Abstract
Previously, we showed that 0.5% quercetin simultaneously decreased serum homocysteine and glutathione (GSH) levels in rats. The aim of the present study was to investigate the effects of 0.5% quercetin on GSH metabolism, related enzymes and signal pathways in rats. Rats were fed the control diet and 0.5% quercetin-supplemented diet for 6 weeks. The results showed that quercetin reduced serum and hepatic content of GSH and the ratio of GSH and oxidized glutathione (GSSG), enhanced hepatic activity and mRNA expression of glutathione S-transferase (GST), inhibited hepatic activity and mRNA expression of glutamate cysteine ligase (GCL), and decreased hepatic glutathione reductase (GR) mRNA expression. Levels of phosphorylated p38 and extracellular signal-regulated kinase (ERK) 1/2 mitogen-activated protein kinases (MAPKs) increased, while that of nuclear factor E2-like 2 (Nrf2) protein decreased after quercetin treatment. However, no significant hepatotoxicity was noted. We concluded that quercetin treatment altered hepatic GSH metabolism by modulating GSH metabolic enzyme activities and mRNA expression in rats, and p38, ERK1/2 MAPKs, and Nrf2 were involved in modulating GSH metabolism-related enzymes.
Keywords: quercetin, glutathione, glutamate cysteine ligase, Nrf2, MAPKs
Introduction
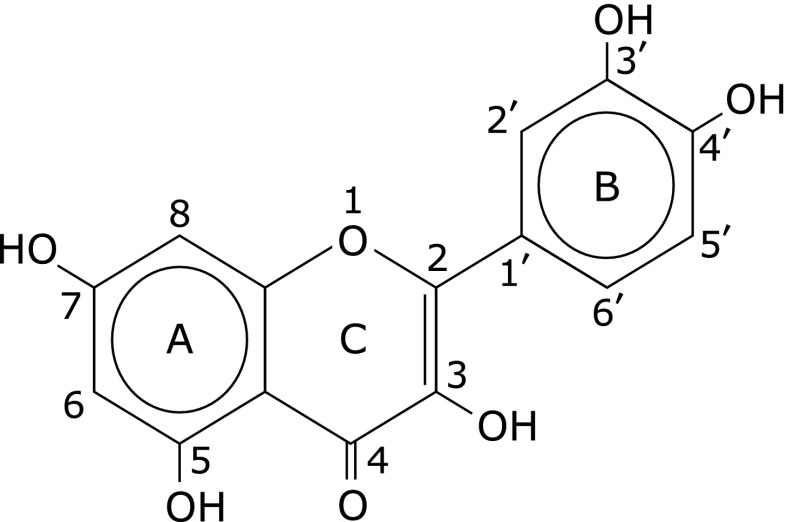
Quercetin (3,3',4',5,7-pentahydroxyflavone) is one of the common dietary polyphenols present in fruits, vegetables, beverages, and teas,(1–4) as well as in various medicinal herbs (Fig. 1).(5,6) Quercetin has both medicinal and nutritional values such as antioxidant, anti-inflammatory, immuno-stimulatory, and anti-chronic-disease properties.(7,8) Previously, we fed rats with diets containing 1% methionine in combination with 0.1%, 0.5% and 2.5% quercetin. It was found that 0.5% and 2.5% quercetin displayed a significant impact on glutathione (GSH) homeostasis, in which hepatic level of GSH was notably decreased. A significant hepatotoxic action was demonstrated when 2.5% quercetin was supplied.(9) Chen et al.(10) also found that 0.05% quercetin decreased serum GSH content, and increased GSH-Px activity in rats. However, the underlying mechanisms are not well clarified yet.
Fig. 1.
Chemical structure of quercetin.
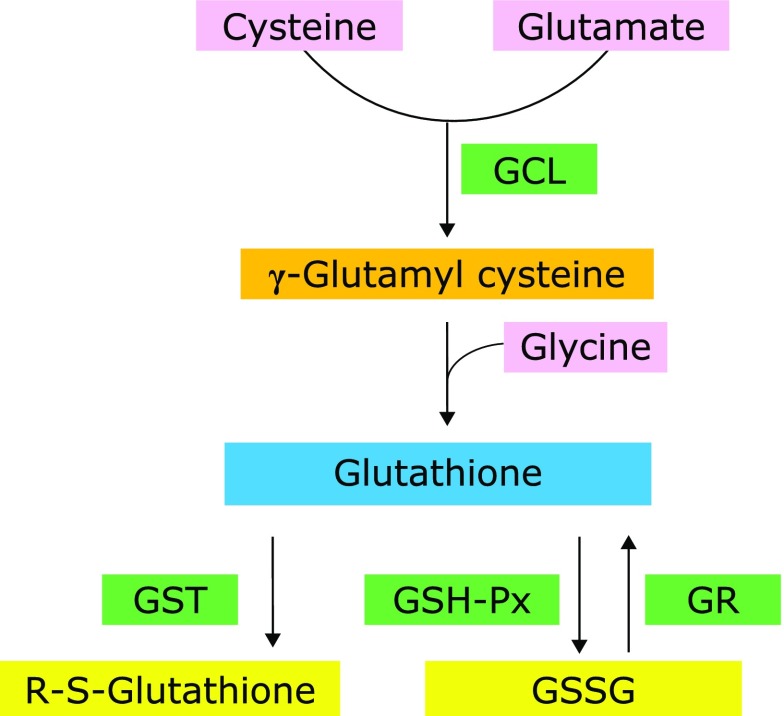
GSH, a tripeptide composed of glycine, cysteine, and glutamate, is the most abundant antioxidant in vivo and considered one of the most important determinants of the antioxidant defense system.(11) GSH quenches reactive hydroxyl free radicals and oxygen-centered free radicals by providing the protons for the antioxidant enzymes or conjugates with endogenous substances and xenobiotics by the reactions catalyzed by detoxication enzymes such as glutathione S-transferase (GST).(12,13) Glutathione peroxidase (GSH-Px) catalyzes reductive processes for lipid hydroperoxides, using GSH as an electron donor.(14) GST catalyzes the reaction of GSH with endogenous substances or diverse xenobiotics by forming mixed disulfides.(15) In those processes, GSH itself is converted to its oxidative form GSSG, which is regenerated to two GSH molecules by glutathione reductase (GR). Glutamate cysteine ligase (GCL), also known as γ-glutamylcysteine synthetase, catalyzes the first rate-limiting enzymatic step in GSH synthesis.(16) Among the four enzymes participating in GSH metabolism, GSH-Px and GST consume GSH, while GR and GCL are responsible for GSH regeneration and synthesis, respectively (Fig. 2).
Fig. 2.
Glutathione metabolic pathways. GCL, glutamate cysteine ligase; GST, glutathione S-transferase; GSH-Px, glutathione peroxidase; GR, glutathione reductase; GSSG, oxidized glutathione.
Two signaling pathways play critical roles in modulating the expression of GSH metabolic enzymes. Kelch-like erythroid cell-derived-associated protein 1 (Keap1)/nuclear factor E2-like 2 (Nrf2) is responsible for transcriptional activation of a large number of genes that are regulated by antioxidant response elements in their promoters. The Keap1/Nrf2 signal pathway has been demonstrated to play a role in regulating the expression of GSH metabolic enzymes.(17,18) Mitogen-activated protein kinases (MAPKs) comprise a family of signaling proteins that convert extracellular stimuli into intracellular transduction pathways via phosphorylation of a cascade of substrates. The MAPK signal pathway is also involved in the regulation of GSH metabolism.(19)
The liver plays a critical role in metabolic processes and is the principal detoxifying organ involved in the clearance of toxic chemicals and metabolic waste products from the body.(20–22) The liver also serves as an important organ in GSH metabolism and redox reactions depending partially on GSH availability.(15,16) We hypothesize that quercetin at high intake levels alters GSH homeostasis in the liver through modulating related signaling pathways. To test this hypothesis, we investigated the effects of 0.5% quercetin on hepatic GSH metabolic enzymes and explored the changes in the Keap1/Nrf2 and MAPKs pathways.
Materials and Methods
Animals, diets, and experimental protocol
Animal handling was performed according to the current Chinese legislation on the care and use of laboratory animals. The experimental protocol was approved by the Ethical Committee of the Department of Scientific Management of the institute. Twenty male Wistar rats, weighing 180–200 g, were purchased from the Laboratory Animal Center, Chinese Academy of Military Medical Sciences (Beijing, China) and housed individually in stainless-steel cages. The room temperature was between 22 and 24°C, and relative humidity between 40% and 60%, with 12 h light/dark cycles. Food and tap water were provided ad libitum. Dietary intake was recorded every day, and body weight once a week. After being acclimatized on a polyphenol-free semisynthetic diet (AIN-93 formula) for 5 days,(23) the rats were divided randomly into two groups based on body weight and maintained on the control diet (AIN-93 diet) and 0.5% quercetin (Sigma-Aldrich, St. Louis, MO) supplemented AIN-93 diet (0.5%Q), respectively, for 6 weeks. At the end of the experiment, all rats were fasted overnight, and blood samples were collected from the orbital plexus under ether anesthetization. The serum was separated and stored at −80°C. Liver tissues were sampled immediately, cleaned up in ice-cold saline, and frozen at −80°C until use.
Contents of quercetin and its methylated metabolites in the serum and liver
Quercetin and its methylated metabolites in the serum and liver were determined after enzymatic deconjugation by a high performance liquid chromatography (HPLC) procedure described previously by Chen et al.(24)
Serum activities of alanine aminotransferase and aspartate aminotransferase
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were detected using commercial kits purchased from the Biosino Bio-Technology and Science Inc. (Beijing, P.R. China).
Hepatic contents of GSH, GSSG, and malondialdehyde
The contents of hepatic GSH and GSSG were measured using assay kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu). Malondialdehyde (MDA) was assayed spectrophotometrically by the reaction with thiobarbituric acid.(25) Protein concentration was determined using a bicinchonininc acid (BCA) reagent kit (Sangon Biotech, Shanghai, China).
Hepatic activities of GSH-Px, GST, GR and GCL
Activities of hepatic GSH-Px, GST, GR and GCL were assayed by commercial kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The kits numbers of GSH-Px, GST, GR and GCL were A005, A004, A062 and A120, respectively. All measurements were performed strictly in line with the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction analysis
Hepatic mRNA expressions of GSH metabolic enzymes and extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK) were determined by quantitative real-time polymerase chain reaction (qPCR).(26) Total hepatic RNA was extracted using the TRIzol reagent (Roche, Basel, Switzerland). The first cDNA strand was synthesized using a cDNA Synthesis kit (Roche). qPCR was performed after cDNA synthesis in a FastStart Universal SYBR Green Master mix (Roche). The primers used for the genes studied are listed in Table 1. Finally, melting curve analysis was performed by slowly cooling the PCR mixture with simultaneous measurement of the SYBR Green I signal intensity by Takara PCR Thermal Cycler Dice Real Time System (Takara TP800; Takara, Tokyo, Japan). The Δ threshold cycle (Ct) method was used to evaluate the relative quantification, and β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as reference.
Table 1.
Sequences of the primers used in quantitative real-time polymerase chain reaction (qPCR) analysis
| Genes | Primers | |
|---|---|---|
| GSH-Px | F | 5'-AGGAGAATGGCAAGAATGAAGAGA-3' |
| R | 5'-GGAAGGTAAAGAGCGGGTGAG-3' | |
| GST | F | 5'-TTCTGACCTCTTTCCCTCTGCT-3' |
| R | 5'-CTTGAAAACCTTCCTTGCTTCTTC-3' | |
| GR | F | 5'-TCTACTCGACCGCCTTCACC-3' |
| R | 5'-GCCAACCACCTTCTCCTCTTT-3' | |
| GCL | F | 5'-CTGCACATCTACCACGCAGTCA-3' |
| R | 5'-ATCGCCGCCATTCAGTAACAA-3' | |
| JNK | F | 5'-GGATTTGGAGGAGCGAACTA-3' |
| R | 5'-TGACAGACGGCGAAGAGAC-3' | |
| ERK1 | F | 5'-ATCTGTGATTTTGGCCTTGC-3' |
| R | 5'-GCCCTTGGAGTTAAGCATGA-3' | |
| ERK2 | F | 5'-GCCGCGCTACACTAATCTCT-3' |
| R | 5'-AGGTCTGGTGCTCAAAAGGA-3' | |
| p38 | F | 5'-GGCACACTGATGACGAAATG-3' |
| R | 5'-CCACGGACCAAATATCCACT-3' | |
| β-Actin | F | 5'-GTCCCTCACCCTCCCAAAA-3' |
| R | 5'-GCTGCCTCAACACCTCAACCC-3' | |
| GAPDH | F | 5'-TGATTCTACCCACGGCAAGTT-3' |
| R | 5'-TGATGGGTTTCCCATTGATGA-3' |
GSH-Px, glutathione peroxidase; GST, glutathione S-transferase; GR, glutathione reductase; GCL, glutamate cysteine ligase; JNK, c-Jun NH2-terminal kinase; ERK1, extracellular signal-regulated kinase 1; ERK2, extracellular signal-regulated kinase 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. F, forward; R, reverse.
Western blotting analysis
Hepatic protein expression of Keap1, Nrf2, ERK1/2, phosphorylated-ERK (p-ERK), p38, p-p38, JNK and p-JNK were analyzed by western blotting.(26) The software Image-Pro Plus 6 (Rockville, MD) was used to analyze the protein bands, and the results were determined by the ratio of target protein density to that of control after being corrected by GAPDH. Anti-JNK, anti-p-JNK, anti-p38, anti-p-p38, anti-ERK1/2, anti-p-ERK1/2 and anti-Keap1 were purchased from Cell Signaling Technology Ltd. (Boston, MA), anti-Nrf2 from Abcam Ltd. (Cambridge, UK), anti-GAPDH from KangChen Ltd. (Shanghai, China), horseradish peroxidase (HRP)-labeled goat anti-mouse IgG and HRP-labeled goat anti-rabbit IgG from Bomeike Bio Ltd. (Beijing, China), and HRP-labeled donkey anti-goat IgG from Santa Cruz Biotechnology (Santa Cruz, CA).
Statistical analysis
Data are presented as means ± SD. The statistical analysis was performed using the SPSS 10.01 software (SPSS Inc., Chicago, IL). Student’s t test was performed to compare the differences between the control and the quercetin supplemented group. Differences between two groups were considered statistically significant at p<0.05.
Results
Dietary intake and body weight
The rats fed the quercetin-supplemented diet were similar to those in the control group in both food intake and body weight gain, indicating that 0.5% quercetin supplementation had no effect on dietary consumption and growth (data not shown).
Quercetin and its methylated metabolites
No peaks corresponding to quercetin aglycone or its methylated metabolites were detected on HPLC chromatograms for the serum and liver samples from the control rats. On the other hand, distinct peaks corresponding to quercetin aglycone, tamarixetin, and isorhamnetin were identified in the quercetin-supplemented group. The serum contents of quercetin aglycone, tamarixetin, and isorhamnetin were 52.32 ± 11.27, 253.58 ± 37.54, and 253.18 ± 37.48 µmol/L, and hepatic contents were 22.55 ± 3.46, 36.42 ± 4.21, and 20.21 ± 2.33 nmol/g protein, respectively.
Serum ALT and AST activity, and hepatic lipid peroxidation
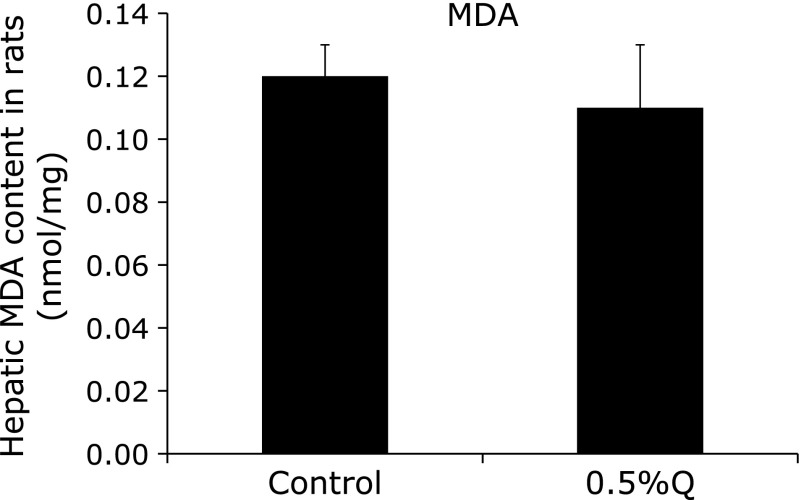
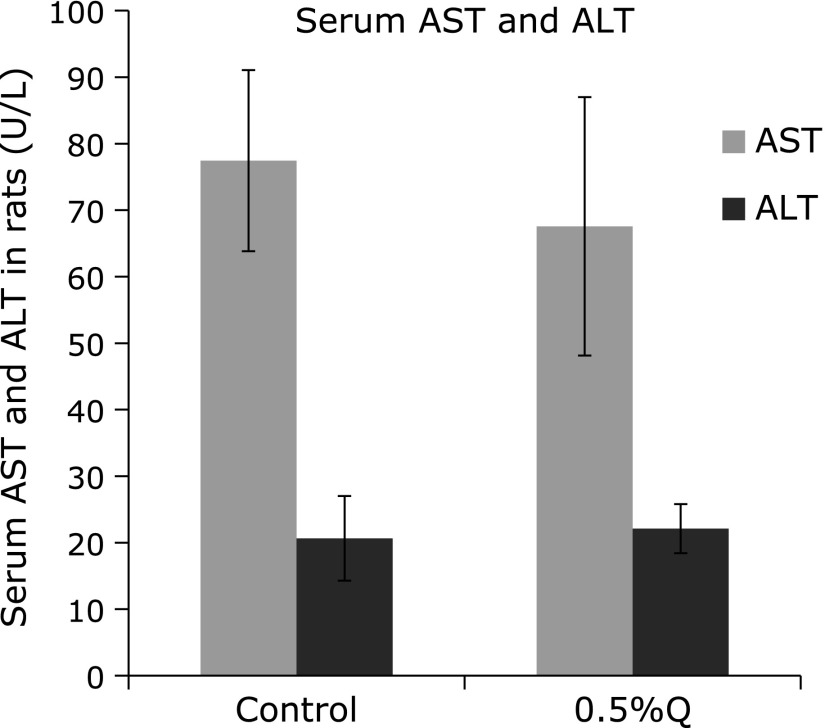
There was no significant difference between the control and the quercetin-supplemented group in hepatic MDA content and serum ALT and AST activities, suggesting that no significant hepatotoxicity was detected after 0.5% quercetin supplementation (Fig. 3 and 4).
Fig. 3.
Hepatic malondialdehyde (MDA) content in rats. MDA, malondialdehyde; 0.5%Q, 0.5% quercetin-supplemented group. Results are expressed as mean and standard deviation (n = 10).
Fig. 4.
Serum activities of aspartate aminotransferase (ALT) and alanine aminotransferase (AST) in rats. AST, aspartate aminotransferase; ALT, alanine aminotransferase; 0.5%Q, 0.5% quercetin-supplemented group. Results are expressed as mean and standard deviation (n = 10).
Serum and hepatic GSH, GSSG, and ratio of GSH/GSSG
The serum content of GSH and the ratio of GSH/GSSG decreased significantly after 0.5% quercetin supplementation compared to that in the control (p<0.05). The hepatic contents of GSH and GSSG and the ratio of GSH/GSSG also decreased significantly in the quercetin-supplemented group (p<0.05) (Table 2). Hepatic GSH was reduced by 48.7% and GSSG by 10.5%, indicating that hepatic GSH content declined more than GSSG content in response to the quercetin treatment.
Table 2.
Serum and hepatic contents of glutathione (GSH) and oxidized glutathione (GSSG), and the ratio of GSH to GSSG in rats
| Group | Serum |
Liver |
|||||
|---|---|---|---|---|---|---|---|
| GSH (µmol/g prot) | GSSG (µmol/g prot) | GSH/GSSG | GSH (µmol/g prot) | GSSG (µmol/g prot) | GSH/GSSG | ||
| Control | 45.62 ± 10.81 | 3.87 ± 0.91 | 12.27 ± 3.01 | 1.19 ± 0.23 | 1.91 ± 0.21 | 0.86 ± 0.19 | |
| 0.5%Q | 8.12 ± 3.23* | 3.55 ± 0.68 | 1.75 ± 0.68* | 0.61 ± 0.28* | 1.71 ± 0.14* | 0.47 ± 0.21* | |
Values are presented as mean ± standard deviation (n = 10). *p<0.05, compared with control. Serum GSH and GSH/GSSG, and hepatic GSH, GSSG and GSH/GSSG all decreased significantly, and and p value were 0.000, 0.000, 0.000, 0.023 and 0.000 respectively. GSH, glutathione; 0.5%Q, 0.5% quercetin-supplemented group.
Hepatic mRNA expression and activity of GSH-Px, GST, GCL and GR
An increase in GSH-Px activity and a decrease in GR activity in the liver were noted in the quercetin-supplemented rats but without statistical significance (Table 3). Hepatic activity and mRNA expression of GST significantly increased, whereas GCL expression significantly decreased in rats fed the quercetin-supplemented diet compared to that in rats fed the control diet (p<0.05). Hepatic mRNA expression of GR was significantly inhibited after quercetin treatment (p<0.05) (Table 4).
Table 3.
Hepatic activities of glutathione (GSH) metabolic enzymes in rats
| Group | GSH-Px (U/mg prot) | GST (U/mg prot) | GR (U/g prot) | GCL (U/mg prot) |
|---|---|---|---|---|
| Control | 847.38 ± 54.05 | 100.79 ± 6.72 | 37.91 ± 12.13 | 0.28 ± 0.05 |
| 0.5%Q | 886.10 ± 72.40 | 122.25 ± 5.37* | 22.84 ± 10.65 | 0.19 ± 0.02* |
Values are presented as mean ± standard deviation (n = 10). *p<0.05, compared with control. Hepatic activity of GST increased, while GCL decreased significantly after administration of 0.5% quercetin, and p value were 0.000 and 0.002 respectively. GSH-Px, glutathione peroxidase; GST, glutathione S-transferase; GR, glutathione reductase; GCL, glutamate cysteine ligase; 0.5%Q, 0.5% quercetin-supplemented group.
Table 4.
Hepatic mRNA expression of glutathione (GSH) metabolic enzymes in rats
| Group | GSH-Px | GST | GR | GCL |
|---|---|---|---|---|
| Control | 1.01 ± 0.02 | 0.99 ± 0.13 | 0.97 ± 0.06 | 0.91 ± 0.17 |
| 0.5%Q | 1.17 ± 0.16 | 2.49 ± 0.25* | 0.17 ± 0.06* | 0.37 ± 0.06* |
Values are presented as mean ± standard deviation (n = 3). *p<0.05, compared with control. Hepatic mRNA expression of GST increased, while GR and GCL decreased significantly after administration of 0.5% quercetin, and p value were 0.001, 0.000 and 0.006 respectively. GSH-Px, glutathione peroxidase; GST, glutathione S-transferase; GR, glutathione reductase; GCL, glutamate cysteine ligase; 0.5%Q, 0.5% quercetin-supplemented group.
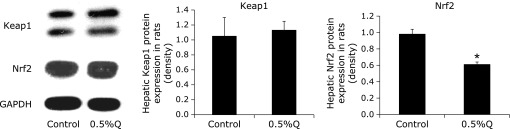
Keap1 and Nrf2 protein expression
The hepatic protein expression of Keap1 was slightly increased in the 0.5% quercetin-supplemented group, whereas Nrf2 level was significantly lower in the 0.5% quercetin-supplemented group than in the control group (p<0.05) (Fig. 5).
Fig. 5.
Hepatic Kelch-like erythroid cell-derived-associated protein 1 (Keap1) and nuclear factor E2-like 2 (Nrf2) protein expression in rat liver (n = 3, x̄ ± s). *Significantly different from the control group.
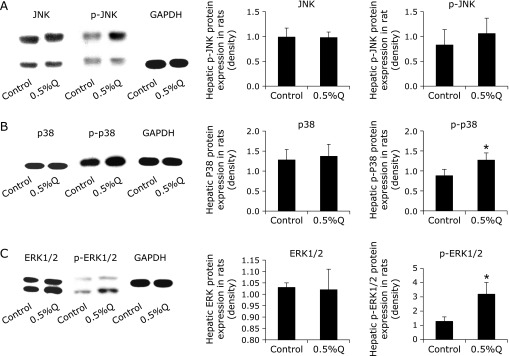
Hepatic ERK, p38, and JNK mRNA and protein expression
Hepatic p-p38 and p-ERK protein expressions were increased significantly in the 0.5% quercetin-supplemented group. Hepatic p-JNK level was also increased but no statistical difference was detected. No remarkable change in JNK, p38, and ERK mRNA and protein expressions in the liver was found after quercetin supplementation (Table 5, Fig. 6).
Table 5.
Hepatic mRNA expression of mitogen-activated protein kinases (MAPKs) in rats.
| Group | ERK1 | ERK2 | JNK | p38 |
|---|---|---|---|---|
| Control | 0.88 ± 0.13 | 1.02 ± 0.29 | 1.06 ± 0.28 | 1.01 ± 0.11 |
| 0.5%Q | 0.99 ± 0.23 | 0.96 ± 0.16 | 1.03 ± 0.49 | 1.01 ± 0.31 |
Values are presented as mean ± SD (n = 3). ERK1, extracellular signal-regulated kinase 1; ERK2, extracellular signal-regulated kinase 2; JNK, c-Jun N-terminal kinase.
Fig. 6.
Hepatic protein expression of MAPKs. (A) Hepatic c-Jun N-terminal kinase (JNK) and phosphorylated-JNK (p-JNK); (B) hepatic p38 and phosphorylated p38 (p-p38) protein expression in rat liver; (C) hepatic extracellular signal-regulated kinase (ERK)1/2 and phosphorylated-ERK1/2 (p-ERK1/2) (n = 3, x̄ ± s). Con: control; 0.5%Q: 0.5% quercetin group. *Significantly different from the control group.
Discussion
In our experiment, hepatic GSH content was significant decreased in rats treated by 0.5% quercetin. This is consistent with the data reported by Choi et al.(27,28) Metodiewa et al.(29) showed that quercetin altered GSH homeostasis directly. The C4-keto moiety and C2=C3 double bond in quercetin structure facilitated the formation of o-quinoid type metabolites such as quinones and quinone methides, in which quercetin initially is oxidized to an o-quinone, which is rapidly isomerized to quinone methides. (30–32) These quinoids are trapped by GSH, and isomeric mixtures of quinoid GSH conjugates are formed. Simultaneously, a decrease in GSH content occurs.(33) However, some studies showed that quercetin could impact GSH homeostasis via other mechanisms. For example, Choi et al.(27) demonstrated that quercetin treatment reduced hepatic GR activity by 34% in rats, which also contributed to the decline of GSH content.
In the present study, we confirmed that 0.5% quercetin treatment induced a significant decrease in the contents of hepatic GSH and GSSG, as well as the ratio of GSH/GSSG. In addition, the decline of GSH level was more significant than that of GSSG. We further investigated the enzymatic activity and mRNA expression of GCL. The results showed that hepatic activity and mRNA expression of GCL were decreased after exposure to quercetin, indicating that GSH synthesis was suppressed. We also found that mRNA expression of GR was significantly reduced in response to quercetin treatment. Since GCL and GR are responsible for GSH synthesis and regeneration, the inhibition of those two enzymes will contribut to the decrease of hepatic GSH level after quercetin treatment. We also showed that hepatic GST activity and mRNA expression were enhanced after quercetin treatment. Similar results were reported by others,(34–38) showing that the administration of quercetin increased the activity of GST in rats, silkworms, and cultured cells. Since GST catalyzes the conjugation of GSH with endogenous compounds or xenobiotics, increased GST activity will require more GSH as a substrate, leading to the consumption of hepatic GSH.
Evidences have showed that GSH depletion was accompanied with oxidative stress even without significant oxidative stimulation. Vaziri et al.(39) found a 3-fold decrease in tissue GSH content after exposure to l-buthionine sulphoximine (BSO), a selective inhibitor of gamma glutamyl cysteine synthetase, and oxidative stress was resulted in Sprague-Dawley rats. Beddowes et al.(40) demonstrated that 8-oxodeoxyguanosine and malondialdehyde deoxyguanosine adducts were significantly increased in HepG2 cells after being treated by BSO to deplete GSH. Armstrong et al.(41) showed that after BSO treatment, there was an early decline in cellular GSH, followed by an increase in reactive oxygen species production in human B lymphoma cell line. Besides BSO, other reagents such as vinylidene chloride, diethylmaleate and doxycycline are also used to induce GSH depletion and oxidative stress is resulted. For example, incubations of liver homogenates from glutathione-depleted mice and rats after exposure to vinylidene chloride and diethylmaleate resulted spontaneously in a large increase of the lipid peroxidation.(42) Down-regulation of glutamyl-cysteine synthetase gene expression by doxycycline resulted in a reduction in mitochondrial GSH levels, which led to increased oxidative stress in PC12 cells.(43) In previous experiments, we found that serum MDA was significantly increased in rats treated by 0.5%–2.5% quercetin for 6 weeks.(9,10) However, in the present study, we do not find significant changes in hepatic MDA level after 0.5% quercetin administration, although serum and hepatic GSH content was distinctly decreased. Thereby, we think that 0.5% quercetin may be a critical level in inducing a pro-oxidant effect. Serum ALT and AST levels also did not increase significantly in response to quercetin treatment. Thereby, 0.5% quercetin supplementation did not induce a significant toxic action in the liver. Previously, it was demonstrated that the administration of quercetin markedly increased the amount of α-tocopherol in rat plasma and liver.(27,44) The adverse effects of quercetin on GSH homeostasis may be compensated by increased α-tocopherol level, because α-tocopherol is also one of the critical antioxidants in vivo. However, the content of hepatic α-tocopherol was not measured in this study. It should be mentioned that quercetin is extensively metabolized after absorption,(45,46) which may limit the action of quercetin on GSH metabolism. In this study, we detected the contents of quercetin and its methylated derivatives in the serum and liver. The results confirmed that quercetin was present largely in the form of its methylated derivatives in the serum and liver. Although quercetin metabolites is relatively weaker in antioxidant capacity,(47) it have been demonstrated that part of quercetin metabolites could be transformed to quercetin aglycon and play an antioxidant role in tissues.(48,49) Therefore, it is possible that quercetin and its metabolites in the tissues can play an antioxidant role and make up partly for the decline of GSH content.
Since the GSH homeostasis is tightly controlled by several upstream signaling pathways, we further investigated the change in the Keap1/Nrf2 and MAPKs pathways. Nrf2 expression plays an important role in modulating the expression of some GSH-related enzymes, especially GCL and GR.(50,51) Nrf2 down-expression blocked the GCL expression induced by isorhamnetin.(52) Down-expression of Nrf2 also decreased GR activity and mRNA expression, and reduced GSH content in fish gill with amino acid phenylalanine deficiency.(53) Moreover, Nrf2 knockout led to a significantly reduction in hepatic GST expression of mRNA, protein, and catalytic activity in mice.(54) In this study, we found that hepatic Nrf2 protein expression was significantly decreased, which was correlated with decreased activities and mRNA expressions of GCL and GR in 0.5% quercetin-treatment group. Thereby, it is likely that down-regulated Keap1/Nrf2 signaling pathway contributes to the change in GSH homeostasis after quercetin exposure. The mammalian MAPK family consists of ERK1/2, p38 and JNK.(55,56) It had been demonstrated that quercetin increased phosphorylation of p38 and ERK1/2 in BV-2 microglial cells and induced phosphorylation of p38 and affected GSH homeostasis in HepG2 cells.(57,58) Quercetin derivatives such as isorhamnetin and rutin were also effective in increasing ERK1/2 phosphorylation.(19,52) In the present study, the hepatic protein expressions of p-ERK1/2 and p-p38 were increased after quercetin treatment, indicating that quercetin altered GSH homeostasis partly via MAPKs signal pathway.
Conclusion
In conclusion, this study demonstrated that 0.5% quercetin treatment reduced hepatic GSH content by enhancing the activity and mRNA expression of GST, and inhibiting the activity and mRNA expression of GCL. This action is associated with the changes of Keap1/Nrf2 and MAPKs signal pathways. More profound changes in GSH homeostasis may be manifested when quercetin is administered at higher doses. Thereby, the safety of quercetin administration still needs to be further investigated.
Acknowledgments
This work was supported financially by grants from Tianjin Research Program of Application Foundation and Advanced Technology (Project no. 13JCQNJC11800) and the National Natural Science Foundation of China (Project no. 81372988, 81172656).
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BCA
bicinchonininc acid
- BSO
l-buthionine sulphoximine
- ERK1/2
extracellular signal-regulated kinase 1/2
- GCL
glutamate cysteine ligase
- GR
glutathione reductase
- GSH
glutathione
- GSH-Px
glutathione peroxidase
- GST
glutathione S-transferase
- HPLC
high performance liquid chromatography
- JNK
c-Jun NH2-terminal kinase
- Keap1
Kelch like ECH-associated protein 1
- MAPKs
mitogen-activated protein kinases
- MDA
malondialdehyde
- Nrf2
nuclear factor erythroid 2-like 2
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Borghetti GS, Carini JP, Honorato SB, Ayala AP, Moreira JCF, Bassani VL. Physicochemical properties and thermal stability of quercetin hydrates in the solid state. Thermochim Acta. 2012;539:109–114. [Google Scholar]
- 2.Ghosh D, Ghosh S, Sarkar S, et al. Quercetin in vesicular delivery systems: evaluation in combating arsenic-induced acute liver toxicity associated gene expression in rat model. Chem Biol Interact. 2010;186:61–71. doi: 10.1016/j.cbi.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 2008;11:733–740. doi: 10.1097/MCO.0b013e32831394b8. [DOI] [PubMed] [Google Scholar]
- 4.Sampson L, Rimm E, Hollman PC, de Vries JH, Katan MB. Flavonol and flavone intakes in US health professionals. J Am Diet Assoc. 2002;102:1414–1420. doi: 10.1016/s0002-8223(02)90314-7. [DOI] [PubMed] [Google Scholar]
- 5.Kelly GS. Quercetin. Monograph. Altern Med Rev. 2011;16:172–194. [PubMed] [Google Scholar]
- 6.Wu H, Chen M, Fan Y, Elsebaei F, Zhu Y. Determination of rutin and quercetin in Chinese herbal medicine by ionic liquid-based pressurized liquid extraction-liquid chromatography-chemiluminescence detection. Talanta. 2012;88:222–229. doi: 10.1016/j.talanta.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Zhang L, Lu S. Evaluation of antioxidant and immunity activities of quercetin in isoproterenol-treated rats. Molecules. 2012;17:4281–4291. doi: 10.3390/molecules17044281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelmoaty MA, Ibrahim MA, Ahmed NS, Abdelaziz MA. Confirmatory studies on the antioxidant and antidiabetic effect of quercetin in rats. Indian J Clin Biochem. 2010;25:188–192. doi: 10.1007/s12291-010-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng B, Gao W, Wei J, Pu L, Tang Z, Guo C. Quercetin increases hepatic homocysteine remethylation and transsulfuration in rats fed a methionine-enriched diet. Biomed Res Int. 2015;2015:815210. doi: 10.1155/2015/815210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M, Gao W, Pu L, Wei J, Guo C, Kang Y. Effects of quercetin on serum glutathione content and activity of related enzymes in rats. Acta Nutr Sin. 2015;37:279–282. (in Chinese) [Google Scholar]
- 11.Hou Y, Wang Y, Wang H, Xu Y. Induction of glutathione synthesis in human hepatocytes by acute and chronic arsenic exposure: differential roles of mitogen-activated protein kinases. Toxicology. 2014;325:96–106. doi: 10.1016/j.tox.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Raheem IT, Abdel-Ghany AA, Mohamed GA. Protective effect of quercetin against gentamicin-induced nephrotoxicity in rats. Biol Pharm Bull. 2009;32:61–67. doi: 10.1248/bpb.32.61. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002;64:1019–1026. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- 14.Harris ED. Regulation of antioxidant enzymes. FASEB J. 1992;6:2675–2683. doi: 10.1096/fasebj.6.9.1612291. [DOI] [PubMed] [Google Scholar]
- 15.Strange RC, Jones PW, Fryer AA. Glutathione S-transferase: genetics and role in toxicology. Toxicol Lett. 2000;112–113:357–363. doi: 10.1016/s0378-4274(99)00230-1. [DOI] [PubMed] [Google Scholar]
- 16.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 17.Shen G, Kong AN. Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharm Drug Dispos. 2009;30:345–355. doi: 10.1002/bdd.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 19.Moghbelinejad S, Nassiri-Asl M, Farivar TN, et al. Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicol Lett. 2014;224:108–113. doi: 10.1016/j.toxlet.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee R, Mitra A. An overview of effective therapies and recent advances in biomarkers for chronic liver diseases and associated liver cancer. Int Immunopharmaco. 2015;24:335–345. doi: 10.1016/j.intimp.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Invernizzi P. Liver auto-immunology: the paradox of autoimmunity in a tolerogenic organ. J Autoimmun. 2013;46:1–6. doi: 10.1016/j.jaut.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Raschzok N, Sallmon H, Pratschke J, Sauer IM. MicroRNAs in liver tissue engineering - New promises for failing organs. Adv Drug Deliver Rev. 2015;88:67–77. doi: 10.1016/j.addr.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 24.Chen CY, Milbury PE, Lapsley K, Blumberg JB. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. J Nutr. 2005;135:1366–1373. doi: 10.1093/jn/135.6.1366. [DOI] [PubMed] [Google Scholar]
- 25.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Xie Z, Gao W, Pu L, Wei J, Guo C. Quercetin regulates hepatic cholesterol metabolism by promoting cholesterol-to-bile acid conversion and cholesterol efflux in rats. Nutr Res. 2016;36:271–279. doi: 10.1016/j.nutres.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Choi EJ, Chee KM, Lee BH. Anti- and prooxidant effects of chronic quercetin administration in rats. Eur J Pharmacol. 2003;482:281–285. doi: 10.1016/j.ejphar.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 28.Choi EJ, Lee BH, Lee K, Chee KM. Long-term combined administration of quercetin and daidzein inhibits quercetin-induced suppression of glutathione antioxidant defenses. Food Chem Toxicol. 2005;43:793–798. doi: 10.1016/j.fct.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Metodiewa D, Jaiswal AK, Cenas N, Dickancaité E, Segura-Aguilar J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radical Bio Med. 1999;26:107–116. doi: 10.1016/s0891-5849(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 30.Awad HM, Boersma MG, Vervoort J, Rietjens IM. Peroxidase-catalyzed formation of quercetin quinone methide-glutathione adducts. Arch Biochem Biophys. 2000;378:224–233. doi: 10.1006/abbi.2000.1832. [DOI] [PubMed] [Google Scholar]
- 31.Rietjens IM, Al Huseiny, Boersma MG. Flavonoids and alkenylbenzenes: new concepts in bioactivation studies. Chem Biol Interact. 2011;192:87–95. doi: 10.1016/j.cbi.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Lee-Hilz YY, Boerboom AM, Westphal AH, Berkel WJ, Aarts JM, Rietjens IM. Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem Res Toxicol. 2006;19:1499–1505. doi: 10.1021/tx060157q. [DOI] [PubMed] [Google Scholar]
- 33.Bolton JL. Quinone methide bioactivation pathway: contribution to toxicity and/or cytoprotection? Curr Org Chem. 2014;18:61–69. doi: 10.2174/138527281801140121123046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olayinka ET, Ore A, Ola OS, Adeyemo OA. Protective effect of quercetin on melphalan-induced oxidative stress and impaired renal and hepatic functions in rat. Chemother Res Pract. 2014;2014:936526. doi: 10.1155/2014/936526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padma VV, Baskaran R, Roopesh RS, Poornima P. Quercetin attenuates lindane induced oxidative stress in Wistar rats. Mol Biol Rep. 2012;39:6895–6905. doi: 10.1007/s11033-012-1516-0. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Miao M, Zhang Y, et al. Quercetin ameliorates liver injury induced with Tripterygium glycosides by reducing oxidative stress and inflammation. Can J Physiol Pharm. 2015;93:427–433. doi: 10.1139/cjpp-2015-0038. [DOI] [PubMed] [Google Scholar]
- 37.Zhang YE, Ma HJ, Feng DD, et al. Induction of detoxification enzymes by quercetin in the silkworm. J Econ Entomol. 2012;105:1034–1042. doi: 10.1603/ec11287. [DOI] [PubMed] [Google Scholar]
- 38.Odenthal J, van Heumen BW, Roelofs HM, et al. The influence of curcumin, quercetin, and eicosapentaenoic acid on the expression of phase II detoxification enzymes in the intestinal cell lines HT-29, Caco-2, HuTu 80, and LT97. Nutr Cancer. 2012;64:856–863. doi: 10.1080/01635581.2012.700994. [DOI] [PubMed] [Google Scholar]
- 39.Vaziri ND, Wang XQ, Oveisi F, Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36:142–146. doi: 10.1161/01.hyp.36.1.142. [DOI] [PubMed] [Google Scholar]
- 40.Beddowes EJ, Faux SP, Chipman JK. Chloroform, carbon tetrachloride and glutathione depletion induce secondary genotoxicity in liver cells via oxidative stress. Toxicology. 2003;187:101–115. doi: 10.1016/s0300-483x(03)00058-1. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong JS, Steinauer KK, Hornung B, et al. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002;9:252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- 42.Younes M, Siegers CP. Lipid peroxidation as a consequence of glutathione depletion in rat and mouse liver. Res Commun Chem Pathol Pharmacol. 1980;27:119–128. [PubMed] [Google Scholar]
- 43.Jha N, Jurma O, Lalli G, et al. Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity. Implications for Parkinson’s disease. J Biol Chem. 2000;275:26096–26101. doi: 10.1074/jbc.M000120200. [DOI] [PubMed] [Google Scholar]
- 44.Frank J, Budek A, Lundh T, et al. Dietary flavonoids with a catechol structure increase alpha-tocopherol in rats and protect the vitamin from oxidation in vitro. J Lipid Res. 2006;47:2718–2725. doi: 10.1194/jlr.M600291-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Moalin M, van Strijdonck GP, Bast A, Haenen GR. Competition between ascorbate and glutathione for the oxidized form of methylated quercetin metabolites and analogues: tamarixetin, 4'O-methylquercetin, has the lowest thiol reactivity. J Agric Food Chem. 2012;60:9292–9297. doi: 10.1021/jf302068v. [DOI] [PubMed] [Google Scholar]
- 46.Bakır T, Sönmezoğlu I, Imer F, Apak R. Antioxidant/prooxidant effects of α-tocopherol, quercetin and isorhamnetin on linoleic acid peroxidation induced by Cu(II) and H2O2. Int J Food Sci Nutr. 2014;65:226–234. doi: 10.3109/09637486.2013.845654. [DOI] [PubMed] [Google Scholar]
- 47.Manach C, Morand C, Crespy V, et al. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998;426:331–336. doi: 10.1016/s0014-5793(98)00367-6. [DOI] [PubMed] [Google Scholar]
- 48.Yeh SL, Lin YC, Lin YL, Li CC, Chuang CH. Comparing the metabolism of quercetin in rats, mice and gerbils. Eur J Nutr. 2016;55:413–422. doi: 10.1007/s00394-015-0862-9. [DOI] [PubMed] [Google Scholar]
- 49.O'Leary KA, Day AJ, Needs PW, Sly WS, O'Brien NM, Williamson G. Flavonoid glucuronides are substrates for human liver beta-glucuronidase. FEBS Lett. 2001;503:103–106. doi: 10.1016/s0014-5793(01)02684-9. [DOI] [PubMed] [Google Scholar]
- 50.de Oliveira MR, Schuck PF, Bosco SMD. Tanshinone I induces mitochondrial protection through an Nrf2-dependent mechanism in paraquat-treated human neuroblastoma SH-SY5Y cells. Mol Neurobiol. 2017;54:4597–4608. doi: 10.1007/s12035-016-0009-x. [DOI] [PubMed] [Google Scholar]
- 51.Ramprasath T, Selvam GS. Potential impact of genetic variants in Nrf2 regulated antioxidant genes and risk prediction of diabetes and associated cardiac complications. Curr Med Chem. 2013;20:4680–4693. doi: 10.2174/09298673113209990154. [DOI] [PubMed] [Google Scholar]
- 52.Yang JH, Shin BY, Han JY, et al. Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes. Toxicol Appl Pharmacol. 2014;274:293–301. doi: 10.1016/j.taap.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 53.Feng L, Li W, Liu Y, et al. Protective role of phenylalanine on the ROS-induced oxidative damage, apoptosis and tight junction damage via Nrf2, TOR and NF-κB signalling molecules in the gill of fish. Fish Shellfish Immunol. 2017;60:185–196. doi: 10.1016/j.fsi.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 54.Anwar-Mohamed A, Degenhardt OS, El Gendy MA, Seubert JM, Kleeberger SR, El-Kadi AO. The effect of Nrf2 knockout on the constitutive expression of drug metabolizing enzymes and transporters in C57Bl/6 mice livers. Toxicol In Vitro. 2011;25:785–795. doi: 10.1016/j.tiv.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Owens DM, Keyse SM. Differential regulation of MAP kinase signaling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Dong C. Regulatory mechanisms of mitogen-activated kinase signaling. Cell Mol Life Sci. 2007;64:2771–2789. doi: 10.1007/s00018-007-7012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun GY, Chen Z, Jasmer KJ, et al. Quercetin attenuates inflammatory responses in BV-2 microglial cells: role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS One. 2015;10:e0141509. doi: 10.1371/journal.pone.0141509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: involvement of p38. Chem Biol Interact. 2012;195:154–164. doi: 10.1016/j.cbi.2011.12.005. [DOI] [PubMed] [Google Scholar]