Abstract
Purpose
Some research has suggested that discussion of prognosis can disrupt the patient-physician relationship. This study assessed whether physician discussion of prognosis is associated with detrimental changes in measures of the strength of the patient-physician relationship.
Methods
This was a longitudinal cohort study of 265 adult patients with advanced cancer who visited 38 oncologists within community- and hospital-based cancer clinics in Western New York and Northern California. Prognostic discussion was assessed by coding transcribed audio-recorded visits using the Prognostic and Treatment Choices (PTCC) scale and by patient survey at 3 months after the clinic visit. Changes in the strength of the patient-physician relationship were computed as differences in patient responses to The Human Connection and the Perceived Efficacy in Patient-Physician Interactions scales from baseline to 2 to 7 days and 3 months after the clinic visit.
Results
Prognostic discussion was not associated with a temporal decline in either measure. Indeed, a one-unit increase in PTCC during the audio-recorded visit was associated with improvement in The Human Connection scale at 2 to 7 days after the visit (parameter estimate, 0.10; 95% CI, −0.02 to 0.23) and 3 months after the visit (parameter estimate, 0.18; 95% CI, 0.02 to 0.35) relative to baseline. Standardized effect sizes (SES) associated with an increase of two standard deviations in the PTCC at each time point were consistent with small beneficial effects (SES, 0.14 [95% CI, −0.02 to 0.29] at 2 to 7 days; SES, 0.24 [95% CI, 0.02 to 0.45] at 3 months), and lower bounds of CIs indicated that substantial detrimental effects of prognostic discussion were unlikely.
Conclusion
Prognostic discussion is not intrinsically harmful to the patient-physician relationship and may even strengthen the therapeutic alliance between patients and oncologists.
INTRODUCTION
Patients with life-limiting illnesses rely on physicians to communicate complex clinical and prognostic information while also attending to patient symptoms and emotions.1 In advanced cancer, patients need accurate prognostic information, yet physicians often emphasize treatment options during patient interactions to the exclusion of prognostic discussion or end-of-life planning.2-6 Although oncologists can estimate life expectancy of a patient with advanced cancer within some bounds of uncertainty,7 many patients who are approaching the end of life maintain overly optimistic estimates of their prognosis.2,8 The inaccuracy of patient perceptions of prognosis may contribute to deferment of hospice services, greater use of intensive hospital-based services at the end of life, higher care costs, lower quality of life for patients at the end of life, more difficult bereavement for caregivers, and potentially shortened survival.8-12
Oncologists have been urged to prioritize the sharing of prognostic information in hope of reducing patient misperceptions of prognosis.1,13,14 However, several lines of evidence suggest that oncologists may avoid frank discussions of prognosis out of fear of disrupting the physician-patient relationship.3 Among patients with stage IV lung or colorectal cancer who had discussed chemotherapy with their oncologists, patients with unrealistic expectations of being cured by chemotherapy were more satisfied with their doctors’ communication.15 When patients with advanced cancer viewed videos of physicians delivering identical treatment information, except for a more versus less optimistic frame, patients rated doctors providing the optimistic frame as more compassionate and more trustworthy.16 A randomized trial of an end-of-life communication curriculum among mostly resident physicians found that the intervention was associated with no difference in the patient-rated quality of communication but an increase in patients’ depressive symptoms.17 In samples of patients without cancer, physician delivery of what patients may perceive to be bad news has been associated with disruption of the doctor-patient relationship.18 Nevertheless, in a recent analysis of data from patients with metastatic cancer in whom at least one chemotherapy regimen failed, there was no significant association between self-reported receipt of prognostic disclosure and patient ratings of their therapeutic alliance with their oncologist.19
The evidence base regarding the influence of prognostic discussion on the doctor-patient relationship has several notable limitations. First, most analyses have been cross-sectional and could not examine the prospective effects of prognostic discussion.15,19 Second, in some analyses,15,17 patients rated their communication with all physicians recently seen, so these analyses could not directly assess the association of specific physicians’ prognostic discussions with patients’ ratings of their therapeutic alliances with these physicians. Third, analyses have either included patient self-reported prognostic discussion19 or inferred the nature of the disclosure on the basis of patients’ attitudes regarding chemotherapy15 but have not included direct observation of these discussions. To address these evidence gaps, we analyzed longitudinal data from a randomized trial of a communication intervention to assess whether two measures of prognostic discussion (one coded from transcripts of outpatient patient-oncologist encounters, and one based on patient self-report) were associated with deleterious changes in ratings by patients with advanced cancer of their relationship with their oncologists.
METHODS
Overview
Data for this study were derived from the Values and Options in Cancer Care (VOICE) cluster randomized clinical trial (RCT), which evaluated whether oncologist- and patient-level interventions improved the quality of communication between oncologists and their patients with advanced cancer and the patients’ caregivers (ClinicalTrials.gov identifier: NCT01485627). Detailed study methods and results are reported elsewhere.20,21 In brief, oncologists were randomly assigned to intervention and control groups using a stratified randomization scheme to ensure balance by site and subspecialty. Patients were assigned to intervention and control groups on the basis of oncologists’ assignments. The oncologist intervention included a video and feedback from standardized patients portraying patients with advanced cancer and caregivers during two separate visits. Patient interventions included a coaching session incorporating a question prompt list and up to three follow-up telephone calls from coaches. We audio recorded the first visit with each patient’s oncologist after either enrollment (for control subjects) or the intervention (for intervention subjects). The patient intervention was not designed to promote patient-physician discussion of prognosis during the audio-recorded visit unless patients endorsed this as a priority during coaching.
In brief, VOICE interventions were associated with significant improvement in the primary outcome of communication quality during the audio-recorded visit but no differences in patient quality of life or, among decedents, in health care use during the last 30 days of life (secondary outcomes).20 The current study examines associations between the extent of prognosis discussion during the audio-recorded visit and pre- to postvisit changes in two measures of the patients’ assessment of the strength of their relationship with the oncologist. Institutional review boards at the University of Rochester and University of California, Davis, approved the study.
Setting and Subjects
Oncologists and patients were recruited from four community-based cancer clinics, three academic medical centers, and three community hospitals in Western New York and Sacramento, CA. Oncologists were eligible if they treated nonhematologic malignancies within the study facilities. Of 52 oncologists who were contacted, 43 enrolled and 38 were randomly assigned to intervention or control groups. Patients of enrolled oncologists were eligible if they were ≥ 21 years of age, able to understand spoken English, provide written informed consent, and had either stage IV nonhematologic cancer, or stage III cancer and if their oncologist “would not be surprised” if the patient died within 12 months.22 Among patients of the 38 randomly assigned oncologists, we identified 453 potentially eligible to participate in the study, 281 of whom provided written informed consent. Of these 281 patients, 265 had audio-recorded visits with enrolled oncologists.
Data Collection
Patients completed in-person or telephone surveys at baseline, between 2 and 7 days after audio-recorded visits, and approximately 3 months after audio-recorded visits. Survey content included questions about the content of recent visits with oncologists and the patient-oncologist relationship.
Dependent Variables
During each survey, patients completed two assessments of the perceived strength of their relationship with their oncologist, The Human Connection (THC) scale and the Perceived Efficacy in Patient-Physician Interactions (PEPPI) scale.23,24 The 16-item THC scale assesses the strength of the patient-physician therapeutic alliance, including the extent to which patients like, trust, and respect their physicians (Cronbach α = 0.90).23 THC scale has a theoretical range of 16 to 64, with higher scores signifying stronger therapeutic alliance. The PEPPI scale is a validated five-item scale that asks patients to rate their confidence in obtaining needed information and attention from physicians regarding their medical concerns (Cronbach α = 0.83).24 Although THC scale directly assesses the extent to which patients like and trust their physicians,23 the PEPPI scale correlates strongly with independent measures of patients’ global satisfaction with physicians and patient satisfaction with physicians’ interpersonal and communication skills.24 Therefore, we consider the PEPPI scale an indirect measure of the patients’ perception of the strength of their therapeutic connection with their physician. The primary outcomes were the change in THC and PEPPI scales from baseline to 2 to 7 days and from baseline to 3 months, which were computed as differences between the respective measures at the times of the two follow-up surveys and at baseline.
Measures of Prognostic Discussion
We adapted the informing subscale of the Prognostic and Treatment Choices scale to measure the extent to which oncologists engaged patients regarding prognosis and treatment options during the audio-recorded visit (hereafter referred to as the prognostic discussion scale [PDS]).25 To derive the PDS, trained graduate and undergraduate students coded audio recordings for the presence of either oncologist or patient statements across nine domains encompassing cancer prognosis, curability, the likelihood of effective treatment, and the transition from active to palliative treatment. For example, within the palliative care domain, coders assessed for oncologist statements such as, “Have you had thoughts about stopping the chemotherapy and focusing more on comfort and quality of life?” Each additional prognosis-related statement or question generated an approximately 1.6-point increase in the total score up to a maximum of five points within each domain, yielding a scale with a theoretical range of 0 to 45, with higher scores indicating greater prognostic discussion. Coders were blinded to study hypotheses or study arm, and coding quality and reproducibility were carefully monitored (intraclass correlation coefficient, 0.75 for the total scale score).
As a patient-level subjective measure of prognostic discussion, we asked the following question during the 3-month follow-up survey: “Please indicate whether or not you discussed your prognosis (life expectancy) with your cancer doctor in the last 3 months.” Patients responded yes, no, or unsure. We classified patients who responded “yes” as having had a prognostic discussion with their oncologist.
Covariates
We adjusted in analyses for covariates that have been associated with physician communication and patient experience ratings in other contexts, including patient age (as a continuous measure), education (high school or less v more than high school), and sex.26 Because we considered that patients with breast cancer might be more activated and, therefore, interact with their physicians differently than those with other malignancies, we classified oncologists by whether > 50% of their study patients had breast cancer. We also adjusted for study arm (intervention v control) and site (New York v California).
In the VOICE trial, study arm was associated with higher overall scores in an aggregate communication measure that was based on coded audio-recorded visits that included the PDS, but differences between control and intervention in the PDS alone—although favoring the intervention—did not reach statistical significance (P = .11).20 The intervention was not associated with differences in THC or the PEPPI scales during follow-up visits. Although all patients had advanced cancers (as detailed above in “Settings and Subjects”), we categorized patients as having more versus less aggressive cancer (less aggressive cancers were defined prospectively by two study oncologists as cancers of the breast, colon, or prostate). We also collected data on income and marital status.
Statistical Analyses
For the primary analyses, we used mixed-effects linear regression to model changes in THC or the PEPPI scales from baseline to the two follow-up surveys (2 to 7 days and 3 months) as a function of the Prognostic and Treatment Choices score during audio-recorded visits. Additionally, we used mixed-effects linear regression to model changes in THC and PEPPI scales from baseline to 3 months as functions of whether patients reported a discussion of prognosis during the previous 3 months. All models included physician-level random effects to correct standard errors for within-physician clustering and adjusted for study arm, site, whether the oncologist subspecialized in breast cancer, patient age, education, and sex. To facilitate interpretation, we report parameter estimates with 95% CIs for the prognostic discussion predictors as well as standardized effect sizes (SES) associated with a two standard deviation (SD)–difference in the PDS. Intuitively, these analyses can be interpreted as comparing adjusted outcomes among oncologists with average values of the PDS, with adjusted outcomes among oncologists with PDS values at the 95% percentile relative to peers.
In additional analyses, we analogously computed effect sizes associated with the presence versus absence of a patient report of a prognostic discussion in the prior 3 months. Effect sizes of approximately 0.20, 0.50, and 0.80 are often considered small, medium, and large, respectively.
We conducted a sensitivity analysis to assess whether the effect of prognostic discussion differed by study arm, potentially because physicians who received the intervention may have used communication techniques that would enhance the doctor-patient relationship to a greater extent compared with control physicians. In this sensitivity analysis, we structured the models as described but also included a study arm by PDS interaction term. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
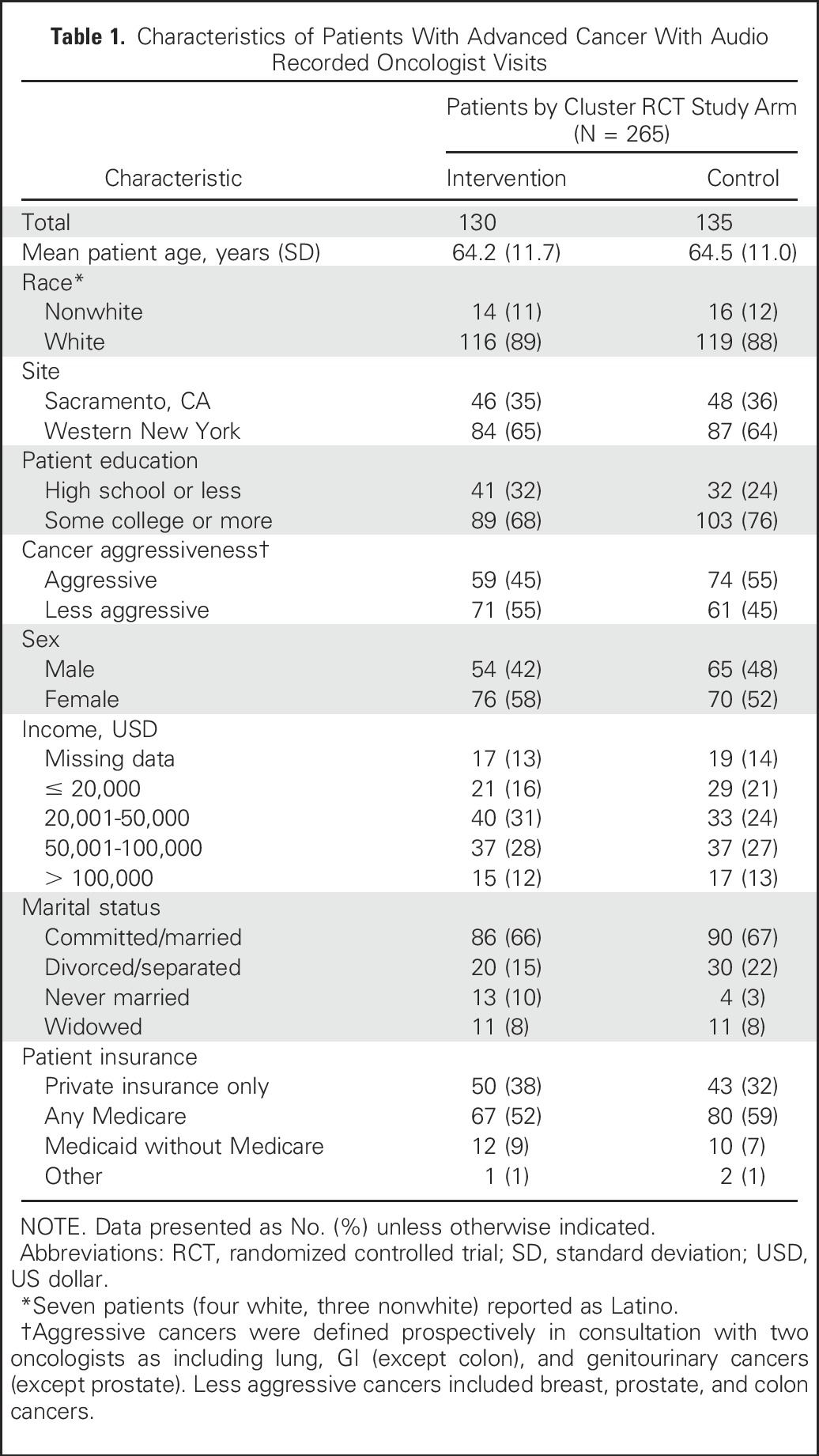
Table 1 provides characteristics of the 265 patients with advanced cancer who were randomly assigned within the cluster RCT and who had audio recorded oncologist visits. Overall, 12% of patients were nonwhite, and approximately half had aggressive cancers. Of the 38 study oncologists, seven (18%) subspecialized in the treatment of breast cancer. Of the 265 patients, 259 (97.7%) responded to surveys at 2 to 7 days after their clinic visit and 216 (81.5%) responded to surveys 3 months after their visit. Of the 49 patients without responses at 3 months, 45 patients (17.0%) had died in the intervening 3 months and four (1.5%) declined further participation. Across the 265 audio-recorded visits, the mean PDS score was 4.1 (SD, 3.9; range, 0 to 19.2), implying that the average visit contained limited discussion of patient prognosis. Of the 216 patients who responded at 3 months after their visit, 79 (36.6%) reported having discussed prognosis with their oncologist during the previous 3 months.
Table 1.
Characteristics of Patients With Advanced Cancer With Audio Recorded Oncologist Visits
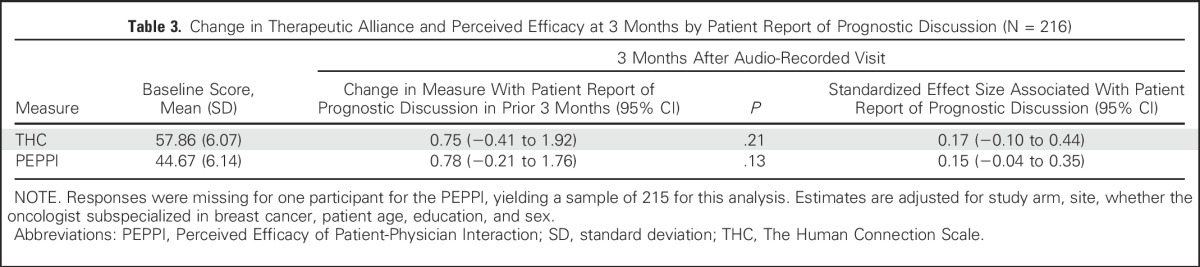
Compared with baseline, a one-unit increase in the PDS during the audio-recorded visit was associated with an increase in THC scale at 2 to 7 days after their visit (parameter estimate, 0.10; 95% CI, −0.02 to 0.23) but this difference did not reach statistical significance (P = .09; Table 2). The standardized effect size associated with a two-SD increase in the PDS was consistent with a small effect (SES, 0.14; 95% CI, −0.02 to 0.29). At 3 months after patients’ clinic visit, a one-unit increase in the PDS was associated with a statistically significant improvement in THC scale (parameter estimate, 0.18; 95% CI, 0.02 to 0.35). The SES associated with a two-SD increase in the PDS was small (0.24; 95% CI, 0.02 to 0.45). At 2 to 7 days, the PDS was not associated with any change in the PEPPI scale from baseline (SES,−0.02; 95% CI, −0.22 to 0.18), nor was there any substantive change at 3 months compared with baseline (SES, −0.04; 95% CI, −0.33 to 0.23). Results were similar in analyses assessing the association between patient report of a prognostic discussion and changes in THC and PEPPI scales from baseline to 3 months after patients’ visits (Table 3). Notably, for the SES at 3 months, the lower bounds of the confidence limits exclude substantial decrements in either scale associated with patient report of a prognostic discussion. Study arm was not significantly associated with changes in THC or PEPPI scales in any analyses, and sensitivity analyses showed no evidence of effective modification by study arm.
Table 2.
Change in Therapeutic Alliance and Perceived Efficacy by Prognostic Discussion With Oncologists
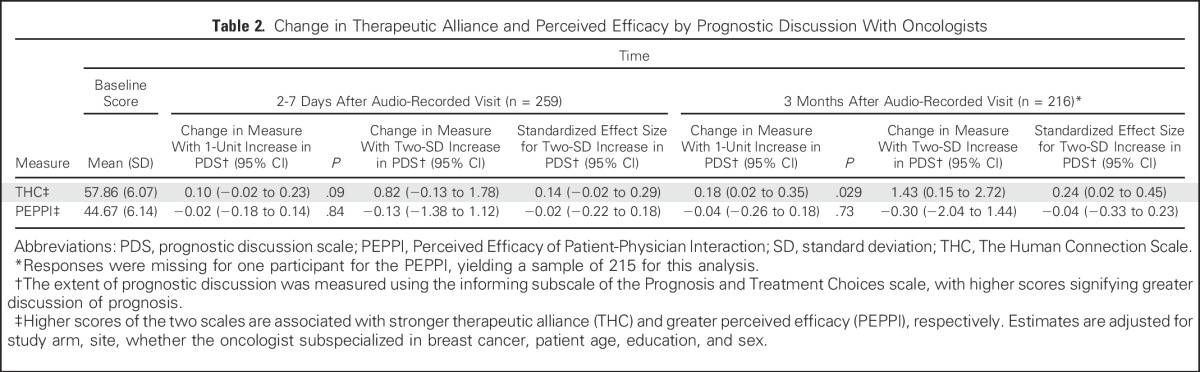
Table 3.
Change in Therapeutic Alliance and Perceived Efficacy at 3 Months by Patient Report of Prognostic Discussion (N = 216)
DISCUSSION
Among patients with advanced cancer, physician discussion of prognosis was not associated with a decline in either of two measures of the patient’s perceived strength of their relationship with their physician. Indeed, based on changes in study measures from baseline to 3 months after audio recorded oncologist visits, patients who had visits with a greater amount of prognostic discussion rated their therapeutic alliance with physicians statistically significantly more favorably than patients whose visits had less prognostic discussion. Based on effect sizes, we estimate that a two-SD increase in prognostic discussion is associated with a small increase in therapeutic alliance. Results were generally consistent in analyses based on patient self-report of prognostic discussion. Notably, the lower bounds of CIs in the latter analyses suggest that it is highly unlikely that prognostic discussions adversely affected patients’ perceptions of their relationship with their oncologists.
Although prior studies have raised concerns that discussion of patient prognosis may disrupt the patient-oncologist relationship,15,16 our study did not suggest an adverse effect of prognostic discussion on the patient-oncologist alliance. These findings are supported by a recent analysis by Enzinger et al19 that found no significant association between prognostic disclosure and therapeutic alliance among patients with advanced cancer whose disease had progressed after at least one round of chemotherapy. Alongside the Enzinger et al19 study results, our study findings suggest prognostic disclosure and discussion are not intrinsically harmful to the doctor-patient relationship but may require understanding the patient’s hopes and aspirations, applying tact and skill in sharing information, inquiring into patients’ emotions, and being sensitive to timing. High-quality communication transmits information accurately without undermining patients’ hopes for the future.27,28 Yet, medical training currently devotes little time and limited assessments of these nuanced communication skills, and deficiencies may go undetected and uncorrected.
In addition to concerns about disrupting the patient-physician relationship, oncologists face other challenges in approaching discussions of prognosis. First, oncologists may address prognosis cursorily when patients are feeling well, perhaps because of the perception that patients or caregivers are not ready to receive prognostic information, but fail to revisit prognosis when cancers progress.3,4,29 Second, oncologists may hasten or defer prognostic discussions in response to their own needs for emotional respite.4 Third, the language or statistics used to communicate prognosis (eg, “response rate”) can be ambiguous or potentially misleading, leading some patients to believe that the availability of any treatment of their advanced cancer implies the possibility of cure.15 Fourth, individual oncologists may lack skill or confidence in the delivery of accurate prognostic information while also supporting healthy optimism and hope.27,28,30
Several limitations of our study warrant consideration. First, our study included RCT participants from two US regions with low representation of nonwhite people; results could differ in other settings with more diverse patient and physician samples. Second, our measures of prognostic discussion occurred soon after patient enrollment, and the timing of these measures may not have coincided with points in patients’ disease trajectories when prognostic discussion would potentially be rife with emotions and heightened risk of misunderstanding. Still, a strength of our study is that we could perform analyses using both observer-coded and patient-reported measures of prognostic discussion, resulting in similar conclusions. Third, we have limited information about the content or quality of the prognostic discussion, including the extent to which physicians were realistic versus overoptimistic. Some of the prognostic discussions in our sample may have been superficial,31,32 failing to address issues that patients might have viewed as threatening or disturbing.
Among patients with advanced cancer, oncologist discussion of patient prognosis during clinical encounters was associated with a small but statistically significant improvement in patients’ ratings of the therapeutic alliance with their physicians, and longitudinal analyses based on patient self-report suggest a substantial detrimental effect of prognostic discussion on the doctor-patient relationship is highly unlikely. Our results provide reassurance that prognostic discussion need not undermine the therapeutic alliance and, in some circumstances, may even strengthen the relationship between patients and oncologists.
Footnotes
Processed as a Rapid Communication manuscript.
Supported by the National Cancer Institute Grant No. R01 CA140419-05 (R.L.K. and R.M.E.), and Grant No. R01 CA168387 (P.R.D.).
AUTHOR CONTRIBUTIONS
Conception and design: Joshua J. Fenton, Paul R. Duberstein, Richard L. Kravitz, Ronald M. Epstein
Financial support: Ronald M. Epstein
Administrative support: Joshua J. Fenton, Paul R. Duberstein, Richard L. Kravitz, Ronald M. Epstein
Collection and assembly of data: Joshua J. Fenton, Paul R. Duberstein, Richard L. Kravitz, Ronald M. Epstein
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Prognostic Discussions on the Patient-Physician Relationship: Prospective Cohort Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Joshua J. Fenton
No relationship to disclose
Paul R. Duberstein
No relationship to disclose
Richard L. Kravitz
No relationship to disclose
Guibo Xing
No relationship to disclose
Daniel J. Tancredi
Research Funding: Merck (Inst)
Kevin Fiscella
No relationship to disclose
Supriya Mohile
Consulting or Advisory Role: Seattle Genetics
Ronald M. Epstein
No relationship to disclose
REFERENCES
- 1.Epstein RM, Street RL, Jr: Patient-Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. Bethesda, MD, National Cancer Institute, 2007 [Google Scholar]
- 2.Epstein AS, Prigerson HG, O’Reilly EM, et al. : Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol 34:2398-2403, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon EJ, Daugherty CK: ‘Hitting you over the head’: Oncologists’ disclosure of prognosis to advanced cancer patients. Bioethics 17:142-168, 2003 [DOI] [PubMed] [Google Scholar]
- 4.The AM, Hak T, Koëter G, et al. : Collusion in doctor-patient communication about imminent death: An ethnographic study. BMJ 321:1376-1381, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henselmans I, Smets EMA, Han PKJ, et al. : How long do I have? Observational study on communication about life expectancy with advanced cancer patients. Patient Educ Couns 100:1820-1827, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Chou WS, Hamel LM, Thai CL, et al. : Discussing prognosis and treatment goals with patients with advanced cancer: A qualitative analysis of oncologists’ language. Health Expect 20:1073-1080, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamont EB, Christakis NA: Prognostic disclosure to patients with cancer near the end of life. Ann Intern Med 134:1096-1105, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Weeks JC, Cook EF, O’Day SJ, et al. : Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 279:1709-1714, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Bakitas M, Lyons KD, Hegel MT, et al. : Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA 302:741-749, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mack JW, Weeks JC, Wright AA, et al. : End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol 28:1203-1208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733-742, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Wright AA, Zhang B, Ray A, et al. : Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 300:1665-1673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Committee on Approaching Death : Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC, The National Academies Press, 2015 [PubMed] [Google Scholar]
- 14.Gilligan T, Coyle N, Frankel RM, et al. : Patient-clinician communication: American Society of Clinical Oncology consensus guideline. J Clin Oncol 35:3618-3632, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Weeks JC, Catalano PJ, Cronin A, et al. : Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med 367:1616-1625, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanco K, Rhondali W, Perez-Cruz P, et al. : Patient perception of physician compassion after a more optimistic vs a less optimistic message: A randomized clinical trial. JAMA Oncol 1:176-183, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Curtis JR, Back AL, Ford DW, et al. : Effect of communication skills training for residents and nurse practitioners on quality of communication with patients with serious illness: A randomized trial. JAMA 310:2271-2281, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redelmeier DA, Yarnell CJ, Thiruchelvam D, et al. : Physicians’ warnings for unfit drivers and the risk of trauma from road crashes. N Engl J Med 367:1228-1236, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Enzinger AC, Zhang B, Schrag D, et al. : Outcomes of prognostic disclosure: Associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol 33:3809-3816, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein RM, Duberstein PR, Fenton JJ, et al. : Effect of a patient-centered communication intervention on oncologist-patient communication, quality of life, and health care utilization in advanced cancer: The VOICE randomized clinical trial. JAMA Oncol 3:92-100, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoerger M, Epstein RM, Winters PC, et al. : Values and options in cancer care (VOICE): Study design and rationale for a patient-centered communication and decision-making intervention for physicians, patients with advanced cancer, and their caregivers. BMC Cancer 13:188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moroni M, Zocchi D, Bolognesi D, et al. : The ‘surprise’ question in advanced cancer patients: A prospective study among general practitioners. Palliat Med 28:959-964, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Mack JW, Block SD, Nilsson M, et al. : Measuring therapeutic alliance between oncologists and patients with advanced cancer: The Human Connection scale. Cancer 115:3302-3311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maly RC, Frank JC, Marshall GN, et al. : Perceived efficacy in patient-physician interactions (PEPPI): Validation of an instrument in older persons. J Am Geriatr Soc 46:889-894, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Shields CG, Coker CJ, Poulsen SS, et al. : Patient-centered communication and prognosis discussions with cancer patients. Patient Educ Couns 77:437-442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenton JJ, Jerant AF, Bertakis KD, et al. : The cost of satisfaction: A national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med 172:405-411, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Hagerty RG, Butow PN, Ellis PM, et al. : Communicating with realism and hope: Incurable cancer patients’ views on the disclosure of prognosis. J Clin Oncol 23:1278-1288, 2005. [Erratum: J Clin Oncol. 2005;23(15):3652] [DOI] [PubMed] [Google Scholar]
- 28.Mack JW, Wolfe J, Cook EF, et al. : Hope and prognostic disclosure. J Clin Oncol 25:5636-5642, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Elkin EB, Kim SH, Casper ES, et al. : Desire for information and involvement in treatment decisions: Elderly cancer patients’ preferences and their physicians’ perceptions. J Clin Oncol 25:5275-5280, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Groopman JE: A strategy for hope: A commentary on necessary collusion. J Clin Oncol 23:3151-3152, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Graugaard PK, Rogg L, Eide H, et al. : Ways of providing the patient with a prognosis: A terminology of employed strategies based on qualitative data. Patient Educ Couns 83:80-86, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Walczak A, Henselmans I, Tattersall MH, et al. : A qualitative analysis of responses to a question prompt list and prognosis and end-of-life care discussion prompts delivered in a communication support program. Psychooncology 24:287-293, 2015 [DOI] [PubMed] [Google Scholar]


