Abstract
Purpose
The incidence of human papilloma virus (HPV)–positive oropharyngeal cancers has risen rapidly in recent decades among men in the United States. We investigated the US population–level effect of prophylactic HPV vaccination on the burden of oral HPV infection, the principal cause of HPV-positive oropharyngeal cancers.
Methods
We conducted a cross-sectional study of men and women 18 to 33 years of age (N = 2,627) within the National Health and Nutrition Examination Survey 2011 to 2014, a representative sample of the US population. Oral HPV infection with vaccine types 16, 18, 6, or 11 was compared by HPV vaccination status, as measured by self-reported receipt of at least one dose of the HPV vaccine. Analyses accounted for the complex sampling design and were adjusted for age, sex, and race. Statistical significance was assessed using a quasi-score test.
Results
Between 2011 and 2014, 18.3% of the US population 18 to 33 years of age reported receipt of at least one dose of the HPV vaccine before the age of 26 years (29.2% in women and 6.9% in men; P < .001). The prevalence of oral HPV16/18/6/11 infections was significantly reduced in vaccinated versus unvaccinated individuals (0.11% v 1.61%; Padj = .008), corresponding to an estimated 88.2% (95% CI, 5.7% to 98.5%) reduction in prevalence after model adjustment for age, sex, and race. Notably, the prevalence of oral HPV16/18/6/11 infections was significantly reduced in vaccinated versus unvaccinated men (0.0% v 2.13%; Padj = .007). Accounting for vaccine uptake, the population-level effect of HPV vaccination on the burden of oral HPV16/18/6/11 infections was 17.0% overall, 25.0% in women, and 6.9% in men.
Conclusion
HPV vaccination was associated with reduction in vaccine-type oral HPV prevalence among young US adults. However, because of low vaccine uptake, the population-level effect was modest overall and particularly low in men.
INTRODUCTION
The incidence of oropharyngeal cancer caused by human papillomavirus (HPV) infection has increased rapidly in recent decades in men in the United States as well as numerous other developed countries worldwide.1 Furthermore, HPV-positive oropharyngeal cancer is projected to become the most common HPV-caused cancer in the United States by 2020, with the majority of the burden in men.1 More than 70% of the approximately 12,000 oropharyngeal cancers diagnosed annually in the United States are caused by HPV, with approximately 90% of HPV-positive oropharyngeal cancers caused by HPV16 and the remainder caused by other oncogenic HPV types.1-3 Given the absence of screening and secondary prevention strategies, prophylactic HPV vaccination has the greatest potential to prevent HPV-positive oropharyngeal cancers.4
Prophylactic HPV vaccination with the bivalent (HPV16/18), quadrivalent (HPV16/18/6/11), or nonavalent (HPV16/18/6/11/31/33/45/52/58) vaccine is currently recommended for US females and males (quadrivalent and nonavalent) ages 9 to 26 years.5 HPV vaccination was recommended for routine use in US females in 2006, permissive use in US males in 2009, and routine use in US males in 2011. These vaccines are indicated for the prevention of genital warts (caused by HPV types 6 and 11) and cervical, anal, vulvar, and vaginal precancers and cancers in females and genital warts and anal precancer and cancer in males.5 Randomized clinical trials demonstrate > 90% vaccine efficacy in the prevention of anogenital HPV infections and precancerous lesions.5,6 Surveillance studies also show significant population-level reductions in genital HPV prevalence among young US females in the postlicensure era.7,8 In contrast, few studies have evaluated the population-level effect of HPV vaccination on oral HPV infections.9-11
Herein, we provide a surveillance report of the population-level effect of prophylactic HPV vaccination on the burden of oral HPV infections in young US women and men.
METHODS
We compared oral HPV prevalence in vaccinated versus unvaccinated men and women 18 to 33 years of age within the National Health and Nutrition Examination Survey (NHANES) cycles 2011 to 2012 and 2013 to 2014.12 The NHANES is a representative cross-sectional, stratified, multistage probability sample of the noninstitutionalized civilian US population.12
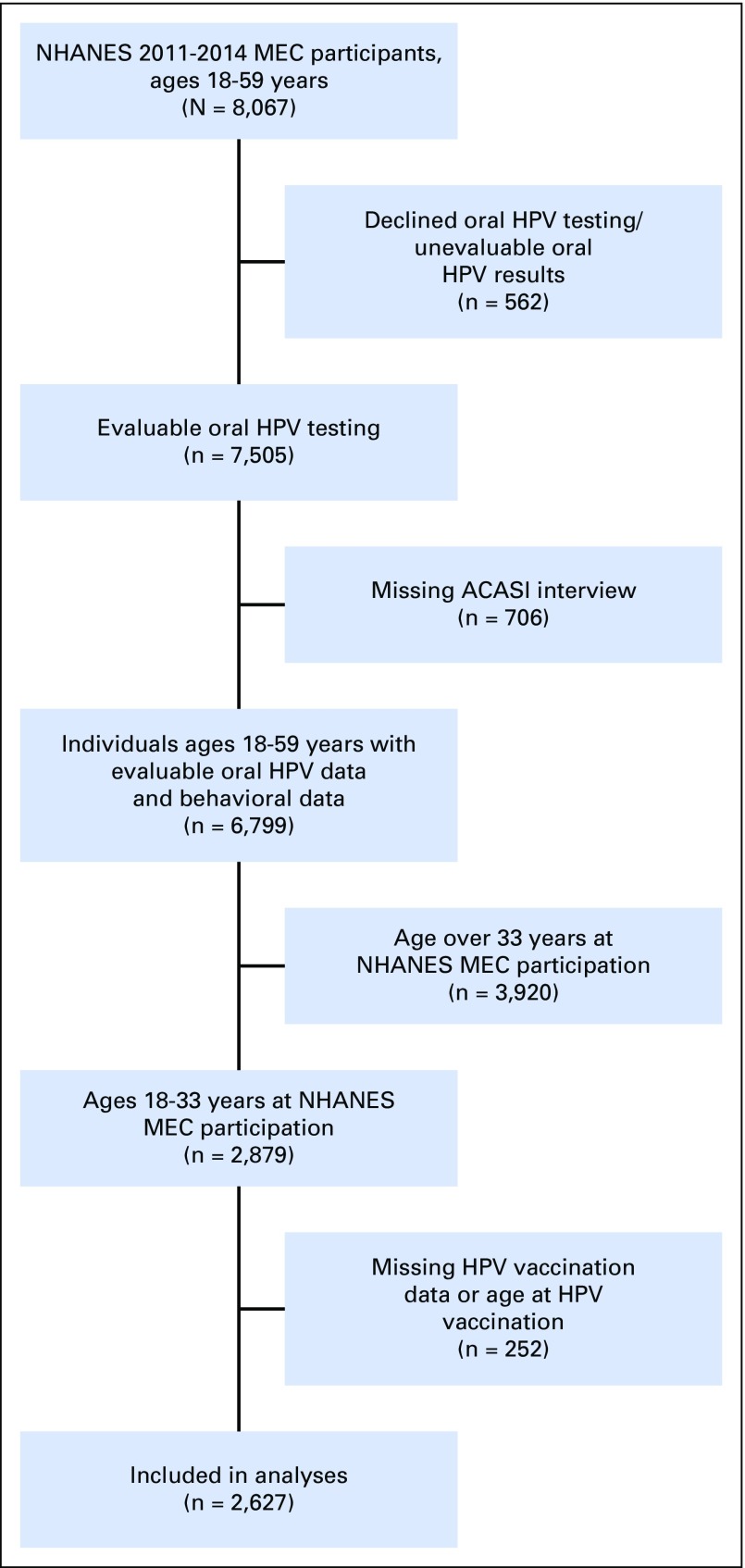
In the NHANES Mobile Exam Center (MEC),13 all participants 14 to 69 years of age provided a 10-mL scope/saline oral rinse and gargle sample. DNA from oral rinses was evaluated for the presence of 37 HPV genotypes using PGMY09/11 polymerase chain reaction and Roche Linear Array genotyping.13 Demographic and behavioral factors were collected through an audio computer-assisted self-interview during the MEC visit.12 Information on HPV vaccination (receipt, age at vaccination, and doses) was collected from participants 9 to 59 years of age by interviewers during the household visit.12 Public-use data on both oral HPV infection and vaccination were available in individuals 18 to 59 years of age (Appendix Fig A1, online only).
Statistical Analyses
Analyses were restricted to 2,627 individuals 18 to 33 years of age; 33 years was the oldest observed age for individuals who were vaccinated through 26 years of age, the oldest recommended age for vaccination in the United States. Individuals who received at least one dose were considered vaccinated.
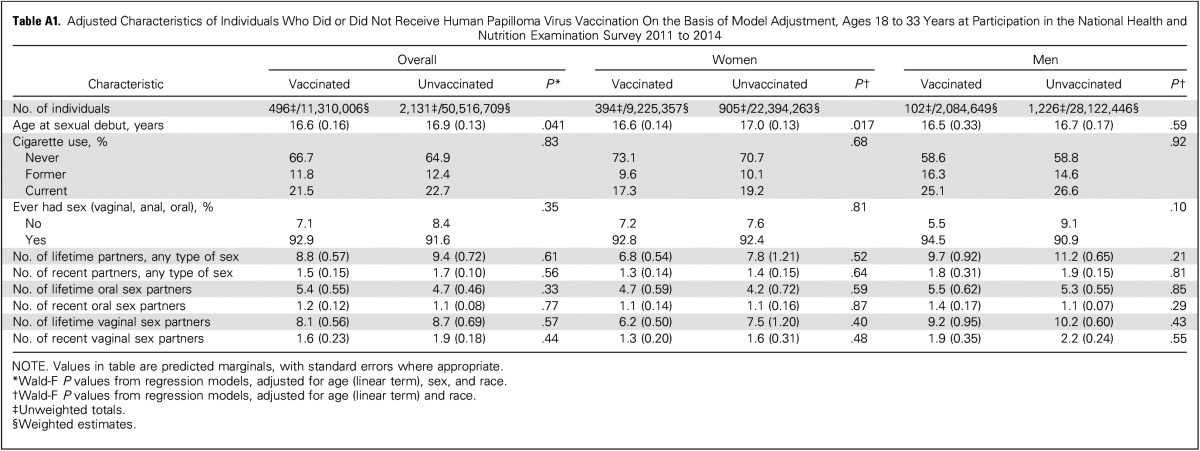
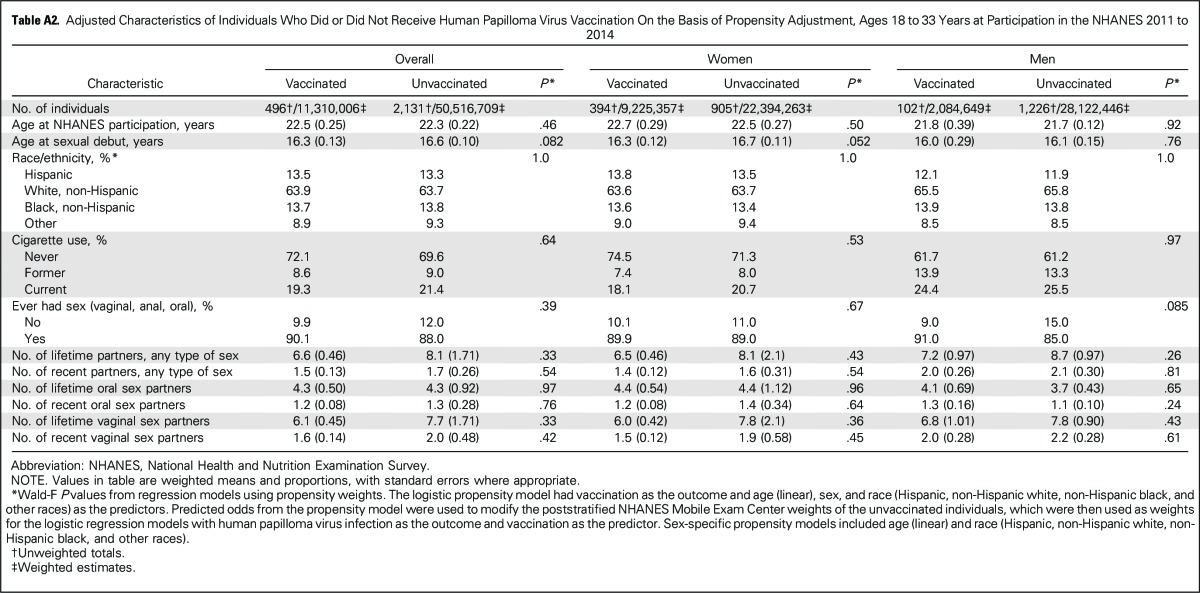
All analyses were conducted in SAS (Cary, NC) and SAS-Callable SUDAAN (RTI International, Raleigh, NC). The primary outcome was oral HPV16/18/6/11 prevalence, given the predominant use of the quadrivalent vaccine in the United States through 2014.5,7 Analyses accounted for the complex sampling design and potential bias from exclusions (Fig A1) through use of MEC sample weights, repost-stratified to match the age-by-sex-by-race US population distribution. Because of the inclusion of two NHANES cycles in the analyses, per NHANES analytic guidelines, we used the sample weights of each NHANES cycles divided by two. Given the nonrandomized comparisons, the vaccinated and unvaccinated groups differed by demographic and behavioral factors (Table 1), which substantially diminished upon adjustment for age (at NHANES participation), sex, and race (Appendix Tables A1 and A2, online only). Thus, oral HPV comparisons by vaccination status were conducted using binary logistic regression, with model adjustment for age, sex, and race. As alternative analyses, we also used propensity weighting (for vaccination by age, sex, and race), which incorporates fewer assumptions but may have lower statistical power than model adjustment.14 Additional adjustment for socioeconomic status factors, such as education, did not materially change the results (data not shown).
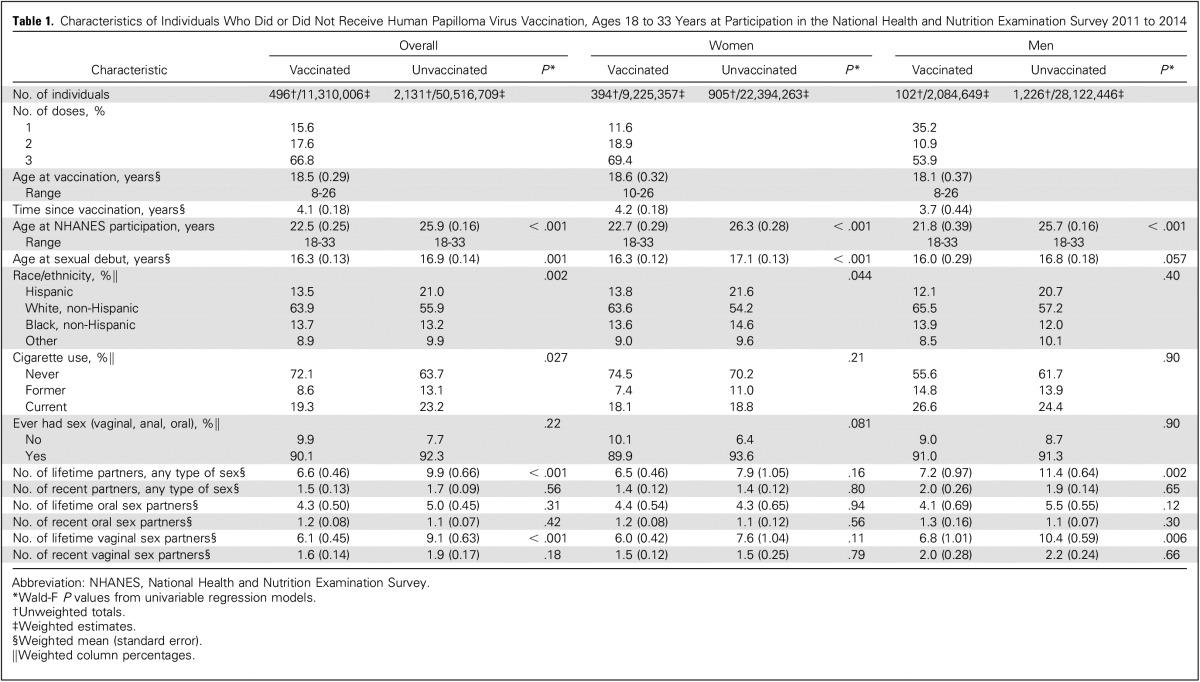
Table 1.
Characteristics of Individuals Who Did or Did Not Receive Human Papilloma Virus Vaccination, Ages 18 to 33 Years at Participation in the National Health and Nutrition Examination Survey 2011 to 2014
Comparisons between vaccinated and unvaccinated individuals are presented as infection prevalence and percent reduction in prevalence ([unvaccinated minus vaccinated/unvaccinated] × 100). Prevaccine-era oral HPV prevalence data are not available in the US population; thus, we used the observed prevalence in unvaccinated individuals as the best-available surrogate for prevaccine-era oral HPV prevalence. We then estimated the total number of infections in the absence of HPV vaccination in the US population (US population size × prevalence in unvaccinated), the number of preventable infections at 100% vaccination levels (US population size × prevalence in vaccinated), and the number of potentially vaccine-prevented infections at current HPV vaccine–uptake levels (US population size of vaccinated individuals × prevalence difference between unvaccinated and vaccinated). As a measure of the population-level effect of HPV vaccination on the burden of oral HPV infection, we estimated the proportion of potentially vaccine-prevented infections at the current HPV vaccine–uptake levels (number of potentially vaccine-prevented infections/total number of infections in the absence of HPV vaccination).
Given the sparsity of oral HPV outcomes in the vaccinated group, statistical comparisons were conducted using a quasi-score test.15 Specifically, the score test and the corresponding P value were computed under the null hypothesis of no difference in oral HPV prevalence between the vaccinated and unvaccinated groups, which allowed pooling of the two groups, thus ameliorating small event rates in either group. Prior simulation studies showed better performance of the score test compared with standard Wald tests for obtaining accurate P values under studies using logistic regression with small event rates, while also accounting for clustered sampling designs.15 We used logistic regression modeling through either model adjustment or propensity adjustment to account for the imbalance in confounders between the vaccinated and unvaccinated individuals; we then computed the score test P value for the comparison of oral HPV prevalence between vaccinated and unvaccinated individuals. Using the model-adjustment approach, predicted margins were computed to obtain adjusted prevalence and prevalence ratios (vaccinated v unvaccinated), which are directly standardized to the distribution in the United States of the covariates used in the logistic modeling. Statistical significance was assessed at a two-sided P < .05.
RESULTS
Between 2011 and 2014, 18.3% of the US population 18 to 33 years of age reported receipt of at least one dose of HPV vaccine through the age of 26 years. Vaccination rates were significantly higher in women than men (29.2% v 6.9%; P < .001). Vaccinated and unvaccinated individuals significantly differed by demographic and behavioral characteristics in unadjusted analyses (Table 1). However, characteristics were similar upon adjustment for age, sex, and race through either model adjustment (Appendix Table A1) or propensity weighting (Appendix Table A2).
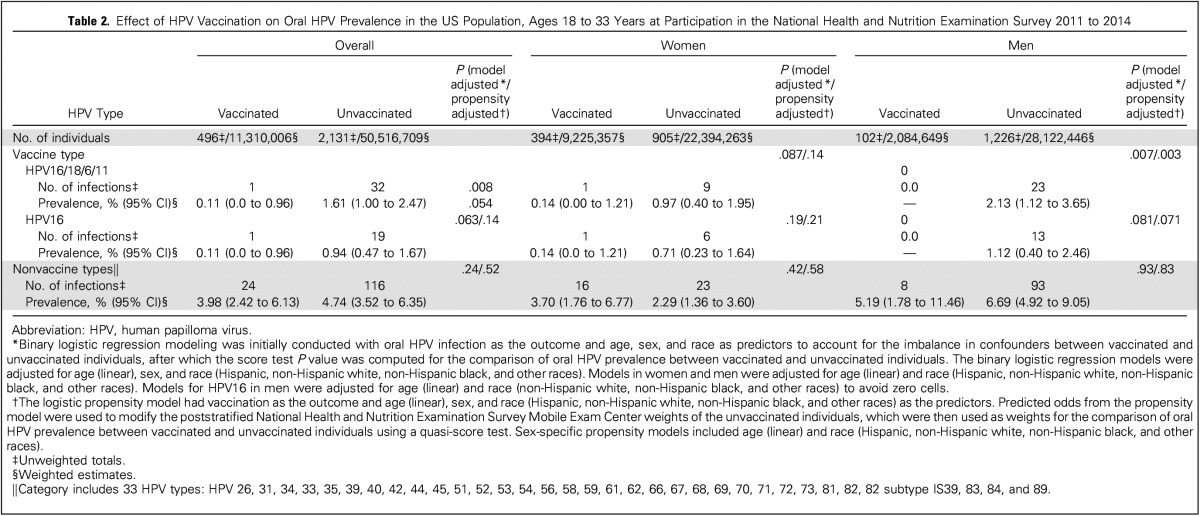
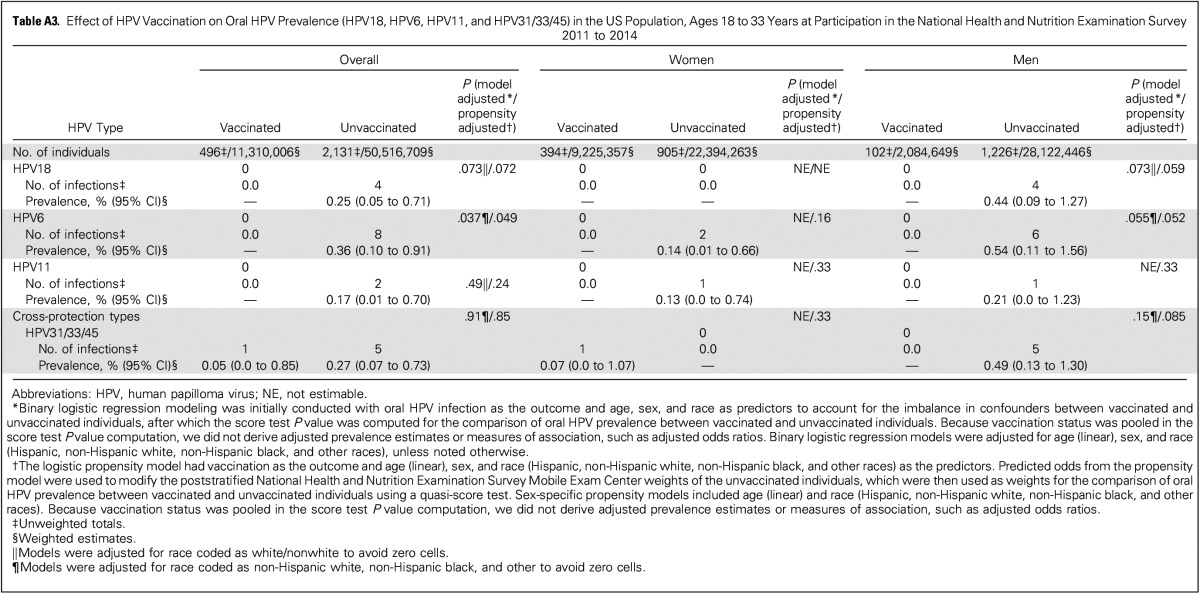
Oral HPV prevalence was assessed at an average of 4.1 years after vaccination. The prevalence of vaccine-type oral HPV infections (HPV16/18/6/11) was significantly reduced in vaccinated versus unvaccinated individuals 18 to 33 years of age (0.11% v 1.61%; model-adjusted P = .008; Table 2). This corresponded to an adjusted (model adjustment for age, sex, and race) prevalence ratio of 8.45 for unvaccinated versus vaccinated individuals (95% CI, 1.06 to 67.22) and an estimated 88.2% (95% CI, 5.7% to 98.5%) reduction in vaccine-type infections among vaccinated individuals. Prevalence of oral HPV16, which accounts for approximately 90% of HPV-positive oropharyngeal cancers, was also nonsignificantly reduced in vaccinated versus unvaccinated individuals (0.11% v 0.94%; model-adjusted P = .063). In contrast, prevalence of 33 nonvaccine HPV types was similar between vaccinated versus unvaccinated individuals (Table 2: 3.98% v 4.74%; model-adjusted P = .24). Event numbers for HPV18, HPV6, HPV11, and types with limited cross protection (HPV31/33/45) were too low for reliable estimation (Appendix Table A3, online only).
Table 2.
Effect of HPV Vaccination on Oral HPV Prevalence in the US Population, Ages 18 to 33 Years at Participation in the National Health and Nutrition Examination Survey 2011 to 2014
Results were similar in sex-stratified as well as propensity-adjusted analyses, albeit at lower significance levels (Table 2). Notably, prevalence of vaccine-type oral HPV infections (HPV16/18/6/11) was significantly reduced in vaccinated men versus unvaccinated men (Table 2: 0.0% v 2.13%; model-adjusted P = .007).
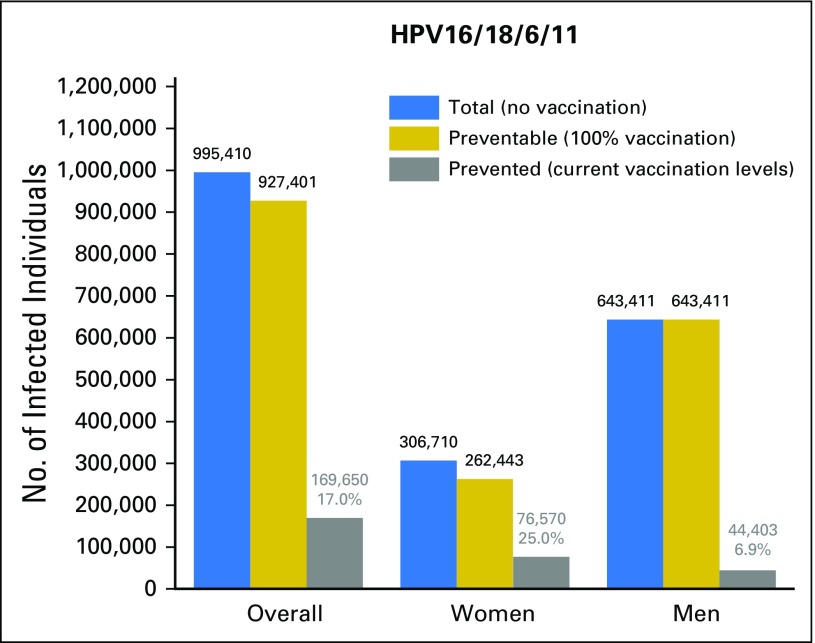
We estimated the population-level effect of HPV vaccination on the burden of vaccine-type oral HPV infections between 2011 and 2014 in the US population of individuals 18 to 33 years of age. These estimates combined the reduction in prevalence among vaccinated individuals with current HPV vaccination rates (Fig 1). HPV vaccination potentially prevented an estimated 169,650 (95% CI, 90,668 to 248,862) oral HPV16/18/6/11 infections, including 76,570 (95% CI, 7,665 to 145,770) among women and 44,403 (95% CI, 19,919 to 68,750) among men. The corresponding estimate of population-level effect was 17.0% overall, 25.0% in women, and 6.9% in men. For HPV16, an estimated 93,873 (95% CI, 27,749 to 158,298) infections were potentially prevented (52,585 [95% CI, −10,185 to 115,241] among women and 23,348 [95% CI, 4,301 to 42,315] among men), corresponding to a population-level effect of 16.2% overall, 23.4% in women, and 6.9% in men.
Fig 1.
Effect of human papillomavirus (HPV) vaccination on the burden of vaccine-type oral HPV infections in the US population of individuals 18 to 33 years of age, National Health and Nutrition Examination Survey 2011 to 2014. The bar graph shows the estimated effect of prophylactic HPV vaccination on the burden of vaccine-type oral HPV infections (HPV16/18/6/11) among individuals 18 to 33 years of age at participation in the National Health and Nutrition Examination Survey 2011 to 2014. Results are shown overall as well as separately in men and women. Results are presented as the total number of infections in the absence of HPV vaccination in the US population: (US population size × prevalence in unvaccinated) = blue bars; the number of preventable infections at 100% vaccination levels (US population size × prevalence in vaccinated) = gold bars; and the number of potentially vaccine-prevented infections at current HPV vaccine–uptake levels (US population size of vaccinated individuals × prevalence difference between unvaccinated and vaccinated) = gray bars. As a measure of the population-level effect of HPV vaccination on the burden of oral HPV infection, we estimated the proportion of potentially vaccine-prevented infections at the current HPV vaccine–uptake levels (number of potentially vaccine-prevented infections/total number of infections in the absence of HPV vaccination). Prevalence estimates used for these computations were not adjusted for any factors. The prevalence of individual vaccine-type HPV infections in the US population of individuals 18 to 33 years of age was as follows: HPV16 = 0.79%, HPV18 = 0.20%, HPV6 = 0.29%, and HPV11 = 0.14%.
DISCUSSION
We evaluated the effect of HPV vaccination on the population-level burden of oral HPV infections in the United States. HPV vaccination was associated with an estimated 88% reduction in prevalence of vaccine-type oral HPV16/18/6/11 infections among vaccinated young adults in the United States. However, because of a vaccination rate of only 18.3% between 2011 and 2014 among individuals 18 to 33 years of age, the population-level effect of HPV vaccination on oral HPV16/18/6/11 infections was a modest 17.0%.
Our results are similar to a prior study in Costa Rica that reported a 93% reduction in HPV16/18 prevalence in women who received the bivalent HPV vaccine.9,11 A recent study by Hirth et al10 also used NHANES data (2009 to 2014, individuals ages 18 to 30 years) and concluded that vaccine-type oral HPV prevalence was lower in individuals who received the HPV vaccine compared with unvaccinated individuals.10 Compared with the study by Hirth et al,10 the unique aspects of our study include the use of statistical methods optimal for the sparse sample sizes of oral HPV infections, adjustment for important confounders (such as age, sex, and race), the conduct of sex-stratified analyses, and an evaluation of the population-level effect of HPV vaccination on the burden of oral HPV infections.
To our knowledge, our study is the first to report a significant reduction (100%) in prevalence of vaccine-type oral HPV infections in vaccinated men. This reduction in vaccine-type oral HPV infections in vaccinated men is of particular importance because HPV-positive oropharyngeal cancer incidence is three to five times higher in men than in women worldwide.4 HPV vaccination is currently the most promising prevention option to stem the rising tide of HPV-positive oropharyngeal cancers in men. Unfortunately, low vaccine uptake in adult men between 2011 and 2014 translated to a low population-level effect of HPV vaccination (approximately 6.9%) in men. We note that vaccine uptake in US boys 14 to 17 years of age increased in 2015, reaching 49.8%.16 Nonetheless, uptake in males has remained significantly lower than in females, which in turn has not achieved the high rates needed for herd immunity to males.16
We note the limitations of our study. The nonrandomized comparisons of infection prevalence between vaccinated and unvaccinated individuals preclude a causal interpretation of our results. Despite the inclusion of two NHANES cycles, our analyses were performed on the basis of sparse sample sizes for oral HPV infections, particularly in vaccinated individuals. These sparse sample sizes partly reflect the restriction of analyses to a narrow age range (predefined on the basis of HPV vaccination recommendations),5 the rarity of oral HPV prevalence compared with anogenital HPV prevalence,7 and importantly, the high efficacy of HPV vaccination in reducing HPV infections.6 The low oral HPV prevalence also precluded key subgroup analyses, such as analyses stratified by age at vaccination, vaccine doses, and time since vaccination as well as analyses of cross protection against nonvaccine HPV types. Because of the sparsity of data, we could not calculate adjusted estimates of the number of oral HPV infections (in the absence of vaccination and at current vaccination levels) as well as of population-level effect. Also, our analyses were performed on the basis of self-reported receipt of vaccination, which may have resulted in misclassification.17 Nevertheless, this misclassification would be expected to be nondifferential by oral HPV prevalence status, which may have biased the effect of vaccination toward the null. Unlike cervical/vaginal HPV infection, for which prevaccine-era prevalence estimates are available in NHANES and other national surveys, there are no prevaccine-era prevalence data for oral HPV infection in the US population. Consequently, we used the observed prevalence of oral HPV infection in unvaccinated individuals as the best-available surrogate for the prevaccine-era prevalence of oral HPV infection, and then modeled the population-level effect of vaccination on oral HPV infections. Despite these limitations, our results have high global public health significance given the rapid increases in recent decades in the incidence of oropharynx cancers, particularly in men in the United States and numerous other developed countries, including Australia, Canada, Denmark, Japan, Sweden, the Netherlands, and the United Kingdom.1
Because of the absence of vaccine-efficacy trials, HPV vaccines are not currently indicated for the prevention of oral HPV infection and HPV-positive oropharyngeal cancers. Previous efforts to conduct efficacy trials were thwarted by regulatory policy that required clinically identifiable surrogate end points, such as precancers, which are difficult to detect in the oropharynx. However, a recent report from a US National Cancer Institute–International Agency for Research on Cancer joint workshop endorsed prevention of incident and persistent oral HPV16 as an acceptable end point for efficacy trials.6 Such efficacy trials would support evidence-based recommendations for the prevention of HPV-positive oropharyngeal cancer and potentially enhance vaccine uptake in males.
In conclusion, HPV vaccination was associated with reduction in vaccine-type oral HPV prevalence among young US adults. However, because of low vaccine uptake, the population-level effect was modest overall and particularly low in men.
Appendix
Fig A1.
Inclusions/exclusions for the analyses of the effect of human papilloma virus (HPV) vaccination on oral HPV infections, National Health and Nutrition Examination Survey (NHANES) 2011 to 2014. MEC, Mobile Exam Center. ACASI, Audio-computer assisted self-interview.
Table A1.
Adjusted Characteristics of Individuals Who Did or Did Not Receive Human Papilloma Virus Vaccination On the Basis of Model Adjustment, Ages 18 to 33 Years at Participation in the National Health and Nutrition Examination Survey 2011 to 2014
Table A2.
Adjusted Characteristics of Individuals Who Did or Did Not Receive Human Papilloma Virus Vaccination On the Basis of Propensity Adjustment, Ages 18 to 33 Years at Participation in the NHANES 2011 to 2014
Table A3.
Effect of HPV Vaccination on Oral HPV Prevalence (HPV18, HPV6, HPV11, and HPV31/33/45) in the US Population, Ages 18 to 33 Years at Participation in the National Health and Nutrition Examination Survey 2011 to 2014
Footnotes
Supported in part by NIDCR R01DE023175 and Intramural Research Program of the National Cancer Institute.
Presented in part at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 5, 2017.
AUTHOR CONTRIBUTIONS
Conception and design: Anil K. Chaturvedi, Barry I. Graubard, Tatevik Broutian, Maura L. Gillison
Financial support: Anil K. Chaturvedi, Maura L. Gillison
Administrative support: Maura L. Gillison
Collection and assembly of data: Anil K. Chaturvedi, Tatevik Broutian, Robert K.L. Pickard, Zhen-Yue Tong, Weihong Xiao, Lisa Kahle, Maura L. Gillison
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Anil K. Chaturvedi
No relationship to disclose
Barry I. Graubard
Stock or Other Ownership: Medtronic
Tatevik Broutian
No relationship to disclose
Robert K.L. Pickard
No relationship to disclose
Zhen-Yue Tong
No relationship to disclose
Weihong Xiao
No relationship to disclose
Lisa Kahle
No relationship to disclose
Maura L. Gillison
Honoraria: Bristol-Myers Squibb
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Celgene, Amgen, AstraZeneca
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), AstraZeneca (Inst)
Travel, accommodation, expenses: Merck, Sanofi
REFERENCES
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. : Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29:4294-4301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saraiya M, Unger ER, Thompson TD, et al. : US assessment of HPV types in cancers: Implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 107:djv086, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2016. CA Cancer J Clin 66:7-30, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Gillison ML, Chaturvedi AK, Anderson WF, et al. : Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 33:3235-3242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrosky E, Bocchini JA, Jr, Hariri S, et al. : Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 64:300-304, 2015 [PMC free article] [PubMed] [Google Scholar]
- 6.Lowy DR, Herrero R, Hildesheim A, et al. : Primary endpoints for future prophylactic human papillomavirus vaccine trials: Towards infection and immunobridging. Lancet Oncol 16:e226-e233, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Markowitz LE, Liu G, Hariri S, et al. : Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics 137:e20151968, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Markowitz LE, Hariri S, Lin C, et al. : Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010. J Infect Dis 208:385-393, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Herrero R, Quint W, Hildesheim A, et al. : Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One 8:e68329, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. doi: 10.1016/j.vaccine.2017.05.025. Hirth JM, Chang M, Resto VA, et al: Prevalence of oral human papillomavirus by vaccination status among young adults (18-30 years old). Vaccine 35:3446-3451, 2017. [DOI] [PubMed] [Google Scholar]
- 11. doi: 10.1093/jnci/djv302. Beachler DC, Kreimer AR, Schiffman M, et al: Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV infection. J Natl Cancer Inst 108:djv302, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC): National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD. U S Department of Health and Human Services, Centers for Disease Control and Prevention, [serial online] 2014. [Google Scholar]
- 13.Gillison ML, Broutian T, Pickard RK, et al. : Prevalence of oral HPV infection in the United States, 2009-2010. JAMA 307:693-703, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridgeway G, Kovalchik SA, Griffin BA, et al. : Propensity score analysis with survey weighted data. J Causal Inference 3:237-249, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunsberger S, Graubard BI, Korn EL: Testing logistic regression coefficients with clustered data and few positive outcomes. Stat Med 27:1305-1324, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. : National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2015. MMWR Morb Mortal Wkly Rep 65:850-858, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Dorell CG, Jain N, Yankey D: Validity of parent-reported vaccination status for adolescents aged 13-17 years: National Immunization Survey-Teen, 2008. Public Health Rep 126:60-69, 2011. (suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]