Abstract
Objective:
To evaluate diagnostic performance of IOTA simple rules plus pattern recognition in predicting tubal cancer.
Methods:
Secondary analysis was performed on prospective database of our IOTA project. The patients recruited in the project were those who were scheduled for pelvic surgery due to adnexal masses. The patients underwent ultrasound examinations within 24 hours before surgery. On ultrasound examination, the masses were evaluated using the well-established IOTA simple rules plus pattern recognition (sausage-shaped appearance, incomplete septum, visible ipsilateral ovaries) to predict tubal cancer. The gold standard diagnosis was based on histological findings or operative findings.
Results:
A total of 482 patients, including 15 cases of tubal cancer, were evaluated by ultrasound preoperatively. The IOTA simple rules plus pattern recognition gave a sensitivity of 86.7% (13 in 15) and specificity of 97.4%. Sausage-shaped appearance was identified in nearly all cases (14 in 15). Incomplete septa and normal ovaries could be identified in 33.3% and 40%, respectively.
Conclusion:
IOTA simple rules plus pattern recognition is relatively effective in predicting tubal cancer. Thus, we propose the simple scheme in diagnosis of tubal cancer as follows. First of all, the adnexal masses are evaluated with IOTA simple rules. If the B-rules could be applied, tubal cancer is reliably excluded. If the M-rules could be applied or the result is inconclusive, careful delineation of the mass with pattern recognition should be performed.
Keywords: Adnexal mass, IOTA simple rules, benign ovarian tumor, ovarian cancer, tubal cancer, ultrasound
Introduction
Primary cancer of the fallopian tube is relatively rare, accounting for 0.5% of all gynecologic malignancies(Schneider et al., 2000). Classically, tubal cancer presents with a triad of abnormal vaginal bleeding, frequently associated with watery vaginal discharge (hydrops tubae profluens) and abdominal pain. Typically, colicky lower abdominal pain is relieved by a profuse, serous, watery, yellow intermittent discharge from the vagina. Nevertheless, such typical syndrome is rare and the correct preoperative diagnosis is very limited (Nordin, 1994). Therefore, preoperative diagnosis of tubal cancer is difficult and rarely made prior to surgery. Since the prognosis is usually associated with the stage of the disease, it is important to be familiar with its sonographic characteristics to establish early diagnosis and thus improve prognosis. Moreover, correct preoperative diagnosis both in terms of malignancy/benignity and the origin of the disease is helpful in counseling and a plan of management. We believe that awareness of the ultrasound characteristics of tubal cancer could be helpful for correct diagnosis before operation, or at least for high suspicion on this relatively rare cancer. Nevertheless, to the best of our knowledge, though several case reports and case series have been published, the effectiveness of ultrasound diagnosis of tubal cancer has never been reported. Therefore, we conducted this study aimed to determine the effectiveness of preoperative ultrasound in predicting tubal cancer, using IOTA simple rules plus pattern recognition. IOTA simple rule was developed mainly for differentiation between malignancy and benign ovarian mass and it is widely used recently (Timmerman et al., 2010; Nunes et al., 2012; Alcazar et al., 2013; Sayasneh et al., 2013a; Sayasneh et al., 2013b; Tantipalakorn et al., 2014). We hypothesized that IOTA simple rule could also be helpful in differentiating tubal cancer from benign tubal disorders as well as differentiating tubal cancer from ovarian cancer, especially when combined with pattern recognition of the tubal cancer.
Materials and Methods
Patients and Methods
This was secondary analysis of a diagnostic study based on the data of our previous prospective studies (Tantipalakorn et al., 2014; Tinnangwattana et al., 2015; Tongsong et al., 2016). The study was undertaken at Maharaj Nakorn Chiang Mai Hospital, Chiang Mai University, between April 2011 and March 2017 with ethical approval of the institute review board. The study population was patients scheduled for elective pelvic surgery because of an adnexal mass. The women participated in the study with written informed consent. Inclusion criteria were as follows: 1) a woman with an adnexal mass which was detected either by prior ultrasound examination or pelvic examination, and 2) no known diagnosis of the masses before surgery, either based on the findings of previous surgery or laparoscopic examination. Exclusion criteria were as follows: 1) the patients undergoing surgery beyond 24 hours after ultrasound examination, and 2) the final diagnosis of a non-adnexal disease such as urinary tract or intestinal disease etc.
All examinations were performed by the authors who had no any clinical information of the patients. Ultrasound examinations were performed, using a real-time machine, Aloka Prosound alpha10 or Hitachi-Aloka model ProSound37 (Hitachi Aloka Medical Ltd, Inc., Tokyo, Japan or Voluson E8 (GE Medical Systems, Zipf, Austria). On sonographic examination, morphology of the masses was assessed using 2D real-time ultrasound, either by transabdominal transducer (2-5 MHz) or transvaginal transducer (5-7.5 MHz), and vascularization was evaluated by color flow mapping (CFM). The sonographic characteristics of the masses were described according to the IOTA simple rules (Timmerman et al., 2008) and classified as malignant (M) or benign (B) as presented in Table 1. A papillary projection was any solid projection into a cystic cavity of a mass of larger than 3 mm. The largest solid component other than a papillary projection was measured in three diameters (orthogonal perpendicular planes). In cases that a solid papillary projection was the largest solid part, the papillary projection was considered both as a papillary projection and as the largest solid component of the mass. The amount of vascularization of the mass was assessed by Doppler color flow mapping (CFM), which were expressed as follows. A CFM score was defined as a score of 1 if no color or power Doppler signals are seen, a score of 2 for a minimal amount of the signals, a score of 3 for a moderate amount, and a score of 4 if abundant signals were detected. If one or more M-rules applied in the absence of a B-rule, the mass was categorized as malignant. If one or more B-rules applied in the absence of an M-rule, it was categorized as benign. If both M-rules and B-rules applied or no rule applies, the mass was categorized as inconclusive. During ultrasound examination, video clips of the masses, both real-time 2D cine and color flow mapping, were also recorded. All ultrasound examinations were performed within 24 hours of operations. The results were not exposed to the clinicians and were not used for any clinical decisions.
Table 1.
The IOTA Simple Rules for Identifying a Benign or Malignant Tumor
| Rules for predicting a malignant tumor (M-rules) | |
|---|---|
| M1 | Irregular solid tumor |
| M2 | Presence of ascites |
| M3 | At least four papillary structures |
| M4 | Irregular multilocular solid tumor with largest diameter ≥100 mm |
| M5 | Very strong blood flow (color score 4) |
| Rules for predicting a benign tumor (B-rules) | |
| B1 | Unilocular |
| B2 | Presence of solid components with the largest diameter <7 mm |
| B3 | Presence of acoustic shadows |
| B4 | Smooth multilocular tumor with largest diameter <100 mm |
| B5 | No blood flow (color score 1) |
Preoperative diagnosis of tubal cancer consisted of the IOTA simple rules indicating M rules and at least one of the followings: 1) visible normal ovary of the ipsilateral side, or 2) pattern recognition of tubal cancer including sausage cysts/masses or areas of incomplete septum (Fig 1-5). A sausage cyst with thickened wall or a cyst without solid part or projection was not considered as malignancy, usually caused by pyo- or hydrosalpinx.
Figure 1.
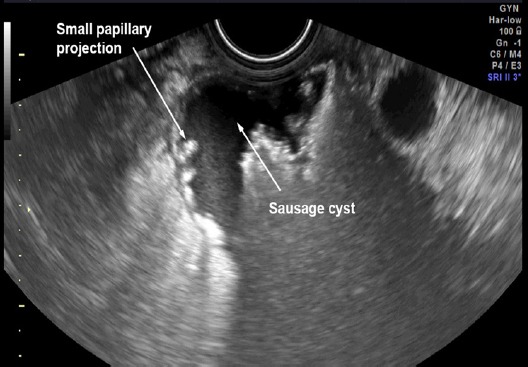
Pattern Recognition: Sausage cyst with Small Papillary Projections
Figure 2.
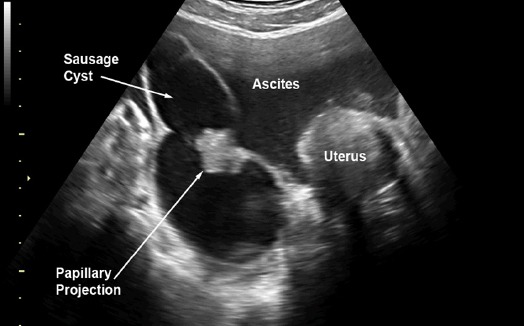
Pattern Recognition: Sausage cyst Large Papillary Projection
Figure 3.
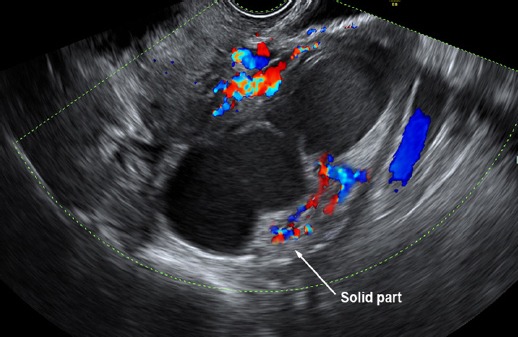
Pattern Recognition: Sausage cyst with Small Area of Solid with High Vasculaa
Figure 4.
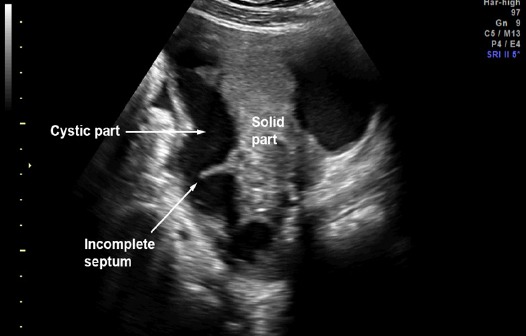
Pattern Recognition: Sausage Solid-Cystic Mass with Incomplete Septum
Figure 5.
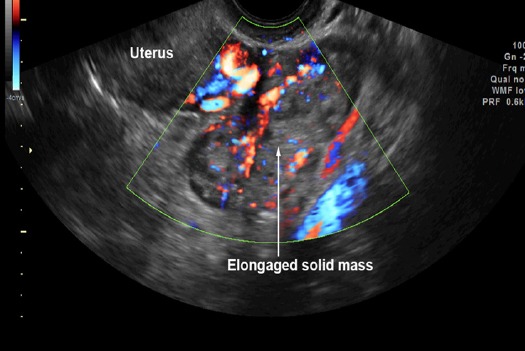
Pattern Recognition: Sausage (Elongated) Solid Mass with High Vascularization
The final diagnosis was based on pathological diagnosis (Figure 6-7) used as a gold standard. In case of some benign masses without pathological specimens, the final diagnosis was based on the conclusion made by the surgeons. All of the masses were categorized into 2 groups as a benign and malignant group. The masses with histological diagnosis of low malignant potential tumors were classified in the malignant group. The patient with bilateral tubal masses of the cancer was considered as one record and data from the larger or more complex mass was used for statistical analysis.
Figure 6.
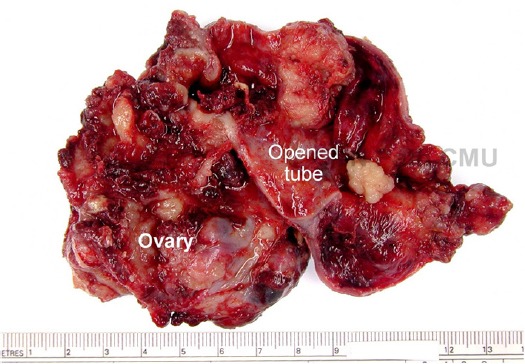
Gross-Pathology of the Mass in Figure 4 (Opened Sausage Mass) (Courtesy by Dr. Surapan Khunamornpong, Department of Pathology, Faculty of Medicine, Chiang Mai University, Thailand
Figure 7.
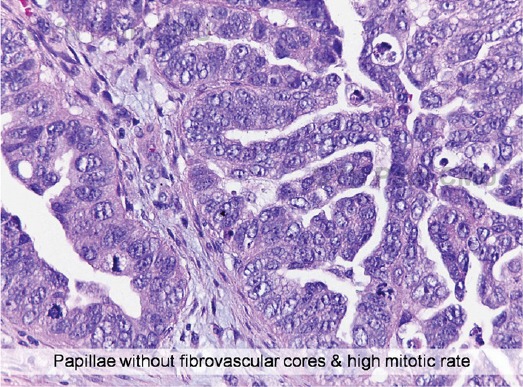
Micro-Histology of the Mass in Figure 4 (Courtesy by Dr. Surapan Khunamornpong, Department of Pathology, Faculty of Medicine, Chiang Mai University, Thailand).
Statistical analysis
The effectiveness of the IOTA simple rules plus pattern recognition in predicting tubal cancer was calculated for sensitivity and specificity. The statistical analyses were performed using IBM SPSS version 21.0 (IBM SPSS Statistics for Windows, Released 2012. Armonk, NY: IBM Corp).
Results
A total of 482 women underwent preoperative ultrasound examinations and pelvic surgery because of adnexal masses with complete data. Fifteen cases were finally proven to be tubal cancer. The remaining 467 women had non-tubal cancers. The mean (+ SD) age of the women was 42.1+12.4 years (range 12-80 years). Most of them (269; 55.8%) were parous women. About two-thirds (317 women, 65.8%) were in reproductive age, 151 (31.3%) were post-menopausal and 14 (2.9%) were in early adolescent (15 years or less).
The prevalence of tubal cancer was approximately 3.1% (15 in 482) of the women with adnexal mass requiring laparotomy. The diagnostic performance of IOTA simple rules with pattern recognition in predicting a tubal cancer had a sensitivity of 86.7% (95% CI: 62.1-96.3%) and a specificity of 97.4% (95% CI: 95.6-98.5%), as presented in Table 2.
Table 2.
Diagnostic Performance of Ultrasound in Predicting a Tubal Cancer
| Pathological Diagnosis | Total | ||
|---|---|---|---|
| Tubal Cancer | Non-tubal Cancer | ||
| Ultrasound diagnosis of tubal cancer | 13 (86.7%) | 12 (2.6%) | 25 (5.2%) |
| Ultrasound diagnosis of non-tubal cancer | 2 (13.3%) | 455 (97.4%) | 457 (94.8%) |
| Total | 15 (100.0%) | 467 (100.0%) | 482 (100.0%) |
Table 3.
Sonographic Characteristics of Tubal Cancer (N, 15 cases)
| Characteristics | n/N (%) |
|---|---|
| IOTA simple rules | |
| M-rules: Malignant | 12 (80%) |
| B-rules: Benign | 0 (0%) |
| Inconclusive | 3 (20.0%) |
| Pattern recognition | |
| Sausage / elongated / ovoid solid-cystic mass predominantly cystic | 7 (46.7%) |
| Sausage / elongated / ovoid solid-cystic mass predominantly solid | 5 (33.3%) |
| Sausage / elongated / completely solid | 3 (20.0%) |
| Incomplete septa | 5 (33.3%) |
| Visible ipsilateral normal ovary | 6 (40.0%) |
| None of the aforementioned | 2 (13.3%) |
The common types of ultrasound appearance by pattern recognition were identified as being typical of tubal cancer were as follows: a sausage-shaped cystic structure with solid tissue protruding into it like a papillary projection (46.7%); a sausage-shaped cystic structure with a large solid component filling part of the cyst cavity (33.3%); an ovoid or elongated completely solid mass (20.0%). Note that most cases had sausage-shaped appearance while incomplete septa and normal ovaries could be identified in 33.3% and 40%, respectively.
Of 12 cases of false positive results, 10 cases were ovarian cancer with sausage-shaped appearance, one had incomplete septa and the other was pyosalpinx with markedly thickened wall. The two missed cases had IOTA applied to M-rules (malignant) but showed no sausage-shaped appearance, no incomplete seta and no visible normal ovary.
Note that IOTA simple rules could effectively screen tubal cancer. None of them was predicted as benign, though three of them were inconclusive. Of the three inconclusive cases, one was sausage-shaped smooth multilocular cyst (5 cm diameter) with small papillary projections. The two remainders showed sausage-shaped solid cystic appearance but had no strong blood flow. Nevertheless, all of the three cases with inconclusive results were preoperatively diagnosed for tubal cancer due to pattern recognition of sausage-shaped solid-cystic appearance as well as visible normal ovaries.
Discussion
Based on this study, sonographic characteristics of a tubal cancer can be summarized as follows: 1) IOTA simple rules indicating malignancy (at least one of the following: irregular solid, presence of ascites, at least four papillary structures, irregular multilocular solid tumor, or very strong blood flow), 2) pattern recognition including sausage shaped mass with a partially or completely solid component or papillary projections, and a mass with incomplete septa, and 3) a visible normal ipsilateral ovary. On examination, attention must be paid to identify the sausage-shaped mass (when demonstrating a rounded or ovoid mass on cross-sectional view of the mass we must rotate the ultrasound transducer to get its longitudinal view to see whether it shows sausage-shaped appearance or incomplete septa or not.) and carefully demonstrate the presence of normal ovaries.
Our findings are consistent with those of several isolated case reports (Yuen et al., 2002; Romagosa et al., 2003; Haratz-Rubinstein et al., 2004; Huang et al., 2005; Ko et al., 2005) and small case series (Slanetz et al., 1997; Mikami et al., 2003; Patlas et al., 2004) as well as the largest retrospective study reported by Ludovisi et al (Ludovisi et al., 2014) which described the sonographic characteristics of tubal cancer, including 79 cases recruited from 13 centers. According to all of those studies, the most typical ultrasound feature of tubal cancer is a sausage-shaped solid or solid-cystic mass or a sausage-shaped cyst with papillary projecting into it. Different from Ludovisi’s series (Ludovisi et al., 2014), which was retrospective and mainly based on subsequent review of still images rather than videoclips, our study was a secondary analysis of our previous prospective studies conducted on the women from a single center and all sonographic diagnoses were made within 24 hours before the operations.
To the best of our knowledge, this is the first study using IOTA simple rules to differentiate malignant from benign adnexal masses and then using pattern recognition to distinguish the tubal origin from others. Additionally, this is the first study evaluating the effectiveness of sonographic diagnosis of tubal cancer including both true and false diagnosis. This study was different from other previous studies, which did not include cases of negative findings and could not assess the diagnostic performance in terms of sensitivity and specificity.
In pattern recognition of tubal cancer, one should keep in mind that in some cases, pyosalpinx and acute salpingitis might possibly be confused with tubal cancer. The folded thickened wall of the enlarged pyosalpinx can sometime be mistaken for a solid part of a tubal cancer. This is due to the fact that protrusions of solid tissue into a cystic tube can mimic the swollen mucosal folds in a pyosalpinx together with that both an acutely inflamed tube and a tubal cancer have high vascularization on color flow mapping. However, with high precaution and familiarity of the IOTA simple rules one could differentiate them without difficulty.
The strengths of this study are as follows: 1) This secondary analysis was based on the prospective nature of the study, in which the diagnosis was made preoperatively. 2) Diagnostic performance in terms of sensitivity and specificity was also assessed. The weaknesses of this study are as follows: 1) The sample size was relatively small because of rarity of the disease, 2) Ultrasound examinations were performed by experienced sonographers. Therefore, the external validity of the test may not be perfect.
In conclusion, IOTA simple rules plus pattern recognition is relatively effective in predicting tubal cancer. Thus, we propose the simple scheme in diagnosis of tubal cancer as follows. First of all, the adnexal masses are evaluated with IOTA simple rules. If the B-rules could be applied, tubal cancer is reliably excluded. If the M-rules could be applied or the result is inconclusive, careful delineation of the mass with pattern recognition should be performed.
Acknowledgements
The authors wish to thank the National Research University Project under Thailand’s Office of the Higher Education Commission for financial support.
Conflict of interest
None.
References
- Alcazar JL, Pascual MA, Olartecoechea B, et al. IOTA simple rules for discriminating between benign and malignant adnexal masses: prospective external validation. Ultrasound Obstet Gynecol. 2013;42:467–71. doi: 10.1002/uog.12485. [DOI] [PubMed] [Google Scholar]
- Haratz-Rubinstein N, Russell B, Gal D. Sonographic diagnosis of Fallopian tube carcinoma. Ultrasound Obstet Gynecol. 2004;24:86–8. doi: 10.1002/uog.1078. [DOI] [PubMed] [Google Scholar]
- Huang WC, Yang SH, Yang JM. Ultrasonographic manifestations of fallopian tube carcinoma in the fimbriated end. J Ultrasound Med. 2005;24:1157–60. doi: 10.7863/jum.2005.24.8.1157. [DOI] [PubMed] [Google Scholar]
- Ko ML, Jeng CJ, Chen SC, et al. Sonographic appearance of fallopian tube carcinoma. J Clin Ultrasound. 2005;33:372–4. doi: 10.1002/jcu.20138. [DOI] [PubMed] [Google Scholar]
- Ludovisi M, De Blasis I, Virgilio B, et al. Imaging in gynecological disease (9): clinical and ultrasound characteristics of tubal cancer. Ultrasound Obstet Gynecol. 2014;43:328–35. doi: 10.1002/uog.12570. [DOI] [PubMed] [Google Scholar]
- Mikami M, Tei C, Kurahashi T, et al. Preoperative diagnosis of fallopian tube cancer by imaging. Abdom Imaging. 2003;28:743–7. doi: 10.1007/s00261-003-0009-y. [DOI] [PubMed] [Google Scholar]
- Nordin AJ. Primary carcinoma of the fallopian tube: a 20-year literature review. Obstet Gynecol Surv. 1994;49:349–61. doi: 10.1097/00006254-199405000-00026. [DOI] [PubMed] [Google Scholar]
- Nunes N, Yazbek J, Ambler G, et al. Prospective evaluation of the IOTA logistic regression model LR2 for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2012;40:355–9. doi: 10.1002/uog.11088. [DOI] [PubMed] [Google Scholar]
- Patlas M, Rosen B, Chapman W, et al. Sonographic diagnosis of primary malignant tumors of the fallopian tube. Ultrasound Q. 2004;20:59–64. doi: 10.1097/00013644-200406000-00004. [DOI] [PubMed] [Google Scholar]
- Romagosa C, Torne A, Iglesias X, et al. Carcinoma of the fallopian tube presenting as acute pelvic inflammatory disease. Gynecol Oncol. 2003;89:181–4. doi: 10.1016/s0090-8258(03)00062-3. [DOI] [PubMed] [Google Scholar]
- Sayasneh A, Kaijser J, Preisler J, et al. A multicenter prospective external validation of the diagnostic performance of IOTA simple descriptors and rules to characterize ovarian masses. Gynecol Oncol. 2013a;130:140–6. doi: 10.1016/j.ygyno.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Sayasneh A, Wynants L, Preisler J, et al. Multicentre external validation of IOTA prediction models and RMI by operators with varied training. Br J Cancer. 2013b;108:2448–54. doi: 10.1038/bjc.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Wight E, Perucchini D, et al. Primary carcinoma of the fallopian tube. A report of 19 cases with literature review. Eur J Gynaecol Oncol. 2000;21:578–82. [PubMed] [Google Scholar]
- Slanetz PJ, Whitman GJ, Halpern EF, et al. Imaging of fallopian tube tumors. AJR Am J Roentgenol. 1997;169:1321–4. doi: 10.2214/ajr.169.5.9353450. [DOI] [PubMed] [Google Scholar]
- Tantipalakorn C, Wanapirak C, Khunamornpong S, et al. IOTA simple rules in differentiating between benign and malignant ovarian tumors. Asian Pac J Cancer Prev. 2014;15:5123–6. doi: 10.7314/apjcp.2014.15.13.5123. [DOI] [PubMed] [Google Scholar]
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31:681–90. doi: 10.1002/uog.5365. [DOI] [PubMed] [Google Scholar]
- Timmerman D, Van Calster B, Testa AC, et al. Ovarian cancer prediction in adnexal masses using ultrasound-based logistic regression models: a temporal and external validation study by the IOTA group. Ultrasound Obstet Gynecol. 2010;36:226–34. doi: 10.1002/uog.7636. [DOI] [PubMed] [Google Scholar]
- Tinnangwattana D, Vichak-Ururote L, Tontivuthikul P, et al. IOTA simple rules in differentiating between benign and malignant adnexal masses by non-expert examiners. Asian Pac J Cancer Prev. 2015;16:3835–8. doi: 10.7314/apjcp.2015.16.9.3835. [DOI] [PubMed] [Google Scholar]
- Tongsong T, Tinnangwattana D, Vichak-Ururote L, et al. Comparison of effectiveness in differentiating benign from malignant ovarian masses between IOTA simple rules and subjective sonographic assessment. Asian Pac J Cancer Prev. 2016;17:4377–80. [PubMed] [Google Scholar]
- Yuen JH, Wong GC, Lam CH. Preoperative sonographic diagnosis of primary fallopian tube carcinoma. J Ultrasound Med. 2002;21:1171–3. doi: 10.7863/jum.2002.21.10.1171. [DOI] [PubMed] [Google Scholar]


