As a consequence of oxidative stress, lipids, proteins, and DNA are endogenously oxidized, and this leads to the formation of modified molecules in tissue and blood.(1–3) Some of these products are excreted in urine. Among these oxidized molecules, lipid peroxidation-derived products (aldehydes) or quinones are highly reactive and form adducts with amine moieties in DNA and amine/thiol moieties in proteins.(3–9) Oxidative stress is considered to be caused by and be a result of disease. Therefore, oxidized molecules could be factors in the initiation, promotion, and development of pathologies such as cardiovascular(10) and neurodegenerative diseases.(11) Therefore, the detection of these molecules could serve as a “torch” for researchers to reveal the picture of such diseases. These modified molecules are a kind of “rust” in living systems and could be indicators of a balance between oxidation and reduction.
We have food every day. Food contains nutrients (proteins, sugars and lipids). Plant-derived food, in particular, is also rich in non-nutrients such as phytochemicals (e.g., polyphenols). This indicates that “xenobiotics”, which are not true nutrients, are incorporated into our daily diets whether we like it or not. Nutritionally, we do not “digest” these xenobiotics (e.g., phytochemicals), but some are circulated through the body either intact or in conjugated forms. These ingested phytochemicals could elicit biological effects. Indeed, epidemiological studies have shown the beneficial effect of polyphenols in, for example, the prevention of cardiovascular diseases.(12) These effects may be triggered by specific or non-specific reactions between ingested xenobiotics and various biological molecules within cells.(13) Plant-derived isothocyanates can form adducts with proteins and thereby induce cellular responses,(14) while some polyphenols can conjugate with proteins via their quinone moieties.(15) These xenobiotics may act as “toxic chemicals” that induce self-defense mechanisms in the body.(16–17) Alternatively, some biological effects could be caused by “indirect” reactions between a phytochemical and a cell via a receptor.(18) This means that the “brain-gut interaction” is a pathway to instantiate functions triggered by the ingestion of fruits and vegetables.
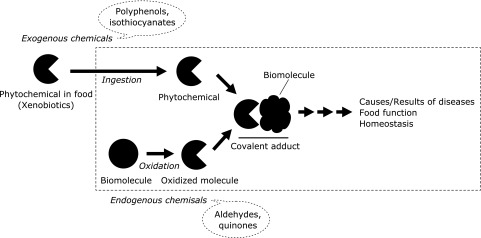
In these serial reviews, current progress in covalent modification of biomolecules by food or oxidatively generated compounds will be reviewed in six separate papers from different points of view. In particular, these reviews will focus on the biological relevance of the interaction of oxidized or ingested molecules with biomolecules and their role in triggering biological functions via shared pathways (Fig. 1).
Fig. 1.
Schematic illustration of two-into-one pathway for covalent adduction that could contribute biological function. Food-derived ingested chemicals and endogenously generated oxidized products may share the pathway for the expression of biological function.
References
- 1.Asahi T, Kondo H, Masuda M, et al. Chemical and immunochemical detection of 8-halogenated deoxyguanosines at early stage inflammation. J Biol Chem. 2010;285:9282–9291. doi: 10.1074/jbc.M109.054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hisaka S, Kato Y, Kitamoto N, et al. Chemical and immunochemical identification of propanoyllysine derived from oxidized n-3 polyunsaturated fatty acid. Free Radic Biol Med. 2009;46:1463–1471. doi: 10.1016/j.freeradbiomed.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Kawai Y, Kato Y, Nakae D, et al. Immunohistochemical detection of a substituted 1,N(2)-ethenodeoxyguanosine adduct by omega-6 polyunsaturated fatty acid hydroperoxides in the liver of rats fed a choline-deficient, L-amino acid-defined diet. Carcinogenesis. 2002;23:485–489. doi: 10.1093/carcin/23.3.485. [DOI] [PubMed] [Google Scholar]
- 4.Blair IA. DNA adducts with lipid peroxidation products. J Biol Chem. 2008;283:15545–15549. doi: 10.1074/jbc.R700051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato Y. The formation of lipid hydroperoxide-derived amide-type lysine adducts on proteins: a review of current knowledge. Subcell Biochem. 2014;77:21–39. doi: 10.1007/978-94-007-7920-4_2. [DOI] [PubMed] [Google Scholar]
- 6.Kato Y, Oki K, Suga N, et al. A novel quinone derived from 5-hydroxyindoleacetic acid reacts with protein: possible participation of oxidation of serotonin and its metabolite in the development of atherosclerosis. Free Radic Biol Med. 2016;101:500–510. doi: 10.1016/j.freeradbiomed.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Kato Y, Ono S, Kitamoto N, Kettle AJ. Covalent modification of cytoskeletal proteins in neuronal cells by tryptamine-4,5-dione. Redox Biol. 2014;2:983–990. doi: 10.1016/j.redox.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai YL, Tomono S, Miyoshi N, Ohshima H. Inhibition of endothelial- and neuronal-type, but not inducible-type, nitric oxide synthase by the oxidized cholesterol metabolite secosterol aldehyde: implications for vascular and neurodegenerative diseases. J Clin Biochem Nutr. 2012;50:84–89. doi: 10.3164/jcbn.11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomono S, Miyoshi N, Shiokawa H, et al. Formation of cholesterol ozonolysis products in vitro and in vivo through a myeloperoxidase-dependent pathway. J Lipid Res. 2011;52:87–97. doi: 10.1194/jlr.M006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugamura K, Keaney JF Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruyama W, Shaomoto-Nagai M, Kato Y, Hisaka S, Osawa T, Naoi M. Role of lipid peroxide in the neurodegenerative disorders. Subcell Biochem. 2014;77:127–136. doi: 10.1007/978-94-007-7920-4_11. [DOI] [PubMed] [Google Scholar]
- 12.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81 (1 Suppl):317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 13.Ohnishi K, Ohkura S, Nakahata E, et al. Non-specific protein modifications by a phytochemical induce heat shock response for self-defense. PLoS One. 2013;8:e58641. doi: 10.1371/journal.pone.0058641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura Y, Miyoshi N. Electrophiles in foods: the current status of isothiocyanates and their chemical biology. Biosci Biotechnol Biochem. 2010;74:242–255. doi: 10.1271/bbb.90731. [DOI] [PubMed] [Google Scholar]
- 15.Ishii T, Mori T, Tanaka T, et al. Covalent modification of proteins by green tea polyphenol (–)-epigallocatechin-3-gallate through autoxidation. Free Radic Biol Med. 2008;45:1384–1394. doi: 10.1016/j.freeradbiomed.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Mattson MP, Cheng A. Neurohormetic phytochemicals: low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular Med. 2008;10:236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamio N, Suzuki T, Watanabe Y, Suhara Y, Osakabe N. A single oral dose of flavan-3-ols enhances energy expenditure by sympathetic nerve stimulation in mice. Free Radic Biol Med. 2016;91:256–263. doi: 10.1016/j.freeradbiomed.2015.12.030. [DOI] [PubMed] [Google Scholar]