Abstract
Introduction:
Location of malignant melanoma lesions depends on environmental, genetic, sociological and demographical factors. Available sources do not provide enough information on such dependencies in various populations. There is no data concerning the role of socio-demographic factors for the population of the Central and Eastern Europe.
Aim:
The aim of this work was to evaluate the anatomical location of the primary malignant melanoma lesion in correlation to patients’ gender and age.
Material and methods:
A retrospective analysis of medical documentation of 363 patients has been performed. The patients had been diagnosed with malignant melanoma and were undergoing treatment in the years 2010-2014 in two Polish oncologic hospitals. The subject group consisted of 199 (55%) females and 164 (45%) males. The age varied between 19 - 90 years, with the median of 62 years.
Results:
In women, the melanoma lesions seem to appear more often in their lower extremities, while in case of men such lesions seem to be more often on their torsos. In both cases, the difference was statistically significant (p<0.01 When the specific locations are considered in women the lesions were more often located on their shins (p<0.01), whereas for men the lesions were located on their backs (p<0.01). It has been observed that there is dependency between lesion localization and age of patients. The lesions located on heads and necks were most common in older patients, and the lesions located in lower extremities were most common in younger ones.
Conclusion:
Differences in location of malignant melanoma lesions may be due to either genetic or environmental reasons. It is often emphasized in literature that correlation between the socio-demographic factors and the process of oncogenesis requires intensive research. In our work, we have tried to fill this gap for the population of Central and Eastern Europe to determine the exact epidemiology of this kind of cancer. This knowledge may be then used for developing cancer prevention methods specific to gender and age.
Keywords: Malignant melanoma, location, gender, age
Introduction
Melanoma is the least common but the deadliest skin cancer, accounting for only about 1% of all cases, but the vast majority of skin cancer death. The number of malignant melanoma cases in Poland, as well as in other countries inhabited mainly by the Caucasian race, is growing (de Vries et al., 2002; Lens and Dawes, 2004), almost tripling in Poland during the last 30 years (Didkowska and Wojciechowska, 2016). In 2012, the standardized cancer detection factor reached 4.9 per 100,000, and 5.1 per 100,000 for Polish men and women, respectively, whereas the standardized cancer-caused deaths factors were 2.31 (men) and 1.48 (women).
From the epidemiologic point of view, it is interesting to determine the factors responsible for the malignant melanoma itself, but also those, which in charge for the location of the lesions. So far, there has been not much research on the relationship between the lesions locations and the sociodemographic characteristics of the patients, such as age and gender. Already performed studies have shown that melanoma lesions most frequently appear on the trunks of men and in the lower extremities of women (Kuciel-Lisieska et al., 2011; Vazquez et al., 2015). It has been also pointed out in these studies that location of the melanoma depends on several socio-demographic factors. Thus, it means that in various populations different locations may be dominant. Also, there has been insufficient research concerning neither the general population of Central and Eastern Europe (Chevalier et al., 2014) nor, specifically, the population of Poland.
Aim
The aim of this work is to evaluate the anatomic location of the primary malignant melanoma lesion in correlation to the patients’ gender and age.
Materials and Methods
The analysis involves 363 patients primarily diagnosed with a malignant melanoma in the years 2010-2014, and undergoing treatment in two oncologic centers in the southern Poland: Holy Cross Cancer Center in Kielce (the subpopulation of 151 patients) and Maria Skłodowska-Curie Memorial Cancer Center and Institute of Oncology in Gliwice (the subpopulation of 212 patients). The patients’ documentation has been analyzed retrospectively with emphasis on patients’ gender, age, general and detailed location of a lesion (accompanied by the information on the side of body that the lesion appeared on).
While the patients belong to two different subpopulations from two different regions of Poland, the authors decided to combine their data, as the analysis is not focused on sub-regions and covers the whole Polish population. Additionally, increasing the number of patients taken into account improves the statistical value of the analysis. It is highly probable that different sub-regions of Poland exhibit slightly different characteristics due to cultural and behavioral differences and it would be highly desirable to perform a sub-region-focused analysis. The authors, however, at the moment do not have access to data that would make such an analysis possible.
In the studied group the female to male ratio was 55% (n=199) vs 45% (n=164) and the patients’ age ranged from 19 to 90 years (median: 62 years).
For the purpose of this research the general and specific anatomical areas of interest have been defined. The group of the general areas involves the following body regions: head and neck, trunk, upper limb (without hand), lower limb (without foot), hand, foot.
In the group of the specific areas the following regions were considered: scalp, forehead, temple, ear, neck. They have been all accounted to the perimeter area. The central area has been defined as consisting of: eyelids, nose, cheeks and chin and lips area.
For the hand and the foot, some specific areas have also been distinguished. In case of the hand, they are: finger, hand lower surface, hand upper surface, wrist area. In case of the foot: toes, sole area and foot upper area together with the ankle.
Figure 1 presents the names of the main and specific anatomical areas considered in the study, whereas the characteristics of the dispersion of tumor lesions (the number of the tumor lesions recorded at a given location) for the main anatomical areas is given in Table 1, and in Tables 2-4 for the specific areas according to sex and age.
Figure 1.
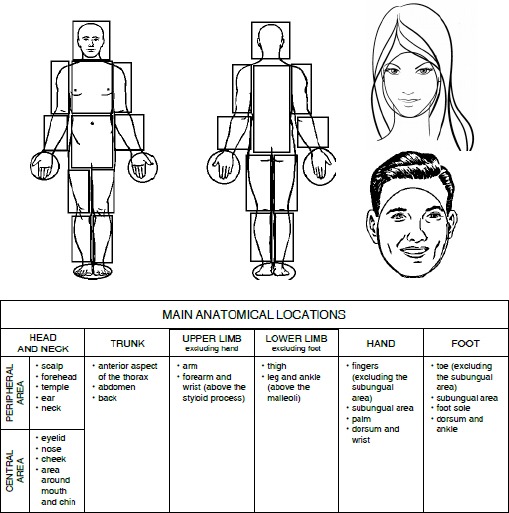
General and Detailed Areas Taken into Consideration
Table 1.
Main Anatomical Locations of Melanoma According to Sex
| Location | n (%) | Men | Women | P-value |
|---|---|---|---|---|
| Head and neck | 65 (17.9%) | 29 (17.7%) | 36 (18.1%) | ns |
| Trunk | 138 (38.0%) | 85 (51.8%) | 53 (26.6%) | <0.01 |
| Upper limb (excluding hand) | 51 (14.1%) | 28 (17.1%) | 23 (11.6%) | ns |
| Lower limb (excluding foot) | 73 (20.1%) | 11 (6.7%) | 62 (31.2%) | <0.01 |
| Hand | 9 (2.5%) | 3 (1.8%) | 6 (3.0%) | ns |
| Foot | 27 (7.4%) | 8 (4.9%) | 19 (9.5%) | ns |
| Total | 363 (100%) | 164 (100%) | 199 (100%) |
Table 2.
Location of Head and Neck Melanoma According to Sex
| Location | Men | Women | Location | Men | Women |
|---|---|---|---|---|---|
| Scalp | 0 | 0 | Peripheral area | ||
| Scalp | 0 | 0 | |||
| Forehead | 0 | 0 | |||
| Temple | 7 | 3 | 18 (62,1%) | 11 (30,6%) | |
| Ear | 4 | 2 | |||
| Neck | 5 | 5 | |||
| Eyelid | 0 | 4 | Central area | ||
| Nose | 2 | 5 | |||
| Cheek | 7 | 16 | 11 (37,9%) | 25 (69,4%) | |
| Area around mouth/chin | 2 | 0 | |||
| Total | 29 | 36 | 29 (100%) | 36 (100%) |
Table 3.
Location of Trunk Melanoma According to Sex
| Location | Men | Women | P-value |
|---|---|---|---|
| Trunk | 85 | 53 | p<0.01 |
| Thorax (anterior) | 17 | 6 | ns |
| Back | 63 | 41 | p<0.01 |
| Abdomen | 5 | 6 | ns |
| Upper limb | 28 | 23 | ns |
| Left | 17 | 12 | ns |
| Right | 11 | 11 | ns |
| Arm | 23 | 19 | ns |
| Left | 14 | 9 | ns |
| Right | 9 | 10 | ns |
| Forearm | 5 | 4 | ns |
| Left | 3 | 3 | ns |
| Right | 2 | 1 | ns |
| Lower Limb * | 11 | 62 | p<0.01 |
| Left | 4 | 36 | ns |
| Right | 6 | 26 | ns |
| Thigh | 7 | 25 | ns |
| Left | 2 | 17 | ns |
| Right | 5 | 8 | ns |
| Leg | 4 | 37 | p<0.01 |
| Left | 2 | 19 | |
| Right | 1 | 18 | |
| Hand | 3 | 6 | ns |
| Finger | 2 | 4 | |
| Palm | 0 | 2 | |
| Dorsum and wrist | 1 | 0 | |
| Foot ** | 8 | 19 | ns |
| Toe | 0 | 2 | |
| Foot sole | 2 | 2 | |
| Dorsum and ankle | 0 | 6 |
, Data are missing on laterality in for 1 man;
, Data are missing for foot precise location for6 men and 9 women.
Table 4.
Laterality of Head and Neck Melanoma According to Sex
| All cases | Cases with central distribution | Cases with peripheral distribution | ||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| Side | ||||||
| Left | 14 (60,9%) | 16 (51,6%) | 3 | 11 | 11 | 5 |
| Right | 9 (39,1%) | 15 (48,4%) | 5 | 13 | 4 | 2 |
| Total | 23 (100%) | 31 (100%) | 8 | 24 | 15 | 7 |
* 5 cases were excluded because of median location and another 5 because of missing data on laterality
All statistical computations have been performed using the StatSoft’s Statistica software package, version 10. The p values less than 0.05 – a predetermined significance level – were accepted as indicating that the observed result would be highly unlikely under the null hypothesis. A nonparametric Mann-Whitney-U test was used and the structure’s two properties difference test.
Results
As revealed from the statistical analysis, the dispersion of lesions location was similar in both sub-populations and thus it made it possible to pull the patients from these two medical centers into one homogeneous group involving 363 patients. The initial analysis confirmed that in women the lesions are most frequently located in the lower extremities (31.2%, m=62, p<0.01) and in men the most frequent location is trunk (51.8%, m=85, p<0.01), which is consistent with literature (Chevalier, 2014). However, for the remaining general anatomic areas there was no statistically significant difference between men and women. Table 1 presents the distribution of the lesions in the discussed general areas.
The median of the patients’ age differed significantly depending on the location of the lesions (p<0.01). The mean of the patients’ age was the highest in case of the head and neck region, and the lowest for the lesions located in lower extremities. The association between lesion location and age is presented in Figure 2.
Figure 2.
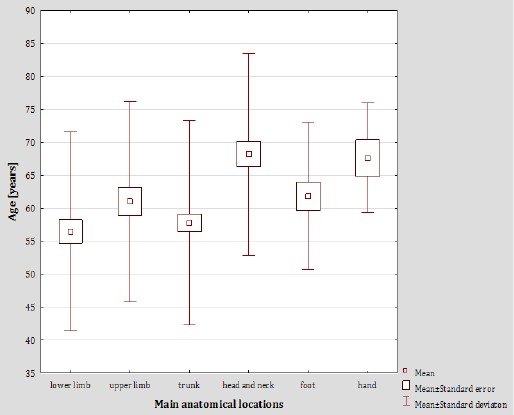
Average Age of Melanoma Patients in Relation to General Anatomic Areas of the Lesions
For the specific anatomical areas a significant difference can be observed between men and women. However, only observations of specific lesion locations for women were statistically significant. For men the melanoma lesions would occur more often in the peripheral area (p=0.2052), whereas for women - in the central area (p=0.03). The detailed information on the distribution of lesion location vs age and gender is presented in Table 2.
The lesions located in the whole upper extremities and within the detailed arm area and in the forearm, were more common in men, the difference being not statistically significant, either. It has not been observed for the lesion to appear more often on either side of the body (see Table 3). However, the lesions locating itself in lower extremities were more common in women, especially in the detailed area of the crus. It has not been observed for such lesions to appear more often in either of two lower extremities (Table 3).
The distribution of lesions location in the detailed areas of the hand and foot are also presented in Table 3.
Torso-located lesions account for 38% of cases (n=138). Among those, in 11 cases the lesions were located in the abdomen skin, in 23 cases in the chest skin and in 104 areas in the back skin. Torso-located lesions were more common in men, and the most frequent location in the specific area was back skin (p<0.01; Table 3). As for the head and neck area of men, the lesions would appear more often on the left side; this difference was not, however, statistically significant (p=0.307;Table 4).
Discussion
The exposure to the UV radiation is the most significant environmental risk factor for the malignant melanoma (El Ghisassi et al., 2009; Marks, 2000; Whiteman et al., 2006). Other factors that influence the risk of melanoma are gender, age, skin phototype, vulnerability to sunburns, job performed, number of skin moles, genetic predispositions, geographic region, economic status and application of UV protection (Gordon et al., 2015).
Location of the lesion is one of the most important prognostic factors. The available publications point out that prognosis is much worse in case of the primary lesion located in the area of head, neck, lower back and in mammary and supramammary areas (Dabouz et al, 2015; Montero et al, 2015; Tseng and Martinez, 2011; Vazquez et al., 2015; Majewski et al., 2015).
The epidemiology of melanoma changes in correspondence to changes in human lifestyle. The most recent publications highlight the raising frequency of melanoma lesions appearing on trunk and extremities, while the lesions appear less likely on head and neck (Butler and Fosko, 2010; Chen et al, 1994; Chevalier et al., 2014; Gordon et al, 2015; Montero et al, 2015; Thörn et al, 1990). This study takes a precise location of a lesion into account, as there has been no such study for the Polish population, and data acquired for the melanoma patients from the whole region of the Central and Eastern Europe is not available (Chevalier et al., 2014).
The women to men ratio in the studied group (55% women vs 45% men)is representative for the distribution of melanoma acquisitions for the whole country (the proportion of cases is about 1:1 between males and females) (Didkowska and Wojciechowska, 2016). As for the analyzed general anatomic areas, the lesions were located mostly in lower extremities in case of women and on the trunk in men, which is consistent with other Polish and world-wide studies. The observed differences may be attributed to the style of clothing: bare torso common among men and bare legs and feet common among women (Gillgren et al., 2005; Gordon et al., 2015; Kuciel-Lisieska et al., 2011; MacKie et al., 2007; Wallingford et al., 2011). Performing work in full sunlight with bare torso, connected with inability to apply UV filters to someone’s own back, could also explain the higher rate of lesions appearing on men’s backs that has been observed in the data.
The average age of the patients was the highest for those with the lesions located on head and neck, and the lowest for those with the lesions in lower extremities, which is also consistent with other sources. The appearance of head and neck lesions and their type (the subtypes as Acral Lentiginous Melanomas (ALM), located on palms, soles, and subungual sites and lentigo malignant melanomas (LMM) usually originating on the face or chronically exposed areas) are characteristic for the prolonged UV exposure. While the most common explanation for the decline in frequency of occurrence of such lesions is the growing awareness of negative aspects of UV exposure, it is probable that there are other factors, such as growing resistance to prolonged UV exposure or DNA repair mechanisms, that also influence this trend. Gilgreen et al point out that biological effects of prolonged but occasional UV exposure may explain this phenomenon. The occasional exposure may lead to specific changes in the DNA and thus change the type and location of eventual lesions in comparison to a prolonged, constant exposure (Dabouz et al., 2015; de Vries et al., 2002; Gillgren et al., 1999; Gillgren et al., 2005; Kuciel-Lisieska et al., 2011; Thörn et al., 1989).
For the head and neck region, the peripheral and central areas have been distinguished. In some studies, it has been pointed out that in men, the lesions tend to be located more frequently in the perimeter area, while the central area is characteristic for women (Gillgren et al., 2000; Lesage et al., 2013; Ringborg et al., 1993). The difference may be explained by the protective role of women’s longer hair, which cover most of the perimeter area reducing its UV exposure by as much as 81% (Green et al., 2006).
Independently from the gender, the head and neck lesions occurred more commonly on the left side of the body. However, the difference between the body sides was not statistically significant. The question of the side preferences in case of melanoma incidents has been raised in several papers recently (Brewster et al., 2007; Bulliard et al., 2008; Clark et al., 2007; Dores et al., 2011; Green et al., 2006; Lesage et al., 2013). Some studies point out that lesions appear more often on the left side of men’s bodies and on the right side of women’s bodies (Green et al., 2006; Lesage et al., 2013), which may be attributed to men being car drivers more often than women and thus being more exposed to UV radiation on their left side, while women, being car passengers, are more exposed on their right side. While this hypothesis seems interesting, it has been undermined by studies on melanoma epidemiology in countries with a left-hand traffic, where lesions appear more commonly on men’s left side, too (Dores et al., 2011). Some researchers point out that left-side lesions are generally more common and the only difference is that among women only the lesions located on their heads and extremities are biased toward the left side (Dores et al., 2011). An interesting theory that may explain this relationship is the phenomenon of asymmetric distribution of melanocytes which takes place during the embryonic stage. Melanocytes derive themselves from their precursor cells, melanoblasts, which are created during the second month of embryonic stage. Melanoblasts travel through the mesenchyme to their target areas. As embryogenesis is not a perfectly symmetrical process, the distribution of melanoblasts may be slightly asymmetric. This hypothesis has not yet undergone an extensive research. However, there exist some publications that describe this phenomenon basing on the embryonic experimental model Xenopus laevis (Pai et al., 2012).
In our study, the lesions located on hands and feet were found to be more frequent in women, however, this difference was not statistically significant. Due to the generally small number of such cases, it is practically impossible to develop these studies. Some researchers claim that the phenomenon of more hand and foot lesions in women can be explained by more transparent footwear more commonly wore by women (Chevalier et al., 2014).
Summing up, our findings are in accordance with the results by other authors. The profile of a Polish melanoma patient is coherent with that obtained for other regions, with similar lesion occurrence in same areas of the body. Reducing the risk of melanoma development involves the same steps as encouraged by other authors, that is: reducing incidental UV exposure and sunburns and prolonged UV exposure, especially head and neck exposure, with special care to bald areas. (Chevalier et al., 2014; Cox et al., 1996; Hemminki et al., 2003; Majewski et al., 2015).
Additionally, social campaigns promoting such safe behavior along with using UV-filters when exposed to sunlight should be performed. While the Polish society is aware of the importance of applying UV filters, melanoma patients often confessed omitting application of UV-filtering lotions. It should be also augmented how important it is to perform self-assessment of skin discolorations and moles, especially on trunk. Considering the fact that most cases analyzed in this paper were lesions in medium and high grade, promoting the need for professional consultation of skin spots that could turn to a melanoma lesions would improve the overall survivability and treatment success rates (Kamińska-Winciorek et al., 2014, 2015).
As for technical means, it would be helpful to equip all car windshields and side windows with layers of UV filtering materials, as people’s growing dependence on car transport forces them to spend hours in direct sunlight every day.
Acknowledgements
Acknowledgements to Professor Stanisław Góźdź and Wojciech Korejba, MD for their help in obtaining and analyzing data concerning cancer patients from the region of Kielce.
References
- Butler ST, Fosko SW. Increased prevalence of left-sided skin cancers. J Am Acad Dermatol. 2010;63:1006–10. doi: 10.1016/j.jaad.2009.11.032. [DOI] [PubMed] [Google Scholar]
- Brewster DH, Horner MJ, Rowan S, et al. Left-sided excess of invasive cutaneous melanoma in six countries. Eur J Cancer. 2007;43:2634–7. doi: 10.1016/j.ejca.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Bulliard JL, Ess S, Bordoni A, Konzelmann I, Levi F. Left-sided excess in the laterality of cutaneous melanoma. Arch Dermatol. 2008;144:556–8. doi: 10.1001/archderm.144.4.556. [DOI] [PubMed] [Google Scholar]
- Chen YT, Zheng T, Holford TR, Berwick M, Dubrow R. Malignant melanoma incidence in Connecticut (United States): time trends and age-period-cohort modeling by anatomic site. Cancer Causes Control. 1994;5:341–50. doi: 10.1007/BF01804985. [DOI] [PubMed] [Google Scholar]
- Chevalier V, Barbe C, Le Clainche A, et al. Comparison of anatomical locations of cutaneous melanoma in men and women: a population-based study in France. Br J Dermatol. 2014;171:595–601. doi: 10.1111/bjd.13052. [DOI] [PubMed] [Google Scholar]
- Clark LN, Shin DB, Troxel AB, et al. Association between the anatomic distribution of melanoma and sex. J Am Acad Dermatol. 2007;56:768–73. doi: 10.1016/j.jaad.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Cox NH, Aitchison TC, Sirel JM, MacKie RM. Comparison between lentigo maligna melanoma and other histogenetic types of malignant melanoma of the head and neck. Scottish Melanoma Group. Br J Cancer. 1996;73:940–4. doi: 10.1038/bjc.1996.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabouz F, Barbe C, Lesage C, et al. Clinical and histological features of head and neck melanoma: a population-based study in France. Br J Dermatol. 2015;172:707–15. doi: 10.1111/bjd.13489. [DOI] [PubMed] [Google Scholar]
- Didkowska J, Wojciechowska U. Zachorowania i zgony na nowotwory złośliwe w Polsce. Krajowy Rejestr Nowotworów, Centrum Onkologii - Instytut im. Marii Skłodowskiej - Curie. 2016. Online registry: http://onkologia.org.pl/k/epidemiologia/
- Dores GM, Huycke MM, Devesa SS. Melanoma of the skin and laterality. J Am Acad Dermatol. 2011;64:193–5. doi: 10.1016/j.jaad.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ghissassi F, Baan R, Straif K, et al. WHO international agency for research on cancer monograph working group: a review of human carcinogens –part D: radiation. Lancet Oncol. 2009;10:751–2. [Google Scholar]
- Gillgren P, Månsson-Brahme E, Frisell J, et al. Epidemiological characteristics of cutaneous malignant melanoma of the head and neck--a population-based study. Acta Oncol. 1999;38:1069–74. doi: 10.1080/028418699432383. [DOI] [PubMed] [Google Scholar]
- Gillgren P, Månsson-Brahme E, Frisell J, et al. A prospective population-based study of cutaneous malignant melanoma of the head and neck. Laryngoscope. 2000;110:1498–504. doi: 10.1097/00005537-200009000-00017. [DOI] [PubMed] [Google Scholar]
- Gillgren P, Brattström G, Frisell J, et al. Effect of primary site on prognosis in patients with cutaneous malignant melanoma. A study using a new model to analyse anatomical locations. Melanoma Res. 2005;15:125–32. doi: 10.1097/00008390-200504000-00007. [DOI] [PubMed] [Google Scholar]
- Gordon D, Gillgren P, Eloranta S, et al. Time trends in incidence of cutaneous melanoma by detailed anatomical location and patterns of ultraviolet radiation exposure: a retrospective population-based study. Melanoma Res. 2015;25:348–56. doi: 10.1097/CMR.0000000000000170. [DOI] [PubMed] [Google Scholar]
- Green AC, Kimlin M, Siskind V, Whiteman DC. Hypothesis: hair cover can protect against invasive melanoma on the head and neck (Australia) Cancer Causes Control. 2006;17:1263–6. doi: 10.1007/s10552-006-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki K, Zhang H, Czene K. Incidence trends and familial risks in invasive and in situ cutaneous melanoma by sun-exposed body sites. Int J Cancer. 2003;104:764–71. doi: 10.1002/ijc.10976. [DOI] [PubMed] [Google Scholar]
- Kamińska-Winciorek G, Calik J, Wydmanski J, Schwartz RA, Czajkowski R. Primary melanoma in rare locations: Clinical and dermatoscopic features. Letter to the editor. Indian J Dermatol Venereol Leprol. 2014;80:369–71. doi: 10.4103/0378-6323.136976. [DOI] [PubMed] [Google Scholar]
- Kamińska-Winciorek G, Gajda M, Wydmański J, Tukiendorf A. What do Web users know about skin self-examination and melanoma symptoms? Asian Pac J Cancer Prev. 2015;16:3051–6. doi: 10.7314/apjcp.2015.16.7.3051. [DOI] [PubMed] [Google Scholar]
- Kuciel-Lisieska G, Godlewski J, Lisieska-Tyszko S, Lachowski A, Licznerska G. Analiza danych epidemiologicznych chorych na czerniaka leczonych w latach 1996-2007 w Warmińsko-Mazurskim Centrum Onkologii w Olsztynie Nowotwory. J Oncol. 2011;61:4. [Google Scholar]
- Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;15:179–85. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- Lesage C, Barbe C, Le Clainche A, et al. Sex-related location of head and neck melanoma strongly argues for a major role of sun exposure in cars and photoprotection by hair. J Invest Dermatol. 2013;133:1205–11. doi: 10.1038/jid.2012.405. [DOI] [PubMed] [Google Scholar]
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003. Br J Cancer. 2007;96:1772–7. doi: 10.1038/sj.bjc.6603801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski W, Stanienda K, Wicherska K, Ulczok R, Wydmanski J. Treatment outcome and prognostic factors for malignant skin melanoma treated with radical surgery. Asian Pac J Cancer Prev. 2015;16:5709–14. doi: 10.7314/apjcp.2015.16.14.5709. [DOI] [PubMed] [Google Scholar]
- Marks R. Epidemiology of melanoma Clinical dermatology. Review article. Clin Exp Dermatol. 2000;25:459–63. doi: 10.1046/j.1365-2230.2000.00693.x. [DOI] [PubMed] [Google Scholar]
- Montero I, Requena C, Traves V, et al. Age-related characteristics of cutaneous melanoma in a Spanish Mediterranean population. Int J Dermatol. 2015;54:778–84. doi: 10.1111/ijd.12496. [DOI] [PubMed] [Google Scholar]
- Pai VP, Vandenberg LN, Blackiston D, Levin M. Neurally derived tissues in xenopus laevis embryos exhibit a consistent bioelectrical left-right asymmetry. Stem Cells. 2012;2012 doi: 10.1155/2012/353491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringborg U, Afzelius LE, Lagerlöf B, et al. Cutaneous malignant melanoma of the head and neck. Analysis of treatment results and prognostic factors in 581 patients: a report from the Swedish Melanoma Study Group. Cancer. 1993;71:751–8. doi: 10.1002/1097-0142(19930201)71:3<751::aid-cncr2820710317>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Thörn M, Adami HO, Ringborg U, Bergström R, Krusemo U. The association between anatomic site and survival in malignant melanoma. An analysis of 12 353 cases from the Swedish Cancer Registry. Eur J Cancer Clin Oncol. 1989;25:483–91. doi: 10.1016/0277-5379(89)90261-7. [DOI] [PubMed] [Google Scholar]
- Thörn M, Bergström R, Adami HO, Ringborg U. Trends in the incidence of malignant melanoma in Sweden, by anatomic site. Am J Epidemiol. 1990;132:1066–77. doi: 10.1093/oxfordjournals.aje.a115749. [DOI] [PubMed] [Google Scholar]
- Tseng WH, Martinez SR. Tumor location predicts survival in cutaneous head and neck melanoma. J Sur Res. 2011;167:192–8. doi: 10.1016/j.jss.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez VL, Silva T, Vieira MA, et al. Melanoma characteristics in Brazil: demographics, treatment, and survival analysis. BMC Res Notes. 2015;8:4. doi: 10.1186/s13104-015-0972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries E, Bray FI, Coebergh JW, Parkin DM. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: Rising trends in incidence and mortality but recent stabilizations in Western Europe and decreases in Scandinavia. Int J Cancer. 2002;107:119–26. doi: 10.1002/ijc.11360. [DOI] [PubMed] [Google Scholar]
- Wallingford SC, Alston RD, Birch JM, Green AC. Increases in invasive melanoma in England, 1979-2006, by anatomical site. Br J Dermatol. 2011;165:859–64. doi: 10.1111/j.1365-2133.2011.10434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172–7. doi: 10.1200/JCO.2006.06.1325. [DOI] [PubMed] [Google Scholar]


