Copy number variants (CNVs) of 1q21.1 are increasingly being recognized due to the widespread use of genetic screening tests for the investigation of developmental disorders and epilepsy. These include microdeletion and microduplication syndromes, associated with a wide variety of pathology including autism spectrum disorders, attention-deficit disorder, learning disabilities, hypotonia, facial dysmorphisms, and schizophrenia. The 1q21.1 region is considered to be genetically unstable because it contains one of the largest areas of identical duplication sequences in the human genome. Epilepsy has been reported in the literature, particularly in microdeletion syndromes, but rarely in association with microduplication syndromes. We report a patient with epilepsy and autism spectrum disorder due to a distal 1q21.1 microduplication and review the available literature and genetic information.
Case report
We present a 10-year-old girl with a low-functioning autism spectrum disorder and focal motor epilepsy. On examination, she has hypertelorism, minimal communicative language skills, and severe macrocephaly (HC = 57 cm, 3.6 SD > 99%). Seizures started at 7 years of age and consisted of head deviation to the left, generalized stiffening, clonic activity of the mouth, and fluttering of the eyelids, lasting for 1–2 minutes. Multiple video EEG recordings showed a right temporal focus with a less active, independent left temporal focus. 3T MRI scan of the brain was normal. Her seizure control was poor despite high doses of oxcarbazepine. She had multiple clusters of seizures after ingestion of large amounts of caffeine in the form of red velvet cookies. She was switched to lamotrigine and was placed on a caffeine-free diet. She has been seizure-free for nearly 1 year on this regimen. Chromosomal single nucleotide polymorphism Affymetrix CytoScan-HD microarray showed a distal 1q21.1-1q21.2 duplication (arr[hg19] 1q21.1q21.2[146,503,349–147,819,438] × 3), 1.3 Mb in size. None of the genes in this region are definitively known to cause neurologic disease, although this duplication is one of the more common CNVs associated with autism spectrum disorders and intellectual disability.1
Discussion
Our patient has many clinical features previously reported with 1q21.1 duplication syndrome, including autism with intellectual disability, hyperactivity and impulsivity, macrocephaly, hypertelorism, and hypotonia. Microduplications have also been reported in “normal” individuals, subsequently often found to have subtle features of the disorder.2 Multiple authors have postulated that clinical expression of the disorder varies widely and that penetrance is incomplete. Duplications of this region have also been associated with a host of non-neurological congenital anomalies with no clear pattern of abnormalities.
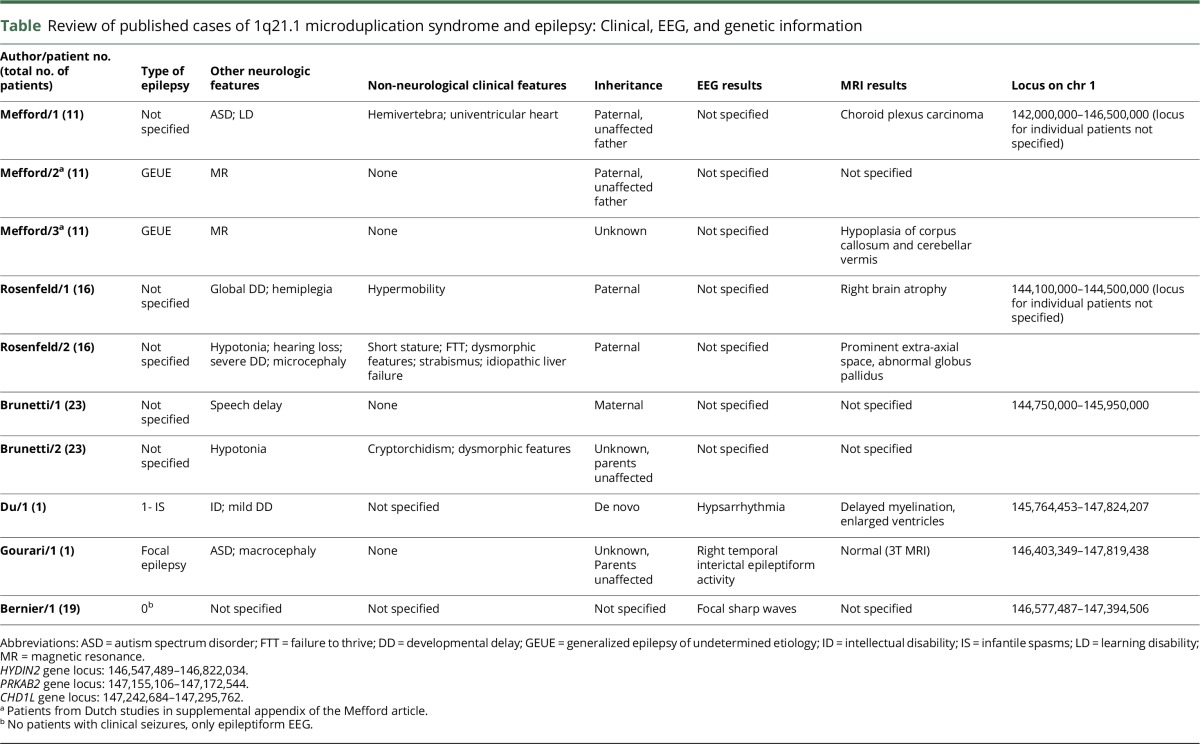
Seizures have rarely been reported in published review articles of 1q21.1 microduplication syndromes (see table for details of reported cases). Numbers of affected patients were small in each study, and very little information was published about types of seizures, EEG findings, etc.
Table.
Review of published cases of 1q21.1 microduplication syndrome and epilepsy: Clinical, EEG, and genetic information
The UCSC Genome Browser lists 2 genes in this region of distal microduplication, which might contribute to epilepsy, CHD1L and PRKAB2. CHD1L encodes a helicase responsible for DNA repair, so far associated only with various cancers. However, it comes from the same family as CHD2, a gene associated with epileptic encephalopathies and a variety of generalized epilepsy syndromes. PRKAB2 encodes a protein responsible for lipid metabolism. It is a regulatory subunit for AMPK (AMP-activated protein kinase). The laforin-malin complex, a set of proteins implicated in Lafora progressive myoclonus epilepsy, promotes ubiquitination of AMPK.3 Further research is needed to determine whether and how this interaction could explain the development of epilepsy. This region also contains the HYDIN2 gene, which was long thought to be a pseudogene, but which was recently shown to be highly transcribed, particularly in neuronal tissue, including the fetal brain. The function of HYDIN2 is currently unknown. It was thought to be related to the head size, but this has been shown to be erroneous. HYDIN2 is involved in 87% of individuals with developmental disabilities and 1q21.1 duplications and 93% of deletions, but CNVs of this gene are extremely rare in normal controls.1 It is possible that abnormalities of this gene contribute to the development of epilepsy.
We reviewed available genetic information of patients with 1q21.1 microduplication syndrome and epilepsy.2,4–7 Review of loci showed 10 patients with proximal microduplications2,4,5 and only 3 patients with pure distal microduplications and epilepsy. There was no section of the duplicated locus ubiquitous to all patients, indicating that most likely there is more than 1 gene causing epilepsy in this population. Further research into the function of genes in the 1q21.1 region is likely to contribute substantially to our understanding of the genetic basis of epilepsy in individuals with autism spectrum disorders.
Author contributions
Ioulia Gourari: study concept and design and acquisition of data. Romaine Schubert: study supervision and acquisition of data. Aparna Prasad: critical revision of the manuscript for important intellectual content.
Study funding
No targeted funding reported.
Disclosure
I. Gourari and R. Schubert report no disclosures. A. Prasad is an employee of Lineagen, Inc. Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NG.
References
- 1.Dougherty ML, Nuttle X, Penn O, et al. The birth of human-specific neural gene by incomplete duplication and gene fusion. Genome Biol 2017;18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunetti-Pierri N, Berg JS, Scaglia F, et al. Recurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat Genet 2008;40:1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno D, Towler M, Hardie DG, et al. The laforin-malin complex, involved in Lafora disease, promotes the incorporation of K63-linked ubiquitin chains into AMP-activated protein kinase beta subunits. Mol Biol Cell 2010;21:2578–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mefford HC, Sharp AJ, Baker C, et al. Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. N Engl J Med 2008;359:1685–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfeld JA, Traylor RN, Schaefer GB, et al. Proximal microdeletions and microduplications of 1q21.1 contribute to variable abnormal phenotypes. Eur J Hum Genet 2012;20:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du X, An Y, Yu L, et al. A genomic copy number variant analysis implicates the MBD5 and HNRNPU genes in Chinese children with infantile spasms and expands the clinical spectrum of 2q23.1 deletion. BMC Med Genet 2014;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernier R, Steinman KJ, Reilly B, et al. Clinical phenotype of the recurrent 1q21.1 copy-number variant. Genet Med Epub 2016;18:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]