Abstract
To clarify the clinical significance of the redox-controlling effects of Kampo, a traditional Japanese herbal medicine, we determined the scavenging activities of various reactive oxygen species in clinically used Kampo formulae using an electron spin resonance-based technique. Formulae containing Rhei Rhizoma (i.e., mashiningan and daiobotanpito) showed high scavenging activity against the alkoxyl radical, and crude extract quantity was significantly correlated with scavenging activity. Hydroxyl radical scavenging activity was positively correlated with the quantity of Zingiberis Rhizoma. Strong hydroxyl radical scavenging activity was also found in formulae containing both Bupleuri Radix and Scutellariae Radix, a widely used anti-inflammatory combination. Formulae containing a clinically common combination of Scutellariae Radix, Coptidis Rhizoma, and Phellodendri Cortex induced high superoxide scavenging activity. Singlet oxygen scavenging activity was high in formulae containing Bupleuri Radix and Glycyrrhizae Radix. In contrast, formulae containing Rehmanniae Radix showed generally low reactive oxygen species scavenging activities, and the quantity of Rehmanniae Radix was negatively correlated with hydroxyl radical and singlet oxygen scavenging activities. These results indicate that the antioxidative effects of Kampo formulae are not uniform but complexly varied against multiple reactive oxygen species. Some formulae have almost no antioxidant effects but may act as pro-oxidants.
Keywords: Kampo, traditional Japanese herbal medicine; hydroxyl radical; multiple radical scavenging activity; Bupleuri Radix; Rehmanniae Radix
Introduction
Traditional herbal medicines have attracted much attention as complementary treatments. Kampo, a traditional Japanese herbal medicine, is an established system of alternative or complementary medicine that has demonstrated beneficial treatment effects and reduced side effects.(1,2) Multiple reports have revealed antioxidative effects for Kampo formulae or other herbal extract prescriptions, and the antioxidative potencies of Kampo formulae are now pharmacologically recognized as an important therapeutic mechanism.(3–8) However, most studies have evaluated antioxidative mechanisms using a few substances or stable end products of oxidative reactions. In contrast to the substantial developments in the analysis of post-cellular reactions, the “upstream” aspect of oxidative stress [i.e., the identification of reactive oxygen species (ROS) that stimulate oxidative stress reactions, or interactions among ROS that generate oxidative stimulators] remains largely unclear.(9,10) Because ROS are not uniform, and individual ROS show specific characteristics during in vivo reactions,(11) analysis of multiple ROS is clearly necessary. To address this problem, we have developed an electron spin resonance (ESR)-based method to analyze multiple ROS scavenging activities (multiple free radical scavenging activities: MULTIS) in biological samples and have used it to analyze the upstream aspect of oxidative stress-related reactions.(12,13)
This study aimed to investigate the multidimensional antioxidative profiles of Kampo formulae by analyzing their relations to clinical effects, and to provide explanations of their Kampo or oriental medical therapeutic effects using Western medical terms. We determined the multiple ROS scavenging activities of 48 clinically used Kampo prescription formulae for prescription. The measured ROS were hydroxyl radical (•OH), alkoxyl radical (RO•, tert-BuO•), alkylperoxyl radical (ROO•, tert-BuOO•), superoxide (O2•−), and singlet oxygen (1O2). We also analyzed the effects of crude drugs on the antioxidative activities of Kampo formulae. Our findings demonstrate previously unknown antioxidative effects in multiple Kampo formulae.
Materials and Methods
Kampo extract preparation
We used 48 Kampo extract prescription formulae widely used in Japan; these are shown in Table 1 and 2. The Kampo extract preparations were donated by Tsumura Co., Ltd (Tokyo, Japan). All preparations were clinical grade extract granules. In clinical use, these extract granules are easily dissolved in approximately 200 ml of hot water or orally administrated as they are. In this study, 2.5 g of the Kampo extract granules were dissolved in ultrapurified water at 90°C and subjected to ESR measurement in 10% aqueous solutions. The relations between ROS scavenging activity and quantity of crude drug components for Kampo preparations were statistically analyzed.
Table 1.
List of Kampo formulae and their abbreviations
| Name | Abbr. | Name | Abbr. | Name | Abbr. |
|---|---|---|---|---|---|
| bakumondoto | BAK | kakkontokasenkyushin'i | KTSS | saikokeishito | SAKT |
| bofutsushosan | BTS | kamikihito | KKT | saikoseikanto | SSET |
| boiohito | BOT | kamishoyosan | KSS | saireito | SRT |
| daikenchuto | DKT | keigairengyoto | KRT | shimbuto | SIT |
| daiobotanpito | DBT | keishibukuryogan | KBG | shimotsuto | SMT |
| daiokanzoto | DKZT | keishikajutsubuto | KSTJB | shokenchuto | SHKT |
| daisaikoto | DST | keishikaryukotsuboreito | KSTRB | shosaikoto | SSK |
| goreisan | GRS | keishito | KST | shoseiryuto | SST |
| goshajinkigan | GJG | maoto | MT | tokiinshi | TKI |
| goshuyuto | GSY | mashiningan | MNG | tokishakuyakusan | TSS |
| hachimijiogan | HJG | ninjinto | NT | tokishigyakukagoshuyushokyoto | TSGST |
| hangekobokuto | HKT | orengedokuto | OGT | tsudosan | TDS |
| hangeshoshinto | HST | rikkunshito | RKT | unseiin | USI |
| hochuekkito | HET | rokumigan | RJG | yokuininto | YT |
| jumihaidokuto | JHT | saibokuto | SBT | yokukansan | YKS |
| juzentaihoto | JTT | saikokaryukotsuboreito | SRB | ||
| kakkonto | KT | saikokeishikankyoto | SAKK |
Abbr.: abbreviation
Table 2.
List of Kampo formulae and their composition of crude extracts. The crude amounts are indicated as daily dose
| Name | Major components (gram per daily dose) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviation | Scutellariae Radix | Phellodendri Cortex | Coptidis Rhizoma | Zingiberis Processum Rhizoma | Glycyrrhizae Radix | Bupleuri Radix | Rehmanniae Radix | Paeoniae Radix | Zingiberis Rhizoma | Rhei Rhizoma | Atractylodes Lancea Rhizome | Processi Aconiti Radix | Ephedrae Herba | |
| bakumondoto | BAK | 2 | ||||||||||||
| bofutsushosan | BTS | 2 | 2 | 1.2 | 0.3 | 1.5 | 1.2 | |||||||
| boiohito | BOT | 1.5 | 1 | 3 | ||||||||||
| daikenchuto | DKT | 5 | ||||||||||||
| daiobotanpito | DBT | 2 | ||||||||||||
| daiokanzoto | DKZT | 2 | 4 | |||||||||||
| daisaikoto | DST | 3 | 6 | 3 | 1 | 1 | ||||||||
| goreisan | GRS | 3 | ||||||||||||
| goshajinkigan | GJG | 5 | 1 | |||||||||||
| goshuyuto | GSY | 1.5 | ||||||||||||
| hachimijiogan | HJG | 6 | 0.5 | |||||||||||
| hangekobokuto | HKT | 1 | ||||||||||||
| hangeshoshinto | HST | 2.5 | 1 | 2.5 | 2.5 | |||||||||
| hochuekkito | HET | 1.5 | 2 | 0.5 | 4 | |||||||||
| jumihaidokuto | JHT | 1 | 3 | 1 | ||||||||||
| juzentaihoto | JTT | 1.5 | 3 | 3 | 3 | |||||||||
| kakkonto | KT | 2 | 2 | 2 | 3 | |||||||||
| kakkontokasenkyushin'i | KTSS | 2 | 2 | 2 | 3 | |||||||||
| kamikihito | KKT | 1 | 3 | 1 | 3 | |||||||||
| kamishoyosan | KSS | 1.5 | 3 | 3 | 1 | 3 | ||||||||
| keigairengyoto | KRT | 1.5 | 1.5 | 1.5 | 1 | 1.5 | 1.5 | 1.5 | ||||||
| keishibukuryogan | KBG | 3 | ||||||||||||
| keishikajutsubuto | KSTJB | 2 | 4 | 1 | 4 | 0.5 | ||||||||
| keishikaryukotsuboreito | KSTRB | 2 | 4 | 1.5 | ||||||||||
| keishito | KST | 2 | 4 | 1.5 | ||||||||||
| maoto | MT | 1.5 | 5 | |||||||||||
| mashiningan | MNG | 2 | 4 | |||||||||||
| ninjinto | NT | 3 | 3 | 3 | ||||||||||
| orengedokuto | OGT | 3 | 1.5 | 2 | ||||||||||
| rikkunshito | RKT | 1 | 0.5 | 4 | ||||||||||
| rokumigan | RJG | 5 | ||||||||||||
| saibokuto | SBT | 3 | 2 | 7 | 1 | |||||||||
| saikokaryukotsuboreito | SRB | 2.5 | 5 | 1 | 1 | |||||||||
| saikokeishikankyoto | SAKK | 3 | 2 | 2 | 6 | |||||||||
| saikokeishito | SAKT | 2 | 2 | 5 | 2 | 1 | ||||||||
| saikoseikanto | SSET | 1.5 | 1.5 | 1.5 | 1.5 | 2 | 1.5 | 1.5 | ||||||
| saireito | SRT | 3 | 2 | 7 | 1 | 3 | ||||||||
| shimbuto | SIT | 3 | 1.5 | 3 | 0.5 | |||||||||
| shimotsuto | SMT | 3 | 3 | |||||||||||
| shokenchuto | SHKT | 2 | 6 | 1 | ||||||||||
| shosaikoto | SSK | 3 | 2 | 7 | 2 | |||||||||
| shoseiryuto | SST | 3 | 3 | 3 | 3 | |||||||||
| tokiinshi | TKI | 1 | 4 | 3 | ||||||||||
| tokishakuyakusan | TSS | 4 | 4 | |||||||||||
| tokishigyakukagoshuyushokyoto | TSGST | 2 | 3 | 1 | ||||||||||
| tsudosan | TDS | 2 | 3 | |||||||||||
| unseiin | USI | 1.5 | 1.5 | 1.5 | 3 | 3 | ||||||||
| yokuininto | YT | 2 | 3 | 4 | 4 | |||||||||
| yokukansan | YKS | 1.5 | 2 | 4 | ||||||||||
Measurements of multiple free radical scavenging activity
MULTIS were measured using a previously described ESR-based method with minor modifications.(12,13) MULTIS measurements were performed three times per each ROS. The ESR spectrometer used was a RR-X1 equipped with 100 kHz field modulation and WIN-RAD operation software (Radical Research Inc., Tokyo, Japan). The compound 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) was used as an ESR spin-trapping reagent. Typical spectrometer settings were field modulation width 0.1 mT; microwave power 10 mW; field scan width and rate ±7.5 mT/2 min; and time constant 0.1 s. Each ROS was produced via in situ illumination with UV/visible light from an illuminator (RUVF-203SR UV illuminator; Radical Research Inc., Tokyo, Japan) equipped with a 200 W medium-pressure mercury/xenon arc lamp and a quartz light-guide connected to the resonator cavity. Table 3 summarizes the light sources, illumination times, precursors, and photosensitizers used to produce ROS. ROS scavenging activities were calculated according to a previously described method and expressed as the unit equivalent to known pure scavengers: glutathione (GSH) for •OH and 1O2, superoxide dismutase (SOD) for O2•−, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (TROLOX) for RO•, and α-lipoic acid for ROO•.(13) To evaluate overall scavenging activity, scavenging activities of each ROS were expressed as relative values with the Kampo formulation tsudosan (TDS) as 1. The scavenging activities were expressed in terms of their daily doses. All samples were prepared in triplicate. Data were expressed as mean values.
Table 3.
Photolytic production methods of multiple reactive oxygen species
| Free radical | Spin trap | Precursor/Sensitizer | UV/VL | Irradiation period | Antioxiidant equivalent |
|---|---|---|---|---|---|
| •OH | CYPMPO | H2O2 10 mM | UV | 5 s | GSH |
| O2•− | CYPMPO | Riboflavin 20 µM | VL | 60 s | SOD |
| RO• | CYPMPO | AAPH 10 mM | UV | 5 s | Trolox |
| ROO• | CYPMPO | tert-BHP 10 mM | UV | 5 s | α-lipoic acid |
| 1O2 | TMPO | Rosebengal 200 µM | VL | 30 s | GSH |
UV, ultraviolet (300–400 nm); VL, visual light (500–600 nm); CYPMPO, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide; AAPH, 2,2'-azobis (2-amidinopropane) hydrochloride; tert-BHP, tert-butyl hydroperoxide; GSH, glutathione; SOD, superoxide dismutase; TMPO, 4-hydroxy-2,2,6,6-tetramethylpiperidine.
Reagents
CYPMPO was obtained from Radical Research Inc. (Tokyo, Japan); hydrogen peroxide, riboflavin, 2,2'-azobis (2-amidinopropane) hydrochloride (AAPH), tert-butyl hydroperoxide, dimethyl sulfoxide (DMSO), rose bengal, and 4-hydroxy-2,2,6,6-tetramethylpiperidine (4-OH-TMPO) were purchased from Tokyo Chemical Industry (Tokyo, Japan) and used without modification. Buffers and biochemical reagents were obtained from Wako Chemical Co. (Osaka, Japan).
Statistical analysis
Statistical analysis was performed using computer software (Prism 6; GraphPad Software Inc., La Jolla, CA). Data were tested using Spearman’s rank correlation coefficient.
Results
Hydroxyl radical scavenging activity of Kampo formulae
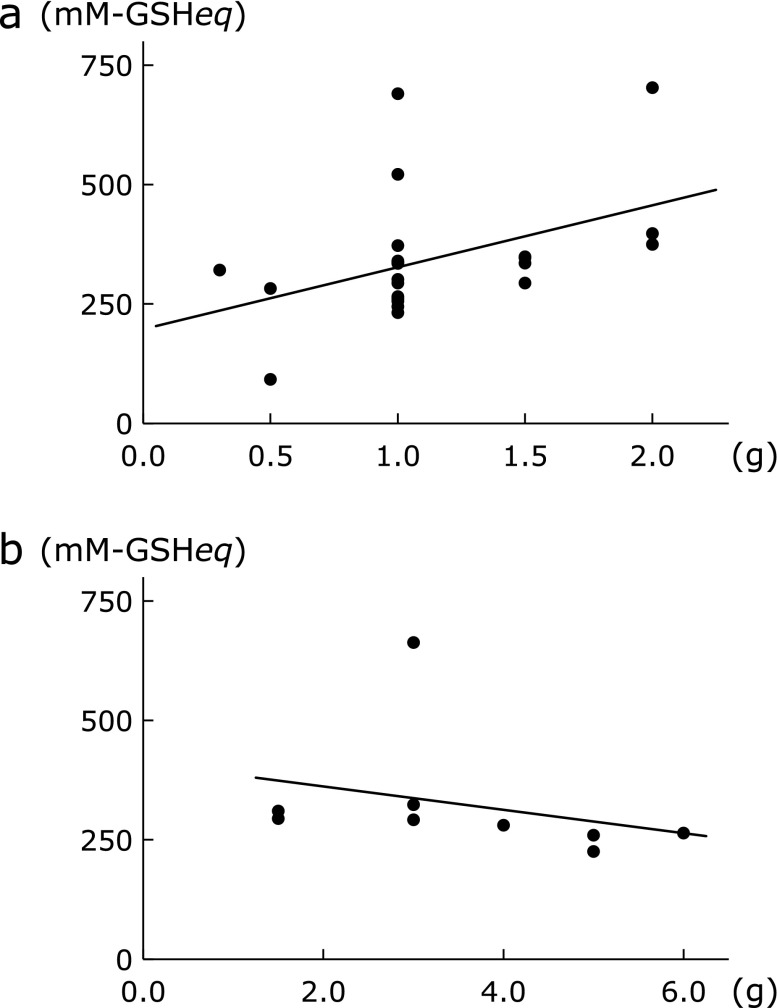
Table 4 shows the •OH scavenging activity of Kampo extract preparations. The formulae shosaikoto (SSK), saikokaryukotsuboreito (SRB), unseiin (USI), and daisaikoto (DST) showed remarkably high •OH scavenging activity. Among them, shosaikoto (SSK), saikokaryukotsuboreito (SRB), and daisaikoto (DST) contain both Bupleuri Radix and Scutellariae Radix as components, which may relate to antioxidative activity [the amount of Bupleuri Radix and Scutellariae Radix were, respectively, 7 g and 3 g in shosaikoto (SSK), 5 g and 2.5 g in saikokaryukotsuboreito (SRB), and 6 g and 3 g in daisaikoto (DST), per daily dose]. Zingiberis Rhizoma showed a significant positive correlation and Rehmanniae Radix showed a significant strong negative correlation between •OH scavenging activity and quantity (r = 0.54, p<0.05 for Zingiberis Rhizoma and r = −0.75, p<0.05 for Rehmanniae Radix, Fig. 1).
Table 4.
Hydroxyl radical scavenging activity of Kampo formulae
| Kampo formula |
•OH scavenging activity (mM-GSHeq) |
|
|---|---|---|
| per daily dose | per gram | |
| shosaikoto | 703.1 | 93.7 |
| saikokaryukotsuboreito | 690.3 | 92.0 |
| unseiin | 663.0 | 88.4 |
| daisaikoto | 521.8 | 69.6 |
| kakkonto | 397.3 | 53.0 |
| daiokanzoto | 393.7 | 52.5 |
| bakumondoto | 382.2 | 42.5 |
| ninjinto | 376.0 | 50.1 |
| kakkontokasenkyushin'i | 375.0 | 50.0 |
| saikokeishito | 372.3 | 49.6 |
| orengedokuto | 349.6 | 46.6 |
| keishito | 348.9 | 46.5 |
| keishikaryukotsuboreito | 347.0 | 46.3 |
| keishikajutsubuto | 339.8 | 45.3 |
| goreisan | 339.8 | 45.3 |
| boiohito | 336.8 | 44.9 |
| shimbuto | 335.6 | 44.7 |
| hangekobokuto | 335.1 | 44.7 |
| mashiningan | 325.1 | 43.3 |
| jyuzendaihoto | 323.9 | 43.2 |
| bofutsushosan | 321.3 | 42.8 |
| saikoseikanto | 310.4 | 41.4 |
| tsudosan | 308.5 | 41.1 |
| daiobotanbito | 301.2 | 40.2 |
| jumihaidokuto | 301.2 | 40.2 |
| yokkansan | 296.8 | 39.6 |
| keigairengyoto | 294.3 | 39.2 |
| shokenchuto | 294.2 | 19.6 |
| goshuyuto | 294.0 | 39.2 |
| shimotsuto | 292.3 | 39.0 |
| saikokeishikankyoto | 292.2 | 39.0 |
| hangeshashinto | 292.1 | 38.9 |
| rikkunshito | 282.5 | 37.7 |
| tokiinshi | 280.4 | 37.4 |
| kamishoyosan | 265.7 | 35.4 |
| hachimijiogan | 264.1 | 35.2 |
| kamikihito | 261.2 | 34.8 |
| goshajinkigan | 259.6 | 34.6 |
| tokishigyakukagoshuyushokyoto | 255.7 | 34.1 |
| tokishakuyakusan | 251.2 | 33.5 |
| keishibukuryogan | 250.2 | 33.4 |
| saibokuto | 244.4 | 32.6 |
| daikenchuto | 242.1 | 16.1 |
| saireito | 232.3 | 25.8 |
| yokuininto | 231.8 | 30.9 |
| maoto | 231.6 | 30.9 |
| rokumigan | 225.7 | 30.1 |
| shoseiryuto | 98.4 | 13.1 |
| hochuekkito | 92.2 | 12.3 |
Fig. 1.
Correlation between hydroxyl radical scavenging activity and the amount of Zingiberis Rhizoma (a) and Rehmanniae Radix (b). r = 0.54, p<0.05 for Zingiberis Rhizoma and r = −0.75, p<0.05 for Rehmanniae Radix.
Alkoxyl radical scavenging activity of Kampo formulae
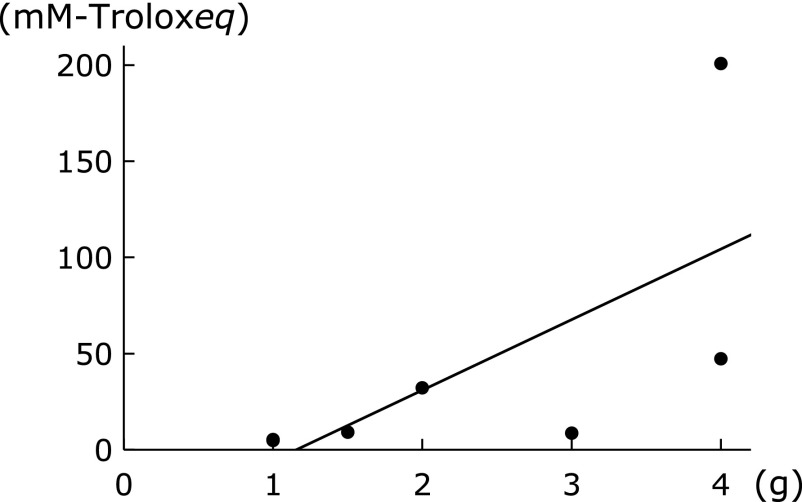
Table 5 shows the RO• scavenging activity of Kampo formulae extracts. Daiokanzoto (DKZT), which contains large amounts of Rhei Rhizoma (4 g per daily dose in the extract), showed remarkably high RO• scavenging activity. There was a significant strong positive correlation between the amount of Rhei Rhizoma and RO• scavenging activity (r = 0.87, p<0.05, Fig. 2). Other formulae containing Rhei Rhizoma, including mashiningan (MNG) and daiobotanpito (DBT), also showed high RO• scavenging activity [the amount of Rhei Rhizoma was 4 g in mashiningan (MNG) and 2 g in daiobotanpito (DBT) per daily dose in the extract]. Formulae that included Coptidis Rhizoma, namely orengedokuto (OGT), unseiin (USI), and saikoseikanto (SSET), also showed high RO• scavenging activity; however, the results were not significant owing to the small number of formulae that contained the component. A previous report found that Kampo formulae containing Rhei Rhizoma showed high oxygen radical absorbance capacity (ORAC) values;(3) our results for RO• scavenging activity generally agree with these previous findings.
Table 5.
Alkoxyl radical scavenging activity of Kampo formulae
| Kampo formula | RO• scavenging activity (mM-TROLOXeq) |
|
|---|---|---|
| per daily dose | per gram | |
| daiokanzoto | 200.7 | 26.8 |
| mashiningan | 47.3 | 6.3 |
| orengedokuto | 32.3 | 4.3 |
| daiobotanbito | 32.2 | 4.3 |
| maoto | 25.7 | 3.4 |
| saikokeishikankyoto | 17.0 | 2.3 |
| unseiin | 14.9 | 2.0 |
| yokuininto | 12.5 | 1.7 |
| saikoseikanto | 11.9 | 1.6 |
| bofutsushosan | 9.2 | 1.2 |
| kakkontokasenkyushin'i | 9.2 | 1.2 |
| tsudosan | 8.7 | 1.2 |
| keigairengyoto | 8.4 | 1.1 |
| hangeshashinto | 6.8 | 0.9 |
| keishibukuryogan | 6.5 | 0.9 |
| saibokuto | 5.9 | 0.8 |
| daisaikoto | 5.3 | 0.7 |
| hochuekkito | 4.8 | 0.6 |
| hangekobokuto | 4.8 | 0.6 |
| saikokaryukotsuboreito | 4.8 | 0.6 |
| ninjinto | 4.5 | 0.6 |
| kakkonto | 4.2 | 0.6 |
| daikenchuto | 4.2 | 0.3 |
| shosaikoto | 4.2 | 0.6 |
| shokenchuto | 4.1 | 0.3 |
| yokkansan | 4.1 | 0.5 |
| saikokeishito | 3.9 | 0.5 |
| keishito | 3.8 | 0.5 |
| saireito | 3.7 | 0.4 |
| keishikaryukotsuboreito | 3.5 | 0.5 |
| keishikajutsubuto | 3.1 | 0.4 |
| boiohito | 3.1 | 0.4 |
| goshuyuto | 3.1 | 0.4 |
| rokumigan | 3.0 | 0.4 |
| tokiinshi | 2.8 | 0.4 |
| jyuzendaihoto | 2.7 | 0.4 |
| rikkunshito | 2.6 | 0.3 |
| kamishoyosan | 2.5 | 0.3 |
| kamikihito | 2.4 | 0.3 |
| shimbuto | 2.4 | 0.3 |
| goshajinkigan | 2.3 | 0.3 |
| shimotsuto | 2.3 | 0.3 |
| shoseiryuto | 2.2 | 0.3 |
| jumihaidokuto | 1.8 | 0.2 |
| bakumondoto | 1.8 | 0.2 |
| tokishigyakukagoshuyushokyoto | 1.7 | 0.2 |
| tokishakuyakusan | 1.7 | 0.2 |
| goreisan | 1.4 | 0.2 |
| hachimijiogan | 0.9 | 0.1 |
Fig. 2.
Correlation between alkoxyl radical scavenging activity and the amount of Rhei Rhizoma (r = 0.87, p<0.05).
Alkylperoxyl radical scavenging activity of Kampo formulae
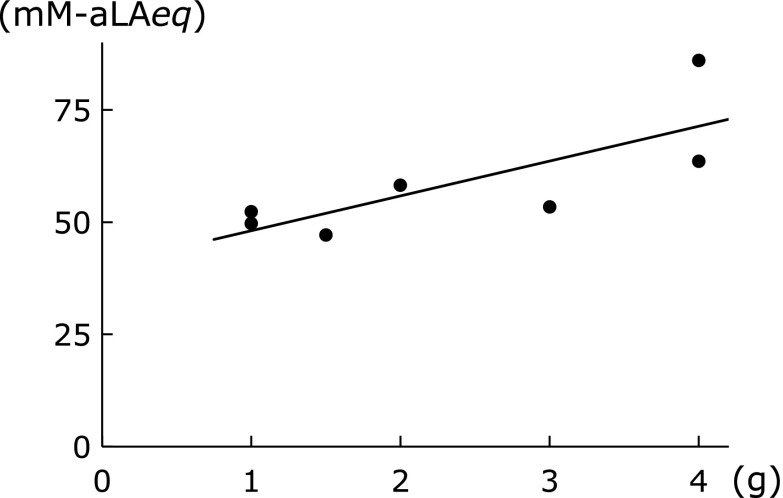
Table 6 shows the ROO• scavenging activity of Kampo formulae extracts. The highest ROO• scavenging activity was found in kakkontokasenkyushin'i (KTSS) and maoto (MT), both of which contain Ephedrae Herba. However, this component had no significant effect on ROO• scavenging activity. The only crude element that significantly contributed to ROO• scavenging activity was Rhei Rhizoma (r = 0.84, p<0.05, Fig. 3). Instead, ROO• scavenging activity was notable in saibokuto (SBT), hangekobokuto (HKT), and goreisan (GRS), whose antioxidative activities were previously unknown. Moreover, many of our results related to ROO• scavenging activity could not be explained by the constitution of herbal ingredients.
Table 6.
Alkylperoxyl radical scavenging activity of Kampo formulae
| Kampo formula | ROO• scavenging activity (mM-aLAeq) |
|
|---|---|---|
| per daily dose | per gram | |
| kakkontokasenkyushin'i | 148.4 | 19.8 |
| maoto | 117.4 | 15.7 |
| keishikaryukotsuboreito | 90.8 | 12.1 |
| saibokuto | 89.6 | 12.0 |
| hangekobokuto | 86.3 | 11.5 |
| mashiningan | 86.0 | 11.5 |
| hochuekkito | 85.6 | 11.4 |
| goreisan | 81.1 | 10.8 |
| keishikajutsubuto | 79.0 | 10.5 |
| saikokeishito | 76.9 | 10.2 |
| yokkansan | 74.1 | 9.9 |
| jyuzendaihoto | 72.5 | 9.7 |
| unseiin | 71.8 | 9.6 |
| ninjinto | 71.7 | 9.6 |
| yokuininto | 70.6 | 9.4 |
| keigairengyoto | 69.8 | 9.3 |
| saikokeishikankyoto | 68.8 | 9.2 |
| daiokanzoto | 63.5 | 8.5 |
| keishito | 62.8 | 8.4 |
| orengedokuto | 59.1 | 7.9 |
| daiobotanbito | 58.2 | 7.8 |
| saikoseikanto | 54.7 | 7.3 |
| rokumigan | 53.5 | 7.1 |
| tsudosan | 53.4 | 7.1 |
| kamikihito | 53.4 | 7.1 |
| shosaikoto | 52.8 | 7.0 |
| saireito | 52.8 | 5.9 |
| saikokaryukotsuboreito | 52.4 | 7.0 |
| jumihaidokuto | 51.3 | 6.8 |
| goshuyuto | 50.3 | 6.7 |
| daisaikoto | 49.7 | 6.6 |
| shimbuto | 49.7 | 6.6 |
| kamishoyosan | 49.3 | 6.6 |
| keishibukuryogan | 49.0 | 6.5 |
| goshajinkigan | 48.7 | 6.5 |
| hachimijiogan | 48.0 | 6.4 |
| tokishakuyakusan | 47.9 | 6.4 |
| tokiinshi | 47.5 | 6.3 |
| bofutsushosan | 47.1 | 6.3 |
| boiohito | 46.6 | 6.2 |
| rikkunshito | 44.1 | 5.9 |
| tokishigyakukagoshuyushokyoto | 42.9 | 5.7 |
| daikenchuto | 42.6 | 2.8 |
| shimotsuto | 42.4 | 5.7 |
| shokenchuto | 41.8 | 2.8 |
| hangeshashinto | 41.0 | 5.5 |
| kakkonto | 41.0 | 5.5 |
| bakumondoto | 36.5 | 4.1 |
| shoseiryuto | 34.6 | 4.6 |
Fig. 3.
Correlation between alkylperoxyl radical scavenging activity and the amount of Rhei Rhizoma (r = 0.84, p<0.05).
Superoxide scavenging activity of Kampo formulae
Table 7 shows the O2•− scavenging activity of Kampo formulae extracts. Formulae containing Scutellariae Radix, Phellodendri Cortex, and Coptidis Rhizoma, namely orengedokuto (OGT), unseiin (USI), and keigairengyoto (KRT), showed high scavenging activity against O2•− [the amounts of Scutellariae Radix, Phellodendri Cortex and Coptidis Rhizoma were, respectively, 3 g, 1.5 g and 2 g in orengedokuto (OGT); 1.5 g, 1.5 g and 1.5 g in unseiin (USI); and 1.5 g, 1.5 g and 1.5 g in keigairengyoto (KRT) per daily dose]. High O2•− scavenging activity was also found in formulae containing Rhei Rhizoma [i.e., daiokanzoto (DKZT) and tsudosan (TDS)] and Ephedrae Herba [i.e., kakkontokasenkyushin'i (KTSS) and maoto (MT)]. Again, no significant results were observed owing to the small number of formulae that contained the component.
Table 7.
Superoxide scavenging activity of Kampo formulae
| Kampo formula | O2•− scavenging activity (U/ml-SODeq) |
|
|---|---|---|
| per daily dose | per gram | |
| orengedokuto | 3,522.3 | 469.6 |
| saikokaryukotsuboreito | 2,499.8 | 333.3 |
| unseiin | 2,321.0 | 309.5 |
| keigairengyoto | 1,482.9 | 197.7 |
| yokkansan | 1,400.1 | 186.7 |
| kakkontokasenkyushin'i | 1,107.3 | 147.6 |
| saireito | 1,036.4 | 115.2 |
| daiokanzoto | 1,023.3 | 136.4 |
| maoto | 991.3 | 132.2 |
| tokiinshi | 834.1 | 111.2 |
| tsudosan | 833.5 | 111.1 |
| saibokuto | 751.0 | 100.1 |
| goshajinkigan | 704.1 | 93.9 |
| shokenchuto | 698.5 | 46.6 |
| daisaikoto | 661.6 | 88.2 |
| kamikihito | 614.7 | 82.0 |
| rokumigan | 604.1 | 80.5 |
| hangeshashinto | 580.5 | 77.4 |
| hochuekkito | 540.2 | 72.0 |
| kakkonto | 538.9 | 71.9 |
| yokuininto | 525.4 | 70.1 |
| daikenchuto | 469.0 | 31.3 |
| mashiningan | 468.5 | 62.5 |
| keishibukuryogan | 454.4 | 60.6 |
| daiobotanbito | 444.4 | 59.3 |
| shoseiryuto | 423.4 | 56.5 |
| saikokeishikankyoto | 406.6 | 54.2 |
| goshuyuto | 394.8 | 52.6 |
| keishito | 382.9 | 51.0 |
| hangekobokuto | 359.8 | 48.0 |
| shosaikoto | 343.1 | 45.7 |
| ninjinto | 333.2 | 44.4 |
| bofutsushosan | 331.8 | 44.2 |
| shimotsuto | 313.5 | 41.8 |
| boiohito | 304.5 | 40.6 |
| keishikaryukotsuboreito | 299.5 | 39.9 |
| tokishigyakukagoshuyushokyoto | 287.8 | 38.4 |
| hachimijiogan | 277.0 | 36.9 |
| saikoseikanto | 269.0 | 35.9 |
| jumihaidokuto | 260.0 | 34.7 |
| keishikajutsubuto | 258.1 | 34.4 |
| goreisan | 251.1 | 33.5 |
| kamishoyosan | 240.0 | 32.0 |
| saikokeishito | 230.9 | 30.8 |
| rikkunshito | 198.4 | 26.5 |
| jyuzendaihoto | 187.4 | 25.0 |
| shimbuto | 179.7 | 24.0 |
| bakumondoto | 161.6 | 18.0 |
| tokishakuyakusan | 143.7 | 19.2 |
Singlet oxygen scavenging activity of Kampo formulae
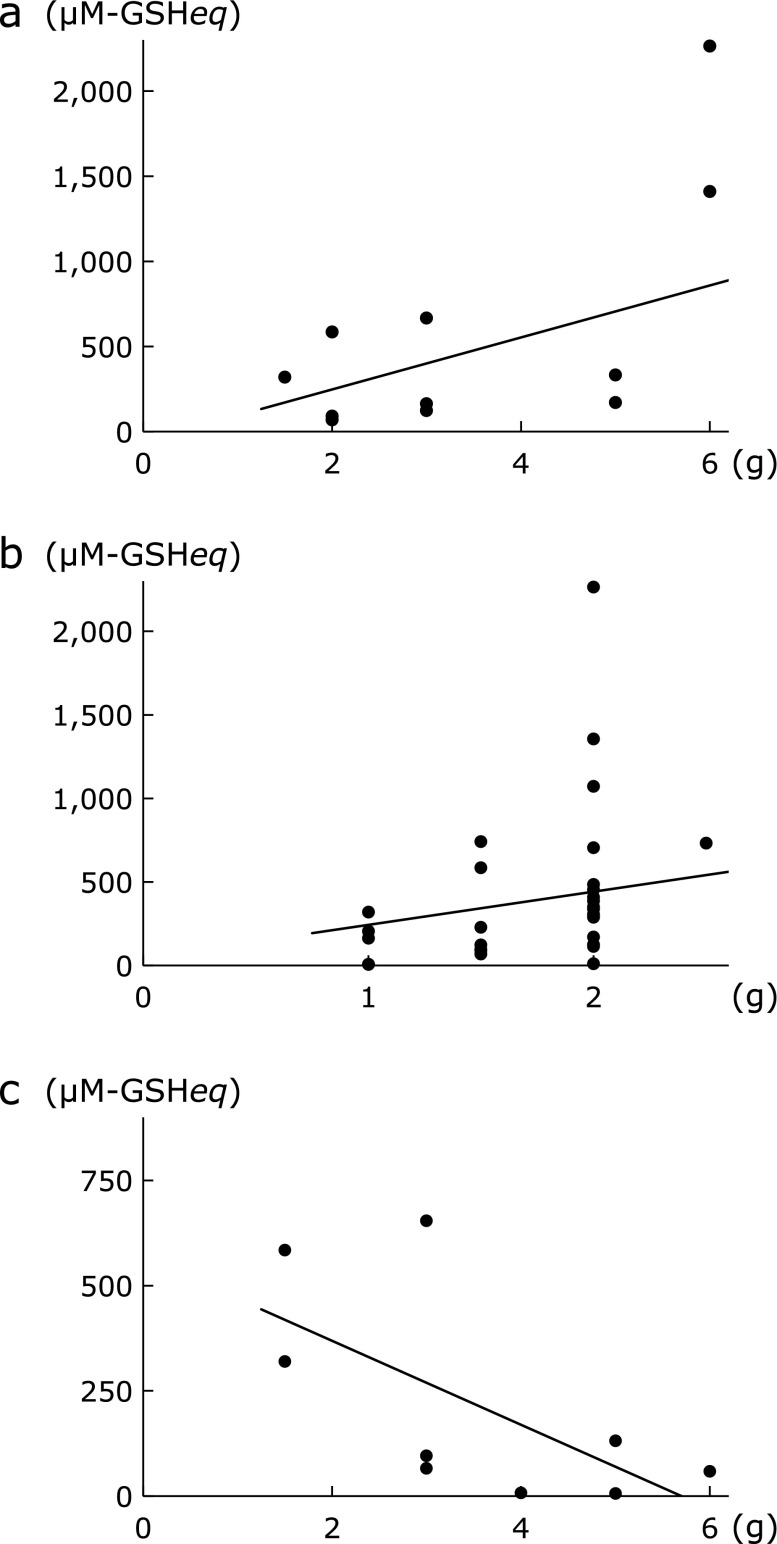
Table 8 lists the Kampo formulae that showed high 1O2 scavenging activity of extracts. Formulae containing Bupleuri Radix, namely saikokeishikankyoto (SAKK), daisaikoto (DST), shosaikoto (SSK), saibokuto (SBT) and saikoseikanto (SSET), showed high scavenging activity against 1O2 [the amount of Bupleuri Radix was 6 g in saikokeishikankyoto (SAKK), 6 g in daisaikoto (DST), 7 g in shosaikoto (SSK), 7 g in saibokuto (SBT) and 6 g in saikoseikanto (SSET) per daily dose] and there was a significant positive correlation between the quantity of Bupleuri Radix and 1O2 scavenging activity (r = 0.57, p<0.05, Fig. 4a). The quantity of Glycyrrhizae Radix was also positively correlated with 1O2 scavenging activity (r = 0.40, p<0.05, Fig. 4b).
Table 8.
Singlet oxygen scavenging activity of Kampo formulae
| Kampo formula |
1O2 scavenging activity (µM-GSHeq) |
|
|---|---|---|
| per daily dose | per gram | |
| saikokeishikankyoto | 2,265.4 | 302.1 |
| daisaikoto | 1,410.9 | 188.1 |
| kakkonto | 1,356.2 | 180.8 |
| shosaikoto | 1,071.4 | 142.9 |
| goshuyuto | 977.9 | 130.4 |
| maoto | 742.3 | 99.0 |
| hangeshashinto | 732.4 | 97.6 |
| saibokuto | 705.7 | 94.1 |
| unseiin | 654.6 | 87.3 |
| saikoseikanto | 584.5 | 77.9 |
| daiobotanbito | 515.0 | 68.7 |
| bofutsushosan | 485.7 | 64.8 |
| keishibukuryogan | 475.1 | 63.4 |
| orengedokuto | 467.3 | 62.3 |
| kakkontokasenkyushin'i | 454.0 | 60.5 |
| hangekobokuto | 451.6 | 60.2 |
| shokenchuto | 408.1 | 27.2 |
| daiokanzoto | 386.4 | 51.5 |
| tsudosan | 350.5 | 46.7 |
| tokishigyakukagoshuyushokyoto | 338.5 | 45.1 |
| saikokaryukotsuboreito | 332.5 | 44.3 |
| keigairengyoto | 319.9 | 42.6 |
| yokuininto | 307.9 | 41.0 |
| saireito | 295.9 | 32.9 |
| keishito | 288.1 | 38.4 |
| ninjinto | 269.0 | 35.9 |
| daikenchuto | 243.8 | 16.3 |
| boiohito | 228.9 | 30.5 |
| rikkunshito | 205.7 | 27.4 |
| shoseiryuto | 172.2 | 23.0 |
| saikokeishito | 171.3 | 22.8 |
| jumihaidokuto | 163.1 | 21.7 |
| rokumigan | 131.6 | 17.5 |
| keishikaryukotsuboreito | 125.6 | 16.8 |
| kamishoyosan | 124.4 | 16.6 |
| keishikajutsubuto | 115.0 | 15.3 |
| jyuzendaihoto | 95.9 | 12.8 |
| hochuekkito | 91.1 | 12.1 |
| shimbuto | 82.6 | 11.0 |
| yokkansan | 68.6 | 9.1 |
| goreisan | 66.8 | 8.9 |
| shimotsuto | 66.0 | 8.8 |
| tokishakuyakusan | 65.4 | 8.7 |
| hachimijiogan | 58.7 | 7.8 |
| mashiningan | 40.8 | 5.4 |
| bakumondoto | 10.5 | 1.2 |
| tokiinshi | 8.0 | 1.1 |
| kamikihito | 6.6 | 0.9 |
| goshajinkigan | 6.0 | 0.8 |
Fig. 4.
Correlation between singlet oxygen scavenging activity and the amount of Bupleuri Radix (a) r = 0.57, p<0.05; Glycyrrhizae Radix (b) r = 0.40, p<0.05; and Rehmanniae Radix (c) r = −0.67, p<0.05).
In contrast, some formulae showed remarkably low 1O2 scavenging activity. The amount of Rehmanniae Radix showed a negative correlation with 1O2 scavenging activity (r = −0.67, p<0.05, Fig. 4c), a finding similar to the results for •OH scavenging activity. Formulae containing high doses of Rehmanniae Radix, namely goshajinkigan (GJG), tokiinshi (TKI) and hachimijiogan (HJG), showed generally low 1O2 scavenging activity. Furthermore, Atractylodis Rhizoma, Processi Aconiti Radix, and Zingiberis Processum Rhizoma also showed a tendency to decrease 1O2 scavenging activity, although these results were not statistically significant (data not shown).
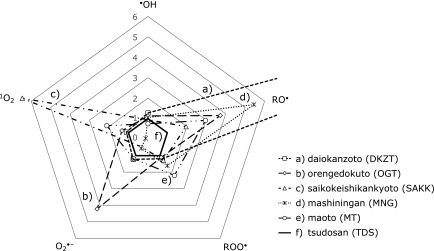
Overall scavenging activities against multiple ROS
Figure 5 summarizes the total ROS scavenging profile of five representative Kampo formulae that exhibited high scavenging activity against each ROS compared with tsudosan (TDS), the formula reported to possess the highest ORAC value.(3) Formulae containing high amounts of Rhei Rhizoma, daiokanzoto (DKZT) and mashiningan (MNG) showed solid RO• and ROO• scavenging activities. The RO• scavenging activity of these two formulae was remarkably strong even when they were compared with tsudosan (TDS). The ROS scavenging activities of orengedokuto (OGT), which contains Scutellariae Radix, Phellodendri Cortex and Coptidis Rhizoma, were remarkable against O2•−. Saikokeishikankyoto (SAKK), a representative formula of a prescription containing Bupleuri Radix, showed high 1O2 scavenging activity.
Fig. 5.
Total ROS scavenging profile of representative Kampo formulae, namely daiokanzoto (DKZT) (a), orengedokuto (OGT) (b), saikokeishikankyoto (SAKK) (c), mashiningan (MNG) (d), maoto (MT) (e), and tsudosan (TDS) (f). The scavenging activities against each ROS are expressed as relative values in comparison with tsudosan.
Discussion
Because ROS possess high reactivity and their reactions are varied and complicated, it is difficult to describe the details of these pathways. This uncertainty is an obstacle for clinical antioxidative therapy and an analysis of multiple ROS pathways is strongly needed.(10) Typically, an oxidative stress-related reaction is initiated by an excess production of primary ROS leading to further radical chain reactions, which then evoke cellular antioxidative responses. It is mainly superoxide that has this initiative role in biological systems. Thereafter, radical chain reactions involving •OH are evoked, leading to lipid hydroperoxide (LOOH) production. In the presence of iron, copper, or heme protein, LOOH is converted to lipid alkoxyl radical (LO•) or lipid alkylperoxyl radical (LOO•), leading to further radical chain reactions. 1O2 also plays an important role in LOOH production.(14) Thus, each ROS shows specific characteristics during in vivo reactions.(11) Because Kampo or other traditional herbal prescriptions contain various active ingredients, the evaluation of multiple ROS dynamics is necessary for detailed analysis of their redox activity.
Oxidative stress is a critical and universal pathological factor that impacts various diseases. Antioxidative effects are recognized as the most important therapeutic mechanism of Kampo formulae. Many studies have demonstrated antioxidative activities for Kampo formulae and other herbal medicines, including their crude components,(3–7) but many details about the mechanisms of these activities remain to be clarified. Our results revealed that Kampo formulae known to present high ORAC values,(3) such as mashiningan (MNG) and daiokanzoto (DKZT), possess high RO• scavenging activity. We also found that Rhei Rhizoma, a key crude drug that shows high ORAC values, showed a positive correlation between its quantity in the formulae and RO• scavenging activity. Therefore, much of the previously demonstrated antioxidative activity of Kampo formulae reflects RO• scavenging activity.
•OH is considered the most toxic oxygen radical because of its extremely high reactivity against biological substances, leading to tissue/organ damage. No specific substances eliminate •OH in vivo; however, •OH scavenging activity is strongly related to the pathophysiology of multiple diseases.(13,15,16) Kampo formulae containing Zingiberis Rhizoma showed high scavenging activity against •OH. Zingiberis Rhizoma contains multiple pharmacologically active components, including zingiberol and shogaol, and exhibits various pharmacological effects.(17) Although Zingiberis Rhizoma shows low antioxidative activity against AAPH-induced oxidative stress,(18) our findings revealed remarkable •OH scavenging activity in formulae containing Zingiberis Rhizoma. Some of its pharmacological effects may be induced by high •OH scavenging activity. In addition, formulae containing both Bupleuri Radix and Scutellariae Radix, namely shosaikoto (SSK), saikokaryukotsuboreito (SRB), and daisaikoto (DST), showed strong •OH scavenging activity [but saibokuto (SBT) did not]. Clinically, Bupleuri Radix and Scutellariae Radix are frequently used in combination and this combination shows stronger anti-inflammatory effects than single usage.(19) Among the formulae used in this study, 69% of formulae containing Scutellariae Radix also contain Bupleuri Radix and their high •OH scavenging activity provides a pharmacological explanation for the traditional use of the crude composition.
We measured the scavenging activity of ROO• using tert-BuOOH; thus, this activity might reflect the lipid peroxidation process. Again, details of the activities of ROO• remain to be clarified, but current research indicates its importance in an animal model of hepatic carcinoma.(20) Unlike the other ROS, ROO• scavenging activity was notable in formulae whose antioxidative activities were previously unknown, such as saibokuto (SBT), hangekobokuto (HKT) and goreisan (GRS). Because saibokuto (SBT) is a blended formula comprising hangekobokuto (HKT) and shosaikoto (SSK), the components of hangekobokuto (HKT) may relate to ROO• scavenging activity. As there were a small number of formulations and many combinations of crude drug ingredients, it was difficult to identify specific important responsible substances. Many results related to ROO• scavenging activity could not be explained by the constitution of herbal ingredients.
The superoxide scavenging activity of Kampo formulae has been widely reported.(21–26) A series of studies by Kohno and colleagues report an extensive screening of O2•− scavenging activity of herbal extracts.(4,5) They found that extracts such as Rheum palmatum, Ephedra sinica, Punica granatum and Caesalpinia sappan show high O2•− scavenging activity. In our analysis of the formulations, we could not obtain a single crude component that enhanced O2•− scavenging activity. However, formulae containing the three crude extracts Scutellariae Radix, Coptidis Rhizoma and Phellodendri Cortex [i.e., orengedokuto (OGT), unseiin (USI) and keigairengyoto (KRT)] showed remarkably high O2•− scavenging activity. Consequently, in contrast to the •OH scavenging activity of Scutellariae Radix, the combination of these three crude extracts may be crucial for O2•− control. Moreover, though unseiin (USI) contains Rehmanniae Radix, which decreases multiple ROS scavenging activities (as described below), O2•− scavenging activity was still high. Therefore, the combination of Scutellariae Radix, Coptidis Rhizoma and Phellodendri Cortex may recover the pro-oxidative effect of Rehmanniae Radix.
1O2 shows high reactivity and toxicity in vivo and leads to lipid peroxidation, inducing further RO• and ROO• production.(14) 1O2 is produced through myeloperoxidase and prostaglandin hydroperoxidase activity, and by the interaction between O2•− and H2O2 during the Haber-Weiss reaction.(27,28) The importance of 1O2 has been shown in several important pathophysiological processes, including ischemia-reperfusion injury and diabetes.(29) We previously reported a crucial change of in vivo 1O2 scavenging activity after an administration of kangen-karyu, a Kampo formula; however, the importance of this activity in Kampo or other herbal medicine remains to be clarified. High 1O2 scavenging activity was detected in the formulae containing Bupleuri Radix; that is, saikokeishikankyoto (SAKK), daisaikoto (DST) and shosaikoto (SSK). The quantity of Bupleuri Radix showed a significant positive correlation with 1O2 scavenging activity. Thus, Bupleuri Radix is responsible for both •OH and 1O2 scavenging activity. In contrast, it is notable that some formulae showed remarkably low 1O2 scavenging activity. In particular, the 1O2 scavenging activity of tokiinshi (TKI), kamikihito (KT) and goshajinkigan (GJG) was less than 1/200 of that of saikokeishikankyoto (SAKK).
Interestingly, the quantity of Rehmanniae Radix showed a significant negative correlation with •OH and 1O2 scavenging activities. Furthermore, formulae containing Rehmanniae Radix [i.e., hachimijiogan (HJG), goshajinkigan (GJG), rokumigan (RUG) and tokiinshi (TKI)] generally revealed low ROS scavenging activities against •OH, RO•, ROO• and 1O, but unseiin (USI) did not. Thus, Rehmanniae Radix may act as a pro-oxidant, rather than an antioxidant. Clinically, formulae that include Rehmanniae Radix are used in “hohou.” Hohou is a revitalizing treatment that stimulates or compensates functions that are lacking and is used to treat chronic diseases, exhaustion, or aging, conditions in which pathophysiology is strongly related to oxidative stress. Kampo emphasizes the concept of equilibrium, which is similar to homeostasis in Western medicine; the concept of oxidative stress is based on the assumption of a pro-oxidant–antioxidant equilibrium.(30) Thus, treatment of oxidative stress by Kampo medicine aims to rebalance the oxidative–antioxidative equilibrium (which is assumed to be tilted toward oxidation) by supplementing antioxidative crude extracts or elements. However, our findings of the low ROS scavenging activities of Rehmanniae Radix, which is a representative crude extract in hohou formulae, totally contradicts this assumption. Therefore, we hypothesize that Kampo oxidative stress treatments do not simply complement antioxidants, but induce internal antioxidative activity by stimulating pro-oxidative elements.
As the “antioxidant paradox” concept indicates, it remains unclear whether treatment of oxidative stress by administration of antioxidants benefits the whole body.(10) Rather, it is highly likely that weak pro-oxidants have better effects on living bodies than antioxidants, through a radiation hormesis-like beneficial effect.(31) However, it is currently difficult to obtain approval for clinical trials using pro-oxidants owing to ethical problems. Kampo is the most systematically organized complementary and alternative medical treatment. Its safety has been established and it is widely used. Therefore, elucidation of the pro-oxidative effects of Kampo might be a promising yet realistic research pathway toward the therapeutic application of pro-oxidants. Further investigations that include oxidative stress evaluations may lead to a new concept of oxidative stress treatment using pro-oxidants.
Acknowledgments
We thank Dr. Burton D. Cohen, Professor Emeritus, Albert Einstein Medical College, for his continual support of our studies. The description of Kampo formulae, abbreviations, and crude drugs follow the Standard Kampo Formula Nomenclature and the regulations of the Japan Society for Oriental Medicine.(32–34) English of this paper was proofread by Diane Williams, PhD, Edanz group.
Abbreviations
Table 1 shows abbreviations of the Kampo formulae. Because Kampo formulae have complex names, we have included both the formal name of Kampo formulae and their abbreviations throughout the manuscript.
- AAPH
2,2'-azobis (2-amidinopropane) hydrochloride
- CYPMPO
5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide
- DMSO
dimethyl sulfoxide
- ESR
electron spin resonance
- GSH
glutathione
- LOOH
lipid hydroperoxide
- MULTIS
multiple free radical scavenging activities
- 4-OH-TMPO
4-hydroxy-2,2,6,6-tetramethylpiperidine
- ORAC
oxygen radical absorbance capacity
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TROLOX
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
Conflict of Interest
This study was funded by the Promotional Project for Sophistication of Education and Research under the Leadership of the President, Tsukuba University of Technology, 2016. The Kampo extract preparations were provided courtesy of Tsumura Co., Ltd. A.H. received research funding in 2013 from Tsumura Co. Ltd. that was unrelated to the current research. The authors declare no other conflicts of interest.
References
- 1.Watanabe K, Matsuura K, Gao P, et al. Traditional Japanese Kampo medicine: clinical research between modernity and traditional medicine-the state of research and methodological suggestions for the future. Evid Based Complement Alternat Med. 2011;2011:513842. doi: 10.1093/ecam/neq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yakubo S, Ito M, Ueda Y, et al. Pattern classification in Kampo medicine. Evid Based Complement Alternat Med. 2014;2014:535146. doi: 10.1155/2014/535146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura K, Osawa T, Watanabe K.Evaluation of oxygen radical absorbance capacity in Kampo medicine. Evid Based Complement Alternat Med 20112011812163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niwano Y, Saito K, Yoshizaki F, Kohno M, Ozawa T. Extensive screening for herbal extracts with potent antioxidant properties. J Clin Biochem Nutr. 2011;48:78–84. doi: 10.3164/jcbn.11-013FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito K, Kohno M, Yoshizaki F, Niwano Y. Extensive screening for edible herbal extracts with potent scavenging activity against superoxide anions. Plant Foods Hum Nutr. 2008;63:65–70. doi: 10.1007/s11130-008-0071-2. [DOI] [PubMed] [Google Scholar]
- 6.Egashira T, Takayama F, Yamanaka Y, Komatsu Y. Monitoring of radical scavenging activity of peroral administration of the Kampo medicine Sho-saiko-to in rats. Jpn J Pharmacol. 1999;80:379–382. doi: 10.1254/jjp.80.379. [DOI] [PubMed] [Google Scholar]
- 7.Taira J, Ikemoto T, Mimura K, Hagi A, Murakami A, Makino K. Effective inhibition of hydroxyl radicals by hydroxylated biphenyl compounds. Free Radic Res Commun. 1993;19 Suppl 1:S71–S77. doi: 10.3109/10715769309056s71. [DOI] [PubMed] [Google Scholar]
- 8.Tomita T, Hirayama A, Matsui H, Aoyagi K. Effect of Keishibukuryogan, a Japanese traditional Kampo prescription, on improvement of microcirculation and Oketsu and induction of endothelial nitric oxide: a live imaging study. Evid Based Complement Alternat Med. 2017;2017:3620130. doi: 10.1155/2017/3620130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70:257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B. The antioxidant paradox: less paradoxical now? Br J Clin Pharmacol. 2013;75:637–644. doi: 10.1111/j.1365-2125.2012.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirayama A, Okamoto T, Kimura S, et al. Kangen-karyu raises surface body temperature through oxidative stress modification. J Clin Biochem Nutr. 2016;58:167–173. doi: 10.3164/jcbn.15-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oowada S, Endo N, Kameya H, Shimmei M, Kotake Y. Multiple free-radical scavenging capacity in serum. J Clin Biochem Nutr. 2012;51:117–121. doi: 10.3164/jcbn.11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalyanaraman B, Feix JB, Sieber F, Thomas JP, Girotti AW. Photodynamic action of merocyanine 540 on artificial and natural cell membranes: involvement of singlet molecular oxygen. Proc Natl Acad Sci U S A. 1987;84:2999–3003. doi: 10.1073/pnas.84.9.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirayama A, Nagase S, Gotoh M, et al. Reduced serum hydroxyl radical scavenging activity in erythropoietin therapy resistant renal anemia. Free Radic Res. 2002;36:1155–1161. doi: 10.1080/1071576021000016418. [DOI] [PubMed] [Google Scholar]
- 16.Nagase S, Aoyagi K, Hirayama A, et al. Favorable effect of hemodialysis on decreased serum antioxidant activity in hemodialysis patients demonstrated by electron spin resonance. J Am Soc Nephrol. 1997;8:1157–1163. doi: 10.1681/ASN.V871157. [DOI] [PubMed] [Google Scholar]
- 17.Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005;12:684–701. doi: 10.1016/j.phymed.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Rhyu DY, Kang KS, Sekiya M, Yokozawa T. Antioxidant effect of Wen-Pi-Tang and its component crude drugs on oxidative stress. Am J Chin Med. 2007;35:127–137. doi: 10.1142/S0192415X07004680. [DOI] [PubMed] [Google Scholar]
- 19.Shen X, Zhao Z, Wang H, Guo Z, Hu B, Zhang G. Elucidation of the anti-inflammatory mechanisms of Bupleuri and Scutellariae Radix using system pharmacological analyses. Mediators Inflamm. 2017;2017:3709874. doi: 10.1155/2017/3709874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada K, Mito F, Matsuoka Y, et al. Fluorescence probes to detect lipid-derived radicals. Nat Chem Biol. 2016;12:608–613. doi: 10.1038/nchembio.2105. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto C, Sekine-Suzuki E, Nyui M, et al. Analysis of the antioxidative function of the radioprotective Japanese traditional (Kampo) medicine, hangeshashinto, in an aqueous phase. J Radiat Res. 2015;56:669–677. doi: 10.1093/jrr/rrv023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakanashi M, Matsuzaki T, Noguchi K, et al. Long-term treatment with san'o-shashin-to, a kampo medicine, markedly ameliorates cardiac ischemia-reperfusion injury in ovariectomized rats via the redox-dependent mechanism. Circ J. 2013;77:1827–1837. doi: 10.1253/circj.cj-12-1434. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Kaneko T, Mizokami Y, et al. Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis. World J Gastroenterol. 2013;19:2718–2722. doi: 10.3748/wjg.v19.i17.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki H, Inadomi JM, Hibi T. Japanese herbal medicine in functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:688–696. doi: 10.1111/j.1365-2982.2009.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dohi K, Satoh K, Ikeda Y, Ohtaki H, Shioda S, Aruga T. Neuroprotective effect from ischemia and direct free radical scavenging activity of Choto-san (kampo medicine) Acta Neurochir Suppl. 2003;86:123–127. doi: 10.1007/978-3-7091-0651-8_27. [DOI] [PubMed] [Google Scholar]
- 26.Saito R, Tamura M, Matsui H, et al. Qing Dai attenuates nonsteroidal anti-inflammatory drug-induced mitochondrial reactive oxygen species in gastrointestinal epithelial cells. J Clin Biochem Nutr. 2015;56:8–14. doi: 10.3164/jcbn.14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toufektsian MC, Boucher FR, Tanguy S, Morel S, de Leiris JG. Cardiac toxicity of singlet oxygen: implication in reperfusion injury. Antioxid Redox Signal. 2001;3:63–69. doi: 10.1089/152308601750100506. [DOI] [PubMed] [Google Scholar]
- 28.Noronha-Dutra AA, Epperlein MM, Woolf N. Reaction of nitric oxide with hydrogen peroxide to produce potentially cytotoxic singlet oxygen as a model for nitric oxide-mediated killing. FEBS Lett. 1993;321:59–62. doi: 10.1016/0014-5793(93)80621-z. [DOI] [PubMed] [Google Scholar]
- 29.Lee JW, Miyawaki H, Bobst EV, Hester JD, Ashraf M, Bobst AM. Improved functional recovery of ischemic rat hearts due to singlet oxygen scavengers histidine and carnosine. J Mol Cell Cardiol. 1999;31:113–121. doi: 10.1006/jmcc.1998.0850. [DOI] [PubMed] [Google Scholar]
- 30.Sies H. Oxidative Stress II. Oxidants and Antioxidants. Academic Press; New York: 1991. [Google Scholar]
- 31.Sies H, Feinendegen LE. Radiation hormesis: the link to nanomolar hydrogen peroxide. Antioxid Redox Signal. 2017;27:596–598. doi: 10.1089/ars.2017.7233. [DOI] [PubMed] [Google Scholar]
- 32.Goda Y. Recommended terminology for Kampo products, conventional crude drug products and crude drugs (part 1). Jpn J Pharmacog. 2008;62:80–90. (In Japanese) [Google Scholar]
- 33.Goda Y. Recommended terminology for Kampo products, conventional crude drug products and crude drugs (part 2) Jpn J Pharmacog. 2009;63:11–21. (In Japanese) [Google Scholar]
- 34.Tsutani K. Standard Kampo Formula Nomenclature: ver.1.0. Natural Med. 2005;59:130–141. (In Japanese) [Google Scholar]