Abstract
The use of blood plasma biomarkers in gastric cancer (GC) management is limited due to a lack of reliable biomarkers. An LC‐MS/MS assay and a bioinformatic analysis were performed to identify blood plasma biomarkers in a GC discovery cohort. The data obtained were verified and validated by western blotting and an ELISA in an independent study cohort. A label‐free quantification analysis of the MS data using PEAKS7 software found that four plasma proteins of apolipoprotein C‐1, gelsolin, sex hormone‐binding globulin (SHBG), and complement component C4‐A were significantly overexpressed in GC patients. A western blot assay of these plasma proteins showed that only SHBG was consistently overexpressed in the patient group. ELISA measurement of SHBG blood plasma levels confirmed that the patient group had significantly higher SHBG levels than the control group. SHBG levels in the patient group remained significantly higher after being stratified by gender, age, and disease stage. These findings show that LC‐MS/MS is powerful and highly sensitive for plasma biomarker discovery, and SHBG could be a potential plasma biomarker for GC management.
Keywords: Gastric cancer, LC‐MS/MS, mass spectrometry, proteomics, SHBG
Introduction
Gastric cancer (GC) is the fifth most common type of cancer with 1 million new cases diagnosed worldwide in 2012. The incidence of GC in men is almost twice that in women 1. GC is also the third most common cause of cancer deaths after lung and liver cancers, and caused approximately 800,000 deaths in 2012 2, 3. With the current treatment strategy, the 5‐year survival rate is only approximately 30% for stage I to stage III and <5% for stage IV 4.
As one of the strategies to reduce the burden of GC, screening has been undertaken in Asian countries such as Japan and South Korea where the incidence of GC is high 5. In Japan, a nationwide screening program using barium meal contrast radiography and endoscopy was introduced in 1960 6, and it is claimed to have reduced GC mortality by 50% 5, 7. In South Korea, biennial screening workup is conducted for people aged ≥40 years old either using barium meal contrast radiography or endoscopy according to their preference 8. Considering that barium meal contrast radiography and endoscopy are invasive and expensive and also require experienced radiologists and endoscopists, these screening methods might not be suitable for resource‐strapped countries. Moreover, mass‐screening for GC using these technologies is not cost‐effective, especially in areas where the incidence of GC is low. Therefore, the availability of simple, less‐invasive, cheap, sensitive, and specific screening tools is an urgent need in order to reduce the burden of GC, and this could be achieved by developing screening tools developed from plasma protein biomarkers.
However, there are very limited numbers of clinically available plasma biomarkers for GC, and currently, no blood plasma biomarker has been recommended for the early detection of GC 9, 10. Serum pepsinogen 11, 12 and its combination with a Helicobacter pylori infection test 13, 14, 15, the serum carcinoembryonic antigen (CEA) level 16, the carbohydrate antigen (CA) 125 level 16, the CA 72‐4 level 16, and a gastrin test 5, 12, 17 are promising tests that have been developed for GC management. However, the sensitivity and specificity of these serum biomarkers are highly variable 9, 13. Moreover, interpretation of the combination of serum pepsinogen and the H. pylori infection test should be done very carefully since the H. pylori eradication therapy changes the pepsinogen serum level in a population 14, which may affect the clinically meaningful test's cutoff point.
With the current advancement in blood plasma biomarker research, proteomic approaches, such as mass spectrometry (MS), have been considered to be promising approaches for plasma protein biomarker discovery 18. Proteomics offer the possibility of analyzing proteome alterations such as posttranslational modifications that cannot be predicted through genomic 19 or transcriptomic 20 approaches. Moreover, the proteome reflects the dynamic relationship between the genome and environment and is directly involved in the pathogenesis of diseases 18. Many studies used proteomic approaches to discover novel plasma biomarkers that can be developed into tools for cancer risk prediction, early detection, tumor classification, and therapeutic drug monitoring 19.
Using proteomic technology, this study aimed to discover novel blood plasma protein biomarkers for GC. Blood plasma samples were collected from both healthy subjects and GC patients, and liquid chromatography tandem MS (LC‐MS/MS) mass spectrophotometry was performed to discover potential plasma biomarker candidates by comparing differentially expressed proteins between healthy subjects and GC patients. Through verification and a validation study, we found serum sex hormone‐binding globulin (SHBG) levels to be a potential candidate for an early diagnostic biomarker that may be applied in GC management.
Materials and Methods
Study subjects and study design
Blood plasma samples collected from 76 GC patients and 86 healthy subjects were provided by the Taipei Medical University Hospital Biobank. Peripheral whole blood of patients and control group was collected into a purple top K2EDTA vacutainer (#367525, BD) and separated plasma by centrifugation for 15 min at 2000 g. Plasma samples were stored in a ‐80°C freezer until being used for the analysis. GC patients included in this study were men and women aged 40–90 years old with the adenocarcinomatous type of GC. Informed consent was obtained from all study subjects. Total subjects were divided into three independent cohorts: discovery, verification, and validation study cohorts. To discover plasma protein biomarker candidates, 24 patients were chosen based on gender, disease stage, and age. They were divided into four different stage groups with each stage group containing six patients with a male–female ratio of 2:1 and similar ratios of GC prevalence in both genders. In the control group, healthy volunteers including six men and three women were selected. Discovered plasma protein biomarker candidates were verified by a western blot analysis in the verification study cohort which consisted of 24 patients and nine controls. The validation study cohort, which consisted of 50 patients (included 22 subjects with age ≤60 years from discovery and verification study cohort) and 68 control subjects, was used to further validate blood plasma levels of plasma protein biomarker candidates validated in the verification study cohort by an enzyme‐linked immunosorbent assay (ELISA). The study was approved by the Taipei Medical University Ethical Committee, and an experimental outline is shown in Figure 1.
Figure 1.
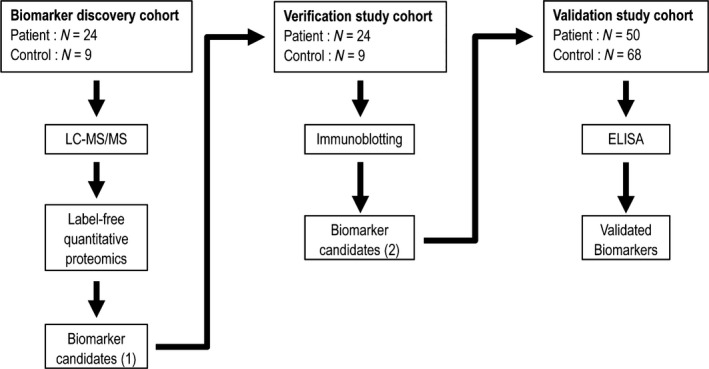
Experimental outline.
Protein concentration determination
A Pierce® Bicinchoninic Acid (BCA) Protein Assay Kit (Thermo Scientific, Vilnius, Lithuania) was used to quantify protein concentrations. Triplicate plasma samples and bovine serum albumin (BSA) standards were added to a flat‐bottomed 96‐well plate which contained 100 μL of working reagent followed by incubation for 30 min at 37°C. The absorbance was measured at 562 nm on an ELISA reader (EZ Read 400 Microplate Reader, Biochrom, UK). A linear standard curve formula for protein quantification was made by plotting the blank‐corrected BSA standard absorbance against the BSA standard concentration. Protein concentrations of samples were calculated by putting the blank‐corrected sample absorbance into the linear standard curve formula.
Immunodepletion of a highly abundant protein (albumin)
The HSA CaptureSelectTM Proteomics Depletion Product (Life Technologies, California) was used to deplete albumin. Albumin immunodepletion was performed according to the manufacturer's manual. In brief, 400 μg of protein resuspended in 50 μL of phosphate‐buffered saline (PBS) from each healthy subject and patient plasma protein was added to an Eppendorf tube containing 100 μL of the HSA antibody followed by incubation on a rotating shaker for 60 min at 4°C. Afterward, the mixture was transferred to a multi‐spin separation column kit (Axygen, California, USA) and centrifuged at 13,500 g for 1 min at 4°C to collect albumin‐depleted plasma samples. In order to check the efficiency of albumin immunodepletion, the original samples and albumin‐depleted samples were run side by side in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) (Data S2).
Protein digestion
An In‐Solution Tryptic Digestion and Guanidination Kit (Thermo Scientific, Vilnius, Lithuania) was used to digest albumin‐depleted samples into peptides. Protein digestion was done according to the manufacturer's instructions. In the reduction step, 15 μL of digestion buffer, 1.5 μL of reducing buffer, and 3 μL of albumin‐depleted samples were mixed. Ultrapure water was added to adjust the final volume to 27 μL followed by incubation at 95°C for 5 min. Three microliters of alkylation buffer containing iodoacetamide was added to the mixture and incubated for 20 min in the dark at room temperature. In the digestion step, 1 μL of activated trypsin was added to the mixture, followed by incubation for 3 h at 37°C. We added an additional 1 μL of activated trypsin to the mixture (with a final volume of 32 μL) and re‐incubated the reaction overnight at 30 °C.
ZipTip C18 procedures
The purpose of the ZipTip procedure was to remove salts which might interfere with the MS analysis. The pH of tryptic‐digested samples was adjusted to <4 by titrating digested samples with 1% or 10% trifluoroacetic acid (TFA) in order to stop the trypsin digestion reaction. For each sample, six Eppendorf tubes were prepared which contained the following solutions: (1) tube 1 had 1 mL of acetonitrile (ACN)/0.1% TFA; (2) tube 2 had 0.5 mL ACN/0.1% TFA plus 0.5 mL H2O/0.1% TFA; (3) tube 3 had 1 mL H2O/0.1% TFA; (4) tube 4 was empty (used for waste after washing); and (5) tubes 5 and 6 each contained 30 μL of tube 2 solution. The ZipTip C18 tip (ZTC18S960, Merck Millipore, California) was equilibrated by pipetting 20 μL of solution into tubes 1–3. Once it was equilibrated, the tip was used to capture the peptides contained in the tryptic‐digested sample solution by pipetting the solution up and down 15 times. Repeated aspiration‐dispensing (5–15 times) of the tube 3 solution was done to wash the captured peptides. The aspirated solution was then dispensed into tube 4. The captured peptides were eluted by pipetting the solution up and down in tubes 5 and 6 five times. The eluted peptides were dried in a centrifugal vacuum concentrator (SpeedVac SC110 Concentrators, Savant Instrument, New York) for 60 min.
Liquid chromatography tandem MS (LC‐MS/MS) conditions
LC was performed on a NanoAquity UPLC system (Waters, MA) coupled to an Orbitrap Elite mass spectrometer (Thermo Electron, MA). Peptide samples were injected into a trap column (2 cm × 180 μm i.d., Symmetry C18, Waters), and then were separated by a 25 cm × 75 μm i.d. BEH130 C18 column (Waters) with a segmented gradient in 120 min from 0% to 85% solvent B at 300 nL/min (buffer A, 0.1% formic acid in H2O; buffer B, 0.1% formic acid in ACN). The Orbitrap was operated in the positive ion mode, with the following acquisition cycle: a full scan (m/z 350–1600) recorded in the orbitrap analyzer at resolution R = 240,000 was followed by MS/MS of the ten most intense peptide ions in the ion trap analyzer. Peptide fragmentation by collision‐induced dissociation was automatically performed in a dynamic data‐dependent mode. All measurements in the orbitrap were performed with the lock mass option to improve the mass accuracy of precursor ions.
Protein database searching and protein identification
All Orbitrap Elite MS raw data files were qualitatively and quantitatively processed using PEAKS Studio (PEAKS 7, Bioinformatic Solution, Ontario, Canada). Settings for the protein database search were as follows: the protein database was the Uniprot‐Human with a decoy database; the enzyme was trypsin with a maximum of two missed cleavage sites; the precursor mass tolerance was 20 ppm; the fragment mass tolerance was 0.8 Da; and the false discovery rate (FDR) value was <1%. Posttranslational modifications (PTMs) were matched with 485 types of PTMs available in the database. Detected peptide‐spectral matches (PSMs) were additionally filtered with the following criteria: a peptide score of ≥20 (in the form of ‐10logP), a multiple of change of ≥1, and unique peptides of ≥1.
LFQ of peptide spectra
Protein intensity quantification was done using a Label‐free quantification (LFQ) method. PEAKS 7 uses an expectation‐maximization (EM)‐based algorithm for feature detection, deconvolution, and refinement. An optimization model for simultaneous feature matching and retention time alignment was used in the analysis. The LFQ parameters used were: a mass error tolerance of 20 ppm; a retention time shift tolerance of 6 min; and an FDR value of <1%. Peptide features and proteins with multiples of change of ≥2.0 and a statistical P < 0.05 were considered significant between patients with GC and healthy controls (HCs). Experimental bias was taken into account by automatic normalization of protein ratios based on the total ion chromatogram (TIC).
Protein separation using SDS‐PAGE
Plasma proteins from original plasma samples were separated using SDS‐PAGE by loading 5 μg of plasma sample proteins on a 12–15% SDS‐polyacrylamide gel (30% acrylamide, sterile water, 1.5 Tris (pH 7.8), 10% SDS, and 10% ammonium persulfate (APS)). Proteins were resolved in Tris‐glycine buffer (0.1% SDS (w/v), 25 mmol/L Tris, and 192 mmol/L glycine, at pH 8.3), and electrophoresis was done at 80 V for 30 min followed by voltage adjustment to 150 V for 60 min.
Immunoblotting assay
An immunoblotting assay was performed in validation study cohort 1 to individually determine plasma protein levels of apolipoprotein C1 (APOC1), gelsolin (GSN), sex hormone‐binding globulin (SHBG), and complement component C4‐A (C4A). The SDS‐PAGE gel‐separated plasma proteins were transferred to Hybond C+ membranes (Amersham, New Jersey) in transfer buffer (20% methanol, 25 mmol/L Tris base, and 192 mmol/L glycine, at pH 8.0) at 100 V for 60 min. Membranes were blocked with 5% skim milk in deionized water with 0.05% Tween‐20. Immunoblotting was done by overnight incubation of the membranes with the following primary antibodies: APOC1 (ab20793, Abcam, Cambridge, UK); GSN (GTX101185, Genetex, Hsinchu City, Taiwan); SHBG (GTX63795, Genetex), and C4A (ab66790, Abcam) followed by incubation with the secondary antibody, a horseradish peroxidase (HRP)‐conjugated rabbit monoclonal immunoglobulin G (IgG) antibody, for 1 h at room temperature. Reactive protein bands were detected using Immobilon ™Western Chemiluminescent HRP Substrate (Merck Millipore). The band intensity was analyzed and estimated using ImageJ software (IJ1.46r revised version, ImageJ developers). Light‐chain IgG immunoblotted with an HRP‐conjugated human monoclonal IgG antibody was used as an internal control, and a sample in the control group was randomly chosen as an external control. An external control was done by loading the same external control sample in every SDS‐PAGE gel. The internal control blotting signal was normalized to the external control blotting signal, and this value was used to calculate the normalized value of each protein expression level in every sample.
Enzyme‐linked immunosorbent assay (ELISA)
An SHBG ELISA assay of the validation cohort was performed with a Human SHBG Quantikine ELISA Kit (R&D Systems, Minnesota, USA) according to the manufacturer's instructions.
Statistical analysis
Data processing and statistical analyses were performed with the Microsoft Excel 2013 and GraphPad Prism 6 statistical software package. The Mann–Whitney U‐test was used for assessing protein relative abundance/intensity differences between control and GC patient groups calculated from LFQ and immunoblotting data. Kruskal–Wallis was used to analyze blotting signal differences among four different stages of GC and three different age groups. A standard error of the mean (SEM) analysis was performed to analyze the mean difference in plasma SHBG levels measured using the ELISA. P < 0.05 were considered statistically significant.
Results
Sample characteristics
Total subjects were divided into three independent cohorts for plasma biomarker discovery, and the verification and validation studies. Table 1 shows study subject characteristics of each study cohort. Details of individual patient characteristics are presented in Data S1.
Table 1.
Characteristics of subjects in three study cohorts
| Characteristics | Biomarker Discovery cohort | Verification study cohort | Validation study cohort | |||
|---|---|---|---|---|---|---|
| Normal subjects | Gastric cancer patients | Normal subjects | Gastric cancer patients | Normal subjects | Gastric cancer patients | |
| Total subjects | 9 | 24 | 9 | 24 | 68 | 50 |
| Male (%) | 6 (67%) | 16 (67%) | 6 (67%) | 15 (63%) | 44 (65%) | 33 (66%) |
| Male–female ratio | 2:1 | 2:1 | 2:1 | 1.7:1 | 1.83:1 | 1.94:1 |
| Median age (years) | 44 | 59 | 44 | 74 | 32 | 51 |
| Men (average) | 43.5 | 59.5 | 43.5 | 74 | 33.7 | 49 |
| Women (average) | 44 | 57 | 44 | 71.5 | 31 | 47.7 |
| Stage I (average) | — | 56.5 | — | 70 | — | 45.3 |
| Stage II (average) | — | 63.5 | — | 77 | — | 53.9 |
| Stage III (average) | — | 55 | — | 73 | — | 47 |
| Stage IV (average) | — | 60.5 | — | 62 | — | 49.9 |
Identification of biomarker candidates using LC‐MS/MS and LFQ analysis
We tested blood plasma from the plasma biomarker discovery cohort which consisted of 24 GC patients and nine healthy subjects. Each blood plasma sample, after albumin immunodepletion, was subjected to in‐solution trypsin digestion followed by the Zip Tip C18 procedure before being processed for LC‐MS/MS. LC‐MS/MS raw data from 24 patients and nine healthy subjects were successfully imported to PEAKS7 bioinformatic software, but one patient's data could not be processed for further analysis as a protein database search could not be generated. Therefore, data of 23 patients and nine healthy subjects were included in the LFQ analysis using PEAKS7 software. LFQ was automatically done by the software after aligning the peptide spectra based on their retention times.
In the discovery cohort, four potential biomarkers were found using LC‐MS/MS and the LFQ analysis. APOC1, GSN, SHBG, and C4A (Table 2) with their significantly higher relative intensities in the patient group are presented in relative abundances as a heat map (Fig. 2). The LC‐MS/MS spectra data of these proteins are shown in Data S3.
Table 2.
The upregulated proteins in plasma gastric cancer patients identified using LC‐MS/MS
| Gene ID | UniProt accession number | Significance (‐10lgP) | Coverage (%) | The best unique peptide sequence | Retention time average | m/z |
|---|---|---|---|---|---|---|
| APOC1 | P02654 | 49.02 | 39 | MREWFSETFQK | 53.04 | 496.9 |
| TPDVSSALDKLKEFGNTLEDK | 71.76 | 577.5 | ||||
| TPDVSSALDKLK | 47.82 | 425.2 | ||||
| DVSSALDKLKEFGNTLEDK | 70.39 | 528.0 | ||||
| GSN | P06396 | 33.84 | 4 | VPFDAATLHTSTAMAAQHGMDDDGTGQK | 47.82 | 719.1 |
| SHBG | P04278 | 31.56 | 3 | IALGGLLFPASNLR | 79.02 | 721.4 |
| C4A | P0C0L4 | 25.74 | 3 | PVAFSVVPTAAAAVSLK | 68.78 | 814.5 |
APOC1, apolipoprotein C‐I; GSN, gelsolin; SHBG, sex hormone‐binding globulin; C4A, complement C4‐A.
Figure 2.

Heat map generated from a PEAKS7 software analysis with the average relative intensity as a reference.
Validation of biomarker candidates using western blotting
A semiquantitative western blotting analysis of APOC1, GSN, SHBG, and C4A was performed in plasma samples from the verification study cohort to confirm the findings in the MS analysis. We selected the control sample, N1, with lowest SHBG protein level as a reference to provide the baseline for protein level comparison. Among the four proteins, only the level of SHBG was consistently higher in patient groups from the verification study cohort (Fig. 3).
Figure 3.
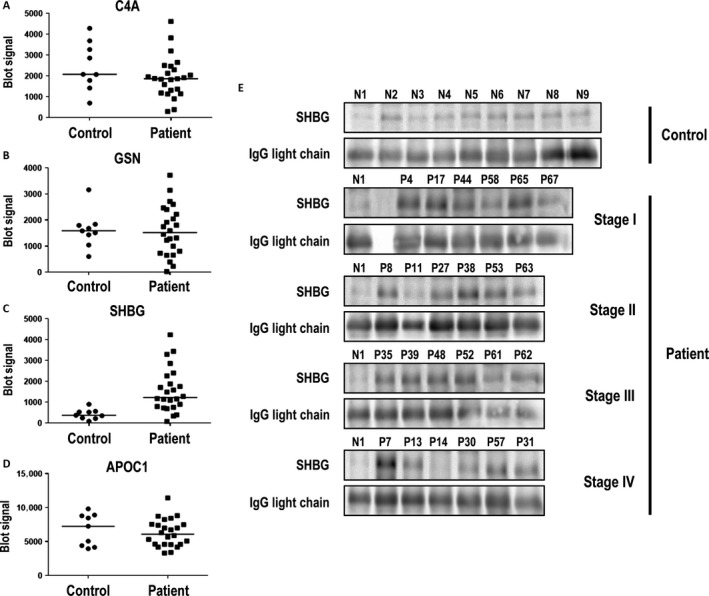
Quantitative analysis of western blotting results of apolipoprotein C‐1 (APOC1) (A), gelsolin (GSN) (B), sex hormone‐binding globulin (SHBG) (C), and complement component C4‐A (C4A) (D) from the verification study cohort. Western blotting images of SHBG (E) are grouped into control and patient groups. The patient group was further divided into four different cancer stages. Human light‐chain immunoglobulin was used as a loading control, and the human light‐chain immunoglobulin blotting signal of N1 was used to normalize the human light‐chain immunoglobulin blotting signal from each sample. The normalized human light‐chain immunoglobulin of each sample was used to calculate the normalized SHBG blotting signal of each sample. Data were analyzed with the Mann–Whitney U‐test.
Validation of blood plasma SHBG levels using an ELISA
Since the plasma level of SHBG consistently had significantly higher expression in GC patients in both the discovery and verification cohorts, an absolute quantification assay by an ELISA to measure plasma SHBG concentrations was performed in an independent study cohort (validation study cohort). However, it was reported that the SHBG levels in women show a decreasing trend during the first six decades followed by a steady increase at around age 60 years onward 21, whereas in men they show an increasing trend with age 22, 23; we stratified the ELISA results based on gender and age, and we only included subjects aged ≤60 years to reduce the variability in the data, especially in women. According to the data from verification study cohort (mean and SD σ), we had performed an A priori test with calculated Effect size d = 1.1316042, α err prob = 0.05, and Power (1‐β err prob) = 0.95. These numbers suggested that at least 22 samples per group for the proposed ELISA analysis. The sample size of validation study cohort (Control = 68, Patient = 50) met the criteria for a successful analysis.
Blood SHBG concentrations in GC patients were about twofold higher than those of the control group (P < 0.0001, Fig. 4A). After stratification by age, SHBG concentrations remained significantly higher in the patient group in subjects aged both 20–40 (P < 0.0001) and 41–60 years (P < 0.0004, Fig. 4B). After subjects were stratified by gender, SHBG concentrations remained significantly higher in the patient group in both male (P < 0.0001) and female (P < 0.0001) subjects (Fig. 4C). SHBG concentrations were significantly higher (P < 0.0001) in every stage of the disease compared to the control group, but did not increase as the stage advanced (Fig. 4D).
Figure 4.
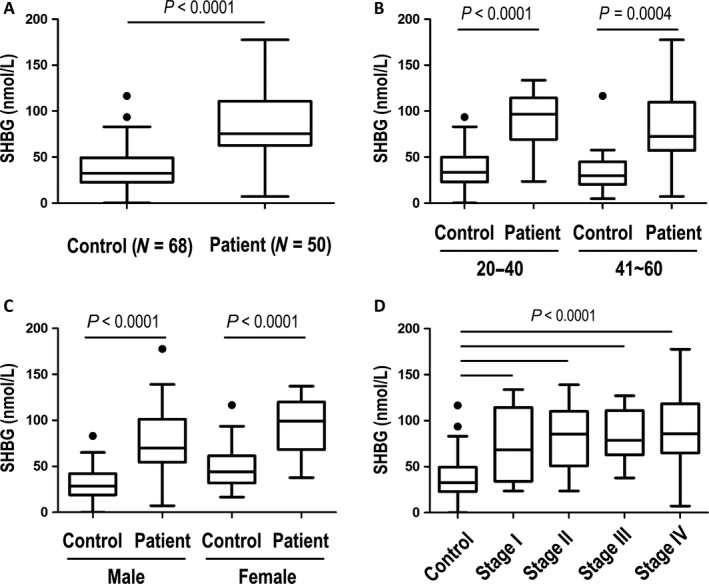
ELISA measurement results of plasma sex hormone‐binding globulin (SHBG) from the validation study cohort. ELISA results were compared between patients and healthy control subjects (A) and were stratified by age (B), gender (C), and disease stage (D). All panels are presented as box plots showing the median value (line), the interquartile range (box), and Tukey whiskers embracing data within 1.5‐fold of the interquartile range; all data outside the range of the Tukey whiskers are presented as individual data points.
Discussion
Using LC‐MS/MS, we found four blood plasma proteins which were significantly overexpressed in plasma of GC patients. A validation study of different cohorts was carried out to confirm blood plasma levels of APOC1, GSN, SHBG, and C4A. Only SHBG expression was significantly higher in the patient group, while expressions of the other three proteins showed no significant difference between the patient and control groups. Therefore, ELISA measurement of plasma SHBG levels in the other independent study cohort was conducted to further validate the findings. We found that blood plasma SHBG levels were significantly higher in the patient group. The plasma SHBG level remained significantly higher in the patient group after subjects were stratified by age, gender, and disease stage. This finding supports a recently published study which reported a 2.3‐fold increase in plasma SHBG level in GC patients compared to a control group as measured by iTRAQ‐based quantitative MS. However, that study did not validate plasma SHBG level in different study cohorts 24.
SHBG is a glycoprotein mainly produced by the liver and secreted into the circulation. The main function of SHBG is as a carrier protein of the sex hormones, testosterone and estrogen, competing with the same binding site, while testosterone had higher binding affinity than estrogen. The concentrations of SHBG are the result of a balanced effect of stimulatory and inhibitory factors. In the clinical androgen replacement study, treatments with testosterone enanthate in normal men and Klinefelter's syndrome patients for three months showed significant reduction of SHBG in both groups 25. While in the study of postmenopausal estrogen replacement therapy, oral conjugated estrogen can increase SHBG levels during therapy 26. These evidences might partly explain different SHBG levels between men and women, in which the level of SHBG in women is higher than in men 27. Moreover, longitudinal studies found that SHBG levels in women show a decreasing trend during the first six decades followed by a steady increase at around age 60 years onward 21, whereas in men, they show an increasing trend with age 22, 23. However, the exact molecular events that regulate SHBG expression are still poorly understood 28.
Relationships between reproductive parameters and GC in women were studied in a prospective study 29. There is a strong association between GC and age at menopause in women. Women who entered menopause at a younger age had a higher risk of developing GC. From a meta‐analysis study among sex hormones, hormonal interventions and GC risk in women subjects, it had suggested longer exposure with estrogen from either ovarian or exogenous origin reduced the risk of GC 30. Higher SHBG levels in women may reduce the level of free estrogen and related to higher risk of GC.
In analyzing the Survival Epidemiology and End Results registries dataset from 1977 to 2004, a reduced risk of developing GC following prostate cancer was observed, and androgen deprivation therapy may reduce the incidence of GC 31. Another case–control study found that free testosterone and dihydrotestosterone (DHT) had positive associations with Barret's esophagus, a precursor lesion of adenocarcinoma of the esophagus. The higher the levels of free testosterone and DHT, the higher the risk was of developing Barret's esophagus 32. It is of note that in a cross‐sectional study of eugonadal men, higher SHBG are unaltered or showed even slightly higher levels of free testosterone 33. In addition to the hypothalamo‐pituitary‐gonadal axis regulation, the ratio of free androgen/estrogen and physiological conditions have also affected the interaction between SHBG and sex hormone utility. Besides, exogenous pulsed treatment of sex hormone may present different activities in balancing the homeostasis in comparison with normal physiological regulation. Although the exact mechanisms still need further elucidation, higher SHBG levels in men may be associated with increasing levels of free testosterone and higher risk of GC.
The above findings suggested the role of SHBG in cancer development, since plasma SHBG directly modulates the bioavailability of unbound sex hormones such as testosterone and estrogen. Our study supported that the role of reproductive hormones in cancer pathogenesis might not only be limited to cancers of reproductive organs but also include other organs such as gastrointestinal organs. Moreover, regulation of reproductive hormones between men and women differs, which might partly explain the difference in GC incidences in the two sexes. The incidence of GC in men is almost twice that in women 1. It should be noted that the increase in SHBG levels in GC patients was not associated with the advanced stages in this study. Although without direct evidence, this data still imply the possibility that alternation of SHBG levels may not be the consequence, but instead it may be the cause, of further GC progression in the GC patients. However, further investigation is needed to elucidate the roles of reproductive hormones and SHBG in GC pathogenesis.
Although the other three proteins did not exhibit potential as GC biomarkers, several studies reported the promising use of these proteins as biomarkers in other types of cancer. A recent study reported that APOC1 was highly expressed in late‐stage lung cancer tissues 34 and was proposed as one of the diagnostic and prognostic biomarkers for lung cancer. Blood plasma APOC1 is also used in combination with other blood plasma biomarkers to predict breast cancer metastasis 35. That study concluded that a combination of several blood plasma biomarkers was a significant independent predictor in a multivariate analysis for predicting breast cancer metastasis. Another observational study reported the potential use of the apolipoprotein family in predicting disease‐free survival of stage II colon cancer patients 36. Several apolipoprotein family members, such as apolipoprotein A‐1 37, 38 and apolipoprotein E 39, were reported to be potential GC biomarkers. C4A is an isotype of complement C4, a member of the complement system. The complement system is part of the innate immune system, which is activated in response to unspecific antigens. Activation of the complement system results in formation of membrane attack complexes that destroy antigens. In cancer, chronic inflammation and many abnormal proteins or metabolites may be produced, which eventually trigger activation of the complement system. A study found that in GC patients, there is an increase in the serum complement C4‐B (C4B) precursor 40; however, a further validation study was not conducted due to the unavailability of a C4B antibody. GSN is an intracellular protein member of the actin‐binding protein family. It has been linked to carcinogenesis and plays a role in dynamic changes of the actin cytoskeleton for cell motility. The secretoric GSN level is significantly increased in advanced‐stage colorectal cancer 41. No study has reported an association between GSN and GC.
To the best of our knowledge, this is the first study to validate SHBG as a potential GC biomarker. However, limitations of this study have to be pointed out. First of all, the sample size is relatively small, although this study included discovery, verification and validation study cohorts, the study findings should be further verified in larger cohorts. Second, it is well known that cancer development is a complex process involving dysregulation of multiple cellular mechanisms. It had been suggested that altered glucose metabolism is a hallmark of GC. There is a significant inverse association between levels of SHBG and fasting serum insulin in the two sexes 42. Diabetic patients showed higher risk of GC, but the use of insulin is not related to the increased risk in a population‐based analysis of the Taiwanese population43. In addition, thyroid hormones also influence SHBG production and significant associations between GC and thyroid disorders were also reported 44. Therefore, the levels of SHBG may not be solely used for diagnosis of GC without knowing the physiological conditions, such as obesity, diabetes, weight control, thyroid disorders, etc. Finally, the levels of total and free testosterone and estrogen in the blood samples were not determined; studies with complete demographic data could render our results more convincing.
However, since information/knowledge on the relationship between SHBG and GC is still very limited, more studies are needed to further validate the use of SHBG in managing GC. In conclusion, this study identified that plasma SHBG levels could be a potential early diagnostic biomarker for GC.
Conflict of Interest
The authors declare that no competing interests exist.
Supporting information
Data S1: Figure S1: The albumin immunodepletion. In the albumin‐depleted samples (N11d, N6Fd, P2d, P42d); the albumin was removed from the original plasma sample (N11, N6F, P2, and P42).
Data S2. Individual characteristics of the patients in three different cohorts.
Data S3. LC‐MS/MS spectra of four upregulated proteins.
Acknowledgments
We thank the TMU Joint Biobank and the Proteomics Core Facility of the Institute of Biomedical Sciences, Academia Sinica for clinical sample collection and LTQ‐Orbitrap mass spectrometry analysis, respectively. The work was supported by the MOST 104‐2320‐B‐038‐056‐MY3 and MOST 105‐2314‐B‐038‐085 grants to C.‐C.C. and Y.‐R.L. We thank Dr. Jin‐Hua Chen, Research Center of Biostatistics, College of Management of Taipei Medical University, Taiwan, who provided consultancy services for power calculations.
Cancer Medicine 2018; 7(1):64–74
The work was supported by the MOST 104‐2320‐B‐038‐056‐MY3 and MOST 105‐2314‐B‐038‐085 grants to C.‐C.C. and Y.‐R.L. We thank Dr. Jin‐Hua Chen, Research Center of Biostatistics, College of Management of Taipei Medical University, Taiwan, who provided consultancy services for power calculations.
Contributor Information
Hsiu‐Ming Shih, Email: hmshih@ibms.sinica.edu.tw.
Ching‐Yu Lin, Email: cylin@tmu.edu.tw.
References
- 1. Chiang, C. J. , Chen Y. C., Chen C. J., You S. L., and Lai M. S.. 2010. Cancer trends in Taiwan. Jpn. J. Clin. Oncol. 40:897–904. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay, J. , Soerjomataram I., Ervik M., Dikshit R., Eser S., and Mathers C., et al. 2012. GLOBOCAN v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 3. Bray, F. , Ren J., Masuyer E., and Ferlay J.. 2013. Estimates of global cancer prevalence for 27 sites in the adult population in 2008. Int. J. Cancer 132:1133–1145. [DOI] [PubMed] [Google Scholar]
- 4. Dassen, A. E. , Dikken J. L., Bosscha K., Wouters M. W. J. M., Cats A., van de Velde C. J. H., et al. 2014. Gastric cancer: decreasing incidence but stable survival in the Netherlands. Acta Oncol. 53:138–142. [DOI] [PubMed] [Google Scholar]
- 5. Leung, W. K. , Wu M. S., Kakugawa Y., Kim J. J., Yeoh K.‐g., Goh K. L., et al. 2008. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 9:279–287. [DOI] [PubMed] [Google Scholar]
- 6. Asaka, M. , and Mabe K.. 2014. Strategies for eliminating death from gastric cancer in Japan. Proc. Jpn.Acad. Ser B Phys. Biol. Sci. 90:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hisamichi, S. , and Sugawara N.. 1984. Mass screening for gastric cancer by X‐ray examination. Jpn. J. Clin. Oncol. 14:211–223. [PubMed] [Google Scholar]
- 8. Choi, K. S. , Kwak M. S., Lee H. Y., Jun J. K., Hahm M. I., and Park E. C.. 2009. Screening for gastric cancer in Korea: population‐based preferences for endoscopy versus upper gastrointestinal series. Cancer Epidemiol. Biomark. Prev. 18:1390–1398. [DOI] [PubMed] [Google Scholar]
- 9. Hamashima, C. , Shibuya D., Yamazaki H., Inoue K., Fukao A., Saito H., et al. 2008. The Japanese guidelines for gastric cancer screening. Jpn. J. Clin. Oncol. 38:259–267. [DOI] [PubMed] [Google Scholar]
- 10. Shimada, H. , Noie T., Ohashi M., Oba K., and Takahashi Y.. 2014. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the task force of the Japanese Gastric Cancer Association. Gastric Cancer 17:26–33. [DOI] [PubMed] [Google Scholar]
- 11. Cao, X. Y. , Jia Z. F., Jin M. S., Cao D. H., Kong F., Suo J., et al. 2012. Serum pepsinogen II is a better diagnostic marker in gastric cancer. World J. Gastroenterol. 18:7357–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao, Q. , Ran Z. H., and Xiao S. D.. 2007. Screening of atrophic gastritis and gastric cancer by serum pepsinogen, gastrin‐17 and Helicobacter pylori immunoglobulin G antibodies. J. Dig. Dis. 8:15–22. [DOI] [PubMed] [Google Scholar]
- 13. Miki, K. 2006. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer 9:245–253. [DOI] [PubMed] [Google Scholar]
- 14. Miki, K. , and Urita Y.. 2007. Using serum pepsinogens wisely in a clinical practice. J. Dig. Dis. 8:8–14. [DOI] [PubMed] [Google Scholar]
- 15. Kaise, M. , Miwa J., Fujimoto A., Tashiro J., Tagami D., Sano H., et al. 2013. Influence of Helicobacter pylori status and eradication on the serum levels of trefoil factors and pepsinogen test: serum trefoil factor 3 is a stable biomarker. Gastric Cancer 16:329–337. [DOI] [PubMed] [Google Scholar]
- 16. Emoto, S. , Ishigami H., Yamashita H., Yamaguchi H., Kaisaki S., and Kitayama J.. 2012. Clinical significance of CA125 and CA72‐4 in gastric cancer with peritoneal dissemination. Gastric Cancer 15:154–161. [DOI] [PubMed] [Google Scholar]
- 17. Haj‐Sheykholeslami, A. , Rakhshani N., Amirzargar A., Rafiee R., Shahidi S. M., Nikbin B., et al. 2008. Serum pepsinogen I, pepsinogen II, and gastrin 17 in relatives of gastric cancer patients: comparative study with type and severity of gastritis. Clin. Gastroenterol. Hepatol. 6:174–179. [DOI] [PubMed] [Google Scholar]
- 18. Rifai, N. , Gillette M. A., and Carr S. A.. 2006. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat. Biotech. 24:971–983. [DOI] [PubMed] [Google Scholar]
- 19. Faca, V. , Wang H., and Hanash S.. 2009. Proteomic global profiling for cancer biomarker discovery In Pasa‐Tolic L., Lipton M., eds. Mass spectrometry of proteins and peptides methods and protocols. 2 ed. Humana Press: New York. [Google Scholar]
- 20. Horgan, R. P. , and Kenny L. C.. 2011. ‘Omic’ technologies: genomics, transcriptomics, proteomics and metabolomics. Obstetrician Gynaecologist 13:189–195. [Google Scholar]
- 21. Maggio, M. , Lauretani F., Basaria S., Ceda G. P., Bandinelli S., Metter E. J., et al. 2008. Sex hormone binding globulin levels across the adult lifespan in women‐The role of body mass index and fasting insulin. J. Endocrinol. Invest. 31:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feldman, H. A. , Longcope C., Derby C. A., Johannes C. B., Araujo A. B., Coviello A. D., et al. 2002. Age trends in the level of serum testosterone and other hormones in middle‐aged men: longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metabol. 87:589–598. [DOI] [PubMed] [Google Scholar]
- 23. Harman, S. M. , Metter E. J., Tobin J. D., Pearson J., and Blackman M. R.. 2001. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. The Journal of clinical endocrinology and metabolism 86:724–731. [DOI] [PubMed] [Google Scholar]
- 24. Subbannayya, Y. , Mir S. A., Renuse S., Manda S. S., Pinto S. M., and Puttamallesh V. N., et al. 2015. Identification of differentially expressed serum proteins in gastric adenocarcinoma. J. Proteomics. 127 (Part A):80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Plymate, S. R. , Leonard J. M., Paulsen C. A., Fariss B. L., and Karpas A. E.. 1983. Sex hormone‐binding globulin changes with androgen replacement. J. Clin. Endocrinol. Metabol. 57:645–648. [DOI] [PubMed] [Google Scholar]
- 26. Serin, I. S. , Ozcelik B., Basbug M., Aygen E., Kula M., and Erez R.. 2001. Long‐term effects of continuous oral and transdermal estrogen replacement therapy on sex hormone binding globulin and free testosterone levels. Eur. J. Obstet. Gynecol. Reprod. Biol. 99:222–225. [DOI] [PubMed] [Google Scholar]
- 27. Engström, B. E. , Karlsson F. A., and Wide L.. 1998. Marked gender differences in ambulatory morning growth hormone values in young adults. Clin. Chem. 44:1289–1295. [PubMed] [Google Scholar]
- 28. Pugeat, M. , Nader N., Hogeveen K., Raverot G., Dechaud H., and Grenot C.. 2010. Sex hormone‐binding globulin gene expression in the liver: drugs and the metabolic syndrome. Mol. Cell. Endocrinol. 316:53–59. [DOI] [PubMed] [Google Scholar]
- 29. Green, J. , Roddam A., Pirie K., Kirichek O., Reeves G., and Beral V.. 2012. Reproductive factors and risk of oesophageal and gastric cancer in the Million Women Study cohort. Br. J. Cancer 106:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Camargo, M. C. , Goto Y., Zabaleta J., Morgan D. R., Correa P., and Rabkin C. S.. 2012. Sex hormones, hormonal interventions, and gastric cancer risk: a meta‐analysis. Cancer Epidemiol. Biomarkers Prev. 21:20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cooper, S. C. , and Trudgill N. J.. 2012. Subjects with prostate cancer are less likely to develop esophageal cancer: analysis of SEER 9 registries database. Cancer Causes Control 23:819–825. [DOI] [PubMed] [Google Scholar]
- 32. Cook, M. B. , Wood S. N., Cash B. D., Young P., Acosta R. D., Falk R. T., et al. 2015. Association between circulating levels of sex steroid hormones and Barrett's esophagus in men: a case‐control analysis. Clin. Gastroenterol. Hepatol. 13:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Ronde, W. , van der Schouw Y. T., Muller M., Grobbee D. E., Gooren L. J., Pols H. A., et al. 2005. Associations of sex‐hormone‐binding globulin (SHBG) with non‐SHBG‐bound levels of testosterone and estradiol in independently living men. J. Clin. Endocrinol. Metabol. 90:157–162. [DOI] [PubMed] [Google Scholar]
- 34. Ko, H. L. , Wang Y. S., Fong W. L., Chi M. S., Chi K. H., and Kao S. J.. 2014. Apolipoprotein C1 (APOC1) as a novel diagnostic and prognostic biomarker for lung cancer: a marker phase I trial. Thoracic. Cancer 5:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goncalves, A. , Esterni B., Bertucci F., Sauvan R., Chabannon C., Cubizolles M., et al. 2005. Postoperative serum proteomic profiles may predict metastatic relapse in high‐risk primary breast cancer patients receiving adjuvant chemotherapy. Oncogene 25:981–989. [DOI] [PubMed] [Google Scholar]
- 36. Vargas, T. , Moreno‐Rubio J., Herranz J., Cejas P., Molina S., González‐Vallinas M., et al. 2014. Genes associated with metabolic syndrome predict disease‐free survival in stage II colorectal cancer patients. A novel link between metabolic dysregulation and colorectal cancer. Mol. Oncol. 8:1469–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu, J. Y. , Cheng C. C., Wang J. Y., Wu D. C., Hsieh J. S., Lee S. C., et al. 2014. Discovery of tumor markers for gastric cancer by proteomics. PLoS ONE 9:e84158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chong, P. K. , Lee H., Zhou J., Liu S. C., Loh M. C. S., So J. B. Y., et al. 2010. Reduced plasma APOA1 level is associated with gastric tumor growth in MKN45 mouse xenograft model. J. Proteomics. 73:1632–1640. [DOI] [PubMed] [Google Scholar]
- 39. Penno, M. A. S. , Klingler‐Hoffmann M., Brazzatti J. A., Boussioutas A., Putoczki T., Ernst M., et al. 2012. 2D‐DIGE analysis of sera from transgenic mouse models reveals novel candidate protein biomarkers for human gastric cancer. J. Proteomics. 77:40–58. [DOI] [PubMed] [Google Scholar]
- 40. Liu, W. , Liu B., Xin L., Zhang Y., Chen X., Zhu Z., et al. 2007. Down‐regulated expression of complement factor I: a potential suppressive protein for gastric cancer identified by serum proteome analysis. Clin. Chim. Acta 377(1–2):119–26. [DOI] [PubMed] [Google Scholar]
- 41. Tsai, M. H. , Wu C. C., Peng P. H., Liang Y., Hsiao Y. C., Chien K. Y., et al. 2012. Identification of secretory gelsolin as a plasma biomarker associated with distant organ metastasis of colorectal cancer. J. Mol. Med. (Beri). 90:187–200. [DOI] [PubMed] [Google Scholar]
- 42. Daka, B. , Rosen T., Jansson P. A., Rastam L., Larsson C. A., and Lindblad U.. 2013. Inverse association between serum insulin and sex hormone‐binding globulin in a population survey in Sweden. Endocr. Connect. 2:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tseng, C. H. 2013. Diabetes, insulin use, and gastric cancer: a population‐based analysis of the Taiwanese. J. Clin. Gastroenterol. 47:e60–64. [DOI] [PubMed] [Google Scholar]
- 44. Selva, D. M. , and Hammond G. L.. 2009. Thyroid hormones act indirectly to increase sex hormone‐binding globulin production by liver via hepatocyte nuclear factor‐4alpha. J. Mol. Endocrinol. 43:19–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Figure S1: The albumin immunodepletion. In the albumin‐depleted samples (N11d, N6Fd, P2d, P42d); the albumin was removed from the original plasma sample (N11, N6F, P2, and P42).
Data S2. Individual characteristics of the patients in three different cohorts.
Data S3. LC‐MS/MS spectra of four upregulated proteins.


