Abstract
Background:
Breast cancer may be induced by activation of protooncogenes to oncogenes and in many cases inactivation of tumor suppressor genes. Ataxia telangiectasia mutated (ATM) is an important tumor suppressor gene which plays central roles in the maintenance of genomic integrity by activating cell cycle checkpoints and promoting repair of double-strand breaks of DNA. In breast cancer, decrease ATM expression correlates with a poor outcome; however, the molecular mechanisms underlying downregulation are still unclear. Promoter hypermethylation may contribute in downregulation. Hence the present investigation was designed to evaluate promoter methylation and expression of the ATM gene in breast cancer cases, and to determine links with clinical and demographic manifestations, in a South Indian population.
Methods:
Tumor biopsy samples were collected from 50 pathologically confirmed sporadic breast cancer cases. DNA was isolated from tumor and adjacent non-tumorous regions, and sodium bisulfite conversion and methylation-specific PCR were performed using MS-PCR primers for the ATM promoter region. In addition, ATM mRNA expression was also analyzed for all samples using real-time PCR.
Results:
Fifty eight percent (58%) of cancer tissue samples showed promoter hypermethylation for the ATM gene, in contrast to only 4.44% of normal tissues (p= 0.0001). Furthermore, ATM promoter methylation was positively associated with age (p = 0.01), tumor size (p=0.045) and advanced stage of disease i.e. stages III and IV (p =0.019). An association between promoter hypermethylation and lower expression of ATM mRNA was also found (p=0.035).
Conclusion:
We report for the first time that promoter hypermethylation of ATM gene may be useful as a potential new biomarker for breast cancer, especially in the relatively young patients.
Keywords: Breast cancer, epigenetics, MS-PCR, promoter hypermethylation, ATM, mRNA expression, DSB
Introduction
During the past few decades, breast cancer has emerged as one of the top cancer killers amongst women globally. Recent data showed 1384155 newly diagnosed breast cancer (BC) cases which were reported globally every year, estimating 22.9% of all cancers in women. In India 144,937 (27%) women were detected with breast cancer for the year 2012. Among those 70,218 died with breast cancer (http://globocan.iarc.fr), while in 2008 about 115251 (22.2%) breast cancer cases were detected in India (http://globocan.iarc.fr). According to World Health Organization (WHO) by 2020, worldwide 70% of all breast-cancer cases are predicted to be in developing countries like India.
Breast cancer arises from a multi-step process whereby the affected cell acquires a series of mutant gene products initiating a cascade of pathophysiological events which include continuous non-stoppable cell growth and increased angiogenesis, tissue invasion, and finally loss of genomic stability. These changes further lead to activation of protooncogene to oncogenes and also in many cases inactivation of tumor suppressor genes (Ting et al., 2006) which hastens the tumorigenesis process. It has been shown that epigenetic alterations are also responsible for carcinogenesis in breast cancer; similar to other types of cancer (Polyak, 2007; Widschwendter and Jones, 2002). There are various mechanisms for epigenetic alterations like DNA methylation, histone modifications, alterations in chromatin condensation and RNA interference. Epigenetic changes disrupt gene function without changing DNA sequence and can be inherited. The role of DNA methylation in cancer is well established (Esteller, 2007; Feinberg et al., 2006). DNA methylation occurs in 5’ position of cytosine in a CpG dinucleotide. The enzyme DNA methyl transferase (DNMT) adds methyl group in the 5’ position, which clusters in the promoter region. This promoter hypermethylation inactivates the tumor suppressor genes during tumorigenesis (Baylin et al., 1997).
Several genes have previously been shown to be aberrantly methylated in breast cancer. A large number of studies of breast cancer tissue have been conducted showing the frequent methylation of genes involved in cell cycle regulation: p16INK4A, p14ARF, p15, CCDN2, DAPK; DNA repair: MGMT, hMLH1; transformation: GSTP1; signal transduction: RARâ2, APC, ERâ; and adhesion and metastasis: CDH1, CDH13 (Brooks et al., 2009). Radpour et al., (2009) reported methylation signature of 42,528 CpG sites of 22 breast cancer candidate gene and among them 10 were shown to be hypermethylated genes (APC, BIN1, BMP6, BRCA1, CST6, ESRb, GSTP1, P16, P21 and TIMP3) in cancerous breast. Radpour et al., (2011) also showed hypermethylation of the two additional genes involved in T regulatory pathway in the breast cancer cases. Zeinab et al., (2012) reported that the quantitative DNA methylation analysis of the candidate genes showed higher methylation proportion in the primary tumor tissue compared to the matched normal tissue and the differences were significant for the APC, BIN1, BMP6, BRCA1, CST6, ESR-b, P16, PTEN and TIMP3 in breast cancer.
Ataxia telangiectasia mutated (ATM) gene is a tumor suppressor gene which encodes a serine/threonine protein kinase. It is located on long arm of chromosome 11(11q22.3) and is a large gene with 66 exons. ATM plays main role in the repair of DNA double-strand breaks (DSB), which can be induced by ionizing radiation, chemotherapy drugs, or oxidative stress, or occur during normal physiologic events like meiotic recombination or rearrangement of antibody genes during B-cell maturation (Bednarski and Sleckman, 2012; Lieber, 2010). The various responses of DNA damage includes, recognition of damaged DNA, recruitment of repair proteins, signaling to cell cycle checkpoints, transcriptional regulation and activation of apoptosis. ATM gene plays an important role in many of these processes. In normal cells ATM exists as inert dimers or multimers. ATM dissociates to highly active monomers, in response to double-strand DNA breaks (Bakkenist and Kastan, 2003). The ATM kinase directly phosphorylates different downstream proteins including p53. Incorrect mechanisms of the ATM dependent p53 pathway may lead to pathological changes like uncontrolled proliferation and tumor growth and transmitted to daughter cells, the proper functioning of this pathway is required for cellular survival (Lavin, 2008). This protein is the principal mediator of cell-cycle arrest, senescence and apoptosis in response to cellular damage. In response to cellular damage it induces G1/S arrest and promotes apoptosis
In our knowledge, the epigenetic status of ATM gene in South Indian patients with BC has not been studied. Hence in the present study, our objective was to analyze the promoter methylation of ATM gene in sporadic breast cancer patients from South Indian population. MS-PCR was used to study the methylation status of ATM promoter.
Materials and Methods
Study population
This study included 50 sporadic breast cancer patients from South Indian population. Informed consent was obtained from all patients. The study was approved by the Institutional Ethics Committee for Biomedical Research, Bhagwan Mahavir Medical Research Centre and experiments have been performed in accordance with the ethical standards as laid down in the 1964 declaration of Helsinki and its later amendments or comparable ethical standards. Demographic and Clinico-pathological data was collected by direct interviews in a structured Performa, and also with the help of co-investigator.
Criteria for selection of study group
Inclusion criteria: All patients were selected at the time of first diagnosis by the oncologist. None of these cases belonged to the category of co-morbidities. All the cases were above 30 years and not pregnant.
Exclusion criteria
All patients who were undergoing Chemotherapy were excluded, and all those patients which were suffering from additional other diseases.
Sample collection
Total 95 tissue samples (50 malignant and 45 corresponding adjacent tissue- non cancerous tissue areas) were collected from 50 patients with sporadic breast cancer from a tertiary surgical oncology department during 2014 January to 2015 July. The breast cancer patients ranged in the age group of 32 to 81 years, with a median of 54.42 years. None of the studied cases had a hereditary form of breast cancer. Patients were classified on the basis of tumor size, nodal status, tumor stage etc. The samples collected were frozen immediately and stored at minus 80 °C until use.
DNA extraction
DNA extraction was performed from 0.01 - 0.02 g of tissue sample. In brief, the tissue was digested with cell lysis buffer and proteinase K solution (1mg/ml) at 55 °C for 4 hour. The DNA was purified with normal Phenol chloroform method and precipitated in ethyl alcohol. The isolated DNA was eluted in TE buffer and kept in -20 °C. Purity of the DNA was checked by nanodrop method.
Bisulphite modification and MSP
Purified DNA samples were bisulphite-converted using Methylcode bisulfite conversion kit (Invitrogen) according to the manufacturer’s protocol.
MS-PCR was performed using primers specific for methylated and unmethylated DNA for ATM. Primers were taken from previous study (Ai et al, 2004) (and listed in Table 1. MS-PCR was performed using Invitrogen Amplitaq gold pcr master mix. 20 µl Reaction mixtures contained 10 picomole primers, 1.5 µl template DNA, and 10 µl master mix. PCR condition was as follows- hot start at 95 °C for 10 min and the following cycling parameters: 35 cycles of 96 °C for 3 s, X °C for 20 s, 68 °C for 10 s, and 72 °C for 1 min, and 4 °C to cool. After amplification, PCR products were then loaded and electrophoresed on 2% agarose gels, stained with ethidium bromide and visualized under UV illumination. The presence of a product in the methylated or unmethylated reaction indicated the presence or absence of methylated or unmethylated promoter.
Table 1.
Primer Sequences for Methylated and Unmethylated DNA Template
| Gene | Primer sequence | Annealing temperature (°C) | Amplicon size |
|---|---|---|---|
| ATM | MF 5’-GGAGTTCGAGTCGAAGGGC-3’ | 59 | 239bp |
| MR 5’-CTACCTACTCCCGCTTCCGA-3’ | |||
| UF 5’GTTTTGGAGTTTGAGTTGAAGGGT-3’ | 56 | 246bp | |
| UR 5’-AACTACCTACTCCCACTTCCAA-3’ |
Promoter prediction CpG island analysis
Promoter sequences of ATM and gene were retrieved from Transcriptional Regulatory Element Database, TRED (http://rulai.cshl.edu/cgi-bin/TRED/tred.cgi?process=home). Then both the sequences were submitted to the MethPrimer and CpG plot programmes (EMBOSS) for analysis of the CpG islands.
Real-time qRT–PCR for ATM mRNA expression
We extracted total RNA from tissue using trizol method. The cDNA synthesis kit (Invitrogen) was used for converting 1 μg of total RNA to cDNA according to the manufacturer’s instructions. We selected glyceraldehyde-3-phosphate dehydrogenize (GAPDH) as an endogenous control. Real time-PCR of ATM, and GAPDH genes were performed using SYBR green assay by 7300 Real-Time PCR System (Applied Biosystems). Results are expressed as N-fold differences in ATM gene expression relative to the GAPDH gene and termed ‘NATM’ were determined as NATM= 2∆ct sample, where the ∆Ct value of the sample was determined by subtracting the Ct value of the ATM gene from the Ct value of the GAPDH gene. The NATM values of the samples were subsequently normalized such that the median of the NATM values for the control was one.
Statistical analysis
Statistical analyses were performed by using SPSS 16.0 software package and Graph Pad Prism 5.0 (Graph Pad Software Inc., La Jolla, CA, USA). The χ2 test was used to determine associations between methylation of ATM gene promoter and various clinicopathological features of breast cancer. All p values were derived from two-tailed statistical tests. p values of < 0.05 (95 % significant level) were considered in this study. The distributions of ATM mRNA levels were characterized by median value. Relationships between ATM mRNA and clinicopathological parameters, were identified using nonparametric tests, Mann-Whitney test. Association between of ATM mRNA expression and clinicopathological parameter was done by parameters by χ2 tests. Assaying relative gene expression between methylated and unmethylated promoter were also done by Mann-Whitney test. Significance level was set at p < 0.05 for all tests.
Results
The clinical characteristics of the 50 cancer patients at the time of surgery are summarized in Table 2b. Among these patients, the medium age was 54 years (ranging from 32 to 81 years). We evaluated promoter methylation of ATM of cancerous and non-cancerous (normal) tissue in the study group i.e. BC patients. Methylation of the ATM promoter was detected in 29 (58%) out of the 50 tumors examined in which all 29 tumor samples were positive for methylated reaction. Where as in normal tissue only 2 (4.4%) samples showed promoter hypermethylation in ATM gene. Figure 1 shows representative methylation status of ATM promoter by methylation specific PCR. The difference in methylation frequency between cancerous and normal tissue was statistically significant (p = 0.0001)
Table 2a.
Methylation Frequency between Cancerous and Normal Tissue
| Patients (n=50) | Controls (n=45) | p-value | |
|---|---|---|---|
| Methylated | 29 | 2 | 0.0001 |
| Unmethylated | 21 | 43 |
Table 2b.
Associations between ATM Promoter Methylation and Clinicopathological Features of Breast Cancer
| Characteristics | Case n=50 | ATM promoter methylation | p-value | ATM mRNA expression | Expression fold change | p-value | ||
|---|---|---|---|---|---|---|---|---|
| Present n=29 | Absent n=21 | n=17 | Down regulation n=9 | Up regulation n=8 | ||||
| Age(year) | ||||||||
| < 50years | 20 (40%) | 16 (80 %) | 4 (20%) | 0.01 | 6 (35%) | 6 (100%) | 0 (0%) | 0.009 |
| ≥ 50 years | 30 (60%) | 13 (43 %) | 17 (57 %) | 11 (65%) | 3 (33%) | 8 (67%)) | ||
| Histological type | ||||||||
| Non-ductal | 3 (6%) | 1 (33 %) | 2 (67%) | 0.379 | 2 (12%) | 0 (0%) | 2 (100%) | 0.206 |
| Ductal | 47 (94%) | 28 (60%) | 19 (40%) | 15 (88%) | 9 (60%) | 6 (40%) | ||
| Nodal involvement | ||||||||
| Negative | 9 (18%) | 3 (32%) | 6 (68%) | 0.098 | 3 (18%) | 2 (67%) | 1 (33%) | 1 |
| Positive | 41 (82%) | 26 (63%) | 15 (37%) | 14 (82%) | 7 (50%) | 7 (50%) | ||
| TNM Stage | ||||||||
| I/II(early) | 26 (52%) | 11 (42%) | 15 (58%) | 0.019 | 10 (59%) | 4 (40%) | 6 (60%) | 0.335 |
| III/IV(Advance) | 24 (48%) | 18 (75%) | 6 (25%) | 7 (41%) | 5 (71%) | 2 (29%) | ||
| Metastasis | ||||||||
| Yes | 3 (6%) | 2 (67%) | 1 (33%) | 0.754 | - | - | ||
| No | 47 (94%) | 27 (57%) | 20 (43%) | - | ||||
| Tumor size | ||||||||
| ≤20 mm | 10 (20%) | 3 (30%) | 7 (70%) | 0.045 | 4 (23.5%) | 1 (25%) | 3 (75%) | 0.294 |
| >20 mm | 40 (80%) | 26 (65 %) | 14 (35% | 13 (76.5%) | 8 (61.5%) | 5 (52.9%) | ||
| Menopausal status | ||||||||
| Pre | 11 (22%) | 7 (64%) | 4 (36%) | 0.668 | 3 (18%) | 3 (100%) | 0 (0%) | 0.206 |
| Post | 39 (78%) | 22 (56 %) | 17 (44%) | 14 (82%) | 6 (43 %)) | 8 (57.%) | ||
Figure 1.
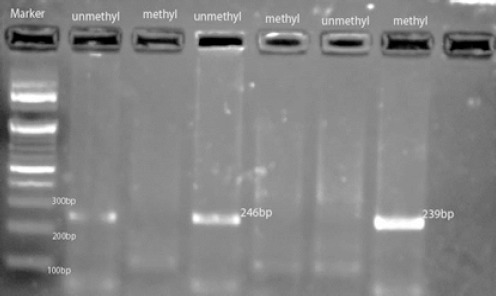
Representative MSP Result of ATM gene Using 2% Agarose Gel Electrophoresis. 100 bp Markers, Unmethylated, Methylated DNA.
Clinicopathological Association of ATM promoter methylation in breast tumors tissue
Associations between ATM promoter methylation and clinicopathological features of breast tumors in this study are shown in Table 2b. A significant association was found between the ATM promoter hypermethylation and patient age (p=0.01), advanced stage (p=0.019) and tumor size (p=0.045). ATM gene aberrant promoter methylation was more frequent in the age group below 50years (80%), advanced stage of cancer (75%) and tumor size >20 mm(65%). There was no significant association between ATM gene promoter hypermethylation with the lymph node involvement of the disease although an increased percentage can be observed (63%) in lymph node positive disease.
Clinicopathological Association of ATM gene expression in breast tumors tissue
ATM mRNA expression level was assessed in the 17 breast tumors and 13 controls. Associations between ATM mRNA expression and clinicopathological features of breast tumors in this study are shown in Table 2b. ATM mRNA expression level was associated with age (p=0.009) of the patient. Lower ATM mRNA expression was found in patients below the age of 50 years (median value=0.310565) compared to patient age ≥ 50 (median value=1.538059121) (p=0.009). ATM mRNA expression showed no significant association with other parameter like histology, TNM stage, tumor size, menopausal status etc. Association between promoter hypermethylation and lower ATM mRNA expression was also found (p=0.035).
Discussion
Eukaryotic cells are exposed continuously to the genotoxic stress that may result in the formation of different types of DNA damages, such as double-strand breaks (DSBs). DNA DSBs may be caused by external factors, like ionizing radiation (IR) and some clastogenic drugs or formed endogenously, e.g. during DNA replication (Lindah and Barnes, 2000). In response to DNA damage, hundreds of proteins are phosphorylated at Ser/Thr-Glu motifs and additional sites by ATM dependent activation (Matsuoka et al., 2007; Smolka et al., 2007;Stokes et al., 2007; Bensimon et al., 2010; Beli et al., 2012). These responses involve the activation of cell cycle Chk factors (Checkpoints factors), DNA repair and Apoptosis. Its downstream targets are Chk1 (Cell Cycle Checkpoint Kinase-1), Chk2 (Cell Cycle Checkpoint Kinase-2), tumor suppressors like and BRCA1 (Breast Cancer gene). ATM is primarily activated by double-stranded DNA breaks (DSBs). The exact mechanism of ATM activation is not fully established, but has been shown to involve dissociation of ATM homodimers into active monomers, autophosphorylation of ATM at Ser1981 and other sites, and acetylation (Bakkenist and Kastan, 2003; Lavin and Kozlov, 2007; Mochan et al., 2003). Once activated, ATM phosphorylates many downstream protein, which in turn phosphorylate their own targets (Choi et al., 2016). The Phosphatidylinositol 3-kinase-related kinase(PI3KK) domain of ATM recognizes serine-glutamine (SQ) and threonineglutamine (TQ) motifs of many proteins, including ones involved in cell-cycle checkpoint arrest (e.g., Chk1 and Chk2), DNA repair (BRCA1 and RAD51), and apoptosis (p53:Blasius and Bartek, 2013). It also has been determined that there are in fact over 700 targets phosphorylated following DSBs, and that ATM modulates networks not immediately involved in DNA repair like the insulin-like growth factor or other metabolic and stress-response pathways (Matsuoka et al., 2007).
ATM dependent phosphorylation of p53 protein activates expression of genes which are involved in the activation of cell cycle checkpoints (a cell survival mechanism) but also genes that promote programmed cell death. These two mechanisms presumably determine cell fate and are influenced by the type and extent of the damage (Shiloh and Ziv, 2013).
Puszynski et al., (2014) presented a mathematical model of the ATM signaling pathway which plays a role of the double-strand breaks (DSB) detector. According to this model, in normal cells after the induction of DSBs, the levels of ATM and following Chk2 protein levels raise quickly in turn, MDM2 is degraded and p53 activated. At this stage, cell cycle is blocked, because of the elevated phosphorylated p53 and p21 level, and DNA repair occurs. Meanwhile, WIP1 molecules cumulate. The role of WIP1 is to inactivate proteins involved in the DDR (DNA damage response) after the successful repair, so the apoptotic signal and the cell cycle blockade signal could be turned off. Various reports have shown the association of ATM mutation with risk of different malignancies like breast, prostate, and ovarian cancers, mantle cell lymphoma, and B-cell chronic lymphocytic leukemia (Forbes et al., 2015). Hypermethylation promoter of ATM gene causes silence of gene expression and subsequently cannot repair damaged DNA and cell may become malignant.
One small study by Vo et al., (2004) identified increased ATM promoter methylation associated with decreased expression in locally advanced sporadic breast cancers. There are few reports suggesting gene-body hypermethylation of ATM in the DNA of peripheral blood samples in breast cancer (Kevin et al., 2012;Flanagan et al., 2009). But numerous other reports suggest no evidence of promoter methylation of this gene in breast cancers (Allinen et al., 2002; Kontorovich et al., 2008; Krasteva et al., 2014;Treilleux et al., 2007; Dejeux et al., 2010; Soukupova et al., 2008). In breast cancer, ATM downregulation has been described at both the mRNA and protein levels which were associated with poor outcome (Rondeau et al., 2015). Rondeau et al., (2015) reported that ATM mRNA expression in unilateral invasive breast tumors using real-time quantitative reverse-transcription PCR (qRT–PCR) and ATM protein levels by reverse-phase protein arrays (RPPA) varied significantly in the expression patters of ATM gene. Their result also showed association of various pathological parameters with ATM mRNA and protein level. Hallajian et al, (2017) reported association between reduce ATM mRNA expression in Iranian sporadic breast cancer patients.
In south India, to the best of our knowledge no study has been done on ATM promoter methylation in breast cancer patients, hence this is the first study reporting hypermethylation of the ATM gene. Our result showed ATM promoter hypermethylation was associated with sporadic breast cancer. The significant associations between ATM promoter methylation and advanced stage of cancer (p=0.019) and tumor size (p=0.045) indicated that ATM methylation may be involved in tumor invasion mechanism. Although nodal involvement was not significantly associated with ATM methylation but the percentage of ATM promoter methylation in lymph node positive cancer cases was high (63%) as compare to lymph node negative cancer cases. We found metastasis in only 3 cases out of which 2 (67%) cases were showed promoter hypermethylation in ATM gene. Association between promoter hypermethylation and lower expression of ATM mRNA was also found (p=0.035). Given these aforementioned correlations it is not surprising that ATM promoter hypermethylation may serve as an indicator of worst outcome.
However, a few limitations of our study point to the fact that we have included only promoter methylation of ATM gene. Methylation in intragenic sequences may also be important in tissue specific gene expression, but interestingly our results on aberrant promoter methylation of ATM genes were highly statistically significant so we could suggest this as novel biomarker strategy for detecting breast cancer in young age groups patients. Further analysis of other novel CpG Island of other genes may be useful to create a panel of genes which could then be listed as associated with breast cancer. Obviously, additional studies are needed to further elucidate the role of other epigenetic mechanism like micro RNA expression or histone modification along with DNA methylation in gene expression in breast cancer. Research with larger sample size may further help to confirm biomarkers for early detection and strategies for prevention of the disease.
Our result also showed, hypermethylation of the ATM promoter appeared to be more prominent in women in the age group below 50 yrs (p=0.001) and a significant association between lower ATM mRNA expression and patient age below 50 yrs (p=0.009) was also found. In our study we have used biopsy samples where we do have non-malignant and malignant portions separated from the biopsy samples, and used them to study their methylation processes. And we found more methylation in the malignant portion of the biopsy as compared to the non-malignant portion. Hence we believe that this finding is novel and useful as a biomarkers as we found a clear difference between the non-malignant and malignant tissues.
Largely used current screening diagnostic methods for breast cancer detection are Magnetic resonance imaging and digital mammography. However, these techniques lack sensitivity and specificity. As an alternative approach, tumor biomarkers could be used as valuable method for early breast cancer detection. High-sensitivity and specificity biomarkers can be detected in accessible tissues or body fluids (Pouliot et al., 2015). Development of DNA methylation-based biomarkers for breast cancer can be helpful, because DNA methylation pattern alterations are one of the earliest modifications occurring in the process of cancer development. Hence hypermethylation of ATM gene promoter may be useful as a potential new biomarker for breast cancer detection. So we may conclude that early detection of young breast cancer patients using this biomarker i.e. aberrant ATM promoter methylation may be useful for clinicians to modifying treatment strategies for longer survival of the patients. We also conclude that inactivation of ATM /p53 signaling pathway by promoter hypermethylation of ATM gene may affect DNA repair mechanism which may lead to tumor progression in breast cancer.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare that they have no conflict of interest associated with this publication and we conducted this research with personal funds.
Acknowledgements
We would like to thank Jawaharlal Nehru Institute of Advanced Studies (JNIAS) for providing facilities and also thank all the doctors of tertiary surgical oncology department for giving valuable tumor sample and patients clinicopathological data.
References
- Ai L, Vo QN, Zuo C, et al. Ataxia-telangiectasia-mutated (ATM) gene in head and neck squamous cell carcinoma: promoter hypermethylation with clinical correlation in 100 cases. Cancer Epidemiol Biomarkers Prev. 2004;13:150–6. doi: 10.1158/1055-9965.epi-082-3. [DOI] [PubMed] [Google Scholar]
- Allinen M, Peri L, Kujala S, et al. Analysis of 11q21–24 loss of heterozygosity candidate target genes in breast cancer: indications of TSLC1 promoter hypermethylation. Genes Chromosomes Cancer. 2002;34:384–9. doi: 10.1002/gcc.10079. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1997;72:141–96. [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Bednarski JJ, Sleckman BP. Integrated signaling in developing lymphocytes: the role of DNA damage responses. Cell Cycle. 2012;11:4129–34. doi: 10.4161/cc.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimon A, Schmidt A, Ziv Y, et al. ATM -dependent and -independent dynamics of the nuclear phosphoproteome after DNA damage. Sci Signal. 2010;3:rs3. doi: 10.1126/scisignal.2001034. [DOI] [PubMed] [Google Scholar]
- Beli P, Lukashchuk N, Wagner SA, et al. Proteomic investigations reveal a role for RNA processing factor THRAP3 in the DNA damage response. Mol Cell. 2012;46:212–25. doi: 10.1016/j.molcel.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius M, Bartek J. ATM targets hnRNPK to control p53. Cell Cycle. 2013;12:1162–3. doi: 10.4161/cc.24485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J, Cairns P, Zeleniuch-Jacquotte A. Promoter Methylation and the detection of breast cancer. Cancer Causes Control. 2009;20:1539–50. doi: 10.1007/s10552-009-9415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Kipps T, Kurzrock R. ATM mutations in cancer: Therapeutic implications. Mol Cancer Ther. 2016;15:1781–91. doi: 10.1158/1535-7163.MCT-15-0945. [DOI] [PubMed] [Google Scholar]
- Dejeux E, Rønneberg JA, Solvang H, et al. DNA methylation profiling in doxorubicin treated primary locally advanced breast tumours identifies novel genes associated with survival and treatment response. Mol Cancer. 2010;9:68. doi: 10.1186/1476-4598-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nature Rev Genet. 2007;8:286–98. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- Flanagan JM, Munoz-Alegre M, Henderson S, et al. Gene-body hypermethylation of ATM in peripheral blood DNA of bilateral breast cancer patients. Hum Mol Gen. 2009;18:1332–42. doi: 10.1093/hmg/ddp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:805–11. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nature Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- Hallajian Z, Mahjoubi F, Nafissi N. Simultaneous ATM / BRCA1/RAD51 expression variations associated with prognostic factors in Iranian sporadic breast cancer patients. Breast Cancer. 2017 doi: 10.1007/s12282-016-0750-z. doi:10.1007/s12282-016-0750-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kevin B, Montserrat G, Nick O, et al. Intragenic ATM methylation in peripheral blood DNA as a biomarker of breast cancer risk. Cancer Res. 2012;72:2304–13. doi: 10.1158/0008-5472.CAN-11-3157. [DOI] [PubMed] [Google Scholar]
- Kontorovich T, Cohen Y, Nir U, Friedman E. Promoter methylation patterns of ATM ATR BRCA1 BRCA2 and as putative cancer risk modifiers in Jewish BRCA1 / BRCA2 mutation carriers. Breast Cancer Res Treat. 2008;116:195–200. doi: 10.1007/s10549-008-0121-3. [DOI] [PubMed] [Google Scholar]
- Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6:931–42. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- Lavin MF. Ataxia-telangiectasia: From a rare disorder to a paradigm for cell signalling and cancer. Nature Rev Mol Cell Biol. 2008;9:759–69. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindah T, Barnes DE. Repair of endogenous DNA damage. Cold Spring Harb Symp Quant Biol. 2000;65:123–7. doi: 10.1101/sqb.2000.65.127. [DOI] [PubMed] [Google Scholar]
- Krasteva ME, Antov GG, Gospodinova ZI, et al. Aberrant promoter methylation in p53 and ATM genes was not associated with sporadic breast carcinogenesis in Bulgarian patients. J Bio Sci Biotech. 2014;3:105–9. [Google Scholar]
- Matsuoka S, Ballif BA, Smogorzewska A, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–6. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- Mochan TA, Venere M, DiTullio RAJ, Halazonetis TD. 53BP1 and NFBD1 / MDC1-Nbs1 function in parallel interacting pathways activating ataxiatelangiectasia mutated (ATM) in response to DNA damage. Cancer Res. 2003;63:8586–91. [PubMed] [Google Scholar]
- Polyak K. Breast cancer: origins and evolution. J Clin Invest. 2007;117:3155–63. doi: 10.1172/JCI33295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot MC, Labrie Y, Diorio C, Durocher F. The role of methylation in breast cancer susceptibility and treatment. Anticancer Res. 2015;35:4569–74. [PubMed] [Google Scholar]
- Puszynski K, Gandolfi A, d'Onofrio A. The Pharmacodynamics of the p53-Mdm2 targeting drug nutlin: The role of gene-switching noise. PLoS Comput Biol. 2014;10:e1003991. doi: 10.1371/journal.pcbi.1003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radpour R, Kohler C, Haghighi MM, et al. Methylation profiles of 22 candidate genes in breast cancer using highthroughput MALDI-TOF mass array. Oncogene. 2009;28:2969–78. doi: 10.1038/onc.2009.149. [DOI] [PubMed] [Google Scholar]
- Radpour R, Barekati Z, Kohler C, et al. Hypermethylation of tumor suppressor genes involved in critical regulatory pathways for developing a blood-based test in breast cancer. PLoS One. 2011;6:e16080. doi: 10.1371/journal.pone.0016080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondeau S, Vacher S, Koning LD, et al. ATM has a major role in the double-strand break repair pathway dysregulation in sporadic breast carcinomas and is an independent prognostic marker at both mRNA and protein levels. Br J Cancer. 2015;112:1059–66. doi: 10.1038/bjc.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MB, Albuquerque CP, Chen SH, Zhou H. Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc Natl Acad Sci. 2007;104:10364–9. doi: 10.1073/pnas.0701622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukupova J, Dundr P, Kleibl Z, Pohlreich P. Contribution of mutations in ATM to breast cancer development in the Czech population. Oncol Rep. 2008;19:1505–10. [PubMed] [Google Scholar]
- Stokes MP, Rush J, Macneill J, et al. Profiling of UV-induced ATM / ATR signaling pathways. Proc Natl Acad Sci. 2007;104:19855–860. doi: 10.1073/pnas.0707579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nature Rev Mol Cell Biol. 2013;14:197–210. [PubMed] [Google Scholar]
- Ting AH, McGarvey KM, Baylin SB. The cancer epigenome –components and functional correlates. Genes Dev. 2006;20:3215–31. doi: 10.1101/gad.1464906. [DOI] [PubMed] [Google Scholar]
- Treilleux I, Chapot B, Goddard S, et al. The molecular causes of low ATM protein expression in breast carcinoma;promoter methylation and levels of the catalytic subunit of DNA-dependent protein kinase. Histopathology. 2007;51:63–9. doi: 10.1111/j.1365-2559.2007.02726.x. [DOI] [PubMed] [Google Scholar]
- Vo QN, Kim WJ, Cvitanovic L, et al. The ATM gene is a target for epigenetic silencing in locally advanced breast cancer. Oncogene. 2004;23:9432–7. doi: 10.1038/sj.onc.1208092. [DOI] [PubMed] [Google Scholar]
- Widschwendter M, Jones PA. DNA methylation and breast carcinogenesis. Oncogene. 2002;21:5462–82. doi: 10.1038/sj.onc.1205606. [DOI] [PubMed] [Google Scholar]
- Zeinab B, Ramin R, Qing L, et al. Methylation signature of lymph node metastases in breast cancer patients. BMC Cancer. 2012;12:244. doi: 10.1186/1471-2407-12-244. [DOI] [PMC free article] [PubMed] [Google Scholar]


