Abstract
Background
The National Wilms Tumor Study (NWTS) approach to treating stage III favorable-histology Wilms tumor (FHWT) is Regimen DD4A (vincristine, dactinomycin, and doxorubicin) and radiation therapy. Further risk stratification is required to improve outcomes and reduce late effects. We evaluated clinical and biologic variables for patients with stage III FHWT without combined loss of heterozygosity (LOH) at chromosomes 1p and 16q treated in the Children’s Oncology Group protocol AREN0532.
Methods
From October 2006 to August 2013, 588 prospectively treated, centrally reviewed patients with stage III FHWT were treated with Regimen DD4A and radiation therapy. Tumor LOH at 1p and 16q was determined by microsatellite analysis. Ineligible patients (n = 5) and those with combined LOH 1p/16q (n = 40) were excluded.
Results
A total of 535 patients with stage III disease were studied. Median follow-up was 5.2 years (range, 0.2 to 9.5). Four-year event-free survival (EFS) and overall survival estimates were 88% (95% CI, 85% to 91%) and 97% (95% CI, 95% to 99%), respectively. A total of 58 of 66 relapses occurred in the first 2 years, predominantly pulmonary (n = 36). Eighteen patients died, 14 secondary to disease. A better EFS was associated with negative lymph node status (P < .01) and absence of LOH 1p or 16q (P < .01), but not with gross residual disease or peritoneal implants. In contrast, the 4-year EFS was only 74% in patients with combined positive lymph node status and LOH 1p or 16q. A total of 123 patients (23%) had delayed nephrectomy. Submitted delayed nephrectomy histology showed anaplasia (n = 8; excluded from survival analysis); low risk/completely necrotic (n = 7; zero relapses), intermediate risk (n = 63; six relapses), and high-risk/blastemal type (n=7; five relapses).
Conclusion
Most patients with stage III FHWT had good EFS/overall survival with DD4A and radiation therapy. Combined lymph node and LOH status was highly predictive of EFS and should be considered as a potential prognostic marker for future trials.
INTRODUCTION
The event-free survival (EFS) and overall survival (OS) estimates for patients with favorable-histology Wilms tumor (FHWT) are excellent. However, certain subgroups of patients have inferior survival estimates related to stage and biologic risk factors.1-5 The National Wilms Tumor Study Group (NWTS), and subsequently the Children’s Oncology Group (COG), have adopted an approach of up-front nephrectomy when feasible, followed by stage- and biology-directed treatment.6,7 This is distinct from the International Society of Pediatric Oncology (SIOP) approach, where chemotherapy is given before nephrectomy in most patients, which has been shown to alter the proportion designated as stage III.8-11 Thus, definitions and outcomes for patients with stage III disease are not strictly comparable between SIOP and NWTS/COG. Both cooperative groups use treatments associated with long-term adverse effects; thus, strategies to further refine risk stratification are warranted.
Under the auspices of the COG, patients with stage III disease are conventionally treated with Regimen DD4A (vincristine, dactinomycin, and doxorubicin) for 24 weeks with either flank or whole abdominal radiation. Relapse after stage III treatment is associated with an OS of only 50% despite intensive salvage chemotherapy and/or autologous bone marrow transplantation12-15; those who do survive are predicted to have a high rate of late effects, including early mortality.16-19 It is thus highly desirable to identify patients who need augmentation of initial therapy with the hope of preventing relapse.
Conversely, doxorubicin and abdominal radiation therapy used in front-line therapy are associated with long-term cardiotoxicity and second malignancies. Omission of doxorubicin has recently shown an acceptable EFS and OS in some SIOP patients.20 It would be advantageous to identify subgroups of patients treated using a COG approach that may not require doxorubicin.
Several advances provide important biologic insights into the etiology of Wilms tumor.21-25 Some of these may be predictive of relapse but typically affect a small proportion of patients. Loss of heterozygosity (LOH) of 1p and 16q was validated by Grundy et al7 to be a marker of both inferior EFS and OS, especially if LOH 1p and 16q were combined. Numerous studies have now demonstrated the adverse impact of 1q gain.4,26-29 In addition, a number of clinicopathologic markers have been identified in patients with NWTS-5 stage III disease, including tumor involvement of lymph nodes.5 This latter finding requires validation.
We report the outcomes of patients with stage III disease in the COG AREN0532 study. We sought to confirm an EFS > 85% and OS > 95% for a new, more highly defined group of patients and to further refine and validate clinical and biologic prognostic factors.
METHODS
All participants or their legally authorized guardians provided consent. The National Institutes of Health Central Institutional Review Board approved the protocol, which facilitated local institutional review board approval. In jurisdictions without Central Institutional Review Board agreements, local research ethics boards provided approval.
Clinical Samples
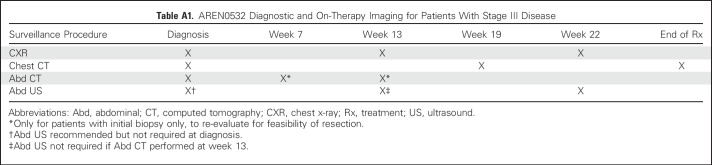
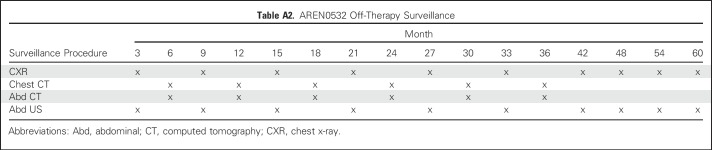
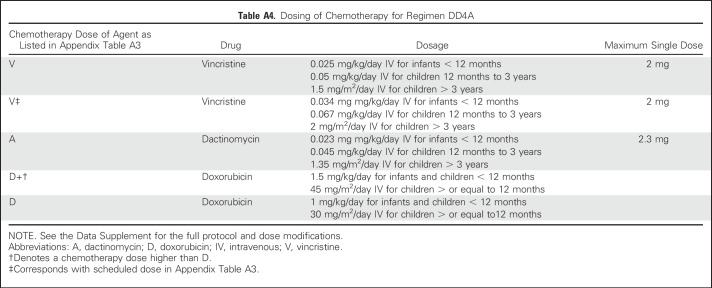
The COG AREN03B2 biology and classification protocol was the portal for access to this study. (Details on the study population and COG quality assurance are provided in the Appendix, online only.) Expert central review of representative pathology, surgical summaries, and protocol-dictated diagnostic imaging occurred for all patients. Patients were given an initial risk assignment of stage III before AREN0532 study (Appendix) enrollment. Stage III disease criteria (Table 1) were as previously described for NWTS-5, except that patients with stage II disease who underwent percutaneous needle or open biopsy before nephrectomy or had intraoperative tumor spillage were considered to have stage III disease. In addition, contrary to NWTS-5, round, noncalcified lung nodules not in a fissure visible on chest computed tomography were considered stage IV, regardless of size, unless histologically proven not to be Wilms tumor.3,5,7 Patients began chemotherapy no later than day 14 postnephrectomy or diagnostic biopsy, unless there was a medical contraindication to initiating treatment. The standard operation was a unilateral uteronephrectomy with lymph node sampling. The decision to biopsy versus attempt nephrectomy was at the institutional surgeon’s discretion on the basis of an assessment of safety and feasibility, for which there are published criteria.30 Patients had to be younger than 30 years old, not have received previous chemotherapy, have a Karnofsky or Lansky score ≥ 50, and have adequate liver and cardiac function. Patients identified to have combined LOH 1p and 16q were taken off protocol by week 6 and offered a different study (to be reported elsewhere). Submission of delayed nephrectomy specimens was requested but not mandatory and classified using the SIOP grading schema as anaplastic, high risk/blastemal, intermediate or low risk/necrotic.31 No treatment changes were made based on these assessments except for patients with anaplasia, who were removed from AREN0532 protocol therapy. All patients underwent protocol-specified surveillance (Appendix Tables A1 and A2, online only).
Table 1.
COG Staging System Used to Define Stage III Wilms Tumor in AREN0532
Treatment
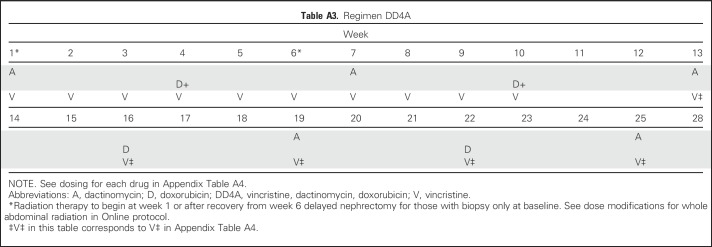
All patients received Regimen DD4A with vincristine, doxorubicin, and dactinomycin32 (Appendix Tables A3 and A4, online only). Dose modifications were specified by protocol. Radiation therapy was delivered as previously described concurrently with initiation of chemotherapy after either primary or delayed nephrectomy (expected 1 to 2 weeks).33,34 Radiation was delivered regardless of postnephrectomy histology. Depending on initial clinicopathologic status, patients received flank (10.8 Gy) or whole abdominal (10.5 Gy) radiation, with a 10.8- or 10.5-Gy boost to gross residual tumor, respectively. Radiation therapy was delivered at COG-approved centers. The detailed treatment plan and dosimetry were required to be submitted to the Quality Assurance Review Center.
Biomarkers
Tumor specimens, blood, and urine were obtained at the time of initial nephrectomy or biopsy, snap-frozen, and subsequently stored at the COG reference laboratory at −80°C. DNA was extracted using standard techniques, and 1p and 16q LOH was performed prospectively using microsatellite analysis by the COG Biopathology Center per protocol.
Statistics
The primary goal of the study was to document continued excellent outcome (4-year EFS > 85% and OS > 95%) for patients with stage III FHWT without LOH of 1p and 16q treated with Regimen DD4A. The EFS for stage III FHWT tumors without LOH was compared with that for similar patients who received the same therapy in NWTS-5. The AREN0532 study had several treatment arms; the driver for accrual for the full study was the sample size requirements for the very-low–risk arm (previously published).35 The study was monitored by an independent data safety monitoring board.
Associations between prognostic biomarkers and the survival outcomes were tested using the log-rank test. Both the EFS and OS were calculated from the time of study enrollment. An event included relapse, second malignant neoplasm, and death, whichever occurred first. All analyses were a priori except for the assessment of lymph node and LOH 1p/16q interaction. The SEs of the EFS and OS were estimated using the Peto-Peto method. Data frozen on December 31, 2016, were used except where indicated. Missing data were less than 1%.
RESULTS
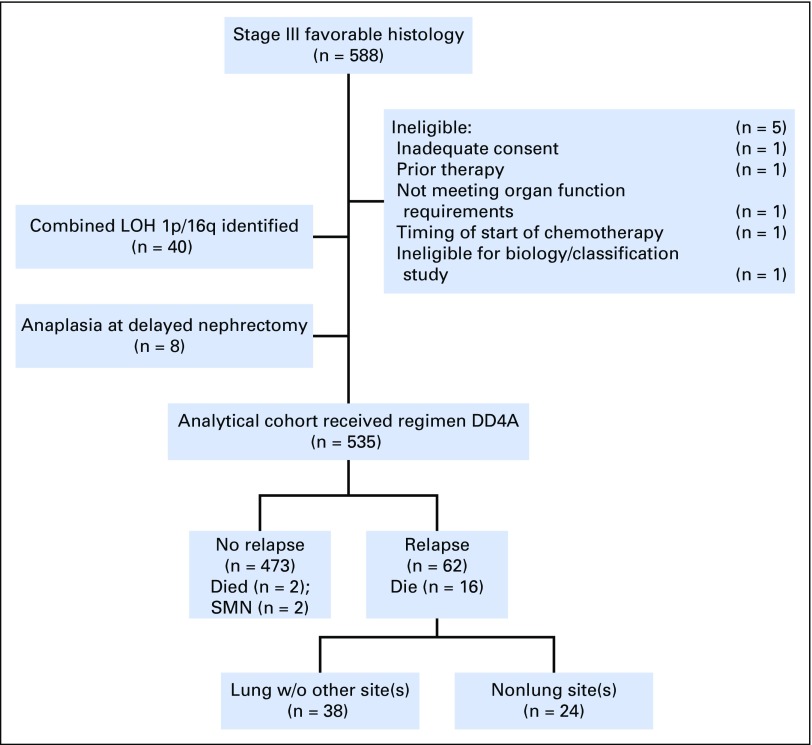
There were 588 patients with stage III favorable-histology tumors enrolled in AREN0532 (Fig 1). Five patients were declared ineligible for the following reasons: inadequate consent procedures (n = 1); not meeting organ function requirements (n = 1); ineligible for AREN03B2 (n = 1); prior therapy (n = 1); and timing of start of protocol therapy (n = 1). In addition, per pre-established protocol criteria, 40 patients withdrew from the study when the presence of combined LOH 1p and 16q was identified, and eight patients were excluded because of anaplasia first identified at delayed nephrectomy. Thus, 535 patients were evaluable for survival analysis (EFS and OS). The median follow-up for patients who were still alive as of December 31, 2016, was 5.2 years (range, 0.2 to 9.5 years).
Fig 1.
Flow diagram of study demonstrating exclusions for lack of eligibility at enrollment and reasons patients were off protocol therapy resulting in 535 patients available for the final analysis. DD4A, vincristine, dactinomycin, doxorubicin; LOH, loss of heterozygosity; SMN, second malignant neoplasm; w/o, without.
Demographics and Tumor Characteristics
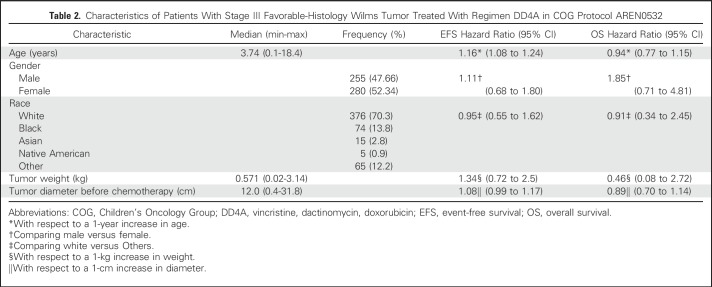
The time to start of therapy for those having up-front nephrectomy was slower (median, 12 days; range, 0 to 31 days) compared with those who had a biopsy at diagnosis (median, 6 days; range, 0 to 19 days). Patient and tumor characteristics are listed in Table 2. We determined the staging criteria that contributed to the patient being designated as having stage III disease (patients could have more than one criterion): delayed nephrectomy after preoperative chemotherapy (n = 116), lymph nodes positive (n = 151), margins positive (n = 189), peritoneal implants (n = 24), tumor rupture (n = 256; 128 intraoperatively, 128 preoperatively), or renal biopsy immediately followed by nephrectomy (n = 8). We examined the median tumor diameter as a surrogate for the ability to perform primary resection. This showed no difference in size, with a median tumor diameter of 12.8 cm (range, 1 to 22 cm) for those with delayed nephrectomy compared with 12 cm (range, 0.4 to 31.8 cm) for those with up-front nephrectomy.
Table 2.
Characteristics of Patients With Stage III Favorable-Histology Wilms Tumor Treated With Regimen DD4A in COG Protocol AREN0532
Outcomes
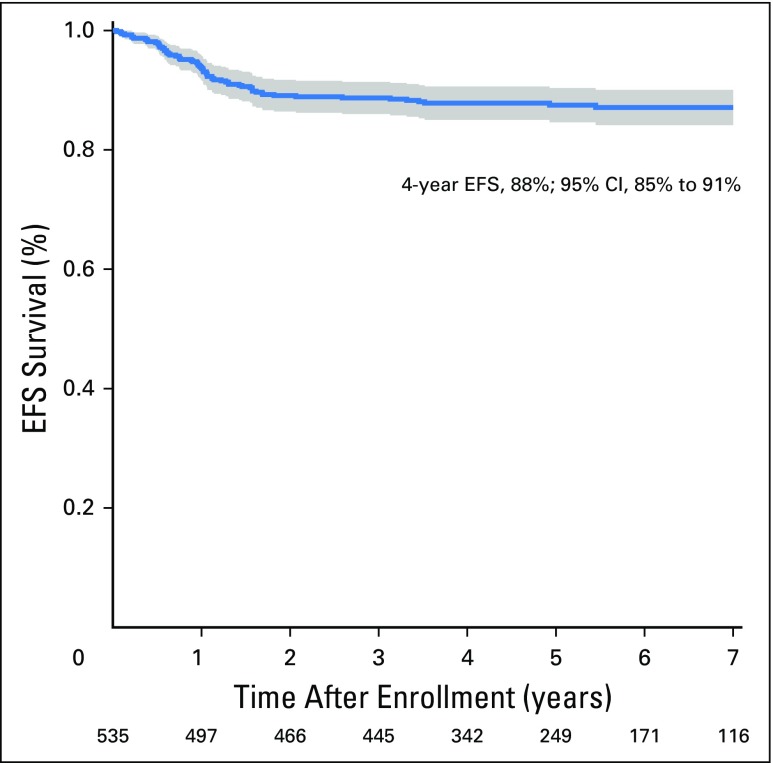
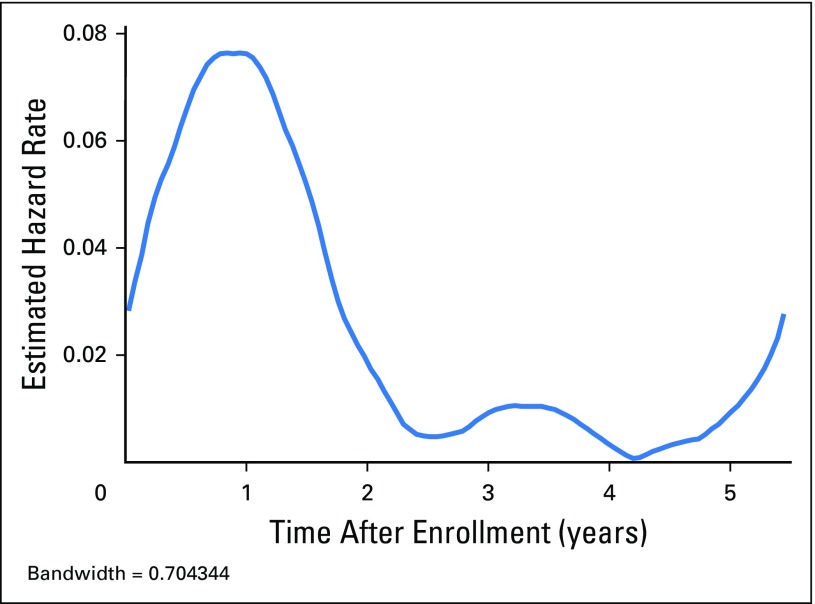
The 4-year EFS and OS estimates were 88% (95% CI, 85% to 91%; Fig 2) and 97% (95% CI, 95% to 98%), respectively. There were 66 first events and 18 deaths. The smoothed hazard function is shown in Appendix Figure A1 (online only). The majority (58 of 66) of the events occurred in the first 2 years after diagnosis. First events consisted of relapse (n = 62) or death from other cause (n = 2). There were two second malignancies as a first event (bladder papillary carcinoma, T-cell leukemia). The majority (50 of 62; 80.6%) of relapses were confirmed by biopsy. By institutional report, the relapse was suspected clinically before routine surveillance imaging in 18 of 62 patients (29%). The median time to relapse among relapsing patients was 11.9 months from study entry (range, 0.5 to 65.4 months). Ten patients relapsed within 7 months postnephrectomy (thus, receiving therapy or identified at the end of therapy evaluations).
Fig 2.
The 4-year event-free survival (EFS) rates for stage III disease in AREN0532.
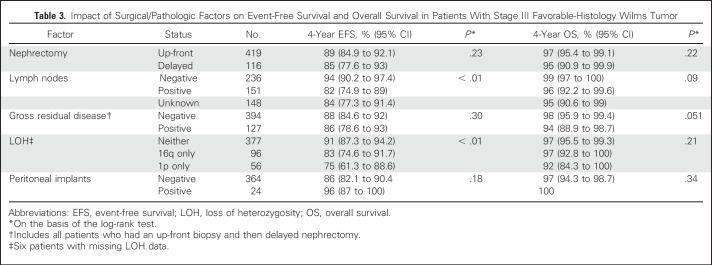
We examined the influence of clinicopathologic factors (positive lymph nodes by pathology, gross residual disease, peritoneal implants, and type of biopsy) on 4-year EFS and OS (Table 3). There was a pattern of poorer 4-year EFS for patients with positive lymph nodes (n = 151; EFS, 82%; 95% CI, 74.9% to 89%) compared with those with negative lymph nodes (n = 236; EFS, 94%; 95% CI, 90.2% to 97.4%) but not a large effect on OS (95% CI overlapped). Patients without lymph node sampling (n = 148) showed a trend toward a lower 4-year EFS of 84% (95% CI, 77.3 to 91.4%); compared with those with lymph node sampling (n = 387), 4-year EFS was 89% (95% CI, 85.6% to 92.8%). There was no difference for patients who underwent up-front nephrectomy (n = 419; 4-year EFS, 89%; 95% CI, 85% to 92.1%) compared with those who received preoperative chemotherapy (n = 116; EFS, 85%; 95% CI, 77.6% to 93.0%), nor was there a difference in 4-year OS (95% CI overlapped). Similarly, there was no impact on 4-year EFS for patients who had gross residual disease, including those who received preoperative chemotherapy (n = 127; EFS, 86%; 95% CI, 78.6% to 93.0%) versus those who had no gross residual disease (n = 394; EFS, 88%; 95% CI, 84.6% to 92%).
Table 3.
Impact of Surgical/Pathologic Factors on Event-Free Survival and Overall Survival in Patients With Stage III Favorable-Histology Wilms Tumor
Among 116 patients who had delayed nephrectomy, 80 (69%) were submitted for central pathology review as of June 30, 2017. These were classified according to the SIOP histologic classification system36 as low risk (n = 7; zero relapses; 4-year EFS, 100%; OS, 100%), intermediate risk (n = 63; six relapses; 4-year EFS, 90.5%; 95% CI, 81.7% to 99.2%; OS, 94.6%; 95% CI, 87.9% to 100%), high-risk/blastemal (n = 7; five relapses; 4-year EFS, 28.6%; 95% CI, 0% to 75.9%; OS, 83.3%; 95% CI, 53.5% to 100%), and indeterminate (n = 3; two relapses). The eight patients with anaplasia are reported separately.
The presence of LOH at either 1p or 16q was found to influence EFS but not OS (Table 3). When lymph node status and LOH status were combined, a strong predictor of excellent EFS and OS emerged when both were absent, and conversely, a relatively poorer outcome was identified if both were present (Table 4). This conclusion still holds whether or not patients with nodes identified at delayed nephrectomy (n = 5) were included.
Table 4.
Combined LOH and Lymph Node Status and Impact on Outcome in Patients With Stage III Favorable-Histology Wilms Tumor Treated With Regimen DD4A and Radiation Therapy
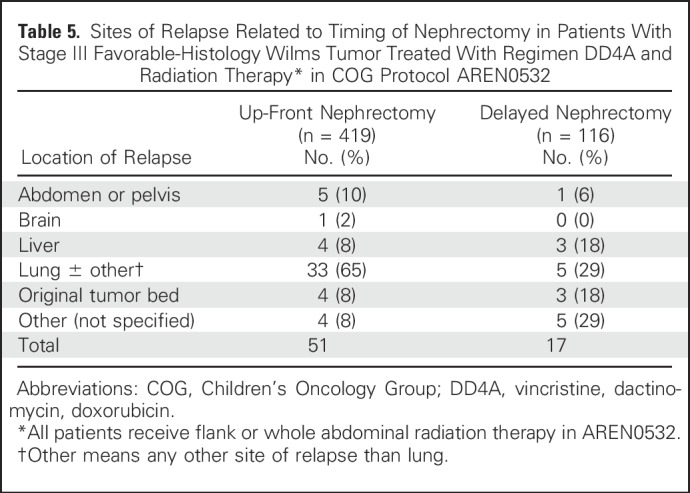
The majority of relapses occurred solely in the lung (n = 33) or in the lung with another site (n = 5). There was a correlation between the site of relapse and up-front or delayed nephrectomy status (Fisher’s exact test P = .035; Table 5).
Table 5.
Sites of Relapse Related to Timing of Nephrectomy in Patients With Stage III Favorable-Histology Wilms Tumor Treated With Regimen DD4A and Radiation Therapy* in COG Protocol AREN0532
Common Terminology Criteria for Adverse Events (version 4.0) grade 4 (n = 34) and 5 (n = 18) events were in the realm of previously reported adverse events and expected progressive disease. One patient developed renal failure.
We compared outcomes of the AREN0532 patients with outcomes of similar patients from the NWTS-5 studied and followed cohorts who received the same therapy in NWTS-5 (n = 716) by the log-rank test. The 4-year EFS in NWTS-5 was 86.5% (95% CI, 83.8% to 89.1%; P =.39) and 4-year OS was 94.5% (95% CI, 92.8% to 96.3%; P = 0.08).
DISCUSSION
This study identified a composite of clinical, pathologic, and molecular features that correlated with prognosis in patients with stage III FHWT. We confirmed the observation from NWTS-5 that lymph node involvement confers inferior EFS.5 We observed a trend toward inferior EFS in patients who did not undergo lymph node sampling, consistent with previous reports.1,36,37 Grundy et al7 reported the lack of impact of LOH 1p or 16q on relapse-free survival and OS in 488 patients with stage III disease treated in NWTS-5. We observed an inferior EFS if either LOH 1p or 16q was present but, likewise, saw no significant impact on OS.
A novel finding in our study was the remarkably strong predictive value of combining LOH and lymph node status. We found that the relapse rate was exceptionally low among patients with tumors that were LOH- and lymph node–negative. However, we demonstrated that those with combined lymph node involvement and LOH 1p or 16q had a significantly worse 4-year EFS outcome of 74%. There is a trend toward a poorer 4-year OS in this comparison; however, it is not statistically different. Longer follow-up is needed to be confident that OS is truly not affected.12,15,38 These findings were the product of a post hoc analysis and thus should be interpreted with caution. Moderately intensified up-front chemotherapy in these poorer risk patients may therefore reduce the risk of relapse.
Approximately two thirds of patients had delayed-nephrectomy tissue submitted for central pathology review. Most patients with blastemal-type Wilms tumor but none of seven patients with low-risk/completely necrotic Wilms tumor experienced relapse, consistent with the findings of SIOP that histologic response to preoperative chemotherapy plays an important role in predicting outcome.9,31,39,40
Avoidance of relapse is clearly desirable for stage III patients where the salvage rate is at best 50%6,13,15,41 and where survivors are at high risk for late effects, such as cardiomyopathy, pulmonary fibrosis, second malignancy, and renal insufficiency.16,17,42,43 Conversely, DD4A therapy carries with it a risk of late effects related to anthracycline and radiation exposure; avoidance of anthracycline would be of potential clinical benefit if there was minimal impact on survival. The SIOP-2001 study demonstrated that omitting doxorubicin in stage II and III intermediate-risk histology nephroblastoma was associated with slightly more frequent relapses, but no difference in OS.20 These findings are not immediately translatable to the COG experience for several reasons. First, the radiation dosing guidelines differ between COG and SIOP protocols. Higher radiation doses delivered in SIOP protocols may have compensated for the omission of doxorubicin. Second, SIOP treatment consisted of 31 weeks of chemotherapy, compared with the 25-week COG regimen. Finally, the SIOP approach of preoperative chemotherapy allows selection of patients with intermediate-risk histology to not receive doxorubicin; patients with blastemal-type histology were excluded. However, we do identify a subset of patients without LOH 1p or 16q, without lymph node involvement at diagnosis, and with completely necrotic tumors at delayed nephrectomy who should be considered for therapy reduction, including avoidance of radiation therapy.
There is strong evidence that 1q gain confers inferior survival.4,26-29,44 We did not attempt to replicate this in our study. There is also a strong correlation of LOH 1p/16q with 1q gain due to common genetic mechanisms (including isochromosome 1q and translocations involving chromosomes 1 and 16).4,45 The weight of evidence in recent publications strongly suggests these markers be incorporated in future risk stratification.
A few features of this study may have affected the outcome of patients.2,46 First, we included as stage III patients previously classified as stage II who had tumor spillage as the sole reason for the stage III assignment. This represented a small number of patients enrolled in this study and would therefore be unlikely to influence the overall results. In addition, in contrast to NWTS-5, patients with local stage III disease and nodules visible only on computed tomography were all treated as stage IV unless proven on biopsy not to represent malignancy; these patients with stage IV disease were not eligible for the AREN0532 study. We also noted a trend toward a higher rate of delayed nephrectomy.47-49 Some of these patients may have had local stage I or II disease and were converted to stage III by virtue of biopsy and delay in nephrectomy. Despite these limitations that could be considered stage migration, we are able, with these analyses, to identify particular subgroups that may be tested for therapy intensification or reduction.
The strengths of this study include prospective, real-time central surgical, pathologic, and radiologic review. To our knowledge, this is the largest reported cohort of patients with local stage III FHWT without the potential confounding influence of patients with local stage III presenting with disseminated disease. We have central review of more than two thirds of patients with delayed nephrectomy and have been able to correlate EFS with histology. Some caution is merited because our study was not powered to correlate histology after delayed nephrectomy with outcomes. Limitations include that we did not have 1q gain status for this cohort, because the strong importance of 1q gain was identified after the conclusion of this study. Lastly, we found attempts at classification of microscopic disease as somewhat subjective and thus could not confidently test this as a risk factor for relapse.
In summary, we described the overall good outcome of patients with stage III FHWT using DD4A with radiation therapy and identified an association of combined lymph node and LOH status, as well as postchemotherapy, delayed nephrectomy histology, with EFS. Given the serious potential late effects of doxorubicin, we believe it essential to examine strategies to eliminate anthracyclines in patients with an excellent prognosis. At the same time, prevention of relapse in higher risk patients is an important goal. Taken together, the COG Renal Tumors Committee is strongly considering using a prognostic algorithm of up-front nephrectomy when feasible, incorporating LOH 1p/16q status and 1q gain to identify patients who merit more intensive therapy. We are also planning to study the omission of doxorubicin in patients who lack these risk factors. For those with delayed nephrectomy, we propose to validate the SIOP-type strategy of treatment on the basis of postnephrectomy histology in the context of a COG-based induction regimen. These proposed research strategies are not yet proven.
ACKNOWLEDGMENT
We thank the parents and children who enrolled in this study and the investigators of the Children’s Oncology Group and the many pathologists, surgeons, pediatricians, radiation oncologists, diagnostic imagers, statisticians, and other health professionals who manage the children entered in the Children’s Oncology Group renal tumor studies. We also thank the following research and protocol coordinators who worked on the project over the years: Celeste Sabinske, Ellen Tsan, Tanya Wallas, Laetitia Goddet, Meera Raman, Teni Karimimian, and Mary Bancroft.
Appendix
Children’s Oncology Group Operations Related to AREN0532
The Children’s Oncology Group (COG) renal tumors committee is a multidisciplinary committee (oncology, surgery, radiation therapy, diagnostic imaging, pathology, biology, nursing, clinical research associate, statistics) that oversees the conduct of therapeutic trials. The study protocol is developed by the renal committee and approved by the Scientific Council of senior leadership of COG and then by the Clinical Trials Evaluation Program of the National Institutes of Health. The protocol is then reviewed by the independent National Cancer Institute–sponsored central pediatric Institutional Review Board, where regulations allow (US sites). These reviews are compliant with the US Code of Federal Regulations (Common Rule). The protocol is approved by the local institutional research ethics board in jurisdictions where US regulations do not apply.
External validity.
The COG currently encompasses approximately 220 member institutions. As of June 30, 2015, 174 COG institutions (of 218 active) had active institutional review board approval to participate in the study. This fluctuated by a few institutions over the course of the study. Each institution is assessed by COG for membership on the basis of a minimum number of clinical trial enrollments per year on the basis of a rolling average of 12 per year over 3 years and adequate clinical trials and regulatory infrastructure to support children with cancer.
The vast majority of institutions that treat children with cancer in North America (> 90%), as well as a number of institutions in Australia, New Zealand, Switzerland, and Israel, participated in this trial. Thus, this study is not a population-based study but approximates one in North America.
Internal validity.
All institutions are subject to an in-person COG regulatory audit that occurs on a minimum of a 3-year rotating basis. Data quality is the responsibility of the institutional principal investigator (PI) and selected study data are examined by the COG auditors. COG sends out regular reminders to the lead clinical research associate and PI at each institution noting delinquent data form submissions, has an online delinquency annotation (called a Scheduled and Delinquency Data [SADD] list) and compiles an institutional delinquency report quarterly. The compliance with the SADD list in the majority of institutions exceeds 98%. Major violations with compliance may lead to institutional suspension. Inconsistent or missing data are queried and cleaned.
All patients enrolled in the AREN0532 study had to first enroll in the biology and classification study AREN03B2 study, which had central review of original pathology slides, institutional pathology reports, surgical reports, and submitted diagnostic imaging by an expert review committee of the COG renal tumors committee. All patients enrolled in this study were further secondarily reviewed from these original reports by the study PI (C.V.F.) to confirm eligibility. This is recorded in a study-specific study chair eligibility form.
During the follow-up, significant events, such as relapse, progression, second malignancy neoplasm, and death, were monitored. In the article, median duration of follow-up was reported for patients who were still alive as of December 31, 2016. The schedule for statistical follow-up is every 6 months for the first 3 years (after off-protocol therapy) and annually thereafter until the eighth anniversary of study entry.
Surveillance Imaging Required by AREN0532 Protocol
Fig A1.
Epanechnikov kernel-smoothed hazard function.
Table A1.
AREN0532 Diagnostic and On-Therapy Imaging for Patients With Stage III Disease
Table A2.
AREN0532 Off-Therapy Surveillance
Regimen: Vincristine, Dactinomycin, Doxorubicin (July 26, 2017)
Table A3.
Regimen DD4A
Table A4.
Dosing of Chemotherapy for Regimen DD4A
Footnotes
Supported by grants No. U10CA180886, U10CA180899, U10CA098543, U10CA098413, and U24CA114766 from the National Cancer Institute, National Institutes of Health, to support the Children’s Oncology Group. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Also supported by St Baldrick’s Foundation.
Presented in part at the 2015 American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 29-June 2, 2015.
Clinical trial information: NCT00352534.
AUTHOR CONTRIBUTIONS
Conception and design: Conrad V. Fernandez, Elizabeth A. Mullen, Peter F. Ehrlich, Elizabeth J. Perlman, John A. Kalapurakal, Thomas E. Hamilton, Fredric A. Hoffer, Robert C. Shamberger, James I. Geller, James R. Anderson, Paul E. Grundy, Jeffrey S. Dome
Administrative support: Jeffrey S. Dome
Provision of study materials or patients: Jeffrey S. Dome
Collection and assembly of data: Conrad V. Fernandez, Elizabeth A. Mullen, Yueh-Yun Chi, Peter F. Ehrlich, Elizabeth J. Perlman, John A. Kalapurakal, Geetika Khanna, Arnold C. Paulino, Thomas E. Hamilton, Kenneth W. Gow, Zelig Tochner, Fredric A. Hoffer, Janice S. Withycombe, James I. Geller, James R. Anderson, Paul E. Grundy, Jeffrey S. Dome
Data analysis and interpretation: Conrad V. Fernandez, Elizabeth A. Mullen, Yueh-Yun Chi, Peter F. Ehrlich, Elizabeth J. Perlman, John A. Kalapurakal, Geetika Khanna, Thomas E. Hamilton, Robert C. Shamberger, Yeonil Kim, James I. Geller, Paul E. Grundy, Jeffrey S. Dome
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Outcome and Prognostic Factors in Stage III Favorable-Histology Wilms Tumor: A Report From the Children’s Oncology Group Study AREN0532
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Conrad V. Fernandez
No relationship to disclose
Elizabeth A. Mullen
No relationship to disclose
Yueh-Yun Chi
No relationship to disclose
Peter F. Ehrlich
No relationship to disclose
Elizabeth J. Perlman
No relationship to disclose
John A. Kalapurakal
No relationship to disclose
Geetika Khanna
No relationship to disclose
Arnold C. Paulino
Employment: MD Anderson Cancer Center
Patents, Royalties, Other Intellectual Property: Royalty from Elsevier for book on PET/CT in radiotherapy treatment planning
Travel, Accommodations, Expenses: Henry Ford Hospital
Thomas E. Hamilton
No relationship to disclose
Kenneth W. Gow
Consulting or Advisory Role: BARD
Zelig Tochner
Consulting or Advisory Role: Mevion Medical Systems, P-Cure
Speakers' Bureau: Mevion Medical Systems
Travel, Accommodations, Expenses: Mevion Medical Systems, P-Cure
Fredric A. Hoffer
No relationship to disclose
Janice S. Withycombe
No relationship to disclose
Robert C. Shamberger
No relationship to disclose
Yeonil Kim
No relationship to disclose
James I. Geller
No relationship to disclose
James R. Anderson
Employment: Merck
Paul E. Grundy
No relationship to disclose
Jeffrey S. Dome
Patents, Royalties, Other Intellectual Property: Rockland Immunochemicals
REFERENCES
- 1.Irtan S, Jitlal M, Bate J, et al. : Risk factors for local recurrence in Wilms tumour and the potential influence of biopsy - The United Kingdom experience. Eur J Cancer 51:225-232, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Green DM, Breslow NE, D’Angio GJ, et al. : Outcome of patients with stage II/favorable histology Wilms tumor with and without local tumor spill: A report from the National Wilms Tumor Study Group. Pediatr Blood Cancer 61:134-139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dome JS, Perlman EJ, Graf N: Risk stratification for Wilms tumor: Current approach and future directions. Am Soc Clin Oncol Educ Book 34:215-223, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Gratias EJ, Jennings LJ, Anderson JR, et al. : Gain of 1q is associated with inferior event-free and overall survival in patients with favorable histology Wilms tumor: A report from the Children’s Oncology Group. Cancer 119:3887-3894, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrlich PF, Anderson JR, Ritchey ML, et al. : Clinicopathologic findings predictive of relapse in children with stage III favorable-histology Wilms tumor. J Clin Oncol 31:1196-1201, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green DM, Cotton CA, Malogolowkin M, et al. : Treatment of Wilms tumor relapsing after initial treatment with vincristine and actinomycin D: A report from the National Wilms Tumor Study Group. Pediatr Blood Cancer 48:493-499, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Grundy PE, Breslow NE, Li S, et al. : Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol 23:7312-7321, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Kaste SC, Dome JS, Babyn PS, et al. : Wilms tumour: Prognostic factors, staging, therapy and late effects. Pediatr Radiol 38:2-17, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Reinhard H, Semler O, Bürger D, et al. : Results of the SIOP 93-01/GPOH trial and study for the treatment of patients with unilateral nonmetastatic Wilms Tumor. Klin Padiatr 216:132-140, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Weirich A, Ludwig R, Graf N, et al. : Survival in nephroblastoma treated according to the trial and study SIOP-9/GPOH with respect to relapse and morbidity. Ann Oncol 15:808-820, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Dome JS, Graf N, Geller JI, et al. : Advances in Wilms Tumor treatment and biology: Progress through international collaboration. J Clin Oncol 33:2999-3007, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mavinkurve-Groothuis AM, van den Heuvel-Eibrink MM, Tytgat GA, et al. : Treatment of relapsed Wilms tumour (WT) patients: Experience with topotecan. A report from the SIOP Renal Tumour Study Group (RTSG). Pediatr Blood Cancer 62:598-602, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Malogolowkin M, Spreafico F, Dome JS, et al. : Incidence and outcomes of patients with late recurrence of Wilms’ tumor. Pediatr Blood Cancer 60:1612-1615, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Ha TC, Spreafico F, Graf N, et al. : An international strategy to determine the role of high dose therapy in recurrent Wilms’ tumour. Eur J Cancer 49:194-210, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Malogolowkin M, Cotton CA, Green DM, et al. : Treatment of Wilms tumor relapsing after initial treatment with vincristine, actinomycin D, and doxorubicin. A report from the National Wilms Tumor Study Group. Pediatr Blood Cancer 50:236-241, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Termuhlen AM, Tersak JM, Liu Q, et al. : Twenty-five year follow-up of childhood Wilms tumor: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 57:1210-1216, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasso G, Greco N, Murino P, et al. : Late toxicity in Wilms tumor patients treated with radiotherapy at 15 years of median follow-up. J Pediatr Hematol Oncol 32:e264-e267, 2010 [DOI] [PubMed] [Google Scholar]
- 18. doi: 10.1200/JCO.2008.18.6981. Cotton CA, Peterson S, Norkool PA, et al: Early and late mortality after diagnosis of Wilms tumor. J Clin Oncol 27:1304-1309, 2009 [Erratum: J Clin Oncol 27:1304-1309, 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright KD, Green DM, Daw NC: Late effects of treatment for Wilms tumor. Pediatr Hematol Oncol 26:407-413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pritchard-Jones K, Bergeron C, de Camargo B, et al. : Omission of doxorubicin from the treatment of stage II-III, intermediate-risk Wilms’ tumour (SIOP WT 2001): An open-label, non-inferiority, randomised controlled trial. Lancet 386:1156-1164, 2015 [DOI] [PubMed] [Google Scholar]
- 21. doi: 10.1016/j.ccell.2015.01.003. Walz AL, Ooms A, Gadd S, et al: Recurrent DGCR8, DROSHA, and SIX homeodomain mutations in favorable histology Wilms tumors. Cancer Cell 27:286-297, 2015 [Erratum: Cancer Cell 27:286-297, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlton J, Pavasovic V, Pritchard-Jones K: Biomarkers to detect Wilms tumors in pediatric patients: Where are we now? Future Oncol 11:2221-2234, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Wegert J, Ishaque N, Vardapour R, et al. : Mutations in the SIX1/2 pathway and the DROSHA/DGCR8 miRNA microprocessor complex underlie high-risk blastemal type Wilms tumors. Cancer Cell 27:298-311, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Sredni ST, Gadd S, Huang CC, et al. : Subsets of very low risk Wilms tumor show distinctive gene expression, histologic, and clinical features. Clin Cancer Res 15:6800-6809, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Anglesio MS, O’Sullivan M, et al. : The E3 ligase HACE1 is a critical chromosome 6q21 tumor suppressor involved in multiple cancers. Nat Med 13:1060-1069, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Gratias EJ, Dome JS, Jennings LJ, et al. : Association of chromosome 1q gain with inferior survival in favorable-histology Wilms tumor: A report from the Children’s Oncology Group. J Clin Oncol 34:3189-3194, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chagtai T, Zill C, Dainese L, et al. : Gain of 1q as a prognostic biomarker in Wilms tumors (WTs) treated with preoperative chemotherapy in the International Society of Paediatric Oncology (SIOP) WT 2001 trial: A SIOP Renal Tumours Biology Consortium Study. J Clin Oncol 34:3195-3203, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segers H, van den Heuvel-Eibrink MM, Williams RD, et al. : Gain of 1q is a marker of poor prognosis in Wilms’ tumors. Genes Chromosomes Cancer 52:1065-1074, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Hing S, Lu YJ, Summersgill B, et al. : Gain of 1q is associated with adverse outcome in favorable histology Wilms’ tumors. Am J Pathol 158:393-398, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchey ML, Shamberger RC, Haase G, et al. : Surgical complications after primary nephrectomy for Wilms’ tumor: Report from the National Wilms’ Tumor Study Group. J Am Coll Surg 192:63-68, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Weirich A, Leuschner I, Harms D, et al. : Clinical impact of histologic subtypes in localized non-anaplastic nephroblastoma treated according to the trial and study SIOP-9/GPOH. Ann Oncol 12:311-319, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Green DM, Breslow NE, Beckwith JB, et al. : Effect of duration of treatment on treatment outcome and cost of treatment for Wilms’ tumor: A report from the National Wilms’ Tumor Study Group. J Clin Oncol 16:3744-3751, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Kalapurakal JA, Dome JS, Perlman EJ, et al. : Management of Wilms’ tumour: Current practice and future goals. Lancet Oncol 5:37-46, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Kalapurakal JA, Li SM, Breslow NE, et al. : Influence of radiation therapy delay on abdominal tumor recurrence in patients with favorable histology Wilms’ tumor treated on NWTS-3 and NWTS-4: A report from the National Wilms’ Tumor Study Group. Int J Radiat Oncol Biol Phys 57:495-499, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Fernandez CV, Perlman EJ, Mullen EA, et al. : Clinical outcome and biological predictors of relapse after nephrectomy only for very low-risk Wilms tumor: A report from Children’s Oncology Group AREN0532. Ann Surg 265:835-840, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vujanić GM, Sandstedt B, Harms D, et al. : Revised International Society of Paediatric Oncology (SIOP) working classification of renal tumors of childhood. Med Pediatr Oncol 38:79-82, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Shamberger RC, Guthrie KA, Ritchey ML, et al. : Surgery-related factors and local recurrence of Wilms tumor in National Wilms Tumor Study 4. Ann Surg 229:292-297, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spreafico F, Pritchard-Jones K, Bergeron C, et al. : Value and difficulties of a common European strategy for recurrent Wilms’ tumor. Expert Rev Anticancer Ther 9:693-696, 2009 [DOI] [PubMed] [Google Scholar]
- 39.van den Heuvel-Eibrink MM, van Tinteren H, Bergeron C, et al. : Outcome of localised blastemal-type Wilms tumour patients treated according to intensified treatment in the SIOP WT 2001 protocol, a report of the SIOP Renal Tumour Study Group (SIOP-RTSG). Eur J Cancer 51:498-506, 2015 [DOI] [PubMed] [Google Scholar]
- 40.de Kraker J, Graf N, van Tinteren H, et al. : Reduction of postoperative chemotherapy in children with stage I intermediate-risk and anaplastic Wilms’ tumour (SIOP 93-01 trial): A randomised controlled trial. Lancet 364:1229-1235, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Pein F, Michon J, Valteau-Couanet D, et al. : High-dose melphalan, etoposide, and carboplatin followed by autologous stem-cell rescue in pediatric high-risk recurrent Wilms’ tumor: A French Society of Pediatric Oncology study. J Clin Oncol 16:3295-3301, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Lange JM, Takashima JR, Peterson SM, et al. : Breast cancer in female survivors of Wilms tumor: A report from the national Wilms tumor late effects study. Cancer 120:3722-3730, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knijnenburg SL, Mulder RL, Schouten-Van Meeteren AY, et al. : Early and late renal adverse effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev 10:CD008944, 2013 [DOI] [PubMed] [Google Scholar]
- 44. doi: 10.1002/gcc.22250. Pritchard-Jones K, Williams R, Segers H, et al: Response to the letter to the editor: 1q gain is a frequent finding in preoperatively treated Wilms tumors, but of limited prognostic value for risk satisfaction in the SIOP2009/Gesellschaft fur Padiatrische Onkologie und Hamatologie (GPOH) trial. Genes Chromosomes Cancer 54:397-399, 2015. [DOI] [PubMed] [Google Scholar]
- 45.Bown N, Cotterill SJ, Roberts P, et al. : Cytogenetic abnormalities and clinical outcome in Wilms tumor: A study by the U.K. cancer cytogenetics group and the U.K. Children’s Cancer Study Group. Med Pediatr Oncol 38:11-21, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Green DM: Considerations in the diagnosis and management of pediatric patients with favorable histology Wilms tumor who present with only pulmonary nodules. Pediatr Blood Cancer 63:589-592, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powis M, Messahel B, Hobson R, et al. : Surgical complications after immediate nephrectomy versus preoperative chemotherapy in non-metastatic Wilms’ tumour: Findings from the 1991-2001 United Kingdom Children’s Cancer Study Group UKW3 Trial. J Pediatr Surg 48:2181-2186, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Green DM: Controversies in the management of Wilms tumour - Immediate nephrectomy or delayed nephrectomy? Eur J Cancer 43:2453-2456, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Mitchell C, Pritchard-Jones K, Shannon R, et al. : Immediate nephrectomy versus preoperative chemotherapy in the management of non-metastatic Wilms’ tumour: Results of a randomised trial (UKW3) by the UK Children’s Cancer Study Group. Eur J Cancer 42:2554-2562, 2006 [DOI] [PubMed] [Google Scholar]