Abstract
Background:
Anthracosis of the lung occurs due to the deposition of carbon and silica in the mucosa and submucosa, manifested as black lesions. The association of anthracosis with lung cancer has remained to be clearly elucidated The current study aimed to assess the P16, CDH1 and LUNX genes expression level to evaluate the association of anthracotic lesions in the lungs with the occurrence of non-small cell lung cancer.
Methods:
Forty biopsy samples were taken from the center and 40 from the margins of black anthracotic lesions in the lungs; RNA was extracted from the samples and cDNA was synthesized. Real-time reverse-transcription polymerase chain reaction (RT-PCR) was performed to assess the expression of P16, CDH1 and LUNX genes. All steps were performed in triplicate.
Results:
A significant reduction in P16 gene expression was noted at the center compared to the margins of the lesions (P<0.001).expression level of CDH1 at the center of lesions was significantly lower than margins (P<0.001). However, LUNX gene had significantly higher expressionlevel at the center compared to margins (P<0.001).
Conclusion:
Decreased expression of P16 and CDH1 and increased expression of LUNX tumor genes were noted at the center of anthracotic lesions. Significant increase in expression of LUNX gene in NSCLC indicates an association between anthracosis and NSCLC, according to which, anthracotic patients may carry a high risk for NSCLC.
Keywords: P16- CDH1- LUNX- anthracotic- non, small cell lung cancer
Introduction
Pulmonary anthracosis is characterized by the formation of black nodules in bronchial mucosa (Touhidi et al., 2002). Repeated exposure to carbon, silica or quartz dust (as in workplaces) may cause pulmonary anthracosis; however, cases with no history of occupational exposure have also been reported (Naeye, 1994). Anthracosis can be diagnosed by bronchoscopy. In some cases, anthracosis may progress and cause bronchial obstruction (Chung et al., 1998). Anthracosis may damage the bronchial mucosa and impair mucociliary clearance of the bronchi; this can predispose patients to pulmonary infection (Sonnenberg et al., 2000; Castranova et al., 2002). Also, some studies have shown an association between anthracosis and development of lung cancer (Hou et al., 1998), 17. Lung cancer has high rate of morbidity and mortality. Treatment of lung cancer is challenging because effective treatment strategies and specific markers for early detection of lung cancer do not exist (Cheng et al., 2008; Ghadimi et al., 2017; Karimi et al., 2017; Moshref et al., 2017). Several tumor markers have been tested for diagnosis and prognosis of lung cancer such as CEA, CK19, VEGF and LUNX (Wieskopf et al., 1995; Iwao et al., 2001; Lantuéjoul et al., 2003; Snead et al., 2003; Chanin et al., 2004; Wallace et al., 2005; Karimi et al., 2015). Lung cancers are divided into two types of small cell lung cancer and NSCLC; NSCLC is the most important and most common form of lung cancer. It is an epithelial tumor, which may metastasize at initial stages and expand (Wieskopf et al., 1995; Iwao et al., 2001).
P16 as tumor suppressor impedes tumor growth by inhibiting cell cycle (Pisani et al., 1997). Expression of P16 regularly occurs in old cells and increases with age (Collado et al., 2007). Activation of P16 prevents the occurrence of cancer by stopping cell growth(Esteller et al., 2001). Inactivation of P16 by its elimination, point mutation or methylation of its promoter results in out of control cell proliferation, which eventually results in cancer. Methylation of P16 promoter and turn-off of this gene has been reported in many cancers (Esteller et al., 2001; Payá et al., 2009). In addition, the CDH1 (E-cadherin) is a member of the cadherin family and is among the most important molecules enabling cell attachment (Hulpiau and Van Roy, 2009). It is capable of inhibiting metastasis and invasion of cancer cells(Hulpiau and Van Roy, 2009). Most cancer cells in human tissues relatively or completely lose their E-cadherin molecules as they progress towards malignancy (Sundfeldt, 2003; Strumane et al., 2004; Naora and Montell, 2005). Significant reduction in expression of E-cadherin has been reported on the surface of cancer cells (Kleer et al., 2001). Moreover, LUNX gene is exclusively expressed in human lungs and can be used as an efficient marker in body fluids or tissues for detection of patients with NSCLC (Karimi et al., 2015). The exact mechanism of action of this gene is not clear; however, it may play a role in innate immunity (Karimi et al., 2015). Thus, its assessment may be helpful for detection of NSCLC (Karimi et al., 2015).
This study aimed to assess P16, CDH1 and LUNX genes expression level in black anthracotic lesions and adjacent healthy tissues to evaluate the correlation of anthracosis with NSCLC.
Materials and Methods
This study was approved by the ethics committee of Masih Daneshvari Hopsital, affiliated to Shahid Beheshti University of Medical Sciences. After obtaining ethical code to No.IR.SBMU.NRITLD.REC.1394.137, forty anthracotic patients were selected and enrolled after signing written informed consent forms. Tissue samples were obtained from the center and margins of anthracotic lesions. Biopsy samples were transferred to a laboratory and subjected to RNA extraction using Cinna Pure RNA kit (Cat No: PR891620-S). First, 20-25 mg of the fresh tissue was milled; 400 μL of the lysis solution present in the kit was added to the milled tissue, mixed and homogenized. The precipitation solution was added to the homogenized mixture and the obtained solution was transferred to the extraction column and centrifuged. washing buffers were poured on the column and centrifuged. Eventually, 50 μL of RNase free water was poured on the column and pure RNA present in the column was extracted. The quality of RNA was then assessed by NanoDrop. In the next step, cDNA was synthesized using Viva 2-step RT-PCR (Cat No. RTPL12); 15μL of RNA obtained according to the instructions provided in the kit was used for cDNA synthesis. Each RNA sample was used for cDNA synthesis three times and three cDNA vials were synthesized and controlled with NanoDrop. Primers were obtained using allele ID7 software, controlled and synthesized. Sequences of primers for the real-time RT-PCR reaction are shown in Table 1.
Table 1.
Primers and Their Sequences for the Real-Time RT-PCR Reaction
| Gene | ||||
|---|---|---|---|---|
| P16 | CDH1 | LUNX | 18s rRNA | |
| Forward primer | ACCCTGGATGTCCTCTATGG | TGCCATAGATGAATTGAAGGAATG | CCACCGTCTCTATGTCACCA | GTAACCCGTTGAACCCCATT |
| Primer length | 20 | 24 | 20 | 20 |
| Reverse primer | CAGGCATAGGTCCCGTTATTA | TGTCATATATTAATTGCATAAACACCTCA | GCCAAGTCCATCAAGCAGA | CCATCCAATCGGTAGTAGCG |
| Primer length | 21 | 29 | 19 | 20 |
Real-time RT-PCR
Real-time RT-PCR was carried out using Hot Tag EvaGreen q PCR Mix kit. The cDNA aliquots were then utilized in qPCR reactions for P16, CDH1 and LUXN genes, with 18s rRNA used as the endogenous reference gene. The reaction components were template (2μL), master mix (4μL) and F and R primers (0.5μL). The final volume was reached to 20μl by deionized distilled water. The positive and negative controls were also used.
Statistical analysis
The data were statistically analyzed using SPSS version 20 and analyzed using t-test followed by Bonferroni’s post-hoc test. The differences were considered significant when the P-values were lower than 0.001.
Results
A total of 40 patients with mean age of 49±10 years were evaluated (six females and 34 males).18s rRNA gene was chosen as the reference gene. Comparison of the mean Ct values of the samples obtained from the center and margins of the lesions showed non-significant difference (P=0.234), indicating that selection of this gene as the reference gene was appropriate selection.
Expression of P16, CDH1 and LUNX genes was positive at the center of lesions in nine out of 40 (22.5%), 18 out of 40 (45%) and 19 out of 40 (47.5%) patients, respectively. Expression of P16, CDH1 and LUNX genes was positive at the margins of lesions in 25 out of 40 (62.5%), 27 out of 40 (67.5%) and six out of 40 (15%) patients, respectively. Comparison of the rate of positivity of P16 CDH1 and LUNX genes at the center and margins of lesions showed a significant difference (P<0.001) (Figure 1).
Figure 1.
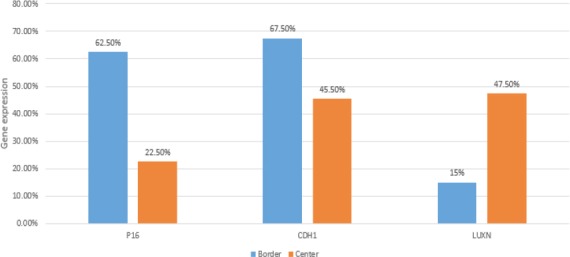
Evaluation of CDH1, LUXN and P16 mRNA Expression in the Center and Borders of the Lesions by RT PCR
To increase sensitivity, all tests were performed in triplicate to obtain more accurate results.
Difference in expression level of P16, CDH1 and LUNX genes at the center and borders of lesions
The obtained value for P16 was 1.51, which indicated that the number of primary transcripts of this marker at the center of lesions was 1.51 times lower than the rate at the margins. The obtained value for CDH1 was 1.23, which indicated that the number of primary transcripts of this marker at the center of lesions was 1.23 times lower than the rate at the margins. This value for LUNX was 2.92; indicating that the primary transcripts for this marker at the center of lesions were 2.92 times higher than those at the margins (Figure 2).
Figure 2.
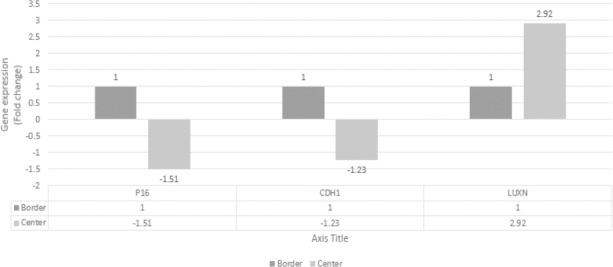
Difference in Expression of P16, CDH1 and LUNX Genes at the Center and Borders of Lesions
Discussion
Anthracosis refers to accumulation of coal particles and their black pigments (mainly composed of carbon) in the lungs. These black pigments are also found in the form of black lesions in the bronchi of coal miners, workers in some specific industries and those living in metropolitan areas (Castranova et al., 2002; Searl et al., 2002).
Although anthracosis is a simple indicator of air pollution or smoking, accurate estimation of the amount of deposited particles is difficult (Seto et al., 1994). Thus, in general, inhalation of carbon particles present in polluted air of cities or direct exposure to smoke in workplaces may result in anthracosis (Seto et al., 1994; Konno et al., 2004)
Previous studies have shown a correlation between anthracosis and clinical characteristics of pulmonary adenocarcinoma (Hou et al., 1998; Konno et al., 2004). DNA methylation at the promoter plays an important role in development of cancer and tumor growth via the expression of some specific genes (Konno et al., 2004). However, methylation is not always seen in a specific type of cancer but it occurs in specific tissues (Konno et al., 2004).
This study sought to assess the correlation between black anthracotic lesions and NSCLC. Thus, three genes with changed expression in cancer were chosen. Based on previous studies, LUNX gene, which is an exclusive gene in NSCLC was chosen to better elucidate this correlation (Karimi et al., 2015). Expression of this gene at the center and margins of lesions was detected using real-time RT-PCR, which is accurate and sensitive technique and is commonly used for assessment of expression of genes and biomarkers (Karimi et al., 2015; Mohamadnia et al., 2016; Bahrami et al., 2016). Genes evaluated in this study were P16, CDH1 and LUNX. First, tissue samples were taken from patients, total RNA was extracted, and cDNA was synthesized and assessed by real-time RT-PCR. All tests were done in triplicate to increase sensitivity and specificity (Castaldo et al., 1997; Sun et al., 2007).
P16 protein in most cancers undergoes genetic changes such as mutation and hyper methylation of promoter, causing a reduction in expression of P16 protein and uncontrolled growth, eventually enhancing the occurrence of cancer (Shima et al., 2011). Our findings indicated a significant reduction in expression of P16 gene at the center of anthracotic lesions compared to the margins. In line with our findings, previous studies have demonstrated a down regulation of P16 in cancerous tissues compared to adjacent healthy tissues (Tada et al., 2002).
It has also been reported a reduction in expression of P16 gene in 38% of the patients with colorectal cancer (Zou et al., 2002). The findings of research also have demonstrated lymph node metastasis in tumors with decreased expression of P16 (Tada et al., 2002).
CDH1 molecules are adhesion molecules that regulate cell cycle and interactions with other cells. They also play a role in tumor invasion and metastasis (Waki et al., 2003). In our study, it was revealed that expression level of CDH1 gene significantly decreased at the center of lesions compared to the margins. Reduction in expression of CDH1 (e-cadherin) was also observed in cell lines causing invasion and metastasis (Cano et al., 2000). Evaluation of breast cancers has demonstrated that the expression of CDH1 was significantly lower on the surface of cancer cells (Kashiwagi et al., 2010), a finding which was closely in accordance with our findings.
In the present study, we have shown increased expression level of LUNX gene at the center of lesions compared to the margins. The previous studies have shown that LUNX gene was up regulated in NSCLC patients compared to healthy individuals (Iwao et al., 2001). It has also been emphasized that LUNX gene is a specific marker for lung cancer (Karimi et al., 2015), confirming our results.
In conclusion, decreased expression of P16 and CDH1 and increased expression of LUNX tumor genes were noted at the center of anthracotic lesions. Significant increase in expression of LUNX gene in NSCLC indicates an association between anthracosis and NSCLC, according to which, anthracotic patients may carry a high risk for NSCLC.
Ethical approval
This study was approved by the ethics committee of Masih Daneshvari Hopsital, affiliated to Shahid Beheshti University of Medical Sciences. After obtaining ethical code to No.IR.SBMU.NRITLD.REC.1394.137, Forty anthracotic patients were selected and enrolled after signing written informed consent forms.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
None of the authors had any conflicts of interest to announce in connection with this work.
Acknowledgements
We appreciate National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran for their support.
References
- Bahrami N, Gholami M, Jamaati HR, et al. Expression of two essential mRNA biomarker in the peripheral blood as possible biomarkers for diagnosis of non-small cell lung carcinoma. Minerva Pneumol. 2016;55:31–6. [Google Scholar]
- Cano A, Pérez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Castaldo G, Tomaiuolo R, Sanduzzi A, et al. Lung cancer metastatic cells detected in blood by reverse transcriptase-polymerase chain reaction and dot-blot analysis. J Clin Oncol. 1997;15:3388–93. doi: 10.1200/JCO.1997.15.11.3388. [DOI] [PubMed] [Google Scholar]
- Castranova V, Porter D, Millecchia L, et al. In 'oxygen/nitrogen radicals: Cell injury and disease'. Springer; 2002. Effect of inhaled crystalline silica in a rat model: time course of pulmonary reactions; pp. 177–84. [PubMed] [Google Scholar]
- Chanin TD, Merrick DT, Franklin WA, et al. Recent developments in biomarkers for the early detection of lung cancer: perspectives based on publications 2003 to present. Curr Opin Pulm Med. 2004;10:242–7. doi: 10.1097/01.mcp.0000130321.11513.13. [DOI] [PubMed] [Google Scholar]
- Cheng M, Chen Y, Yu X, et al. Diagnostic utility of LunX mRNA in peripheral blood and pleural fluid in patients with primary non-small cell lung cancer. BMC Cancer. 2008;8:1. doi: 10.1186/1471-2407-8-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MP, Kim H, Rhee CH, et al. Bronchial stenosis due to anthracofibrosis. Chest. 1998;113:344–50. doi: 10.1378/chest.113.2.344. [DOI] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Esteller M, Corn PG, Baylin SB, et al. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–9. [PubMed] [Google Scholar]
- Ghadimi K, Bahrami N, Fathi M, et al. Diagnostic value of LunX mRNAand CEA mRNA expression in pleural fluid of patients with non-small cell lung cancer. Minerva Pneumol. 2017;56:90–5. [Google Scholar]
- Hou M, Morishita Y, Iijima T, et al. The implication of anthracosis in the development of pulmonary adenocarcinoma. Jpn J Cancer Res. 1998;89:1251–6. doi: 10.1111/j.1349-7006.1998.tb00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulpiau P, Van Roy F. Molecular evolution of the cadherin superfamily. Int J Biochem Cell Biol. 2009;41:349–69. doi: 10.1016/j.biocel.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Iwao K, Watanabe T, Fujiwara Y, et al. Isolation of a novel human lung-specific gene, LUNX, a potential molecular marker for detection of micrometastasis in non-small-cell lung cancer. Int J Cancer. 2001;91:433–7. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1059>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Karimi S, Bahrami N, Sharifi K, et al. Investigating gene expression level of MUC1 and CEA in pleural fluid of NSCLC lung cancer patients with real-time RT-PCR method. Minerva Pneumol. 2017;56:18–24. [Google Scholar]
- Karimi S, Mohamadnia A, Nadji SA, et al. Expression of two basic mRNA biomarkers in peripheral blood of patients with non-small cell lung cancer detected by real-time rt-PCR, individually and simultaneously. Iran Biomed J. 2015;19:17. doi: 10.6091/ibj.1397.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi S, Yashiro M, Takashima T, et al. Significance of E-cadherin expression in triple-negative breast cancer. Br J Cancer. 2010;103:249–55. doi: 10.1038/sj.bjc.6605735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleer CG, van Golen KL, Braun T, et al. Persistent E-cadherin expression in inflammatory breast cancer. Mod Pathol. 2001;14:458–64. doi: 10.1038/modpathol.3880334. [DOI] [PubMed] [Google Scholar]
- Konno S, Morishita Y, Fukasawa M, et al. Anthracotic index and DNA methylation status of sputum contents can be used for identifying the population at risk of lung carcinoma. Cancer Cytopathol. 2004;102:348–54. doi: 10.1002/cncr.20643. [DOI] [PubMed] [Google Scholar]
- Lantuéjoul S, Constantin B, Drabkin H, et al. Expression of VEGF, semaphorin SEMA3F, and their common receptors neuropilins NP1 and NP2 in preinvasive bronchial lesions, lung tumours, and cell lines. J pathol. 2003;200:336–47. doi: 10.1002/path.1367. [DOI] [PubMed] [Google Scholar]
- Mohamadnia A, Karimi S, Yadegar Azari R, et al. Expression of CK19 gene in patients with lung cancer and Its comparison with carcinoembryonic antigen in peripheral blood. Payavard Salamat. 2016;9:459–68. [Google Scholar]
- Moshref Behzad N, Bahrami N, Farzanegan B, et al. Expression of CK19-mRNA and CEA -mRNA biomarkers in pleural fluid of patients with non-small cell lung cance. Minerva Pneumologica. 2017:56. [Google Scholar]
- Naeye R. Pathology of pulmonary disease. Philadelphia: JB Lippincott; 1994. The pneumoconiosis;coal worker's pneumoconiosis; pp. 369–85. [Google Scholar]
- Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nature Reviews Cancer. 2005;5:355–66. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- Payá A, Alenda C, Pérez-Carbonell L, et al. Utility of p16 immunohistochemistry for the identification of Lynch syndrome. Clinical Cancer Research. 2009;15:3156–62. doi: 10.1158/1078-0432.CCR-08-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani P, Parkin DM, Muñoz N, et al. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiology Biomarkers & Prevention. 1997;6:387–400. [PubMed] [Google Scholar]
- Searl A, Nicholl A, Baxter P. Assessment of the exposure of islanders to ash from the Soufriere Hills volcano, Montserrat, British West Indies. Occupational and environmental medicine. 2002;59:523–31. doi: 10.1136/oem.59.8.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H, Ohkubo T, Watanabe N, et al. Black dust matter in human lung and causes of the deposition. Ann. Rep. Tokyo Metrop. Res. Lab. PH. 1994;45:139–48. [Google Scholar]
- Shima K, Nosho K, Baba Y, et al. Prognostic significance of CDKN2A (p16) promoter methylation and loss of expression in 902 colorectal cancers: Cohort study and literature review. International journal of cancer. 2011;128:1080–94. doi: 10.1002/ijc.25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead D, Perunovic B, Cullen N, et al. hnRNP B1 expression in benign and malignant lung disease. The Journal of pathology. 2003;200:88–94. doi: 10.1002/path.1292. [DOI] [PubMed] [Google Scholar]
- Sonnenberg P, Murray J, Glynn J, et al. Risk factors for pulmonary disease due to culture-positive M. tuberculosis or nontuberculous mycobacteria in South African gold miners. European Respiratory Journal. 2000;15:291–6. doi: 10.1034/j.1399-3003.2000.15b12.x. [DOI] [PubMed] [Google Scholar]
- Strumane K, Berx G, Van Roy F. In 'Cell adhesion'. Springer; 2004. Cadherins in cancer; pp. 69–103. [DOI] [PubMed] [Google Scholar]
- Sun S, Schiller JH, Spinola M, et al. New molecularly targeted therapies for lung cancer. The Journal of clinical investigation. 2007;117:2740–50. doi: 10.1172/JCI31809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundfeldt K. Cell–cell adhesion in the normal ovary and ovarian tumors of epithelial origin;an exception to the rule. Molecular and cellular endocrinology. 2003;202:89–96. doi: 10.1016/s0303-7207(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Tada T, Watanabe T, Kazama S, et al. Reduced p16 expression correlates with lymphatic invasion in colorectal cancers. Hepato-gastroenterology. 2002;50:1756–60. [PubMed] [Google Scholar]
- Touhidi M, Keshmiri M, Ataran D, et al. Tuberculus bronchostenosis presenting as anthracofibrosis. 2002 [Google Scholar]
- Waki T, Tamura G, Sato M, et al. Promoter methylation status of DAP-kinase and RUNX3 genes in neoplastic and non-neoplastic gastric epithelia. Cancer science. 2003;94:360–4. doi: 10.1111/j.1349-7006.2003.tb01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MB, Block MI, Gillanders W, et al. Accurate molecular detection of non-small cell lung cancer metastases in mediastinal lymph nodes sampled by endoscopic ultrasound-guided needle aspiration. CHEST Journal. 2005;127:430–7. doi: 10.1378/chest.127.2.430. [DOI] [PubMed] [Google Scholar]
- Wieskopf B, Demangeat C, Purohit A, et al. Cyfra 21-1 as a biologic marker of non-small cell lung cancer: evaluation of sensitivity, specificity, and prognostic role. Chest. 1995;108:163–9. doi: 10.1378/chest.108.1.163. [DOI] [PubMed] [Google Scholar]
- Zou H-Z, Yu B-M, Wang Z-W, et al. Detection of aberrant p16 methylation in the serum of colorectal cancer patients. Clinical Cancer Research. 2002;8:188–91. [PubMed] [Google Scholar]


