Abstract
Background
The objective of this systematic review was to examine for the first time the associations between sleep duration and a broad range of health indicators in children aged 0 to 4 years.
Methods
Electronic databases were searched with no limits on date or study design. Included studies (published in English or French) were peer-reviewed and met the a priori determined population (apparently healthy children aged 1 month to 4.99 years), intervention/exposure/comparator (various sleep durations), and outcome criteria (adiposity, emotional regulation, cognitive development, motor development, growth, cardiometabolic health, sedentary behaviour, physical activity, quality of life/well-being, and risks/injuries). The quality of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework. Due to high levels of heterogeneity across studies, narrative syntheses were employed.
Results
A total of 69 articles/studies (62 unique samples) met inclusion criteria. Data across studies included 148,524 unique participants from 23 countries. The study designs were randomized trials (n = 3), non-randomized interventions (n = 1), longitudinal studies (n = 16), cross-sectional studies (n = 42), or longitudinal studies that also reported cross-sectional analyses (n = 7). Sleep duration was assessed by parental report in 70% of studies (n = 48) and was measured objectively (or both objectively and subjectively) in 30% of studies (n = 21). Overall, shorter sleep duration was associated with higher adiposity (20/31 studies), poorer emotional regulation (13/25 studies), impaired growth (2/2 studies), more screen time (5/5 studies), and higher risk of injuries (2/3 studies). The evidence related to cognitive development, motor development, physical activity, and quality of life/well-being was less clear, with no indicator showing consistent associations. No studies examined the association between sleep duration and cardiometabolic biomarkers in children aged 0 to 4 years. The quality of evidence ranged from “very low” to “high” across study designs and health indicators.
Conclusions
Despite important limitations in the available evidence, longer sleep duration was generally associated with better body composition, emotional regulation, and growth in children aged 0 to 4 years. Shorter sleep duration was also associated with longer screen time use and more injuries. Better-quality studies with stronger research designs that can provide information on dose-response relationships are needed to inform contemporary sleep duration recommendations.
Electronic supplementary material
The online version of this article (10.1186/s12889-017-4850-2) contains supplementary material, which is available to authorized users.
Keywords: Adiposity, Emotional regulation, Cognitive development, Motor development, Growth, Cardiometabolic health, Physical activity, Sedentary behaviour, Quality of life, Well-being, Injuries, Newborns, Infants, Toddlers, Preschoolers
Background
Sleep is essential for healthy cognitive, psychosocial, and physical health [1, 2]. Healthy sleep is generally defined by adequate duration, appropriate timing, good quality, and the absence of sleep disturbances or disorders [3]. Sleep-wake regulation and sleep states evolve rapidly during the first year of life, with continued maturation across childhood [4]. For example, newborns (0–3 months) do not have an established circadian rhythm [5]; this begins to emerge at around 10–12 weeks of age, with sleep becoming more nocturnal between ages 4–12 months [6]. Children continue to take daytime naps between 1 and 4 years of age, and night wakings are common in infancy and early childhood [7]. By age 5, daytime napping typically ceases and overnight sleep duration gradually declines throughout childhood, in part due to a shift to later bedtimes and unchanged wake times [7].
Sleep patterns can vary between individuals and are explained by a complex interplay between genetic, environmental, behavioural, and social factors. For example, factors such as parenting practices and expectations, family routines, cultural preferences, and daycare schedules can all influence sleep [4]. Findings from a recent systematic review of 69,542 infants, toddlers, and preschoolers from 18 countries showed mean reference values and ranges (± 1.96 SD) of 12.8 h/day (9.7–15.9) for infants (< 2 years), and 11.9 h/day (9.9–13.8) for toddlers/preschoolers (ages 2–5 years) [8]. These international normative data can help to determine the normative distribution of sleep duration, but cannot identify duration associated with health benefits.
Although many studies have confirmed the importance of sleep duration for individual health outcomes, to our knowledge no study has attempted to systematically and comprehensively examine the literature on the associations between sleep duration and a broad range of health indicators in children aged 0–4 years. A systematic review can help to determine whether the available evidence supports existing sleep duration recommendations. The National Sleep Foundation recommends that for every 24-h cycle, newborns (0–3 months) obtain 14–17 h of sleep, infants (4–11 months) obtain 12–15 h of sleep, toddlers (1–2 years) obtain 11–14 h of sleep, and preschoolers (3–5 years) obtain 10–13 h of sleep [9]. Similarly, the American Academy of Sleep Medicine recommends that infants (4–11 months) sleep 12–16 h/day, children 1 to 2 years of age sleep 11–14 h/day, and children 3 to 5 years of age sleep 10–13 h/day on a regular basis (including naps) to promote optimal health [10]. Although the ideal amount of sleep may vary from one person to another, sleep duration recommendations are important for surveillance and to inform public policies, interventions, and the general public of healthy sleep behaviours [11, 12].
Therefore, the present work aims to provide a global picture of how sleep duration relates to, or affects, a broad set of health indicators in children aged 0–4 years, and findings from this review will help to better inform sleep duration recommendations for this population and identify future research needs. More specifically, the objective of this systematic review is to examine the relationships between sleep duration and various health indicators in children aged 0–4 years.
Methods
Protocol and registration
This review was registered a priori with the International Prospective Register of Systematic Reviews (PROSPERO; Registration no. CRD42016040096; available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016040096), and was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews and meta-analyses [13].
Eligibility criteria
The Participants, Interventions, Comparisons, Outcomes, and Study design (PICOS) framework [14] was followed to identify key study concepts in the research question a priori, and to facilitate the search process.
Population
The population included apparently healthy (i.e., general populations, including those with overweight/obesity, but with no diagnosed medical condition) children aged 1 month to 4.99 years for at least one exposure measurement point. Clinical populations (e.g., patients with sleep apnea) were excluded. Subgroups were defined as: newborns (0–3 months), infants (4–11 months), toddlers (1–2 years), and preschoolers (3–4.99 years).
Intervention (exposure)
The intervention or exposure was sleep duration. Studies were included if they used objective (e.g., polysomnography, actigraphy/accelerometry) or subjective (e.g., proxy-report) measures of sleep duration (or both). This could include actual sleep duration or even time in bed, depending on how it was reported in the studies. Experimental studies were included only if the intervention targeted sleep duration exclusively and not multiple health behaviours (e.g., interventions that targeted both sleep and diet).
Comparison
Various sleep durations were used for comparison. A comparator or control group was not required for inclusion.
Outcomes (health indicators)
Ten health indicators were chosen based on the literature, expert input and consensus, and recognition of the importance of including a broad range of health indicators. Five health indicators were identified as critical (primary outcomes) by expert agreement: (1) adiposity (e.g., overweight, obesity, body mass index, skinfold thickness, body fat); (2) emotional regulation (e.g., mood, social-emotional problems, stress, hyperactivity/impulsivity); (3) cognitive development (e.g., learning, memory, attention, concentration, language development); (4) motor development (e.g., gross motor skills, fine motor skills, locomotor and object control); and (5) growth. Five health indicators were identified as important (secondary outcomes) by expert agreement: (1) cardiometabolic health (e.g., blood pressure, blood lipids, glucose, insulin); (2) sedentary behaviour (e.g., screen time); (3) physical activity (e.g., moderate- to vigorous-intensity physical activity); (4) quality of life/well-being; and (5) risks/injuries.
Study designs
All study designs, except case studies, were eligible for inclusion in this systematic review. In longitudinal studies, any follow-up length was allowed; however, the exposure had to be assessed at least once during the identified age range. There were no sample size restrictions for studies included in this systematic review. Published peer-reviewed original manuscripts and “in press” articles were eligible for inclusion, as were studies with results posted to a trial registry. Grey literature, book chapters, dissertations and conference abstracts were excluded.
Information sources and search strategy
A research librarian with expertise in systematic review searching created the electronic search strategy. A second research librarian peer-reviewed it. See Additional file 1: Table S1 for the complete search strategies. The following databases were searched using the Ovid interface, initially in June and again in November 2016: MEDLINE (1946 to November 1, 2016), EMBASE (1980 to 2016 Week 44), PsychINFO (1806 to 2016 October Week 4), and the Cochrane Central Register of Controlled Trials (CENTRAL) (September 2016). Trial registries (https://clinicaltrials.gov and http://who.int/ictrp/en) were searched for registered clinical trials that met our inclusion/exclusion criteria and where results were posted online. Reference lists of relevant reviews were also checked. Studies were included if they were published in English or French.
Study selection
References were extracted as text files and imported into the Reference Manager Software (Thompson Reuters, San Francisco, CA, USA) for removal of duplicate references. Titles and abstracts of potentially relevant articles were imported to DistillerSR (Evidence Partners, Ottawa, ON, Canada), and were screened independently by two reviewers. Exclusion by both reviewers was needed for a study to be excluded at the first level screening. A full-text copy of each article that met initial screening criteria was obtained, and the same two reviewers independently examined all full-text manuscripts (level 2 screening). Any discrepancies were resolved with a discussion and consensus between the two reviewers. If the reviewers were unable to reach consensus, a third reviewer was asked to examine the article.
Data extraction
Data extraction was completed in Excel (Microsoft) and checked for accuracy by a second reviewer. Results from the most fully adjusted models were extracted for studies that reported findings from multiple models. Important study features (i.e., author, publication year, study design, country, sample size, age and sex of participants, measure of sleep duration and health outcomes, results, and confounders) were extracted.
Risk of bias and study quality assessment
A risk of bias assessment was completed for all included studies, as described in the Cochrane Handbook [15]. Briefly, the risk of bias assessment identifies methodological features of each study that can impact confidence in the overall estimate of effect for an outcome. The quality of evidence for each outcome by type of study design was determined using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework [16]. The GRADE framework categorizes the quality of evidence into four groups (high, moderate, low, and very low). The quality of evidence rating starts at “high” for randomized studies and at “low” for all other studies (e.g., non-randomized experiments or observational studies). The quality of evidence can be downgraded if there are serious limitations across studies (e.g., serious risk of bias, inconsistency of relative treatment effects, indirectness, imprecision, or other factors) [16]. The quality of evidence assessment was conducted by the lead author (J.-P. Chaput) and verified by the larger review team, including systematic review methodology experts (M. Sampson and A. Jaramillo). Disagreements were resolved by discussion among the team members, if needed.
Synthesis of results
A meta-analysis was planned in the event that findings were found to be sufficiently homogenous in terms of methodological, statistical, and clinical characteristics. If not sufficiently homogeneous, narrative syntheses were planned.
Results
Description of studies
As shown in Fig. 1, a total of 1382 records were identified through database searches and an additional three unique records were identified through reference list searches and through the review team and collaborators. Trial registries did not yield any eligible studies. After removing duplicates, a total of 1154 records remained. After titles and abstracts were screened, 133 full-text articles were obtained for further review and 69 articles/studies met the inclusion criteria (from 62 unique samples). Reasons for excluding articles were: not reporting sleep duration as it relates to a health outcome (n = 23), no measure of sleep duration (n = 15), ineligible age (n = 10), participants not apparently healthy (n = 8), sleep duration was treated as a covariate or outcome only (n = 5), intervention not targeting sleep duration (n = 1), not with human participants (n = 1), and not original research (i.e., review paper) (n = 1). Some studies were excluded for multiple reasons.
Fig. 1.
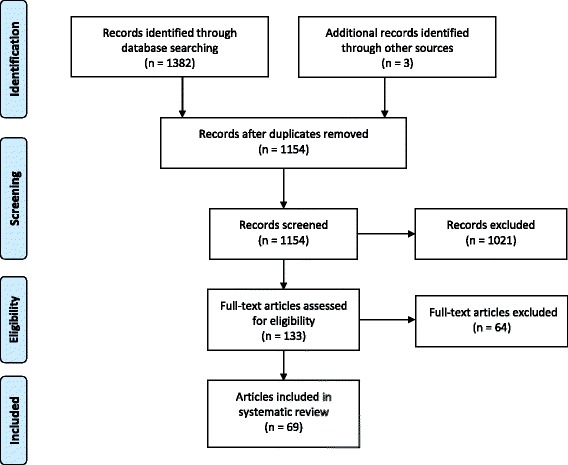
PRISMA flow diagram for the identification, screening, eligibility, and inclusion of studies
Characteristics of studies sorted by outcome indicator are summarized in Additional file 2: Table S2. Data across studies involved 148,524 unique participants. Studies were conducted in 23 different countries from five continents (North America, South America, Europe, Australia/Oceania, and Asia); however, studies were predominantly from North America with White/Caucasian ethnicity. Studies were published between 1992 and 2016, although most were published in the past 5 years. The study designs were randomized trials (n = 3), non-randomized interventions (n = 1), longitudinal studies (n = 16), cross-sectional studies (n = 42), or longitudinal studies that also reported cross-sectional analyses (n = 7). Sleep duration was measured objectively (polysomnography or actigraphy/accelerometry) in 10 studies, subjectively (parent-report) in 48 studies, and by both actigraphy/accelerometry and a sleep log in 11 studies. It was determined by the review team that a meta-analysis was not possible because of high levels of heterogeneity across studies (see Additional file 2: Table S2), and narrative syntheses were employed instead. All studies are given equal weight in a narrative synthesis of the evidence.
Data synthesis
Adiposity
A total of 31 studies examined the association between sleep duration and adiposity indicators (Table 1 and Additional file 2: Table S2). Among the 13 longitudinal studies, 10 reported that shorter sleep duration was associated with adiposity gain [17–26], 2 reported null findings [27, 28], and 1 reported that longer sleep duration predicted adiposity gain [29]. The quality of evidence remained at “low” for the longitudinal studies. Among the 18 cross-sectional studies, 10 reported a significant association between shorter sleep duration and adiposity [23, 26, 30–37], 7 reported null findings [24, 25, 27, 28, 38–40], and 1 reported that sleep duration was unfavourably associated with adiposity [41]. The quality of evidence remained at “low” for the cross-sectional studies.
Table 1.
Association between sleep duration and adiposity in children aged 0–4 years
| No of studies | Design | Quality Assessment | No of participants | Absolute effect | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other | |||||
| Mean age ranged between 0 and 4.9 years. Data were collected cross-sectionally and up to 9.5 years of follow-up. Sleep duration was assessed by actigraphy or parent report. Adiposity was assessed objectively as body weight, body mass index (absolute, z-score or percentile), waist-for-length ratio, weight status (different definitions for underweight, normal weight, overweight, obese) or % body fat/fat mass/fat mass index (bioelectrical impedance, dual-energy X-ray absorptiometry, skinfolds). | |||||||||
| 13 | Longitudinal studya | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 31,482 | Out of 13 longitudinal analyses, 10 reported a significant association between shorter sleep duration and adiposity gain [17–26], 2 reported null findings [27, 28], and 1 reported that longer sleep duration predicted adiposity gain [29]. | LOW |
| 18 | Cross-sectional studyb | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 30,829 | Out of 18 cross-sectional analyses, 10 reported a significant association between shorter sleep duration and adiposity [23, 26, 30–37], 7 reported null findings [24, 25, 27, 28, 38–40], and 1 reported that sleep duration was positively associated with BMI z-scores [41]. | LOW |
Emotional regulation
A total of 25 studies examined the association between sleep duration and emotional regulation (Table 2 and Additional file 2: Table S2). The 2 randomized studies (both randomized cross-over trials) showed better self-regulation strategies and emotional responses in the routine sleep versus the sleep restriction condition [42, 43]. The quality of evidence remained at “high” for the randomized trials. There was also 1 non-randomized trial showing a reduced morning cortisol awakening response after sleep restriction [44]. The quality of evidence was downgraded from “low” to “very low” because of a serious risk of imprecision. Among the 5 longitudinal studies, 2 reported that shorter sleep duration was associated with poorer emotional regulation at follow-up [45, 46], while 3 reported null findings [47–49]. The quality of evidence remained at “low” for the longitudinal studies. Among the 17 cross-sectional studies, 8 reported that shorter sleep duration was associated with poorer emotional regulation [50–57], 7 reported null findings [38, 49, 58–62], and 2 reported opposite associations [63, 64]. The quality of evidence was downgraded from “low” to “very low” due to a serious inconsistency in the findings.
Table 2.
Association between sleep duration and emotional regulation in children aged 0–4 years
| No of studies | Design | Quality Assessment | No of participants | Absolute effect | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other | |||||
| Mean age ranged between 1 month and 4.7 years. Intervention studies were between 1 day and 25 days (in-home protocol), and longitudinal studies were up to 6 years. Sleep duration was assessed by actigraphy, polysomnography or parent report. Emotional regulation was assessed through various instruments (e.g. video-recording, cortisol response, or questionnaires). | |||||||||
| 2 | Randomized triala | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 22 | Nap deprivation resulted in moderate-to-large effects on self-regulation strategies, with decreases in skepticism (d = 0.77; 7% change), negative self-appraisal (d = 0.92; 5% change) and increases in physical self-soothing (d = 0.68; 10% change), focus on the puzzle piece that would not fit (perseveration; d = 0.50; 9% change) and insistence on completing the unsolvable puzzle (d = 0.91; 10% change). After losing daytime sleep, toddlers were less able to engage effectively in a difficult task and reverted to less mature self-regulation strategies than when they were well rested [42]. When sleep restricted, children displayed less confusion in response to neutral pictures, more negativity to neutral and negative pictures, and less positivity to positive pictures. Sleep restriction also resulted in a 34% reduction in positive emotion responses (solvable puzzle), as well as a 31% increase in negative emotion responses and a 39% decrease in confused responses (unsolvable puzzle) [43]. |
HIGH |
| 1 | Non-randomized trialb | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious imprecisionc | None | 7 | The cortisol awakening response was robust after nighttime sleep, diminished after sleep restriction, and smaller but distinct after morning and afternoon (not evening) naps. Cortisol remained elevated 45 min after morning and afternoon naps [44]. | VERY LOW |
| 5 | Longitudinal studyd | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 46,959 | Out of 5 longitudinal analyses, 2 reported that shorter sleep duration was associated with poorer emotional regulation at follow-up [45, 46] while 3 reported null findings [47–49]. | LOW |
| 17 | Cross-sectional studye | No serious risk of bias | Serious inconsistencyf | No serious indirectness | No serious imprecision | None | 16,536 | Out of 17 cross-sectional analyses, 8 reported that shorter sleep duration was associated with poorer emotional regulation [50–57], 7 reported null findings [38, 49, 58–62], and 2 reported opposite associations [63, 64]. | VERY LOW |
Due to heterogeneity in the measurement of sleep and emotional regulation, a meta-analysis was not possible
aIncludes 2 randomized cross-over studies [42, 43]
bIncludes 1 non-randomized intervention [44]
cOnly one study was published with a sample size of N = 7 so the risk of imprecision is high (the quality of evidence was downgraded from “low” to “very low”)
dIncludes 5 longitudinal studies [45–49]
eIncludes 17 cross-sectional studies [38, 49–64]
fStudies reported mixed findings (the quality of evidence was downgraded from “low” to “very low”)
Cognitive development
A total of 16 studies examined the association between sleep duration and cognitive development (Table 3 and Additional file 2: Table S2). One randomized trial examined this association [65] and found that the number of correct answers in an explicit recognition task was significantly higher in the nap condition compared to the wake (sleep restriction) condition; however, implicit memory (priming task) did not differ between conditions. The quality of evidence remained at “high” for this randomized trial. The 4 longitudinal studies that examined the relationships between sleep duration and cognitive development provided mixed findings, although they had mainly favourable associations or null findings [66–69]. The quality of evidence for longitudinal studies remained at “low”. Finally, of 11 cross-sectional studies, 7 reported null findings [38, 51, 55, 70–73], 3 reported that shorter sleep duration was associated with poorer cognitive function [57, 74, 75], and 1 reported opposite associations [76]. The quality of evidence remained at “low” for the cross-sectional studies.
Table 3.
Association between sleep duration and cognitive development in children aged 0–4 years
| No of studies | Design | Quality Assessment | No of participants | Absolute effect | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other | |||||
| Mean age ranged between 6 months and 4.9 years. Data were collected cross-sectionally and up to 3 years of follow-up. Sleep duration was assessed by actigraphy or parent report. Cognition was measured by various instruments including memory tasks, imitation tasks, neuropsychological tests, interviews, scales of intelligence or questionnaires. | |||||||||
| 1 | Randomized triala | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 23 | The number of correct answers in an explicit recognition task was significantly higher in the nap (control) compared to the wake (sleep-restricted) condition, whereas implicit memory (priming task) did not differ between conditions [65]. | HIGH |
| 4 | Longitudinal studyb | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 438 | Children getting higher proportions of their sleep at night as infants (i.e. 1 year) were found to perform better on executive functions, but did not show better general cognition [66]. Higher proportions of total sleep occurring at night time, at both 12 and 18 months, were associated with better performance on executive tasks, especially those involving a strong impulse control component. However, the total sleep duration at 12 and 18 months was not associated with executive functioning at 18 and 26 months. Sleep duration at 12 months was not correlated with 18 month working memory (r = −0.11, p > 0.05), 26 month conflict executive functioning (r = −0.10, p > 0.05) or 26 month impulse control (r = −0.06, p > 0.05). Sleep duration at 18 months was not correlated with 18 month working memory (r = −0.16, p > 0.05), 26 month conflict executive functioning (r = 0.09, p > 0.05) or 26 month impulse control (r = −0.16, p > 0.05) [67]. The number of daytimenaps was positively associated with both predicted expressive (p = 0.062) and receptive vocabulary growth (p = 0.006), whereas the length of nighttime sleep was negatively associated with rate of predicted expressive vocabulary growth (p = 0.045) [68]. Children who had 8 h or more of sleep had significantly higher General Conceptual Ability (GCA) scores than those with 7 h or less of sleep by 35.53 points at age 3. Children with more than 10 h of sleep had higher GCA scores at age 3 compared to children with 8–9 h or less of sleep (233.91 vs. 203.92, respectively) [69]. |
LOW |
| 11 | Cross-sectional studyc | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 10,838 | Out of 11 cross-sectional analyses, 7 reported null findings [38, 51, 55, 70–73], 3 reported that shorter sleep duration was associated with poorer cognitive function [57, 74, 75], and 1 reported opposite associations [76]. | LOW |
Motor development
Two cross-sectional studies examined the association between sleep duration and motor development (Table 4 and Additional file 2: Table S2). Both studies reported no associations between sleep duration, and gross and fine motor skills [38, 51]. The quality of evidence remained at “low” for the cross-sectional studies.
Table 4.
Association between sleep duration and motor development in children aged 0–4 years
| No of studies | Design | Quality Assessment | No of participants | Absolute effect | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other | |||||
| Mean age ranged between 7.4 months and 13 months. Data were collected cross-sectionally only. Sleep duration was assessed by actigraphy or parent report. Motor development was assessed using the Ages and Stages Questionnaire in both studies. | |||||||||
| 2 | Cross-sectional studya | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 1403 | Sleep duration was not associated with gross and fine motor skills [38, 51]. | LOW |
Growth
Two studies examined the relationship between sleep duration and linear growth (Table 5 and Additional file 2: Table S2). The longitudinal study by Lampl et al. [29] showed that higher total daily sleep hours and number of sleep bouts were significantly associated with growth in infant length. The quality of evidence was downgraded from “low” to “very low” for this study because of a serious risk of bias. In the cross-sectional study [77], sleep was assessed both objectively and subjectively in 6-month-old infants. The authors reported that shorter actigraphy-measured sleep duration was associated with higher weight-for-length ratio in girls only. The results also showed that, in the total sample, shorter night sleep duration (as reported by parents) was associated with higher weight-for-length ratio and weight above the expected weight for length. The quality of evidence was downgraded from “low” to “very low” due to a serious risk of imprecision.
Table 5.
Association between sleep duration and growth in children aged 0–4 years
| No of studies | Design | Quality Assessment | No of participants | Absolute effect | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other | |||||
| Mean age ranged between 4 months and 17 months. Data were collected cross-sectionally and up to 13 months. Sleep duration was assessed by actigraphy or parent report. Growth was assessed using the maximum stretch technique and using weight above the expected weight for length. | |||||||||
| 1 | Longitudinal studya | Serious risk of biasb | No serious inconsistency | No serious indirectness | No serious imprecision | None | 23 | Saltatory length growth was associated with increased total daily sleep hours (p < 0.001) and number of sleep bouts (p = 0.001). Subject-specific probabilities of a growth saltation associated with sleep included a mean odds ratio of 1.20 for each additional hour of sleep (n = 8, 95% CI 1.15–1.29) and 1.43 for each additional sleep bout (n = 12, 95% CI 1.21–2.03) [29]. | VERY LOW |
| 1 | Cross-sectional studyc | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious imprecisiond | None | 139,305 | Using actigraphy, sleep duration was associated with weight-to-length ratio (r = −0.47, p < 0.01) in girls only. Using the questionnaire, night sleep duration was associated with weight-to-length ratio (r = −0.26, p < 0.05) and weight above the expected weight for length (r = −0.25, p < 0.05) in the total sample [77]. | VERY LOW |
aIncludes 1 longitudinal study [29]
bSleep duration was parent-reported with no psychometric properties reported. Therefore, the quality of evidence was downgraded from “low” to “very low”
cIncludes 1 cross-sectional study [77]
dOnly one study was published, including a convenience sample of infants and showing differences between boys and girls with the use of actigraphy, so the risk of imprecision is high. Therefore, the quality of evidence was downgraded from “low” to “very low”. Due to the fact that only two studies were published on sleep duration and growth, a meta-analysis was not possible
Cardiometabolic health
No studies examined the association between sleep duration and cardiometabolic biomarkers in children aged 0–4 years.
Sedentary behaviour
A total of 5 studies (1 longitudinal study and 4 cross-sectional studies) examined the association between sleep duration and screen time (Table 6 and Additional file 2: Table S2). The longitudinal study showed that longer sleep duration at 4 years of age was associated with less television viewing and computer use at 6 years of age [22]. The quality of evidence was downgraded from “low” to “very low” due to a serious risk of bias. The 4 cross-sectional studies [31, 78–80] showed that shorter sleep duration was associated with more screen time. The quality of evidence was downgraded from “low” to “very low” because of a serious risk of bias.
Table 6.
Association between sleep duration and sedentary behaviour in children aged 0–4 years
| No of studies | Design | Quality Assessment | No of participants | Absolute effect | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other | |||||
| Mean age ranged between 6 months and 4.5 years. Data were collected cross-sectionally and up to 4 years. Sleep duration was assessed by parent report. Sedentary behaviors (screen time) were assessed using time-use diaries or questionnaires. | |||||||||
| 1 | Longitudinal studya | Serious risk of biasb | No serious inconsistency | No serious indirectness | No serious imprecision | None | 2984 | Sleep duration at 4 years of age was inversely associated with television viewing (β = −0.07, p = 0.003) and computer use (β = −0.04, p = 0.001) at 6 years of age [22]. | VERY LOW |
| 4 | Cross-sectional studyc | Serious risk of biasd | No serious inconsistency | No serious indirectness | No serious imprecision | None | 42,751 | Short sleep duration was associated with time spent watching TV (OR: 1.65, 95% CI 1.23–2.21 per additional hour/24 h) in boys. In girls, the association was not significant (p = 0.75) [31]. Infants who were exposed to screen media in the evening at 12 months of age had a 28-min lower nighttime sleep duration on weekdays. Moreover, infants who were exposed to screen media in the evening at age 6 months and 12 months had shorter 12-month nighttime sleep duration compared with those who were not exposed to screen media after 7 pm at both ages [78]. Watching more than an hour of TV in the evening was associated with short sleep duration (OR = 1.89, 95% CI 1.26–2.84). However, the association was not significant with watching more than an hour of TV in the morning (OR = 1.13, 95% CI 0.80–1.58) [79]. Short sleep duration was associated with longer hours spent watching television (OR = 1.91, 95% CI 1.26–2.90 for ≥4 h/day) and playing computer games (OR = 1.62, 95% CI 1.18–2.23 for ≥2 h/day) compared to not watching/playing [80]. |
VERY LOW |
Due to heterogeneity in the measurement of sleep and sedentary behaviors, a meta-analysis was not possible
aIncludes 1 longitudinal study [22]
bSleep duration was parent-reported with no psychometric properties reported. Therefore, the quality of evidence was downgraded from “low” to “very low”
cIncludes 4 cross-sectional studies [31, 78–80]
dSleep duration was parent-reported with no psychometric properties reported. Therefore, the quality of evidence was downgraded from “low” to “very low”
Physical activity
Four studies (1 longitudinal study and 3 cross-sectional studies) examined the association between sleep duration and physical activity (Table 7 and Additional file 2: Table S2). The longitudinal study showed that sleep duration at 4 years of age was not associated with level of physical activity at 6 years of age [22]. The quality of evidence was downgraded from “low” to “very low” due to a serious risk of bias. The 3 cross-sectional studies [30, 31, 81] showed either favourable (i.e., longer sleep duration was associated with more physical activity) or null findings. The quality of evidence remained at “low” for the cross-sectional studies.
Table 7.
Association between sleep duration and physical activity in children aged 0–4 years
| No of studies | Design | Quality Assessment | No of participants | Absolute effect | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other | |||||
| Mean age ranged between 20 months and 4.5 years. Data were collected cross-sectionally and up to 4 years. Sleep duration was assessed by parent report. Physical activity was assessed using accelerometers, time-use diaries or questionnaires. | |||||||||
| 1 | Longitudinal studya | Serious risk of biasb | No serious inconsistency | No serious indirectness | No serious imprecision | None | 2984 | Sleep duration at 4 years of age was not associated with physical activity at 6 years of age (β = −0.02, 95% CI −0.09-0.03) [22]. | VERY LOW |
| 3 | Cross-sectional studyc | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 2272 | Longer nighttime sleep duration was associated with more physical activity (MVPA min/day: r = 0.19, p = 0.012; activity counts: r = 0.21, p = 0.006). In multivariable models, nighttime sleep duration was positively associated with physical activity (β = 0.332, p = 0.017) [30]. Sleep duration was not associated with physical activity in either boys (p = 0.89) or girls (p = 0.41) [31]. Total daily sleep duration was positively associated with physical activity in boys only (OR = 1.04, 95% CI 1.02–1.07) [81]. |
LOW |
Due to heterogeneity in the measurement of sleep and physical activity, a meta-analysis was not possible
aIncludes 1 longitudinal study [22]
bSleep duration was parent-reported with no psychometric properties reported. Therefore, the quality of evidence was downgraded from “low” to “very low”
Quality of life/well-being
Only 1 study examined the association between sleep duration and quality of life/well-being (Table 8 and Additional file 2: Table S2). This longitudinal study found that short sleep duration at 3 years of age (< 10 h versus > 11 h) was not associated with poor quality of life at ~13 years of age [82]. The quality of evidence was downgraded from “low” to “very low” because of a serious risk of bias.
Table 8.
Association between sleep duration and quality of life/well-being in children aged 0–4 years
| No of studies | Design | Quality Assessment | No of participants | Absolute effect | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other | |||||
| Children were 3 years of age and followed until first-year junior high school (approximately 13 years old). Data were collected longitudinally (approximately a 10-year follow-up period). Sleep duration was assessed by parent report. Quality of life was assessed using the Dartmouth Primary Care Cooperative Project (COOP) charts. | |||||||||
| 1 | Longitudinal studya | Serious risk of biasb | No serious inconsistency | No serious indirectness | No serious imprecision | None | 9674 | Short sleep duration at 3 years of age (< 10 h vs. > 11 h) was not associated with quality of life at age ~13 years (OR = 1.15, 95% CI 0.99–1.33, p = 0.06) [82]. | VERY LOW |
Due to the fact that only one study was published on sleep duration and quality of life/well-being, a meta-analysis was not possible
aIncludes 1 longitudinal study [82]
bSleep duration was parent-reported with no psychometric properties reported. Therefore, the quality of evidence was downgraded from “low” to “very low”
Risks/injuries
Three cross-sectional studies examined the association between sleep duration and risks/injuries in children aged 0–4 years (Table 9 and Additional file 2: Table S2). Koulouglioti et al. [83] reported that children with shorter sleep duration sustained a higher number of medically attended injuries. Likewise, Boto et al. [84] reported that a sleep duration shorter than 8 h per day was associated with an increased risk of accidental falls. In contrast, Owens et al. [85] did not find an association between sleep duration and injury risk. The quality of evidence remained at “low” for the cross-sectional studies.
Table 9.
Association between sleep duration and risks/injuries in children aged 0–4 years
| No of studies | Design | Quality Assessment | No of participants | Absolute effect | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other | |||||
| Mean age ranged between 18 months and 4.9 years. Data were collected cross-sectionally only. Sleep duration was assessed by parent report. Risks/injuries were assessed using medical record data, the Injury Behavior Checklist, interviews, or chart reviews of injuries. | |||||||||
| 3 | Cross-sectional studya | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 2382 | Children with shorter sleep duration sustained a higher number of medically attended injuries (b = 0.1759, p < 0.05) [83]. Usual sleep duration shorter than 8 h was associated with an increased risk of accidental falls (OR = 2.7, 95% CI 1.2–6.1) [84]. The Children’s Sleep Habits Questionnaire (CSHQ) sleep duration score did not significantly differ between the high injury and low injury groups (5.93 ± 1.03 vs. 6.36 ± 0.96, respectively, p = 0.09). Also, the CSHQ sleep duration score did not significantly differ between the high-injury-behavior and the low-injury-behavior groups (5.73 ± 2.10 vs. 4.32 ± 1.92, respectively, p not provided) after Bonferroni correction. The Pearson correlation coefficient between sleep duration and the total Injury Behavior Checklist score was r = 0.32, p = 0.005. To specifically examine the relationship between parent-reported sleep duration and injuries and injury behavior, they divided the group by median split for sleep duration (low sleep < 690 min, high sleep ≥690 min). There were no significant differences in the number of injuries in the past 2 years or in the Injury Behavior Checklist total score [85]. |
LOW |
Summary of findings
A high-level summary of findings by health outcome can be found in Table 10. Overall, studies tended to show favourable associations between sleep duration and adiposity (20/31 studies), emotional regulation (13/25 studies), growth (2/2 studies), screen time (5/5 studies), and risks/injuries (2/3 studies). However, no association was found between sleep duration and motor development (only 2 studies) and quality of life (only 1 study), and the evidence was mixed for cognitive development and physical activity indicators. It is difficult to establish the optimal amount of sleep associated with favourable health outcomes based on the available evidence. Most of the evidence was correlational in nature or compared groups with different cut-points for short and long sleep duration. However, longer sleep durations, when compared to shorter sleep durations, were generally associated with better outcomes in the studies synthesized herein, and the pattern of associations did not differ by the age group examined (i.e., infants, toddlers, and preschoolers).
Table 10.
High-level summary of findings by health indicator
| Health Indicator | # of studies | Quality of Evidence | Summary of Findings |
|---|---|---|---|
| Critical | |||
| Adiposity | 31 | Low |
N = 20 studies reported a significant association between shorter sleep duration and adiposity. N = 9 studies reported null findings. N = 2 studies reported a significant association between longer sleep duration and adiposity. |
| Emotional Regulation | 25 | Very Low to High |
N = 13 studies reported a significant association between shorter sleep duration and poorer emotional regulation. N = 10 studies reported null findings. N = 2 studies reported a significant association between longer sleep duration and poorer emotional regulation. |
| Cognitive Development | 16 | Low to High |
N = 6 studies reported a significant association between shorter sleep duration and poorer cognitive function. N = 8 reported null findings. N = 2 study reported a significant association between longer sleep duration and poorer cognitive function. |
| Motor Development | 2 | Low | N = 2 studies reported null findings. |
| Growth | 2 | Very Low | N = 2 studies reported better growth with longer sleep duration. |
| Important | |||
| Sedentary Behavior | 5 | Very Low | N = 5 studies reported shorter sleep duration was associated with more screen time. |
| Physical Activity | 4 | Low to Very Low |
N = 2 studies reported longer sleep duration was associated with more physical activity. N = 2 studies reported null findings. |
| Risks/Injuries | 3 | Low |
N = 2 studies reported a higher risk of injuries with shorter sleep duration. N = 1 study reported null findings. |
| Quality of Life/Well-Being | 1 | Very Low | N = 1 study reported null findings. |
| Cardio-Metabolic Health | 0 | N/A | N/A |
The number of studies is more than N = 69 because some papers had more than one outcome measure and/or study design
Discussion
This systematic review synthesized peer-reviewed scientific evidence from 69 articles/studies examining the relationships between sleep duration and key health indicators in children aged 0–4 years. The overall quality of evidence ranged from “very low” to “high” across study designs and health indicators. Collectively, shorter sleep duration was generally associated with higher adiposity, poorer emotional regulation, impaired growth, more screen time, and higher risk of injuries. However, the evidence was mixed for cognitive development and physical activity, and null findings were reported for motor development and quality of life. Also, no studies examined the association between sleep duration and cardiometabolic biomarkers in this population. Overall, this comprehensive assessment of available evidence should encourage efforts aimed at promoting the importance of sleep duration for overall health in children aged 0–4 years.
Adiposity (n = 31 studies) and emotional regulation (n = 25 studies) were the health indicators with the highest number of studies in the present systematic review. This is in agreement with our previous systematic review examining the associations between sleep duration and health indicators in school-aged children and youth [2]. However, the findings from these two health indicators in the current paper are more mixed than those found in the children and youth review. Potential reasons to explain this difference include: (1) differences in measurement tools used to assess sleep duration and health outcomes; (2) differences in confounding factors; (3) differences in development stages; (4) differences in the robustness of study designs; and (5) the likelihood that it is more difficult to find associations with adverse health indicators in a younger and healthier population of children, as the outcomes explored in this review are likely to manifest over time if short sleep duration is prolonged .
Many tools have been used to assess emotional regulation in the studies reviewed herein. These included video-recording, various questionnaires, and even cortisol response. It is debatable whether cortisol awakening response (CAR) is an emotional regulation indicator, but it fit our inclusion/exclusion criteria as a stress marker. The non-randomized intervention that examined CAR after sleep restriction [44] showed that CAR was robust after nighttime sleep, diminished after sleep restriction, and was smaller but still distinct after morning and afternoon naps. Although the authors indicated in their article that reduced CAR after shortened sleep suggests a decreased ability to deal with environmental stressors, this viewpoint is not unanimous in the scientific community.
Findings of studies included in the present systematic review are consistent with current sleep duration recommendations, and we are not advocating that they should be changed. However, based on the results of our review—which gathered the best evidence available in this field of research—it is clear that, currently, the evidence being used to inform sleep duration recommendations in the early years is weak, suggesting that expert opinion is needed until more and better research is conducted. There is an urgent need for higher-quality studies that can help to better inform recommendations for sleep duration in this population. For example, the available evidence relies heavily on cross-sectional studies that use parent-reported sleep durations. Multiple age groups were also grouped together, despite obvious differences in development. Most importantly, the current evidence is largely correlational in nature, and there is a clear need for dose-response curves with multiple time points of sleep duration that can provide a better idea of optimal sleep duration ranges. In an experimental context, this means examining how health indicators change in response to sleep restriction/extension interventions. In observational studies, this means comparing several categories of sleep duration in relation to health indicators rather than using continuous data in order to have a better sense of dose-response gradient. Ideally, results would be reported for narrower age groups that are aligned with the current sleep duration recommendations (i.e., newborns [0–3 months], infants [4–11 months], toddlers [1–2 years], preschoolers [3–5 years]); development progresses rapidly in the early years and many factors can confound the associations (e.g., growth, eating habits, environment, locomotion).
The National Sleep Foundation in the USA recommends that in each 24-h cycle, newborns (0–3 months) obtain 14–17 h of sleep, infants (4–11 months) obtain 12–15 h of sleep, toddlers (1–2 years) obtain 11–14 h of sleep, and preschoolers (3–5 years) obtain 10–13 h of sleep [9]. The American Academy of Sleep Medicine issued similar recommendations in 2016 [10]. To develop their guidelines, both organizations relied on a multidisciplinary expert panel to evaluate the latest scientific evidence, including a consensus and voting process. The present systematic review will also help to inform the Canadian 24-Hour Movement Guidelines for the Early Years (0–4 Years): An Integration of Physical Activity, Sedentary Behaviour, and Sleep [86].
Existing sleep duration recommendations provide ranges and imply that a U-shaped association exists between sleep duration and health outcomes, with one side of the “U” representing short sleep duration and the other side representing long sleep duration. It is increasingly recognized that the two sides of this U-shape have different health impacts [87]. While insufficient sleep is a stressor for the metabolism and is consistently associated with adverse health outcomes, excessively long sleep duration may interfere with children’s exploration of their physical and social environment and thereby possibly impede their optimal development [88]. Thus, it is logical for public health sleep duration guidelines to recommend a range to represent “healthy” or “optimal” sleep durations rather than a threshold value.
The present systematic review focused on sleep duration only. However, many other important factors beyond sleep quantity should be considered in the development of sleep recommendations, including aspects of sleep quality such as sleep efficiency (i.e., proportion of the sleep opportunity actually spent in sleep), timing (i.e., bedtime/wake-up times and naps), sleep architecture (i.e., sleep stages), consistency (i.e., day-to-day variability, seasonal changes), and sleep consolidation (i.e., organization of sleep across the night). The National Sleep Foundation recently published evidence-based recommendations and guidance to the public regarding indicators of good sleep quality across the lifespan [89]. Overall, the panel members agreed that most sleep continuity variables (e.g., sleep latency, number of awakenings >5 min, wake after sleep onset, and sleep efficiency) were appropriate indicators of good sleep quality. However, there was less or no consensus regarding sleep architecture or nap-related variables as indicators of good sleep quality.
Sleep duration in the early years is generally comprised of both daytime and nighttime sleep. However, it has been reported that daytime sleep and nighttime sleep may not have the same effects on health, with positive effects of sleep duration suggested to relate to the stage in sleep transition from polyphasic to monophasic sleep (the stage at which naps cease) [90]. The same systematic review also concluded that beyond the age of 2 years, napping is associated with later sleep onset at night as well as reductions in both sleep quality and duration, suggesting that clinicians should investigate napping patterns in children who present with sleep problems [90]. As discussed extensively in previous papers, many healthy sleep practices can help to achieve age-appropriate amounts of sleep, including having a consistent bedtime routine and removing screens from children’s bedrooms [88, 91].
It is also well-known that parent-reported sleep duration overestimates actual sleep duration compared with objective measures [92]. This can have implications for sleep duration recommendations if future studies rely more heavily on objective assessments of sleep, because this will require extrapolation of objectively measured sleep durations to real-world conditions. Most of the studies synthesized in the present work utilized subjective measures of sleep with no psychometric properties, and results may have more ecological validity.
A number of limitations and research gaps have been highlighted in the discussion section already. However, other limitations of the present systematic review should be mentioned. First, the high level of heterogeneity across studies precluded conducting meta-analyses, and all studies were weighted equally. Second, the present systematic review included only articles published in English or French, meaning any relevant studies published in other languages were excluded. Third, the risk of publication bias (i.e., an over-representation of studies with significant findings) is always a possibility in science. Finally, many studies did not adjust for important confounding factors (e.g., diet when examining adiposity as an outcome measure), thereby impacting our confidence in the estimates of effect for the outcome measures.
Conclusions
The present article was the first to systematically examine the associations between sleep duration and a broad range of key health indicators in children aged 0–4 years. We provide support for previous evidence showing that shorter sleep duration is associated with adverse health indicators in some areas of physical and mental health; however, the synthesized evidence relies heavily on cross-sectional study designs and parent-reported sleep durations, and combines multiple ages together despite clear differences in development. To better inform sleep recommendations, scientists should conduct and publish higher-quality studies in this population to have a better idea of dose-response relationships. Robust sleep guidelines should be based on the best available evidence, expert consensus, stakeholder consultation, and consideration of values and preferences, applicability, feasibility, resource use, and equity [86]. There is a clear need for more and better studies in this young age group, which is an important time for growth and development.
Additional files
Search strategy for the systematic review. (DOC 175 kb)
Summary of studies included in the systematic review sorted by (whenever possible) outcome indicator, study design, age group, and sleep assessment (objective, then subjective). (DOC 254 kb)
Acknowledgements
We would like to thank Alejandra Jaramillo, Linda Slater, Holly Livock, Laura Callender, and Sheniz Eryuzlu for providing help with the screening process and the methodology.
Funding
This study has been made possible through funding from the Public Health Agency of Canada, the Canadian Society for Exercise Physiology, and the Healthy Active Living and Obesity Research Group at the Children’s Hospital of Eastern Ontario Research Institute. Publication charges for this article have been funded by the Public Health Agency of Canada. VC was supported by a Canadian Institutes of Health Research (CIHR) New Investigator Salary Award.
Availability of data and materials
Not applicable
About this supplement
This article has been published as part of BMC Public Health Volume 17 Supplement 5, 2017: 24-Hour Movement Guidelines for the Early Years: An Integration of Physical Activity, Sedentary Behaviour, and Sleep. The full contents of the supplement are available online at https://bmcpublichealth.biomedcentral.com/articles/supplements/volume-17-supplement-5.
Abbreviations
- CAR
Cortisol awakening response
- CENTRAL
Cochrane Central Register of Controlled Trials
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- PICOS
Participants, interventions, comparisons, outcomes, and study design
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
Authors’ contributions
JPC, CEG, VJP, VC, RG, CSB, JEM, SA, MS, and MST participated in the conception of the article. JPC, CEG, and SA screened the articles. JPC extracted the data and SA checked the extracted data. JPC wrote the first version of the manuscript. All authors participated in the revisions of the manuscript, and read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12889-017-4850-2) contains supplementary material, which is available to authorized users.
Contributor Information
Jean-Philippe Chaput, Phone: +1 613 737 7600, Email: jpchaput@cheo.on.ca.
Casey E. Gray, Email: casgray@cheo.on.ca
Veronica J. Poitras, Email: vepoitras@cheo.on.ca
Valerie Carson, Email: vlcarson@ualberta.ca.
Reut Gruber, Email: reut.gruber@douglas.mcgill.ca.
Catherine S. Birken, Email: catherine.birken@sickkids.ca
Joanna E. MacLean, Email: joanna.maclean@ualberta.ca
Salomé Aubert, Email: saubert@cheo.on.ca.
Margaret Sampson, Email: msampson@cheo.on.ca.
Mark S. Tremblay, Email: mtremblay@cheo.on.ca
References
- 1.Institute of Medicine (US) Committee on Sleep Medicine and Research . In: Sleep disorders and sleep deprivation: an unmet public health problem. Colten HR, Altevogt BM, editors. Washington, DC: The National Academies Press; 2006. [PubMed] [Google Scholar]
- 2.Chaput JP, Gray CE, Poitras VJ, Carson V, Gruber R, Olds T, et al. Systematic review of the relationships between sleep duration and health indicators in school-aged children and youth. Appl Physiol Nutr Metab. 2016;41(Suppl 3):266–282. doi: 10.1139/apnm-2015-0627. [DOI] [PubMed] [Google Scholar]
- 3.Gruber R, Carrey N, Weiss SK, Frappier JY, Rourke L, Brouillette RT, et al. Position statement on pediatric sleep for psychiatrists. J Can Acad Child Adolesc Psychiatry. 2014;23:174–195. [PMC free article] [PubMed] [Google Scholar]
- 4.McLaughlin Crabtree V, Williams NA. Normal sleep in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2009;18:799–811. doi: 10.1016/j.chc.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Davis KF, Parker KP, Montgomery GL. Sleep in infants and young children: part one: normal sleep. J Pediatr Health Care. 2004;18:65–71. doi: 10.1016/S0891-5245(03)00149-4. [DOI] [PubMed] [Google Scholar]
- 6.Sheldon SH. Sleep in infants and children. In: Lee-Chiong TL, Sateia MJ, Carskadon MA, editors. Sleep Medicine. Philadelphia: Hanley and Belfus Inc.; 2002. pp. 99–103. [Google Scholar]
- 7.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 8.Galland BC, Taylor BJ, Elder DE, Herbison P. Normal sleep patterns in infants and children: a systematic review of observational studies. Sleep Med Rev. 2012;16:213–222. doi: 10.1016/j.smrv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1:233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785–786. doi: 10.5664/jcsm.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matricciani L, Olds TS, Blunden S, Rigney G, Williams MT. Never enough sleep: a brief history of sleep recommendations for children. Pediatrics. 2012;129:548–556. doi: 10.1542/peds.2011-2039. [DOI] [PubMed] [Google Scholar]
- 12.Matricciani L, Blunden S, Rigney G, Williams MT, Olds TS. Children’s sleep needs: is there sufficient evidence to recommend optimal sleep for children? Sleep. 2013;36:527–534. doi: 10.5665/sleep.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 14.Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0. The Cochrane Collaboration. 2010. http://www.cochrane-handbook.org. Accessed 13 June 2017.
- 16.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence – study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr. 2004;145:20–25. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonuck K, Chervin RD, Howe LD. Sleep-disordered breathing, sleep duration, and childhood overweight: a longitudinal cohort study. J Pediatr. 2015;166:632–639. doi: 10.1016/j.jpeds.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touchette E, Petit D, Tremblay RE, Boivin M, Falissard B, Genolini C, et al. Associations between sleep duration patterns and overweight/obesity at age 6. Sleep. 2008;31:1507–1514. doi: 10.1093/sleep/31.11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speirs KE, Liechty JM, CF W. Strong kids research team. Sleep, but not other daily routines, mediates the association between maternal employment and BMI for preschool children. Sleep Med. 2014;15:1590–1593. doi: 10.1016/j.sleep.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Magee C, Caputi P, Iverson D. Lack of sleep could increase obesity in children and too much television could be partly to blame. Acta Paediatr. 2014;103:e27–e31. doi: 10.1111/apa.12447. [DOI] [PubMed] [Google Scholar]
- 23.Diethelm K, Bolzenius K, Cheng G, Remer T, Buyken AE. Longitudinal associations between reported sleep duration in early childhood and the development of body mass index, fat mass index and fat free mass index until age 7. Int J Pediatr Obes. 2011;6:e114–e123. doi: 10.3109/17477166.2011.566338. [DOI] [PubMed] [Google Scholar]
- 24.Carter PJ, Taylor BJ, Williams SM, Taylor RW. Longitudinal analysis of sleep in relation to BMI and body fat in children: the FLAME study. BMJ. 2011;342:d2712. doi: 10.1136/bmj.d2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butte NF, Puyau MR, Wilson TA, Liu YL, Wong WW, Adolph AL, et al. Role of physical activity and sleep duration in growth and body composition of preschool-aged children. Pediatr Obes. 2016;24:1328–1335. doi: 10.1002/oby.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharf RJ, DeBoer MD. Sleep timing and longitudinal weight gain in 4- and 5-year-old children. Pediatr Obes. 2014;10:141–148. doi: 10.1111/ijpo.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiscock H, Scalzo K, Canterford L, Wake M. Sleep duration and body mass index in 0-7-year olds. Arch Dis Child. 2011;96:735–739. doi: 10.1136/adc.2010.204925. [DOI] [PubMed] [Google Scholar]
- 28.Klingenberg L, Christensen LB, Hjorth MF, Zangenberg S, Chaput JP, Sjodin A, et al. No relation between sleep duration and adiposity indicators in 9-36 months old children: the SKOT cohort. Pediatr Obes. 2012;8:E14–E18. doi: 10.1111/j.2047-6310.2012.00109.x. [DOI] [PubMed] [Google Scholar]
- 29.Lampl M, Johnson ML. Infant growth in length follows prolonged sleep and increased naps. Sleep. 2011;34:641–650. doi: 10.1093/sleep/34.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hager ER, Calamaro CJ, Bentley LM, Hurley KM, Wang Y, Black MM. Nighttime sleep duration and sleep behaviors among toddlers from low-income families: associations with obesogenic behaviors and obesity and the role of parenting. Child Obes. 2016;12:392–400. doi: 10.1089/chi.2015.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plancoulaine S, Lioret S, Regnault N, Heude B, Charles MA, Eden Mother-Child Cohort Study Group Gender-specific factors associated with shorter sleep duration at age 3 years. J Sleep Res. 2015;24:610–620. doi: 10.1111/jsr.12308. [DOI] [PubMed] [Google Scholar]
- 32.Dev DA, McBride BA, Fiese BH, Jones BL, Cho H. Risk factors for overweight/obesity in preschool children: an ecological approach. Child Obes. 2013;9:399–408. doi: 10.1089/chi.2012.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones BL, Fiese BH, STRONG Kids Team Parent routines, child routines, and family demographics associated with obesity in parents and preschool-aged children. Front Psychol. 2014;5:374. doi: 10.3389/fpsyg.2014.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang F, Zhu S, Yan C, Jin X, Bandla H, Shen X. Sleep and obesity in preschool children. J Pediatr. 2009;154:814–818. doi: 10.1016/j.jpeds.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 35.Sijtsma A, Koller M, Sauer PJ, Corpeleijn E. Television, sleep, outdoor play and BMI in young children: the GECKO Drenthe cohort. Eur J Pediatr. 2015;174:631–639. doi: 10.1007/s00431-014-2443-y. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe E, Lee JS, Kawakubo K. Associations of maternal employment and three-generation families with pre-school children’s overweight and obesity in Japan. Int J Obes. 2011;35:945–952. doi: 10.1038/ijo.2011.82. [DOI] [PubMed] [Google Scholar]
- 37.Dieu HT, Dibley MJ, Sibbritt D, Hanh TT. Prevalence of overweight and obesity in preschool children and associated socio-demographic factors in Ho Chi Minh City, Vietnam. Int J Pediatr Obes. 2007;7:40–50. doi: 10.1080/17477160601103922. [DOI] [PubMed] [Google Scholar]
- 38.Gibson R, Elder D, Gander P. Actigraphic sleep and development progress of one-year-old infants. Sleep Biol Rhythms. 2012;10:77–83. doi: 10.1111/j.1479-8425.2011.00525.x. [DOI] [Google Scholar]
- 39.Fisher A, McDonald L, van Jaarsveld CHM, Llewellyn C, Fildes A, Schrempft S, et al. Sleep and energy intake in early childhood. Int J Obes. 2014;38:926–929. doi: 10.1038/ijo.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardon G, De Bourdeaudhuij I, Iotova V, Latomme J, Socha P, Koletzko B, et al. Health related behaviours in normal weight and overweight preschoolers of a large pan-European sample: the ToyBox-Study. PLoS One. 2016;11:e0150580. doi: 10.1371/journal.pone.0150580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuzik N, Carson V. The association between physical activity, sedentary behavior, sleep, and body mass index z-scores in different settings among toddlers and preschoolers. BMC Pediatr. 2016;16:100. doi: 10.1186/s12887-016-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller AL, Seifer R, Crossin R, Lebourgeois MK. Toddler’s self-regulation strategies in a challenge context are nap-dependent. J Sleep Res. 2015;24:279–287. doi: 10.1111/jsr.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berger RH, Miller AL, Seifer R, Cares SR, Lebourgeois MK. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. J Sleep Res. 2012;21:235–246. doi: 10.1111/j.1365-2869.2011.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gribbin CE, Watamura SE, Cairns A, Harsh JR, LeBourgeois K. The cortisol awakening response (CAR) in 2- to 4-year old children: effects of acute nighttime sleep restriction, wake time, and daytime napping. Dev Psychobiol. 2012;54:412–422. doi: 10.1002/dev.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jansen PW, Saridjan NS, Hofman A, Jaddoe VWV, Verhulst FC, Tiemeier H. Does disturbed sleeping precede symptoms of anxiety or depression in toddlers? The generation R study. Psychosom Med. 2011;73:242–249. doi: 10.1097/PSY.0b013e31820a4abb. [DOI] [PubMed] [Google Scholar]
- 46.Bouvette-Turcot AA, Pluess M, Bernier A, Pennestri MH, Levitan R, Sokolowski MB, et al. Effects of genotype and sleep on temperament. Pediatrics. 2015;136:e914–e921. doi: 10.1542/peds.2015-0080. [DOI] [PubMed] [Google Scholar]
- 47.Saenz J, Yaugher A, Alexander GM. Sleep in infancy predicts gender specific social-emotional problems in toddlers. Front Pediatr. 2015;3:42. doi: 10.3389/fped.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayaski K, Yorifujji T, Yamakawa M, Oka M, Inoue S, Yoshinaga H, et al. Poor toddler-age sleep schedules predict school-age behavioral disorders in a longitudinal survey. Brain and Development. 2015;37:572–578. doi: 10.1016/j.braindev.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Shinohara H, Kodama H. Relationship between duration of crying/fussy behavior and actigraphic sleep measures in early infancy. Early Hum Dev. 2012;88:847–852. doi: 10.1016/j.earlhumdev.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Kaley F, Reid V, Flynn E. Investigating the biographic, social and temperamental correlates of young infants’ sleeping, crying and feeding routines. Infant Behav Dev. 2012;35:596–605. doi: 10.1016/j.infbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Mindell JA, Lee C. Sleep, mood and development in infants. Infant Behav Dev. 2015;41:102–107. doi: 10.1016/j.infbeh.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Scher A, Epstein R, Sadeh A, Tirosh E, Lavie P. Toddlers’ sleep and temperament: reporting bias or a valid link? A research note. J Child Psychol Psychiatry. 1992;33:1249–1254. doi: 10.1111/j.1469-7610.1992.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 53.Hysing M, Sivertsen B, Garthus-Niegel S, Eberhard-Gran M. Pediatric sleep problems and social-emotional problems. A population-based study. Infant Behav Dev. 2016;42:111–118. doi: 10.1016/j.infbeh.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Lavigne JV, Arend R, Rosenbaum D, Smith A, Weissbluth M, Binns HJ, et al. Sleep and behavior problems among preschoolers. J Dev Behav Pediatr. 1999;20:164–169. doi: 10.1097/00004703-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Keefe-Cooperman K, Brady-Amoon P. Preschooler sleep patterns related to cognitive and adaptive functioning. Early Educ Dev. 2014;25:859–874. doi: 10.1080/10409289.2014.876701. [DOI] [Google Scholar]
- 56.Scharf RJ, Demmer RT, Silver EJ, Stein REK. Nighttime sleep duration and externalizing behaviors of preschool children. J Dev Behav Pediatr. 2013;34:384–391. doi: 10.1097/DBP.0b013e31829a7a0d. [DOI] [PubMed] [Google Scholar]
- 57.Vaughn BE, Elmore-Staton L, Shin N, El-Sheikh M. Sleep as a support for social competence, peer relations, and cognitive functioning in preschool children. Behav Sleep Med. 2015;13:92–106. doi: 10.1080/15402002.2013.845778. [DOI] [PubMed] [Google Scholar]
- 58.Scher A, Tirosh E, Lavie P. The relationship between sleep and temperament revisited: evidence for 12-months-olds: a research note. J Child Psychol Psychiatry. 1998;39:785–788. doi: 10.1017/S0021963098002546. [DOI] [PubMed] [Google Scholar]
- 59.Gibson R, Gander P, Elder D. Factors differentiating infants identified by parents as problem sleepers, and those that are not. Sleep Biol Rhythms. 2012;10:46–52. doi: 10.1111/j.1479-8425.2011.00517.x. [DOI] [Google Scholar]
- 60.Komada Y, Abe T, Okajima I, Asaoka S, Matsuura N, Usui A, et al. Short sleep duration and irregular bedtime are associated with increased behavioral problems among Japanese preschool-age children. J Exp Med. 2011;224:127–136. doi: 10.1620/tjem.224.127. [DOI] [PubMed] [Google Scholar]
- 61.Molfese VJ, Rudasill KM, Prokasky A, Champagne C, Holmes M, Molfese DL, et al. Relations between toddler sleep characteristics, sleep problems, and temperament. Dev Neuropsychol. 2015;40:138–154. doi: 10.1080/87565641.2015.1028627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yokomaku A, Misao K, Omoto F, Yamagishi R, Tanaka K, Takada K, et al. A study of the association between sleep habits and problematic behaviors in preschool children. Chronobiol Int. 2008;25:549–564. doi: 10.1080/07420520802261705. [DOI] [PubMed] [Google Scholar]
- 63.Spruyt K, Aitken RJ, So K, Charlton T, Adamson M, RSC H. Relationship between sleep/wake patterns, temperament and overall development in term infants over the first year of life. Early Hum Dev. 2008;84:289–296. doi: 10.1016/j.earlhumdev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Liu Z, Wang G, Geng L, Luo J, Li N, Owens J. Sleep patterns, sleep disturbances, and associated factors among Chinese urban kindergarten children. Behav Sleep Med. 2016;14:100–117. doi: 10.1080/15402002.2014.963581. [DOI] [PubMed] [Google Scholar]
- 65.Giganti F, Arzilli C, Conte F, Toselli M, Viggiano MP, Ficca G. The effect of a daytime nap on priming and recognition tasks in preschool children. Sleep. 2014;37:1087–1093. doi: 10.5665/sleep.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bernier A, Beauchamp MH, Bouvette-Turcot AA, Carlson SM, Carrier J. Sleep and cognition in preschool years: specific links to executive functioning. Child Dev. 2013;84:1542–1553. doi: 10.1111/cdev.12063. [DOI] [PubMed] [Google Scholar]
- 67.Bernier A, Carlson SM, Bordeleau S, Carrier J. Relations between physiological and cognitive regulatory systems: infant sleep regulation and subsequent executive functioning. Child Dev. 2010;81:1739–1752. doi: 10.1111/j.1467-8624.2010.01507.x. [DOI] [PubMed] [Google Scholar]
- 68.Horvath K, Plunkett K. Frequent daytime naps predict vocabulary growth in early childhood. J Child Psychol Psychiatry. 2016;57:1008–1017. doi: 10.1111/jcpp.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jung E, Molfese VJ, Beswick J, Jacobi-Vessels J, Molnar A. Growth of cognitive skills in preschoolers: impact of sleep habits and learning-related behaviors. Early Educ Dev. 2009;20:713–731. doi: 10.1080/10409280802206890. [DOI] [Google Scholar]
- 70.Konrad C, Herbert JS, Schneider S, Seehagen S. The relationship between prior night’s sleep and measures of infant imitation. Dev Psychobiol. 2016;58:450–461. doi: 10.1002/dev.21387. [DOI] [PubMed] [Google Scholar]
- 71.Scher A. Infant sleep at 10 months of age as a window to cognitive development. Early Hum Dev. 2005;81:289–292. doi: 10.1016/j.earlhumdev.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 72.Lukowski AF, Milojevich HM. Sleeping like a baby: examining relations between habitual infant sleep, recall memory, and generalization across cues at 10 months. Infant Behav Dev. 2013;36:369–376. doi: 10.1016/j.infbeh.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Hoyniak CP, Petersen IT, McQuillan ME, Staples AD, Bates JE. Less efficient neural processing related to irregular sleep and less sustained attention in toddlers. Dev Neuropsychol. 2015;40:155–166. doi: 10.1080/87565641.2015.1016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lam JC, Mahone EM, Mason T, Scharf SM. The effects of napping on cognitive function in preschoolers. J Dev Behav Pediatr. 2011;32:90–97. doi: 10.1097/DBP.0b013e318207ecc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scott N, Blair PS, Emond AM, Fleming PJ, Humphreys JS, Henderson J, et al. Sleep patterns in children with ADHD: a population-based cohort study from birth to 11 years. J Sleep Res. 2013;22:121–128. doi: 10.1111/j.1365-2869.2012.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nathanson AI, Fries PT. Television exposure, sleep time, and neuropsychological function among preschoolers. Media Psychol. 2014;17:237–261. doi: 10.1080/15213269.2014.915197. [DOI] [Google Scholar]
- 77.Tikotzky L, De Marcas G, Har-Toov J, Dollberg S, Bar-Haim Y, Sadeh A. Sleep and physical growth in infants during the first 6 months. J Sleep Res. 2010;19:103–110. doi: 10.1111/j.1365-2869.2009.00772.x. [DOI] [PubMed] [Google Scholar]
- 78.Vijakkhana N, Wilaisakditipakorn T, Ruedeekhajorn K, Pruksananonda C, Chonchaiya W. Evening media exposure reduces night-time sleep. Acta Paediatr. 2015;104:306–312. doi: 10.1111/apa.12904. [DOI] [PubMed] [Google Scholar]
- 79.McDonald L, Wardle J, Llewellyn CH, van Jaarsveld CH, Fisher A. Predictors of shorter sleep in early childhood. Sleep Med. 2014;15:536–540. doi: 10.1016/j.sleep.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ikeda M, Kaneita Y, Kondo S, Itani O, Ohida T. Epidemiological study of sleep habits among four-and-a-half-year-old children in Japan. Sleep Med. 2012;13:787–794. doi: 10.1016/j.sleep.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 81.Hinkley T, Salmon J, Okely AD, Hesketh K, Crawford D. Correlates of preschool children’s physical activity. Am J Prev Med. 2012;42:159–167. doi: 10.1016/j.amepre.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 82.Wang H, Sekine M, Chen X, Yamagami T, Kagamimori S. Lifestyle at 3 years of age and quality of life (QOL) in first-year junior high school students in Japan: results of the Toyama Birth Cohort Study. Qual Life Res. 2008;17:257–265. doi: 10.1007/s11136-007-9301-6. [DOI] [PubMed] [Google Scholar]
- 83.Koulouglioti C, Cole R, Kitzman H. Inadequate sleep and unintentional injuries in young children. Public Health Nurs. 2008;25:106–114. doi: 10.1111/j.1525-1446.2008.00687.x. [DOI] [PubMed] [Google Scholar]
- 84.Boto LR, Crispim JN, de Melo IS, Juvandes C, Rodrigues T, Azeredo P, et al. Sleep deprivation and accidental fall risk in children. Sleep Med. 2012;13:88–95. doi: 10.1016/j.sleep.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 85.Owens JA, Fernando S, Mc Guinn M. Sleep disturbance and injury risk in young children. Behav Sleep Med. 2005;3:18–31. doi: 10.1207/s15402010bsm0301_4. [DOI] [PubMed] [Google Scholar]
- 86.Tremblay MS, Chaput JP, Adamo KB, Aubert S, Barnes JD, Choquette L, et al. Canadian 24-Hour Movement Guidelines for the Early Years (0-4 years): An Integration of Physical Activity, Sedentary Behaviour, and Sleep. 2017;17(5) [in press]. [DOI] [PMC free article] [PubMed]
- 87.Knutson KL, Turek FW. The U-shaped association between sleep and health: the 2 peaks do not mean the same thing. Sleep. 2006;29:878–879. doi: 10.1093/sleep/29.7.878. [DOI] [PubMed] [Google Scholar]
- 88.Allen SL, Howlett MD, Coulombe JA, Corkum PV. ABCs of SLEEPING: a review of the evidence behind pediatric sleep practice recommendations. Sleep Med Rev. 2016;29:1–14. doi: 10.1016/j.smrv.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 89.Ohayon M, Wickwire EM, Hirshkowitz M, Albert SM, Avidan A, Daly FJ, et al. National Sleep Foundation’s sleep quality recommendations: first report. Sleep Health. 2017;3:6–19. doi: 10.1016/j.sleh.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 90.Thorpe K, Staton S, Sawyer E, Pattinson C, Haden C, Smith S. Napping, development and health from 0 to 5 years: a systematic review. Arch Dis Child. 2015;100:615–622. doi: 10.1136/archdischild-2014-307241. [DOI] [PubMed] [Google Scholar]
- 91.Mindell JA, Li AM, Sadeh A, Kwon R, Goh DY. Bedtime routines for young children: a dose-dependent association with sleep outcomes. Sleep. 2015;38:717–722. doi: 10.5665/sleep.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Girschik J, Fritschi L, Heyworth J, Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;5:462–468. doi: 10.2188/jea.JE20120012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy for the systematic review. (DOC 175 kb)
Summary of studies included in the systematic review sorted by (whenever possible) outcome indicator, study design, age group, and sleep assessment (objective, then subjective). (DOC 254 kb)
Data Availability Statement
Not applicable


