Abstract
Background and aims:
The insulin pathway may play a role in development of colorectal cancer (CRC). In this study, we investigated associations between CRC and obesity in Egyptians with reference to single nucleotide polymorphisms (SNPs) in the insulin-like growth factor-1 (IGF-I) gene. We also studied serum levels of IGF-1in Egyptian CRC patients with different BMI values.
Methods:
This prospective study included 66 CRC patients and 30 healthy individuals, for whom body mass index (BMI) was estimated, patients and controls being categorized into overweight or obese in one group and average weight in the other. Serum levels of IGF-1 were assessed by ELISA and SNPs in the IGF-I gene at rs6214C/T, rs6220 T/C and rs35767 C/T were examined by PCR- RFLP.
Results:
Serum levels of IGF-1 were significantly lower in both CRC average weight and overweight cases. IGF-1 could negatively predict CRC at a cut-off of 154 ng/ml with 87.5% sensitivity and 72.6 specificity. IGF-1 rs6214 CT and TT (T allele) genotypes were associated with a significantly increased risk of CRC. Univariate logistic regression showed that CRC risk significantly decreases by 0.14 for each one unit increase in IGF1.
Conclusion:
BMI could be considered as effect modifier for CRC risk. IGF-1 SNP rs6214 (TT and CT) are significantly associated with risk regardless of the BMI.
Keywords: Colorectal cancer, insulin like growth factor binding protein, single nucleotide polymorphism, Obesity
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in males coming after lung and prostate and the second in females after breast cancer worldwide, It is considered the fourth cause of cancer related death all over the world (Ferlay et al., 2013). Obesity, diabetes mellitus and physical inactivity have been linked to the CRC through different mechanisms, the most important of which was insulin pathway (Chapelle, 2004; Chung et al., 2008; lee et al., 2013).
Insulin like Growth Factor 1 (IGF-1) is an insulin related peptide that mediates the effect of growth hormone on the growing tissues. It is secreted mainly by the liver and produces its effect either systemically or by paracrine action via its receptor IGF-1 receptor (IGF-1 R) expressed in different tissues. Approximately 99% of serum IGF is found in circulation bound to its binding proteins (IGFBP) mainly IGFBP-3 (Clemmons, 2016). Levels of IGF are influenced by circulating insulin levels which in turn alter the level of IGF binding protein thus increasing the bio-availability of IGF. The insulin-like growth factors are involved in enhancement of cell proliferation, differentiation and apoptosis and have been implicated in carcinogenesis (Brahmkhatri et al., 2015).
Several epidemiologic studies have shown that IGF-1 is positively associated with the risk of colorectal cancer, and experimental studies have shown that IGF-1 has mitogenic and antiapoptotic actions on colorectal cancer cells; also clinical studies showed higher incidence of CRC in diabetic patients and Acromegalic patients through higher serum levels IGF (Giovannucci, 2001) The increased IGF-1 bioavailability may, over time, increase the risk of colorectal cancer. IGF axis through its components may have role in colorectal carcinogenesis, also diet and other associated factors like physical activity and obesity may increase the risk of this cancer (sax et al., 2014).
However other studies couldn’t establish such finding (Rinaldi et al., 2010; Karimi et al., 2013). The aim of current study was to clarify the link between obesity and colorectal carcinogenesis through insulin pathway in Egyptian patients through studying the genetic mutations in IGF-I gene and IGFBP gene as well as the serum levels of IGF-1 and IGFBP-3 in patient with different BMI.
Materials and Methods
Patients and methods
This case-control study was approved by the clinical research ethics committee in the Endemic Medicine Department, Cairo University. The study was done in accordance with the Helsinki Declaration and all participants gave written informed consent before being involved in the study. We recruited 96 subjects from those presented to the Endoscopy unit, Kasr Al Ainy hospitals, Cairo University during the period from February 2015 to December 2016. All participants were either presenting with lower gastrointestinal symptoms and indicated for colonoscopy or coming for screening colonoscopy. Sixty six patients showed colonoscopic evidence of colorectal cancers and histopathologically confirmed adenocarcinoma were involved in the study as two groups, the main exclusion criteria were Patients with CRC on top of Inflammatory bowel disease, Patients with Familial Polyposis Coli, Patients receiving sex hormones, Patients with decompensated liver disease and Patients had previous bariatric surgery.
BMI was estimated in all participants using the following AQ7 formula: weight (kg)/height (m2). Regardless of whether they were cases or controls, participants were divided according to their BMI into two groups: the first group included participants with BMI of at least 25 kg/m2 (the overweight and obese group) and comprised 36 cases of confirmed CRC, and the second group included patients with BMI of less than 25 kg/m2 (the average weight group) and included 30 cases of confirmed CRC. Thirty participants without colonic lesions were enrolled as controls. CRC cases and controls were age and sex matched.
Colonoscopy
After patient preparation for 48 hours with magnesium salt, full colonoscopic examination was done using Olympus X 240 Videoscope under conscious sedation (using benzodiazepines), minimum withdrawal time was 10 minutes to allow visualization of all colonic segments, Exact localization of the site of the lesion, The gross nature is clearly identified either Malignant ulcer, Mass lesion, Diffuse infiltration or Malignant stricture, Biopsy taking by Avulsion technique or Push biopsy approach.
Serum level IGF-1: Assessment of IGF-1 blood level was conducted according to manufacturer’s protocol using ELISA kit supplied by R&D systems (Minneapolis, USA), inc. Catalogue number DC140; Minneapolis, Minnesota, USA).
Assessment of genetic polymorphisms
Single nuclutide polymorphisms (SNPs) at three different genes coding for IGF-1 were assessed in all subjects: rs6214 C/T, rs6220 T/C and rs35767 C/T.
Five ml of blood was collected in EDTA tube from each AQ9 participant. An equal volume of Ficoll solution (5 ml) was added to the blood sample. The samples were centrifuged at 3000 rpm for 10 min to collect the mononuclear cell layer that was aspirated and stored at – 201C. DNA was extracted from the mononuclear cell layer using QIAamp kit supplied by Qiagen (catalogue number AQ10 51306; USA) according to manufacturer’s specifications.
DNA was amplified using TaqPCR Master Mix Kit supplied by Qiagen (catalogue number 201443). Polymorphism analysis was performed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique. All PCR reactions were performed in a total volume of 25 μl containing 150-ng genomic DNA, 2X Taq Green PCR Master Mix, 25 pM of each forward and reverse primers (Biosearch technologies). PCR amplification was carried out in the DNA thermal cycler (PTC programmable thermal controller, MJ Research, Watertown, MA). Amplification conditions were initial denaturation at 95°C for 5 min followed by 35 cycles of; 95°C for 45 s, * for 45 s, and 72°C for 30 s, with final extension for 7 min at 72°C. The amplified PCR products were visualized by 2% agarose gel electrophoresis under UV light. Primers sequences used were as follows;
rs6214 (IGF-1)
F: 50-AAT TAT TCC CTC TCA ACA AAA CTT TAT AGG-3’
R: 50-TGA AGG AAA TAA GTC ATA GAC ACT CTT AGA A-3’
rs6220 (IGF-1)
F: 50-AAC AAA GAG ATT TCT ACC AGT GAA AGG-3’
R: 50-GCC TAG AAA AGA AGG AAT CAT TGT G-3’
rs35767 (IGF-1)
F: 50-AGA GTA GGA TTT CAA GCA GAA CTG TGT-3’
R: 50-TGG AAA TAA CCT GGA CCT TGA ATT-3’
Statistical methods
Data were entered, validated and analyzed using STATA 14 software. Patients’ data were expressed by mean (± standard deviation)or median (interquartile range (IQR) for continues variables and as number (percent) for categorical variables Receiver operator characteristic (ROC) curves were constructed to assess the performance of IGF-1and IGFBP-3 markers in predictability of CRC and to detect best cutoff values. Gentoypes and polymorphism patterns of all genes were compared amoung cases and controls by univariate logistic regression with reported ODDs ration (OR), 95% confidence interval (95% CI) and P value.
Univariate and multivariate logistic regression were done for important predictors for CRC among the studied patients. The independent variables include age, gender, BMI and serum markers. In all tests, p value was considered significant if less than 0.05
Results
Colorectal cancer cases were older (50.20 ± 11.84) than controls with more men than women interviewed shown in Table 1.
Table 1.
Demographic Characteristics of the Studied Groups: IGF-1, Insulin-Like Growth factor-1.
| Total | P value | Average weight | P value | Overweight | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Cases | Control | Cases | Control | Case | ||||
| N=30 | N=66 | N=16 | N=30 | N=14 | N= 36 | ||||
| Mean age (yrs.) | 46.97 ± 9.65 | 50.20 ± 11.84 | 0.194 | 44.6 ± 12.3 | 51.8 ± 13.5 | 0.08 | 49.6 ± 4.3 | 48.9 ±10.2 | 0.78 |
| Gender | |||||||||
| Male | 16 (53.3) | 45 (68.2) | 0.16 | 9 (56.3) | 24 (80) | 0.088 | 7(50) | 21(58.3) | 0.594 |
| Female | 14 (46.7) | 21 (31.8) | 7 (43.8) | 6 (20) | 7(50) | 15 (41.8) | |||
| IGF1* (ng/ml) (Median&IQR) | 184.6 (157, (200.5) | 129.7 (81.3, 156.9) | < 0.001 | 162.4 (154.7, 194.7) | 129.9 (78.9, 158.0) | 0.003 | 188.6 (175.2, 202.3) | 119.2 (83.6, 150.20) | 0.005 |
The main presenting symptoms in our cases were abdominal pain, constipation (recent onset), loss of weight or bleeding per rectum and about 70% of patients presented with constipation (recent onset), and the least percentage of patients presented with bleeding per rectum, most of the patients had more than one symptom.
Also our study showed that there is statistically significant difference observed in the levels of cholesterol and triglycerides between the CRC and the control group and also between the average weight and overweight, Table 2.
Table 2.
Serum Markers of Metabolic Syndrome
| Control (NO=30) | CRC (NO=66) | |||
|---|---|---|---|---|
| BMI<25 | BMI>25 | BMI<25 | BMI>25 | |
| (Mean and SD) | (Mean and SD) | (Mean and SD) | (Mean and SD) | |
| (NO=14) | (NO=16) | (NO=30) | (NO=36) | |
| Cholesterol | 189 (±58)AB | 200 (±53)AB | 170 (±38)A | 223 (±77)B |
| TGs | 131 (±50)A | 158 (±51)B | 112 (58)A | 162 (±69)C |
| FBS | 90.7 (±22) | 88.2 (±8) | 87 (±12) | 102 (±34) |
| PPBS | 126 (±38) | 116 (±22) | 113 (±26) | 126 (±39) |
| HBA1C | 5.73 (±0.7) | 6.11 (±1.1) | 5.74 (±0.5) | 6.12 (±1.1) |
TGs, triglycerides; FBS, fasting blood sugar; PPBS, postprandial blood sugar; HBA1C, glycated HB Values marked with different letters mean significant difference (p value less than 0.05) in each raw
Our study showed that there was no significant difference in the degree of tumor progression (Liver and LNs metastasis) detected by abdominal ultrasound and CT scan between the overweight and average weight patients having CRC, but exact tumour staging couldn’t be done duo to inability to detect the degree of wall invasion exactly in most of the cases to detect the TNM stage of each case.
There were overall significant lower levels of IGF-1 in CRC cases compared to normal controls (129.7ng/ml vs. 184.6ng/ml). Receiver operator characteristics (ROC) curve showed that at a cutoff of 154 ng/ml, IGF-1yielded 87.5% sensitivity and 72.6 specificity in prediction of CRC cases (AUC=0.79 and P value 0.006 with 95% CI; 0.99 - 1) (Figure 1). Such lower level remains significant in average weight group (129.9ng/ml vs. 162.4ng/ml) and overweight group (119.2 ng/ml vs. 188.6ng/ml) with p value of <0.001, 0.003 and 0.005 respectively.
Figure 1.
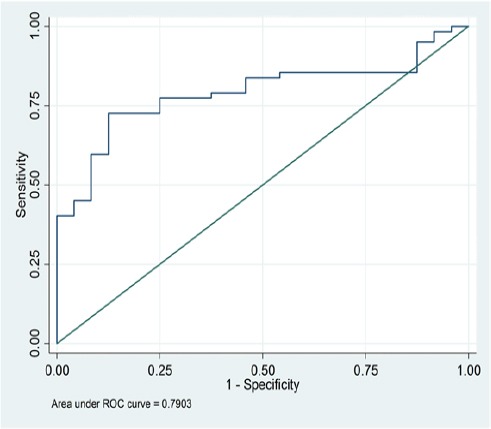
ROC Curve of IGF1
Analysis of genetic mutation in IGF-1 showed that having at least one copy of the non wild types rs6214 gene (i.e., CT or TT genotypes) was associated with a significantly increased risk of CRC (OR 17.68, 95% CI; 2.27 - 137.99) at any BMI. Similar mutation among overweight group was also associated with CRC risk (OR 10.40, 95% CI; 1.23-88.18). None of IGFBP3 variants was associated independently with colon cancer (Table 3).
Table 3.
Genotype Distribution of the SNPs in IGF1 and IGFBP3 (Polymorphism es/no)
| Total | Average weight | Overweight | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| control | case | Or (95%CI) | control | case | Or (95%CI) | control | case | Or (95%CI) | ||
| rs 6214 | CC | 29 | 41 | 1.41 (0.88 - 2.27) | 16 | 21 | 1.31 (0.68 - 2.52) | 13 | 20 | 1.54(0.77 - 3.09) |
| CT or TT | 1 | 25 | 17.68 (2.27 - 137.99 ) | 0 | 9 | empty | 1 | 16 | 10.40 (1.23- 88.18) | |
| rs 6220 | TT | 8 | 28 | 3.50 (1.60 - 7.68) | 4 | 7 | 1.75 (0.51- 5.98) | 4 | 21 | 5.25 ( 1.80 - 15.29) |
| TC or CC | 22 | 38 | 0.49( 0.19 - 1.27) | 12 | 23 | 1.10 ( 0.27 - 4.50 | 10 | 15 | 0.29 (0.08- 1.09) | |
| rs 35767 | CC | 21 | 37 | 1.76 (1.03 -3.01) | 11 | 19 | 1.73 (0.82 -3.63) | 10 | 18 | 1.80( 0.83 -3.90) |
| CT or TT | 9 | 29 | 1.83 (0.73 - 4.59) | 5 | 11 | 1.27( 0.35 4.64) | 4 | 18 | 2.50 (0.66- 9.46) | |
Univariate logistic regression model for important predictors (e.g. age, gender, BMI, IGF-1 and IGFBP-3) of CRC among the studied cohort was constructed with stratification of patients according to BMI. Overall, the log odds for CRC significantly decreases by 0.14 for each one unit increase in IGF-1 (95%CI; 0.04-0.46, p value; <0.001) (Table 4).
Table 4.
Univariate Logistic Regression Model for Individual Predictors of CRC
| Total | Average weight | Overweight | ||||
|---|---|---|---|---|---|---|
| Uni-variate logistic regression model | ||||||
| Variable | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value |
| Age | 1.03 (0.99 - 1.07) | 0.19 | 1.04 ( 0.99 - 1.10) | 0.09 | 0.99 (0.92-1.06) | 0.78 |
| Gender | 0.53(0.22 - 1.29) | 0.16 | 0.32 ( 0.08 -1.22) | 0.1 | 0.71 (0.21- 2.47 | 0.59 |
| BMI | 1.08 (0.96 - 1.20 | 0.2 | 1.67( 1.07 - 2.61) | 0.02 | 0.96(0.66- 1.41) | 0.85 |
| IGF1* | 0.14 (0.04-0.46) | <0.001 | 0.14 ( 0.03- 0.73) | 0.02 | 0.15 ( 0.03-0.79) | 0.03 |
Significance p < 0.05
BMI could be considered as confounder and effect modifier for CRC risk (crude OR 1.08, adjusted OR in average weight group 1.67 and in overweight group) (Table 4).
Discussion
CRC represents about 4% of total cancers in both sexes in Egypt. Dietary factors, physical activity and obesity rates play role in its incidence (Zeeneldin et al., 2012).
In the current study, the mean age for our patients with CRC was 50.2 +11.8 years, with higher incidence in males than females (68.2% / 31.8% respectively) that matches the American cancer society, CRC incidence increases with older age with majority of cases occurring in people around age of fifty.
We found higher predilection to distal colonic segments with about 66% of lesions was seen in the recto-sigmoid region. Similarly, a large study included 2,462 patients showed that approximately 70% of colorectal cancers are localized in the distal or left large bowel, between the splenic flexure and the lower rectum (ponz et al., 2004).
Our study proved a statistically significant difference observed in the levels of cholesterol and triglycerides between the CRC and the control group and also between the average weight and overweight and this partially matches the results of Martine (2007) who stated that the data on hyperlipidemia and colon cancer are some-what heterogeneous. In one study, there was a significant positive association between serum cholesterol levels, triglycerides levels, and colorectal carcinoma, in other studies there was an inverse correlation between colon cancer risk and hypercholesterolemia.
Many studies showed a direct correlation between IGF-1 and CRC accusing its involvement in enhancement of cell proliferation, differentiation, apoptosis and tumorigenesis (Giovannucci, 2008; Davies et al., 2006; Durai et al., 2005).
In the current study, serum levels of the IGF1 were lower in patients with CRC (both groups) than control (129.7ng/ml vs. 184.6ng/ml). Similarly, other studies showed that the mean serum levels of IGF-1 were significantly lower (123±47) in CRC cases than controls (P <0.01) (Probst-Hensch et al., 2001; Pankaj et al., 2015), However, other studies that included 438, 134 and 375 CRC cases showed no association between serum levels of IGF-I and CRC (Gunter et al., 2008; Max et al., 2008; Otani et al., 2007). These lower levels of IGF1 may be attributed to malnourishment or advanced stages of malignancy as more than 50% of our cases had either regional LNs involvement or hepatic deposits which denote advanced stages of tumour (table 5).
Table shows that more than 50% of Cases had Either Regional LNs Involvement or Hepatic Deposits
| CT scan | |||
|---|---|---|---|
| BMI>25 | BMI<25 | P Value | |
| (No =36) | (No =30) | ||
| Mass lesion | 15 (41.7%) | 15 (41.7%) | 0.73 |
| Wall thickening | 17 (47.2%) | 11 (36.7%) | 0.53 |
| Regional LNs | 17 (47.2%) | 12 (40.0%) | 0.82 |
| Liver metastasis | 4 (12.7%) | 2 (6.9%) | 0.63 |
| Abdominal US | |||
| FATTY LIVER | 14 (38.9%) | 3 (10.0%) | 0.02 |
| HFLs | 2 (6.1%) | 1 (3.3%) | 0.43 |
| Regional LNs | 1 (2.8%) | 1 (3.3%) | 0.76 |
Palmqvist et al., (2002) showed that cases of rectal cancer had a significantly lower mean IGF-1 level than their matched controls while values for colon cancer cases were slightly higher than their respective controls. It is interesting to note that 66.7% of the cases in our study had CRC in the recto-sigmoid region, so this may explain the low levels of IGF-1 found in our study. Moreover, low levels of IGF-1 can be explained by the malnutrition complicating advanced CRC cases (Pollak, 2000)
In the current study, we found that SNP rs6214 CT/TT genotypes were significantly associated with colorectal cancer regardless of BMI. While no significant association was found between polymorphisms of IGF-I rs6220 and rs35767. This was evident in a large case–control study (CORSA), where a statistically significant association was found between colorectal cancer risk and IGF-I rs6214 TT genotype, on the other hand, none of the other investigated SNPs of IGF1 showed a significant association with colorectal cancer (Feik et al., 2010).
The small number of patients is one of the main drawbacks for our study especially for the genetic testing that needs large sample size to assess the prevalence of the different genotypes in a studied community. Another defect is the presence of large number of patients with advanced malignancy especially those with hepatic deposits that may affect the results and interpretations of the IGF levels however on the other hand such advance stages will not affect the results of the genetic testing.
In conclusion, our results suggested that both BMI could be considered as confounder and effect modifier for CRC risk. Moreover, having at least one copy of the IGF-1 rs6214 gene (CT or TT genotypes) was associated with an increased risk of CRC.
Clinical Practice Points
The presence of at least one copy of the IGF-1 rs6214 non wild genotypes (CT or TT genotypes) may increase the risk of developing CRC regardless of BMI.
There was an overall significant lower level of IGF-1 in CRC cases compared to normal controls. At a cutoff of 154 ng/ml, IGF-1 can predict CRC with 87.5% sensitivity and 72.6 specificity (AUC=0.79 and P value 0.006 with 95% CI; 0.99 - 1) but larger studies are recommended to prove this association.
The risk for CRC significantly decreases by 0.14 for each one unit increase in IGF-1.
References
- Brahmkhatri VP, Prasanna C, Atreya HS. Insulin-like growth factor system in cancer: novel targeted therapies. Biomed Res Int. 2015 doi: 10.1155/2015/538019. 538019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer. 2004;4:769–80. doi: 10.1038/nrc1453. [DOI] [PubMed] [Google Scholar]
- Chung YW, Han DS, Park KH, et al. Insulin therapy and colorectal adenoma risk among patients with Type 2 diabetes mellitus: a case-control study in Korea. Dis Colon Rectum. 2008;51:593–7. doi: 10.1007/s10350-007-9184-1. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. Role of IGF binding proteins in regulating metabolism. Trends Endocrinol Metab. 2016;27:375–91. doi: 10.1016/j.tem.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Davies M, Gupta S, Goldspink G, Winslet M. The insulin-like growth factor system and colorectal cancer: clinical and experimental evidence. Int J Colorectal Dis. 2006;21:201–8. doi: 10.1007/s00384-005-0776-8. [DOI] [PubMed] [Google Scholar]
- Durai R, Yang W, Gupta S, et al. The role of the insulin-like growth factor system in colorectal cancer: review of current knowledge. Int J Colorectal Dis. 2005;20:203–20. doi: 10.1007/s00384-004-0675-4. [DOI] [PubMed] [Google Scholar]
- Feik E, Baierl A, Hieger B, Führlinger G, et al. Association of IGF1 and IGFBP3 polymorphisms with colorectal polyps and colorectal cancer risk. Cancer Causes Control. 2010;21:91–7. doi: 10.1007/s10552-009-9438-4. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, et al. Globocan 2012 v1.0, Cancer incidence and mortality worldwide: IARC cancer base No. 11. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109–20. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008;68:329–37. doi: 10.1158/0008-5472.CAN-07-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi K, Mahmoudi T, Karimi N, et al. Is there an association between variants in candidate insulin pathway genes IGF-I, IGFBP-3, INSR, and IRS2 and risk of colorectal cancer in the Iranian population? Asian Pac J Cancer Prev. 2013;14:5011–6. doi: 10.7314/apjcp.2013.14.9.5011. [DOI] [PubMed] [Google Scholar]
- Lee DH, Kim JY, Lee MK, et al. Effects of a 12-week home-based exercise program on the level of physical activity, insulin, and cytokines in colorectal cancer survivors: a pilot study. Support Care Cancer. 2013;21:2537–45. doi: 10.1007/s00520-013-1822-7. [DOI] [PubMed] [Google Scholar]
- Martine Extermann. Interaction between comorbidity and cancer: Cancer control January, 14, No. 1. 2007 doi: 10.1177/107327480701400103. [DOI] [PubMed] [Google Scholar]
- Max JB, Limburg PJ, Ogunseitan A, et al. IGF-I IGFBP-3, and IGF-I/IGFBP-3 ratio: no. association with incident colorectal cancer in the alphatocopherol beta-carotene. Cancer Epidemiol Biomarkers Prev. 2008;17:1832–4. doi: 10.1158/1055-9965.EPI-08-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T, Iwasaki M, Sasazuki S, et al. Japan public health center-based prospective study group Plasma C-peptide, insulin-like growth factor-I, insulin-like growth factor binding proteins and risk of colorectal cancer in a nested case-control study: the Japan public health center-based prospective study. Int J Cancer. 2007;120:12. doi: 10.1002/ijc.22556. [DOI] [PubMed] [Google Scholar]
- Ponz de Leon M, Marino M, Benatti P, et al. Trend of incidence, subsite distribution and staging of colorectal neoplasms in the 15-year experience of a specialised cancer registry. Ann Oncol. 2004;15:940–6. doi: 10.1093/annonc/mdh224. [DOI] [PubMed] [Google Scholar]
- Probst-Hensch NM, Yuan JM, Stanczyk FZ, et al. IGF-1, IGF-2 and IGFBP-3 in prediagnostic serum: association with colorectal cancer in a cohort of Chinese men in Shanghai. Br J Cancer. 2001;85:1695–9. doi: 10.1054/bjoc.2001.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist R, Hallmans G, Rinaldi S, et al. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut. 2002;50:642–6. doi: 10.1136/gut.50.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. Insulin-like growth factor physiology and cancer risk. Eur J Cancer. 2000;36:1224–8. doi: 10.1016/s0959-8049(00)00102-7. [DOI] [PubMed] [Google Scholar]
- Pankaj J, Kumari JR, Kim W, Lee SA. Insulin-like growth factor-1, IGF-binding protein-3, C-peptide and colorectal cancer: a case-control study. Asian Pac J Cancer Prev. 2015;16:3735–40. doi: 10.7314/apjcp.2015.16.9.3735. [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Cleveland R, Norat T, et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer. 2010;126:1702–15. doi: 10.1002/ijc.24927. [DOI] [PubMed] [Google Scholar]
- Sax AT, Jenkins DG, Devin JL, et al. The insulin-like growth factor axis: A biological mechanism linking physical activity to colorectal cancer survival. Cancer Epidemiol. 2014;38:455–9. doi: 10.1016/j.canep.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Zeeneldin AA, Saber MM, El-din IS, et al. Colorectal carcinoma in gharbiah district, Egypt: Comparison between the elderly and non-elderly. J Solid Tumor. 2012;2:13–23. [Google Scholar]


