Abstract
Background and Purpose
Although we have recently demonstrated that spinal astrocyte gap junctions mediate the development of mirror‐image pain (MIP), it is still unclear which astrocyte‐derived factor is responsible for the development of MIP and how its production is controlled. In the present study, we focused on the role of ipsilateral versus contralateral D‐serine in the development of MIP and investigated the possible involvement of σ1 receptors and gap junctions in astrocyte D‐serine production.
Experimental Approach
Following carrageenan injection, mechanical allodynia was tested at various time points to examine the effect of individual drugs. Immunohistochemistry and Western blot analyses were performed to clarify the expression levels of spinal D‐serine, serine racemase, σ1 receptors and connexin 43.
Key Results
The expression of ipsilateral D‐serine was up‐regulated during the early phase of inflammation, while contralateral D‐serine increased during the later phase of inflammation. The pharmacological inhibition of D‐serine during the early phase blocked the development of both ipsilateral and contralateral mechanical allodynia. However, the inhibition of D‐serine during the later phase of inflammation blocked contralateral, but not ipsilateral mechanical allodynia. Furthermore, the inhibition of σ1 receptors during the earlier phase of inflammation inhibited the increase in ipsilateral D‐serine. Conversely, the blockade of astrocyte gap junctions suppressed the up‐regulation of contralateral D‐serine during the later phase of inflammation.
Conclusion and Implications
Spinal astrocyte D‐serine plays an important role in the development of mirror‐image pain. Furthermore, σ1 receptors and astrocyte gap junction signalling mediate ipsilateral and contralateral D‐serine production respectively.
Abbreviations
- BD1047
N‐[2‐(3,4‐dichlorophenyl)ethyl]‐N‐methyl‐2‐(dimethylamino) ethylamine dihydro‐bromide
- carbenoxolone
carbenoxolone
- Cx43
connexin 43
- DAAO
D‐amino acid oxidase
- Gap26
43Gap26
- GFAP
glial fibrillary acid protein
- Iba‐1
ionized calcium binding adaptor molecule‐1
- LSOS
L‐serine O‐sulfate potassium
- MIP
mirror‐image pain
- NeuN
neuronal nucleus
- NP
nucleus proprius
- PWF
paw withdrawal frequency
- SDH
superficial dorsal horn
- SeRa
serine racemase
- σ1 receptor
sigma non‐opioid intracellular receptor 1
Introduction
Inflammation or trauma on one side of body can induce pathological pain on the contralateral non‐injured side, called ‘mirror‐image pain (MIP)’ (Cheng et al., 2014; Choi et al., 2015). MIP is typically characterized by a delayed onset as compared to the ipsilateral side, and it is generally observed as a form of mechanical hypersensitivity (Schreiber et al., 2008). While the mechanisms responsible for the development of MIP are still under investigation, recent studies have focused on astrocyte sensitization and the involvement of the astrocyte network in the spread of nociceptive signals from the ipsilateral to the contralateral side of the spinal cord (Spataro et al., 2004; Choi et al., 2016a). We hypothesize that the astrocytic network is responsible for the development of MIP, and thus, the present study is designed to determine if astrocyte‐derived D‐serine is responsible for the development of MIP and to examine how the production of this factor is controlled.
D‐serine is synthesized from L‐serine through activation of serine racemase (SeRa) in astrocytes, and it serves as an endogenous co‐agonist for the glycine site on the NMDA receptor (Panatier et al., 2006; Moon et al., 2015). Because NMDA receptors are expressed in both astrocytes and neurons (Verkhratsky and Kirchhoff, 2007), activation of NMDA receptors amplifies postsynaptic activity (Porres et al., 2011; Hunt and Castillo, 2012) and facilitates neuron‐glial intercellular communication by stimulating neighbouring neurons and glial cells (Lalo et al., 2006; Moon et al., 2015). In this regard, astrocyte Ca2+ transients evoke a long‐term potentiation of local neuronal responses, which depends on the astrocyte‐induced release of D‐serine and ATP (Bazargani and Attwell, 2016). As a result, it has been proposed that astrocyte‐derived D‐serine facilitates astrocyte and/or neuronal NMDA receptor potentiation (Henneberger et al., 2010; Lefevre et al., 2015) and by extension, promotes the interaction between astrocytes and neurons resulting the development of mechanical allodynia (Ren and Dubner, 2008). These findings imply the possibility that activated astrocyte‐derived D‐serine can be a key factor for the development of MIP.
The sigma non‐opioid intracellular receptor 1 (σ1 receptor) is a unique ligand‐operated molecular chaperone receptor predominantly localized to astrocytes in the spinal cord (Moon et al., 2014). Because σ1 receptors have been implicated in Ca2+‐dependent second messenger cascades, the direct activation of σ1 receptors can mediate various biological processes including an initiation of mechanical allodynia (Roh et al., 2008; Maurice and Su, 2009). Previous studies from our laboratories have demonstrated that activation of σ1 receptor provokes the mechanical allodynia in a neuropathic pain model through the up‐regulation of D‐serine production from astrocytes (Moon et al., 2015; Choi et al., 2016b). These observations provide evidence for our second hypothesis that activation of σ1 receptors modulates the production of D‐serine.
From a communication standpoint, astrocytes are connected to one another by gap junctions and form an astrocyte network in the CNS. This distinguishing property of astrocytes enables the astrocyte network to serve as an important pathway for local spinal cord nociceptive signal spreading to distance sites, including the contralateral side (Hansson, 2006; Del Valle et al., 2009). Increased intracellular IP3 molecules or free‐Ca2+ ions can diffuse through astrocyte gap junctions, termed Ca2+ oscillations, which can alter the biological activity of astrocytes located at distant sites and ultimately influence synaptic activity or glia‐neuronal interactions at these sites (Scemes et al., 2000; Munoz et al., 2015). In this regard, astrocyte gap junction‐mediated communication has been proposed as a potential mechanism for D‐serine production at distant locations (Henneberger et al., 2010).
The present study used a rodent carrageenan‐induced inflammatory pain model to investigate our hypotheses and to determine (1) the potential role of astrocyte D‐serine in the development of MIP and (2) the roles that σ1 receptors and astrocyte gap junctions play in astrocyte D‐serine production in MIP.
Methods
Animal preparation
Male Sprague–Dawley rats (280–290 g) were purchased from The Laboratory Animal Centre of Seoul National University (SNU). The rats had free access to food and water throughout the investigation and were housed in standard temperature and light controlled rooms (24 ± 2°C, 12/12 h light/dark cycle with lights on at 07:00 h) for at least 1 week prior to beginning any experiments. All of the animal experimental procedures used in this study were approved and reviewed by the SNU Animal Care and Use Committee and were performed according to guidelines established by NIH (NIH publication No. 86–23, revised 1985). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Peripheral inflammation was induced by intraplantar injection of 200 μL of 2% λ‐carrageenan (Sigma, St. Louis, MO, USA) into the left hindpaw. Sham animals received 200 μL of sterile PBS into the left hindpaw. Intraplantar injection of carrageenan or PBS was performed under light anaesthesia with 3% isoflurane in a mixture of N2O/O2 gas.
Drugs and intrathecal injection
The following drugs were used: D‐serine (1, 10 and 100 μg; Sigma); D‐amino acid oxidase (DAAO) (0.01 and 0.1 U; Sigma), an endogenous D‐serine degrading enzyme; L‐serine O‐sulfate potassium (LSOS) (10 and 100 nmol; Santa Cruz, CA, USA), a SeRa inhibitor; N‐[2‐(3,4‐dichlorophenyl)ethyl]‐N‐methyl‐2‐(dimethylamino) ethylamine dihydro‐bromide (BD1047; Tocris Bioscience, Bristol, UK) (100 nmol), a σ1 receptor antagonist; carbenoxolone (CBX) (0.04 μmol; Sigma), a potent gap junctional decoupler; and 43Gap26 (Gap26) (0.1 nmol; Anaspec, Fremont, CA, USA), a specific connexin 43 (Cx43) mimetic blocking peptide. The doses of D‐serine were selected based on doses previously reported in the literature (Yoon and Yaksh, 1999; Lefevre et al., 2015). Two different doses of DAAO and LSOS were selected, and the doses used were based on those used in previous studies from our laboratories. The two doses of each drug were chosen because they were found to have either no effect (low dose) or a pharmacological effect (higher dose) in previous studies from our laboratories in which these drugs were tested at a variety of doses (Moon et al., 2015; Choi et al., 2016b). The doses carbenoxolone and Gap26 used in this study were based on from those used in previous studies (Roh et al., 2010; Choi et al., 2015). The dose of BD1047 was identical to that used in previous reports from our laboratories (Roh et al., 2008; Moon et al., 2015). All drugs were dissolved in 10 μL of PBS (pH 7.4) and were injected i.t. as previously described (Moon et al., 2015). Briefly, rats were anaesthetized with 3% isoflurane in a mixture of N2O/O2 gas and injection was performed when the animal did not show any voluntary movement. A 26‐gauge needle connected to a 100 μL Hamilton syringe was inserted at a 45° angle to the vertebral column from the caudal direction immediately lateral to the L6 spinous process into the i.t. space; and then 10 μL of drug or vehicle was injected slowly over a 5 s period. A flick of the tail was considered indicative of a successful i.t. administration. Control animals received an i.t. injection of 10 μL of PBS (vehicle). Drugs were administered twice a day on post‐carrageenan injection days 0 to 3 (early phase) or on post‐carrageenan injection days 4 to 7 (late phase). Animals were randomly assigned to experimental groups, and subsequent drug treatment and analyses were performed blind.
Assessment of mechanical allodynia
After carrageenan injection, the degree of mechanical allodynia was measured using a 4.0 g von Frey filament (North Coast Medical, Morgan Hill, CA, USA). Based on previous studies from our laboratories, the 4.0 g filament was selected, because it normally does not elicit a behavioural withdrawal response in naïve animals when applied to the plantar surface of the hindpaw (0 or 1 withdrawals out of 10 applications) (Moon et al., 2012). Rats were placed on a metal mesh grid under a plastic chamber, and the von Frey filament was applied 10 times from underneath the metal mesh flooring to each left (ipsilateral) or right (contralateral) hindpaw with each application separated by a 10 s interval. The number of paw withdrawal responses to each set of 10 von Frey stimuli was then counted. The results obtained from this mechanical response testing were expressed as a % of the paw withdrawal response frequency (PWF, %) for both the ipsilateral and contralateral hindpaws. PWFs were determined for all rats before carrageenan injection at day 0 to obtain normal baseline values of withdrawal response to mechanical stimuli. Rats were then tested at 3 h post‐carrageenan injection on day 0 and subsequently tested again daily to determine if there were changes in PWFs over time.
Immunohistochemistry
Spinal cord immunohistochemistry was performed as previously described (Moon et al., 2015). Animals were deeply anaesthetized with 3% isoflurane in a mixture of N2O/O2 gas and perfused transcardially with calcium‐free Tyrode's solution followed by a fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The spinal cords were removed immediately after perfusion, post‐fixed for 2 h and then placed in 30% sucrose in PBS (pH 7.4) 48 h at 4°C. Serial transverse sections (40 μm) of the L3–5 spinal cord region were cut using a cryostat (Leica Biosystems, Nussloch, Germany). These serial sections were preblocked with 3% normal donkey serum with 0.3% Triton X‐100 in PBS at room temperature for 1 h. The following primary antibodies were used: mouse anti‐glial fibrillary acid protein (GFAP; 1:1000; Millipore, Billerica, mechanical allodynia, USA); mouse anti‐neuronal nucleus (NeuN; 1:1000; Millipore); goat anti‐ionized calcium binding adaptor molecule‐1 (Iba‐1; 1:500; Abcam, Cambridge, United Kingdom); rabbit anti‐D‐serine (1:500; Abcam); and rabbit anti‐SeRa (1:500; Santa Cruz). The tissue sections were incubated in the primary antibodies overnight at room temperature. Following several rinses, the tissue sections were then further incubated with the following secondary antibodies: Alexa488‐conjugated donkey anti‐mouse IgG (1:400; Invitrogen, Carlsbad, CA, USA), Alexa488‐conjugated donkey anti‐goat IgG (1:400; Invitrogen) and Alexa568‐conjugated donkey anti‐rabbit IgG (1:400; Invitrogen). The immunosignal for SeRa was further amplified with biotinylated goat anti‐rabbit antibody (1:200; Vector, Burlingame, CA, USA) and Alexa568‐conjugated streptavidin (1:400; Invitrogen).
Image analysis
Image analysis was conducted as described previously (Choi et al., 2015). L3–5 spinal segments were mounted on slides, and three to five spinal cord sections were randomly selected from each animal. The individual sections were visualized with Eclipse TE200 confocal microscope (Nikon, Tokyo, Japan) and were analysed using a computer‐assisted image analysis system (Metamorph version 7.7.2; Molecular Devices, Sunnyvale, CA, USA). To maintain a constant threshold for each image and to compensate for subtle variabilities in the immunostaining, we only counted pixels that were at least 90% brighter than the average level of each image after background subtraction and shading correction. The spinal dorsal horn was divided into the following three areas for analysis: (1) the superficial dorsal horn (SDH; laminae I and II); (2) the nucleus proprius (NP; laminae III and IV); and (3) the neck (NECK; laminae V and VI) as previously described (Seo et al., 2008). All analytical and quantitative procedures described above were performed blind without knowledge of the experimental conditions.
Western blot assay
After carrageenan injection, the rats were killed and the spinal cords were removed and collected to examine the potential change in the expression level of SeRa. The L3–5 spinal cord segments were extracted following laminectomy of the spinal vertebrae, and the segments were then separated into left (ipsilateral) and right (contralateral) halves under a neurosurgical microscope. The left and right dorsal quadrants of these spinal L3–5 segment halves were isolated and then homogenized in buffer containing 20 mM Tris [pH 7.5], 1 mM EDTA, 1 mM EGTA, 1% Triton X‐100, 1 mg·mL−1 aprotinin, 1 mM PMSF and 0.5 mM sodium orthovanadate. The total amount of protein in each sample was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA) prior to loading on PAGs. The spinal cord homogenates (20 μg protein) were separated using 10% SDS‐PAGE and transferred to PVDF membranes. After the blots had been washed with Tris‐buffered saline with Tween 20 (10 mM Tris–HCl [pH 7.6], 150 mM NaCl and 0.01% Tween‐20), the membranes were blocked with 5% skimmed milk for 1 h and incubated at 4°C overnight with primary antibodies specific for rabbit anti‐SeRa (1:1000; Santa Cruz), rabbit anti‐σ1 receptor (1:1000; Abcam), rabbit anti‐Cx43 (1:2000; Invitrogen) and mouse anti‐β‐actin (1:5000, loading control; Sigma). After reaction with secondary HRP‐conjugated antibodies (1:10 000; Santa Cruz), the bands were visualized by exposing the membrane to enhanced chemiluminescence (Amersham Pharmacia Biotech; Buckinghamshire, UK). The positive pixel area of specific bands was measured with a computer‐assisted image analysis system and normalized against the corresponding β‐actin loading control bands. The intensity of the control band for each blot was set to 100%, and the intensity of the experimental bands are represented relative to the control group.
Statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). All data are presented as the mean ± SEM. Statistical analysis was performed using Prism 5.0 (Graph Pad Software, San Diego, CA, USA). Two‐way ANOVA was performed to determine overall differences in the time course of PWF (%). Post hoc analysis was performed using the Bonferroni's multiple comparison test in order to determine the P‐value among experimental groups. One‐way ANOVA followed by the Newman–Keuls multiple comparison tests or Student's two‐tailed t‐test was used to determine differences across all experimental groups (immunohistochemistry and Western blot assay). F values for these comparisons were then calculated. A value of P < 0.05 was considered to be statistically significant.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c).
Results
Temporal changes in the expression of ipsilateral and contralateral spinal D‐serine and serine racemase in carrageenan rats
To investigate the potential involvement of spinal D‐serine in carrageenan‐induced inflammation, we examined the temporal changes of both D‐serine and SeRa expression in the ipsilateral and contralateral spinal cord dorsal horn using immunohistochemistry to detect changes in D‐serine expression and a Western blot assay to detect changes in SeRa expression. The expression of ipsilateral spinal D‐serine (Figure 1A) and SeRa (Figure 1B) were both significantly increased at 3 days post‐carrageenan injection as compared to that of sham animal controls. The ipsilateral PWF also significantly increased (indicating the development of mechanical allodynia) at 3 h post‐carrageenan injection, and this persisted throughout the 10 day experimental period as compared to that of sham control animals (Figure 1C). In contrast, the expression of contralateral spinal D‐serine (Figure 1A) and SeRa (Figure 1B) was only significantly up‐regulated at 5 and 10 day post‐carrageenan injection. Similarly, the contralateral PWF was first significantly increased on day 5 post‐carrageenan injection and persisted throughout the remainder of the 10 day experimental period compared to that of sham animals (Figure 1C). A separate set of experiments were performed to clarify the changes in D‐serine and SeRa expression at the 3 h post‐carrageenan injection time point (Supporting Information Figure S1). The expression of ipsilateral spinal D‐serine was significantly increased as compared to that of sham animal controls (Supporting Information Figure S1A), while the expression levels of ipsilateral and contralateral SeRa was unchanged compared to that of sham animals (Supporting Information Figure S1B).
Figure 1.
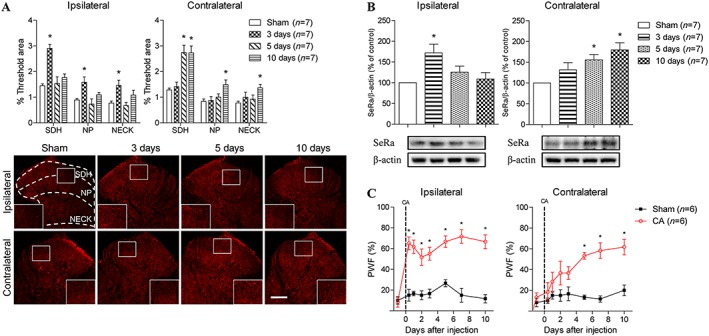
Graphs and photomicrographs illustrating the effect of intraplantar carrageenan (CA) injection on the temporal pattern of D‐serine and serine racemase (SeRa) expression in the spinal cord dorsal horn and on the PWF. (A) Ipsilateral D‐serine expression was significantly up‐regulated by day 3 post‐CA (F SDH = 15.36, F NP = 5.534, F NECK = 4.973). In contrast, contralateral D‐serine expression was increased between days 5 and 10 post‐CA injection (F SDH = 13.66, F NP = 4.739, F NECK = 2.803). (B) Similarly, the expression of ipsilateral SeRa was significantly up‐regulated by day 3 post‐CA injection (F = 4.894), while contralateral SeRa expression was significantly increased between days 5 and 10 post‐CA injection (F = 6.099). (C) CA injection induced a significant increase in ipsilateral PWF as early as 3 h post‐injection (F = 8.343). However, contralateral PWF showed a different developmental time course and was only significantly increased beginning on day 5 post‐CA injection (F = 7.526). *P < 0.05 as compared to that of sham animals. Scale bar = 150 μm.
Cellular distribution of serine racemase and D‐serine in the spinal cord dorsal horn
In a separate set of experiments, we performed double‐labelling immunohistochemistry to determine the cellular distribution of SeRa and D‐serine in the contralateral spinal cord dorsal horn 10 day post‐carrageenan injection. The SeRa‐positive cells were double stained with GFAP which is indicative of astrocytes, but not with NeuN (indicative of neuronal staining, Figure 2B3) or Iba‐1 (indicative of microglia staining, Figure 2C3). By comparison, D‐serine‐positive cells co‐localized with both GFAP (Figure 2D3) and NeuN (Figure 2E3), but not with Iba‐1 (Figure 2F3), indicating that D‐serine is present in both astrocytes and neurons.
Figure 2.
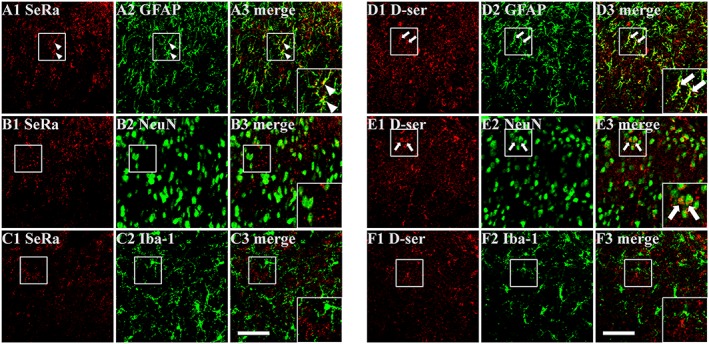
Representative photomicrographs depicting the cellular localization of serine racemase (SeRa) and D‐serine (D‐ser) immunoreactivity in the contralateral spinal cord dorsal horn at 10 days post‐carrageenan injection. (A–C) SeRa (A1, B1 and C1)‐immunoreactive cells were observed to co‐contain GFAP‐immunoreactivity (A3, arrow head), but not co‐contain NeuN (B3) or Iba‐1 (C3) immunoreactivity indicating that SeRa is localized to astrocytes. (D–F) D‐ser (D1, E1 and F1) immunoreactive cells co‐contained both GFAP (D3, arrow) and NeuN (E3, arrow) immunoreactivity indicating that both astrocytes and neurons contain D‐ser. Scale bar = 50 μm.
Differential involvement of ipsilateral and contralateral D‐serine in the development of contralateral mechanical allodynia in carrageenan‐injected rats
To determine the potential involvement of spinal D‐serine in the development of mechanical allodynia, we first performed a single i.t. injection of D‐serine (1, 10 and 100 μg) in normal animals and examined the potential changes in PWFs at 15 and 30 min and 1, 2 and 4 h following the injection (Figure 3A). I.t. injection of 10 and 100 μg of D‐serine dose‐dependently produced a significant increase in PWFs at 15and 30 min and 1 and 2 h post‐injection compared to that of vehicle‐treated rats (Figure 3A) indicating that increases in spinal D‐serine can produce mechanical allodynia. Next, to investigate the roles of ipsilateral versus contralateral spinal D‐serine in the development of carrageenan‐induced contralateral mechanical allodynia, we i.t. administrated DAAO (0.01 or 0.1 U) or LSOS (10 and 100 nmol) on days 0–3 post‐carrageenan injection (an early time period during which ipsilateral D‐serine expression is significantly up‐regulated, Figure 3B, C) or on days 4–7 post‐carrageenan injection (a later time period when contralateral D‐serine is significantly increased, Figure 3D, E). I.t. administration of DAAO 0.1 U (Figure 3B) or LSOS 100 nmol (Figure 3C) on days 0–3 post‐carrageenan injection significantly blocked the carrageenan‐induced increase in both the ipsilateral and contralateral PWFs compared to that of vehicle‐treated carrageenan rats. However, i.t. treatment with these drugs when administered 4–7 day post‐carrageenan injection had no effect on the carrageenan‐induced increase in ipsilateral PWF, but it effectively suppressed the increase in contralateral PWF compared to that of vehicle‐treated carrageenan rats, and this contralateral inhibition persisted throughout the remainder of the 10 day experimental period (Figure 3D, E).
Figure 3.
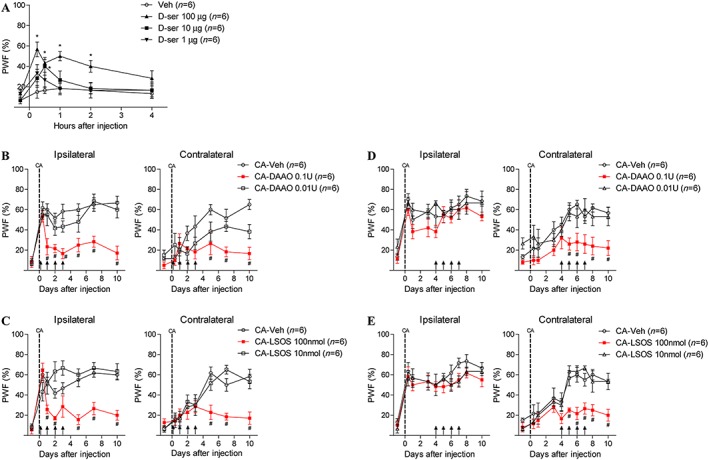
Graphs illustrating the potential role of D‐serine on the PWF in carrageenan (CA)‐treated rats. Normal animals were injected i.t. with D‐serine (at doses of 1, 10 and 100 μg, single treatment). DAAO (0.01 and 0.1 U), LSOS (10 and 100 nmol) or vehicle (Veh) was administered i.t. twice a day on days 0–3 or from days 4–7 post‐CA injection. (A) I.t. injection of D‐serine (10 and 100 μg) dose‐dependently increased the PWFs to innocuous mechanical stimuli at 15 and 30 min and 1 and 2 h post‐injection (F 100ug = 4.607, F 10ug = 2.506). (B) Treatment with DAAO 0.1 U from days 0–3 post‐CA injection significantly blocked the increase in both ipsilateral (F = 6.349) and contralateral PWFs (F = 6.386). (C) Administration of LSOS 100 nmol on days 0–3 post‐CA injection also significantly inhibited the increase in ipsilateral (F = 5.000) and contralateral PWFs (F = 7.392). (D) Treatment with DAAO 0.1 U on days 4–7 post‐CA injection only blocked the increase in contralateral PWF (F = 2.798) but had no effect on the ipsilateral PWF. (E) Similarly, administration of LSOS 100 nmol on days 4–7 post‐CA injection also significantly blocked the increase in contralateral PWF (F = 3.722), whereas the ipsilateral PWF was not affected by LSOS treatment. *P < 0.05 as compared to that of vehicle‐treated normal rats (Veh). #P < 0.05 as compared to that of vehicle‐treated carrageenan rats (CA‐Veh).
Involvement of spinal σ1 receptors in carrageenan rats and the role of σ1 receptors in ipsilateral spinal D‐serine expression
To clarify whether σ1 receptors can control the ipsilateral and/or contralateral spinal D‐serine expression in carrageenan rats, we initially performed a Western blot analysis to investigate the temporal changes in spinal σ1 receptor expression. The expression level of σ1 receptors was significantly increased in the ipsilateral spinal dorsal horn at day 3 post‐carrageenan injection, while contralateral σ1 receptor expression was not changed at this post‐injection time point (Figure 4A). We next performed a separate set of experiments using both immunohistochemical and Western blot analyses to identify whether σ1 receptors affect ipsilateral spinal cord D‐serine production. Spinal cord samples were collected at the 3 day time point following daily i.t. treatment with 100 nmol BD1047 from day 0–3 post‐carrageenan injection, a time period when ipsilateral spinal D‐serine and SeRa expression are increased. Immunohistochemical and Western blot data showed that i.t. treatment with 100 nmol BD1047 during the 0–3 day post‐carrageenan injection period drastically inhibited the carrageenan‐induced increase in ipsilateral D‐serine expression (Figure 4B) and SeRa expression (Figure 4C) compared to that of vehicle‐treated carrageenan rats.
Figure 4.
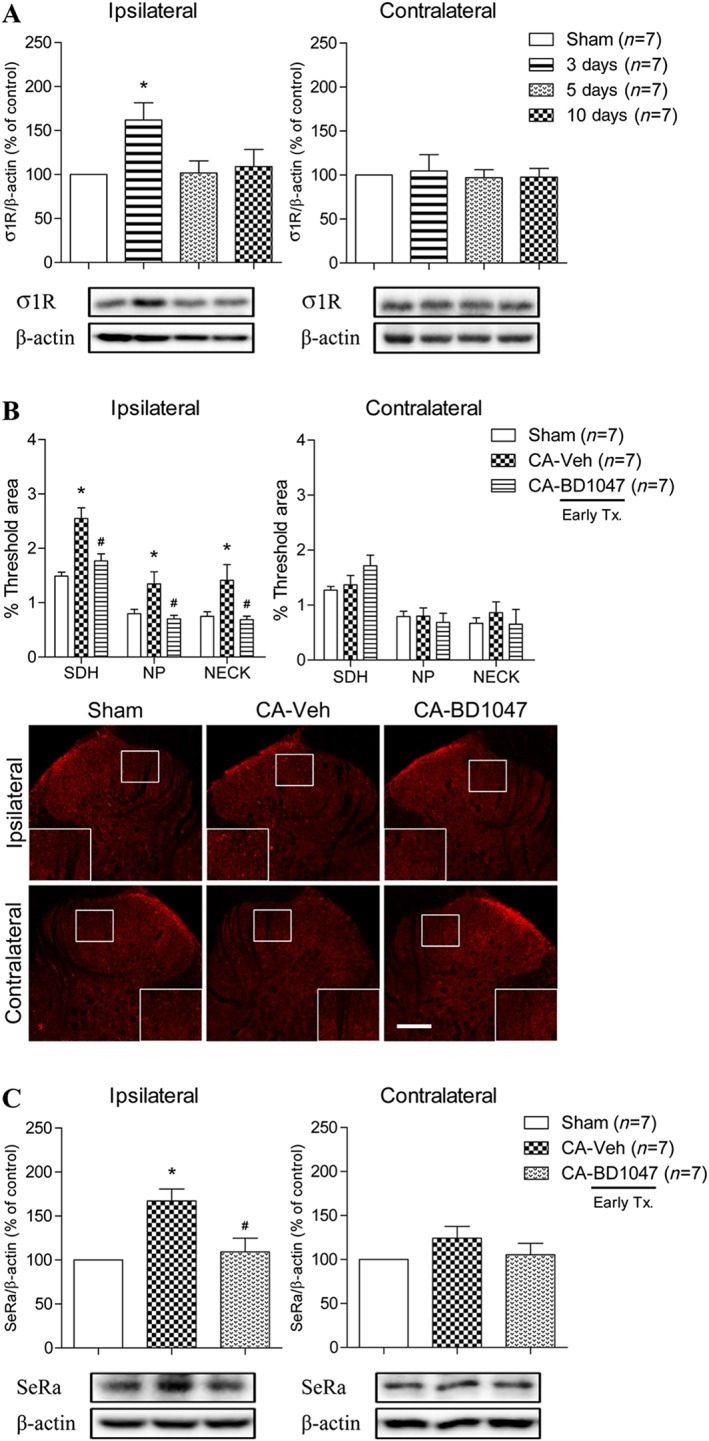
Graphs and photomicrographs displaying the effect of carrageenan (CA) inflammation on σ1 receptor expression and the effect of σ1 receptor inhibition on ipsilateral D‐serine and SeRa expression. (A) The expression level of σ1 receptors was found to be significantly increased by 3 day post‐CA injection, but only in the ipsilateral spinal cord dorsal horn (F = 3.893). (B) I.t. administration of BD1047 (CA + BD1047) significantly blocked the up‐regulation of ipsilateral D‐serine expression compared to that of vehicle‐treated CA rats (CA + Veh) (F SDH = 16.32, F NP = 5.398, F NECK = 4.436). (C) Moreover, i.t. treatment with BD1047 (CA + BD1047) also significantly inhibited the CA‐induced up‐regulation of ipsilateral SeRa expression compared to that of vehicle‐treated CA rats (CA + Veh) (F = 9.365). *P < 0.05 as compared to that of sham group and #P < 0.05 as compared to that of CA + Veh group. Scale bar = 150 μm.
In the next experiment, immunohistochemical and Western blot analyses were performed to determine whether σ‐1 receptors also control the expression of contralateral spinal D‐serine. Spinal cord samples collected at the 7 day time point following i.t. treatment with BD1047 from days 4–7 post‐carrageenan injection, a time period when contralateral spinal D‐serine and SeRa expression are increased. Immunohistochemical and Western blot data indicate that the inhibition of σ1 receptors with BD1047 during the day 4–7 post‐carrageenan injection period did not suppress the carrageenan‐induced up‐regulation of either contralateral D‐serine (Figure 5A) or SeRa (Figure 5B) expression. Moreover, treatment with BD1047 between days 4 and 7 post‐carrageenan injection had no effect on either ipsilateral or contralateral PWFs compared to that of vehicle‐treated CA rats (Figure 5C).
Figure 5.
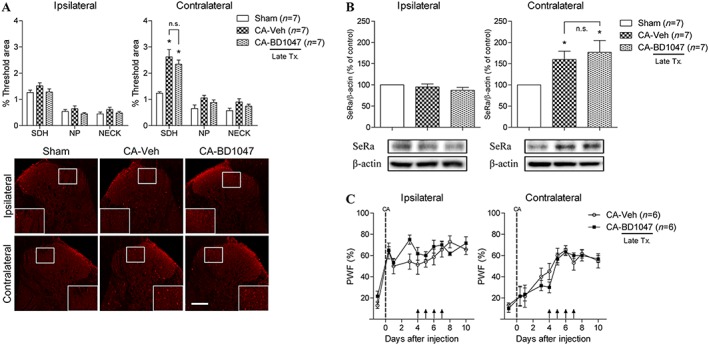
Graphs and photomicrographs illustrating the effect of σ1 receptor inhibition on contralateral D‐serine and SeRa expression during the later phase of carrageenan inflammation. BD1047 (100 nmol) or vehicle (Veh) was administered twice a day on days 4–7 post‐CA injection. (A) I.t. administration of BD1047 (CA + BD1047) did not affect the CA‐induced increase in contralateral D‐serine expression levels compared to that of vehicle‐treated CA rats (CA + Veh) (F SDH = 15.25). (B) Similarly, administration of BD1047 to CA‐treated rats (CA + BD1047) had no effect on the up‐regulated expression of contralateral SeRa (F = 4.721). (C) Treatment with BD1047 (CA + BD1047) on days 4–7 post‐CA injection did not affect either ipsilateral or contralateral PWF compared to that of vehicle‐treated CA rats (CA + Veh). *P < 0.05 as compared to that of sham group. Scale bar = 150 μm.
The role of astrocyte gap junctions in the up‐regulation of contralateral spinal D‐serine expression in carrageenan‐induced nociception
We next examined whether the activation of astrocyte gap junctions is responsible for the increase in spinal D‐serine expression observed in the ipsilateral and contralateral spinal cord in carrageenan‐injected rats. Western blot analysis showed that the expression of the astrocyte gap junctional protein Cx43 was significantly increased in both the ipsilateral and contralateral spinal dorsal horns at 5 and 10 day post‐carrageenan injection (Figure 6A). Immunohistochemical and Western blot data from spinal cord samples collected 7 day post‐carrageenan injection showed that the i.t. injection of the gap‐junction inhibitor carbenoxolone (0.04 μmol) during 4–7 day post‐carrageenan injection period (a time period when contralateral spinal D‐serine and SeRa expression are significantly increased) blocked both the increase in contralateral D‐serine expression (Figure 6B) and the increase in contralateral SeRa expression (Figure 6C). Similarly, i.t. injection of 0.1 nmol of Gap26 4–7 day post‐carrageenan injection also significantly inhibited the increase in both contralateral D‐serine expression (Figure 6D) and SeRa expression (Figure 6E). Furthermore, administration of either carbenoxolone or Gap26 on days 4–7 post‐carrageenan injection significantly blocked the increase in contralateral PWF, while the ipsilateral PWF was not affected by either carbenoxolone or Gap26 treatment (Figure 6F).
Figure 6.
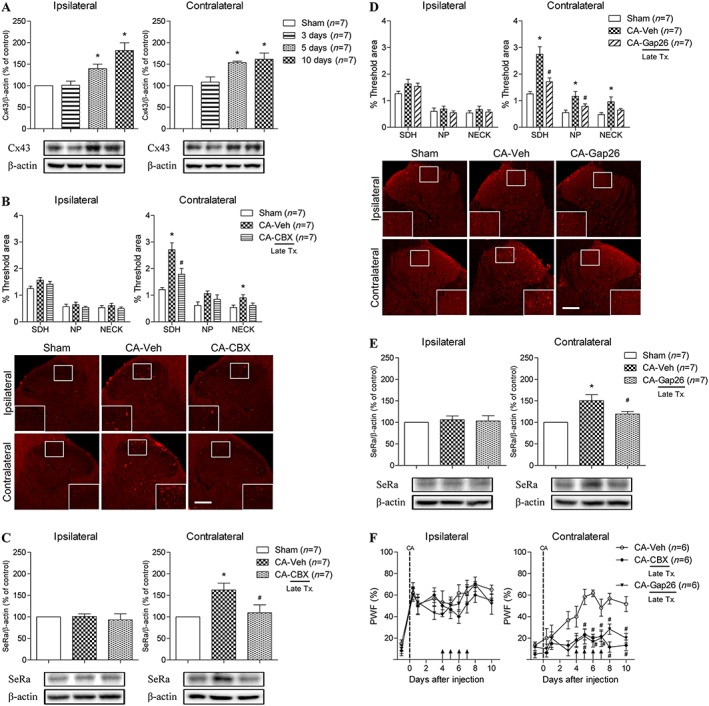
Graphs and photomicrographs displaying the effect of inhibiting astrocyte gap junctions on the expression of contralateral D‐serine and SeRa during the later phase of carrageenan inflammation. Carbenoxolone (CBX; 25 μg), Gap26 (0.1 nmol) or vehicle (Veh) was administered twice a day on days 4–7 post‐CA injection. (A) The expression level of Cx43 (an astrocyte gap junctional protein) was found to be significantly increased between days 5 and 10 post‐CA injection in both the ipsilateral (F = 12.17) and contralateral spinal cord dorsal horns (F = 11.49). (B) I.t. administration of carbenoxolone (CA + CBX) significantly blocked the up‐regulation of contralateral D‐serine expression compared to that of vehicle‐treated CA rats (CA + Veh) (F SDH = 14.32, F NECK = 3.949). (C) Moreover, i.t. treatment with carbenoxolone (CA + CBX) also inhibited the up‐regulation of contralateral SeRa expression compared to that of vehicle‐treated CA rats (CA + Veh) (F = 6.189). (D) Similarly, i.t. treatment with Gap26 (CA + Gap26) significantly blocked the increase in contralateral spinal D‐serine expression compared to that of vehicle‐treated CA rats (CA + Veh) (F SDH = 16.41, F NP = 6.100, F NECK = 4.249). (E) Furthermore, Gap26 treatment (CA + Gap26) dramatically inhibited the increase in contralateral SeRa expression as compared to that of vehicle‐treated CA rats (CA + Veh) (F = 9.363). (F) Administration of either carbenoxolone (CA + CBX) or Gap26 (CA + Gap26) on days 4–7 post‐CA injection significantly blocked the carrageenan‐induced increase in contralateral PWF (F CBX = 3.993, F Gap26 = 3.425), whereas ipsilateral PWF was not affected by carbenoxolone or Gap26 treatment. *P < 0.05 as compared to that of sham group and #P < 0.05 as compared to that of CA + Veh group. Scale bar = 150 μm.
In the next set of experiments, we used both immunohistochemical and Western blot analyses of spinal cord samples collected at the 3 day post‐carrageenan time point to determine whether astrocyte gap junctions also control ipsilateral spinal D‐serine expression. Immunohistochemical and Western blot data showed that i.t. injection of 0.04 μmol of carbenoxolone administered during the 0–3 day post‐carrageenan injection period (when ipsilateral spinal D‐serine and SeRa expression are increased) did not inhibit the carrageenan‐induced increase in ipsilateral D‐serine expression (Figure 7A) or SeRa (Figure 7B). Similarly, we found that i.t. injection of 0.1 nmol of Gap26 during the 0–3 day post‐carrageenan injection period also failed to suppress the increase in both ipsilateral D‐serine (Figure 7C) and ipsilateral SeRa (Figure 7D) expression.
Figure 7.
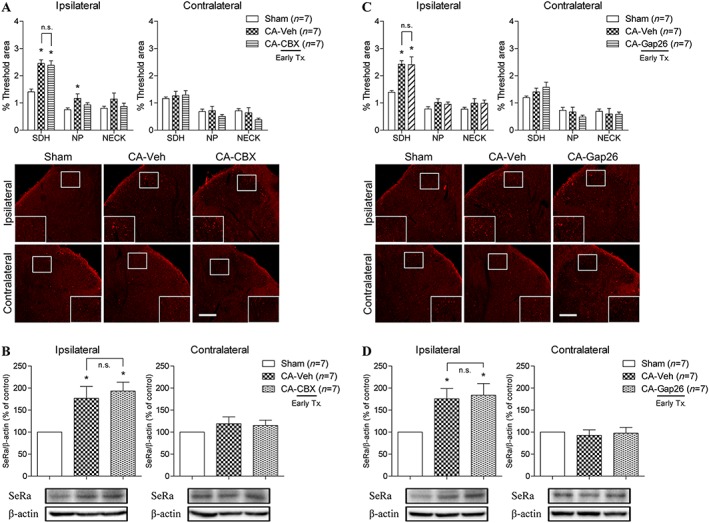
Graphs and photomicrographs illustrating the effect of astrocyte gap junction inhibition on ipsilateral D‐serine and SeRa expression during the earlier phase of carrageenan inflammation. Carbenoxolone (CBX; 25 μg), Gap26 (0.1 nmol) or vehicle (Veh) was administered twice a day on days 0–3 post‐CA injection. (A) I.t. administration of carbenoxolone (CA + CBX) did not change the CA‐induced increase in ipsilateral D‐serine expression as compared to that of vehicle‐treated CA rats (CA + Veh) (F SDH = 18.03). (B) Similarly, i.t. administration of carbenoxolone (CA + CBX) had no effect on the CA‐induced increase in ipsilateral spinal SeRa expression (CA + Veh) (F = 6.536). (C) I.t. treatment with Gap26 (CA + Gap26) also did not affect the increased ipsilateral D‐serine expression levels as compared to that of vehicle‐treated CA rats (CA + Veh) (F SDH = 10.03). (D) Furthermore, i.t. treatment with Gap26 (CA + Gap26) did not change the CA‐induced increased in ipsilateral SeRa expression levels (CA + Veh) (F = 5.674). *P < 0.05 as compared to that of the sham group. Scale bar = 150 μm.
Discussion and conclusions
Previously, we have reported that spinal astrocytes play a critical role in the development of mirror‐image pain (Choi et al., 2015; 2016a). However, it is still not clear which astrocyte‐derived factor contributes to the development of MIP nor how the expression of this factor is controlled. In the present study, we have taken an important step forward in elucidating some of the mechanistic details underlying the development of MIP. In this regard, we demonstrate that spinal astrocyte D‐serine plays an important role in the development of contralateral mechanical allodynia. Moreover, we showed that the production of ipsilateral versus contralateral D‐serine is modulated by different astrocytic associated mechanisms; thus, σ1 receptors induce ipsilateral D‐serine production, while gap junctional communication is responsible for contralateral D‐serine production.
Recent studies have demonstrated that astrocyte‐derived D‐serine is an important mediator that activates the NMDA receptor through the PKC‐dependent phosphorylation of the NR1 subunit leading to the development of mechanical allodynia (Dieb and Hafidi, 2013; Choi et al., 2016b). Our current results demonstrated that the expression of ipsilateral versus contralateral D‐serine mimics the developmental time course of ipsilateral versus contralateral carrageenan‐induced mechanical allodynia respectively. The pharmacological inhibition of D‐serine or SeRa during the early time period (0–3 day post‐carrageenan injection) effectively blocked the development of both ipsilateral and contralateral mechanical allodynia, while administration of D‐serine or SeRa inhibitors at a later time point (4–7 day post‐carrageenan injection) only blocked the development of contralateral mechanical allodynia. Moreover, immunohistochemical analyses demonstrated that the increase in both ipsilateral and contralateral D‐serine expression occurred predominantly in the SDH region (laminae I and II) of the spinal cord dorsal horn. This region is a significant site of pain transmission and modulation and receives nociceptive input from skin, muscle and visceral organs and thus is important in the initial processing of nociceptive information (Gutierrez‐Mecinas et al., 2017). Thus, the early increase in D‐serine in ipsilateral SDH and followed by a delayed increase in contralateral SDH suggests that the earlier increase in the ipsilateral SDH contributes to the initial development of ipsilateral mechanical allodynia, while the later increase in the contralateral SDH is associated with the delayed development of contralateral mechanical allodynia.
A number of studies have demonstrated that ipsilateral and contralateral mechanical allodynia develop through different mechanisms (Chacur et al., 2001; Radhakrishnan et al., 2003). Carrageenan induces acute noxious inflammation in a restricted area peripherally and various algogenic substances (e.g. prostaglandins, bradykinins and cytokines) derived from the ensuing local inflammation stimulate local C‐fibre nerve endings producing an immediate increase of mechanical sensitivity (Levine et al., 1993). In this regard, the carrageenan‐induced early development of ipsilateral mechanical allodynia has been reported to be induced by direct stimulation of nociceptive neurons in the ipsilateral spinal cord (Zhang et al., 2002). Adding to the information from these studies, the present results demonstrate that ipsilateral astrocyte D‐serine also plays a crucial role in the development of ipsilateral mechanical sensitivity. Therefore, we hypothesize that direct synaptic transmission between primary afferent fibre and spinal nociceptive second‐order neurons in combination with the early increase in ipsilateral astrocyte‐derived D‐serine contribute to the development of ipsilateral mechanical allodynia.
In contrast to the ipsilateral side, the development of contralateral mechanical allodynia has been reported to be mediated centrally, but not peripherally (Sluka et al., 2001; Spataro et al., 2004). In particular, contralateral nociception develops when the initial ipsilateral spinal cord nociceptive intensity reaches a level that induces nociceptive signal spreading to the opposite side of the spinal cord, which in turn induces both biochemical and structural changes on the contralateral side (Chacur et al., 2001; Radhakrishnan et al., 2003). It has been shown that chemical or mechanical stimulation of astrocytes triggers elevations of free cytosolic Ca2+ that spread through astroglial networks in the form of propagating Ca2+ waves via astrocyte gap junctions, mainly composed Cx43, and that this is an important pathway for the signal spreading over long distances (Scemes et al., 2000; Spataro et al., 2004). Because persistent local noxious stimulation induced by various factors (including D‐serine, glutamate, ATP and cytokines) triggers Ca2+ or IP3 entry from the extracellular compartment into astrocytes or Ca2+ release from intracellular Ca2+ storage compartments (Gao et al., 2013), the intense and long‐lasting increase in intracellular Ca2+ or IP3 levels in ipsilateral astrocytes can be propagated along astrocyte processes and spread through gap junctions to contralateral astrocytes (Hansson, 2006). Our present results support these studies by showing that i.t. treatment with DAAO or LSOS during the early (0–3 days) post‐carrageenan injection period reduced ipsilateral mechanical allodynia and significantly blocked the development of contralateral mechanical allodynia. Conversely, when we i.t. administer DAAO or LSOS during the late phase (4–7 day post‐carrageenan injection), there is a selective blockade of contralateral mechanical allodynia, implying that increased contralateral D‐serine is an important factor in the development of contralateral mechanical allodynia. Collectively, the present results suggest that the increase in ipsilateral spinal cord D‐serine during the earlier phase of peripheral inflammation not only plays a critical role in the development of ipsilateral mechanical allodynia but also indirectly mediates the development of contralateral mechanical allodynia by initiating the spread of astrocytic signalling to the opposite side of the spinal cord. Furthermore, our results indicate that up‐regulated contralateral D‐serine during the later phase of inflammation serves as a key factor that directly contributes to the development of contralateral mechanical allodynia.
The σ1 receptor has been shown to play an important role in the initiation of mechanical allodynia, but not in the maintenance of mechanical allodynia (Roh et al., 2008; Moon et al., 2013). One mechanism by which σ1 receptors facilitate mechanical allodynia is through the regulation of D‐serine expression in the astrocytes (Moon et al., 2015). Previous reports from our laboratories have demonstrated that activated σ1 receptors can increase the phosphorylation of p38 mechanical allodyniaPK in astrocytes during the induction phase of neuropathic pain (Moon et al., 2013). Therefore, these results suggest that one possible early mechanism through which activated σ1 receptors facilitate SeRa expression is via a p38‐dependent pathway resulting in increased D‐serine production. Moreover, it has been shown that activation of σ1 receptors increases the intracellular Ca2+ concentration through an IP3‐induced Ca2+ efflux from the endoplasmic reticulum (Su et al., 2010). Because several studies have reported that SeRa is activated by the direct binding of Ca2+ ions (Cook et al., 2002; Baumgart et al., 2007) and that the release of D‐serine is facilitated through Ca2+ and SNARE protein‐dependent pathways (Mothet et al., 2005), it is possible that σ1 receptor‐mediated increases in intracellular Ca2+ ions lead to an up‐regulation of ipsilateral D‐serine production via astrocyte SeRa activation or up‐regulation. This hypothesis is supported by our immunohistochemical and Western blotting results performed at the 3 h post‐carrageenan injection time point showing that although the expression of ipsilateral SeRa was not changed, the expression levels of ipsilateral D‐serine were significantly increased compared to that of sham animals (see Supporting Information Figure S1). Collectively, these findings strongly suggest that early activation of the ipsilateral σ1 receptor plays as an important role in modulating ipsilateral SeRa up‐regulation and consequentially D‐serine production.
The temporal pattern of contralateral D‐serine production and SeRa expression and the mechanisms underlying the development of contralateral mechanical allodynia appear to be considerably different from that on the ipsilateral side (Choi et al., 2015). This implies that contralateral spinal D‐serine production is controlled through different mechanisms then direct σ1 receptor activation as occurs on the ipsilateral side. The literature together with our own results suggest that the up‐regulation of contralateral SeRa expression and D‐serine production are caused by astrocyte gap junction‐mediated Ca2+ or IP3 diffusion. Thus, it seems reasonable to focus the discussion of the contralateral spinal mechanisms underlying changes in SeRa expression and D‐serine production on gap junctions and their elaborated intracellular calcium signalling and high degree of intercellular communication. A significant number of reports have now demonstrated that activation of JNK, mechanical allodyniaPK and subsequent downstream signalling in astrocytes play critical roles in regulating nociceptive processing (Zhuang et al., 2006; Katsura et al., 2008). Increased intracellular Ca2+ concentration via gap junction Ca2+ waves is reported to be one of the stimuli that activates JNK (Gao et al., 2013). Furthermore, other studies have demonstrated that activated JNK can increase AP‐1 activity, which in turn can up‐regulate SeRa promoter activity (Wu and Barger, 2004; Barger, 2016). Collectively, information from the literature together with the present results suggest that astrocyte gap junctional Ca2+ waves and possibly subsequent JNK activation in contralateral astrocytes represent an important mechanism underlying contralateral SeRa up‐regulation and D‐serine production. However, clearly more work is required to solidify our understanding of the precise mechanisms underlying ipsilateral versus contralateral D‐serine and SeRa up‐regulation.
In conclusion, the present study shows that astrocyte D‐serine serves as a critical mediator that plays a key role in the development of contralateral mechanical allodynia in carrageenan‐induced inflammatory pain model. We also demonstrated that the expression of ipsilateral D‐serine is controlled by activation of σ1 receptors and that it indirectly affects the development of contralateral mechanical allodynia. Furthermore, we showed that astrocyte gap junctions play a key role in transmitting nociceptive signals to the contralateral spinal cord and that the transmitted signals affect both contralateral D‐serine expression and the development of contralateral mechanical allodynia (Figure 8). These novel findings provided evidence that expands our understanding of the mechanisms underlying the development of contralateral mechanical allodynia and further suggest the possibility of targeting astrocyte D‐serine as a therapeutic approach to treating mirror image pain.
Figure 8.
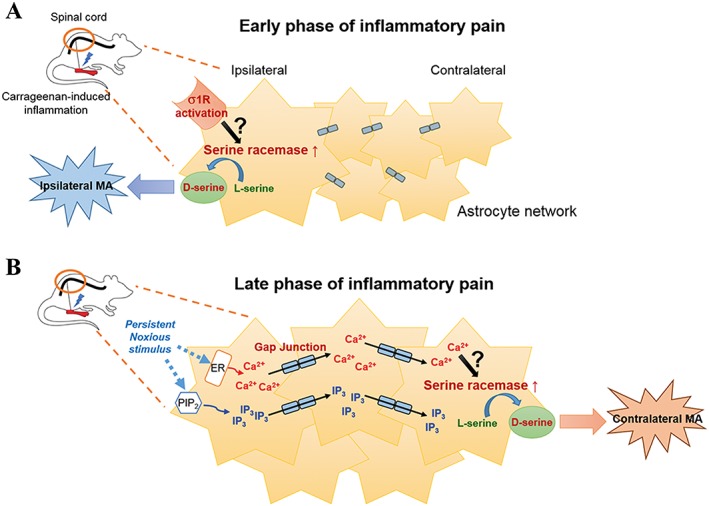
Schematic diagram summarizing the proposed mechanisms for D‐serine‐induced development of ipsilateral versus contralateral mechanical allodynia (MA) and modulation of astrocyte SeRa and D‐serine up‐regulation in carrageenan rats. (A) During the early phase of inflammation, activated ipsilateral spinal σ1 receptors up‐regulate the expression of SeRa and D‐serine, which contribute to the development of ipsilateral mechanical allodynia. (B) During the late phase of inflammation, the persistent ipsilateral noxious stimulation causes an increase in Ca2+ ion or IP3 concentration in ipsilateral astrocytes. This generates a wave of Ca2+ ions or IP3 spread (Ca2+ waves) to distant astrocytes through astrocytic gap junctions. The increased Ca2+ ion or IP3 concentration in contralateral astrocytes increases the expression of SeRa and results in increased D‐serine production. The up‐regulated contralateral D‐serine subsequently contributes to the development of contralateral mechanical allodynia.
Author contributions
H.S.C. designed and performed experiments and prepared the manuscript. D.H.R. and S.Y.Y. gave technical support and guided the design of the study and drafting of the manuscript. S.R.C, S.G.K., S.Y.K. and J.Y.M. gave technical support in experiments and helped with analysis, interpretation of data and drafting of the manuscript. H.J.H., A.J.B. and J.H.L. gave conceptual advice and assisted with the study design, interpretation of the data and the writing of the manuscript. All authors have read and approved the final manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Graphs and photomicrographs illustrating the effect of intraplantar carrageenan (CA) injection on the D‐serine and serine racemase (SeRa) expression in the spinal cord dorsal horn at 3 hour post‐CA injection. (A) Ipsilateral D‐serine expression was significantly upregulated at 3 hour post‐CA (FSDH= 8.584), while contralateral D‐serine expression was not changed compared to that of sham animals. (B) In contrast, the expression of SeRa was not significantly changed in either ipsilateral or contralateral spinal cord. *P < 0.05 as compared to that of sham group. Scale bar=150 μm.
Acknowledgements
This research was supported by a grant the National Research Foundation of Korea grant funded by the Republic of Korean Government (NRF‐2017R1A2A2A05001402).
Choi, H.‐S. , Roh, D.‐H. , Yoon, S.‐Y. , Choi, S.‐R. , Kwon, S.‐G. , Kang, S.‐Y. , Moon, J.‐Y. , Han, H.‐J. , Beitz, A. J. , and Lee, J.‐H. (2018) Differential involvement of ipsilateral and contralateral spinal cord astrocyte D‐serine in carrageenan‐induced mirror‐image pain: role of σ1 receptors and astrocyte gap junctions. British Journal of Pharmacology, 175: 558–572. doi: 10.1111/bph.14109.
References
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Overview. Br J Pharmacol 174: S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol 174: S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Other ion channels. Br J Pharmacol 174: S195–S207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger SW (2016). Gene regulation and genetics in neurochemistry, past to future. J Neurochem 139: 24–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart F, Mancheno JM, Rodriguez‐Crespo I (2007). Insights into the activation of brain serine racemase by the multi‐PDZ domain glutamate receptor interacting protein, divalent cations and ATP. FEBS J 274: 4561–4571. [DOI] [PubMed] [Google Scholar]
- Bazargani N, Attwell D (2016). Astrocyte calcium signaling: the third wave. Nat Neurosci 19: 182–189. [DOI] [PubMed] [Google Scholar]
- Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang HC, Tracey KJ et al (2001). A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri‐sciatic immune activation in rats. Pain 94: 231–244. [DOI] [PubMed] [Google Scholar]
- Cheng CF, Cheng JK, Chen CY, Lien CC, Chu DC, Wang SY et al (2014). Mirror‐image pain is mediated by nerve growth factor produced from tumor necrosis factor alpha‐activated satellite glia after peripheral nerve injury. Pain 155: 906–920. [DOI] [PubMed] [Google Scholar]
- Choi HS, Roh DH, Yoon SY, Moon JY, Choi SR, Kwon SG et al (2015). Microglial interleukin‐1beta in the ipsilateral dorsal horn inhibits the development of mirror‐image contralateral mechanical allodynia through astrocyte activation in a rat model of inflammatory pain. Pain 156: 1046–1059. [DOI] [PubMed] [Google Scholar]
- Choi HS, Roh DH, Yoon SY, Kwon SG, Choi SR, Kang SY et al (2016a). The role of spinal interleukin‐1beta and astrocyte connexin 43 in the development of mirror‐image pain in an inflammatory pain model. Exp Neurol 287: 1–13. [DOI] [PubMed] [Google Scholar]
- Choi SR, Moon JY, Roh DH, Yoon SY, Kwon SG, Choi HS et al (2016b). Spinal D‐serine increases PKC‐dependent GluN1 phosphorylation contributing to the sigma‐1 receptor‐induced development of mechanical allodynia in a mouse model of neuropathic pain. J Pain 18: 415–427. [DOI] [PubMed] [Google Scholar]
- Cook SP, Galve‐Roperh I, del Pozo AM, Rodriguez‐Crespo I (2002). Direct calcium binding results in activation of brain serine racemase. J Biol Chem 277: 27782–27792. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle L, Schwartzman RJ, Alexander G (2009). Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain Behav Immun 23: 85–91. [DOI] [PubMed] [Google Scholar]
- Dieb W, Hafidi A (2013). Astrocytes are involved in trigeminal dynamic mechanical allodynia: potential role of D‐serine. J Dent Res 92: 808–813. [DOI] [PubMed] [Google Scholar]
- Gao K, Wang CR, Jiang F, Wong AYK, Su N, Jiang JH et al (2013). Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c‐Jun/AP‐1 pathway and switch on GFAP expression. Glia 61: 2063–2077. [DOI] [PubMed] [Google Scholar]
- Gutierrez‐Mecinas M, Bell AM, Marin A, Taylor R, Boyle KA, Furuta T et al (2017). Preprotachykinin A is expressed by a distinct population of excitatory neurons in the mouse superficial spinal dorsal horn including cells that respond to noxious and pruritic stimuli. Pain 158: 440–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson E (2006). Could chronic pain and spread of pain sensation be induced and maintained by glial activation? Acta Physiol 187: 321–327. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SHR, Rusakov DA (2010). Long‐term potentiation depends on release of D‐serine from astrocytes. Nature 463: 232–U120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt DL, Castillo PE (2012). Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol 22: 496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura H, Obata K, Miyoshi K, Kondo T, Yamanaka H, Kobayashi K et al (2008). Transforming growth factor‐activated kinase 1 induced in spinal astrocytes contributes to mechanical hypersensitivity after nerve injury. Glia 56: 723–733. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A (2006). NMDA receptors mediate neuron‐to‐glia signaling in mouse cortical astrocytes. J Neurosci 26: 2673–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre Y, Amadioa A, Vincent P, Descheemaeker A, Oliet SHR, Dallel R et al (2015). Neuropathic pain depends upon D‐serine co‐activation of spinal NMDA receptors in rats. Neurosci Lett 603: 42–47. [DOI] [PubMed] [Google Scholar]
- Levine JD, Fields HL, Basbaum AI (1993). Peptides and the primary afferent nociceptor. J Neurosci 13: 2273–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Su TP (2009). The pharmacology of sigma‐1 receptors. Pharmacol Ther 124: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JY, Song S, Yoon SY, Roh DH, Kang SY, Park JH et al (2012). The differential effect of intrathecal Nav1.8 blockers on the induction and maintenance of capsaicin‐ and peripheral ischemia‐induced mechanical allodynia and thermal hyperalgesia. Anesth Analg 114: 215–223. [DOI] [PubMed] [Google Scholar]
- Moon JY, Roh DH, Yoon SY, Kang SY, Choi SR, Kwon SG et al (2013). Sigma‐1 receptor‐mediated increase in spinal p38 MAPK phosphorylation leads to the induction of mechanical allodynia in mice and neuropathic rats. Exp Neurol 247: 383–391. [DOI] [PubMed] [Google Scholar]
- Moon JY, Roh DH, Yoon SY, Choi SR, Kwon SG, Choi HS et al (2014). sigma 1 receptors activate astrocytes via p38 MAPK phosphorylation leading to the development of mechanical allodynia in a mouse model of neuropathic pain. Br J Pharmacol 171: 5881–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JY, Choi SR, Roh DH, Yoon SY, Kwon SG, Choi HS et al (2015). Spinal sigma‐1 receptor activation increases the production of D‐serine in astrocytes which contributes to the development of mechanical allodynia in a mouse model of neuropathic pain. Pharmacol Res 100: 353–364. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G (2005). Glutamate receptor activation triggers a calcium‐dependent and SNARE protein‐dependent release of the gliotransmitter D‐serine. Proc Natl Acad Sci U S A 102: 5606–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz MF, Puebla M, Figueroa XF (2015). Control of the neurovascular coupling by nitric oxide‐dependent regulation of astrocytic Ca2+ signaling. Front Cell Neurosci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA et al (2006). Glia‐derived D‐serine controls NMDA receptor activity and synaptic memory. Cell 125: 775–784. [DOI] [PubMed] [Google Scholar]
- Porres CP, Meyer EM, Grothe B, Felmy F (2011). NMDA currents modulate the synaptic input‐output functions of neurons in the dorsal nucleus of the lateral lemniscus in Mongolian gerbils. J Neurosci 31: 4511–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan R, Moore SA, Sluka KA (2003). Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain 104: 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R (2008). Neuron‐glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol 21: 570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Kim KW et al (2008). Intrathecal injection of the sigma(1) receptor antagonist BD1047 blocks both mechanical allodynia and increases in spinal NR1 expression during the induction phase of rodent neuropathic pain. Anesthesiology 109: 879–889. [DOI] [PubMed] [Google Scholar]
- Roh DH, Yoon SY, Seo HS, Kang SY, Han HJ, Beitz AJ et al (2010). Intrathecal injection of carbenoxolone, a gap junction decoupler, attenuates the induction of below‐level neuropathic pain after spinal cord injury in rats. Exp Neurol 224: 123–132. [DOI] [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Spray DC (2000). Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci 20: 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber KL, Beitz AJ, Wilcox GL (2008). Activation of spinal microglia in a murine model of peripheral inflammation‐induced, long‐lasting contralateral allodynia. Neurosci Lett 440: 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Kim HW, Roh DH, Yoon SY, Kwon YB, Han HJ et al (2008). A new rat model for thrombus‐induced ischemic pain (TIIP); development of bilateral mechanical allodynia. Pain 139: 520–532. [DOI] [PubMed] [Google Scholar]
- Sluka K, Kalra A, Moore S (2001). Unilateral intramuscular injections of acidic saline produce a bilateral, long‐lasting hyperalgesia. Muscle Nerve 24: 37–46. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spataro LE, Sloane EM, Milligan ED, Wieseler‐Frank J, Schoeniger D, Jekich BM et al (2004). Spinal gap junctions: potential involvement in pain facilitation. J Pain 5: 392–405. [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE (2010). The sigma‐1 receptor chaperone as an inter‐organelle signaling modulator. Trends Pharmacol Sci 31: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Kirchhoff F (2007). NMDA receptors in glia. Neuroscientist 13: 28–37. [DOI] [PubMed] [Google Scholar]
- Wu SZ, Barger SW (2004). Induction of serine racemase by inflammatory stimuli is dependent on AP‐1. Ann N Y Acad Sci 1035: 133–146. [DOI] [PubMed] [Google Scholar]
- Yoon MH, Yaksh TL (1999). The effect of intrathecal gabapentin on pain behavior and hemodynamics on the formalin test in the rat. Anesth Analg 89: 434–439. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Ji GC, Wu GC, Zhao ZQ (2002). Excitatory amino acid receptor antagonists and electroacupuncture synergetically inhibit carrageenan‐induced behavioral hyperalgesia and spinal fos expression in rats. Pain 99: 525–535. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR et al (2006). A peptide c‐Jun N‐terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci 26: 3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Graphs and photomicrographs illustrating the effect of intraplantar carrageenan (CA) injection on the D‐serine and serine racemase (SeRa) expression in the spinal cord dorsal horn at 3 hour post‐CA injection. (A) Ipsilateral D‐serine expression was significantly upregulated at 3 hour post‐CA (FSDH= 8.584), while contralateral D‐serine expression was not changed compared to that of sham animals. (B) In contrast, the expression of SeRa was not significantly changed in either ipsilateral or contralateral spinal cord. *P < 0.05 as compared to that of sham group. Scale bar=150 μm.


