aseptate or septate, variable in shape.
Type genus: Spadicoides S. Hughes (as Xenospadicoides Hern.-Restr. et al., Stud. Mycol. 86: 92. 2017)
Key to genera accepted in the Xenospadicoidales
| 1. | Ascospores brown prior to discharge ………………………. Calyptosphaeria |
| 1. | Ascospores hyaline prior to discharge ……………………………………….. 2 |
| 2. | Ascospores thick-walled, smooth-walled, ascomatal neck without sulcations …………………………………………………………………….… Torrentispora |
| 2. | Ascospores thin-walled, smooth-walled or ornamented, ascomatal neck with or without sulcations …………………………………………………….... 3 |
| 3. | Ascospores longitudinally striate, conidiogenesis holoblastic-denticulate ………………………………………………………………………… Lentomitella |
| 3. | Ascospores smooth-walled or delicately verrucose, conidiogenesis tretic and holoblastic-denticulate ……………………………………….. Spadicoides |
Calyptosphaeria Réblová & A.N. Mill., gen. nov. MycoBank MB821760
Etymology: Kalyptó (Gr.) meaning hide, conceal or envelop, referring to this taxon, which remained hidden within Lentomitella until its position could be resolved with DNA sequence data.
Sexual morph: Ascomata perithecial, non-stromatic, immersed, partially erumpent becoming superficial with only bases immersed, scattered or grouped, varying in position from upright to nearly horizontal; venter globose, subglobose to conical, clothed with hairs. Neck conical, cylindrical or rostrate with 3–4 deep sulcations or roughened lacking sulcations, dark brown, upright or slightly decumbent. Ostiole periphysate. Ascomatal wall fragile, two-layered. Paraphyses becoming partially disintegrated, septate. Asci unitunicate, cylindrical, short-stipitate, 8-spored; apex with a distinct, non-amyloid apical annulus. Ascospores ellipsoidal or ellipsoidal-fusiform, sometimes flattened on one side, hyaline becoming pale brown or dull brown prior to discharge, aseptate with a delayed formation of three transverse septa when still within the ascus, smooth-walled, without sheath or appendages. Asexual morph: unknown.
Type species: Calyptosphaeria tenebrosa Réblová & A.N. Mill.
Notes: Huhndorf et al. (2008) expanded the generic concept of Lentomitella by including two species with ellipsoidal or ellipsoidal-fusiform, smooth-walled ascospores that turn brown prior to discharge, i.e. L. pallibrunnea and L. tropica, and showed their sister relationship to L. cirrhosa and L. crinigera using partial nucLSU sequence data. Based on the combined analysis of six nuclear markers and morphology of ascospores, the new genus Calyptosphaeria is segregated from Lentomitella to accommodate L. pallibrunnea and L. tropica and two other morphologically similar taxa. The formation of septa is delayed and mature ascospores remain mostly aseptate and, in some cases, visible but indistinct cytoplasmic bands appear in areas where septa would be expected to form (Barr 1986 and this study). The ascospores are uniseriate or overlapping uniseriate within the asci, although sometimes they can be partially biseriate in the middle of the sporiferous part and asci appear slightly clavate.
Key to the species accepted in Calyptosphaeria
| 1. | Ascomatal neck roughened lacking sulcations, ascospores (10–)10.5–12(–12.5) × 4.5–5 μm ……………………………………… C. collapsa |
| 1. | Ascomatal neck sulcate ………………………………………………………... 2 |
| 2. | Ascospore longer than 16 μm, (16–)17–20(–21) × 6–7 μm ……………….……………………………………………………………..………… C. tenebrosa |
| 2. | Ascospores up to 16 μm long …………………………………………..…….. 3 |
| 3. | Ascospores 11–14.5(–16) × 4–5 μm …………………..….. C. subdenudata |
| 3. | Ascospores 14.5–16 × 5–6 μm ………………………………..…... C. tropica |
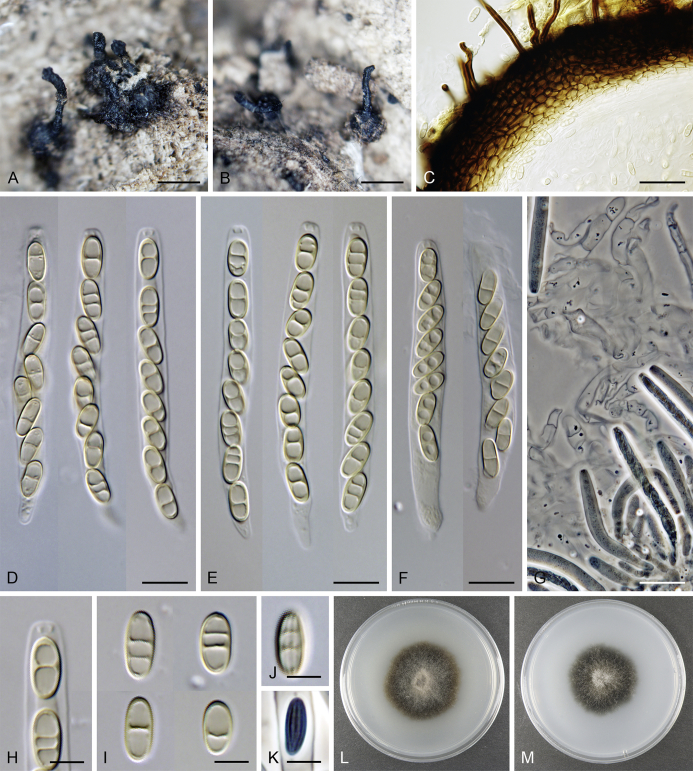
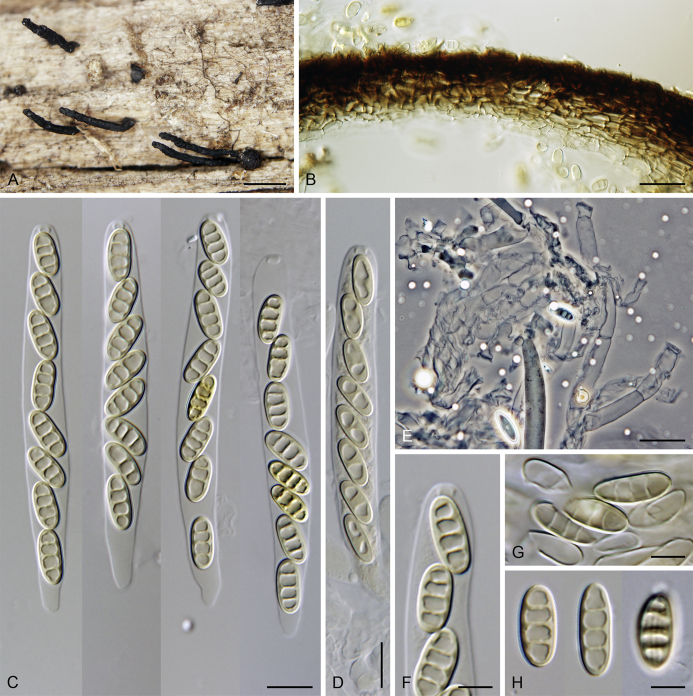
Calyptosphaeria collapsa Réblová & A.N. Mill., sp. nov. MycoBank MB821761. Fig. 5.
Fig. 5.
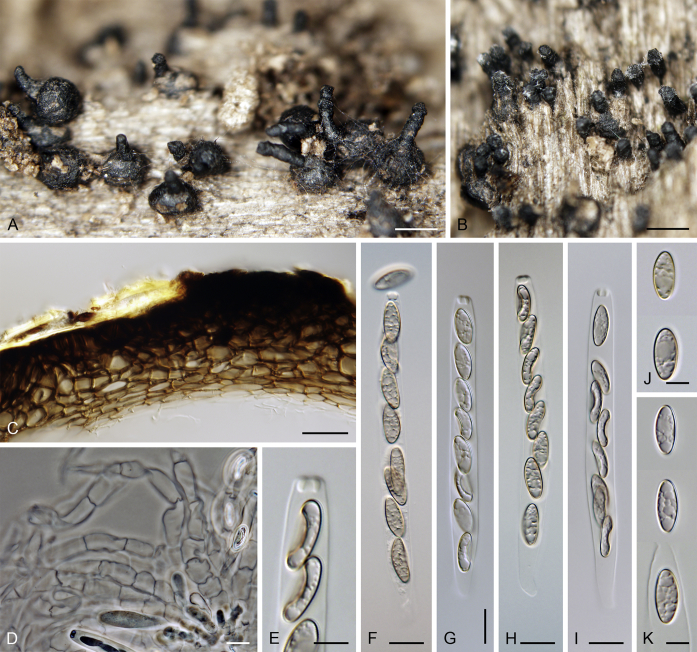
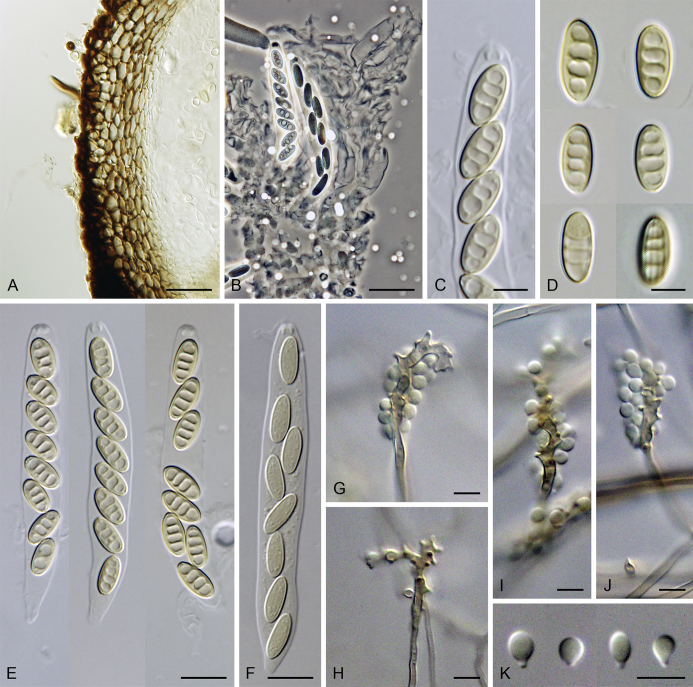
Calyptosphaeria collapsa. A, B. Ascomata. C. Longitudinal section of the ascomal wall. D. Paraphyses. E. Ascal apex with apical annulus. F–I. Asci. J, K. Ascospores. A–K from PRA-12743. Scale bars: A, B = 500 μm, C = 20 μm, E, J, K = 5 μm, D, F–I = 10 μm.
Etymology: Collapsus (L.) meaning collapsed, referring to ascospores which collapse laterally upon aging.
Sexual morph: Ascomata immersed, partially erumpent becoming superficial with only bases immersed, closely grouped. Venter 350–490 μm diam, 400–500 μm high, subglobose, upright or sometimes lying horizontally on the host, dark brown to black, with brown, septate hairs 2–3 μm wide sparsely covering the sides and bottom. Neck central, 120–140 μm wide, up to 700 μm long, cylindrical, upright, apex roughened, without sulcations. Ostiole periphysate. Ascomatal wall fragile to leathery, 44–62(–75) μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura prismatica to textura epidermoidea with opaque walls; cells tending to be darker towards the outside, more flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, slightly constricted at the septa, hyaline, 6.5–9.5 μm wide, tapering to ca. 3 μm apically. Asci 82–96(–100) × (7–)7.5–8.5(–9) μm (mean ± SD = 89.4 ± 5.3 × 8 ± 0.4 μm), 67–72(–86) μm (mean ± SD = 77.2 ± 5.1 μm) long in the sporiferous part, truncate at the apex, cylindrical, with a short stipe; with 8 overlapping uniseriate or partly biseriate ascospores; apical annulus 3.5–4 μm wide, ca. 2.5 μm high. Ascospores (10–)10.5–12(–12.5) × 4.5–5 μm (mean ± SD = 11.3 ± 0.6 × 4.8 ± 0.3 μm), ellipsoidal to fusiform, sometimes slightly flattened on one side, often collapsing laterally upon aging, aseptate, later with up to three indistinct cytoplasmatic bands, pale brown prior to discharge, smooth-walled. Asexual morph: unknown.
Specimens examined: Czech Republic, Southern Moravia, Lednice, Nejdek old Slavic settlement, area close to the pagan burial grounds, on decaying wood of Carpinus betulus, 16 Nov. 2014, M. Réblová M.R. 3881 (holotype, PRA-12743); ibid., M.R. 3882, M.R. 3884.
Notes: Calyptosphaeria collapsa differs from other species of the genus by a roughened ascomatal neck without sulcations. In the absence of an asexual morph (Calyptosphaeria spp. did not germinate in vitro) it is difficult to find any other morphological differences between them. It resembles C. subdenudata and C. tropica in the morphology of ascospores, but the ascospores are shorter in C. collapsa. It further differs from C. subdenudata in having longer asci and from C. tropica in having a cylindrical neck vs. conical rostrate ascomatal apex in the latter species. The collapsing ascospores were observed in water, lactic acid and Melzer's reagent.
Calyptosphaeria collapsa was found on strongly decaying wood of several fallen trunks of Carpinus betulus in the Czech Republic, the remains of old growth trees that were more than one hundred years old.
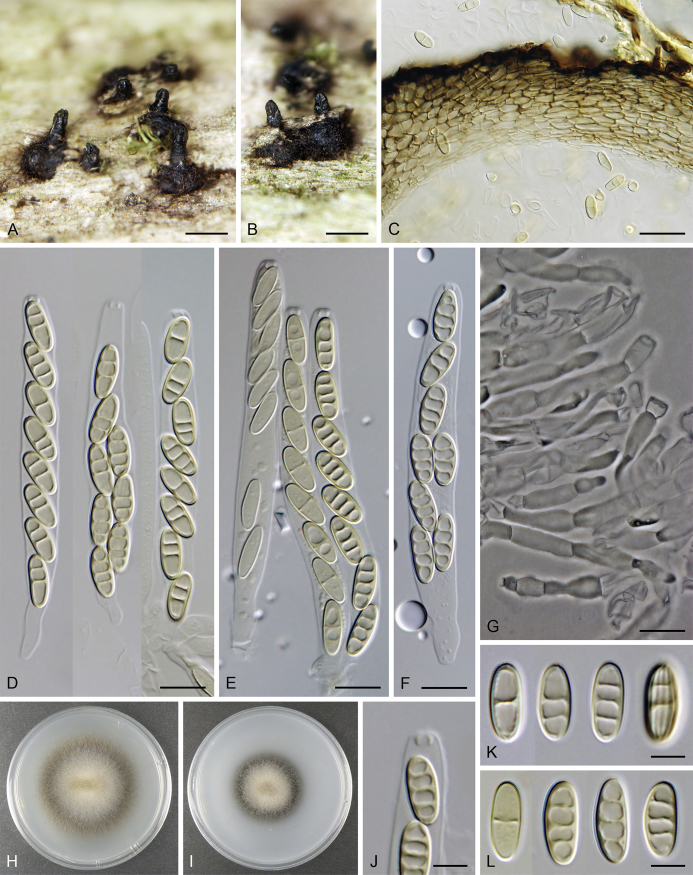
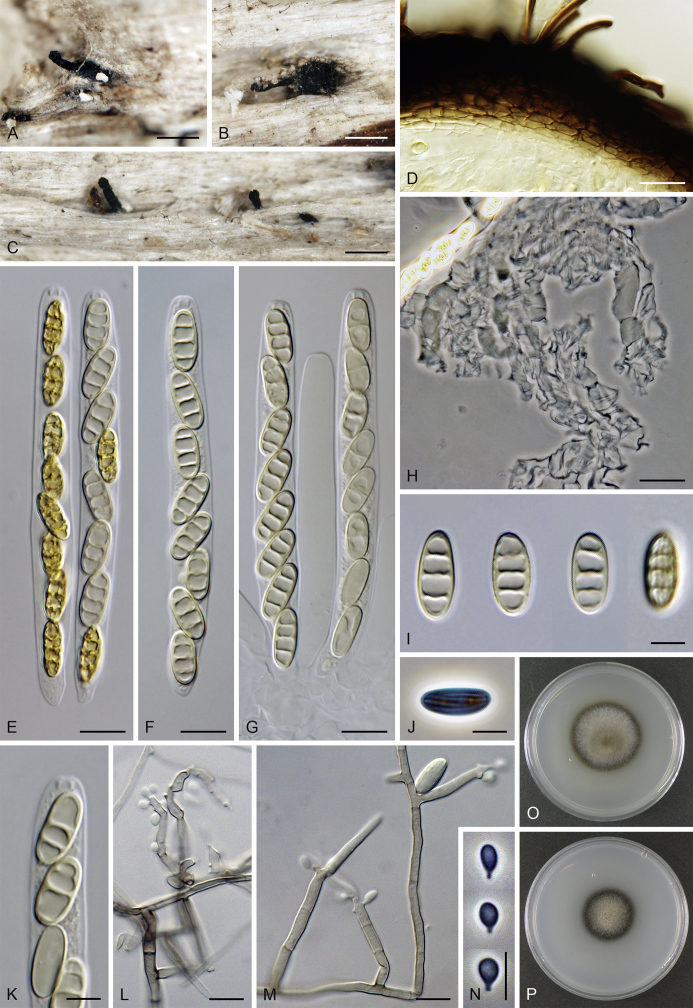
Calyptosphaeria subdenudata (Peck) Réblová & A.N. Mill., comb. nov. MycoBank MB821762. Fig. 6.
Fig. 6.
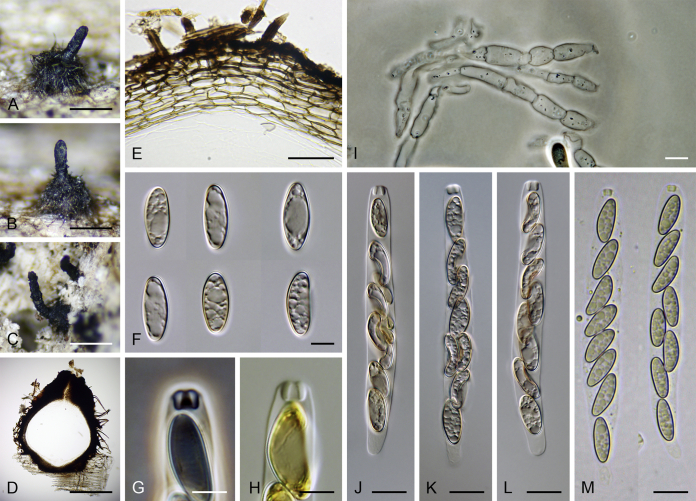
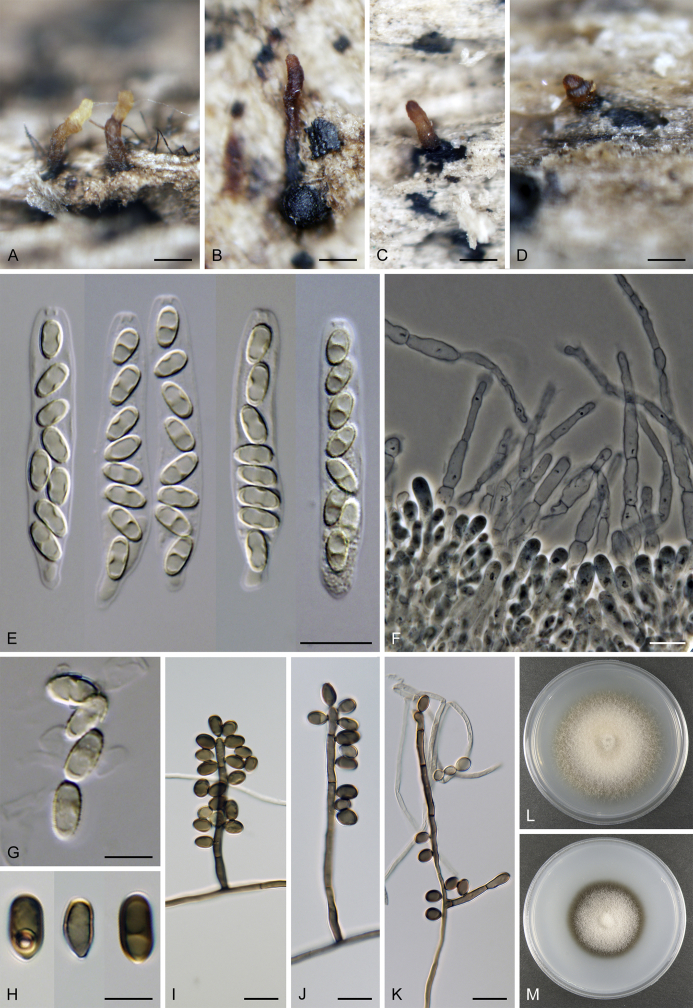
Calyptosphaeria subdenudata. A–C. Ascomata. D, E. Longitudinal section of the ascomal wall. F. Ascospores. G, H. Ascal apex with apical annulus. I. Paraphyses. J–M. Asci. A–M from JF 16082. Scale bars: A–C = 500 μm, D = 250 μm, E = 20 μm, F–H = 5 μm, I–M = 10 μm.
Basionym: Sphaeria subdenudata Peck, Ann. Rep. N.Y. St. Mus. nat. Hist. 32: 52. 1880 (1879).
Synonyms: Ceratostoma subdenudatum (Peck) Sacc., Syll. fung. 9: 481. 1891.
Ceratostomella subdenudata (Peck) M.E. Barr, Bull. N.Y. St. Mus. 459: 44. 1986.
Wegelina subdenudata (Peck) M.E. Barr, Cryptog. Bryol.-Lichénol. 19: 172. 1998.
Lentomitella pallibrunnea Huhndorf et al., Mycologia 100: 948. 2008.
Xylomelasma moderata Lar.N. Vassiljeva & S.L. Stephenson, Mycosphere 5: 223. 2014.
Sexual morph: Ascomata immersed, partially erumpent with protruding necks becoming superficial with only bases immersed, scattered or in groups. Venter 390–500 μm diam, 400–520 μm high, subglobose, upright, more often lying horizontally on the host, dark brown to black, with reddish brown, septate hairs ca. 3.5 μm diam sparsely covering the sides. Neck central, 100–130 μm wide, up to 500 μm long, cylindrical, upright, slightly roughened, apex with several deep sulcations. Ostiole periphysate. Ascomatal wall fragile, 37–40 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses sparse, becoming disintegrated with age, septate, slightly constricted at the septa, hyaline, 5–6 μm wide. Asci (50–)60–85(–90) × (7.5–)8–10 μm (mean ± SD = 72.5 ± 3.2 × 9 ± 0.4 μm), truncate to broadly rounded at the apex, cylindrical, with a short stipe; with 8 overlapping uniseriate or biseriate ascospores; apical annulus ca. 3 μm wide, 2.5–3 μm high. Ascospores 11–14.5(–16) × 4–5 μm (mean ± SD = 13.3 ± 1.4 × 4.7 ± 0.4 μm), ellipsoidal, often slightly flattened on one side and slightly curved, aseptate or with several indistinct cytoplasmatic bands, hyaline becoming light dull brown, smooth-walled. Asexual morph: unknown.
Specimens examined: French West Indies, Martinique, Sainte-Marie, La Philippe, coastal mesophilic rainforest, on decaying wood, 3 Aug. 2016, J. Fournier J.F. 16082. USA, New York, Delaware County, Griffins', Catskill Mts., on decaying wood, Sep. 1877, C.H. Peck (holotype of Sphaeria subdenudata, NYS); ibid., Indian Lake, on decaying wood, Oct. 1878, C.H. Peck (NYS). Michigan. Berrien County, Warren Woods, south end of trail, through picnic area, up to creek, on 12 cm thick branch on the ground, 8 Nov. 1998, F.A. Fernández & A.N. Miller, S.M.H. 3877 (holotype of Lentomitella pallibrunnea, F).
Notes: Calyptosphaeria subdenudata is known from French West Indies (Martinique) and North America (Illinois, Massachusetts, Michigan and New York), based on recently collected material and the revision of the holotype and other herbarium material of Lentomitella pallibrunnea (Huhndorf et al. 2008) and Sphaeria subdenudata (Peck, 1879, Barr, 1986). Xylomelasma moderata (Vassiljeva & Stephenson 2014) fits well within the species concept based on the ascoma, ascus and ascospore morphology. Both latter species are placed in synonymy under C. subdenudata. Barr (1986) revised Peck's material of S. subdenudata and noted that ascospores become 1–5-pseudoseptate at maturity. Up to three cytoplasmic bands were observed in areas where septa would be expected to form during our revision of the type material of S. subdenudata; the paraphyses were already disintegrated. The ascospores of L. pallibrunnea were described as aseptate (Huhndorf et al. 2008).
Calyptosphaeria subdenudata is similar to C. collapsa in the ellipsoidal, brown ascospores, but it differs in having shorter asci, longer ascospores and a neck with deep sulcations.
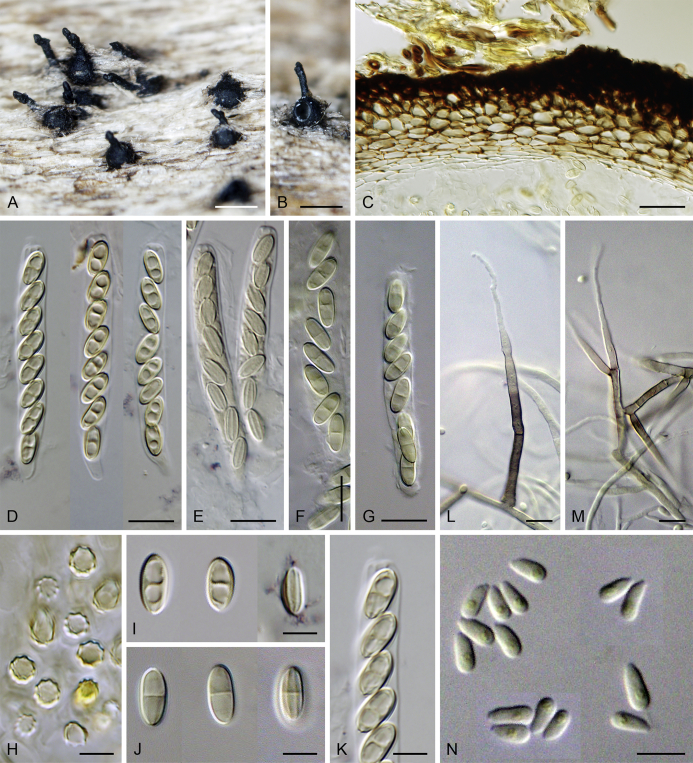
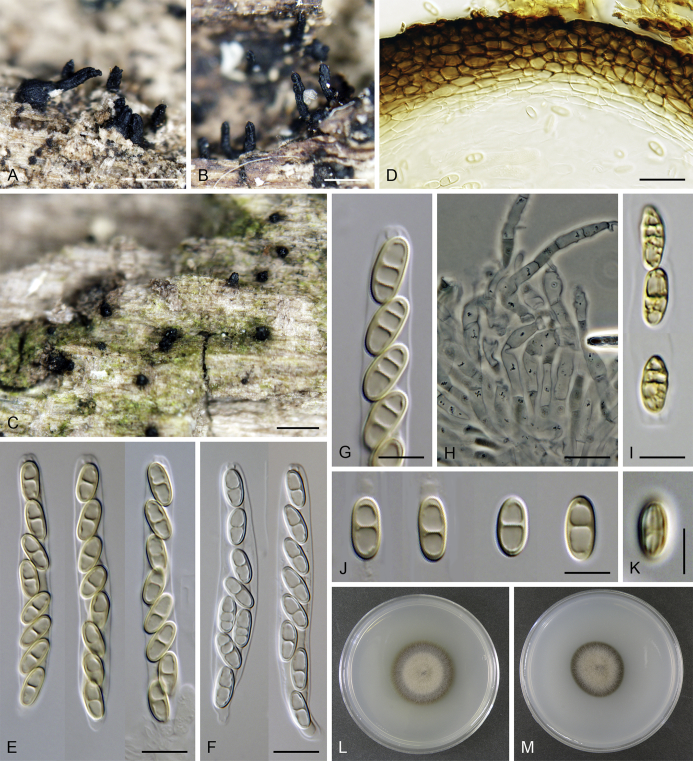
Calyptosphaeria tenebrosa Réblová & A.N. Mill., sp. nov. MycoBank MB821763. Fig. 7.
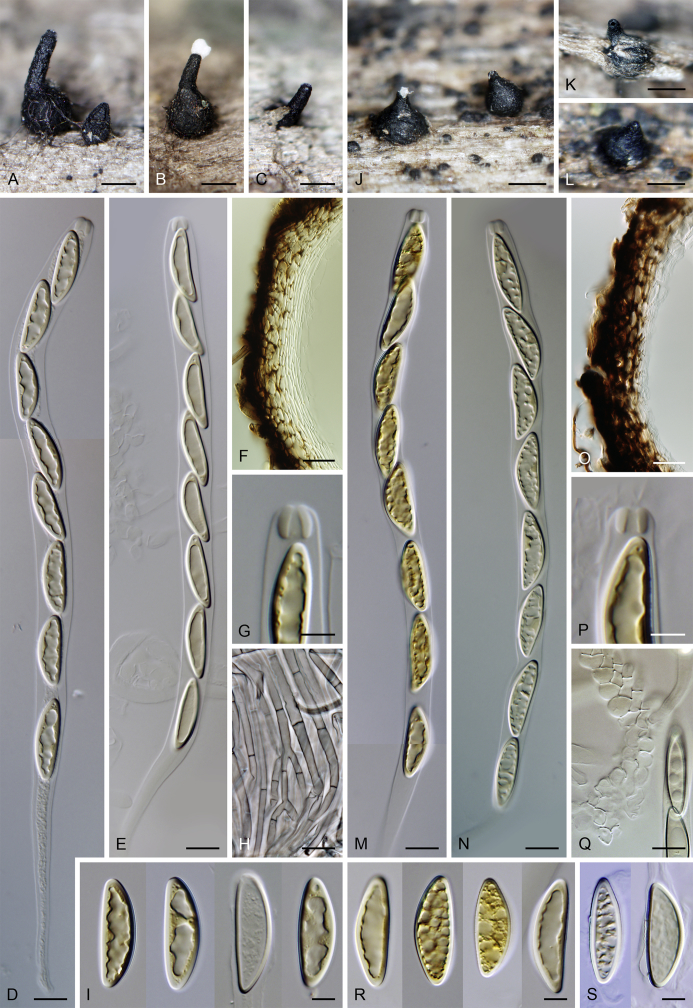
Fig. 7.
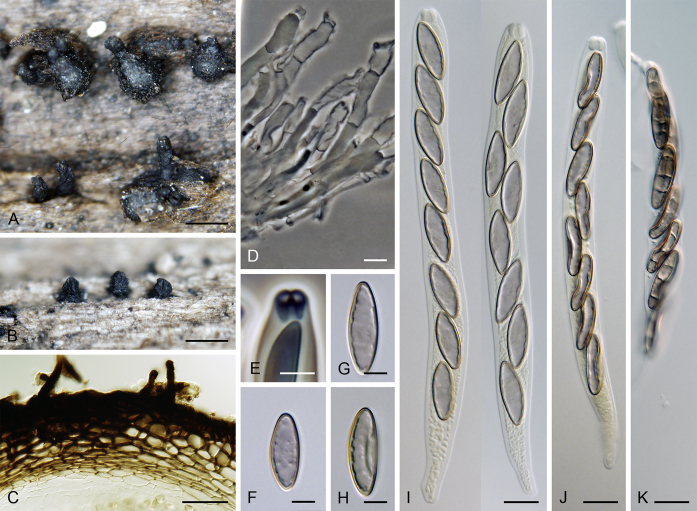
Calyptosphaeria tenebrosa. A, B. Ascomata. C. Longitudinal section of the ascomal wall. D. Paraphyses. E. Ascal apex with apical annulus. F–H. Ascospores. I–K. Asci. A, D, J, K from PRA-12742, C, G, F, I from PRA-12741, B, E, H from PRA-12740. Scale bars: A, B = 500 μm, C = 20 μm, D, I–K = 10 μm, E–H = 5 μm.
Etymology: Tenebrosus (L.) meaning dark, referring to the colour of the ascospores.
Sexual morph: Ascomata immersed, partially erumpent with protruding necks or becoming superficial with only bases immersed, scattered or grouped sometimes in rows. Venter 310–550 μm diam, 390–650 μm high, subglobose to conical, straight or more often lying horizontally on the host, dark brown to black, with brown, septate hairs 4.5–5 μm diam sparsely covering the lower part. Neck central, 120–140 μm wide, up to 600 μm long, cylindrical, upright, straight, often roughened, apex slightly widened with 3–4 deep sulcations. Ostiole periphysate. Ascomatal wall fragile to leathery, 55–65 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura prismatica to textura angularis with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, slightly constricted at the septa, hyaline, 5.5–9.5 μm wide, tapering to 2.5–3.5 μm. Asci (119–)122–150 × 9–10(–11) μm (mean ± SD = 134.7 ± 8.5 × 9.5 ± 0.3 μm), (105–)110–130 μm (mean ± SD = 116.7 ± 6.4 μm) long in the sporiferous part, truncate at the apex, cylindrical, with a short stipe; with 8 uniseriate or partly biseriate ascospores; apical annulus 4.5–5 μm wide, 4–4.5 μm high. Ascospores (16–)17–20(–21) × 6–7 μm (mean ± SD = 18.6 ± 1.1 × 6.4 ± 0.3 μm), ellipsoidal-fusiform, tapering towards the ends, rarely inequilateral, sometimes collapsing laterally upon aging, aseptate, three transverse septa sometimes present in old ascospores, hyaline to yellowish becoming pale brown prior to discharge, smooth-walled. Asexual morph: unknown.
Specimens examined: Czech Republic, Central Bohemia, Křivoklátsko Protected landscape area, Nižbor, Vůznice Nature reserve, on decaying wood of C. betulus, 20 Oct. 2004, M. Réblová M.R. 2923. Northern Moravia, Podhoří ‘Podhorn’ near Hranice, on decaying wood of Fagus sylvatica, Mar. 1914, F. Petrak (as Ceratostoma operculatum, Fl. Bohem. Morav. Exs. No. 968, PRM 655798). Southern Moravia, Lednice, Nejdek, forested area on the right side of the Nejdek crossroads, on decaying wood of Acer campestre, 15 Nov. 2014, M. Réblová M.R. 3871 (holotype, PRA-12740); ibid., M.R. 3869; ibid., Nejdek, old Slavic settlement near river Zámecká Dyje, on decaying wood of Carpinus betulus, 27 Oct. 2014, M. Réblová M.R. 3867 (PRA-12741); ibid., Valtice, Rendez-vous National nature monument, on decaying wood of Quercus cerris, 18 Nov. 2012, M. Réblová M.R. 3704. France, Ariège, Lescure, Bois du Pas du Baup, 500 m. a.s.l., on decaying wood of Alnus glutinosa, 24 Feb. 2004, J. Fournier J.F. 04021 (PRA-12742). New Zealand, Westlands, Victoria Forest Park, Rough Creek Road, 4 km S of Inangahua, on decaying wood, 22 Apr. 2005, M. Réblová M.R. 2967/NZ 810.
Notes: Calyptosphaeria tenebrosa can be distinguished from other species of the genus by longer asci with the largest apical annulus known in the genus and longer ellipsoidal-fusiform ascospores. It occurs on decaying wood of various hardwood trees and it is known from several localities in the Czech Republic, France and New Zealand.
Calyptosphaeria tropica (Huhndorf et al.) Réblová & A.N. Mill., comb. nov. MycoBank MB821764.
Basionym: Lentomitella tropica Huhndorf et al., Mycologia 100: 948. 2008.
Notes: For description and illustration see Huhndorf et al. (2008). Calyptosphaeria tropica can be distinguished from other species of the genus by size of asci and ascospores, conical rostrate ascomatal apex and tropical distribution (Costa Rica and Puerto Rico).
Lentomitella Höhn., Annls mycol. 3: 552. 1906.
Sexual morph: Ascomata perithecial, non-stromatic, immersed or partially erumpent with protruding necks or becoming superficial, solitary or in rows or groups. Venter globose to subglobose, clothed by brown or reddish-brown hairs. Neck central, cylindrical, upright to slightly decumbent, glabrous, apex sulcate. Ostiole periphysate. Ascomatal wall fragile to leathery, two-layered. Paraphyses longer than the asci, becoming disintegrated with age, septate, constricted at the septa, hyaline. Asci cylindrical to clavate, broadly rounded or truncate at the apex, with a short stipe; with 8 uniseriate or obliquely uniseriate or overlapping, sometimes partly biseriate ascospores; with a distinct, non-amyloid apical annulus. Ascospores ellipsoidal, suboblong or ellipsoidal-fusiform, sometimes inequilateral, hyaline, longitudinally striate, 1–3-septate with 2–4 large drops, with a delayed formation of second and third septum. Asexual morph: A phaeoisaria-like asexual morph is sometimes formed in vitro. Conidiophores macronematous, mononematous, unbranched or branched apically, arising from aerial hyphae, brown near the base, subhyaline to hyaline towards the tip. Conidiogenous cells terminal or intercalary, hyaline, subcylindrical to slender flask-shaped, with a rachis bearing minute denticles, conidiogenesis holoblastic-denticulate, conidial heads slimy, inconspicuous, transparent. Conidia globose, ellipsoidal, clavate to obovate, slightly apiculate at the base, hyaline, aseptate, smooth-walled.
Type species: Lentomitella vestita Höhn.
Notes: Lentomitella forms a strongly supported monophyletic clade in the phylogenetic analysis based on six nuclear markers. It is well-distinguished from other genera of the Xenospadicoidales by hyaline, septate, longitudinally striate ascospores, distinct but relatively small apical annulus (2.5–3 μm wide, 1.5–2 μm high) and ascomata with a cylindrical neck with usually 3–4 deep sulcations at the apex and venter clothed by dark interwoven hairs that can disappear upon aging. Lentomitella comprises 11 species; L. cirrhosa, L. crinigera, L. unipretoriae and L. vestita are accepted in the genus, two new combinations are proposed for L. conoidea and L. investita, and five species are described as new to science, i.e. L. magna, L. obscura, L. sulcata, L. striatella and L. tenuirostris. The phaeoisaria-like asexual morph has been experimentally proven only for L. investita, L. sulcata, and Lentomitella sp. The ascospores do not germinate easily. Germinating tubes appear in ca. 1–2 wk after isolation on water agar. Despite the lack of known asexual morphs, we observed that the colonies of individual species differ macroscopically at the margin. The margin consists either of densely branched hyphae, i.e. in L. conoidea, L. crinigera and L. magna, or unbranched or sparsely branched hyphae of the substrate mycelium in the other species.
Key to the species accepted in Lentomitella
| 1. | Ascospores shorter than 11 μm …..…………………………………….…. 2 |
| 1. | Ascospore longer than 11 μm …………………………………………….... 8 |
| 2. | Ascospores 1-septate; (5.5–)6–7 × 3–3.5 μm …………………. L. vestita |
| 2. | Ascospores 1–3-septate, longer than 7 μm ………………………...……. 3 |
| 3. | Asci (46–)50–56(–65) × 6–7 μm, ascospores (7–)7.5–9 × 3.5–4(–4.5) μm ………………..………………………………………………... L. investita |
| 3. | Asci longer than 56 μm ………………..……………………………………. 4 |
| 4. | Ascospores usually 1–2-septate, old ascospores 3-septate …………… 5 |
| 4. | Ascospores 3-septate early in ontogeny ………………………………… 7 |
| 5. | Ascospores 1(–3)-septate, first-formed septum in the middle, 8–10 × 4.5–5 μm …………………………………………..…… L. cirrhosa |
| 5. | Ascospores usually 1–2-septate, first-formed septum in the middle or slightly above or below the middle ………………………………………… 6 |
| 6. | Ascospores up to 9.5 μm long; aerial mycelium on MLA woolly, woolly-floccose or almost cobwebby at the margin; margin of the colony consisting of densely branched hyphae ………………………. L. conoidea |
| 6. | Ascospores usually slightly longer, 9–10.5 μm; aerial mycelium on MLA cottony to felty having a more compact appearance; margin of the colony consisting of unbranched or sparsely branched hyphae ….. L. tenuirostris |
| 7. | Ascospores (8–)9–10.5(–11) × 4–5 μm, asci 72–81 × 6.5–7.5 μm …………….………………………………………………………… L. unipretoriae |
| 7. | Ascospores longer than 10.5 μm ………………………………………… 8 |
| 8. | Ascospores (12.5–)13–15 × 5.5–6.5 μm ………….…………… L. magna |
| 8. | Ascospores shorter than 13 μm …………………………………………… 9 |
| 9. | Preferring coniferous wood; ascomata up to 600 μm diam with a neck up to 170 μm wide; ascospores (10–)10.5–13 × 4–5.5 μm, asci (66–)70–88(–90) × (7.5–)8–9.5(–10) μm …………..……………... L. crinigera |
| 9. | Species without substrate preferences, ascomata and necks smaller …………………………………………………………………………………….. 10 |
| 10. | Asci up to 8 μm wide, ascospores up to 5 μm wide; asci (70–)72–80(–82) × 7.5–8(–8.5) μm, ascospores (10–)11–12 × 4–5(–5.5) μm ……………………………….………………………………… L. obscura |
| 10. | Asci wider than 8 μm, ascospores wider than 5 μm …….……………… 11 |
| 11. | Asci length/width ratio 9.4, ascospores (10.5–)11–12(–13) μm long ………………………………………………………..……………... L. sulcata |
| 11. | Asci length/width ratio 8.6, ascospores 11.5–13(–14) μm long ……………………………………………………………………... L. striatella |
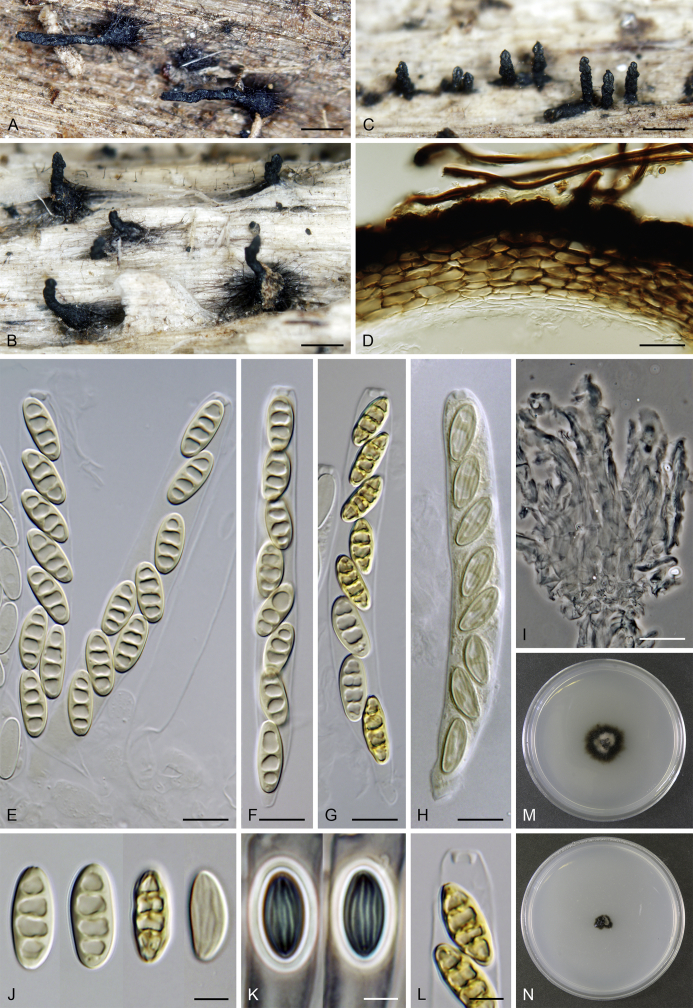
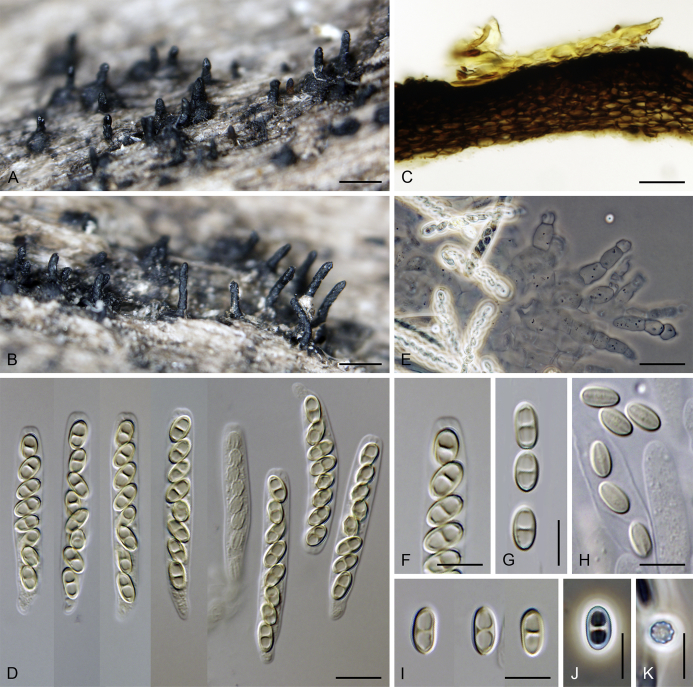
Lentomitella cirrhosa (Pers.: Fr.) Réblová, Mycologia 98: 82. 2006. Fig. 8, Fig. 9.
Fig. 8.
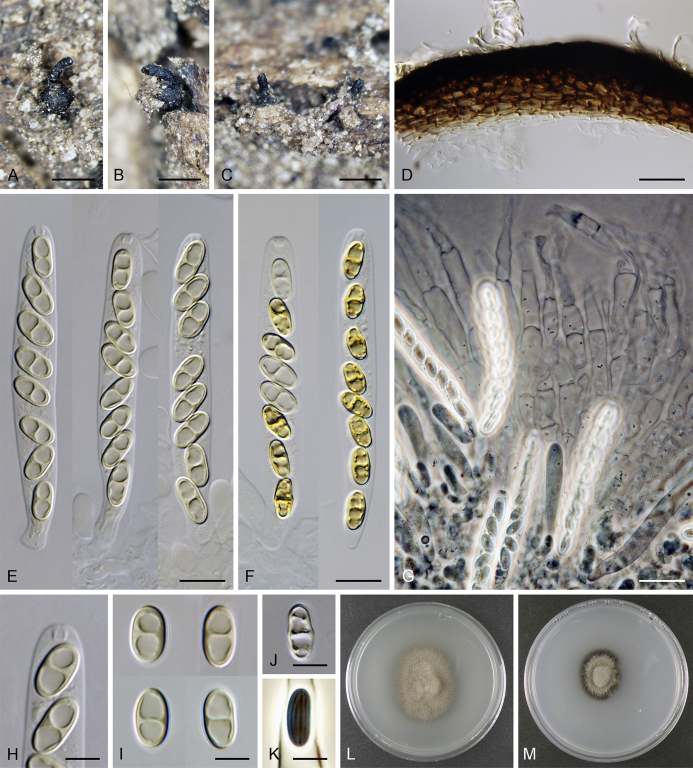
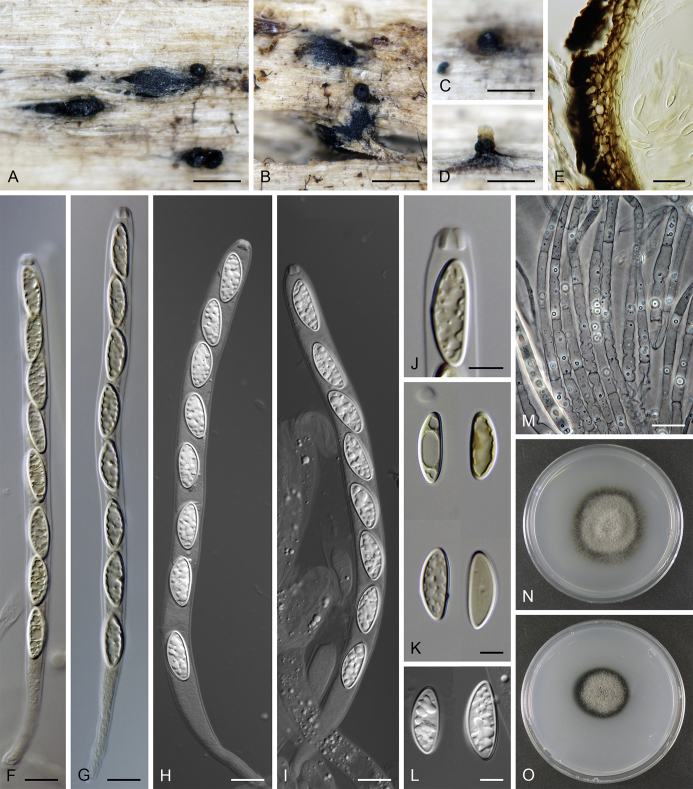
Lentomitella cirrhosa. A–C. Ascomata. D. Longitudinal section of the ascomal wall. E, F. Asci. G. Paraphyses. H. Ascal apex with apical annulus. I–K. Ascospores. L, M. Colonies on MLA and PCA after 28 d. A–M from ICMP 15131. Scale bars: A–C = 500 μm, D, G = 20 μm, E, F = 10 μm, H–K = 5 μm.
Fig. 9.
Lentomitella cirrhosa. A. Description of Sphaeria cirrhosa (Persoon 1801). B. Illustration (lectotype) of S. cirrhosa (Persoon 1808).
Basionym: Sphaeria cirrhosa Pers., Syn. Meth. Fung. p. 59. 1801 : Fries, Syst. Mycol. 2: 475. 1823.
Synonyms: Ceratostoma cirrhosum (Pers.: Fr.) Fuckel, Jahr. nassau. Ver. Naturk. 23–24: 127. 1870.
Cerastoma cirrhosum (Pers.: Fr.) Quél., Mém. Soc. Émul. Montbéliard, Sér. 2 5: 522. 1875.
Ceratostomella cirrhosa (Pers.: Fr.) Sacc., Michelia 1: 370. 1878.
Amphitrichum cirrhosum (Pers.: Fr.) Kuntze, Revis. gen. pl. 3(2): 443. 1898.
Endoxyla cirrhosa (Pers.: Fr.) Arx & E. Müll., Beitr. Krypt-Fl. Schweiz 11(1): 355. 1954.
Sexual morph: Ascomata immersed to partially erumpent becoming superficial, solitary or in small groups. Venter 300–330 μm diam, 300–340 μm high, globose to subglobose, dark brown to black, covered with sparse, dark brown, septate hairs ca. 3.5 μm wide. Neck central, 90–100 μm wide, up to 500 μm long, cylindrical, upright, straight or slightly flexuous, glabrous, tapering, apex sulcate. Ostiole periphysate. Ascomatal wall fragile to leathery, 25–40 μm thick, two-layered. Outer layer consisting of thick-walled, brown, polyhedral cells of textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, constricted at the septa, hyaline, 7–10 μm wide, tapering to ca. 2.5–3 μm. Asci (62–)64–70(–73) × 6.5–7.5(–8.5) μm (mean ± SD = 66.7 ± 3.0 × 7.1 ± 0.4 μm), (55–)58–62(–66) μm (mean ± SD = 60.2 ± 2.8 μm) long in the sporiferous part, broadly rounded at the apex, cylindrical to clavate with a short stipe; with 8 uniseriate or obliquely uniseriate ascospores; apical annulus ca. 2.5 μm wide, 1.5 μm high. Ascospores 8–10 × 4.5–5 μm (mean ± SD = 9.4 ± 0.5 × 4.6 ± 0.3 μm), ellipsoidal, hyaline, longitudinally striate, 1–3-septate, usually 1-septate with two large drops becoming 3-septate upon aging. Asexual morph: unknown.
Culture characteristics: Colonies on MLA 16–19 mm diam after 14 d (19–23 mm after 21 d, 20–25 after 28 d) at 25 °C, circular, convex. Aerial mycelium abundant, cottony, margins filamentous, colony surface pale brown to cocoa brown; reverse black. Colonies on PCA 10–12 mm diam after 14 d (13–15 mm after 21 d, 14–16 mm after 28 d) at 25 °C, circular, convex. Aerial mycelium abundant, woolly, loose to cobwebby towards the margin, colony surface beige-grey with olive-brown inner ring and conspicuous dark brown marginal ring of submerged hyphae; reverse black. Vegetative hyphae branched, septate, medium to dark brown, smooth-walled. Margin of the colony consisting of unbranched or sparsely branched hyphae of substrate mycelium. Sporulation not observed.
Lectotype; designated by Réblová (2006): (Illustration) Persoon, Icones pictae specierum rariorum Fungorum in Synopsi Methodica Descriptarum. fasc. 4, tab. 24, fig. 3. 1808.
Specimens examined: New Zealand, South Island, Southland, Southland Distr., Fiordland National park, Kaherekoau Mts., Blue cliffs, Hump track 15 km W of Papatotara, on decaying wood, 12 Mar. 2005, M. Réblová M.R. 2952/NZ 481; ibid., West Coast, Westland Distr., Hokitika, Mananui Point, Lake Mahinapua, Swimmers Beach walk, on decaying wood of Podocarpus totara, 5 Mar. 2003, M. Réblová M.R. 2705/NZ 206 (epitype, PDD 81434, culture ex-epitype ICMP 15131).
Notes: It is challenging to interpret L. cirrhosa in the absence of a type specimen in Persoon's herbarium (L). Persoon (1801) described Sphaeria cirrhosa just with a few words. The illustration (Persoon 1808) showing black ascomata with immersed venter clothed with sparse hairs and emerging glabrous neck represents the only original element left and is reproduced here as Fig. 9; the figure caption of tab. 24 in Persoon (1808) incorrectly refers to pictures 1, 2 but S. cirrhosa is depicted in picture 3. In the absence of a type specimen, this illustration (Persoon 1808) was selected as lectotype [Réblová 2006, incorrectly cited as Persoon (1800), corrected here], and in order to stabilise the species concept of L. cirrhosa, a collection from New Zealand on decayed wood of Podocarpus totara (PDD 81434) was designated as epitype (Réblová 2006).
Fuckel (1870) accepted S. cirrhosa in the genus Ceratostoma as C. cirrhosum and provided a description based on his own material published in Fungi Rhen. Exs. No. 1804 [= Lentomitella vestita, this study]. Saccardo (1882) interpreted this species based on various collections. He transferred S. cirrhosa to Ceratostomella and described the ascomata as sparse, ca. 400 μm diam, immersed becoming partially erumpent, subglobose, clothed with hairs, with a long glabrous neck, asci cylindrical-clavate 65–75 × 7–9 μm, containing eight ellipsoidal to oblong, hyaline ascospores 9–12 × 3.5 μm, with 1–4, mostly two drops.
The species concept of Ceratostomella cirrhosa presented by von Arx (1952) included 12 species synonyms and was based on a revision of type and other herbarium material from Europe and North America. The synonymy was partly adopted by Réblová (2006) and C. cirrhosa was transferred to the reinstated Lentomitella. The species description of L. cirrhosa was based on the epitype, other herbarium material collected in Europe and on the revision of holotypes of Sphaeria investita, Ceratostomella vestita var. vestita and var. varvicensis and Eriosphaeria conoidea. In the light of phylogenetic analysis of six nuclear markers and re-evaluation of morphological characters of ascospores and asci, they are not conspecific with L. cirrhosa and belong to three different species recognised in this study as L. conoidea, L. investita (including C. vestita var. varvicensis as its synonym) and L. vestita.
Although L. cirrhosa and L. vestita differ significantly in the size of asci and ascospores, and the ascospores of the latter species are regularly 1-septate vs. 1–3-septate in L. cirrhosa, von Arx (1952) regarded them as conspecific. He considered the holotype of L. vestita to be insufficiently developed and therefore ignored the smaller size of ascospores (5.5–)6–7 × 3–3.5 μm and asci 43–47(–55) × 5.5–6 μm in his description of C. cirrhosa [asci 50–80 μm long in the sporiferous part with stipe 20–40 μm long, ascospores 8–10 × 3.5 μm fide von Arx (1952)]. Another eight Ceratostomella species synonymised by von Arx (1952) with L. cirrhosa, and previously transferred to Amphitrichum by Kuntze (1898), were revised; they belong to the genera Ceratostomella (Réblová 2006), Natantiella (Réblová & Štěpánek 2009) and other fungi discussed below.
The ascospores of L. cirrhosa in the epitype (PDD 81434) are mostly 1-septate with two large drops, but old ascospores released from the asci possess three septa and four drops. The closest relative to L. cirrhosa is L. striatella, which differs by longer and wider asci and slightly longer and wider, regularly 3-septate ascospores.
Lentomitella cirrhosa is also similar to L. investita and L. conoidea in morphology of ascospores. It shares with L. investita mostly 1-septate ascospores with the first-formed septum positioned always in the middle and a delayed formation of two additional septa. Lentomitella investita differs from L. cirrhosa by shorter and narrower asci and slightly smaller ascospores. Lentomitella conoidea differs from L. cirrhosa by slightly longer, mostly 1–2-septate ascospores with the first septum formed in the middle or slightly above or below the middle.
Lentomitella conoidea (Feltg.) Réblová, comb. nov. MycoBank MB821765. Fig. 10.
Fig. 10.
Lentomitella conoidea. A, B. Ascomata. C. Longitudinal section of the ascomal wall. D–F. Asci. G. Paraphyses. H. Ascal apex with apical annulus. I–K. Ascospores. L, M. Colonies on MLA and PCA after 28 d. A, B, J, K from CBS 141370, C, E from M.R. 3135, F from LUX 043455, D, G, H, I, L, M from CBS 131660. Scale bars: A, B = 500 μm, C, G = 20 μm, D–F = 10 μm, H–K = 5 μm.
Basionym: Eriosphaeria conoidea Feltg., Vorstud Pilzfl. Luxemb., Nachtr. 3: 282. 1903.
Sexual morph: Ascomata immersed to partially erumpent becoming superficial, solitary or in groups. Venter 350–400 μm diam, 370–450 μm high, globose to subglobose, dark brown to black, covered by dark brown to reddish brown, septate hairs 3–4 μm wide. Neck central, 100–110 μm wide, up to 800 μm long, cylindrical, upright, straight or slightly flexuous, glabrous, tapering, apex sulcate. Ostiole periphysate. Ascomatal wall leathery, 45–57 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura angularis to textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, constricted at the septa, hyaline, 5–9 μm wide, tapering to ca. 3.5 μm. Asci 62–70(–75) × 6.5–7.5 μm (mean ± SD = 67.3 ± 2.9 × 7.1 ± 0.2 μm), 58–63(–66) μm (mean ± SD = 61.3 ± 2.3 μm) long in the sporiferous part, truncate at the apex, subcylindrical to clavate, with a short stipe; with 8 partially overlapping, obliquely uniseriate or partly biseriate ascospores; apical annulus ca. 2.5 μm wide, 1.5 μm high. Ascospores 8.5–9.5(–10.5) × 4–4.5 μm (mean ± SD = 9.0 ± 0.5 × 4.5 ± 0.2 μm), ellipsoidal to oblong, hyaline, longitudinally striate, 1–3-septate, commonly only 1–2-septate, the first-formed septum in the middle or slightly below or above the middle, third septum developing rarely. Asexual morph: unknown.
Culture characteristics: Colonies on MLA 13–15 mm diam after 14 d (18–21 mm after 21 d, 24–25 mm after 28 d) at 25 °C, circular, convex. Aerial mycelium abundant, woolly to cottony becoming loose to woolly-floccose at the margin, colony surface brown-grey turning brown towards the margin; reverse black. Colonies on PCA 8–9 mm after 14 d (10–11 mm after 21 d, 10–12 after 28 d) at 25 °C, circular or slightly irregular, raised. Aerial mycelium abundant, woolly to cottony, loose or almost cobwebby towards the margin, colony surface brown-grey at the centre turning dark olive-brown towards the margin; reverse black. Vegetative hyphae branched, septate, medium to dark brown, smooth-walled. Margin of the colony consisting of densely branched hyphae of substrate mycelium. Sporulation not observed.
Specimens examined: Belgium, Moersdorf, on bark of Pyrus communis, 25 May 1902, J. Feltgen (holotype of Eriosphaeria conoidea, LUX 043455). Czech Republic, Southern Bohemia, Šumava Mts. National park, Stožec, Medvědice Mt., on decaying deciduous wood, 17 Sep. 2007, M. Réblová M.R. 2998 (culture CBS 141370). Denmark, Sjælland, Jægerspris Nordskov, distr. 44, on very rotten wood of Quercus sp., 4 Nov. 1963, A. Munk (C); ibid., Ermelunded, on old bark of Fraxinus excelsior, 7 Dec. 1964, A. Munk (C); ibid., Bernstorffsparken, on very rotten wood, 24 Mar. 1965, A. Munk (C); ibid., Suserup Skov, on wood of Sorbus sp., 15 Dec. 1995, T. Læssøe TL-4062 (C 34511). Italy, Lazio, province Viterbo, Farnese, Selva di Lamone Nature reserve, on decaying wood of Quercus cerris, 19 Mar. 2010, M. Réblová M.R. 3135; ibid., 3 Apr. 2011, M.R. 3607 (culture CBS 131660), M.R. 3611 (culture CBS 131481).
Notes: The examination of the holotype of Eriosphaeria conoidea (Feltgen 1903) revealed a fungus that fits well the description of Lentomitella and represents a distinct species. Therefore, it is transferred to this genus and a new combination is proposed. Höhnel (1906b) examined the holotype and concluded that E. conoidea is conspecific with Ceratostomella debaryana (Saccardo 1882) and that it shows remarkable similarity to L. vestita and L. investita. Although C. debaryana shares similar size of asci and ascospores with L. conoidea, in the protologue (Auerswald 1869) the ascospores are described and illustrated as aseptate with granulose content, and the width of ascomata (140 μm) is too small to match the size of any accepted species of Lentomitella. The type material of C. debaryana could not be located. The holotype of E. conoidea deviates slightly in the length of the asci from recently collected material from the Czech Republic and Denmark; the asci are 57–66 × 6.5–7.5 μm, although in the protologue of E. conoidea they were described as longer, 62–72 × 6–8 μm by Feltgen (1903). Similar length of asci of E. conoidea (ca. 70 μm) was reported also by Höhnel (1906b).
Lentomitella conoidea is represented by four strains in our phylogeny. The ascospores are mostly 1–2-septate, the first septum is formed in the middle or slightly above or below the middle. Ascospores with three septa were observed only in the collections from Denmark. The length of the neck and presence of hairs covering the venter vary among collections.
Lentomitella conoidea is most similar to L. tenuirostris in morphology of asci and ascospores, but it differs by slightly shorter ascospores, slightly longer asci and larger ascomata. In culture, the aerial mycelium of L. conoidea is woolly to cottony, loose to woolly-floccose or almost cobwebby at the margin, while in L. tenuirostris the aerial mycelium is cottony to felty resulting in more compact aerial hyphae; the colony surface is paler, because substrate mycelium and the brown hue in the agar do not show through loose aerial hyphae as is the case of L. conoidea. Moreover, the margin of the colony of L. conoidea consists of densely branched hyphae of substrate mycelium, while the margin of L. tenuirostris is formed of unbranched or sparsely branched hyphae. Most specimens of L. conoidea were collected on various hardwoods in deciduous forests in lowlands, while specimens of L. tenuirostris were collected in the mountain regions of the Czech Republic and France. For further comparison see Discussion and also comments of L. tenuirostris.
Lentomitella conoidea can be also compared to L. investita, but the latter differs by shorter asci and ascospores that possess the first-formed septum always in the middle and two additional septa that form symmetrically.
Lentomitella crinigera (Cooke) Réblová, Mycologia 98: 83. 2006. Fig. 11.
Fig. 11.
Lentomitella crinigera. A, B. Ascomata. C. Longitudinal section of the ascomal wall. D–F. Asci. G. Paraphyses. H, I. Colonies on MLA and PCA after 28 d. J. Ascal apex with apical annulus. K, L. Ascospores. A–D, G–I, K from CBS 138678, E, J, L from M.R. 1671, F from M.R. 1526. Scale bars: A, B = 500 μm, C, G = 20 μm, D–F = 10 μm, J–L = 5 μm.
Basionym: Sphaeria crinigera Cooke, Grevillea 1: 156. 1873.
Synonyms: Ceratosphaeria crinigera (Cooke) Sacc. Syll. fung. 2: 227. 1883.
Ceratostomella crinigera (Cooke) Cooke, Grevillea 17: 49. 1889.
Ceratostomella triseptata Petr., Annls mycol. 23: 135. 1925.
Sexual morph: Ascomata immersed with protruding necks or partially erumpent becoming superficial with base immersed, solitary, in rows or small groups. Venter 450–600 μm diam, 510–650 μm high, globose to subglobose, dark brown, with dark brown, septate hairs 3.5–5 μm wide. Neck central, 120–170 μm wide, up to 1 mm long, cylindrical, upright, straight or slightly flexuous, glabrous, tapering; apex sulcate. Ostiole periphysate. Ascomatal wall fragile to leathery, 35–55(–65) μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura angularis to textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, constricted at the septa, hyaline, 6.5–10.5 μm wide, tapering to 3.5–4.5 μm. Asci (66–)70–88(–90) × (7.5–)8–9.5(–10) μm (mean ± SD = 78.7 ± 4.4 × 8.5 ± 0.6 μm), (62–)68–76 μm (mean ± SD = 69.1 ± 4.4 μm) long in the sporiferous part, truncate at the apex, cylindrical to clavate, with a short stipe; with 8 obliquely uniseriate or biseriate ascospores; apical annulus ca. 2.5–3 μm wide, 1.5–2 μm high. Ascospores (10–)10.5–13 × 4–5.5 μm (mean ± SD = 11.5 ± 0.3 × 4.8 ± 0.3 μm), ellipsoidal-fusiform, slightly inequilateral, hyaline, longitudinally striate, 1–3-septate. Asexual morph: unknown.
Culture characteristics: Colonies on MLA 10–13 mm diam after 14 d (13–15 mm after 21 d, 15–17 after 28 d) at 25 °C, circular, convex. Aerial mycelium abundant, woolly to cottony, margin filamentous, colony surface pale brown to beige with a dark brown marginal ring; reverse black. Colonies on PCA 4–5 mm diam after 14 d (5–7 mm after 21 d, 6–8 mm after 28 d) at 25 °C, circular, convex. Aerial mycelium abundant, cottony, loose towards the margins, margin filamentous, colony surface beige to pale brown, with a brown ring of submerged hyphae at the margin; reverse black. Vegetative hyphae branched, septate, medium to dark brown, smooth-walled. Margin of the colony consisting of densely branched hyphae of substrate mycelium. Sporulation not observed.
Specimens examined: Czech Republic, Northern Moravia, Podhoří ‘Podhorn’ near Hranice, on decaying coniferous wood, Nov. 1913, F. Petrak (holotype of Ceratostomella triseptata, W 18093). Southern Bohemia, Novohradské hory Mts., Dobrá voda, Hojná voda National nature monument, on decaying wood, 4 Oct. 2012, M. Réblová M.R. 3742 (culture CBS 138678). Southern Bohemia, Šumava Mts. National park, Železná Ruda, glacial cirque of the Čertovo jezero Lake National nature reserve, on decaying wood of Abies alba, 28 Aug. 1997, M. Réblová, M.R. 997, M.R. 1034; ibid., on decaying wood of Picea abies 12 Aug. 1999, M.R. 1544, M.R. 1585; 21 Aug. 2000, M.R. 1672; ibid., glacial cirque of the Černé jezero Lake National nature reserve, on decaying wood of Abies alba, 13 Aug. 1999, M.R. 1526, M.R. 1546; ibid., Boubínský prales National nature reserve, on decaying wood of Picea abies, 17 Aug. 1999, M.R. 1611; ibid., Prášily, Ždanidla Mt., 24 Aug. 2000, on decaying wood of Picea abies, M.R. 1671.; ibid., Modrava, Ptačí nádrž, on decaying wood of Picea abies, 14 Aug. 1999, M.R. 1457; ibid., Modrava, Modravské slatě, Pytlácký roh, on decaying wood of Picea abies, 14 Aug. 1999, M.R. 1652. Sweden, Fries' Scleromyceti Sueciae No. 346, on decaying wood (UPS). UK, England, Norfolk, King's Lynn, on decaying pine wood, C.B. Plowright (holotype of Sphaeria crinigera, K 84422).
Notes: Lentomitella crinigera is characterised by 1–3-septate, ellipsoidal-fusiform ascospores, with all three septa developed early in the ontogeny. Compared to other Lentomitella spp. the ascomata of L. crinigera are larger, exceeding 500 μm diam, and the neck is wider, 120–170 μm. It is probably the only member of the genus which expresses a clear preference for coniferous wood. The examination of the holotype of Ceratostomella triseptata revealed that this name is a synonym of L. crinigera.
Lentomitella crinigera is most similar to L. obscura, L. sulcata and L. striatella in morphology and size of ascospores and asci. Based on phylogenetic evidence, none of these three species is closely related to L. crinigera and their distinction is supported at the RNA structural level. However, in the absence of DNA sequence data, morphological distinction of these species is challenging. Both L. striatella and L. sulcata are known only from New Zealand and were collected on decaying wood of Nothofagus sp.; the length of their asci is in the upper range typical of L. crinigera. On the other hand, the asci of L. obscura are shorter than those of L. sulcata and L. striatella and their length is in the lower range of L. crinigera. Lentomitella obscura can be distinguished from L. crinigera in having slightly shorter ascospores and by occurrence on deciduous wood; all three strains of L. obscura originate from various deciduous hardwoods from three localities in Ariège, southern France. Therefore, the host determination is important to aid the identification of these morphologically similar species.
The substrate of the specimen of L. crinigera sequenced in this study (M.R. 3742, culture CBS 138678) has not been determined. The wood is very rotten and was collected in a locality with stands of Fagus sylvatica, Picea abies and old fallen trunks of Abies alba. Our attempts to isolate DNA from herbarium material or cultivate L. crinigera from identified coniferous substrates were not successful.
Lentomitella investita (Schw.) Réblová, comb. nov. MycoBank MB821766. Fig. 12.
Fig. 12.
Lentomitella investita. A, B. Ascomata. C. Longitudinal section of the ascomal wall. D–G. Asci. H–J. Ascospores. K. Ascal apex with apical annulus. L, M. Conidiophores. N. Conidia. A–E, H, I, K–N from PDD 110876, F, G, J from PH 01016198. Scale bars: A, B = 500 μm, C = 20 μm, D–G = 10 μm, H–N = 5 μm.
Basionym: Sphaeria investita Schw., Trans. Amer. Phil. Soc. 2, Vol. 4: 216. 1834.
Synonyms: Ceratostoma investitum (Schw.) Ellis & Everh., North Amer. Pyrenom. p. 193. 1892.
Ceratostomella investita (Schw.) Starbäck, Bih. Kongl. Svenska Vet.-Akad. Handl. 19(2): 26. 1894.
Amphitrichum investitum (Schw.) Kuntze, Revis. Gen. Pl. 3(2): 443. 1898.
Ceratostomella vestita Sacc. var. varvicensis Grove, J. Bot. 23: 131. 1885.
Ceratostomella maderensis Petr., Bot. Jahrb., Beiblt. 142: 98. 1929.
Sexual morph: Ascomata immersed to partially erumpent with protruding necks becoming superficial with only base immersed, solitary or in groups. Venter 300–350(–400) μm diam, 320–360 μm high, globose to subglobose, dark brown to black, with sparse, brown to reddish-brown, septate hairs 3.5–4 μm wide at the lower part. Neck central, 90–110 μm wide, up to 800 μm long, cylindrical, upright, straight or slightly flexuous, glabrous, tapering, apex sulcate. Ostiole periphysate. Ascomatal wall fragile to leathery, 37–56 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura angularis to textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses becoming disintegrated with age, septate, constricted at the septa, hyaline, 4.5–10 μm wide, tapering to ca. 3.5 μm. Asci (46–)50–56(–65) × 6–7 μm (mean ± SD = 54.3 ± 2.3 × 6.7 ± 0.3 μm), 45–52 μm (mean ± SD = 49.3 ± 2.3 μm) long in the sporiferous part, truncate at the apex, cylindrical to clavate, with a short stipe; with 8 obliquely uniseriate, partly overlapping ascospores; apical annulus ca. 2.5–3 μm wide, 1.5 μm high. Ascospores (7–)7.5–9 × 3.5–4(–4.5) μm (mean ± SD = 8.1 ± 0.3 × 3.7 ± 0.3 μm), ellipsoidal or ellipsoidal-fusiform, slightly inequilateral, hyaline, longitudinally striate, 1(–3)-septate, first-formed septum in the middle, with two large drops. Asexual morph: A phaeoisaria-like asexual morph was formed in vitro. Sporulation sparse, widespread throughout the colony. Conidiophores macronematous, mononematous, arising terminally or laterally from aerial hyphae, unbranched or branched apically, brown near the base, subhyaline to hyaline towards the tip, 64–80 × 2–2.5 μm. Conidiogenous cells terminal or intercalary, pale brown to subhyaline at the bottom, hyaline in the conidiogenous part, subcylindrical or slender flask-shaped, tapering toward the apex, with a rachis 16–20 × 1.5–2 μm at the tip; conidiogenesis holoblastic-denticulate, conidial heads slimy, inconspicuous, transparent. Conidia clavate to obovate, slightly apiculate at the base, broadly rounded at the apical end, 4.5–7 × 2 (mean ± SE = 5.2 ± 0.9 × 2) μm, hyaline, aseptate, smooth.
Specimens examined: Germany, Nassau, on decaying wood, in autumn 1894, L. Fuckel (as Ceratostomella rhenana, Herb. Barbey-Boissier 603, G). Great Britain, Sutton, Driffold Lane, on decaying wood, 25 Oct. 1884, W.B. Grove (holotype of Ceratostomella vestita var. varvicensis, K 124145). Madeira, Ribeiro frio, on decaying wood of Laurus novocanariensis, 3 Jun. 1926, A. Ade (holotype of Ceratostomella maderensis, W 03981). New Zealand, South Island, Southland, Southland Distr., Fiordland National park, Lake Monowai 40 km S of Manapouri, Borland Nature Walk 300 m NW of Borland Lodge, on decaying wood of Nothofagus sp., 9 Mar. 2005, M. Réblová M.R. 2951/NZ 443 (PDD 110876); ibid., West Coast, Buller Distr., Victoria Forest Park, Big River Inanganua track 14 km SE of Reefton, on decaying wood of Nothofagus sp., 6 Mar. 2003, M. Réblová M.R. 2721/NZ 222, M.R. 2726/NZ 227; ibid., Westland Distr., Arthur's Pass National Park, Kelly Shelter 5 km W of Otira, Cockayane Nature Walk, on decaying wood of Nothofagus sp., 16 Mar. 2003, M. Réblová M.R. 2829/NZ 339. USA, Pennsylvania, Northhampton County, Bethlehem, on decaying wood, L.D. Schweinitz, S.F. 1621.476 (holotype of Sphaeria investita, PH 01016198).
Notes: Lentomitella investita is characterised by ellipsoidal, mostly 1-septate ascospores with the septum positioned in the middle, with two additional septa developed symmetrically but rarely upon aging only in the collections from New Zealand. The longitudinally striate walls of the ascospores are conspicuous and individual ridges are often seen protruding at the poles. The holotypes of Ceratostomella vestita var. varvicensis (Grove 1885) and Ceratostomella maderensis (Petrak 1929) were examined. Based on the morphology of ascomata, asci and ascospores they are considered conspecific with L. investita. The holotype of C. maderensis deviates slightly in the length of the asci from the holotype of L. investita and other recently collected material; they are 52–63(–68) × 6–7.5 μm. Our specimens from New Zealand fit well the description of L. investita, although the ascospores in PDD 110876 are more tapering towards the ends. This collection was isolated into axenic culture, which is, unfortunately, no longer available. The fungus formed in vitro a phaeoisaria-like asexual morph, which is consistent with asexual morphs observed in L. sulcata and Lentomitella sp. The DNA extraction from herbarium material was not successful.
Lentomitella investita, L. vestita and L. cirrhosa were formerly treated as conspecific (Von Arx, 1952, Réblová, 2006). Based on molecular evidence the two latter are accepted as separate species in our study. Although no DNA sequence data of L. investita are available, given the size of asci and ascospores, the species is intermediate between L. vestita and L. cirrhosa and therefore regarded as distinct. Lentomitella cirrhosa differs from L. investita by longer and wider ascospores and longer asci. On the other hand, L. vestita is well-distinguished from L. investita by shorter, regularly 1-septate ascospores and shorter asci.
Lentomitella investita resembles L. conoidea in the size of ascospores, but the latter differs by longer asci and mostly 1–2-septate ascospores with the first-formed septum in the middle or slightly above or below the middle.
Lentomitella magna Réblová, sp. nov. MycoBank MB821767. Fig. 13.
Fig. 13.
Lentomitella magna. A–C. Ascomata. D. Longitudinal section of the ascomal wall. E–H. Asci. I. Paraphyses. J, K. Ascospores. L. Ascal apex with apical annulus. M, N. Colonies on MLA and PCA after 28 d. A–N from ICMP 18371. Scale bars: A–C = 500 μm, D, I = 20 μm, E–H = 10 μm, J–L = 5 μm.
Etymology: Magnus (L.) meaning big, large, referring to the large ascospores, which are the largest of the known Lentomitella species.
Sexual morph: Ascomata immersed to partially erumpent becoming superficial with only base immersed, solitary. Venter 300–390 μm diam, 310–380 μm high, globose to subglobose, dark brown to black, with dark brown, septate hairs 3–3.5 μm wide growing at the exposed sides. Neck central, 90–110 μm wide, up to 2 000 μm long, cylindrical, upright or decumbent, flexuous, glabrous, tapering, sometimes laterally flattened, apex sulcate. Ostiole periphysate. Ascomatal wall fragile to leathery, 50–56 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura angularis to textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, constricted at the septa, hyaline, 5.5–7.5 μm wide, tapering to ca. 3 μm. Asci (89–)93–102(–105) × (9.5–)10–12 μm (mean ± SD = 97.5 ± 4.7 × 10.7 ± 1.4 μm), (75–)88–95 μm (mean ± SD = 88.1 ± 6.0 μm) long in the sporiferous part, truncate at the apex, cylindrical to clavate, with a short stipe; with 8 obliquely uniseriate or partly biseriate ascospores; apical annulus 3.5–4 μm wide, 2 μm high. Ascospores (12.5–)13–15 × 5.5–6.5 μm (mean ± SD = 13.7 ± 0.9 × 6 ± 0.4 μm), ellipsoidal to ellipsoidal-fusiform, inequilateral, hyaline, with longitudinal ridges that are often discontinuous, 3-septate with four large drops. Asexual morph: unknown.
Culture characteristics: Colonies on MLA 10–12 mm diam after 14 d (13–14 mm after 21 d, 15–16 after 28 d) at 25 °C, circular to slightly irregular, mostly flat, raised at the centre. Aerial mycelium sparse, cottony at the centre surrounded by a wide zone of loose to almost cobwebby mycelium, margin filamentous, colony surface beige in the centre with olive-brown ring of submerged hyphae at the margin; reverse black. Colonies on PCA 3–4 mm diam after 14 d (4–6 mm after 21 d, 5–7 mm after 28 d) at 25 °C, circular to slightly irregular, flat, raised at the centre. Aerial mycelium sparse, cottony to woolly, cobwebby towards the margin, margin filamentous, colony surface pale brown with dark olive-brown ring of submerged hyphae at the margin; reverse black. Vegetative hyphae branched, septate, medium to dark brown, smooth-walled. Margin of the colony consisting of densely branched hyphae of substrate mycelium. Sporulation not observed.
Specimen examined: New Zealand, South Island, West Coast, Westland Distr., Westland Tai Poutini National park, Lake Matheson, 5 km SW from Fox Glacier, on decaying wood, 13 Apr. 2005, M. Réblová M.R. 2961/NZ 781 (holotype, PDD 110877, culture ex-type ICMP 18371).
Notes: Lentomitella magna is characterised by 3-septate, ellipsoidal-fusiform, inequilateral ascospores and asci, which are the largest of all Lentomitella species accepted in this study. It is known only from a single collection from New Zealand. The ascomata are mostly superficial with decumbent or upright necks and horizontally lying venter covered with dark brown hairs. The position of ascomata and necks on the substrate was influenced by their growth under decaying, partly peeled off bark. The longitudinal ridges in the ascospore wall are partly discontinuous giving the ascospore wall a reticulate appearance.
Lentomitella obscura Réblová, sp. nov. MycoBank MB821768. Fig. 14.
Fig. 14.
Lentomitella obscura. A–C. Ascomata. D. Longitudinal section of the ascomal wall. E, F. Asci. G. Paraphyses. H. Ascal apex with apical annulus. I–K. Ascospores. L, M. Colonies on MLA and PCA after 28 d. A–C, F, G, I from CBS 138735, D, E, H, J–M from CBS 138736. Scale bars: A–C = 500 μm, D, G = 20 μm, E, F = 10 μm, H–K = 5 μm.
Etymology: Obscure (L.) meaning indistinct, obscure, referring to the morphology of ascospores.
Sexual morph: Ascomata immersed with protruding necks to partially erumpent or becoming superficial, solitary or densely aggregated. Venter 380–500 μm diam, 370–510 μm high, globose to subglobose, dark brown to black, with brown to reddish-brown, septate hairs 3.5–4 μm wide. Neck central, 120–150 μm wide, up to 1 500 μm long, cylindrical, upright or partly decumbent, straight or slightly flexuous, glabrous, tapering, apex sulcate. Ostiole periphysate. Ascomatal wall fragile to leathery, 35–50 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura angularis to textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, constricted at the septa, hyaline, 5–8 μm wide, tapering to ca. 3 μm. Asci (70–)72–80(–82) × 7.5–8(–8.5) μm (mean ± SD = 76.2 ± 3.2 × 8.1 ± 0.2 μm), 65–76 μm (mean ± SD = 71.8 ± 3.4 μm) long in the sporiferous part, truncate at the apex, cylindrical to clavate, with a short stipe; with 8 obliquely uniseriate or partly biseriate ascospores; apical annulus ca. 3 μm wide, 1.5–2 μm high. Ascospores (10–)11–12 × 4–5(–5.5) μm (mean ± SD = 11.7 ± 0.6 × 5 ± 0.4 μm), ellipsoidal-fusiform, hyaline, longitudinally striate, 1–3-septate. Asexual morph: unknown.
Culture characteristics: Colonies on MLA 19–23 mm diam after 14 d (30–32 mm after 21 d, 37–42 after 28 d) at 25 °C, circular, convex. Aerial mycelium abundant, woolly, loose towards the margins, margin filamentous, colony surface pale brown-beige with a brown ring of submerged hyphae at the margin; reverse black. Colonies on PCA 7–10 mm diam after 14–28 d in CBS 138736; 10–13 mm after 14 d in CBS 138735 and CBS 137799 (20–21 mm after 21 d, 27–28 mm after 28 d) at 25 °C; circular to irregular, convex. Aerial mycelium abundant, cottony to woolly, loose towards the margins, margin filamentous, colony surface beige-grey with olive-brown ring of submerged hyphae at the margin; reverse black. Vegetative hyphae branched, septate, medium to dark brown, smooth-walled. Margin of the colony consisting of unbranched or sparsely branched hyphae of substrate mycelium. Sporulation not observed.
Specimens examined: France, Ariège, Rimont, Las Muros, banks of the Peyrau brook 440 m a.s.l., on decaying wood of Pinus sp., 30 Sep. 2013, M. Réblová M.R. 3707 (PRA-12736, culture CBS 138735); ibid., banks of La Maille brook, 550 m a.s.l., on decaying wood, 2 Oct. 2013, M. Réblová M.R. 3784 (holotype, PRA-12737, culture ex-type CBS 138736); ibid., along D18 1.5 km S of the village, banks of Le Baup brook, on decaying wood of Quercus sp., 3 Oct. 2013, M. Réblová M.R. 3801 (culture CBS 137799).
Notes: The specimens of L. obscura were collected in three different localities in the Ariège region in France. The ascomatal necks vary greatly in length within one collection. They are elongated up to 1 500 μm in more humid areas, especially in cracks of wood, and much shorter (ca. 600 μm) when growing on the surface of the wood, where they are more exposed to air and sun. The hairs, which abundantly cover the venter, disappear with age leaving the ascomata almost glabrous, but remnants of hairs can be seen as a tomentum tightly attached to the base of ascomata and wood.
Although all three strains of L. obscura form a strongly supported monophyletic clade and their DNA sequences are identical, the morphological delimitation of this species was hindered by different stages of their growth and maturation resulting in a variable morphology of the ascospores. Two specimens, i.e. M.R. 3707 (culture CBS 138735) and M.R. 3801 (culture CBS 137799), showed mostly ellipsoidal, 1–2-septate ascospores with the third septum developing rarely and mostly in the shrinking ascospores. Moreover, their ascospores measuring 9–10(–11) × 4–4.5(–5) μm and asci 65–72(–75) × 6.5–7.5 μm were shorter than those of the third strain, which is designated as holotype (PRA-12737, ex-type strain CBS 138736). The holotype of L. obscura exhibits a perfectly mature stage with ellipsoidal-fusiform ascospores that are regularly 3-septate. In vitro, the colonies of all three strains have identical appearance on MLA and PCA media except that the ex-type strain grows slower on PCA (7–10 mm vs. 27–28 mm after 28 d). Given these circumstances, it is extremely difficult to identify the strains with shorter ascospores based on sexual characters only; in such case at least ITS sequences should be produced for further comparison.
In the phylogenetic tree (Fig. 1), L. obscura is placed with Lentomitella sp., L. magna and L. sulcata in a highly supported subclade; all these species having ellipsoidal-fusiform, 3-septate ascospores. The latter three species all originate from New Zealand. Lentomitella obscura is similar to L. sulcata in morphology and size of ascospores, but the latter differs in longer asci and it is delimited at the RNA structural level by a unique CBC (Fig. 4), see Discussion.
Lentomitella striatella Réblová, sp. nov. MycoBank MB821769. Fig. 15.
Fig. 15.
Lentomitella striatella. A. Ascomata. B. Longitudinal section of the ascomal wall. C, D. Asci. E. Paraphyses. F. Ascal apex with apical annulus. G, H. Ascospores. A from M.R. 2694, B–H from ICMP 18369. Scale bars: A = 500 μm, B, E = 20 μm, C, D = 10 μm, F–H = 5 μm.
Etymology: Diminutive of Striatus (L.) meaning striped, referring to a fine linear marking in the longitudinally striate ascospores.
Sexual morph: Ascomata immersed to partially erumpent with protruding necks, solitary or in groups. Venter 330–400 μm diam, 310–410 μm high, globose to subglobose, dark brown to black, with sparse, dark brown, septate hairs ca. 3.5 μm wide. Neck central, 90–110 μm wide, 600–1 500 μm long, cylindrical, upright, straight, glabrous, tapering, apex sulcate. Ostiole periphysate. Ascomatal wall fragile to leathery, 40–44 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, constricted at the septa, hyaline, 6.5–9.5 μm wide, tapering to ca. 4 μm. Asci (75–)82–88 × 9–10.5 μm (mean ± SD = 85 ± 3.9 × 9.8 ± 0.6 μm), 68–78(–81) μm (mean ± SD = 75.8 ± 4.4 μm) long in the sporiferous part, truncate at the apex, cylindrical to clavate, with a short stipe; with 8 obliquely uniseriate or partly biseriate ascospores; apical annulus 3–3.5 μm wide, 1.5–2 μm high. Ascospores 11.5–13(–14) × 5–5.5 μm (mean ± SD = 12 ± 0.7 × 5.2 ± 0.3 μm), ellipsoidal, slightly inequilateral, hyaline, longitudinally striate, 3-septate. Asexual morph: unknown.
Culture characteristics: Colonies on MLA 12–16 mm diam after 14 d (15–19 mm after 21 d, 18–21 after 28 d) at 25 °C, circular, raised. Aerial mycelium abundant, cottony, loose towards the margin, colony surface brown to dark olive-brown turning brown towards the margin; reverse black. Colonies on PCA 11–12 mm diam after 14 d (13–16 mm after 21 d, 14–18 mm after 28 d) at 25 °C, circular, raised. Aerial mycelium abundant, cottony to woolly, loose towards the margin, colony surface brown-grey with olive-brown ring of submerged hyphae at the margin; reverse black. Vegetative hyphae branched, septate, medium to dark brown, smooth-walled. Margin of the colony consisting of unbranched or sparsely branched hyphae of substrate mycelium. Sporulation not observed.
Specimens examined: New Zealand, South Island, West Coast, Buller Distr., Victoria Forest Park, Duffy´s Creek Track, 30 km SE from Reefton, on decaying wood of Nothofagus sp., 7 Apr. 2005, M. Réblová M.R. 2959/NZ 751 (holotype, PDD 110878, culture ex-type ICMP 18369); ibid., Palmer´s Hut 18 km SW of Springs Junction on unpaved road, Lake Christabel track, on decaying wood of Nothofagus sp., 1 Mar. 2003, M. Réblová M.R. 2694/NZ 195; ibid., Westland Distr., Arthur's Pass National park, Arthur's Pass village, on decaying wood of Nothofagus solandri, 17 Mar. 2003, M. Réblová M.R. 2870/NZ 387.
Notes: Lentomitella striatella appears as sister to L. cirrhosa and together they form a strongly supported monophyletic subclade at the base of the Lentomitella clade. It differs from L. cirrhosa in having longer and wider asci and longer, 3-septate ascospores. The septa are formed early in ontogeny still within the asci, while ascospores of L. cirrhosa are mostly 1-septate with two additional septa observed occasionally in released and shrinking ascospores.
In morphology of ascospores, L. striatella resembles L. crinigera, L. magna, L. obscura and L. sulcata. Lentomitella crinigera and L. sulcata differ from L. striatella by shorter ascospores and slightly longer asci, and L. magna is well-distinguished from L. striatella by longer ascospores and asci, while L. obscura possesses much shorter asci. The ascospore wall of L. striatella is longitudinally striate, but the ridges are shallow and appear less conspicuous compared to other species.
Lentomitella sulcata Réblová, sp. nov. MycoBank MB821770. Fig. 16.
Fig. 16.
Lentomitella sulcata. A–C. Ascomata. D. Longitudinal section of the ascomal wall. E–G. Asci. H. Paraphyses. I, J. Ascospores. K. Ascal apex with apical annulus. L, M. Conidiophores. N. Conidia. O, P. Colonies on MLA and PCA after 28 d. A–P from ICMP 15124. Scale bars: A–C = 500 μm, D, H = 20 μm, E–G = 10 μm, I–N = 5 μm.
Etymology: Sulcatus (L.) meaning furrowed or grooved, referring to the apex of the ascomatal neck, which has several deep sulcations.
Sexual morph: Ascomata immersed to partially erumpent with protruding necks, solitary. Venter 300–400 μm diam, 310–400 μm high, globose to subglobose, dark brown to black, with sparse dark brown to reddish-brown, septate hairs 3–4 μm wide covering the lower part. Neck central, 100–120 μm wide, up to 700 μm long, cylindrical, upright, straight or slightly flexuous, glabrous, apex sulcate. Ostiole periphysate. Ascomatal wall fragile to leathery, 30–45 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura angularis to textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses longer than the asci, becoming disintegrated with age, septate, constricted at the septa, hyaline, 8–12.5 μm wide. Asci 79–90(–93) × 8.5–9.5(–10) μm (mean ± SD = 86.4 ± 4.0 × 9.2 ± 0.4 μm), 72–84(–86) μm (mean ± SD = 80.3 ± 4.1 μm) long in the sporiferous part, broadly rounded to truncate at the apex, cylindrical to clavate, with a short stipe; with 8 obliquely uniseriate ascospores; apical annulus ca. 3 μm wide, 2 μm high. Ascospores (10.5–)11–12(–13) × 5–5.5 μm (mean ± SD = 11.7 ± 0.5 × 5.3 ± 0.3 μm), ellipsoidal to ellipsoidal-fusiform, slightly inequilateral, hyaline, longitudinally striate, 1–3-septate with four large drops, when vital. Asexual morph: Conidiophores in vitro macronematous, mononematous, arising terminally or laterally from aerial hyphae, unbranched or rarely branched apically, pale brown near the base, subhyaline to hyaline towards the tip, 24–45 × 2–2.5 μm. Conidiogenous cells terminal or intercalary, hyaline, cylindrical, tapering toward the apex, with a rachis 9–20(–25) × 2–2.5 μm at the tip bearing 2–10 hyaline denticles 0.5–1 × 0.5–1 μm; conidiogenesis holoblastic-denticulate, conidial heads slimy, inconspicuous, transparent. Conidia ellipsoidal to globose, apiculate at the base, 4–6 × 2–2.5 μm (mean ± SE = 5.2 ± 0.2 × 2.2 ± 0.3 μm), hyaline, aseptate, smooth.
Culture characteristics: Colonies on MLA 19–22 mm diam after 14 d (23–25 mm after 21 d, 25–26 after 28 d) at 25 °C, circular, convex. Aerial mycelium abundant, cottony to woolly, margin filamentous, colony surface beige-brown with olive-brown marginal ring of submerged hyphae; reverse black. Colonies on PCA 12–15 mm diam after 14 d (15–17 mm after 21 d, 18–19 mm after 28 d) at 25 °C, circular, convex. Aerial mycelium abundant, woolly, loose towards the margin, margin filamentous, colony surface beige-grey with dark olive-brown marginal ring of submerged hyphae; reverse black. Vegetative hyphae branched, septate, medium to dark brown, smooth-walled. Margin of the colony consisting of unbranched or sparsely branched hyphae of substrate mycelium. Sporulation copious, widespread throughout the colony.
Specimen examined: New Zealand, South Island, West Coast, Buller Distr., Victoria Forest park, Reefton, Lake Stream track, 30 km SE of Reefton, on decaying wood of Nothofagus sp., 27 Feb. 2003, M. Réblová M.R. 2659/NZ 145 (holotype, PDD 81435, culture ex-type ICMP 15124 = CBS 113655).
Notes: Although the specimen PDD 81435 was formerly treated under L. crinigera (Réblová 2006), it is introduced as a new species, L. sulcata, in this study. Their distinction as two different species, though morphologically difficult, is corroborated by molecular and RNA structural data.
Lentomitella sulcata is nested together with L. magna, L. obscura and Lentomitella sp. in a monophyletic clade. It is characterised by 3-septate, longitudinally striate ascospores and it forms simple, rarely branched conidiophores with globose to ellipsoidal, apiculate conidia in vitro. The asexual morph is most similar to Lentomitella sp. and also comparable to that produced by L. investita.
Lentomitella tenuirostris Réblová, sp. nov. MycoBank MB821771. Fig. 17.
Fig. 17.
Lentomitella tenuirostris. A–C. Ascomata. D. Longitudinal section of the ascomal wall. E, F. Asci. G. Ascal apex with apical annulus. H. Paraphyses. I–K. Ascospores. L, M. Colonies on MLA and PCA after 28 d. A, B, E, G from CBS 141371, C, D, H, J–M from CBS 138734, F, I from M.R. 3712. Scale bars: A–C = 500 μm, D, H = 20 μm, E, F = 10 μm, G, I–K = 5 μm.
Etymology: Tenuis (L.) meaning thin, rostrum (L.) meaning beak, referring to ascomata with a slender projecting neck.
Sexual morph: Ascomata immersed to partially erumpent with protruding necks, becoming superficial, solitary, sometimes in rows or small groups. Venter 300–410 μm diam, 300–420 μm high, globose to subglobose, black, upright sometimes positioned horizontally towards the substrate, with sparse, dark brown, septate hairs 3–4 μm wide growing from the bottom and lower part, disappearing with age, venter later appears almost glabrous. Neck central, 90–110 μm wide, up to 900 μm long, cylindrical, upright, straight or slightly flexuous, tapering, apex sulcate. Ostiole periphysate. Ascomatal wall fragile to leathery, 32–42 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, constricted at the septa, hyaline, 5–9 μm wide, tapering to ca. 3.5 μm. Asci (58–)60–66(–69) × 7–7.5(–8) μm (mean ± SD = 62.9 ± 3.0 × 7.4 ± 0.5 μm), 52–62 μm (mean ± SD = 57.7 ± 3.2 μm) long in the sporiferous part, truncate at the apex, cylindrical, with a short stipe; with 8 uniseriate partially overlapping or obliquely uniseriate ascospores; apical annulus ca. 2.5 μm wide, 1.5 μm high. Ascospores (8.5–)9–10.5 × 4–4.5 μm (mean ± SD = 9.8 ± 0.6 × 4.3 ± 0.3 μm), ellipsoidal to suboblong, slightly inequilateral, sometimes slightly curved, hyaline, longitudinally striate, 1–3-septate, mostly 1–2-septate, the first-formed septum in the middle or slightly above or below the middle, formation of the third septum delayed, visible in old ascospores. Asexual morph: unknown.
Culture characteristics: Colonies on MLA 10–13 mm diam after 14 d (12–14 mm after 21 d, 19–20 mm after 28 d) at 25 °C, circular, convex to pulvinate. Aerial mycelium abundant, cottony to felty, loose to floccose towards the margin, margin filamentous, colony surface beige to brown-grey with outer olivaceous ring and darker olive-brown ring of submerged hyphae at the margin; reverse black. Colonies on PCA 7–10 mm diam after 14 d (9–10 mm after 21 d, 10–12 after 28 d) at 25 °C, circular, convex. Aerial mycelium abundant, cottony to felty, floccose towards the margin, margin filamentous, colony surface beige-grey with dark olive-brown ring consisting of submerged hyphae at the margin; reverse black. Margin of the colony consisting of unbranched or sparsely branched hyphae of substrate mycelium. Sporulation not observed.
Specimens examined: Czech Republic, Southern Bohemia, Novohradské hory Mts., Pohoří na Šumavě, Myslivna Mt., on decaying wood of Sorbus aucuparia, 6 Oct. 2012, M. Réblová M.R. 3771 (holotype, PRA-12738, culture ex-type CBS 138734); ibid., Dobrá voda, Hojná voda National nature monument, on decaying wood of Fagus sylvatica, 4 Oct. 2012, M.R. 3735; ibid., 28 Sep. 2014, M.R. 3859. Šumava Mts. National park, Železná Ruda, glacial cirque of the Černé jezero lake National nature reserve, on decaying wood of Picea abies, 22 Oct. 1998, M.R. 858; ibid., Boubínský prales National nature reserve, on decaying wood of Fagus sylvatica, 14 Aug. 1999, M.R. 1533; ibid., Mt. Spáleniště near Stožec, on decaying wood of Fraxinus excelsior, 16 Aug. 1999, M.R. 1545; ibid., Povydří National nature reserve, Čeňkova Pila, on decaying wood of Ulmus glabra, 27 Aug. 2000, M.R. 1677. France, Ariège, Rimont, Las Muros, banks of the Peyrau brook 440 m a.s.l., on decaying wood, 30 Sep. 2013, M. Réblová M.R. 3715 (culture CBS 141371), M.R. 3712, M.R. 3714.
Notes: Lentomitella tenuirostris and L. conoidea form a pair of closely related and morphologically highly similar species. There are only a few subtle differences in ascus and ascospore characters and the colony appearance in vitro, which make their correct identification challenging in the absence of molecular data. Lentomitella tenuirostris differs from L. conoidea by slightly shorter asci, slightly longer ascospores and generally smaller ascomata clothed by sparse dark brown to black hairs, but the venter soon becomes glabrous. The development of the third septum in ascospores of L. tenuirostris was observed in shrinking ascospores still within the asci only in specimen M.R. 3712 collected in the same locality and on the same day as specimen M.R. 3715, whose culture CBS 141371 was sequenced in our study. Only two strains of L. tenuirostris were isolated in axenic culture. Several other specimens from France and the Czech Republic fit well the description of this species.
Lentomitella tenuirostris can be distinguished from the morphologically similar L. investita by shorter asci and shorter, mostly 1-septate ascospores of the latter. Lentomitella unipretoriae (Marincowitz et al. 2008) has similar ascospore size, but it differs from L. tenuirostris by regularly 3-septate ascospores and longer asci.
Lentomitella unipretoriae M.J. Wingf. et al., CBS Biodiversity Ser. 7: 60. 2008.
Notes: For description and illustration see Marincowitz et al. (2008). The authors emphasized the evanescent nature of paraphyses as a unique character of this species, however, this is a character common to all members of Lentomitella. Lentomitella unipretoriae originates from South Africa where it was collected on a senescent flower head of Protea lepidocarpodendron. It is most similar to L. crinigera, L. magna, L. striatella and L. sulcata in 3-septate, ellipsoidal ascospores, but differs from them by smaller ascospores whose length does not exceed 11 μm, (8–)9–10.5(–11) × 4–5 μm fide Marincowitz et al. (2008). No DNA sequence data nor a living culture of this species are available.
Lentomitella vestita (Sacc.) Höhn., Annls mycol. 3: 548. 1906. Fig. 18.
Fig. 18.
Lentomitella vestita. A, B. Ascomata. C. Longitudinal section of the ascomal wall. D. Asci. E. Paraphyses. F. Ascal apex with apical annulus. G–K. Ascospores. A–K from PRA-12739. Scale bars: A, B = 500 μm, C, E = 20 μm, D = 10 μm, F–K = 5 μm.
Basionym: Ceratostomella vestita Sacc., Michelia 1: 370. 1878.
Synonyms: Cerastomis vestita (Sacc.) Clem., Gen. Fungi p. 259. 1931.
Endoxyla vestita (Sacc.) Munk, Bot. Tidsskr. 61: 64. 1965.
Sexual morph: Ascomata immersed to partially erumpent with protruding necks or becoming superficial, solitary or in small groups. Venter 300–380 μm diam, 310–400 μm high, globose to subglobose, dark brown to black, with dark brown, septate hairs 4‒4.5 μm wide covering the lower part. Neck central, 90–110 μm wide, up to 600 μm long, cylindrical, upright, straight, glabrous, apex sulcate. Ostiole periphysate. Ascomatal wall fragile to leathery, 33–40 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura angularis to textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, constricted at the septa, hyaline, 6–10 μm wide, tapering to 3.5–4 μm. Asci (40–)42–51(–55) × (5.5–)6–7 μm (mean ± SD = 47 ± 3.9 × 6.5 ± 0.3 μm), 36–45(–48) μm (mean ± SD = 41.2 ± 3.1 μm) long in the sporiferous part, truncate at the apex, cylindrical to clavate, with a short stipe; with 8 obliquely uniseriate ascospores; apical annulus 2–2.5 μm wide, 1.5 μm high. Ascospores (5.5–)6–7 × 3–3.5 μm (mean ± SD = 6.7 ± 0.4 × 3.3 ± 0.2 μm), ellipsoidal, broadly rounded at both ends but sometimes slightly tapering, hyaline, longitudinally striate, 1-septate. Asexual morph: unknown.
Specimens examined: Czech Republic, Central Bohemia, Lysá nad Labem, Byšičky, Hrbáčkovy tůně National nature reserve, on decaying wood of Quercus sp., 11 Nov. 2012, M. Réblová M.R. 3677 (PRA-12739). Denmark, Fyn, Snarup Mose SW of Kværndrup, on inner side of bark of Betula sp., 1 Apr. 1999, J. Vesterholt JV99-015 (C 45296); ibid., Sjælland, Charlottenlund skov, on decaying wood, 10 Nov. 1964, A. Munk (C); ibid., Dyrehaven, on decaying wood, 26 Nov. 1964, A. Munk (C); ibid., on decaying wood of Fagus sylvatica, 2 Dec. 1963, A. Munk (C). Germany, Oestrich forest, on decaying wood, autumn, L. Fuckel (Fungi Rhen. Exs. No. 1804, G); ibid., on decaying wood of Fagus sylvatica, L. Fuckel (as Ceratostomella rostrata, Herbarium Barbey-Boissier, G). Italy, on decaying wood, Sep. 1878. P.A. Saccardo (holotype of Ceratostomella vestita, PAD).
Notes: Lentomitella vestita is well-distinguished from other Lentomitella species by the shortest ascospores and asci in the genus and the 1-septate ascospores that do not develop additional septa. The neck in the holotype does not appear sulcate, i.e. the tip of the neck is without longitudinal ridges and instead it appears only slightly roughened. However, the material is old and the ridges could have disappeared upon aging and some of the tips were broken. All other collections of L. vestita that were examined in this study have ascomata with necks distinctly sulcate at the tip, which is a character common to all Lentomitella species.
Lentomitella vestita resembles L. investita in 1-celled ellipsoidal ascospores, but the latter differs in having longer asci and longer and wider ascospores that occasionally develop two additional septa upon aging.
Despite our numerous attempts we did not obtain L. vestita in culture. Therefore, the DNA was extracted from herbarium material of our collection PRA-12739 that matches the holotype. Some ascospores in this collection seem to taper slightly at the poles, which is caused to a certain extent by protrusion of the fine longitudinal ridges, a character we also observed in L. investita.
Lentomitella sp. Fig. 19.
Fig. 19.
Lentomitella sp. A. Longitudinal section of the ascomal wall. B. Paraphyses. C. Ascal apex with apical annulus. D. Ascospores. E, F. Asci. G–J. Conidiophores. K. Conidia. A–K from M.R. 2953. Scale bars: A, B = 20 μm, C, D, G–K = 5 μm, E, F = 10 μm.
Sexual morph: Ascomata immersed to partially erumpent with protruding necks, solitary. Venter 390–480 μm diam, 370–500 μm high, globose to subglobose, dark brown to black, with sparse brown, septate hairs 3.5–4 μm wide. Neck central, 110–130 μm wide, up to 700 μm long, cylindrical, upright, straight or slightly flexuous, glabrous, tapering, apex sulcate. Ostiole periphysate. Ascomatal wall fragile to leathery, 35–43 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura angularis to textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, becoming disintegrated with age, septate, constricted at the septa, hyaline, 4.5–8 μm wide. Asci (61–)63–70(–75) × (7.5)8–9.5 μm (mean ± SD = 67.1 ± 4.5 × 8.7 ± 0.7 μm), (55–)60–63 μm (mean ± SD = 61.1 ± 4.7 μm) long in the sporiferous part, truncate at the apex, cylindrical to clavate, with a short stipe; with 8 obliquely uniseriate or partly biseriate ascospores; apical annulus ca. 3 μm wide, 1.5–2 μm high. Ascospores 9.5–11 × 4.5–5 μm (mean ± SD = 10.5 ± 0.5 × 4.8 ± 0.3 μm), ellipsoidal-fusiform, inequilateral, hyaline, longitudinally striate, 1–3-septate. Asexual morph: Sporulation in vitro sparse, widespread throughout the colony. Conidiophores macronematous, mononematous, unbranched or rarely branched apically, arising terminally or laterally from aerial hyphae, pale brown, subhyaline towards the tip, 40–45 × 2–2.5 μm. Conidiogenous cells terminal or intercalary, pale brown or subhyaline, cylindrical, slightly tapering toward the apex, with a rachis 15–25 × 2(–2.5) μm, at the tip bearing numerous hyaline denticles 0.5–1 μm wide, 0.5–1 μm long, conidiogenesis holoblastic-denticulate, conidial heads slimy, inconspicuous, transparent. Conidia ellipsoidal to globose, apiculate at the base, 2–3(–4) × 2–3 (mean ± SE = 3.1 ± 0.9 × 2.6 ± 0.4) μm, hyaline, aseptate, smooth.
Specimen examined: New Zealand, South Island, Southland Distr., Catlins Coastal Rain Forest park, Haldane, Kiwi Walk along the Waipohatu stream, at the end of Waipohatu Road, 11 km W of Waikawa, 14 Mar. 2005, on decaying wood, M.R. 2953/NZ 500.
Notes: This collection from New Zealand is grouped in a monophyletic clade consisting of this and the three species L. magna, L. obscura and L. sulcata; all are characterised by 3-septate, ellipsoidal-fusiform, slightly inequilateral ascospores. It is most similar to L. sulcata, which also originates from New Zealand, in morphology of ascospores and conidia but differs in shorter ascospores and asci. For comparison of this clade at the RNA structural level see Discussion. The specimen M.R. 2953 was scarce and contained only a few ascomata; because no material is left after isolation and examination, and the culture is no longer available, this specimen is labelled Lentomitella sp. for the time being and its morphological description, illustration and DNA sequences are published in this study.
Spadicoides S. Hughes, Canad. J. Bot. 36: 805. 1958; emend. Réblová & A.N. Mill.
Synonyms: Xenospadicoides Hern.-Restr. et al., Stud. Mycol. 86: 92. 2017.
Pseudodiplococcium Hern.-Restr. et al., Stud. Mycol. 86: 92. 2017.
Sexual morph: Ascomata perithecial, non-stromatic, immersed to partially erumpent with protruding necks, solitary, in short rows or grouped. Venter globose or subglobose, often pinched laterally upon drying, dark brown to black, clothed with septate dark hairs; surface sometimes covered by a bright waxy layer staining the surrounding substrate. Neck central, upright, sometimes slightly decumbent, glabrous, the projecting part dark brown, light fulvous to subhyaline, cylindrical, apex smooth or with several sulcations. Ostiole periphysate. Ascomatal wall fragile to leathery, two-layered. Paraphyses becoming disintegrated apically, anastomosing, septate. Asci cylindrical to clavate, with a short stipe, 8-spored, apex with a distinct, non-amyloid apical annulus. Ascospores ellipsoidal to ovoid, often inequilateral, aseptate or with a middle septum usually developed upon aging, hyaline, smooth-walled or delicately verrucose. Asexual morph: Colonies dark, effuse, stroma absent. Conidiophores macronematous, mononematous, unbranched, occasionally with branches, erect or ascending, straight or flexuous, septate, brown, paler towards the apex. The upper three-quarters or less of the conidiophores are usually conidiogenous. Conidiogenous cells terminal or intercalary in the upper part of the conidiophore, cylindrical, with numerous small pores in the wall, conidia formed singly or in chains at the apex and laterally, conidiogenesis tretic. The number of pores per conidiogenous cell usually 5–15 causing the conidia to entirely envelope the conidiophore in a dry mass. Conidia oblong, ellipsoidal or obovoid, aseptate or several-septate, brown, borne singly or occasionally in chains at the apex and laterally in the position of pores and secede readily. Synasexual morph: A selenosporella-like is sometimes formed in vitro and in vivo. Conidiophores macronematous or semimacronematous, mononematous, branched or unbranched, often reduced to conidiogenous cells. Conidiogenous cells discrete, terminal or intercalary, subcylindrical to flask-shaped, polyblastic, sympodially proliferating with a short terminal rachis, often arising from vegetative hyphae or directly from conidia, sometimes reduced to a few denticles. Conidia clavate, oblong or narrow fusiform, hyaline, aseptate, smooth-walled.
Type species: Spadicoides bina (Corda) S. Hughes
Notes: Based on a revision of the holotypes of the two Ceratostomella species, their novel DNA sequence data and cultures, and nucLSU sequence of the ex-type strain of Pseudodiplococcium ibericum (Hernández-Restrepo et al. 2017), three new combinations are proposed in Spadicoides, namely S. fuscolutea with Lentomitella tomentosa and S. grovei as synonyms, S. hyalostoma and S. iberica.
The sexual-asexual relationship of Spadicoides was experimentally proven in our study for S. bina, S. fuscolutea and S. hyalostoma. Spadicoides atra and S. iberica remain asexual. A selenosporella-like synasexual morph has been encountered for S. bina and S. fuscolutea in vitro (see Discussion). Given these results, the description of Spadicoides is expanded to include sexual and asexual characters.
The Spadicoides sexual morphs, especially S. bina and S. fuscolutea, are strongly reminiscent of Lentomitella in morphology of ascomata with a sulcate neck and venter clothed by dark interwoven hairs and cylindrical short-stipitate asci with a distinct apical annulus. However, Lentomitella differs from Spadicoides by 1–3-septate, longitudinally striate vs. aseptate or rarely 1-septate, smooth-walled or verrucose ascospores.
The main mode of conidiogenesis of Spadicoides is tretic, rarely accompanied by a holoblastic-denticulate conidiogenesis of a selenosporella-like synasexual morph. A key to holomorphic Spadicoides is provided below. Keys to asexual morphs of Spadicoides were published in Ellis, 1963, Wang, 1976, Holubová-Jechová, 1982, Goh and Hyde, 1996 and Ma et al. (2016).
Key to sexually reproducing Spadicoides species
| 1. | Ascospores delicately verrucolose ………………………….... S. hyalostoma |
| 1. | Ascospores smooth-walled ……………………………………………………. 2 |
| 2. | Ascospores (7.5–)8–9.5 × 3.5–4.5 μm ……………………………… S. bina |
| 2. | Ascospores (13–)14–16(–17) × (5–)5.5–6(–7) μm ………... S. fuscolutea |
Spadicoides bina (Corda) S. Hughes [as ‘binum’], Canad. J. Bot. 36: 806. 1958. Fig. 20.
Fig. 20.
Spadicoides bina. A, B. Ascomata. C. Longitudinal section of the ascomal wall. D. Asci. E. Paraphyses. F. Ascospores. G. Shrinking ascospores with a middle septum (see arrow). H. Conidia. I, K. Selenosporella-like synasexual morph. J, L–N. Conidiophores with conidia (arrows indicate conidia in short chains). O, P. Colonies on MLA and PCA after 28 d. A–G from PRA-13420, H–P from CBS 137794. Scale bars: A, B = 500 μm. C = 20 μm, D, E, I, J, L–N = 10 μm, F, G, H, K = 5 μm.
Basionym: Helminthosporium binum Corda [as ‘Helmisporium’], in Zobel, Icon. Fung. 6: 9. 1854.
For full synonymy see Hughes (1958).
Sexual morph: Ascomata non-stromatic, immersed to partially erumpent with protruding necks, solitary, in rows or small groups. Venter 420–510 μm diam, 390–500 μm high, globose to subglobose, slightly pinched laterally upon drying, upright or lying horizontally, dark brown to black, with dark brown, septate hairs 3–3.5 μm wide covering the lower part. Neck central, 100–120 μm wide, up to 800 μm long, rostrate, glabrous, upright, apex sulcate becoming deeply roughened with age. Ostiole periphysate. Ascomatal wall fragile to leathery, 45–55(–63) μm, two-layered; outer layer consisting of brown, polyhedral cells of textura prismatica to textura angularis with opaque walls; cells tending to be darker towards the outside, and becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming slightly disintegrated at the apex, septate, constricted at the septa, hyaline, 4–5 μm wide, tapering to ca. 2.5 μm. Asci 60–67(–73) × (7.5–)8–9 μm (mean ± SD = 65.1 ± 1.5 × 8.4 ± 0.4 μm), 52–58 μm (mean ± SD = 54.8 ± 2.6 μm) long in the sporiferous part, truncate at the apex, cylindrical to clavate, with a short stipe, ascospores overlapping uniseriate or partly biseriate; with 8 uniseriate to partially biseriate ascospores; apical annulus ca. 2.5 μm wide, 1.5 μm high. Ascospores (7.5–)8–9.5 × 3.5–4.5 μm (mean ± SD = 8.8 ± 0.5 × 3.9 ± 0.4 μm), ellipsoidal to ovoid, aseptate, rarely with a cytoplasmic band in the middle or a middle septum developed in old and shrinking ascospores, hyaline, with one or two large drops, smooth-walled. Asexual morph: Remnants of conidiophores and conidia identical to those developed in vitro were observed in the intimate juxtaposition to ascomata; conidia aseptate 6.5–7 × 3.5 μm, 1-septate 7.5–8 × 3.5 μm. Conidiophores in vitro macronematous, 30–75 μm long, 2.5–3 μm wide, unbranched or with short branches, erect, straight or flexuous, septate, brown, paler towards the apex, smooth-walled. Conidiogenous cells integrated, terminal or intercalary in the upper part of the conidiophore, 2.5–3 μm wide, cylindrical, polytretic with numerous small pores in the wall. Conidia mostly aseptate, 5–6 × 3–3.5 μm (mean ± SD = 5 ± 0.5 × 3.4 ± 0.3 μm), pale to brown, ellipsoidal, rounded at the apical end, with a slightly apiculate basal scar, thick-walled, formed singly through pores or in short chains, at maturity becoming 1-septate, 7–9.5 × 3.5–4 μm (mean ± SD = 7.9 ± 1.1 × 3.8 ± 0.2 μm), pale brown to dark brown, ellipsoidal to oblong, with the middle septum obscured by a black band, slightly constricted at the septum, smooth-walled. Synasexual morph: A selenosporella-like was formed on MLA and PCA in 28 d at 25 °C. Conidiophores semimacronematous, branched or unbranched, pale brown to subhyaline, often reduced to conidiogenous cells. Conidiogenous cells discrete or integrated, terminal, intercalary, often arising directly from dark brown, aseptate conidia, hyaline, subcylindrical or narrowly flask-shaped, 6.5–11.5 μm long, ca. 2 μm wide, tapering to 1–1.2 μm, apically slightly swollen or elongated, polyblastic, sympodially proliferating with a short terminal rachis. Conidia 3.5–4.5 × 1–1.5 μm (mean ± SD = 3.8 ± 0.3 × 1.3 ± 0.2 μm), clavate, oblong, slightly curved, hyaline, aseptate, smooth-walled.
Culture characteristics: Colonies on MLA 14–15 mm diam after 14 d (20–23 mm after 21 d, 34–35 mm after 28 d) at 25 °C, circular, raised. Aerial mycelium abundant, cottony to woolly, margins filamentous, colony surface beige-brown with a thin brown margin of submerged hyphae, the margin disappears as colony ages and is overgrown by aerial hyphae; reverse dark brown. Colonies on PCA 15–16 mm diam after 14 d (19–20 mm after 21 d, 22–23 mm after 28 d) at 25 °C, circular, flat. Aerial mycelium abundant, cottony to felty, margins filamentous, colony surface beige with a brown margin of submerged hyphae, margin disappears as colony ages and is overgrown by aerial hyphae; reverse dark brown. Vegetative hyphae branched, septate, pale brown, 1.5–2.5 μm wide, smooth-walled. Sporulation in 21 d at the margin of the colony.
Specimen examined: Czech Republic, Southern Moravia, Valtice, Rendez-vous National nature monument, on decaying wood of Quercus cerris, 17 Nov. 2012, M. Réblová M.R. 3686 (PRA-13420, culture CBS 137794).
Notes: Spadicoides bina is most similar to S. fuscolutea in morphology of ascomata, asci and ascospores, but the latter differs in presence of orange-red pigment on the outer ascomatal wall layer, larger ascospores, asci and obovate or clavate, 3-septate conidia.
The conidia of S. bina are 0–1-septate, occasionally a second septum can develop (Ellis 1963). Hughes (1973a) examined the type, “cotype” (syntype) and other authentic material and concluded that the number of septa in conidia can vary in different collections. Ellis (1963) illustrated 1-septate conidia of S. bina as usually oblong to oval, non-constricted or slightly constricted with a conspicuous reddish-brown band obscuring the middle septum. Hughes (1973a) noted that 1-septate conidia are “generally waisted” at the middle septum. The 1-septate conidia observed in vivo and those formed in vitro in the strain CBS 137794 were always slightly constricted at the middle septum. The formation of the septum was delayed, conidia were mostly aseptate. Spadicoides bina resembles S. canadensis (Hughes 1973b) in 1-septate, brown, oblong to ellipsoidal conidia, but the latter species differs by somewhat wider conidia (5.2–6.3 μm fide Hughes 1973b) and a septum formed slightly below the middle of the conidium. Spadicoides bina occurs on decaying wood and bark of various deciduous and coniferous trees (Ellis, 1963, Hughes, 1973a, Holubová-Jechová, 1982).
Spadicoides fuscolutea (Rehm) Réblová, comb. nov. MycoBank MB821772. Fig. 21.
Fig. 21.
Spadicoides fuscolutea. A, B. Ascomata. C, D. Asci. E, F. Acospores. G. Longitudinal section of the ascomal wall. H. Paraphyses. I, J. Selenosporella-like synasexual morph. K–M. Conidiophores with conidia. N, O. Colonies on MLA and PCA after 28 d. A–C, G from PRM 902274, D–F, H, I, J, N, O from CBS 141263, K–M from CBS 141262. Scale bars: A, B = 500 μm, C–F, H–M = 10 μm, G = 20 μm.
Basionym: Ceratostomella fuscolutea Rehm, Annls mycol. 6: 320. 1908.
Synonyms: Lentomitella tomentosa Réblová & J. Fourn., Mycologia 98: 86. 2006.
Spadicoides grovei M.B. Ellis, Mycol. Pap. 93: 12. 1963.
Diplococcium grovei (M.B. Ellis) R.C. Sinclair et al., Trans. Br. mycol. Soc. 85: 736. 1986.
Sexual morph: Ascomata non-stromatic, immersed with protruding necks or becoming superficial, solitary, in short rows or groups. Venter 350–500 μm diam, 400–550 μm high, globose to subglobose, pinched laterally upon drying, dark brown to black, with septate, pale brown to reddish-brown hairs ca. 3–5 μm wide; surface of the venter covered by an orange to orange-red waxy layer up to 13 μm thick sometimes disappearing with age; granules of the same pigment also attached to the surface of hairs and also staining the surrounding substrate. Neck central, 100–150(–180) μm wide, 500–700 μm long, cylindrical, upright, tapering, apex with 3–5 deep sulcations. Ostiole periphysate. Ascomatal wall fragile to leathery, 45–55 μm, two-layered; outer layer consisting of brown, thick-walled, polyhedral cells of textura prismatica to textura angularis with opaque walls. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses becoming disintegrated apically, anastomosing, septate, constricted at the septa, hyaline, 4.5–6 μm wide, tapering to 2.5–3 μm. Asci (75–)80–102(–110) × 9.5–11 μm (mean ± SD = 89.1 ± 4.5 × 10.6 ± 1.1 μm), (65–)70–85(–90) μm (mean ± SD = 73.9 ± 4.2 μm) long in the sporiferous part, broadly rounded or truncate at the apex, cylindrical to clavate, with a short stipe; with 8 obliquely uniseriate or biseriate ascospores in the upper sporiferous part; apical annulus 3–3.5 μm wide, 2–2.5 μm high. Ascospores (13–)14–16(–17) × (5–)5.5–6(–7) μm (mean ± SD = 14.5 ± 0.4 × 5.8 ± 0.3 μm), ellipsoidal to ovoid, sometimes inequilateral, aseptate, rarely with a middle septum, hyaline, smooth-walled. Asexual morph: Conidiophores in vitro macronematous, 45–100 μm long, 3–4 μm wide, unbranched, rarely with short branches, erect, straight or flexuous, septate, dark brown, smooth-walled. Conidiogenous cells integrated, intercalary, terminal, positioned in the upper two-thirds of the conidiophore, ca. 3.5(–4) μm wide, cylindrical, with 1–3 pores in the wall visible after the secession of conidia. Conidia 16.5–20(–21) × (7.5–)8–10(–11) μm (mean ± SD = 18.8 ± 1.3 × 8.8 ± 1.0 μm), formed singly through the pores, obovate or clavate, rounded at the apical end, conico-truncate at the base, brown to dark brown, 3-septate, often only with two septa developed; septa thick and dark brown due to the band of colour on the wall. Synasexual morph: A selenosporella-like was formed on MLA in 8 wk at 25 °C. Conidiophores macronematous or semimacronematous, branched, often reduced to conidiogenous cells, pale brown to hyaline, 10–55 μm long, 1.5–2 μm wide. Conidiogenous cells integrated, terminal or intercalary, subhyaline to hyaline, subcylindrical, 10–15 μm long, ca. 2.5 μm wide near the base, tapering to 1.5 μm, polyblastic, sympodially proliferating with a short terminal rachis. Conidia 4–5.5 × 1.5(–2) μm (mean ± SD = 4.6 ± 0.5 × 1.4 ± 0.2 μm), clavate or oblong, slightly curved, hyaline, aseptate, smooth-walled.
Culture characteristics: Colonies on MLA 8–10 mm diam after 14 d (16–17 mm after 21 d, 18–20 mm after 28 d) at 25 °C, circular, convex. Aerial mycelium abundant, woolly, margins filamentous, colony surface beige grey, brown olivaceous towards the margin; reverse black to dark brown. Colonies on PCA 4–5 mm diam after 14 d (6–7 mm after 21 d, 13–14 mm after 28 d) at 25 °C, circular, convex. Aerial mycelium abundant, woolly, margins filamentous, colony surface brown-grey, brown-olivaceous towards the margin; reverse black. Sporulation in 21 d at the margins of the colony.
Specimens examined: Czech Republic, Southern Bohemia, Novohradské hory Mts., Pohoří na Šumavě, Myslivna Mt., on decaying wood of Fagus sylvatica, 6 Oct. 2012, M. Réblová M.R. 3776 (culture CBS 141263); ibid., Horní Stropnice, Hojná Voda National nature monument, on decaying wood of Fagus sylvatica, 4 Oct. 2012, M. Réblová M.R. 3744 (culture CBS 141262); ibid., 13 Oct. 2013, M.R. 3813 (associated with Spadicoides hyalostoma). Denmark, Sjæland, Dyrehaven, on decaying wood of a stump, 3 Oct. 1964, A. Munk (C); ibid., 20 Mar. 1965, A. Munk (C). Silkeborg, Spring area near Almindsø, on decaying wood of Alnus glutinosa, 4 Sep. 1953, A. Munk (C). France, Finistère, Plohars, Forêt de Carnoët, Pont Douar, 40 m a.s.l., on decaying wood of Fagus sylvatica, 26 Oct. 2002, J. Fournier J.F. 02196, associated with the asexual morph (holotype of Lentomitella tomentosa, PRM 902274). Germany, Swabia, Allgäu, Hochgrat Mt., on decaying wood of Fagus sylvatica, 1881, M. Britzelmayer [holotype of Ceratostomella fuscolutea, F11132 (S)].
Notes: Spadicoides fuscolutea is distinguished from other members of the genus by ascomata, which are covered by a thin layer of orange-red pigment that also stains the surrounding wood and 3-septate, brown to dark brown, obovate or clavate conidia, which are truncate at the base and arise singly or in short chains from polytretic conidiogenous cells. The bright pigment dissolves in KOH.
The asexual morph was originally described by Ellis (1963) as Spadicoides grovei from decaying wood of Fagus sylvatica and other trees, while the sexual morph was introduced as Ceratostomella fuscolutea (Rehm 1908) and Lentomitella tomentosa (Réblová 2006), both from wood of F. sylvatica. The link between the two morphs was experimentally proven by ascospore isolation of two specimens M.R. 3744 (culture CBS 141262) and M.R. 3776 (culture CBS 141263) collected on beech wood in the Czech Republic. Both collections fit well into the species concept of C. fuscolutea. Identical conidia and remnants of conidiophores were also observed in the holotype of L. tomentosa (PRM 902274) and in M.R. 3776 around ascomata on the wood or trapped among the ascomatal hairs. Based on the evidence from a taxonomic revision of the holotypes of C. fuscolutea and L. tomentosa and cultivation data, C. fuscolutea is transferred to Spadicoides and S. grovei and L. tomentosa are accepted as its synonyms.
The development of the orange-red layer covering the venter varies. For example, in the holotype of L. tomentosa (Réblová 2006), this layer is conspicuous, while in specimens M.R. 3774 and M.R. 3776 the orange layer almost disappeared probably as a result of aging, but the orange pigment staining the surrounding wood is still visible. In the holotype of Ceratostomella fuscolutea (F11132, S), the orange-pigmented wood surrounding the ascomata is also prominent.
The asexual morph resembles S. xylogena in 3-septate, brown conidia, but the latter taxon differs by longer, oval to broadly ellipsoidal conidia with narrower black bands at the septa (Ellis, 1963, Hughes, 1973c). Based on DNA sequence data, S. xylogena is related to the Pleosporales (Shenoy et al. 2010). Spadicoides constricta, S. klotzschii, and S. obovata resemble S. fuscolutea in clavate, obovate or sometimes also ellipsoidal conidia; S. constricta differs from S. fuscolutea by longer conidia constricted at the septa (Wang & Sutton 1982), while S. klotzschii and S. obovata differ by conidia that are 2-septate, shorter and narrower (Hughes, 1973d, Hughes, 1973e, Holubová-Jechová, 1982).
Spadicoides hyalostoma (Munk) Réblová, comb. nov. MycoBank MB821773. Fig. 22.
Fig. 22.
Spadicoides hyalostoma. A–D. Ascomata. E. Asci. F. Paraphyses. G. Ascospores. H. Conidia. I–K. Conidiophores. L, M. Colonies on MLA and PCA after 28 d. A–D from CBS 137793, E, G from CBS 139771, H–M from CBS 138688. Scale bars: A–D = 250 μm, E, F, I–K = 10 μm, G, H = 5 μm.
Basionym: Endoxyla hyalostoma Munk, Bot. Tidsskr. 61: 62. 1965.
Synonym: Ceratostomella hyalostoma (Munk) Unter., Mycologia 85: 307. 1993.
Sexual morph: Ascomata non-stromatic, immersed to partially erumpent with protruding necks, solitary or grouped. Venter 270–350 μm diam, 280–380 μm high, subglobose, often laterally pinched upon drying, dark brown, glabrous with brown, septate hyphae at the base. Neck central, 90–130 μm wide, 130–170 μm at the widest part, slightly narrower at the base, 250–800 μm long, cylindrical, upright, straight or slightly flexuous, the projecting part subhyaline to light fulvous with darker zones. Ostiole periphysate. Ascomatal wall leathery, 35–42 μm, two-layered; outer layer consisting of brown, polyhedral cells of textura prismatica with opaque walls. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Cells in the neck of textura porrecta with thick-walled clavate cells on the surface. Paraphyses abundant, persistent, becoming disintegrated at the apex, septate, constricted at the septa, hyaline, 3.5–5 μm wide, tapering to ca. 2.5 μm. Asci (36–)40–45(–48) × (5–)5.5–7(–8) μm (mean ± SD = 43.5 ± 2.9 × 6.1 ± 0.4 μm), truncate at the apex, cylindrical to clavate, non-stipitate or with a short narrowly rounded stipe; with 8 obliquely uniseriate or partially biseriate ascospores in the lower sporiferous part; apical annulus ca. 2 μm wide, 1.5–2 μm high. Ascospores (5.5–)6–7 × 3–3.5 μm (mean ± SD = 6.1 ± 0.4 × 3.1 ± 0.2 μm), ellipsoidal to ovoid, 0–1-septate, with one or two large drops, hyaline, delicately verruculose. Asexual morph: Conidiophores in vitro macronematous, 30–130 μm long, 2.5–3 μm wide, unbranched or with short branches, upright, septate, brown, subhyaline or pale brown when young, dark brown at maturity, smooth-walled. Conidiogenous cells integrated, terminal, intercalary, positioned in the upper two thirds of the conidiophore, ca. 2.5 μm wide, cylindrical, polytretic with numerous pores dispersed per cell. Conidia 4.5–6 × 3.5–4(–4.5) μm (mean ± SD = 5.4 ± 0.5 × 3.7 ± 0.4 μm), formed singly through the pores, subglobose to ellipsoidal, sometimes inequilateral, tapering towards base and truncate, aseptate, hyaline becoming dark brown.
Culture characteristics: Colonies on MLA 16–17 mm diam after 14 d (29–30 mm after 21 d, 40–41 mm after 28 d) at 25 °C, circular, raised. Aerial mycelium abundant, cottony to woolly, margins filamentous, colony surface beige with a brown margin of submerged hyphae; reverse dark brown. Colonies on PCA 15–18 mm diam after 14 d (24–26 mm after 21 d, 27–28 mm after 28 d) at 25 °C, circular, raised. Aerial mycelium abundant, cottony to woolly, margins filamentous, colony surface beige-grey, with a brown-olivaceous margin of submerged mycelium; reverse dark brown. Vegetative hyphae branched, septate, smooth-walled, 2–3.5 μm wide, hyaline; hyphae on which the conidiophores arise brown. Periodically arranged clusters of cells bearing short branches are present on submerged hyphae at the colony margin. Sporulation observed after 2 mo at the margins of the colony.
Specimens examined: Czech Republic, Central Bohemia, Křivoklátsko Protected Landscape Area, Karlova Ves, Vysoký Tok Nature Reserve, on decaying wood of Quercus sp., 29 Sep. 2012, M.R. 3662 (culture CBS 137793). Southern Bohemia, Novohradské hory Mts., Horní Stropnice, Hojná Voda National nature monument, on decaying wood of Fagus sylvatica, 13 Oct. 2013, M. Réblová M.R. 3813, associated with S. fuscolutea (culture CBS 138688). Northern Moravia, Podhoří ‘Podhorn’ near Hranice, on decaying wood, 7 Aug. 1923, F. Petrak (as Ceratostomella subpilosa, Fl. Bohem. Morav. Exs. No. 1809, PRM 481212). Southern Moravia, Lednice, Nejdek old Slavic settlement, on decaying wood of Fraxinus excelsior, 27 Oct. 2014, M. Réblová M.R. 3866 (culture CBS 139771); ibid., Břeclav, obora Soutok near Lanžhot, on decaying wood, 23 Oct. 2004, M. Réblová M.R. 2597. Denmark, Sjaelland, Ermelunden forest near Copenhagen, on decaying wood, 15 Dec. 1963, A. Munk (holotype of Endoxyla hyalostoma, collection no. 14, C); ibid., Høje Sandbjerg, decaying wood, 18 Dec. 1964, A. Munk (C); ibid., Dyrehaven, on decaying wood, 3 Nov. 1964, A. Munk (C); ibid., Lellinge, decaying wood, 23 May 1964, A. Munk (C); ibid., Bernstorffsparken, on decaying wood, 23 Mar. 1965, A. Munk (C); ibid., Boserup, on decaying wood of Ulmus sp., soc. Capronia pilosella. 1 Apr. 1963, A. Munk (C); ibid., Rude Skov, on decaying wood of Alnus sp., 4 Feb. 1964, A. Munk (C); ibid., on decaying wood of Fagus sylvatica, 17 Nov. 1963, A. Munk (C); ibid., forest W of Frederikssun, on decaying wood, soc. Ruzenia spermoides, A. Munk (C). Italy, Lazio, province Viterbo, Caprarola, Mt. Venere, on decaying wood of Fagus sylvatica, 2 Apr. 2011, M. Réblová M.R. 3610 (culture CBS 131268).
Notes: Spadicoides hyalostoma can be distinguished from other species of the genus by subhyaline to light fulvous necks with darker zones and verruculose, aseptate ascospores. A selenosporella-like synasexual morph has not been observed in vitro or on the natural substrate in any examined collection.
The asexual morph of S. hyalostoma strongly resembles S. atra in the morphology of conidia. Although the size of conidia of both species overlap, i.e. conidia of S. atra 4–6.5 × 3–4 μm fide Ellis (1963), 3.7–6.3 × 2.5–4.6 μm fide Hughes (1973f) and 4–6.5 (7.5) × 2.5–3.5 (4.5) μm fide Holubová-Jechová (1982), the latter species is distinguished by conidia rounded at both ends. Spadicoides atra is represented in our phylogeny by the strain CBS 489.77 (Czech Republic, Lány, Lánská obora game park, on decaying wood of Quercus petraea, 12 Jun. 1976, V. Holubová-Jechová) and it is shown basal to other Spadicoides species. Spadicoides hyalostoma is also highly similar to S. subsphaerica, but the latter species differs in having shorter, subglobose, globose or broadly ellipsoidal conidia rounded at both ends (Li 2010). It can be also compared with several other Spadicoides species with aseptate, brown conidia, i.e. S. arengae, S. cuneata, S. macrocontinua, and S. sphaerosperma. These taxa differ from S. hyalostoma by larger, differently shaped conidia.
Spadicoides iberica (Hern.-Restr. et al.) Réblová & A.N. Mill., comb. nov. MycoBank MB823341
Basionym: Pseudodiplococcium ibericum Hern.-Restr. et al., Stud. Mycol. 86: 92. 2017.
Notes: For description and illustration see Hernández-Restrepo et al. (2012, 2017). This species produces branched conidiophores and (0–)1-septate conidia in long often branched chains.
Torrentispora K.D. Hyde et al., Mycol. Res. 104: 1399. 2000; emend. Shearer & F.R. Barbosa, Mycologia 105: 338. 2013.
Synonyms: Pseudoannulatascus Z.L. Luo et al., Phytotaxa 239: 179. 2015.
Fusoidispora Vijaykr. et al., Sydowia 57: 272. 2005.
Sexual morph: Ascomata perithecial, non-stromatic, immersed, partially erumpent becoming superficial with only bases immersed, scattered or grouped, varying in position from upright to nearly horizontal. Venter globose, subglobose to conical, glabrous or sparsely clothed with hairs. Neck rostrate or cylindrical, without sulcations at the apex, dark brown, glabrous or hairy, upright or lying horizontally on the host. Ostiole periphysate. Ascomatal wall fragile, two-layered, with layers of cylindrical cells in surface view. Paraphyses becoming partially disintegrated, tapering, septate. Asci unitunicate, cylindrical, stipitate, 8-spored, apex with a non-amyloid, massive, refractive apical annulus. Ascospores ellipsoidal, ellipsoidal-fusiform or fusiform, or rarely cymbiform to cylindrical, often flattened on one side, sometimes slightly curved, hyaline, aseptate or with several transverse septa at maturity, thick-walled, smooth-walled or with a fibrillar sheath. Asexual morph: unknown.
Type species: Torrentispora fibrosa K.D. Hyde et al.
Notes: Torrentispora comprises eight species from freshwater and terrestrial environments. It is characterised by immersed to partially erumpent or almost superficial, mostly glabrous ascomata with a rostrate or cylindrical neck, disintegrating paraphyses, hyaline, ellipsoidal to fusiform to elongate-fusiform ascospores and a distinct apical annulus. Barbosa et al. (2013) emended the generic description by including taxa with glabrous or hairy ascomata, asci with simple or bipartite apical rings and ascospores with or without a gelatinous sheath that become septate at maturity. Four previously described species are accepted, i.e. T. crassiparietis, T. fibrosa, T. fusiformis and T. pilosa (Hyde et al., 2000, Fryar and Hyde, 2004, Barbosa et al., 2013). Based on novel DNA sequences and morphology, T. calembola and T. novae-zelandiae are described as new to science and three new combinations in Torrentispora are proposed below for Ceratostomella dubia, Fusoidispora aquatica and Pseudoannulatascus biatriisporus. The genera Fusoidispora and Pseudoannulatascus are synonymised with Torrentispora.
Torrentispora can be distinguished from other members of the Xenospadicoidales in having ascomatal necks that are never sulcate, a massive apical annulus and hyaline, thick-walled ascospores (except in T. aquatica, see Discussion). The delayed development of septa in ascospores observed in T. biatriispora, T. crassiparietis and T. pilosa (Barbosa et al. 2013) is also typical of the closely related Calyptosphaeria, Lentomitella and Spadicoides. A relatively large range in the ascospore length of Torrentispora spp. causes frequent overlapping in the size of ascospores of individual species. The proportion between the length of the sporiferous part and stipe of the ascus may vary slightly regarding the position and spacing of ascospores.
The asexual morph of Torrentispora is unknown. Two species, T. fibrosa and T. novae-zelandiae were obtained in axenic culture, but only sterile dematiaceous mycelium is formed.
Key to species of Torrentispora
| 1. | Ascospores longer than 40 μm ……………………………………..……... 2 |
| 1. | Ascospores shorter than 40 μm …………………………...……………..... 3 |
| 2. | Ascospores elongate-fusiform with slightly swollen ends enclosed in a thin, irregular mucilaginous sheath, 40–58 × 8–10 μm …………………………………………………………………………………….. T. biatriispora |
| 2. | Ascospores cymbiform to fusiform or cylindrical, with globose mucilaginous pads at both ends, 42–50 × 4–6 um ………..… T. aquatica |
| 3. | Ascospores longer than 20 μm …………………………………………… 4 |
| 3. | Ascospore length range from below 20 μm ………...…………………….. 8 |
| 4. | Ascospores 20–30 μm long ……………………………………………… 5 |
| 4. | Ascospores longer than 30 μm …………………………………………… 7 |
| 5. | Ascomatal neck glabrous ………………………………………………… 6 |
| 5. | Ascomatal neck covered by short hairs, ascospores 21–30 × 7–8 μm …………………………………………………..……………………. T. pilosa |
| 6. | Neck rostrate, ascospores (19–)20–25(–27.5) × (6.5–)7–8 μm ………………………………………………………………………………… T. dubia |
| 6. | Neck cylindrical, ascospores 22–25(–27) × 6.5–8(–8.5) μm ……………….……………………………………………………………... T. calembola |
| 7. | Ascospores 32–48 × 8–14 μm, wall 2–3 μm thick at sides, 3–4 μm at the ends ……………………..……………………………… T. crassiparietis |
| 7. | Ascospores 24–32.5 × 6–9 μm, thinner-walled …………..… T. fusiformis |
| 8. | Ascospores 13.5–19.5 × 5–7 μm ………………………..……… T. fibrosa |
| 8. | Ascospores (17.5–)18–25(–26) × (6–)7–8.5 μm …. T. novae-zelandiae |
Torrentispora aquatica (Vijaykr. et al.) Réblová & A.N. Mill., comb. nov. MycoBank MB821774.
Basionym: Fusoidispora aquatica Vijaykr. et al., Sydowia 57: 272. 2005.
Notes: For description and illustration see Vijaykrishna et al. (2005). Based on the partial nucLSU sequence data of the holotype of Fusoidispora aquatica, this species grouped in Torrentispora and a new combination is proposed. Torrentispora aquatica was originally collected on wood submerged in fresh water in Hong Kong, China. The main feature distinguishing T. aquatica from other members of this genus are thin-walled, elongate-fusiform to cymbiform, septate ascospores with globose mucilaginous pads at both ends. The pads disappear soon after ascospores are released from the asci (Vijaykrishna et al. 2005).
Torrentispora biatriispora (K.D. Hyde) Réblová & A.N. Mill., comb. nov. MycoBank MB821775.
Basionym: Annulatascus biatriisporus K.D. Hyde, Nova Hedw. 61: 120 (1995)
Synonym: Pseudoannulatascus biatriisporus (K.D. Hyde) Z.L. Luo et al., Phytotaxa 239: 179. 2015.
Notes: For description and illustration see Hyde (1995) and Barbosa et al. (2013). Based on DNA sequence data of a freshwater specimen of P. biatriisporus from Costa Rica, this species was nested in the Torrentispora clade and a new combination is proposed. Torrentispora biatriispora is distinguished from other species of the genus by long fusiform ascospores surrounded by thin irregular mucilage mostly at the poles and weakly swollen ends. It was reported from submerged wood from the tropics in the southern and northern hemispheres including Australia (Hyde 1995), China (Tsui et al. 2002), Costa Rica (Barbosa et al. 2013) and the Seychelles (Hyde & Goh 1998).
Torrentispora calembola Réblová & A.N. Mill., sp. nov. MycoBank MB821776. Fig. 23A–I.
Fig. 23.
Torrentispora calembola and T. dubia. A–I.Torrentispora calembola. A–C. Ascomata (with ascoma primordium on A). D, E. Asci. F. Longitudinal section of the ascomal wall. G. Apical annulus. H. Paraphyses. I. Ascospores. J–S.Torrentispora dubia. J–L. Ascomata. M, N. Asci. O. Longitudinal section of the ascomal wall. P. Apical annulus. Q. Ascogenous hyphae. R, S. Ascospores. A–C from PRA-12745, D–I from PRA-12744, J–R from PRA-12746, S from PAD. Scale bars: A–C, J–L = 250 μm, D, E, H, M, N, Q = 10 μm, F, O = 20 μm, G, I, P, R, S = 5 μm.
Etymology: Cal- from kalós (Gr.) meaning beautiful, émbolon (Gr.) meaning the beak of a ship, referring to the long necks that give the fungus a decorative look.
Sexual morph: Ascomata immersed to partially erumpent with protruding necks becoming superficial, solitary or grouped, venter 300–420 μm diam, 310–450 μm high, subglobose to conical, upright, dark brown to black, with brown, septate hairs 2–3 μm wide, sparsely covering the sides. Neck central, 100–140 μm wide, up to 700 μm long, cylindrical, upright, flexuous, tapering, apex roughened without sulcations. Ostiole periphysate. Ascomatal wall fragile to leathery, 38–58 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura prismatica to textura angularis with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, slightly constricted at the septa, hyaline, 5–6.5 μm wide, tapering to 2.5–3 μm. Asci (195–)207–236 × (9–)10–11.5 μm (mean ± SD = 216.4 ± 10.2 × 10.7 ± 0.7 μm), 162–191(–196) μm (mean ± SD = 171.6 ± 11.1 μm) long in the sporiferous part, truncate at the apex, cylindrical, stipitate; with 8 uniseriate ascospores; apical annulus 5–5.5 μm wide, 4–5 μm high. Ascospores 22–25(–27) × 6.5–8(–8.5) μm (mean ± SD = 23.2 ± 1.3 × 7.1 ± 0.5 μm), ellipsoidal to fusiform, flattened on one side, hyaline, aseptate, smooth- and thick-walled (0.8–0.9 μm), usually with a large drop when fresh. Asexual morph: unknown.
Specimens examined: Czech Republic, Southern Bohemia, Šumava Mts. National park, Železná Ruda, glacial cirque of the Čertovo jezero lake, on decaying wood of Fagus sylvatica, 23 Oct. 1996, M. Réblová M.R. 903. Southern Moravia, Břeclav distr., Valtice, U tří Grácií, on decaying wood of Quercus sp., 15 Nov. 2012, M. Réblová M.R. 3679; ibid., Valtice, Rendez-vous National nature monument, on decaying wood of Quercus cerris, 28 Nov. 2013, M. Réblová M.R. 3843. France, Ariège, Montségur, shores of the Le Lesset stream along D9 road, 880–890 m a.s.l., on decaying wood, 1 Oct. 2013, M. Réblová M.R. 3726 (holotype, PRA-12744); ibid., Rimont, La Maille brook, ca. 550 m a.s.l., on submerged wood of Fraxinus excelsior staining deep green, 13 Mar. 2014, incubated in moist chamber until 28 Apr. 2014, J. Fournier J.F. 14027 (PRA-12745).
Notes: The five specimens of T. calembola from terrestrial and freshwater habitats in the Czech Republic and France fit well within the concept of the genus and are introduced as a new species supported by molecular DNA data. Torrentispora calembola closely resembles T. fusiformis and T. pilosa in morphology and size of ascospores, but it can be distinguished from T. pilosa in that the latter possesses shorter and narrower asci and hairy ascomatal necks. The main features distinguishing T. fusiformis from T. calembola are smaller ascomata and longer ascospores exceeding 30 μm. Torrentispora fusiformis is known only from a freshwater habitat in Brunei. Because the ascospores of T. calembola did not germinate in vitro, the DNA was extracted directly from the holotype.
Torrentispora crassiparietis Fryar & K.D. Hyde, Cryptog. Mycol. 25: 255. 2004.
Notes: For description and illustration see Fryar & Hyde (2004) and Barbosa et al. (2013). This species is known from submerged wood in freshwater habitats from Brazil, Brunei and Costa Rica. Torrentispora crassiparietis can be distinguished from other members of the genus by having ascospores with considerably thicker walls, 2–3 μm thick at sides, 3–4 μm at the ends fide Barbosa et al. (2013). These authors also enlarged its description based on material from Brazil and Costa Rica including hairy ascomata and larger asci and ascospores that stain blue in aqueous nigrosine and become 2–3-septate at maturity.
Torrentispora dubia (Sacc.) Réblová & A.N. Mill., comb. nov. MycoBank MB821777. Fig. 23J–S.
Basionym: Melanomma dubium Sacc., Fungi Veneti novi vel critici. Series III. Michelia 1: 449. Fungi Italici Autographice Delineati. Fasc. 5–8. fig. 299. 1878.
Synonyms: Zignoëlla dubia (Sacc.) Sacc., Michelia 1: 346. 1878.
Ceratostomella dubia (Sacc.) Sacc., Syll. fung. 1: 410. 1882.
Amphitrichum dubium (Sacc.) Kuntze, Revis. gen. pl. 3(2): 443. 1898.
Sexual morph: Ascomata partially erumpent becoming superficial with only base immersed, solitary or grouped. Venter 300–370(–420) μm diam, 300–350(–450) μm high, subglobose to conical, upright or lying slightly horizontally on the host, dark brown to black, glabrous or with brown, septate hairs 2–3 μm diam, sparsely covering the bottom and exposed sides. Neck central, 90–110 μm wide, up to 500 μm long, conical to rostrate, upright, apex without sulcations. Ostiole periphysate. Ascomatal wall fragile to leathery, 37–43 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura prismatica to textura angularis with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses sparse, longer than the asci, becoming disintegrated with age, septate, slightly constricted at the septa, hyaline, 8–7 μm wide, tapering to 3–3.5 μm. Asci 180–240 × (9–)9.5–10.5 μm (mean ± SD = 209.2 ± 5.9 × 9.8 ± 0.5 μm), 150–182(–192) μm (mean ± SD = 175.3 ± 6.0 μm) long in the sporiferous part, truncate at the apex, cylindrical, stipitate; with 8 uniseriate ascospores; apical annulus 4.5–5.5 μm wide, 3.5–4.5 μm high. Ascospores (19–)20–25(–27.5) × (6.5–)7–8 μm (mean ± SD = 22.9 ± 1.3 × 7.6 ± 0.5 μm), ellipsoidal to fusiform, flattened on one side, hyaline, aseptate, smooth- and thick-walled (0.6 μm), filled with one large or numerous small drops. Asexual morph: unknown.
Specimens examined: Czech Republic, Southern Bohemia, Šumava Mts. National park, Prášily, Mt. Ždanidla, on decaying wood of Fagus sylvatica, 24 Aug. 2000, M. Réblová M.R. 1681; ibid., Stožec, Oslí vrch Mt., on decaying wood of Sorbus aucuparia, 16 Oct. 2010, M. Réblová M.R. 2985 (PRA-12744). Germany, Eberbach, on decaying wood of Fagus sylvatica, Herb. Barbey-Boissier No. 620 (G). Italy, Treviso, Cansiglio, on decaying wood of Fagus sylvatica, Oct. 1874, P.A. Saccardo (holotype of Melanomma dubium, PAD).
Notes: A revision of the type material of Melanomma dubium (Saccardo 1878b), although in poor condition and containing only a few perithecia on a piece of wood of Fagus sylvatica, revealed a fungus that matches well the description of Torrentispora. A specimen from Barbey-Boissier herbarium No. 620 (G) and both collections from the Czech Republic correspond most closely to M. dubia. The transfer of M. dubia to Torrentispora is supported by novel DNA sequences of the Bohemian specimen PRA-12744. All examined collections occur on strongly decayed wood of deciduous trees with a preference for F. sylvatica.
Torrentispora dubia is easily distinguished by ascomata with a conical rostrate neck vs. cylindrical neck in other members of Torrentispora. It is most similar to T. calembola in morphology of the ascospores, but it differs in shorter and narrower asci and anatomy of the neck. Both species are known from terrestrial habitats.
Torrentispora fibrosa K.D. Hyde et al., Mycol. Res. 104: 1399. 2000. Fig. 24.
Fig. 24.
Torrentispora fibrosa. A–D. Ascomata. E. Longitudinal section of the ascomal wall. F–I. Asci (H, I in India ink). J. Apical annulus. K, L. Ascospores (L in India ink). M. Paraphyses. N, O. Colonies on MLA and PCA after 28 d. A–M from PDD 110879, N, O from ICMP 15147). Scale bars: A–D = 250 μm, E = 20 μm, F–I, M = 10 μm, J, K, L = 5 μm.
Sexual morph: Ascomata immersed to partially erumpent with protruding necks, solitary or grouped. Venter 300–340 μm diam, 310–360 μm high, subglobose, upright or lying horizontally on the host, dark brown to black, with brown, septate hairs 2.5–4 μm wide, sparsely covering the exposed sides and bottom. Neck central, 90–110 μm wide, up to 600 μm long, cylindrical, upright or decumbent, apex without sulcations. Ostiole periphysate. Ascomatal wall fragile to leathery, 32–45 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura prismatica to textura angularis with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, slightly constricted at the septa, hyaline, 4.5–9 μm wide, tapering to ca. 3 μm. Asci (148–)152–186(–204) × 7.5–9 × 6–9 μm (mean ± SD = 168.3 ± 14.7 × 8.3 ± 0.5 μm), (107–)116–140(–162) μm (mean ± SD = 128.8 ± 11.8 μm) long in the sporiferous part, truncate at the apex, cylindrical, stipitate, with 8 uniseriate, ascospores; apical annulus 4–4.5 μm wide, 2.5–3 μm high. Ascospores (14.5–)15–18(–19) × 5.5–7 μm (mean ± SD = 15.8 ± 1.1 × 6.0 ± 0.3 μm), hyaline, ellipsoidal to fusiform, often flattened on one side, aseptate, wall 0.5–0.6 μm thick, smooth or with a thin fibrillar sheath visible in India ink or with the SEM, filled with one large or numerous small drops. Asexual morph: unknown.
Culture characteristics: Colonies on MLA 19–24 mm diam after 14 d (22–25 mm after 21 d, 26–29 after 28 d) at 25 °C, circular, raised. Aerial mycelium abundant, cottony, margins filamentous, colony surface olive-grey in the centre, dark olivaceous-grey towards margin; reverse black. Colonies on PCA 14–16 mm diam after 14 d (16–18 mm after 21 d, 18–19 mm after 28 d) at 25 °C, circular, raised. Aerial mycelium abundant, cottony, margins filamentous, colony olive-grey with dark olivaceous-grey to almost black margin; reverse black. Sporulation not observed.
Specimens examined: Czech Republic, Southern Bohemia, Šumava Mts. National park, Železná Ruda, glacial cirque of the Černé jezero lake, on decaying wood of Fagus sylvatica, 13 Aug. 1999, M. Réblová M.R. 1586. New Zealand, South Island, West Coast, Buller Distr., Victoria Forest park, Palmer´s Hut 18 km SW of Springs Junction on unpaved road, Lake Christabel track, on decaying wood of Nothofagus sp., 28 Feb. 2003, M. Réblová M.R. 2671/NZ 160; ibid., Westland Distr., Saltwater Forest, Poerua River valley 28 km W of Hari Hari, on dried driftwood, 12 Mar. 2003, M. Réblová M.R. 2796/NZ 306 (PDD 110879, culture ICMP 15147).
Notes: The holotype was not accessible for study (pers. comm., Wenfeng Zhang, IFRD). The ascospores of T. fibrosa were described to have a thin fibrillar sheath visible in India ink or with the SEM (Hyde et al. 2000). The ascal apex and ascospores of T. fibrosa from a non-holotype specimen were studied by Lee et al. (2004) at the ultrastructural level. Some ascospores were surrounded by an electron-transparent mucilage-like matrix clearly visible towards the end of ascospores when they were still inside the ascus. Moreover, they detected an additional wall layer inside the mesosporium, which is also present in the ascospores of T. biatriispora, but absent in the morphologically similar genus, Annulatascus.
Our specimens from New Zealand and the Czech Republic fit well the description and illustration of T. fibrosa from Hong Kong, China, provided by Hyde et al. (2000), except that the ascospores do not possess a fibrillar sheath. The occurrence on water-saturated decayed wood (ICMP 15147, collected as dried driftwood on the shores of a river) is consistent with the ecology of other freshwater species classified in this genus. The material from the Czech Republic was collected in the glacial cirque in the Šumava Mts., a unique locality with specific microclimatic conditions. The ascomata in our collections were larger than ascomata in the type collection (135–255 μm diam fide Hyde et al. 2000). The material collected in New Zealand was isolated in axenic culture, but only sterile dematiaceous mycelium was produced.
Considering that cryptic speciation appears to be common in this lineage, the morphological differences in the ascospore wall, ecological differences, and the absence of molecular data from collections originating from the area of original description of T. fibrosa, the present collections are tentatively identified as this species, pending further investigations of accessions from the area of its description.
Torrentispora fusiformis Fryar & K.D. Hyde, Cryptog. Mycol. 25: 256. 2004.
Notes: For description and illustration see Fryar & Hyde (2004). This species is known only from Brunei on submerged wood in brackish and freshwater environments. Torrentispora fusiformis closely resembles T. calembola in the morphology of the ascospores and immersed ascomata with upright cylindrical necks, but differs in having longer ascospores exceeding 30 μm, and narrower asci.
Torrentispora novae-zelandiae Réblová & A.N. Mill., sp. nov. MycoBank MB821778. Fig. 25.
Fig. 25.
Torrentispora novae-zelandiae. A–C. Ascomata. D. Longitudinal section of the ascomal wall. E, F. Asci. G. Apical annulus. H–J. Ascospores. K. Ascogenous hyphae. L. Paraphyses. M, N. Colonies on MLA and PCA after 28 d. A–L from PDD 110880, M, N from ICMP 18368. Scale bars: A–C = 250 μm, D = 20 μm, E, F, J–L = 10 μm, G–I = 5 μm.
Etymology: Referring to New Zealand, the country where the fungus was collected.
Ascomata immersed, with only tips of necks protruding, solitary. Venter 350–450 μm diam, 350–420 μm high, subglobose, upright or lying horizontally on the host, dark brown to black, glabrous, with brown, septate hairs ca. 2.5 μm wide, sparsely growing at the base. Neck central, 100–110 μm wide, up to 600 μm long, cylindrical, upright or slightly decumbent, apex without sulcations. Ostiole periphysate. Ascomatal wall fragile, 25–33 μm thick, two-layered; outer layer consisting of thick-walled, brown, polyhedral cells of textura prismatica with opaque walls; cells tending to be darker towards the outside, becoming flattened and paler towards the interior. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, longer than the asci, becoming disintegrated with age, septate, slightly constricted at the septa, hyaline, 4.5–6.5 μm wide, tapering to 2–2.5 μm. Asci (170–)200–213(–225) × 8.5–10.5 μm (mean ± SD = 203 ± 18.3 × 9.5 ± 0.9 μm), 140–167(–197) μm (mean ± SD = 166.3 ± 18.6 μm) long in the sporiferous part, truncate at the apex, cylindrical, stipitate with 8 uniseriate ascospores, apical annulus 5–5.5 μm wide, 4–4.5 μm high. Ascospores (17.5–)18–25(–26) × (6–)7–8.5 μm (mean ± SD = 22.9 ± 3.2 × 7.4 ± 0.7 μm), hyaline, fusiform, often flattened on one side, aseptate, smooth- and thick-walled (0.5–0.6 μm), filled with a large drop or numerous small drops.
Culture characteristics: Colonies on MLA 20–23 mm diam after 14 d (24–26 mm after 21 d, 27–30 after 28 d) at 25 °C, circular, raised. Aerial mycelium abundant, present mostly in the centre of the colony, cottony, margins filamentous, colony surface pale grey to whitish in the centre, dark olivaceous-grey towards margin formed by substrate mycelium with a black hue; reverse black. Colonies on PCA 11–14 mm diam after 14 d (12–15 mm after 21 d, 15–18 mm after 28 d) at 25 °C, circular, raised. Aerial mycelium abundant, cottony, margins filamentous, colony surface beige-brown in the centre, dark olivaceous-grey towards margin; reverse black. Sporulation not observed.
Specimens examined: New Zealand, South Island, West Coast, Buller Distr., Victoria Forest Park, Big River Inanganua track, 14 km SE of Reefton, on decaying wood, 5 Apr. 2005, M. Réblová M.R. 2956/NZ 719 (holotype, PDD 110880, culture ex-type ICMP 18368); ibid., M.R. 3438/NZ 718.
Notes: Torrentispora novae-zelandiae is similar to T. calembola in having long-necked immersed ascomata and asci of similar length, but differs from the latter in narrower asci and shorter ascospores. The ascospores in the non-type collection (M.R. 3438/NZ 718) are 19–22 × (5.5–)6–7 μm, the asci are 199–210 × 8.5–9.5 μm.
Torrentispora pilosa Shearer & F.R. Barbosa, Mycologia 105: 339. 2013.
Notes: For description and illustration see Barbosa et al. (2013). Torrentispora pilosa is known from submerged wood in a tropical stream in Costa Rica and is similar to T. fusiformis in ascospore morphology, but differs from the latter in having larger, hairy ascomata, shorter asci and ascospores that can develop up to two septa.
Discussion
Soon after Ceratostomella was established with the simple diagnosis “Perithecia et asci Ceratostomatis. Sporidia continua, hyalina” (Saccardo 1878a), it became a large, heterogeneous group of morphologically similar species whose highly polyphyletic nature was revealed with molecular data (e.g. Réblová, 2006, Réblová, 2011, Huhndorf and Fernández, 2005, Huhndorf et al., 2008, Huhndorf et al., 2009, Réblová and Štěpánek, 2009, De Beer et al., 2013a, De Beer et al., 2013b, De Beer et al., 2014, Réblová et al., 2015a, Réblová et al., 2015b). These studies have challenged the traditional divisions separating species in this fungal complex based on characters of ascoma anatomy, ascospore morphology (colour), branching pattern of ascogenous hyphae, and conidiogenesis. The “Ceratostomella phenotype” apparently represents an ecological adaptation, and so it has evolved numerous times throughout the Sordariomycetes.
Our efforts to clarify phylogenetic relationships among taxa in this fungal complex focused on those Ceratostomella species with hyaline or pale brown ascospores and persistent asci that show similarity to Lentomitella and Torrentispora. Based on revision of their holotypes, recently collected material, living cultures and novel DNA sequences, Ceratostomella dubia, C. fuscolutea, C. hyalostoma and C. subdenudata were confirmed to be distantly related to Ceratostomella and were transferred to three other genera in this study. Furthermore, revision of holotypes of other Ceratostomella species resembling Lentomitella, i.e. C. investita, C. maderensis, C. triseptata and C. vestita var. varvicensis, confirmed their placement in Lentomitella. Other species such as C. cirrhosa and C. crinigera had already been allocated to Lentomitella (Réblová 2006). The taxonomic status of other Ceratostomella spp. with persistent asci, their published details, and where available, phylogenetic data, are listed below.
The combined six-gene phylogenetic analysis of these Ceratostomella spp., the ex-type strains of Fusoidispora aquatica and Pseudodiplococcium ibericum, non-type specimens of Pseudoannulatascus biatriisporus, Spadicoides bina and Xenospadicoides atra, and several other undescribed taxa revealed a robust, strongly-supported monophyletic clade (Fig. 1). It represents the Xenospadicoidales and contains four genera, i.e. Calyptosphaeria, Lentomitella, Spadicoides and Torrentispora. It is embedded in a large subclade (70/0.99) within the Sordariomycetidae comprising Atractosporales, Papulosaceae, Sporidesmiaceae and numerous genera (as incertae sedis) with a prevailing mode of holoblastic conidiogenesis (Fig. 2).
Hernández-Restrepo et al. (2017) introduced Xenospadicoidales based on partial nucLSU sequence data for two monotypic dematiaceous hyphomycete genera Xenospadicoides and Pseudodiplococcium. A part of this order was introduced by Zhang et al. (2017) as the monotypic family Lentomitellaceae for L. cirrhosa and L. crinigera based on existing ITS, nucLSU, nucSSU and rpb2 sequence data (Réblová, 2006, Réblová et al., 2016). However, the sampling in both studies was insufficient, either members of Lentomitella or Spadicoides were absent from the phylogenetic analyses and the relationship between Lentomitella and Torrentispora (as P. biatriisporus and F. aquatica) in Zhang et al. (2017) was not statistically supported. Based on results of our ML and BI analyses of six- and three-gene datasets and in accordance with the principle of priority, the monotypic Lentomitellaceae (Zhang et al. 2017) is synonymised with the Xenospadicoidaceae (Hernández-Restrepo et al. 2017).
The Xenospadicoidales phylogenetic tree (Fig. 1) revealed a topology that is consistent with ascospore morphology and conidiogenesis shared by members of this order. These taxa are characterised by non-stromatic, dark ascomata with a central, cylindrical or rostrate neck with or without sulcations at the tip, persistent asci with a distinct, non-amyloid apical annulus and partially disintegrating paraphyses. The asexual morphs are dematiaceous hyphomycetes with sympodially proliferating holoblastic conidiogenous cells, phaeoisaria-like in Lentomitella, or with a tretic mode of asexual spore production in Spadicoides accompanied by holoblastic-denticulate conidiogenesis of the selenosporella-like synasexual morph. Other members of the order produce mostly sterile mycelium in axenic culture, but their ascospores often do not germinate in vitro. They are cosmopolitan and occur on decaying wood, bark and other plant material in freshwater and terrestrial habitats.
The morphology of ascospores, and to a certain extent also of the ascomatal neck, are the main diagnostic features to distinguish genera in the Xenospadicoidales. In Calyptosphaeria the ascospores are dull brown prior to discharge, smooth-walled, ellipsoidal to fusiform with a tendency to collapse within the asci, while the ascomatal neck is sulcate or rarely roughened without sulcations. In Lentomitella the ascospores are hyaline, longitudinally striate, ellipsoidal or ellipsoidal-fusiform, and the neck is sulcate. In Torrentispora the ascospores are hyaline, smooth-walled or occasionally with a fibrillar sheath, distinctly thick-walled, fusiform or elongated fusiform, rarely cymbiform, and the neck is smooth or roughened without sulcations. The sexual morphs of Spadicoides have hyaline, ellipsoidal to ovoid ascospores; in species with verruculose ascospore walls the ascomatal neck is partly light fulvous to subhyaline without sulcations (S. hyalostoma), or the ascospores are smooth-walled and the ascomatal neck is dark and sulcate (S. bina, S. fuscolutea). In all genera except Lentomitella we observed a delayed formation of septa in aseptate ascospores within or outside the asci. In Lentomitella the ascospores are distinctly 1–3-septate early in ontogeny. In addition to the ascospore and ascomatal neck morphology, the apical annulus also appears to have taxonomic value. In all genera it is distinct, refractive, but differs in size. The relatively smallest apical ring occurs in Lentomitella (2.5‒3 × 1.5‒2 μm, width × height) and Spadicoides (2‒3.5 × 1.5‒2 μm), medium-sized apical rings occur in Calyptosphaeria (3‒5 × 2.5‒4.5 μm) and the largest apical rings are present in members of Torrentispora (4‒8 × 3.5‒4.5 μm).
Calyptosphaeria
Calyptosphaeria, typified with C. tenebrosa, is segregated from Lentomitella based on evidence from phylogenetic analyses and morphology of ascospores. The genus comprises three other species, i.e. C. collapsa, C. subdenudata (Peck, 1879, Barr, 1986, as L. pallibrunnea in Huhndorf et al. 2008) and C. tropica (as L. tropica in Huhndorf et al. 2008). The asexual morph is unknown and since the ascospores do not germinate in vitro, all sequence data were generated from DNA extracted directly from herbarium material (Huhndorf et al. 2008 and this study). Members of Calyptosphaeria inhabit decaying wood and bark and have been reported from terrestrial or rarely freshwater biotopes in tropical and temperate zones of southern and northern hemispheres.
Calyptosphaeria is closely related to Lentomitella, which differs by hyaline, longitudinally striate ascospores. Calyptosphaeria also shows a certain resemblance to Xylomelasma (Réblová 2006) in having ascomata with a sulcate neck and brown smooth-walled ascospores, but the latter genus differs by aseptate, slightly apiculate ascospores and presence of discrete ascogenous cells, which simultaneously produce several lateral and terminal dehiscent cells from which asci arise. Based on DNA sequence data, Calyptosphaeria and Xylomelasma are unrelated (Fig. 2).
Lentomitella
New collection data, living cultures and phylogeny based on novel DNA sequences of six nuclear ribosomal and protein-coding loci revealed a strongly supported Lentomitella clade (Fig. 1). Its members occur on decaying wood and bark in terrestrial habitats in temperate zones of both hemispheres. They are widely distributed on hardwoods, occasionally on wood of fruit trees (L. conoidea, Feltgen 1903) or senescent flower heads of Protea lepidocarpodendron (L. unipretoriae, Marincowitz et al. 2008), and some species like L. crinigera exhibit a clear preference for coniferous wood.
Routine sequencing of ITS and in-depth comparative analysis of the ITS2 2D structure revealed a novel genetic variation among Lentomitella isolates. Eleven species are accepted in the genus, nine of which are included in our phylogenies. No DNA sequence data or cultures of L. investita (Schweinitz 1832) and L. unipretoriae (Marincowitz et al. 2008) exist. Based on results from phylogenetic analyses and revision of morphological characters, three species are excluded from Lentomitella. Lentomitella pallibrunnea and L. tropica (Huhndorf et al. 2008) with pale brown, aseptate, smooth-walled ascospores are transferred to Calyptosphaeria as C. subdenudata and C. tropica, while L. tomentosa (Réblová 2006) with hyaline, aseptate and smooth-walled ascospores is transferred to Spadicoides as S. fuscolutea.
The asexual morphs of Lentomitella spp. are phaeoisaria-like dematiaceous hyphomycetes formed only in vitro. Interestingly, sporulation was observed only in collections of L. investita, L. sulcata and Lentomitella sp., all originating from New Zealand. Lentomitella sulcata and Lentomitella sp. belong to the same subclade and produce globose to ellipsoidal conidia on minute but conspicuous denticles. The asexual morph of L. investita produces clavate to obovate conidia on a long rachis containing numerous but indistinct denticles. The cultures derived from ascospores of European collections remain sterile.
The main diagnostic feature to distinguish among species of Lentomitella is the morphology of ascospores. The ascospores are 1–3-septate, often with a delayed formation of the second and third septum prior to discharge, which makes the correct identification sometimes difficult. Therefore, it is important to examine as many ascospores as possible and also look for old shrinking ascospores released from the asci, which may contain additional septa. Only L. vestita, the type species, has truly 1-septate ascospores. Other species such as L. crinigera, L. magna, L. striatella, L. sulcata and L. unipretoriae have ascospores 3-septate early in ontogeny. Alternatively, L. conoidea, L. investita and L. tenuirostris often develop 1(–2)-septate ascospores with the third septum delayed and sometimes not formed at all.
The presence of longitudinal ridges in ascospores is a good character to distinguish this genus from other morphologically similar taxa. The ridges are usually shallow, but well visible, however two interesting cases were observed. In ascospores of L. investita and L. vestita the individual ridges are more conspicuous than in other species and often can be seen protruding at the poles. In L. magna the longitudinal ridges sometimes become discontinuous giving the ascospore wall a reticulate appearance.
Ascomatal morphology is highly variable within collections of the same species and among collections of different species, and thus, is of limited value for distinguishing species. Ascomatal characters include the degree of immersion in the wood or bark, presence and abundance of hairs growing from the venter and elongation of the neck and its position (upright or slightly decumbent). These characters are often influenced by humid conditions; for example, longer necks often develop when ascomata grow in cracks in wood or are positioned under the bark. The neck can sometimes appear slightly wider at the tip, which is often caused by a rupture of the deeply sulcate ostiolum. In some cases, the sulcations may disappear upon aging, leaving the surface of the neck roughened.
The longitudinally striate, hyaline ascospores of Lentomitella resemble those of Phomatospora (Barr, 1994, Cai et al., 2006), but the latter taxon differs by ascomata developed under a thin clypeus, aseptate ascospores and occurrence on damp or submerged wood or herbaceous material.
Lentomitella and the CBC species concept
The study of interspecific relationships of Lentomitella is corroborated by morphology, phylogenetic data and the ITS2 2D structure using the CBC species concept (Coleman, 2000, Coleman, 2003, Coleman, 2007, Coleman and Vacquier, 2002). A less conservative modification to this concept was proposed by Müller et al. (2007). According to these authors any CBC in the ITS2 is informative. Although the multicopy nature of ITS2 sequences may pose a potential danger of existence of intragenomic CBC and executing the CBC species concept, Wolf et al. (2013) demonstrated that the probability that there is no intragenomic CBC is ∼0.99. Species delimited by CBCs are further characterised by hCBCs and non-CBCs. However, genotypes of two organisms differing by a single hCBC indicate that they can theoretically interbreed. The rapidly evolving hCBCs and short-lived non-CBCs substitutions occur more frequently than CBCs and may facilitate faster ecological adaptations of organisms followed by changes in morphology (Caisová et al., 2011, Réblová et al., 2013, Réblová et al., 2015a).
The identification of canonical pairs that undergo reciprocal substitution (C=G ↔ G=C, A-U, U-A) in helices I–III aided us in discriminating among species of Lentomitella. The distinction of Lentomitella species is supported by seven CBCs (Fig. 3, Fig. 4). The 14th base pair of the helix III of ITS2 is a particularly interesting site because changes among Lentomitella spp. occurring at this position include the full evolution of the reciprocal substitutions involving CBC, hCBC and non-CBC such as U-A → G/A → G/U → G=C. Furthermore, in search for hCBCs and non-CBCs we identified ten such events on helix II, all linked to species originating from New Zealand (L. cirrhosa, L. magna, L. striatella, L. sulcata) except one hCBC unique for L. crinigera and L. obscura both from European material.
The CBCs identified in helices I and III support the distinction of all Lentomitella species except the two species pairs, i.e. L. conoidea and L. tenuirostris, L. cirrhosa and L. striatella. They illustrate a situation when two closely related species are distinguished in the absence of a CBC between them. A close relationship between L. conoidea and L. tenuirostris is strongly supported by individual ITS, tub2, and rpb2 sequence data and by the combined analysis of all six genes. There are only a few subtle characters of ascomata, asci, ascospores and macroscopic colony characters that distinguish these two species, which make their correct identification in the absence of molecular data challenging. Their ITS sequences show 94 % similarity, but the differences between their sequences are not associated with helices I–III of the ITS2. A second case was observed between L. cirrhosa and L. striatella, which also lack a CBC between them. Both species originate from New Zealand and are positioned at the base of the Lentomitella clade. Lentomitella striatella differs from L. cirrhosa in longer and wider asci and slightly longer 3-septate ascospores, while ascospores of L. cirrhosa are predominantly 1-septate and additional septa develop later and are rarely visible in old ascospores. Their ITS sequences show 96 % similarity.
The third interesting case concerns three morphologically highly similar species, L. obscura, L. sulcata and Lentomitella sp. that form a monophyletic clade, which is delimited from other species by a CBC on the 8th base pair of the helix III of the ITS2 (Fig. 4). Although L. obscura and L. sulcata are further distinguished by a unique CBC between them, the distinction between L. obscura represented by three European strains and Lentomitella sp. based on a single New Zealand strain is not supported by any CBC. Their ITS sequences show 97 % similarity. The only difference between them is the occurrence of a non-CBC in the 13th base pair of the helix III in the ITS2.
Spadicoides
Spadicoides, typified by S. bina, was introduced by Hughes (1958) for a group of dematiaceous hyphomycetes occurring as saprobes on decaying wood or plants remnants. For the first time we show the sexual-asexual morph relationship between Spadicoides and perithecial ascomycetes. Hughes (1958) considered unbranched conidiophores as one of the key diagnostic characters to separate Spadicoides from the morphologically similar Diplococcium. His concept was adopted by Ellis (1963, Ellis, 1971, Holubová-Jechová, 1982 and Wang (1976). Sinclair et al. (1985) considered the formation of single vs. catenate conidia as the main diagnostic criterion, superior to the simple/branched conidiophores, to distinguish Spadicoides from Diplococcium. He abandoned the generic concept of Spadicoides sensu Hughes (1958) and transferred four Spadicoides species with conidia formed regularly or occasionally in short chains to Diplococcium.
Using partial nucLSU sequences, Shenoy et al. (2010) suggested that Spadicoides and Diplococcium are polyphyletic and unrelated to each other. In the same nucLSU phylogeny (Shenoy et al. 2010), the strain CBS 113708 of S. bina (nucLSU sequence EF204507) occurred in the Cordanales as sister to Cordana pauciseptata (as Porosphaerella cordanophora, the sexual morph; strain M.R. 1150, nucLSU sequence AF178563) with 100 % bootstrap support, while morphologically similar Spadicoides atra CBS 489.77 was shown as sister to Lentomitella. Spadicoides bina and C. pauciseptata share 1-septate, brown, ellipsoidal conidia of comparable size born terminally or laterally on upright, macronematous, dematiaceous conidiophores, but they differ in the mode of conidiogenesis. It is tretic in Spadicoides while in Cordana conidia are borne on minute denticles from intercalary and terminal swellings. The examination of a specimen [Sweden, Uppland, Dalby par., Jerusalem, on decaying wood of Picea abies, 17 Apr. 1986, K. & L. Holm 3980, F-540504 (UPS)], used for isolation of the “S. bina” strain CBS 113708 and preparation of the dried culture [(1992.02) F-540502 (UPS)] revealed that the fungus present on the wood and in the dried culture is C. pauciseptata (Fig. 26). It is obvious that the fungus was originally misidentified, which was merely followed by Shenoy et al. (2010) and Hernández-Restrepo et al. (2017), who segregated S. atra from Spadicoides into Xenospadicoides. It was distinguished from morphologically similar Pseudodiplococcium by arrangement of conidia, solitary in Xenospadicoides and catenate in Pseudodiplococcium.
Fig. 26.
Cordana pauciseptata. A, B. Conidiophores. C–E. Conidia. A–C from dried culture F-540502 (UPS), D, E from F-540504 (UPS). Scale bars: A–E = 10 μm.
Spadicoides bina was recently recollected and obtained in axenic culture (CBS 137794) from isolated ascospores of an undescribed lentomitella-like species (PRA-13420). A strongly supported Spadicoides clade containing S. bina, S. fuscolutea, S. hyalostoma and X. atra was recovered in our six-gene phylogeny (Fig. 1) and ITS-nucLSU analysis (Supplementary Fig. 1), which included also P. ibericum. Based on molecular DNA data and morphology of conidia, conidiogenous cells and conidiophores, X. atra and P. ibericum are accepted in Spadicoides; a new combination is proposed for the later species, and Pseudodiplococcium and Xenospadicoides are synonymised with Spadicoides. Based on these results, the generic description of Spadicoides is emended to include both sexual and asexual morphs. The genus is characterised by ascomata with long necks, hyaline, aseptate or 1-septate, delicately verruculose or smooth-walled ascospores, unbranched or branched conidiophores and dark brown conidia formed singly or in a chain, and the selenosporella-like synasexual morph.
Selenosporella is a hyphomycete genus (Sordariomycetes, incertae sedis) with pale brown to subhyaline conidiophores producing usually clavate, fusiform, obclavate to falcate, hyaline conidia (MacGarvie 1968). However, the exact mode of conidiogenesis has been the subject of a broad discussion. Although MacGarvie (1968) described the conidiogenesis of S. curvispora, the type species, as holoblastic-denticulate with conidia arising sympodially in basipetal succession from minute denticles, Ellis (1971) and Hughes (1979) interpreted the conidiogenesis as phialidic or possibly phialidic based on study of other Selenosporella and selenosporella-like species. Onofri & Castagnola (1982) studied S. curvispora with electron microscopy and concluded that the conidiogenesis is holoblastic-denticulate. It is likely that selenosporella-like asexual morphs or synasexual morphs occuring in various taxonomic groups have different modes of conidiogenesis, which is difficult to observe with light microscopy.
The selenosporella-like synasexual morph observed in 4–8-wk-old axenic cultures of S. bina and S. fuscolutea also was reported for other species of Spadicoides, i.e. S. heterocolorata (Castañeda et al. 1997), S. obclavata (Kuthubutheen & Nawawi 1991a) and S. wufengensis (Li et al. 2010). Furthermore, it was described as a synasexual morph of dematiaceous hyphomycetes such as Ceratosporium (Hughes 1964), Teratosperma (Hughes, 1951, Matsushima, 1975), and also Diplococcium, e.g. D. hughesii (Wang & Sutton 1998), D. dimorphosporum and D. singulare (Hernández-Restrepo et al. 2012). The selenosporella-like synasexual morph was reported also for Endophragmiella, e.g. E. dimorphospora (Awao and Udagawa, 1974, Matsushima, 1975), E. subolivacea (Matsushima 1975), and E. theobromae (Hughes 1979), including Endophragmiella spp. linked with sexual morphs such as Echinosphaeria canescens and Lasiosphaeria punctata (Hughes, 1979, Sivanesan, 1983) or Ruzenia spermoides (Gams, 1973, Miller and Huhndorf, 2004b). Oxydothis selenosporellae (Samuels & Rossman 1987) and Iodosphaeria (Samuels et al. 1987) are additional sexual morphs linked with a selenosporella-like asexual morph. Fungi with a selenosporella-like phenotype were described as part of the life cycle of several other dematiaceous hyphomycetes such as Acrodictys bambusicola (Matsushima 1975), Arachnophora excentrica (Hughes 1979), Polytretophora calcarata (= Spadicoides calcarata, Kuthubutheen & Nawawi 1991b) and Quadracaea mediterranea (Lunghini et al. 1996).
The position of Spadicoides verrucosa (Rao & de Hoog 1986) is in agreement with Shenoy et al. (2010). In our analysis this species is also placed in the Sordariomycetidae but in a separate clade near members of the Phomatosporales and Magnaporthales. According to Shenoy et al. (2010) Spadicoides xylogena is related to Curvularia brachyspora (ATCC 58872) (Pleosporales, Dothideomycetes); these fungi show remarkable similarity. Considering the obvious polyphyly of Spadicoides, the genus requires taxonomic revision.
The asexual morph of Spadicoides is most similar to Diplococcium in pigmented, macronematous conidiophores, polytretic conidiogenous cells and dark conidia, but it differs in having mostly unbranched or rarely branched conidiophores and is linked to morphologically different sexual morphs. Diplococcium has been linked to five Helminthosphaeria species (Helminthosphaeriaceae, Sordariomycetes) as a presumed asexual morph based on juxtaposition of conidiophores and ascomata (Samuels et al., 1997, Réblová, 1999), while Diplococcium spicatum, the type species, was positioned in the Helotiales (Leotiomycetes) based on molecular DNA data (Shenoy et al. 2010).
Torrentispora
Torrentispora, typified by T. fibrosa, was erected as a monotypic genus in the Annulatascaceae based on its massive, non-amyloid apical annulus (Hyde et al. 2000). It was introduced for taxa morphologically similar to Annulatascus, but distinct in ascomatal wall having irregular rows of cylindrical cells in surface view and smaller ascospores (< 20 μm) vs. textura epidermoidea in surface view and larger ascospores (> 20 μm) in Annulatascus (Hyde 1992). Torrentispora was further distinguished from Annulatascus by morphology of the ascospores at the ultrastructural level; the ascospores of Torrentispora lack episporial verrucose ornamentation and possess an additional wall layer inside the mesosporium (Lee et al. 2004).
In the phylogeny based on three nuclear markers (Fig. 2), Torrentispora is shown unrelated to Annulatascus, but forms a monophyletic, strongly supported subclade nested in the Xenospadicoidales. To date, the genus comprises four species mostly from freshwater habitats from subtropical, tropical and temperate zones, i.e. T. crassiparietis, T. fibrosa, T. fusiformis and T. pilosa (Hyde et al., 2000, Fryar and Hyde, 2004, Barbosa et al., 2013). Based on the evidence from DNA sequence data we introduce T. calembola and T. novae-zelandiae as new species mainly from terrestrial habitats and we propose three new combinations (T. aquatica, T. biatriispora and T. dubia).
Torrentispora fibrosa was originally collected on submerged wood in streams in Hong Kong, China where the climate is subtropical tending towards temperate for nearly half of the year (Hyde et al., 2000, Ho et al., 2001). Other collections have been reported from Florida USA (Raja et al. 2003), but no states north of Florida (Shearer, 1993, Shearer, 2001). Our collections originate from temperate zones of Europe and New Zealand, but differ from the type species by absence of the fibrillar sheath in ascospores. Ingold (1966) speculated on the latitudinal distribution of aquatic hyphomycetes by examining aquatic spores in samples of stream foam at a distance of every 15° latitude. Conversely, it has been supported by molecular data that some freshwater ascomycetes are not restricted to a certain latitude, e.g. collections of the freshwater species Annulusmagnus triseptatus (Annulatascales) from Canada, France, Hong Kong and Venezuela form a strongly supported monophyletic clade (Campbell and Shearer, 2004, Dayarathne et al., 2016).
Pseudoannulatascus was introduced as a monotypic genus (Luo et al. 2015) to include Annulatascus biatriisporus (Hyde 1995) based on partial nucLSU sequence data. Pseudoannulatascus biatriisporus is most similar to Torrentispora in the morphology of ascomata, asci and ascospores. Their close relationship was revealed in our phylogeny using DNA sequences from a non-type collection of P. biatriisporus from Costa Rica [specimen A 464-3, Raja et al. (2003)], our collection of T. fibrosa from New Zealand (ICMP 15147) and other Torrentispora species. Therefore, Pseudoannulatascus is synonymised with Torrentispora.
The monotypic genus Fusoidispora was described for a freshwater lignicolous species, F. aquatica (Vijaykrishna et al. 2005). We analysed the partial nucLSU sequence of F. aquatica [AY780365, holotype HKU(M) 17484, Vijaykrishna et al. (2005)] in the nucLSU (not shown) and six-gene (Fig. 1) phylogenies. This species was nested in the Torrentispora clade with high statistical support. Despite the obvious difference in the ascospore morphology, the DNA sequence data of the holotype suggest that F. aquatica is a member of Torrentispora and therefore a new combination is proposed.
Torrentispora aquatica is most similar to Pisorisporium cymbiforme (Pisorisporiales, Sordariomycetidae) (Réblová et al. 2015c) in morphology and size of ascomata, which lie horizontally on the host, ascomatal wall, asci and morphology of long-fusoid to cymbiform, thin-walled ascospores, including the numerous guttules arranged in a chain within ascospores like peas in a pod. It differs from P. cymbiforme in ascospores with globose mucilaginous pads at both ends and the non-amyloid apical annulus. The DNA of T. aquatica was extracted directly from herbarium material (Vijaykrishna et al. 2005). However, we do not exclude the possibility that the DNA was extracted from another fungus. It is well known that different species of aquatic fungi occur close to each other on the same substrate; we often encountered ascomata of Lentomitella, Spadicoides and Torrentispora next to each other on the natural substrate.
Revision of Ceratostomella spp. with persistent asci
Species of Ceratostomella with evanescent asci that were transferred to members of Microascales and Ophiostomatales are listed in De Beer et al., 2013b, De Beer et al., 2014. The following list includes revised Ceratostomella species with persistent asci in three categories: as accepted species of Ceratostomella s. str., species excluded from Ceratostomella and described in other genera and species of uncertain status. Names in bold refer to the currently accepted classification and are accompanied by short notes, reference to full synonymy and additional published details.
Species accepted in Ceratostomella s. str.
Ceratostomella cuspidata (Fr.) Réblová, Mycologia 98: 77. 2006.
Basionym: Sphaeria cuspidata Fr., Syst. mycol. 2(2): 474. 1823.
Synonym: Ceratostoma cuspidatum (Fr.) Sacc., Syll. fung. 1: 220. 1882.
Ceratostomella pyrenaica Réblová & J. Fourn., in Réblová, Mycologia 98: 78. 2006.
Ceratostomella rhynchophora (De Not.) Réblová, Mycologia 98: 78. 2006
Basionym: Sordaria rhynchophora De Not., Comm. Soc. crittog. Ital. 2, Fasc. 3: 480. 1867.
Ceratostomella rostrata (Tode : Fr.) Sacc., Syll. fung. 1: 408. 1882.
Basionym: Sphaeria rostrata Tode, Fung. mecklenb. sel. 2: 14. 1791 : Fr., Syst. Mycol. 2: 473. 1823.
Notes: For full synonymy, descriptions, illustrations, phylogeny, discussion, nomenclatural notes on C. rostrata and key to Ceratostomella s. str. see Réblová (2006).
Species excluded from Ceratostomella and described in other genera
Barbatosphaeria barbirostris (Dufour : Fr.) Réblová, Mycologia 99: 727. 2007.
Basionym: Sphaeria barbirostris Dufour, Turp. Icon. fig. 1. 1820: Fr., Syst. Mycol. 2: 473. 1823.
Synonyms: Ceratostomella barbirostris (Dufour : Fr.) Sacc., Syll. Fung. 1: 410. 1882.
Ceratostomella trichina (Pers.) Sacc., Syll. fung. 1: 410. 1882.
Ceratostomella dispersa (P. Karst.) Sacc., Syll. Fung. 1: 411. 1882.
Specimens examined: Germany, Rhineland, Vosges, Wasgau, on decaying wood of Quercus sp. (syntype of S. trichina Pers. in Litt., Mougeot & Nestler, Stirp. Crypt. Voges. No. 666, K 147294; C. Roumeguère Fung. Sel. Gal. Exs. No. 187, K 147310).
Notes: Revision of the holotypes of Ceratostomella barbirostris and C. dispersa revealed that these species are conspecific and were transferred to the new genus Barbatosphaeria as B. barbirostris (Réblová 2007). For full synonymy, description, illustration, holotype and other material examined see Réblová (2007), for phylogeny, additional illustrations and key to Barbatosphaeria spp. see Réblová et al. (2015b).
Recently, two collections labelled Sphaeria trichina, with the same locality and host information, were located in the Kew fungarium. The specimen labelled as syntype, K 147294, matches the description of Barbatosphaeria barbirostris. The other specimen, K 147310, although from the same host, contains a different piece of wood containing empty ascomata.
Calyptosphaeria subdenudata (Peck) Réblová & A.N. Mill.
Basionym: Sphaeria subdenudata Peck, Ann. Rep. N.Y. St. Mus. nat. Hist. 32: 52. 1880 (1879).
Synonym: Ceratostomella subdenudata (Peck) M.E. Barr, Bull. N.Y. St. Mus. 459: 44. 1986.
Notes: This study; for full synonymy see above.
Ceratosphaeria lampadophora (Berk. & Broome) Niessl, Verh. nat. Ver. Brünn 14: 203. 1876.
Basionym: Sphaeria lampadophora Berk. & Broome, Ann. Mag. nat. Hist., Ser. 3. 3: 372. 1859.
Synonym: Ceratostomella lampadophora (Berk. & Broome) Cooke, Grevillea 17: 49. 1889.
Notes: For description, illustration and phylogeny of C. lampadophora see Huhndorf et al. (2008), for notes on its harpophora-like asexual morph and additional phylogenetic data see Réblová (2006) and Réblová et al. (2011). Ceratosphaeria, based on C. lampadophora, is a member of the Magnaporthales (Sordariomycetes).
Chaetosphaeria longispora (Sacc.) P.M. Kirk, Index Fung. 120: 1. 2014.
Basionym: Ophioceras longisporum Sacc., Syll. fung. 2: 360. 1883.
Synonyms: Sphaeria longispora Ellis, Bull. Torrey bot. Club 6: 135. 1877 non Currey 1859 nec Karsten 1873. (Nom. illegit., Art. 53.1)
Ceratostomella longispora (Sacc.) Cooke, Grevillea 17: 50. 1889.
Lasiosphaeria ellisii M.E. Barr, Mycotaxon 46: 48. 1993.
Chaetosphaeria ellisii (M.E. Barr) Huhndorf & F.A. Fernández, Fung. Diver. 19: 27. 2005.
Notes: For synonymy see Barr (1993), for description, illustration and phylogeny see Huhndorf & Fernández (2005). Sphaeria longispora (Ellis 1877) is a later homonym of S. longispora Curr. 1859 and S. longispora Karst. 1873. Two replacement names were published for S. longispora. Barr (1993) introduced Lasiosphaeria ellisii for this fungus, later transferred to Chaetosphaeria by Huhndorf & Fernández (2005). Kirk (2014) considered the first combination of S. longispora in Ophioceras by Saccardo (1883) as the earliest legitimate name of the taxon in the same rank (Art. 41.3) in order to replace Sphaeria longispora Ellis. Ophioceras longisporum Sacc. therefore becomes a basionym for all future combinations. Kirk (2014) proposed a new combination of O. longisporum in the genus Chaetosphaeria but erroneously cited S. longispora as the basionym. However, it does not affect the valid publication of this new combination (Art. 41.8c).
Chaetosphaeria myriocarpa (Fr.) C. Booth, Mycol. Pap. 68: 5. 1957.
Basionym: Sphaeria myriocarpa Fr., Syst. mycol. 2(2): 459. 1823.
Synonym: Ceratostomella stevensonii (Berk. & Broome) Sacc., Syll. fung. 1: 412. 1882.
Notes: For full synonymy, description, illustration and revision of the holotype see Booth (1957).
Clohiesia corticola K.D. Hyde, Nova Hedw. 61: 126. 1995.
Synonym: Ceratostomella hyalocoronata Inderb., Mycoscience 41: 167. 2000.
Specimen examined: China, Guangdong Province, Wu Gui Shan, 15 km S of Zhongshan, on decaying branch submerged in a small stream, 8 Nov. 1998, E. M. Leaño & P. Inderbitzin (holotype, UBC F13874).
Notes: The examination of the holotype of C. hyalocoronata (Inderbitzin 2000) revealed a fungus that fits well in the description of Clohiesia (Hyde 1995) and is conspecific with the generic type C. corticola. Using nucLSU partial sequence data, Clohiesia, originally treated as a member of the Annulatascaceae, is related to the Sordariales (Raja et al. 2003) and currently placed there (Kirk et al. 2008).
Daruvedia bacillata (Cooke) Dennis, Belarra 2: 25. 1988.
Basionym: Sphaeria bacillata Cooke, Handb. Brit. Fungi 2: 879. 1871.
Synonym: Ceratostomella bacillata (Cooke) Cooke, Grevillea 17: 50. 1889.
Notes: Dennis (1988) proposed a new monotypic genus, Daruvedia, for Sphaeria bacillata (Cooke 1871). Although he did not find any ascomata in the holotype, Cooke's habit sketches and drawings of the ascomata, ascus and ascospores, the only surviving original elements, enabled him to identify his fresh material as S. bacillata. Hu et al. (2010) designated Dennis's material as epitype and provided full synonymy and detailed description of the species based on additional material. In the absence of DNA sequence data, Daruvedia is either placed in the Pyrenulaceae (Pyrenulales, Eurotiomycetes) (as Pleurotremataceae fide Barr, 1994, Lumbsch and Huhndorf, 2010) or is classified in Dothideomycetes incertae sedis (Eriksson, 2006, Kirk et al., 2008).
Jattaea echinella (Ellis & Everh.) Réblová, Fung. Diver. 49: 182. 2011.
Basionym: Ceratostomella echinella Ellis & Everh., N. Amer. Pyren. p. 195. 1892.
Notes: For description, illustration and revision of the holotype see Réblová (2011). Jattaea is a member of the Calosphaeriales, well-distinguished from Ceratostomella by hyaline, allantoid ascospores in clavate asci lacking an apical annulus and ascomata usually arranged in small valsoid formations.
Jattaea tumidula (Sacc.) Réblová, Fung. Diver. 49: 186. 2011.
Basionym: Calosphaeria tumidula Sacc., Atti Soc. Veneto-Trent. Sci. Nat. Padova 4: 77–100 (Fungi Ven. novi Ser. 4: 20) 1875.
Synonym: Ceratostomella mali Ellis & Everh., Proc. Acad. Nat. Sci. Philad. 42: 225. 1890.
Notes: For full synonymy, description, illustration and holotype information see Réblová (2011).
Lentomitella cirrhosa (Pers.: Fr.) Réblová, Mycologia 98: 82. 2006.
Basionym: Sphaeria cirrhosa Pers., Syn. Meth. Fung. p. 59. 1801 : Fries, Syst. Mycol. 2: 475. 1823.
Synonym: Ceratostomella cirrhosa (Pers.) Sacc., Syll. fung. 1: 408. 1882.
Notes: See Réblová (2006) and this study; for full synonymy see above.
Lentomitella crinigera (Cooke) Réblová, Mycologia 98: 83. 2006.
Basionym: Sphaeria crinigera Cooke, Grevillea 1: 156. 1873.
Synonyms: Ceratostomella crinigera (Cooke) Cooke, Grevillea 17: 49. 1889.
Ceratostomella triseptata Petr., Annls mycol. 23: 135. 1925.
Notes: See Réblová (2006) and this study; for full synonymy see above.
Lentomitella investita (Schw.) Réblová
Basionym: Sphaeria investita Schw., Trans. Amer. Phil. Soc. 2, Vol. 4: 216. 1834.
Synonyms: Ceratostomella investita (Schw.) Starbäck, Bih. Kongl. Svenska Vet.-Akad. Handl. 19(2): 26. 1894.
Ceratostomella vestita Sacc. var. varvicensis Grove, J. Bot. 23: 131. 1885.
Ceratostomella maderensis Petr., Bot. Jahrb., Beiblt. 142: 98. 1929.
Notes: This study; for full synonymy see above.
Lentomitella vestita (Sacc.) Höhn., Annls mycol. 3: 548. 1906.
Basionym: Ceratostomella vestita Sacc., Michelia 1: 370. 1878.
Notes: This study; for full synonymy see above.
Natantiella ligneola (Berk. & Broome) Réblová, Mycol. Res. 113: 996. 2009.
Basionym: Sphaeria ligneola Berk. & Broome, Ann. Mag. nat. Hist., Ser. 3. 3: 372. 1859
Synonyms: Ceratostomella ligneola (Berk. & Broome) Cooke, Grevillea 17: 49. 1889.
Ceratostomella ampullasca (Cooke) Sacc., Syll. Fung. 1: 409. 1882.
Ceratostomella similis Kirschst., Krypt. Flora Brandenb. 7: 245. 1911.
Notes: Revision of the holotypes of Ceratostomella ampullasca, C. ligneola and C. similis revealed that these species are conspecific (Réblová & Štěpánek 2009). They were transferred to a monotypic genus Natantiella supported by DNA sequences. Natantiella ligneola is common on strongly decaying wood and occurs in temperate zones in the northern and southern hemispheres. The genus is placed in incertae sedis position in the Sordariomycetes. In phylogenetic analysis, Natantiella forms a monophyletic, strongly supported clade with members of the Ophiostomatales (this study), and is unrelated to Ceratostomella s. str. For full synonymy, description, illustration, phylogeny and holotype and other material examined see Réblová & Štěpánek (2009).
Phaeoacremonium leptorrhynchum (Durieu & Mont.) D. Gramaje et al., Fung. Biol. 119: 768. 2015.
Basionym: Sphaeria leptorrhyncha Durieu & Mont., Expl. Sci. Alg., Fl. Algér. 1 (livr. 13). p. 510. 1848.
Synonym: Ceratostomella leptorrhyncha (Durieu & Mont.) Sacc., Syll. fung. 1: 412. 1882.
Notes: For full synonymy, illustration and holotype examination see Réblová (2011). Phaeoacremonium leptorrhynchum, known only from Algeria on Chamaerops humilis, is one of the few Phaeoacremonium species that are known only in their sexual morph and only from herbarium material. Based on the revision of the holotype, Réblová (2011) suggested that C. leptorrhyncha and Phaeoacremonium novae-zelandiae (as Togninia) are conspecific and proposed a new combination as Togninia leptorrhyncha, later transferred to Phaeoacremonium by Gramaje et al. (2015), the correct name for the holomorph. We prefer to keep both species separate until representative material of P. leptorrhynchum is recollected, isolated in axenic culture and subjected to phylogenetic analysis. Evidence gathered during our current research calls for caution, when morphologically highly similar material from different continents is compared.
Phomatospora helvetica H. Wegelin, Mitt. thürgau. naturf. Ges. 12: 173. 1894.
Synonyms: Ceratostomella hydrophila Mouton, Bull. Soc. R. Bot. Belg. 26: 171. 1887.
Phomatospora moravica Petr., Annls mycol. 22: 55. 1924.
Phomatospora luteotingens J. Fourn. & Lechat, Mycosphere 1: 40. 2010.
Specimen examined: Belgium, Beaufays near Liège, on submerged decorticated wood in a stream (holotype, BR 93866-67).
Notes: Based on characters of immersed to partially erumpent, globose to conical ascomata 500‒650 μm diam under a thin black clypeus and with a flattened base, cylindrical asci 121‒134 × 8‒9 μm, 100‒120 μm in the sporiferous part, and uniseriate, ellipsoidal, hyaline, aseptate, longitudinally striate ascospores (11.5‒)12‒13.5 × 5‒6 μm, this species fits well the description of Phomatospora helvetica and P. moravica (von Hammer & Scheuer 2008). It was later redescribed as P. luteotingens by Fournier & Lechat (2010). The yellow stain of the woody substrate was observed in the holotype of P. luteotingens and is consistently present in other collections from France and Spain of this species.
To date, the genus Phomatospora includes 120 epithets according to Index Fungorum. It comprises fungi from freshwater, marine and terrestrial habitats on herbaceous debris but also on wood. The ascospores are hyaline, usually aseptate with longitudinally striate walls and often with a mucilaginous sheath and/or bipolar appendages (Barr, 1994, Cai et al., 2006). Based on the habitat and characters of ascospores with and without mucilaginous appendages or sheathes, the genus might be heterogeneous. There has been no monographic study on Phomatospora and very little is known about its asexual morphs (Rappaz 1992). Although C. hydrophila is the oldest name for this species, we refrain from making any formal changes until the genus Phomatospora can be revised.
Pseudorhynchia polyrrhyncha (Penz. & Sacc.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1. 118: 1206. 1909.
Basionym: Ceratostomella polyrrhyncha Penz. & Sacc., Malpighia 11: 408. 1897.
Notes: This tropical species was originally described from Java on Elletaria sp. in a monotypic genus. Höhnel (1909) recollected this taxon in the same locality and on the same host as the type. The third collection was reported by Samuels & Barr (1997) from Venezuela on a dead leaf of Heliconia sp. Samuels & Barr (1997) transferred the genus to the Niessliaceae (Hypocreales). For description and illustration of the type and other representative material see Von Höhnel, 1909, Müller and von Arx, 1962 and Samuels & Barr (1997).
Spadicoides fuscolutea (Rehm) Réblová
Basionym: Ceratostomella fuscolutea Rehm, Annls mycol. 6: 320. 1908.
Notes: This study; for full synonymy see above.
Spadicoides hyalostoma (Munk) Réblová
Basionym: Endoxyla hyalostoma Munk, Bot. Tidsskr. 61: 62. 1965.
Synonym: Ceratostomella hyalostoma (Munk) Unter., Mycologia 85: 307. 1993.
Notes: This study.
Togniniella microspora (Ellis & Everh.) Réblová, Fung. Diver. 49: 193. 2011.
Basionym: Ceratostomella microspora Ellis & Everh., Proc. Acad. nat. Sci. Philad. 45: 444. 1894.
Notes: For description, holotype information, discussion and comparison with the morphologically similar Flabellascus see Réblová (2011) and Réblová et al., 2004, Réblová et al., 2015a.
Torrentispora dubia (Sacc.) Réblová & A.N. Mill.
Basionym: Ceratostomella dubia (Sacc.) Sacc., Syll. fung. 1: 410. 1882.
Notes: This study; for full synonymy see above.
Wallrothiella congregata (Wallr.) Sacc., Syll. Fung. 1: 455. 1882.
Basionym: Sphaeria congregata Wallr., Fl. crypt. Germ. 4: 786. 1833.
Synonym: Ceratostomella sphaerosperma (Fuckel) Sacc., Syll. fung. 1: 412. 1882.
Specimen examined: Germany, Johannisberg, on decaying wood of Pinus sylvestris, spring (holotype, Fungi Rhen Exs. No. 2013, G).
Notes: For synonymy, description, illustration and phylogeny see Réblová & Seifert (2004) and Huhndorf et al. (2009). Based on molecular data, Wallrothiella was placed by Huhndorf et al. (2009) in the Amplistromataceae (Sordariomycetes).
Ceratostomella species of uncertain status
Ceratostomella albocoronata (Ellis) Sacc., Syll. fung. 2, Add. xxx. 1883.
Basionym: Ceratostoma albocoronata Ellis, Am. Nat. 17: 318. 1883.
Specimen examined: USA, New Jersey, Newfield, Gloucester County, on rotten wood, Jun. 1882 (holotype, NY).
Notes: The holotype contained several empty ascomata of a lentomitella-like fungus. They were immersed with short, protruding but mostly broken necks and dark hairs growing from the venter. According to the protologue (Ellis 1883), asci are cylindrical 35 × 7 μm with eight ellipsoidal, hyaline ascospores 7.5–9.5 × 3–3.5 μm with 1–2 drops (as 1–2 nucleate). The dematiaceous hyphomycete mentioned by Ellis (1883), associated with ascomata and scattered over the wood, produces obovate, 2–3-septate, 11.5 × 7.5 μm conidia borne singly on the apices of upright, brown conidiophores. Only a few conidia and remnants of conidiophores were present and they belong to Spadicoides obovata (Cooke and Ellis, 1876, Ellis, 1963). Based on the description, C. albocoronata does not match any known species of Lentomitella. On the other hand, ascospore morphology and the presence of Spadicoides conidia and conidiophores suggest its relationship with species of the latter genus. For an accurate systematic placement, it would be necessary to collect representative material to generate DNA sequences and experimentally prove the connection between the two morphs. To date, the sexual morph of S. obovata is unknown.
Ceratostomella bambusina Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1. 118: 337. 1909.
Specimen examined: Western Malaysia, Indonesia, Java Barat (W. Java), Tjibodas, on decaying bamboo culm, 1907–1908 (holotype, FH 00258773).
Notes: The material contained several non-stromatic, partially erumpent, solitary ascomata with a cylindrical upright neck, persistent, stipitate asci with a non-amyloid apical annulus and eight globose, hyaline, aseptate ascospores. Similar ascospores are a diagnostic feature of Amplistroma and Wallrothiella (Amplistromatales) (Huhndorf et al. 2009) and also occur in Woswasia atropurpurea (incertae sedis) (Jaklitsch et al. 2013), both members of the Sordariomycetidae. While the latter taxon and Amplistroma produce large stromata, Wallrothiella is non-stromatic. However, it has a thick, three-layered ascomatal wall and long-necked ascomata that are solitary or most often confluent. Ceratostomella bambusina can be compared to Wallrothiella congregata, the type species, in characters of ascospores, asci and partly ascomata, but differs in smaller ascomata that develop individually on the substrate and much thinner, two-layered ascomatal walls. DNA sequence data of this species are necessary to confirm its systematic placement.
Ceratostomella canulata (Preuss) Sacc., Syll. fung. 1: 412. 1882.
Basionym: Sphaeria canulata Preuss, Linnaea 26: 714. 1855.
Specimen: Germany, Hoyerswerda, on decaying wood (holotype).
Notes: The type material could not be located in B (pers. comm., B. Hein). The fungus is described with immersed globose ascomata, protruding cylindrical necks with hyphae growing at the base, cylindrical asci and hyaline aseptate ascospores. Based on its description it is not possible to identify the fungus or convincingly suggest its relationship.
Ceratostomella capillaris (Ellis) Sacc., Syll. fung. 2, Add. xxx. 1883.
Basionym: Ceratostoma capillare Ellis, Bull. Torrey bot. Club 9: 20. 1882.
Specimen examined: USA, New York, Gloucester County, on decaying sterile catkins of Alnus serrulata, 28 Jun. 1881 (holotype, NY).
Notes: Based on morphology of minute, globose ascomata with a filiform neck, and asci with a partly evanescent wall containing eight 2‒3-seriate, hyaline to subhyaline, fusiform, slightly curved, aseptate ascospores with gelatinous appendages at both end, this species resembles members of Ophiostoma.
Ceratostomella capilliformis E. Bommer, M. Rousseau & Sacc., Syll. fung. 9: 573. 1891.
Specimen examined: Belgium, Groenendael, on decaying wood of Carpinus betulus (holotype, PAD).
Notes: The type material contained empty ascomata 200 μm diam with cylindrical, upright or decumbent necks ca. 250‒300 μm long. According to the protologue (Saccardo 1891), the asci are 30‒36 × 6‒9 μm, clavate, slightly swollen at the base, paraphyses are present, and ascospores are 8 × 3 μm, ellipsoidal and later curved. Given the morphology of ascomata, asci and ascospores this species resembles species of the genera Barbatosphaeria and Jattaea. The swollen base of the asci may be interpreted as discrete cells arising from ascogenous hyphae, which is one of the diagnostic features of Barbatosphaeria (Réblová et al. 2015b). The bulbose base of the ascus stipe also occurs in members of Jattaea but it is of a different origin (Réblová et al. 2015a). Given the size and especially the width of ascospores, C. capilliformis resembles B. dryina (Réblová et al. 2015b), but the latter taxon differs by larger ascomata 400–600 μm diam, longer asci and 1-septate ascospores. Other species of Barbatosphaeria have usually 1–2 μm wide ascospores. Among Jattaea species, C. capilliformis can be compared to J. discreta (Réblová 2011) in morphology and size of ascomata with a venter 230–400 μm diam, asci (30–)35–45(–50) × 6.5–8 μm and aseptate, suballantoid ascospores (6–)6.5–8 × 1.5–2 μm, which are, however, narrower than those of C. capilliformis. Fresh material that would match the holotype is needed to confirm a systematic placement of this taxon.
Ceratostomella conica (Ellis & Everh.) M.E. Barr, Mycotaxon 46: 60. 1993.
Basionym: Ceratostoma conicum Ellis & Everh., Proc. Acad. nat. Sci. Philad. 42: 226. 1890.
Specimen examined: USA, New Jersey, Newfield, on decaying wood of a pine log, 1888 (holotype, NY).
Notes: The fungus is characterised by immersed to partially erumpent ascomata with a rostrate ostiolum, cylindrical, short-stipitate asci with a massive, non-amyloid apical annulus and fusiform, hyaline 1‒3-septate, smooth-walled ascospores. It resembles Chaetosphaeria, but differs in the morphology of the apical annulus, which is relatively small and shallow in the latter genus. In order to find a correct systematic placement for this species, fresh material should be collected and DNA sequence data generated.
Ceratostomella coprogena Massee, Bull. Misc. Inf., Kew: 105. 1913.
Specimen: Singapore, botanical garden, on animal dung, Burkill (holotype).
Notes: The type material could not be located at Kew (pers. comm., B. Aguirre-Hudson). The coprophilous habitat is rather atypical and it does not occur in any members of the Xenospadicoidales or in other species described in Ceratostomella.
Ceratostomella cyclospora Kirschst., Verh. bot. Ver. Prov. Brandenb. 48: 52. 1907.
Synonym: Calosphaeria cyclospora (Kirschst.) Petr., Annls mycol. 22: 74. 1924.
Specimen: Germany, Grünauer forest near Rathenow, on decaying wood of Pinus sp., 3 Jul. 1904, W. Kirschstein (holotype).
Notes: The holotype is apparently lost (pers. comm., E. Gerhardt, B). According to the protologue (Kirschstein 1907) and a later study of the holotype by Petrak (1924), the ascomata are rarely solitary, mostly congregated in pairs of two or in a valsoid formation, partially or entirely immersed in bark or decorticated wood, 700‒900 μm diam with elongated necks, asci 30‒40 × 4‒6 μm, 20‒25 μm long in the sporiferous part, cylindrical-clavate and tapering at base, thick-walled and containing eight hyaline, aseptate ascospores that are cylindrical, strongly curved, 4 × 1 μm. Petrak (1924) concluded that this species is better placed in Calosphaeria and proposed a new combination in the latter genus. The species fits the description of Barbatosphaeria hippocrepida (Réblová and Štěpánek, 2009, Réblová et al., 2015b), known only from New Zealand, apart from the size of ascomata and asci. It is likely that C. cyclospora represents a species closely related to B. hippocrepida. Without studying the holotype or other representative material we refrain from making any formal changes.
Ceratostomella debaryana (Auersw.) Sacc., Syll. fung. 1: 409. 1882.
Basionym: Gnomonia debaryana Auersw., Mycol. eur. Abbild. Sämmtl. Pilze Eur. 5–6: 23. 1869.
Specimen: Germany, Inselsberge, on decaying wood of Fagus sylvatica, Fleischhak (holotype).
Notes: The type material of C. debaryana could not be located in B (pers. comm., E. Gerhardt). According to the protologue and illustration (Auerswald 1869), the ascomata are immersed, globose, 140 μm diam with subcylindrical protruding necks, cylindrical short-stipitate asci 68 × 6 μm and eight uniseriate, ellipsoidal, hyaline, aseptate ascospores with granulose content. The size of ascomata is too small to match the size of any genus of Xenospadicoidales.
Ceratostomella echinata Ellis & Everh., N. Amer. Pyren. p. 195. 1892. (nom. nud., Art. 32.1).
Notes: Although Index Fungorum lists this name among species of Ceratostomella, this species was not described; page 195 in Ellis & Everhart (1892) refers to C. echinella (see above). No type or authentic material is preserved in NY (pers. comm., B.M. Thiers).
Ceratostomella excelsior Mouton, Bull. Soc. R. Bot. Belg. 36: 12. 1897.
Synonym: Endoxyla excelsior (Mouton) Munk, Bot. Tidsskr. 61: 66. 1965.
Specimen examined: Belgium, Beaufays near Liège, on decaying wood of Fraxinus sp., V. Mouton (holotype, BR-93865-66).
Notes: The species bears a certain resemblance to Jattaea based on the morphology of ascomata, ascospores and tapering asci without a visible apical annulus and with a bulbose base attached to ascogenous hyphae. However, it does not fit the description of any known species of that genus (Damm et al., 2008, Réblová, 2011). Munk (1965) transferred the fungus to the broadly perceived Endoxyla (Untereiner 1993). Representative material should be collected and subjected to phylogenetic analysis in order to correctly classify this taxon.
Ceratostomella hystricina (Cooke) Sacc., Syll. fung. 2, Add. xxx. 1883.
Basionym: Ceratostoma hystricina Cooke, Grevillea 11: 109. 1883.
Specimen examined: USA, South Carolina, Aiken, on bark of Ficus sp., H.W. Ravenel 2676 (syntypes, ex herb. M.C. Cooke, K 147286, K 147288); ibid., H.W. Ravenel Fungi Amer. Exs. No. 674 (K 147290).
Notes: The protologue of C. hystricina and representative herbarium material do not refer to the same fungus. Examination of the three specimens from K cited above revealed a fungus that is in agreement with observations of Ellis & Everhart (1892) and a fungus distributed in Ellis & Everhart's N. Amer. Fungi. Exs. Ser. II. No. 2349 (not seen). These specimens contain a fungus with globose, immersed to superficial, densely aggregated ascomata covered with a dark pink to brown crustose layer, with a cylindrical, upright, glabrous neck and minute, hyaline, aseptate, allantoid ascospores 4‒4.5 × 1‒1.5 μm. Asci are present but indistinct and visible merely as bundles of ascospores. However, in the protologue, the fungus is described with ellipsoidal-lanceolate ascospores 16‒18 × 6 μm (Cooke 1883) and the same illustration is made on the envelope of the syntype (K 147286). Cooke based his description of C. hystricina on H.W. Ravenel Fungi Amer. Exs. No. 674. The fungus present in the examined collections is best placed in the Diaporthales; a species of Valsa fide Ellis & Everhart (1892).
Ceratostomella leiocarpa Sacc. [as ‘lejocarpa’], Michelia 1: 370. 1878.
Specimen: Italy, Cansiglio, on decaying wood of Fagus sylvatica (holotype, PAD).
Notes: The type material could not be located in PAD (pers. comm., R. Marcucci). Based on characters of the ascomata, asci and ascospores cited in the protologue by Saccardo (1878a), this species fits best the description of Lentomitella cirrhosa.
Ceratostomella multirostrata (Fuckel) Sacc., Syll. fung. 1: 411. 1882.
Basionym: Ceratostoma multirostratum Fuckel, Jb. nassau. Ver. Naturk. 23‒24: 129. 1870.
Specimen examined: Germany, Budenheimer Forest, on decaying wood of Pinus sylvestris, spring (holotype, Fungi Rhen. Exs. No. 771, G).
Notes: The holotype contains empty ascomata that are superficial, confluent, with 1‒4 cylindrical upright necks and accompanied by a coelomycete forming stromatic conidiomata with monostichous loculi. Based on the protologue (Fuckel 1870) and illustration accompanying the type material, we cannot conclusively attribute this species to any known genus.
Ceratostomella mycophila Rick, Brotéria, sér. bot. 5: 48. 1906.
Specimen: Brazil, Rio Grande do Sul, in hymenophore of Poria sp., 1905 (holotype, PACA 12733).
Notes: The type material is deposited in Herbarium Anchieta but was not available to our study (pers. comm., M. S. Marchioretto, PACA). According to the protologue (Rick 1906), the fungus fits well the description of Ceratostomella s. str. It resembles C. rostrata in habitat, size and morphology of cylindrical, pale brown ascospores, but differs in shorter asci 25 × 6 μm vs. (26–)30–39 × 5–6 μm in C. rostrata (Réblová 2006). However, some collections of C. rostrata can rarely have shorter asci, e.g. Sclerom. Suec. Exs. No 116, PRM 666367 with asci 23–32 × 5–6 μm. This collection is old, the ascus stipe is partly disintegrated and asci appear slightly wrinkled and therefore shorter. Recollection of fresh material of C. mycophila from its original locality is recommended before it can be accepted in Ceratostomella or confirmed as conspecific with C. rostrata.
Ceratostomella nyssicola (Berk. & M.A. Curtis) Sacc. [as ‘nyssaecola’], Syll. fung. 1: 412. 1882.
Basionym: Sphaeria nyssicola Berk. & M.A. Curtis, [as ‘nyssaecola’] Grevillea 4: 143. 1876.
Specimen examined: USA, Pennsylvania, wood of Nyssa sp., Michener 5166 (holotype, K 155069).
Notes: The holotype is in poor condition and apart from mostly damaged ascomata, only a few pale brown, ellipsoidal and slightly apiculate, aseptate, smooth-walled ascospores 9.5–10.5 × 4.5–5 μm, which have a minute germ pore at each end and a large drop inside, are present. Based on these characters the fungus belongs in Xylomelasma (Réblová 2006), and it is likely an earlier name for X. sordida, the type species.
Ceratostomella rhenana (Auersw.) Sacc., Syll. fung. 1: 409. 1882.
Basionym: Gnomonia rhenana Auersw., Myc. Europ. Pyren. 5–6: 23. 1869.
Specimen examined: Germany, Nassau, on decaying wood, Fuckel ex Herb. Barbey-Boissier No. 603 (K 84430, Fungi Rhen. Exs. No. 1804).
Notes: The type material of C. rhenana is apparently lost (pers. comm., B. Hein, B). Winter (1887) based his description of C. rhenana on a part of Fuckel's exsiccate collection (Fungi Rhen. Exs. No. 1804, pro parte). Our revision of this specimen from Fuckel's herbarium in G revealed Lentomitella vestita, but a fungus sensu Auerswald (1869) and Winter (1887) was not found. However, a specimen of Fungi Rhen. Exs. No. 1804 deposited in Kew (K 84430) contained the fungus, which is in agreement with the protologue of Gnomonia rhenana (Auerswald 1869). The material in Kew is not suitable for typification due to its poor condition and lack of asci. The systematic placement of this fungus is unknown. Other parts of Fungi Rhen. Exs. No. 1804 need to be studied and fresh material of this fungus needs to be recollected to investigate the relationship of C. rhenana with Ceratosphaeria and other morphologically similar taxa. For a detailed discussion on this species and revision of available representative material see Réblová (2009).
Ceratostomella rostrata var. levirostris Sacc., Syll. fung. 1: 408. 1882.
Notes: No type or authentic material of Ceratostomella rostrata var. levirostris could be found in PAD (pers. comm., R. Marcucci). Saccardo (1882) mentioned this taxon briefly in a discussion of C. rostrata: ‘Var. levirostris rostro non v. vix sulcato. Cum specie.’
Ceratostomella stricta (Pers.) Sacc., Syll. fung. 1: 410. 1882.
Basionym: Sphaeria stricta Pers., Syn. meth. fung. 1: 59. 1801.
Notes: The holotype could not be located in L (pers. comm., G. Thijsse). Sphaeria stricta is distributed in Fries's Sclerom. Suec. Exs. No. 148, but this collection was not examined. The fungus was described with just a few words (Persoon 1801), and later redescribed by Saccardo (1882) based on various materials from European localities. Based on Saccardo's description of non-stromatic densely aggregated, globose, glabrous ascomata with a cylindrical neck, cylindrical-clavate asci with a slender stipe and hyaline, subcylindrical, curved ascospores, the fungus would be best placed in Calosphaeria or Jattaea in Calosphaeriales.
Ceratostomella stricta var. cingulata (Fr.) Sacc., Syll. fung. 12: 83. 1897.
Basionym: Sphaeria stricta var. cingulata Fr., Syst. mycol. 2: 474. 1823.
Notes: We could not locate any authentic material for this study.
Ceratostomella stricta var. majuscula Schulzer & Sacc., Hedwigia 23: 42. 1884.
Synonym: Ceratostomella majuscula (Schulzer & Sacc.) Mussat, in Saccardo, Syll. fung. 15: 84. 1901. (Nom. inval., Art. 36.1a, c)
Specimen: Hungary, Vinkovce, on decaying wood of Populus sp. (holotype).
Notes: Type material could not be located in W. The species is described with hyaline ascospores 12–14 × 3 μm with 2–3 guttules (Schulzer & Saccardo 1884). The description is insufficient to transfer this fungus to any known genus. The fungus was also illustrated in Schulzer (1869), cited in Saccardo & Schulzer (1884) as Ill. Fung. Slav. no 845. In the absence of the holotype, the illustration is the only surviving element, which should serve as a lectotype. Schulzer's manuscript with illustrations is deposited at the Hungarian Academy in Budapest. Unfortunately, the illustration could not be located (pers. comm., G. Tóth, Dept. of Manuscripts & Rare Books).
Ceratostomella stylophora (Berk. & Broome) Cooke, Grevillea 17: 49. 1889.
Basionym: Sphaeria stylophora Berk. & Broome, Ann. Mag. nat. Hist., Ser. 3, 7: 453. 1861.
Specimen: UK, Scotland, Mossburnford, on bark of Acer platanoides, A. Jerdon (holotype, K 84425).
Notes: The type material contained non-stromatic, ovoid ascomata densely aggregated in small groups and erumpent through the thin bark of a twig, with upright, cylindrical, partly flattened necks. The asci were mostly disintegrated, only remnants of the upper half with a distinct apical annulus could be seen. The ascospores are hyaline, fusiform, slightly curved, 1-septate with short appendages at each end. The fungus is better placed in the Diaporthales.
Ceratostomella subpilosa (Fuckel) Sacc., Syll. fung. 1: 411. 1882.
Basionym: Ceratostoma subpilosum Fuckel, Jb. nassau. Ver. Naturk. 23–24: 128. 1870.
Specimen examined: Germany, Grünau, on decaying wood of Salix alba (holotype, Fungi Rhen. Exs. No. 2251, G).
Notes: The type material is overmature. The ascomata are subglobose, glabrous with a fragile crumbling wall, immersed with only necks emerging, ascospores 8–9 × 4–5 μm, ellipsoidal slightly tapering towards the ends, hyaline, aseptate, smooth-walled, although some ascospores were observed to be verruculose. Given the poor condition of the specimen, the fungus could not be properly identified.
Ceratostomella subsalsa (P. Crouan & H. Crouan) Sacc., Syll. fung. 1: 412. 1882.
Basionym: Sphaeria subsalsa P. Crouan & H. Crouan, Florule Finistère p. 25. 1867.
Specimen: France, Finistère, on decaying wood of Obione sp. (holotype).
Notes: The holotype material deposited in CO was not examined. The identity and placement of this fungus is unknown; it was described with globose, greenish ascomata 1–2 mm diam, thick-walled clavate asci and ovoid, hyaline ascospores.
Ceratostomella unedonis Fabre, Annls Sci. Nat., Bot., Sér. 6. 15: 34. 1883.
Specimen: France, Vaucluse, on decaying wood of Arbutus unedo (holotype).
Notes: The holotype material deposited in FABR was not examined. The identity and placement of this lignicolous fungus is unclear. Based on the description of Saccardo (1883) the ascomata are sparse, solitary, globose, ca. 330 μm diam, with an upright central neck, short-stipitate asci 70–80 × 8 μm with eight uniseriate, hyaline, aseptate, ovoid ascospores 8–10 × 5 μm. The description is insufficient to identify this taxon.
Acknowledgements
This study was supported by the Project of the National Foundation of the Czech Republic (GACR 506/12/0038, www.gacr.cz), by the MEYS under the project CEITEC 2020 (LQ1601) and as a long-term research development project of the Institute of Botany (No. RVO 67985939) and the Institute of Microbiology (No. RVO 61388971) of the Czech Academy of Sciences. Computational resources were provided by the CESNET LM2015042 and the CERIT Scientific Cloud LM2015085, under the programme “Projects of Large Research, Development, and Innovations Infrastructures”. The field work in New Zealand (March–May 2005) was partly supported by Studienstiftung für mykologische Systematik und Ökologie. We are grateful to Walter Gams for the correction and grammatical review of Greek and Latin epithets. We thank the curators of all cited herbaria for their help in obtaining type and other herbarium material. Peter Johnston is thanked for his assistance to M. R. in obtaining the Manaaki Whenua Fellowship in 2005 and collecting permits for New Zealand. We thank Bevan Weir for his assistance with obtaining strains of Lentomitella from the ICMP culture collection. We are grateful to Hermann Voglmayer for valuable discussion and suggestions on phylogenetic analyses and Jacques Fournier, Sabine Huhndorf, and Carol Shearer for valuable collections of Calyptosphaeria and Torrentispora. Paul M. Kirk and Walter Gams are thanked for providing valuable background for some nomenclatural problems.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.simyco.2017.11.004.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Fig. 1Phylogenetic analysis of members of Spadicoides. Phylogram inferred from the ITS-nucLSU sequences with ML analysis using a GTRCAT model of evolution. Details as in Fig. 1.
References
- Arzanlou M., Groenewald J.Z., Gams W. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology. 2007;58:57–93. doi: 10.3114/sim.2007.58.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerswald B. Synopsis pyrenomycetum europaeorum. In: Gonnerman W., Rabenhorst G.L., editors. Mycologia Europaea. Abbildungen Sämmtlicher Pilze Europa's 5–6. Heinrich; Germany: 1869. pp. 1–30. [Google Scholar]
- Awao T., Udagawa S.I. Endophragmia dimorphospora, a new hyphomycete. Transactions of the Mycological Society of Japan. 1974;15:99–100. [Google Scholar]
- Barbosa F.R., Gusmão L.F.P., Raja H.A. New species and new records of freshwater ascomycetes from Brazil and Costa Rica. Mycologia. 2013;105:335–343. doi: 10.3852/12-054. [DOI] [PubMed] [Google Scholar]
- Barr M.E. Wegelina, a reinstated genus in the Calosphaeriales. Cryptogamie Bryologie Lichénologie. 1986;19:169–173. [Google Scholar]
- Barr M.E. Redisposition of some taxa described by J.B. Ellis. Mycotaxon. 1993;46:45–76. [Google Scholar]
- Barr M.E. Notes on the Amphisphaeriaceae and related families. Mycotaxon. 1994;51:191–224. [Google Scholar]
- Booth C. Studies of Pyrenomycetes: I. Four species of Chaetosphaeria, two with Catenularia conidia. II. Melanopsamma pomiformis and its Stachybotrys conidia. Mycological Papers. 1957;68:1–27. [Google Scholar]
- Cai L., Hyde K.D., Tsui C.K.M. Genera of freshwater fungi. Fungal Diversity Research Series. 2006;18:1–261. [Google Scholar]
- Caisová L., Marin B., Melkonian M. A close-up view on ITS2 evolution and speciation – a case study in the Ulvophyceae (Chlorophyta, Viridiplantae) BMC Evolutionary Biology. 2011;11:262. doi: 10.1186/1471-2148-11-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J., Shearer C.A. Annulusmagnus and Ascitendus, two new genera in the Annulatascaceae. Mycologia. 2004;96:822–833. doi: 10.1080/15572536.2005.11832929. [DOI] [PubMed] [Google Scholar]
- Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Castañeda R.F., Guarro J., Cano J. Notes on conidial fungi. XII. New or interesting hyphomycetes from Cuba. Mycotaxon. 1997;63:169–181. [Google Scholar]
- Castlebury L.A., Rossman A.Y., Jaklitsch W.J. A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia. 2002;94:1017–1031. [PubMed] [Google Scholar]
- Clements F.E., Shear C.L. H.W. Wilson; New York, U.S.A: 1931. The genera of fungi. [Google Scholar]
- Coleman A.W. The significance of a coincidence between evolutionary landmarks found in mating affinity and a DNA sequence. Protist. 2000;151:1–9. doi: 10.1078/1434-4610-00002. [DOI] [PubMed] [Google Scholar]
- Coleman A.W. ITS2 is a double-edged tool for eukaryote evolutionary comparisons. Trends in Genetics. 2003;19:370–375. doi: 10.1016/S0168-9525(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Coleman A.W. Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acids Research. 2007;35:3322–3329. doi: 10.1093/nar/gkm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman A.W. Is there a molecular key to the level of “biological species” in eukaryotes? A DNA guide. Molecular Phylogenetics and Evolution. 2009;50:197–203. doi: 10.1016/j.ympev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Coleman A.W., Vacquier V.D. Exploring the phylogenetic utility of ITS sequences for animals: a test case for abalone (Haliotis) Journal of Molecular Evolution. 2002;54:246–257. doi: 10.1007/s00239-001-0006-0. [DOI] [PubMed] [Google Scholar]
- Cooke M.C. Vol. 2. 1871. pp. 489–981. (Handbook of British fungi). [Google Scholar]
- Cooke M.C. North American fungi. Grevillea. 1883;11:106–111. [Google Scholar]
- Cooke M.C., Ellis J.B. New Jersey fungi (cont.) Grevillea. 1876;5:49–55. [Google Scholar]
- Corda A.C.J. Vol. 1. J.G. Calve; Prague, Czech Republic: 1837. (Icones fungorum hucusque cognitorum). [Google Scholar]
- Damm U., Crous P.W., Fourie P.H. A fissitunicate ascus mechanism in the Calosphaeriaceae, and novel species of Jattaea and Calosphaeria on Prunus wood. Persoonia. 2008;20:39–52. doi: 10.3767/003158508X313940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darty K., Denise A., Ponty Y. VARNA: interactive drawing and editing of the RNA secondary structure. Bioinformatics. 2009;25:1974–1975. doi: 10.1093/bioinformatics/btp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayarathne M.C., Maharachchikumbura S.S.N., Phookamsak R. Morpho-molecular characterization and epitypification of Annulatascus velatisporus. Mycosphere. 2016;7:1389–1398. [Google Scholar]
- De Beer Z.W., Duong T.A., Barnes I. Redefining Ceratocystis and allied genera. Studies in Mycology. 2014;79:187–219. doi: 10.1016/j.simyco.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beer Z.W., Seifert K.A., Wingfield M.J. The ophiostomatoid fungi: their dual position in the Sordariomycetes. CBS Biodiversity Series. 2013;12:1–19. [Google Scholar]
- De Beer Z.W., Seifert K.A., Wingfield M.J. A nomenclator for ophiostomatoid genera and species in the Ophiostomatales and Microascales. CBS Biodiversity Series. 2013;12:245–322. [Google Scholar]
- Dennis R.W.G. A new genus for Sphaeria bacillata Cooke. Belarra. 1988;2:25–28. [Google Scholar]
- Elliott J.A. A cytological study of Ceratostomella fimbriata (E & H) Elliott. Phytopathology. 1925;16:417–422. [Google Scholar]
- Ellis J.B. South Jersey Fungi. Bulletin of the Torrey Botanical Club. 1877;6:133–135. [Google Scholar]
- Ellis J.B. General notes. Botany. The American Naturalist. 1883;17:316–320. [Google Scholar]
- Ellis J.B., Everhart B.M. W.H. Cloyd; New Jersey, USA: 1892. North American Pyrenomycetes. [Google Scholar]
- Ellis M.B. Vol. 93. 1963. Dematiaceous hyphomycetes. V; pp. 1–33. Mycological Papers. [Google Scholar]
- Ellis M.B. CMI Kew; England: 1971. Dematiaceous hyphomycetes. [Google Scholar]
- Eriksson O.E. Outline of Ascomycota. Myconet. 2006;12:1–82. [Google Scholar]
- Farr D.E., Castlebury L.A., Rossman A.Y. Greeneria uvicola, cause of bitter rot of grapes, belongs in the Diaporthales. Sydowia. 2001;53:185–199. [Google Scholar]
- Feltgen J. J. Beffort; Luxemburg: 1903. Vorstudien zu einer Pilzflora des Grossherzogthums Luxemburg. Nachträge III. [Google Scholar]
- Fernández F.A., Lutzoni F.M., Huhndorf S.M. Teleomorph-anamorph connections: the new pyrenomycetous genus Carpoligna and its Pleurothecium anamorph. Mycologia. 1999;91:251–262. [Google Scholar]
- Ferrer A., Miller A.N., Sarmiento C. Three new genera representing novel lineages of Sordariomycetidae (Sordariomycetes, Ascomycota) from tropical freshwater habitats in Costa Rica. Mycologia. 2012;104:865–879. doi: 10.3852/11-111. [DOI] [PubMed] [Google Scholar]
- Fournier J., Lechat C. Phomatospora luteotingens sp. nov., a new aquatic species of Phomatospora from France and Spain. Mycosphere. 2010;1:39–43. [Google Scholar]
- Fries E.M. Vol. 2. G. Bonnier; Copenhagen, Denmark: 1818. (Observationes mycologicae praecipue as illustrandam Flora Suecicam). [Google Scholar]
- Fries E.M. Vol. 2. 1823. (Systema Mycologicum). Lund, Sweden. (Johnson Reprint Corporation, New York, USA, 1952) [Google Scholar]
- Fryar S.C., Hyde K.D. New species and genera of ascomycetes from fresh and brackish water in Brunei: Ayria appendiculata and Sungaiicola bactrodesmiella gen. et spp. nov., Fluviatispora boothii, Torrentispora crassiparietis and T. fusiformis spp. nov. Cryptogamie Mycologie. 2004;25:245–260. [Google Scholar]
- Fuckel L. Symbolae mycologicae. Beiträge zur Kenntniss der Rheinischen Pilze. Jahrbücher des Nassauischen Vereins für Naturkunde. 1870;23–24:1–459. [Google Scholar]
- Gams W. Phialides with solitary conidia? Remarks on conidium ontogeny in some hyphomycetes. Persoonia. 1973;7:161–169. [Google Scholar]
- Gams W., Hoekstra E.S., Aptroot A. 4th ed. Centraalbureau voor Schimmelcultures; Baarn, The Netherlands: 1998. CBS course of mycology. [Google Scholar]
- García D., Stchigel A.M., Cano J. Molecular phylogeny of Coniochaetales. Mycological Research. 2006;110:1271–1289. doi: 10.1016/j.mycres.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Gargas A., Taylor J.W. Phylogeny of Discomycetes and early radiations of the apothecial Ascomycotina inferred from SSU rDNA sequence data. Experimental Mycology. 1995;19:7–15. doi: 10.1006/emyc.1995.1002. [DOI] [PubMed] [Google Scholar]
- Gebhardt H., Begerow D., Oberwinkler F. Identification of the ambrosia fungus of Xyleborus monographus and X. dryographus (Coleoptera: Curculionidae, Scolytinae) Mycological Progress. 2004;3:95–102. [Google Scholar]
- Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh T.K., Hyde K.D. Spadicoides cordanoides sp. nov., a new dematiaceous hyphomycete from submerged wood in Australia, with a taxonomic review of the genus. Mycologia. 1996;88:1022–1031. [Google Scholar]
- Gramaje D., Mostert L., Groenewald J.Z. Phaeoacremonium: from esca disease to phaeohyphomycosis. Fungal Biology. 2015;119:759–783. doi: 10.1016/j.funbio.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Grove W.B. New and noteworthy fungi. Journal of Botany. 1885;23:129–134. [Google Scholar]
- Hafez M., Iranpour M., Mullineux S.T. Identification of group I introns within the SSU rDNA gene in species of Ceratocystiopsis and related taxa. Fungal Biology. 2012;116:98–111. doi: 10.1016/j.funbio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Hall T.A. BioEdit 5.0.9: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hernández-Restrepo M., Gené J., Castañeda-Ruiz R.F. Phylogeny of saprobic microfungi from Southern Europe. Studies in Mycology. 2017;86:53–97. doi: 10.1016/j.simyco.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Restrepo M., Gené J., Mena-Portales J. New species of Cordana and epitypification of the genus. Mycologia. 2014;106:723–734. doi: 10.3852/13-122. [DOI] [PubMed] [Google Scholar]
- Hernández-Restrepo M., Silvera-Simón C., Mena-Portales J. Three new species and a new record of Diplococcium from plant debris in Spain. Mycological Progress. 2012;11:191–199. [Google Scholar]
- Ho W.H., Hyde K.D., Hodgkiss I.J. Fungal communities on submerged wood from streams in Brunei, Hong Kong, and Malaysia. Mycological Research. 2001;105:1492–1501. [Google Scholar]
- Holubová-Jechová V. Lignicolous Hyphomycetes from Czechoslovakia 6. Spadicoides and Diplococcium. Folia Geobotanica et Phytotaxonomica. 1982;17:295–327. [Google Scholar]
- Hu H.L., Fournier J., Jeewon R. Revisiting the taxonomy of Daruvedia bacillata. Mycotaxon. 2010;114:135–144. [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Hughes S.J. Studies on micro-fungi. III. Mastigosporium, Camposporium, and Ceratophorum. Mycological Papers. 1951;36:1–44. [Google Scholar]
- Hughes S.J. Revisiones Hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany. 1958;36:727–836. [Google Scholar]
- Hughes S.J. New Zealand Fungi 1. Ceratosporium Schw. New Zealand Journal of Botany. 1964;2:305–309. [Google Scholar]
- Hughes S.J. 1973. Spadicoides bina; pp. 1–2. Fungi Canadenses No. 4. [Google Scholar]
- Hughes S.J. 1973. Spadicoides canadensis; pp. 1–2. Fungi Canadenses No. 9. [Google Scholar]
- Hughes S.J. 1973. Spadicoides grovei; pp. 1–2. Fungi Canadenses No. 7. [Google Scholar]
- Hughes S.J. 1973. Spadicoides klotzschii; pp. 1–2. Fungi Canadenses No. 8. [Google Scholar]
- Hughes S.J. 1973. Spadicoides obovata; pp. 1–2. Fungi Canadenses No. 6. [Google Scholar]
- Hughes S.J. 1973. Spadicoides atra; pp. 1–2. Fungi Canadenses No. 5. [Google Scholar]
- Hughes S.J. Relocation of species of Endophragmia auct. with notes on relevant generic names. New Zealand Journal of Botany. 1979;17:139–188. [Google Scholar]
- Huhndorf S.M., Fernández F.A. Teleomorph-anamorph connections: Chaetosphaeria raciborskii and related species, and their Craspedodidymum-like anamorphs. Fungal Diversity. 2005;19:23–49. [Google Scholar]
- Huhndorf S.M., Greif M., Miller A.N. Two new genera in the Magnaporthaceae, a new addition to Ceratosphaeria and two new species of Lentomitella. Mycologia. 2008;100:940–955. doi: 10.3852/08-037. [DOI] [PubMed] [Google Scholar]
- Huhndorf S.M., Miller A.N., Fernández F.A. Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae redefined. Mycologia. 2004;96:368–387. [PubMed] [Google Scholar]
- Huhndorf S.M., Miller A.N., Greif M. Amplistroma gen. nov. and its relation to Wallrothiella, two genera with globose ascospores and acrodontium-like anamorphs. Mycologia. 2009;101:904–919. doi: 10.3852/08-213. [DOI] [PubMed] [Google Scholar]
- Hunt J. Taxonomy of the genus Ceratocystis. Lloydia. 1956;19:1–58. [Google Scholar]
- Hyde K.D. Tropical Australian freshwater fungi 2. Annulatascus velatispora gen. et sp. nov., A. bipolaris sp. nov. and Nais aquatica sp. nov. (Ascomycetes) Australian Systematic Botany. 1992;5:117–124. [Google Scholar]
- Hyde K.D. Tropical Australasian fungi. VII. New genera and species of ascomycetes. Nova Hedwigia. 1995;61:119–140. [Google Scholar]
- Hyde K.D., Goh T.K. Fungi on submerged wood in the Riviere St Marie-Louis, the Seychelles. South African Journal of Botany. 1998;64:330–339. [Google Scholar]
- Hyde K.D., Ho W.H., Jones E.B.G. Torrentispora fibrosa gen. et sp. nov. (Annulatascaceae) from freshwater habitats. Mycological Research. 2000;104:1399–1403. [Google Scholar]
- Inderbitzin P. Ceratostomella hyalocoronata, a new pyrenomycete from a stream in southern China. Mycoscience. 2000;41:167–169. [Google Scholar]
- Ingold C.T. The tertaradiate aquatic fungal spore. Mycologia. 1966;58:43–56. [Google Scholar]
- Jaklitsch W.M., Réblová M., Voglmayr H. Molecular systematics of Woswasia atropurpurea gen. et sp. nov. (Sordariomycetidae), a fungicolous ascomycete with globose ascospores and holoblastic conidiogenesis. Mycologia. 2013;105:476–485. doi: 10.3852/12-244. [DOI] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk P.M. Chaetosphaeria longispora. Index Fungorum. 2014;120:1. [Google Scholar]
- Kirk P.M., Cannon P.F., Minter D.W. 10th ed. CAB International; Wallingford, UK: 2008. Ainsworth & Bisby's Dictionary of the Fungi. [Google Scholar]
- Kirschstein W. Neue Märkische Ascomyceten. Verhandlungen des Botanischen Vereins der Provinz Brandenburg. 1907;48:39–61. [Google Scholar]
- Klaubauf S., Tharreau D., Fournier E. Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae) Studies in Mycology. 2014;79:85–120. doi: 10.1016/j.simyco.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntze O. Vol. 3. A. Felix; Leipzig, Germany: 1898. Revisio generum plantarum. [Google Scholar]
- Kuthubutheen A.J., Nawawi A. Two new species of Spadicoides from Malaysia. Mycological Research. 1991;95:163–168. [Google Scholar]
- Kuthubutheen A.J., Nawawi A. Polytretophora dendroidea sp. nov. and P. calcarata (hyphomycetes) from Malaysia. Mycological Research. 1991;95:623–627. [Google Scholar]
- Li D.W. Spadicoides subsphaerica sp. nov. from Connecticut. Mycotaxon. 2010;111:257–261. [Google Scholar]
- Li D.W., Chen J., Wang Y. Two new species of dematiaceous hyphomycetes from Hubei, China. Sydowia. 2010;62:171–179. [Google Scholar]
- Lee T.S.W., Ho W.H., Hyde K.D. Ultrastructure of the asci and ascospores of Torrentispora fibrosa. Fungal Diversity. 2004;16:87–91. [Google Scholar]
- Leontis N.B., Stombaugh J., Westhof E. The non-Watson-Crick base pairs and their associated isostericity matrices. Nucleic Acids Research. 2002;30:3497–3531. doi: 10.1093/nar/gkf481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbsch H.T., Huhndorf S.M. Myconet volume 14. Part One. Outline of Ascomycota—2009. Part Two. Notes on Ascomycete Systematics. Nos. 4751–5113. Fieldiana Life Earth Science. 2010;1:1–64. [Google Scholar]
- Lunghini D., Pinzari F., Zucconi L. Studies on Mediterranean hyphomycetes. III. Quadracaea mediterranea anam.-gen. and sp. nov. Mycotaxon. 1996;60:103–110. [Google Scholar]
- Luo Z.L., Maharachchikumbura S.S.N., Liu X.Y. Annulatascus saprophyticus sp. nov. and Pseudoannulatascus gen. nov. to accommodate Annulatascus biatriisporus (Annulatascales, Sordariomycetes) from Thailand. Phytotaxa. 2015;239:174–182. [Google Scholar]
- Ma J., Zhang K., Zhang X.G. Three new species of Spadicoides from Lushan Mountain, China. Mycological Progress. 2016;15:43. [Google Scholar]
- MacGarvie Q.D. Hyphomycetes on Juncus effusus L. Scientific Proceedings of the Royal Dublin Society. 1968;2:153–161. [Google Scholar]
- Mai J.C., Coleman A.W. The internal transcribed spacer 2 exhibits a common secondary structure in green algae and flowering plants. Journal of Molecular Evolution. 1997;44:258–271. doi: 10.1007/pl00006143. [DOI] [PubMed] [Google Scholar]
- Malloch D. University of Toronto Press; Toronto, Ontario: 1981. Moulds: their isolation, cultivation and identification. [Google Scholar]
- Marincowitz S., Crous P.W., Groenewald J.Z. Microfungi occurring on the Proteaceae in the fynbos. CBS Diversity Series. 2008;60:1–166. [Google Scholar]
- Mason-Gamer R.J., Kellogg E.A. Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae) Systematic Biology. 1996;45:524–545. [Google Scholar]
- Massoumi Alamouti S., Tsui C.K., Breuil C. Multigene phylogeny of filamentous ambrosia fungi associated with ambrosia and bark beetles. Mycological Research. 2009;113:822–835. doi: 10.1016/j.mycres.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Matsushima T. The Nippon Printing & Publishing Co.; Osaka, Japan: 1975. Icones Microfungorum a Matsushima lectorum. [Google Scholar]
- Miller A.N., Huhndorf S.M. Using phylogenetic species recognition to delimit species boundaries within Lasiosphaeria. Mycologia. 2004;96:1106–1127. [PubMed] [Google Scholar]
- Miller A.N., Huhndorf S.M. A natural classification of Lasiosphaeria based on nuclear LSU rDNA sequences. Mycological Research. 2004;108:26–34. doi: 10.1017/s0953756203008864. [DOI] [PubMed] [Google Scholar]
- Miller A.N., Huhndorf S.M. Multi-gene phylogenies indicate ascomal wall morphology is a better predictor of phylogenetic relationships than ascospore morphology in the Sordariales (Ascomycota, Fungi) Molecular Phylogenetics and Evolution. 2005;35:60–75. doi: 10.1016/j.ympev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Moreau C. Coexistence des formes Thielaviopsis et Graphium chez une souche de Ceratocystis major (van Beyma) comb. nov. Revue de Mycologie, Supplement Colonial. 1952;17:17–25. [Google Scholar]
- Müller E., von Arx J.A. Die Gattungen der didymosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz. 1962;11:1–922. [Google Scholar]
- Müller T., Philippi N., Dandekar T. Distinguishing species. RNA. 2007;13:1469–1472. doi: 10.1261/rna.617107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk A. On some species of Endoxyla recently found in Denmark. Botanisk Tidsskrift. 1965;61:56–70. [Google Scholar]
- Nees C.G., Nees T. De plantis nonnulis e mycetoidearum regno. Nova Acta Academiae Caesareae Leopoldino-Carolinae. 1818;9:227–262. [Google Scholar]
- Nylander J. Evolutionary Biology Centre; Uppsala, Sweden: 2008. MrModeltest2 v. 2.3 (Program for selecting DNA substitution models using PAUP*) [Google Scholar]
- O'Donnell K., Cigelnik E. Two different intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- Onofri S., Castagnola M. Scanning electron microscopy on some dematiaceous sympodially proliferating Hyphomycetes. Mycotaxon. 1982;14:125–139. [Google Scholar]
- Peck C.H. Report of the Botanist (1878) Annual Report on the New York State Museum of Natural History. 1879;32:17–72. [Google Scholar]
- Persoon C.H. H. Dieterich; Göttingen, Germany: 1801. Synopsis methodica fungorum. Sistens enumerationem omnium hue usque detectarum specierum, cum brevibus descriptionibus nec non synonymis et observationibus selectis. [Google Scholar]
- Persoon C.H. C.A. König; Paris & Strasbourg, France, Germany: 1808. Icones pictae specierum rariorum Fungorum in Synopsi Methodica Descriptarum. Fasc. 4. [Google Scholar]
- Petrak F. Mykologische Notizen. Annales Mycologici. 1924;24:1–182. [Google Scholar]
- Petrak F. Mykologische Beiträge zur Flora der Kanarishen Inseln. Botanischen Jahrbüchern, Beiblatt. 1929;142:93–160. [Google Scholar]
- Plattner A., Kim J.J., Reid J. Resolving taxonomic and phylogenetic incongruence within species Ceratocystiopsis minuta. Mycologia. 2009;101:878–887. doi: 10.3852/08-132. [DOI] [PubMed] [Google Scholar]
- Raja H.A., Campbell J., Shearer C.A. Freshwater ascomycetes: Cyanoannulus petersenii, a new genus and species from submerged wood. Mycotaxon. 2003;88:1–17. [Google Scholar]
- Ranghoo V.M., Hyde K.D., Liew E.C.Y. Family placement of Ascotaiwania and Ascolacicola based on DNA sequences from the large subunit rRNA gene. Fungal Diversity. 1999;2:159–168. [Google Scholar]
- Rao V.G., de Hoog G.S. New or critical Hyphomycetes from India. Studies in Mycology. 1986;28:1–84. [Google Scholar]
- Rappaz F. Phomatospora berkeleyi, P. arenaria and their Sporothrix anamorphs. Mycotaxon. 1992;45:323–330. [Google Scholar]
- Réblová M. Teleomorph-anamorph connections in Ascomycetes 2. Three new lignicolous species of Helminthosphaeria. Sydowia. 1999;51:210–222. [Google Scholar]
- Réblová M. Molecular systematics of Ceratostomella sensu lato and morphologically similar fungi. Mycologia. 2006;98:68–93. doi: 10.1080/15572536.2006.11832714. [DOI] [PubMed] [Google Scholar]
- Réblová M. Barbatosphaeria gen. et comb. nov., a new genus for Calosphaeria barbirostris. Mycologia. 2007;99:723–732. [PubMed] [Google Scholar]
- Réblová M. Teleomorph of Rhodoveronaea (Sordariomycetidae) discovered and reevaluation of Pleurophragmium. Fungal Diversity. 2009;36:129–139. [Google Scholar]
- Réblová M. New insights into the systematics and phylogeny of the genus Jattaea and similar fungi of the Calosphaeriales. Fungal Diversity. 2011;49:167–198. [Google Scholar]
- Réblová M. Two taxonomic novelties in the Sordariomycetidae: Ceratolenta caudata gen. et sp. nov. and Platytrachelon abietis gen. et comb. nov. for Ceratosphaeria abietis. Mycologia. 2013;105:462–475. doi: 10.3852/12-199. [DOI] [PubMed] [Google Scholar]
- Réblová M., Fournier J., Hyde K.D. Achroceratosphaeria, a new ascomycete genus and re-evaluation of Ceratosphaeria incolorata. Fungal Diversity. 2010;43:75–84. [Google Scholar]
- Réblová M., Fournier J., Štěpánek V. Pisorisporiales, a new order of aquatic and terrestrial fungi for Achroceratosphaeria and Pisorisporium gen. nov. in the Sordariomycetes. Persoonia. 2015;34:40–49. doi: 10.3767/003158515X685544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová M., Fournier J., Štěpánek V. Two new lineages of aquatic ascomycetes: Atractospora gen. nov. and Rubellisphaeria gen. et sp. nov., and a sexual morph of Myrmecridium montsegurinum sp. nov. Mycological Progress. 2016;15:1–18. [Google Scholar]
- Réblová M., Gams W., Seifert K.A. Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Studies in Mycology. 2011;68:163–191. doi: 10.3114/sim.2011.68.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová M., Jaklitsch W., Réblová K. Phylogenetic reconstruction of the Calosphaeriales and Togniniales using five genes and predicted RNA secondary structures of ITS, and Flabellascus tenuirostris gen. et sp. nov. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová M., Mostert L., Gams W. New genera in the Calosphaeriales: Togniniella and its anamorph Phaeocrella, and Calosphaeriophora as anamorph of Calosphaeria. Studies in Mycology. 2004;50:533–550. [Google Scholar]
- Réblová M., Réblová K., Štěpánek V. Molecular systematics of Barbatosphaeria (Sordariomycetes): multigene phylogeny and secondary ITS structure. Persoonia. 2015;35:21–38. doi: 10.3767/003158515X687434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová M., Seifert K.A. Cryptadelphia (Trichosphaeriales), a new genus for holomorphs with Brachysporium anamorphs and clarification of the taxonomic status of Wallrothiella. Mycologia. 2004;96:343–367. doi: 10.1080/15572536.2005.11832981. [DOI] [PubMed] [Google Scholar]
- Réblová M., Seifert K.A. A new fungal genus, Teracosphaeria, with a phialophora-like anamorph (Sordariomycetes, Ascomycota) Mycological Research. 2007;111:287–298. doi: 10.1016/j.mycres.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Réblová M., Seifert K.A., Fournier J. Phylogenetic classification of Pleurothecium and Pleurotheciella gen. nov. and its dactylaria-like anamorph (Sordariomycetes) based on nuclear ribosomal and protein-coding genes. Mycologia. 2012;104:1299–1314. doi: 10.3852/12-035. [DOI] [PubMed] [Google Scholar]
- Réblová M., Štěpánek V. New fungal genera, Tectonidula gen. nov. for Calosphaeria-like fungi with holoblastic-denticulate conidiogenesis and Natantiella gen. nov. for three species segregated from Ceratostomella. Mycological Research. 2009;113:991–1002. doi: 10.1016/j.mycres.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Réblová M., Štěpánek V., Schumacher R.K. Xylochrysis lucida gen. et sp. nov., a new lignicolous ascomycete (Sordariomycetidae) with holoblastic conidiogenesis. Mycologia. 2014;106:564–572. doi: 10.3852/13-266. [DOI] [PubMed] [Google Scholar]
- Réblová M., Untereiner W.A., Réblová K. Novel evolutionary lineages revealed in the Chaetothyriales (Fungi) based on multigene phylogenetic analyses and comparison of ITS secondary structure. PLoS One. 2013;8:e63547. doi: 10.1371/journal.pone.0063547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová M., Untereiner W.A., Štěpánek V. Disentangling Phialophora section Catenulatae: disposition of taxa with pigmented conidiophores and recognition of a new subclass, Sclerococcomycetidae (Eurotiomycetes) Mycological Progress. 2017;16:27–46. [Google Scholar]
- Réblová M., Winka K. Phylogeny of Chaetosphaeria and its anamorphs based on morphological and molecular data. Mycologia. 2000;92:939–954. [Google Scholar]
- Reeb V., Lutzoni F., Roux C. Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Molecular Phylogenetics and Evolution. 2004;32:1036–1060. doi: 10.1016/j.ympev.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Rehm H. Ascomycetes novi. Annales Mycologici. 1908;6:313–325. [Google Scholar]
- Rehmeyer C., Li W., Kusaba M. Organization of chromosome ends in the rice blast fungus, Magnaporthe oryzae. Nucleic Acids Research. 2006;34:4685–4701. doi: 10.1093/nar/gkl588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick J.E. Pilze aus Rio Grande do Sul (Brazilien) Brotéria Série Botânica. 1906;5:4–53. [Google Scholar]
- Saccardo P.A. Fungi Veneti novi vel critici vel mycologiae Venetae addendi. Series IX. Michelia. 1878;1:361–445. [Google Scholar]
- Saccardo P.A. Fungi Veneti novi vel critici. Series III. Michelia. 1878;1:446–452. [Google Scholar]
- Saccardo P.A. Vol. 1. 1882. pp. 1–768. (Sylloge Fungorum). [Google Scholar]
- Saccardo P.A. Vol. 2. 1883. pp. 1–813. (Sylloge Fungorum). [Google Scholar]
- Saccardo P.A. Vol. 9. 1891. pp. 1–1141. (Sylloge Fungorum). [Google Scholar]
- Samuels G.J., Barr M.E. Notes on and additions to the Niessliaceae (Hypocreales) Canadian Journal of Botany. 1997;75:2165–2176. [Google Scholar]
- Samuels G.J., Candoussau F. Heterogeneity in the Calosphaeriales: a new Calosphaeria with ramichloridium- and sporothrix-like synanamorphs. Nova Hedwigia. 1996;62:47–60. [Google Scholar]
- Samuels G.J., Candoussau F., Magni J.F. Fungicolous pyrenomycetes 1. Helminthosphaeria and the new family Helminthosphaeriaceae. Mycologia. 1997;89:141–155. [Google Scholar]
- Samuels G.J., Müller E., Petrini O. Studies on the Amphisphaeriaceae (sensu lato) 3. New species of Monographella and Pestalosphaeria and two new genera. Mycotaxon. 1987;28:473–499. [Google Scholar]
- Samuels G.J., Rossmann A.Y. Studies in the Amphisphaeriaceae (sensu lato) 2. Leiosphaerella cocoës and two new species of Oxydothis on palms. Mycotaxon. 1987;28:461–471. [Google Scholar]
- Schoch C., Sung G.H., López-Giráldez F. The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Systematic Biology. 2009;58:224–239. doi: 10.1093/sysbio/syp020. [DOI] [PubMed] [Google Scholar]
- Schultz J., Maisel S., Gerlach D. A common core of secondary structure of the internal transcribed spacer 2 (ITS2) throughout the Eukaryota. RNA. 2005;11:361–364. doi: 10.1261/rna.7204505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulzer SVM von Müggenburg. 1869. Schwämme und Pilze aus Ungarn und Slavonien. Vinkovce. (In Saccardo's Sylloge Fungorum cited as ‘Illustrationes Fungorum Slavonicorum'). Manuscript. Hungary. [Google Scholar]
- Schulzer SVM von Müggenburg, Saccardo P.A. Micromycetes Slavonici novi. Hedwigia. 1884;23:41–44. [Google Scholar]
- Shearer C.A. The freshwater ascomycetes. Nova Hedwigia. 1993;56:1–33. [Google Scholar]
- Shearer C.A. The distribution of freshwater filamentous ascomycetes. In: Misra J.K., Horn B.W., Robert W., editors. Trichomycetes and other fungal groups. Lichtwardt Commemoration. Science Publishers; Plymouth, UK: 2001. pp. 225–292. [Google Scholar]
- Shenoy B.D., Jeewon R., Wang H. Sequence data reveals phylogenetic affinities of fungal anamorphs Bahusutrabeeja, Diplococcium, Natarajania, Paliphora, Polyschema, Rattania and Spadicoides. Fungal Diversity. 2010;44:161–169. [Google Scholar]
- Shenoy B.D., Jeewon R., Wu W.P. Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycological Research. 2006;110:916–928. doi: 10.1016/j.mycres.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Sinclair R.C., Eicker A., Bhat D.J. Branching in Spadicoides. Transactions of the British Mycological Society. 1985;85:736–738. [Google Scholar]
- Sivanesan A. Studies on Ascomycetes. Transactions of British Mycological Society. 1983;81:313–332. [Google Scholar]
- Smith G.J.D., Liew E.C.Y., Hyde K.D. The Xylariales: a monophyletic order containing 7 families. Fungal Diversity. 2003;13:185–218. [Google Scholar]
- Sone T., Fukiya S., Kodama M. Molecular structure of rDNA repeat unit in Magnaporthe grisea. Bioscience, Biotechnology, and Biochemistry. 2000;64:1733–1736. doi: 10.1271/bbb.64.1733. [DOI] [PubMed] [Google Scholar]
- Spatafora J.W., Blackwell M. Molecular systematics of unitunicate perithecial ascomycetes: The Clavicipitales-Hypocreales connection. Mycologia. 1993;85:912–922. [Google Scholar]
- Spatafora J.W., Johnson D., Sung G.H. A five-gene phylogenetic analysis of the Pezizomycotina. Mycologia. 2007;98:1020–1030. [Google Scholar]
- Spatafora J.W., Volkmann-Kohlmeyer B., Kohlmeyer J. Independent terrestrial origins of the Halosphaeriales (marine Ascomycota) American Journal of Botany. 1998;85:1569–1580. [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Su H.Y., Hyde K.D., Maharachchikumbura S.S.N. The families Distoseptisporaceae fam. nov., Kirschsteiniotheliaceae, Sporormiaceae and Torulaceae, with new species from freshwater in Yunnan Province, China. Fungal Diversity. 2016;80:375–409. [Google Scholar]
- Suh S.O., Blackwell M. Molecular phylogeny of the cleistothecial fungi placed in Cephalothecaceae and Pseudeurotiaceae. Mycologia. 1999;91:836–848. [Google Scholar]
- Sukosd Z., Knudsen B., Kjems J. PPfold 3.0: Fast RNA secondary structure prediction using phylogeny and auxiliary data. Bioinformatics. 2012;28:2691–2692. doi: 10.1093/bioinformatics/bts488. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts: 2002. PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods) [Google Scholar]
- Tang A.M., Jeewon R., Hyde K.D. Phylogenetic utility of protein (RPB2, beta-tubulin) and ribosomal (LSU, SSU) gene sequences in the systematics of Sordariomycetes (Ascomycota, Fungi) Antonie Van Leeuwenhoek. 2007;91:327–349. doi: 10.1007/s10482-006-9120-8. [DOI] [PubMed] [Google Scholar]
- Thongkantha S., Jeewon R., Vijaykrishna D. Molecular phylogeny of Magnaporthaceae (Sordariomycetes) with a new species Ophioceras chiangdaoense from Dracaena loureiroi in Thailand. Fungal Diversity. 2009;34:157–173. [Google Scholar]
- Tsui C.K.M., Ranghoo V.M., Hodgkiss I.J. Three new species of Annulatascus (Ascomycetes) from Hong Kong freshwater habitats. Mycoscience. 2002;43:383–389. [Google Scholar]
- Untereiner W.A. A taxonomic revision of the genus Endoxyla. Mycologia. 1993;85:294–310. [Google Scholar]
- Untereiner W.A., Bogale M., Carter A. Molecular phylogeny of Boliniales (Sordariomycetes) with an assessment of the systematics of Apiorhynchostoma, Endoxyla and Pseudovalsaria. Mycologia. 2013;105:564–588. doi: 10.3852/12-326. [DOI] [PubMed] [Google Scholar]
- Vassiljeva L.N., Stephenson S.L. Notes on pyrenomycetous fungi in the Mountain Lake area of southwestern Virginia. Mycosphere. 2014;5:218–227. [Google Scholar]
- Vijaykrishna D., Jeewon R., Hyde K.D. Fusoidispora aquatica: a new ascomycete from Hong Kong based on morphology and phylogeny inferred from rDNA sequences. Sydowia. 2005;57:267–280. [Google Scholar]
- Von Arx J.A. Über die Ascomycetengattung Ceratostomella Sacc., Ophiostoma Syd. und Rostrella Zimmermann. Antonie van Leeuwenhoek. 1952;18:201–213. doi: 10.1007/BF02538608. [DOI] [PubMed] [Google Scholar]
- Von Hammer C., Scheuer C. Holzbewohnende Pilze aus dem Hartelsgraben (Nationalpark Gesäuse, Steiermark, Österreich) Naturwissenschaftlicher Verein für Steiermark. 2008;137:99–122. [Google Scholar]
- Von Höhnel F.X.R. Mykologishe Fragmente. Annales Mycologici. 1906;3:548–560. [Google Scholar]
- Von Höhnel F.X.R. Revision von 292 der von J. Feltgen aufgestellen Ascomycetenformen auf Grund der Originalexemplare. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften in Wien. Mathematisch-Naturwissenschaftliche Klasse, Abteilung 1. 1906;115:1189–1327. [Google Scholar]
- Von Höhnel F.X.R. Fragmente zur Mykologie: VIII. Mitteilung (Nr. 354 bis 406) Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften in Wien. Mathematisch-Naturwissenschaftliche Klasse, Abteilung 1. 1909;118:1157–1246. [Google Scholar]
- Von Höhnel F.X.R. Mykologische Fragmente. CXCV. Über die Gattung Parodiopsis Maublanc. Annales Mycologici. 1918;7:1–84. [Google Scholar]
- Von Niessl G. Notizen über neue und kritische Pyrenomyceten. Verhandlungen des Naturforschenden Vereines in Brünn. 1876;14:165–218. [Google Scholar]
- Von Schweinitz L.D. Synopsis fungorum in America boreali media degentium. Transactions of the American Philosophical Society. 1832;4:141–316. [Google Scholar]
- Wang C.J.K. Spadicoides in New York. Memoirs of the New York Botanical Garden. 1976;28:218–228. [Google Scholar]
- Wang C.J.K., Sutton B.C. New and rare lignicolous Hyphomycetes. Mycologia. 1982;74:489–500. [Google Scholar]
- Wang C.J.K., Sutton B.C. Diplococcium hughesii sp. nov. with a Selenosporella synanamorph. Canadian Journal of Botany. 1998;76:1608–1613. [Google Scholar]
- Winka K. Umeå University; Sweden: 2000. Phylogenetic relationships within the Ascomycota based on 18S rDNA sequences. Ph.D. Thesis. [Google Scholar]
- Winter H.G. 2nd ed. E. Kummer; Leipzig, Germany: 1887. Die Pilze Deutschlands, Oesterreichs und der Schweiz. 2. Abth. Ascomyceten: Gymnoasceen und Pyrenomyceten. Rabenhorst's Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. [Google Scholar]
- Wolf M., Chen S., Song J. Compensatory base changes in ITS2 secondary structures correlate with the biological species concept despite intragenomic variability in ITS2 sequences – a proof of concept. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Castlebury L.A., Miller A.N. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia. 2007;98:1076–1087. doi: 10.3852/mycologia.98.6.1076. [DOI] [PubMed] [Google Scholar]
- Zhang H., Dong W., Hyde K.D. Towards a natural classification of Annulatascaceae-like taxa: introducing Atractosporales ord. nov. and six new families. Fungal Diversity. 2017;85:75–110. [Google Scholar]
- Zhang N., Zhao S., Shen Q. A six-gene phylogeny reveals the evolution of mode of infection in the rice blast fungus and allied species. Mycologia. 2011;103:1267–1276. doi: 10.3852/11-022. [DOI] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]