Abstract
Objective:
Anthocyanins belong to a class of flavonoids, exhibiting antioxidant and anti-inflammatory actions have been reported to have anti-cancer effects. Here, we investigated whether anthocyanins can inhibit cancer cell proliferation, invasion, and angiogenesis in human lung cancer A549 cells, which are critically involved in cancer metastasis.
Methods:
We used anthocyanins from fruits of Vitis coignetiae Pulliat (AIMs) which has been used in Korean folk medicine for the treatment of inflammatory diseases and cancers. We have performed cell proliferation assays, cell invasion assay, gelatin zymography, wound healing assay and western blotting to examine whether anthocyanins can inhibit cancer cell proliferation, invasion, and angiogenesis in A549 cells.
Result:
AIMs did not inhibit cancer cell proliferation on A549 cells. Also, AIMs suppressed cancer migration, and invasion by supressing MMP-2 and MMP-9 expression. The Immuno-blotting results also revealed that AIMs suppressed the proteins involved in cancer proliferation (COX-2, C-myc, cyclin D1), migration and invasion (MMP-2, MMP-9), anti-apoptosis (XIAP, and c-IAP2), adhesion and angiogenesis (ICAM-1, VEGF).
Conclusion:
This study demonstrates that the anthocyanins isolated from fruits of Vitis coignetiae Pulliat inhibit cancer proliferation, cancer migration, and invasion that is involve in cancer-metastasis. This study provides evidence that AIMs might have anti-cancer effects on human lung cancer.
Keywords: Anthocyanins, Vitis coignetiae Pulliat, invasion, anti-cancer effects, lung cancer
Introduction
Lung cancer is the leading cause of cancer death worldwide (Jung et al., 2014; Song et al., 2016). The main cause of cancer death is metastasis. Therefore, the control of the metastatic activity is crucial for the management of lung cancer. The conventional systemic chemotherapy is a mainstay of lung cancer treatment for the inoperable lung cancer patients, shown to prolong the survival and improve quality of life. However, the most of elderly patients have experienced serious side effects from chemotherapy. Some of these patients die of complications of chemotherapy. As advances in medical science, our lifespan has been extended. The population of elderly cancer patients and their cancer-related mortality are expected to increase because the elderly have a high risk for cancer development. Therefore, it’s essential to find out less toxic agents in controlling cancer for this population. Recently dietary agents are known to safely modulate physiological function and enhance anti-cancer activity (Chun et al., 2003; Liu et al., 2007; Salvioli et al., 2007; Hatcher et al., 2008), and some phytochemicals showed anticancer effects without showing any toxicity to normal cells (Han et al., 2013; Jeong et al., 2015). Vitis coignetiae Pulliat (Meoru in Korea) traditionally has been used in Korean folk medicine for the treatment of inflammatory lesions and cancer. It contains abundance of anthocyanins belonging to a class of flavonoids. Recently, in vitro and in vivo anti-cancer activities of anthocyanins have been demonstrated regarding anti-angiognesis and cancer invasion (Favot et al., 2003; Syed et al., 2008). We previously suggested that the anthocyanins isolated from Vitis coignetiae Pulliat (AIMs) may suppress cancer invasion through suppression of NF-κB pathway (Shin et al., 2009a). However, few studies were conducted to determine whether AIMs inhibit cancer proliferation, migration, invasion, adhesion and angiogenesis that critically determine cancer metastasis. Here, we determine whether AIMs can inhibit cancer cell proliferation, migration, invasion, and angiogenesis which are critically involved in cancer metastasis.
Materials and Methods
Cell culture and chemicals
Human lung cancer cell lines A549 cells from the American type culture collection (Rockville, MD, USA) were cultured in RPMI 1640 medium (Invitrogen Corp, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS) (GIBCO BRL, Grand Island, NY, USA), 1 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2. Caspase activity assay kits were obtained from R and D Systems (Minneapolis, MN, USA). Antibodies against cyclin D1, c-Myc, MMP-2, ICAM-1, and VEGF were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody against β-actin was from Sigma (Beverly, MA). Peroxidase-labeled donkey anti-rabbit and sheep anti-mouse immunoglobulin, and an enhanced chemiluminescence (ECL) kit were purchased from Amersham (Arlington Heights, IL). All other chemicals not specifically cited here were purchased from Sigma Chemical Co. (St. Louis, MO). The anthocyanins were isolated from Vitis coignetiae Pulliat.
Cell proliferation assays
For the cell viability assay, the cells were seeded onto 24-well plates at a concentration of 5× 104 cells/ml, grown to 70% confluence and then treated with the indicated concentration of AIMs for 48 h. Control cells were supplemented with media containing 0.1% DMSO (vehicle control). Following treatment, cell viability was determined by MTT assays.
Cell invasion assay
For the cell invasion assays, A549 cells were cultured in serum-free media overnight. 5 x 104 cells were loaded onto pre-coated Matrigel 24-well invasion chambers (BD Biosciences, San Jose, CA) with and without AIMs (400 μg/ml). Then 0.5 ml of medium containing 20% FBS was added to the wells of the plate to serve as a chemoattractant. The Matrigel invasion chambers were incubated for 18 h. We removed the cells on the Matrigel. Invading cells were fixed with 10% formalin, stained with DAPI, and counted.
Gelatin zymography
The gelatinolytic activities for MMP-2 and MMP-9 in the culture medium were assayed by electrophoresis on 10% polyacrylamide gels containing 1 mg/ml gelatin at 4°C. Polyacrylamide gels were run at 120 V, washed in 2.5% Triton X-100 for 1 h, and then incubated for 16 h at 37°C in activation buffer (50 mM Tris–HCl, pH 7.5, 10 mM CaCl2). After staining with Coomassie blue (10% glacial acetic acid, 30% methanol and 1.5% Coomassie brilliant blue) for 2–3 h, the gel was washed with a solution of 10% glacial acetic acid and 30% methanol without Coomassie blue for 1 h. White lysis zones indicating gelatin degradation were revealed by staining with Coomassie brilliant blue.
Wound Healing Assay
The wound healing assays were conducted according to the methods described previously.13 A549 cells were grown on 35 mm dish plate to 100% confluent monolayer and then scratched to form a 100-µm “wound” using sterile pipette tips. The cells were then cultured in the presence or absence of AFMs in serum-free media for 24 h. The images were recorded at 12h and 24h after scratch using an Olympus photomicroscope.
Western blotting
The procedure was performed as described previously (Kim et al., 2006). In brief, the concentrations of cell lysate proteins were determined by means of the Bradford protein assay (Biorad lab, Richmond, CA, USA) using bovine serum albumin as the standard. The protein (30 μg) was resolved by electrophoresis, electrotransferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA), and then incubated with primary antibodies followed by secondary antibody conjugated to peroxidase. Blots were developed with an ECL detection system.
Statistics
Each experiment was performed in triplicate. The results were expressed as means ± SD. Significant differences were determined using the one-way analysis of variance (ANOVA) with post-test Neuman-Keuls for the cases at least three treatment groups and student t-test for two group comparison. Statistical significance was defined as P<0.05.
Results
AIMs inhibited the cell proliferation and cancer cell invasion in A549 Cells
We assessed the effects of AIMs on the cell growth of A549 cells after 24 h treatment. MTT assay revealed that AIMs did not suppressed the proliferation of the A549 cells in a dose dependent manner (Figure 1A). AIMs at the concentration of 200μg/mL slightly suppressed cell proliferation when compared with controls. To determine whether AIMs inhibit cancer invasion, we performed cell invasion assay, which revealed that AIMs inhibit cancer invasion in a dose-dependent manner (Figure 1B). Next, to find out the molecular mechanism, we performed gelatin zymography, which revealed that AIMs significantly inhibited MMP-2 and MMP-9 expression in a dose-dependent manner (Figure 1C). These results suggest that AIMs have anti-cancer properties on proliferation and cell invasion in A549 cells.
Figure 1.
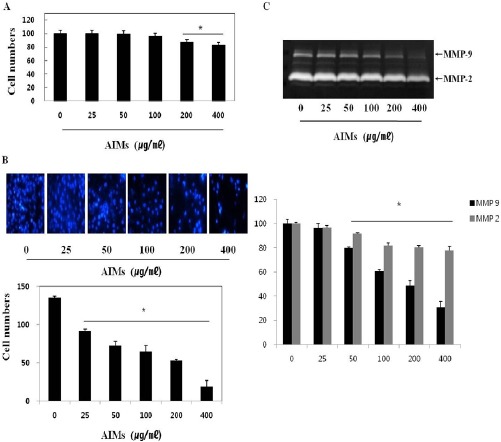
The Inhibitory Effects of Aims on Cancer Cell Proliferation of A549 Lung Cancer Cell. (A) Inhibitory effects of AIMs on cell proliferation were assessed by MTT assay. Cells were treated with indicated concentrations of AIMs for 24h. (B) AIMs greatly influenced the TNF-stimulated cell invasion of A549 cells. (C) A549 cells were treated with TNF. Conditioning medium was used for the measurement of secreted MMP-2 and MMP-9 protein levels by gelatin zymography. The enzyme activities of MMP-2 and MMP-9 in treated cells are expressed as a percentage of their activities in untreated cells. Data are mean ± SD values of three independent experiments. *P< 0.05 versus control.
AIMs inhibited of TNF (tumor necrosis factor)-stimulated migration and invasion by supressing MMP-2 and MMP-9 expression
To show clear anti-cancer effects of AIMs, we use TNF which is known as a cytokine involved in cancer progression and metastasis (Balkwill, 2006). As shown in Figure 2, AIMs suppressed cancer cell migration. In addition, AIMs markedly suppressed the TNF-stimulated cell invasion (Figure 3A). Next, we performed MTT assay, we found that cell viability test, which revealed the TNF did not much stimulated cell proliferation, but AIMs still suppressed cancer cell proliferation (Figure 3B upper). The western blot revealed that TNF upregulated Cyclin D1 and COX-2, which were suppressed by AIMs (Figure 3B lower). Moreover, AIMs suppressed MMP-9 markedly compared to MMP-2, which finding was confirmed in gelatin (Figure 3C). These findings suggest that AIMs inhibited TNF-stimulated cancer cell migration and cancer invasion at least in part by the suppression of MMP-2 and MMP-9 expressions.
Figure 2.
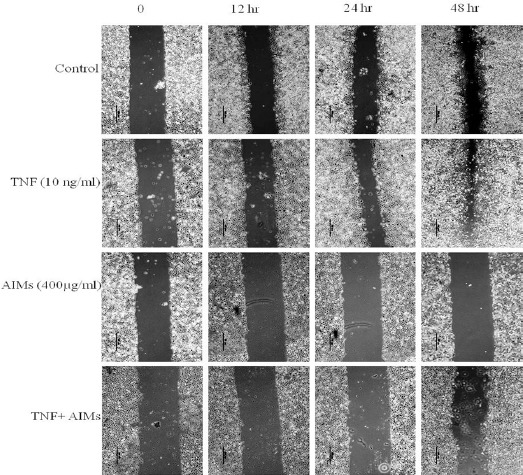
Inhibitory Effects of Aims on Cancer Cell Migration of A549 Lung Cancer Cells. (A) Cells were grown to 100% confluency on 30-mm cell culture dishes coated with rat tail collagen and then treated with or without AIMs (400 μg/ml). For the group treated with AIMs and TNF, cells were pre-treated with AIMs for 1 hour and then treated with TNF (10 ng/mL). A scratch was made through the cell layer using a pipette tip. After washing with PBS, serum-free media with or without AIMs was added. Photographs of the wounded area were taken at the interval of 0 h, 12 h, 24 h, and 48 h after the scratch to evaluate cell movement into the wounded area.
Figure 3.
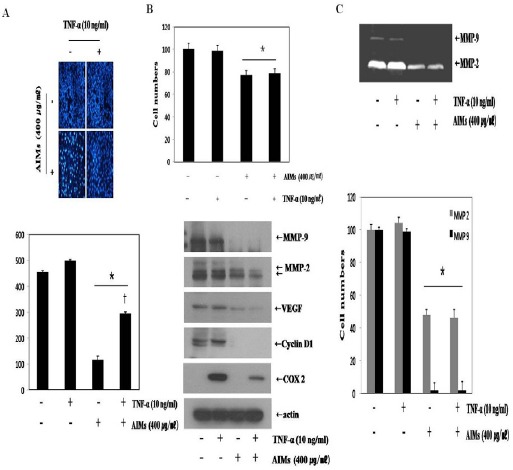
Inhibitory Effects of Aims on the Metastasis-Related Protein Expression of TNF-Stimulated A549 Cells. (A) AIMs greatly influenced the TNF-stimulated cell invasion of A549 cells. (B) A549 cells were treated with TNF. Whole-cell extracts were prepared (30 mg) and subjected to western blot analysis. (C) Conditioning medium was used for the measurement of secreted MMP-2 and MMP-9 protein levels by gelatin zymography. The enzyme activities of MMP-2 and MMP-9 in treated cells are expressed as a percentage of their activities in untreated cells. Data are mean ± SD values of three independent experiments. *P < 0.05 versus control.
AIMs suppress the proteins involved in proliferation, anti-apoptosis, migration, invasion, and angiogenesis
Figure 3B (lower) showed that AIMs inhibited COX-2, cyclin D1, and VEGF. Here, we tested the more proteins stimulated TNF that is involved in cancer metastasis. As shown in Figure 4. AIMs suppressed many genes stimulated TNF that is involved in proliferation (COX-2, c-Myc, and cyclin D1) (Figure 4 A), anti-apoptosis (XIAP, and c-IAP2) (Figure 4B), adhesion and angiogenesis (ICAM-1, and VEGF) (Figure 4C) in cancer. These finding suggest that AIMs suppressed the TNF-induced metastasis-related proteins involved in anti-apoptosis, proliferation, invasion and angiogenesis in A549 cells.
Figure 4.
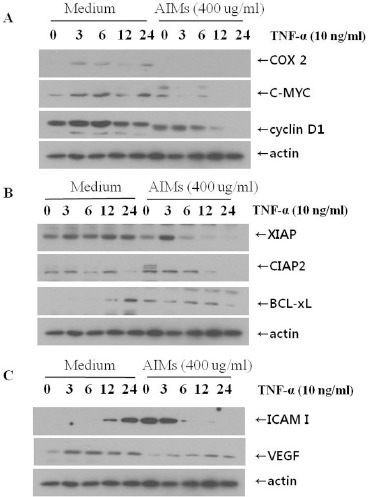
Inhibitory Effects of Aims on the Proteins Involved in Cancer Proliferation, Anti-Apoptosis, Migration, Invasion, and Angiogenesis. Cells (5×104 cells), either left untreated or pre-treated with AIMs for 1 h, were exposed to TNF (10 nM) for indicated times. Whole-cell extracts were prepared, and 30 μg of the whole-cell lysate was analysed by Western blot using antibodies against various TNF-stimulated proteins involved in (A) cancer cell proliferation, (B) anti-apoptosis, and (C) invasion & angiogenesis
Discussion
This study was designed to investigate the anti-cancer effects of AIMs on metastasis-related cellular responses and the protein expressions in lung cancer cells. We found that AIMs inhibited the growth, migration and invasion of A549 cells. Further, AIMs inhibited metastasis-related proteins involved in cell proliferation, anti-apoptosis, invasion, adhesion, and angiogenesis. TNF is known as a NF-κB activator mainly involved in the inflammation, cancer cell progression and metastasis (Aggarwal, 2004; Gilmore, 2006), and it is highly expressed in advanced lung cancer (Tas et al., 2005). Therefore, the use of TNF is not a artificial experimental setting to exaggerate the effects of AIMs. In addition, NF-κB is known to play significant roles in cancer cell invasion and metastasis (Aggarwal, 2004; Gilmore, 2006).
For the metastasis, tumor invasion is the first step, which process includes proteolytic digestion of the ECM, and cell migration through the basement membranes to reach the circulatory system. Particularly, MMP-2 (gelatinase-A) and MMP-9 (gelatinase-B) are involved in the proteolytic digestion of the basement membrane by degrading type IV collagen (Liabakk et al., 1996). In this study, AIMs markedly suppressed MMP-2 and MMP-9, which is consistent with the previous study demonstrating that AIMs had inhibitory activities on the expression of MMP-2 and MMP-9 in hepatocellular carcinoma cells (Shin et al., 2009a; Shin et al., 2009b).
The previous reports showed that AIMs suppress NF-κB activation induced by TNF (Shin et al., 2009c; Yun et al., 2010). The proteins that were suppressed by AIMs are NF-κB down-stream molecules (Aggarwal et al., 2006; Nair et al., 2006; Hafeez et al., 2008). Therefore, it is reasonable that AIMs have anti-metastatic properties.
The limitation of this study is that we did not clarify whether the anticancer effects of AIMs is mainly involved in NF-κB pathway or PI3K-Akt pathway. The previous reports show that anthocyanins suppressed both NF-κB pathway and PI3K-Akt pathway. In addition, a previous report showed that delphinidin inhibited the expression of kinase phospho-IKK which is a upstream kinase of phospho-IκBα. The expression of kinase phospho-IKK is also regulated by many other signals such as PI3K-Akt pathway. According the previous reports, both the signings are involved in the anti-cancer effects of AIMs. Here, we focused to determine whether AIMs exert anti-metastasis effects and to verify the molecular target that is involved in the anti-metastasis effects of AIM.
AIMs suppressed the gene expression involving cell proliferation, anti-apoptosis, invasion, and angiogenesis which are all required to make metastatic lesions.
In conclusion, this study indicated that AIMs have anti-metastatic effects by inhibiting the proteins involved in cell proliferation, anti-apoptosis, invasion, adhesion and angiogenesis (Figure 5). This study provides evidence that AIMs might have anti-cancer effects on human lung cancer.
Figure 5.
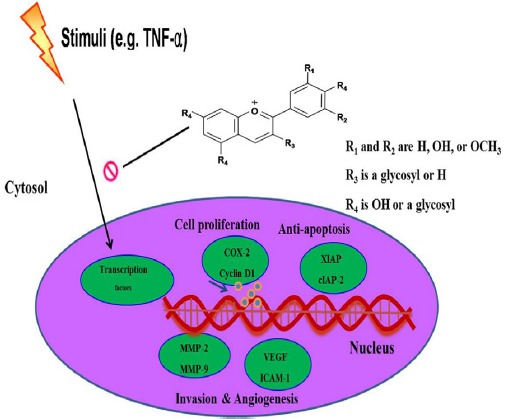
Schematic Representation of the Anti-Cancer Effects of Aims on A549 Lung Cancer Cells. Following treatment of TNF, AIMs inhibited the TNF-stimulated proteins involved in cancer cell proliferation, anti-apoptosis, invasion, adhesion, and angiogenesis. Taken together, these data suggested that AIMs may exert anti-metastatic effects on human lung cancer
Acknowledgements
This study was supported by a grant of the National R and D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (0820050), and in part a grant from the Korean Cancer Research Institute, Republic of Korea.
References
- Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Ichikawa H, Takada Y, et al. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–16. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- Chun KH, Kosmeder JW, Sun S, et al. Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells. J Natl Cancer Inst. 2003;95:291–302. doi: 10.1093/jnci/95.4.291. [DOI] [PubMed] [Google Scholar]
- Favot L, Martin S, Keravis T, et al. Involvement of cyclin-dependent pathway in the inhibitory effect of delphinidin on angiogenesis. Cardiovasc Res. 2003;59:479–87. doi: 10.1016/s0008-6363(03)00433-4. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–4. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Hafeez BB, Siddiqui IA, Asim M, et al. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-kappaB signaling. Cancer Res. 2008;68:8564–72. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MH, Lee WS, Jung JH, et al. Polyphenols isolated from Allium cepa L. induces apoptosis by suppressing IAP-1 through inhibiting PI3K/Akt signaling pathways in human leukemic cells. Food Chem Toxicol. 2013;62:382–9. doi: 10.1016/j.fct.2013.08.085. [DOI] [PubMed] [Google Scholar]
- Hatcher H, Planalp R, Cho J, et al. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee WS, Go SI, et al. Pachymic acid induces apoptosis of EJ bladder cancer cells by DR5 up-regulation, ROS generation, modulation of Bcl-2 and IAP family members. Phytother Res. 2015;29:1516–24. doi: 10.1002/ptr.5402. [DOI] [PubMed] [Google Scholar]
- Jung KW, Won YJ, Kong HJ, et al. Prediction of cancer incidence and mortality in Korea, 2014. Cancer Res Treat. 2014;46:124–30. doi: 10.4143/crt.2014.46.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Tsoy I, Park JM, et al. Anthocyanins from soybean seed coat inhibit the expression of TNF-alpha-induced genes associated with ischemia/reperfusion in endothelial cell by NF-kappaB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett. 2006;580:1391–7. doi: 10.1016/j.febslet.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Liabakk NB, Talbot I, Smith RA, et al. Matrix metalloprotease 2 (MMP-2) and matrix metalloprotease 9 (MMP-9) type IV collagenases in colorectal cancer. Cancer Res. 1996;56:190–6. [PubMed] [Google Scholar]
- Liu BL, Zhang X, Zhang W, et al. New enlightenment of French Paradox: resveratrol's potential for cancer chemoprevention and anti-cancer therapy. Cancer Biol Ther. 2007;6:1833–6. doi: 10.4161/cbt.6.12.5161. [DOI] [PubMed] [Google Scholar]
- Nair AS, Shishodia S, Ahn KS, et al. Deguelin, an Akt inhibitor, suppresses IkappaBalpha kinase activation leading to suppression of NF-kappaB-regulated gene expression, potentiation of apoptosis, and inhibition of cellular invasion. J Immunol. 2006;177:5612–22. doi: 10.4049/jimmunol.177.8.5612. [DOI] [PubMed] [Google Scholar]
- Salvioli S, Sikora E, Cooper EL, et al. Curcumin in cell death processes: a challenge for CAM of age-related pathologies. Evid Based Complement Alternat Med. 2007;4:181–90. doi: 10.1093/ecam/nem043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DY, Lee WS, Kim SH, et al. Anti-invasive activity of anthocyanins isolated from Vitis coignetiae in human hepatocarcinoma cells. J Med Food. 2009a;12:967–72. doi: 10.1089/jmf.2008.1338. [DOI] [PubMed] [Google Scholar]
- Shin DY, Ryu CH, Lee WS, et al. Induction of apoptosis and inhibition of invasion in human hepatoma cells by anthocyanins from meoru. Ann N Y Acad Sci. 2009b;1171:137–48. doi: 10.1111/j.1749-6632.2009.04689.x. [DOI] [PubMed] [Google Scholar]
- Song HN, Go SI, Lee WS, et al. Population-based regional cancer incidence in Korea: Comparison between Urban and Rural areas. Cancer Res Treat. 2016;48:789–97. doi: 10.4143/crt.2015.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed DN, Afaq F, Sarfaraz S, et al. Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol Appl Pharmacol. 2008;231:52–60. doi: 10.1016/j.taap.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas F, Duranyildiz D, Argon A, et al. Serum levels of leptin and proinflammatory cytokines in advanced-stage non-small cell lung cancer. Med Oncol. 2005;22:353–8. doi: 10.1385/MO:22:4:353. [DOI] [PubMed] [Google Scholar]
- Yun JW, Lee WS, Kim MJ, et al. Characterization of a profile of the anthocyanins isolated from Vitis coignetiae Pulliat and their anti-invasive activity on HT-29 human colon cancer cells. Food Chem Toxicol. 2010;48:903–9. doi: 10.1016/j.fct.2009.12.031. [DOI] [PubMed] [Google Scholar]


