Abstract
Functionalized multi-walled carbon nanotube (fMWCNT) development has been intensified to improve their surface activity for numerous applications, and potentially reduce toxic effects. Although MWCNT exposures are associated with lung tumorigenesis in vivo, adverse responses associated with exposure to different fMWCNTs in human lung epithelium are presently unknown. This study hypothesized that different plasma-coating functional groups determine MWCNT neoplastic transformation potential. Using our established model, human primary small airway epithelial cells (pSAECs) were continuously exposed for 8 and 12 weeks at 0.06 µg/cm2 to three-month aged as-prepared-(pMWCNT), carboxylated-(MW-COOH), and aminated-MWCNTs (MW-NHx). Ultrafine carbon black (UFCB) and crocidolite asbestos (ASB) served as particle controls. fMWCNTs were characterized during storage, and exposed cells were assessed for several established cancer cell hallmarks. Characterization analyses conducted at 0 and 2 months of aging detected a loss of surface functional groups over time due to atmospheric oxidation, with MW-NHx possessing less oxygen and greater lung surfactant binding affinity. Following 8 weeks of exposure, all fMWCNT-exposed cells exhibited significant increased proliferation compared to controls at 7 d post-treatment, while UFCB- and ASB-exposed cells did not differ significantly from controls. UFCB, pMWCNT, and MW-COOH exposure stimulated significant transient invasion behavior. Conversely, aged MW-NHx-exposed cells displayed moderate increases in soft agar colony formation and morphological transformation potential, while UFCB cells showed a minimal effect compared to all other treatments. In summary, surface properties of aged fMWCNTs can impact cell transformation events in vitro following continuous, occupationally relevant exposures.
Keywords: Surface properties, aging effects, functionalization, multi-walled carbon nanotubes, cell transformation
Introduction
Past multi-walled carbon nanotube (MWCNT) toxicity studies have primarily focused on assessing risk for pulmonary inflammation, genotoxicity, fibrosis, and tumorigenesis due to aerosolization of MWCNTs during synthesis or subsequent handling. MWCNTs cause aneuploidy via centromere abnormalities in exposed human epithelial cells and are considered a possible human carcinogen (Grosse et al., 2014; Sargent et al., 2014; Siegrist et al., 2014). Furthermore, recent in vivo evidence suggests that MWCNTs directly induce lung tumors and may be a complete carcinogen (Kasai et al., 2016). MWCNTs differ in their physicochemical properties, such as length, diameter, surface area, and surface chemistry, which can drive adverse pulmonary health effects (Donaldson et al., 2013). Expansion of the MWCNT market for a wide array of purposes has resulted in surface functionalization of MWCNTs, either directly during synthesis (i.e., doping) or post-synthesis, with small organic molecules (e.g., carboxylation) or oxidized metals (e.g., aluminum oxide). Functionalization allows for improved dispersion characteristics for incorporation of hydrophobic MWCNTs into hydrophilic polymers or plastics, or provides enhanced electrostatic properties (Kim, 2011). Advances in plasma-grafting technology have given rise to plasma polymer coatings on numerous surfaces, including carbon nanotubes (CNTs; Khelifa et al., 2016). Recent comparisons of fMWCNTs to pristine MWCNTs suggest that carboxyl fMWCNT induce less lung inflammation, toxicity, and fibrosis following pulmonary exposure compared to pMWCNT (Hamilton et al., 2013a,b; Poulsen et al., 2016; Sager et al., 2014). However, other studies report that fMWCNT exhibit equal or greater potency compared to pMWCNT (Dandley et al., 2016; Patlolla et al., 2010; Ursini et al., 2016).
Estimates for the number of U.S. workers who would come in contact with MWCNTs soon after synthesis is small compared to the potential number of downstream users across the MWCNT life cycle (Mackevica & Hansen, 2016). In most instances, MWCNT exposures are expected to occur over long time frames at low levels (Kuempel et al., 2017). Once MWCNTs move downstream from their manufacturing site into additive manufacturing, modifications to their surface, structural integrity, and other physicochemical properties are expected due to their wide spread use in numerous applications, each with their own physical and chemical requirements. Changes or elimination of surface functionalization will not only change the technological abilities, but possibly the transport, transformation, and toxicological effect of MWCNTs if released into the environment (Petersen et al., 2011). To date, few studies have examined the changes in physicochemical properties of MWCNTs across their life cycle (Dahm et al., 2012; Hedmer et al., 2014), and how these changes potentially impact worker pulmonary health (Bishop et al., 2016; Kuempel et al., 2017). No studies have evaluated the life cycle impacts on MWCNT tumorigenic potential.
Aging and transformation of engineered nanomaterials (ENMs) across their life cycle can directly alter unique physicochemical properties (Mitrano et al., 2015), thus affecting not only their nanotechnological application but also toxicological responses. At present, a majority of both exposure and effect toxicological research has evaluated primarily as-produced, pristine ENMs with little consideration of how life cycle transformations affect hazard, release, fate, exposure, and effect. Given ENMs’ unique physicochemical properties that are typically designed for unique applications, it is highly likely that transformation processes across the ENM life cycle will change these physicochemical properties. Transformation of various pristine and functionalized ENMs may increase similarity or result in greater diversity of their physicochemical properties. Since a majority of current ENM risk assessment relies on linking as-produced physicochemical properties of pristine ENM with toxicological effect, it is likely that these predictive estimates may over- or under-estimate toxicological hazard over an ENM’s life cycle (Lowry et al., 2012). Given the large data gaps and uncertainties associated with ENM transformation, release, and potential long-term exposures in the workplace, it is important that research efforts begin to evaluate potential long-term health effects, including carcinogenesis (Becker et al., 2011).
Consistent with this notion, the main objective of this study was to screen and assess different aged fMWCNTs for potential neoplastic transformation ability using primary human small airway epithelial cells (pSAECs). Based on previous fMWCNT literature, we hypothesized that differences in surface properties would impact aged MWCNT neoplastic transformation ability. We monitored the aging of post-synthesis fMWCNTs in laboratory storage conditions and subsequently conducted long-term fMWCNT exposures of human primary small airway epithelial cells. Cancer cell hallmark assays were employed to determine neoplastic transformation potential. We show that three-month aged MW-NHx, with low-percent surface oxygen content and enhanced protein-binding ability, caused a greater cell transformation ability than all other fMWCNTs, suggesting that differences in surface properties of aged fMWCNTs impact their cell transformation potential.
Methods
Additional details on methodology can be found in Supplementary materials.
Nanoparticle characterization and dispersion
‘As-prepared’ (pMWCNT), carboxylated- (MW-COOH), and aminated- (MW-NHx) multi-walled carbon nanotubes, functionalized by plasma gas, were purchased from Perpetuus Carbon Technologies (Ammanford, UK) with equal lengths and surface area containing less than 1% wt. of contaminants. pMWCNT served as the parent particle which was then functionalized in proprietary plasma gas atmospheres to acquire both MW-COOH and MW-NHx. Ultrafine carbon black (UFCB; Elftex 12), Mitsui #7 MWCNT (MWCNT-7), and crocidolite asbestos (ASB; NIEHS standard) were acquired as previously described (Table 1; Wang et al., 2014).
Table 1.
Physicochemical characterization of functionalized MWCNT.
| Particle | UFCB | pMWCNT | MW-COOH | MW-NHx | Crocidolite Asbestos |
|---|---|---|---|---|---|
| Name/Source | Elftex 12, Cabot | Perpetuus | Perpetuus | Perpetuus | NIEHS, Kalahari Desert |
| Synthesisa | Vapor-phase pyrolysis | Argon Plasma | Proprietary Oxygen Blend Plasma | Nitrogen Plasma | Natural |
| Primary Functionalitya | non-functionalized | non-functionalized | COOH | N=H | n/a |
| Other Functionalitiesa | n/a | atmospheric gas | COH, C=O, other oxygen groups | N–H, O=C–N–H2, C=N | n/a |
| Dry Mean Length (µm)a | n/a | 1 to 12 | 1 to 12 | 1 to 12 | 10 |
| Dry Mean Width (nm)a | 37 | 13 to 18 | 13 to 18 | 13 to 18 | 210 |
| Dispersed Mean Length (µm) | n/a | 1.51 ± 0.001 | 1.86 ± 0.18 | 1.50 ± 0.078 | ND |
| Dispersed Mean Width (nm) | 316.6 ± 25.8 | 26.0 ± 5.4 | 26.5 ± 1.0 | 21.6 ± 0.6 | ND |
| BET Surface Area | 43 m2/gb | 214 m2/ga | 214 m2/ga | 214 m2/ga | 9.8 m2/gb |
| Zeta Potential (water) | −7.02 | −24.2 | −30.1 | −32.5 | −35.2 |
| Zeta Potential (medium) | −9.28 | −9.58 | −8.66 | −10.12 | −8.04 |
| % carbon (w/w) | >99% | >99%, 0% ash | >99%, 0% ash | >99%, 0% ash | <1% |
Data represent mean ±SE. ND = not determined.
Supplied by the manufacturer.
Previously reported (Wang et al. 2014).
Upon arrival, all MWCNT were immediately assessed for functionalization presence via Fourier transform infrared spectroscopy (FTIR) analysis using previously described methods (Hamilton et al., 2013a,b). FTIR was chosen as a primary characterization method to monitor time-based changes in graphite surface chemistry (Chang et al., 2016). All MWCNT samples were stored at laboratory room temperature (20–22 °C) and 40% humidity in darkness. FTIR and X-ray photoelectron spectroscopy (XPS) analyses were conducted again at 2 and 12 months post-storage to examine the fMWCNT characteristics over time. MW-COOH were previously reported to possess a significant amount of –COOH functional groups amounting between 7.4 and 7.7% oxygen (NCL NIEHS, 2012). In addition, UFCB and Mitsui #7 MWCNT, our laboratory’s benchmark carbon nanomaterials, were also characterized by FTIR. Dry samples of aged fMWCNTs were shipped to Perpetuus Carbon Technologies for independent validation using scanning electron microscopy (SEM), FTIR, and XPS analyses.
All carbon nanomaterials and ASB were prepared for small airway epithelial cell exposure as previously described (Wang et al., 2014). Briefly, MWCNTs, UFCB, and ASB were weighed and placed into 1 mg/ml suspension in sterile MilliQ water. UFCB and MWCNT particles were diluted to 0.1 mg/ml using water containing 150 µg/ml of Survanta® (Abbott Laboratories, Columbus, OH) or 1% bovine serum albumin (BSA) in water to obtain stock solutions. To disperse each material, UFCB and MWCNT stock solutions containing Survanta® and ASB in water were lightly sonicated. All particle suspensions were serially diluted with SAGM to acquire final exposure concentrations (0.288 µg/ml = 0.06 µg/cm2) for each particle with < 0.3% water. These techniques were previously shown to result in characteristic in vitro neoplastic phenotypes, fibrogenesis, and genomic signatures that correlated well to in vivo models for both MWCNT and ASB (Mishra et al., 2012; Wang et al., 2014). Next, zeta potential was determined for all MWCNT suspensions in sterile MilliQ water and SAGM using a Nanoseries Zetasizer ZS (Malvern). To measure dispersion potential, length/width, and elemental content of particles, freshly sonicated stock solutions were prepared and visualized under field emission SEM (FESEM/EDX) as previously described (Mishra et al., 2012). More than 100 individual MWCNTs per particle type were measured using ImageJ (National Institutes of Health, Bethesda, MD) for dispersed length and width.
To discern differences in the ability of various carbon ENMs ability to bind lung surfactant and protein that may impact toxicological response, each carbon ENM was incubated with Survanta lung surfactant in SAGM medium, analyzed by SDS Page gels, and bound protein quantified using previously described methods (Allegri et al., 2016). Stained protein bands on gels were imaged and quantified using FluoroChem SP.
To identify acellular oxidative potential differences among the particles, dispersed particles were assayed via cell-free dithiothreitol (DTT) depletion reaction as previously described (Roberts et al., 2016). Phosphate-buffered saline and Survanta-only water served as controls. Both DTT consumption vs. particle mass (nM/µg) and surface area (nM/m2) were calculated based on absorbance values at 412 nm.
Long-term pSAEC exposure
pSAECs (Lonza) at 2nd passage were seeded overnight in triplicate into 6-well plates holding SAGM (Lonza) and cultured at 37 °C in a humid atmosphere containing 5% CO2 air throughout the experiment. Following seeding, cells were continuously exposed to 0.06 µg/cm2 of Survanta-dispersed UFCB, pMWCNT, MW-COOH, MW-NHx, or water-suspended ASB for 8 and 12 weeks. fMWCNT particles were aged for 3 months under laboratory conditions prior to the start of the exposure. The chosen dose modeled an expected MWCNT deposition of 30 µg in mouse lung which approximates an estimated human occupational exposure of 10–40 µg/m3 over 27–103 months of light work (Porter et al., 2013). Likewise, it closely matches the deposited dose after inhalation exposure that resulted in MWCNT promotion of lung cancer (Sargent et al., 2014). In addition, this dose gave greater chance for transformation given the shorter life span of pSAECs (<12 weeks; Stueckle et al., 2017) than immortalized SAECs in our previous 0.02 µg/cm2 six month exposure study (Wang et al., 2014). Water and dispersant exposed cells were used as controls. Every three days old medium was removed, adherent cells rinsed in HEPES buffered saline, and fresh SAGM medium with or without particles was administered. All treatments were passaged every 8–10 days following manufacturer’s protocol by washing liberally with saline, incubating with 0.25% trypsin/EDTA, and inactivating trypsin with 2× v/v neutralization buffer. Rinsing attached cells prior to culture medium changes and cell passages, and reseeding at 25% confluence aided in reducing potential bioaccumulation of MWCNT over the exposure time course.
Darkfield enhanced microscopy
Uptake and localization of UFCB, MWCNTs, and ASB in pSAECs was performed and imaged using enhanced darkfield imaging (CytoViva, Auburn, AL) following acute 24 h exposure in low passage pSAECs (Wang et al., 2014).
Cancer hallmarks assessment
To evaluate cancer cell behavioral hallmarks, cells from each exposure replicate were assessed at both 8 and 12 week time points using well-established methods (Creton et al., 2012; OECD 2007; Wang et al., 2014) with minor modifications as described in the Supplementary material. Cell proliferation, transformation and aggressive cell mobility were measured using (a) Trypan blue, WST-1, and colony forming unit (CFU) assays, (b) soft agar colony, and morphological transformation assays, and (c) Transwell migration and invasion chemotaxis assays, respectively.
Statistical analyses
All experimental data sets were evaluated for normal distribution of the residuals and variance homoscedasticity to satisfy ANOVA assumptions. Dispersant-only cells acted as negative passage control in all statistical comparisons. Treatments were compared using one-way ANOVA analyses followed by Dunnett’s or Tukey–Kramer honestly significant difference post-hoc tests (α = 0.05).
Results
Aged MWCNT characterization
Following receipt of shipment, MWCNTs were assessed for differences in surface functionalization using FTIR. All fMWCNTs possessed peaks at 2915 and 2848 cm−1 associated with C–H stretch, and a strong peak at 1385 cm−1 corresponding to the deformation vibration (Figure 1(A)). Peaks at 1627 cm−1 and 1569 cm−1 were associated with MWCNT aromatic ring skeletal vibrations. In addition, a strong peak at 3436 cm−1 was associated with O–H stretch. For the MW-COOH particles, peaks at 1720 cm−1 associated with C = O, 2511 cm−1 was due to carboxylic acid, and the broad 950–1230 cm−1 peak was ascribed to the C–O vibration, respectively, indicated the presence of carboxyl surface functionalization. A peak ranging 1410–1460 cm−1 was assigned to C–O stretching typical of carboxylic groups (Li et al., 2012). For MW-NHx, weak peaks at 1617 and 1643 cm−1 clustered near the 1627 cm−1 peak, along with a strong 1539 cm−1 and weak 1560 cm−1 peak (Supplementary Figure 1A), indicated presence of N–H scissoring and stretching, respectively (Chidawanyika & Nyokong, 2010), which was distinct from all other MWCNTs. A weak 1652 cm−1 peak suggested the presence of saturated amide bond. These particles exhibited a noticeable similarity to the MW-COOH, primarily due to C–O peaks. However, the C–O vibration peak was reduced compared to the other two MWCNT particles, indicating potential reduced presence of oxygen during the nitrogen functionalization process. In addition, a peak at 870 cm−1 associated with a carboxylic or ether group was observed, but relatively absent from pMWCNTs. Negative zeta potentials reflected the nature of the negatively charged plasma gas during synthesis. These characteristics generally supported those reported by the manufacturer (Table 1).
Figure 1.
FTIR characterization of the MWCNT samples. (A) Fresh 0month; (B) 2-month following laboratory aging (n = 3). (C) C1s (left) and O1s (right) XPS spectra for 2 month old MW-COOH.
Prior to exposure, all 2 month aged FTIR spectra were dramatically different compared to freshly shipped material (Figure 1(B)) by experiencing the loss of C–O (950–1230 cm−1), which coincided with the absence of the 1410–1460 cm−1 peak in MW-COOH and MW-NHx samples. MW-COOH retained C = O (1720, and 2511 cm−1) and a weak 1160 cm−1 associated with C–O, respectively, indicating residual carboxyl surface functionalization. Both pMWCNT and MW-COOH, but not MW-NHx, possessed small peaks at 1465 (C–H bend), 1541, and 1570 cm−1 (C aromatic), respectively (Supplemental Figure 1B). The absence of aromatic ring peaks in MW-NHx may have been due to oxidative damage. For MW-NHx, all previously observed nitrogen-associated peaks were absent. In addition, the C–H 1382 and 870 cm−1 peaks were noticeably reduced compared to the other two MWCNT particles. The main peak of C1s from XPS was deconvoluted by fitting into multiple sub-peaks at 284.8 eV (C–C), 285.7 eV (C–O–C), and 286.8 eV (C–OH). XPS analysis (Figure 1(C)) indicated the presence of C–O–C and C = O. The O 1 s peak at 530.7 and 533.0 eV indicated the presence of carboxylic and ether groups (Datsyuk et al., 2008). Percent oxygen content was low compared to previous published studies with similar material, with MW-NHx possessing the lowest content (Table 2). Repeated analysis following 12 months aging showed surface property convergence of all three MWCNTs with C–O–C and C–OH functional groups present and continued reduction in percent oxygen content for all fWMCNTs (Supplementary Figure 2; Table S1). For comparison, FTIR and XPS analyses were also performed on MWCNT-7 which showed O–H and C–O functional groups (Supplementary Figure 3). These findings indicate that the fMWCNT experienced autoxidation during laboratory storage with MW-NHx possessing less percent oxygen than the other MWCNTs.
Table 2.
MWCNT surface elemental analysis from XPS electron signature at 2 months.
| Atomic Element (%) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Particle | Oxygen 1s | Carbon 1s | Nitrogen 1s | Fluorine 1s | Chlorine 2p |
| pMWCNT | 1.82 | 98.09 | 0 | 0.09 | 0 |
| MW-COOH | 2.6 | 97.4 | 0 | 0 | 0 |
| MW-NHx | 1.1 | 98.9 | 0 | 0 | 0 |
SEM analysis of the MWCNT samples showed that all samples comprised particles with CNT morphology with little presence of amorphous carbon (Figure 2). No differences were observed among the different MWCNT particle morphologies once dispersed in Survanta. Polydisperse MWCNT morphologies comprised single MWCNT, nanoropes (~55–60 nm wide), and agglomerates measuring several microns in size (Figure 2). Length and width measurements indicated equivalent short lengths and narrow widths for the various dispersed MWCNT samples tested (Table 1; p > .05). Particle uptake analysis showed that UFCB and all the MWCNT particles co-localized with either the cytoplasm or nucleus of the cells (Figure 3(A)) with minimal evidence that internalized MWCNTs retained a well-dispersed fiber morphology. All MWCNTs appeared as rough spherical particles or loose agglomerates while fiber-shaped MWCNTs were occasionally found.
Figure 2.
FESEM characterization of UFCB and aged MWCNT samples in dry powder (left) and lung surfactant suspension (right). All MWCNT samples exhibited poly-disperse fibers consisting of singlets (white arrows), nanoropes, and loose agglomerates.
Figure 3.
Particle localization comparison of MWCNTs compared to UFCB and crocidolite asbestos in primary small airway epithelial cells at 24 h. (A) White arrows in enhanced dark field images indicate particle co-localization with nucleus. Dashed arrow and inset (lower left panel) = suspended CNT singlet. (B) Protein-binding comparison of carbon ENMs incubated in lung surfactant and culture medium. Lane order (left) matches sample order (right). Bars represent mean ±SE. * and ** indicate significant differences among treatments (n = 3; p < .05).
Protein corona and surface oxidant potential
Next, UFCB and MWCNTs were characterized for their ability to bind lung surfactant and SAGM protein to assess differences in agglomeration and ability to interact with biomolecules. All carbon ENMs bound significantly more protein than lung dispersant only following 1 h incubation in SAGM medium (Figure 3(B)). Moreover, MW-NHx bound significantly more total protein (13-fold) than UFCB and the other MWCNTs (5.8-fold) compared to control. Separate quantification of the high molecular weight, low molecular weight, and strong band 75–90 kD proteins resulted in no significant differences among the carbon ENMs (data not shown). Lastly, all three polydisperse MWCNTs showed equivalent moderate surface oxidant potential (nM/µg), which was significantly lower on a surface area basis, but not a mass basis, than UFCB (Supplementary Figure 4), possibly indicating that gas absorption of dry powder does not adequately reflect structure surface area in suspension (Table 1 and Figure 2).
Cell transformation assessment
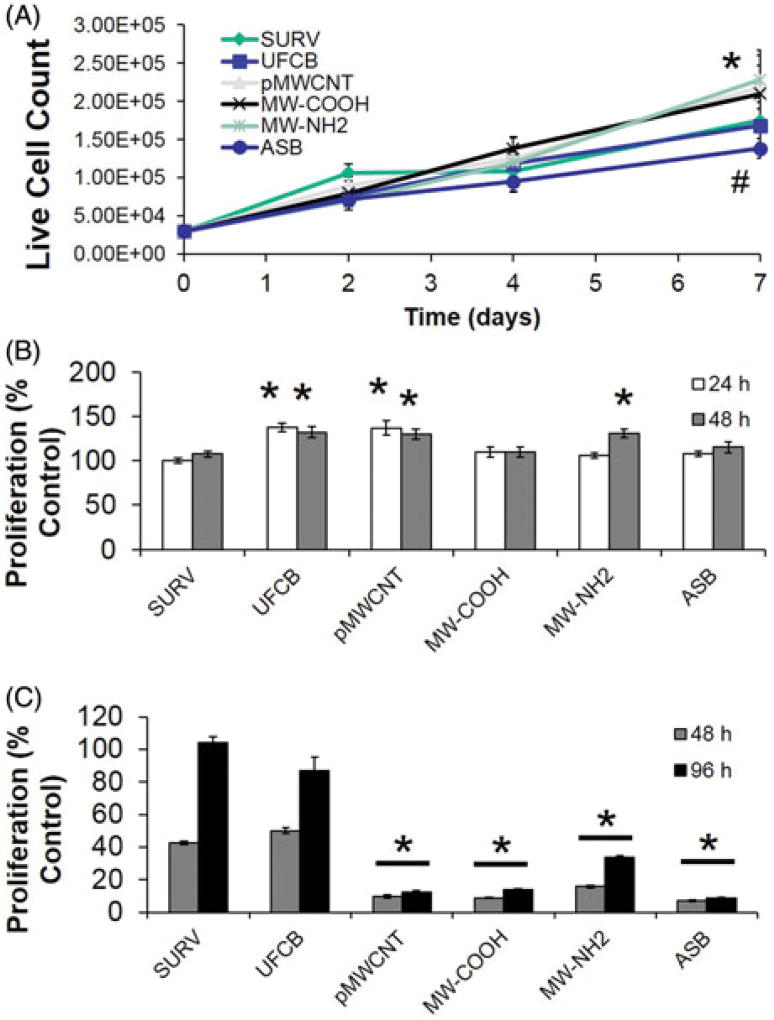
Cell proliferation was monitored every two weeks as a marker for both stimulation of cell growth signaling and an early marker for cell transformation. After 8 weeks of particle exposure, all MWCNT-exposed cells showed a significant increase in live cells at Day 7 post-seeding in particle-free medium compared to unexposed and UFCB-exposed cells (Figure 4(A)). In contrast, ASB exposure reduced live cell number. Next, UFCB and pMWCNT at both time points, and MW-NHx 48 h after an 8 week exposure, displayed subtle, but significantly increased mitochondrial metabolism compared to passage control cells (Figure 4(B)), while ASB-exposed cells showed no difference from control. In the CFU assay, none of the exposed cells showed decreased viability compared to dispersant-only controls (Supplementary Figure 5). pMWCNT and MW-COOH exposed cells showed significant moderate increase in attached CFUs. Passaging of pSAECs over 8 weeks appeared to strengthen CFU ability since saline-exposed cells showed reduced ability. Continuation of the exposure to 12 weeks resulted in the loss of cell proliferation ability for all cells compared to 8-week data. Passage control and UFCB displayed a significant 50–60% reduction in proliferation ability while all fiber particle-exposed cells showed a 75–90% loss (Figure 4(C)). All passaged cells lost proliferation ability and exhibited senescence by 14 weeks in culture.
Figure 4.
Cell proliferation of human pSAECs following long term MWCNT exposure compared to UFCB and asbestos. (A) Trypan blue live cell count after 8 week exposure. All MWCNT-exposed cells exhibited significantly greater proliferation than all other treatments (n = 9; p ≤ .05), while ASB-treated cells exhibited significantly diminished proliferation at 7 d, respectively. (B) 8-week and (C) 12-week WST-1 mitochondrial metabolism (n = 9). Data were standardized to 8-week passaged SURV controls in Panel B. Points and bars represent mean ±SE. * and # indicate significant differences from SURV control and other fiber-exposed cells, respectively (p ≤ .05).
Neoplastic transformation of epithelial cells requires escape from single layer growth morphology, avoiding cell death, and gaining the ability to form both attached, multilayered foci and unattached soft agar colonies (Creton et al., 2012; OECD, 2007). At 8 weeks, only UFCB and MW-NHx cells exhibited significantly increased number of soft agar colonies (Figure 5(A,B)). This effect was modest since colony count per well was low compared to our previous data involving pSAEC and SAEC-hTERT cells exposed to MWCNT-7 (Stueckle et al., 2017; Wang et al., 2014). Attempts to collect individual colonies and reestablish clones in culture (Stueckle et al., 2017) resulted in low survival or cell senescence. Mild morphological transformation was observed only in fiber-exposed cells with multiple Type I foci and occasional Type II foci (Figure 5(C)). MW-NHx cells showed the highest number of Type II foci while passage control and UFCB-exposed cells displayed minimal evidence of Type I foci (Table S2). These data suggested that MW-NHx-exposed cells underwent cell transformation.
Figure 5.
Attachment-independent growth and morphological transformation assessment of MWCNTs. (A) and (B) UFCB and MW-NHx at 8 weeks displayed significant increase in number of soft agar colonies compared to controls (n = 9). Bar = 100 µm. Bars represent mean ±SE. (C) Foci in a morphological transformation assay (n = 9). White arrow = Type II focus; Bar = 500 µm. * indicate significant difference vs. SURV control (p ≤ .05).
Transformed cells undergoing carcinogenesis towards an aggressive phenotype typically exhibit retention of the ability to migrate and invade neighboring tissue. To assess carbon ENM-exposed cells for this phenotype, cells after 8 and 12 weeks exposure were assayed for enhanced mobility. At 8 weeks, UFCB-, pMWCNT-, and MW-COOH-exposed cells displayed a significant 2-to-4-fold increase in invasion ability (Figure 6). A significant proportion of the UFCB-exposed cell invasion ability was due to enhanced cell mobility. MW-NHx and ASB-exposed cells showed no difference compared to passage control cells. To ascertain retention of this ability, a sub-passage of cells were cultured in particle-free medium for one week and re-assayed. All cells showed an increase in migration ability and a decrease in invasion ability compared to 8-week-exposed cells. MW-NHx and ASB cells showed significantly lower migration ability. UFCB-exposed cells still showed a modest, yet significant, elevated invasion ability. At 12 weeks, all cells, except UFCB cells, showed a drastic reduction in both migration and invasion ability compared to saline-exposed pSAEC cells in a sister study done in parallel (Stueckle et al., 2017). Unexpectedly, UFCB-exposed cells showed a significant enhanced invasion ability. These data suggested that observed increases in migration and invasion ability were particle presence-dependent and that the MW-NHx cell transformation was early in neoplastic development.
Figure 6.
Transient invasion and migration ability of pSAECs following 8 and 12 week long term exposure (0.06 µg/cm2) to MWCNT, UFCB, and asbestos. (A) Representative invasion micrographs at 8 weeks of exposure. Quantification of migration and invasion (B) immediately after exposure for 8 weeks, (C) one-week post-8 week exposure, and (D) migration and invasion at 12-week exposure. Bars represent mean ±SE. * and # indicates treatments significantly increased or decreased, respectively, from SURV control (n = 9; p ≤ .05).
Discussion
Surface functionalization of MWCNTs is known to enhance aqueous dispersion for incorporation into nano-enabled products and change its toxicological response following pulmonary exposures. The inherent surface redox potential is thought to influence CNT interactions with cellular components, thus impacting toxicity and eventual risk for disease. Changes to an ENM’s surface reactivity due to aging, either during storage or release into the environment, are expected during its life cycle. However, little research has evaluated how these changes may impact disease development (Mitrano et al., 2015). The aim of the present study was to evaluate how differences in surface properties of aged fMWCNTs impacted neoplastic transformation potential in a long-term exposure primary epithelial cell screening model. We found that three-month aged MW-NHx, with relatively low amounts of surface oxygen and enhanced protein-binding ability harbored a greater cell transformation ability compared to pMWCNT, MW-COOH, and UFCB. These data highlight that plasma fMWCNTs experience atmospheric aging over relatively short time frames during their life cycle and that resulting surface properties can influence measurable toxicological effects.
Numerous techniques for surface modification of CNTs allow a wide variety of enhanced dispersion, improved biocompatibility, technological flexibility, and application for CNTs. However, such advances have induced additional uncertainty into toxicological effects and relative health risks associated with workplace exposure. Different surface functionalization strategies, storage environments, product incorporations, and potential release scenarios represent factors in the ‘toxicological evolution’ (Liu et al., 2015) of CNTs over their life cycle. Although chemical oxidation of MWCNTs allows control over amount and maintenance of surface functionalization, acid treatment can shorten and damage MWCNTs, which can interfere with technological application (Datsyuk et al., 2008), and has complicated efforts to correlate physicochemical properties with pulmonary toxicological effects. For example, recent in vivo studies reported that short-term MW-COOH exposure resulted in reduced inflammation, cytotoxicity, rapid clearance, and fibrosis compared to parent MWCNTs (Hamilton et al., 2013a,b; Jain et al., 2011; Sager et al., 2014). Anionic –COOH functionalization reduced epithelial, fibroblast, and macrophage cytotoxicity and inflammasome activation (Sayes et al., 2006), while strong cationic surfaces increased surface reactivity, resulting in strong inflammasome activation and fibrogenic potential (Li et al., 2013b). Other studies, however, found no difference between –COOH and –NH2 functionalized MWCNT toxicity, since functional group presence caused increased protein binding and agglomeration (Allegri et al., 2016; Coccini et al., 2013).
Conversely, other studies suggest that functionalization allows for better MWCNT dispersion in biological fluids, thus increasing their toxicity potential. For example, aspiration of well-dispersed –COOH functionalized single-walled CNTs (fSWCNTs) caused greater inflammation than parent particle in CD1 mice (Saxena et al., 2007). Intraperitoneal injection of MW-COOH caused greater clastogenic potential in collected bone marrow cells than poorly dispersed parent MWCNTs (Patlolla et al., 2010). Although no differences between functionalized –COOH, –NH2 vs. parent MWCNTs to induce inflammation and airway damage were found, a follow-up study identified –NH2 causing more pronounced PCNA and IL-6 expression in exposed rat alveolar tissue than other MWCNTs (Coccini et al., 2013; Roda et al., 2011). In vitro administered fSWCNTs and fMWCNTs with strong negative charge also increased genotoxicity, hampered natural killer cell function, and inhibited cell division. Of note, an increasing number of recent studies show that fCNTs exhibit enhanced genotoxicity and apoptosis induction compared to parent CNTs, but the opposite for cellular damage and cytotoxicity assays, possibly due to uptake route (Alam et al., 2016; Mrakovcic et al., 2015; Ursini et al., 2012).
Alternatively, plasma gas functionalization allows for a rapid and clean method for surface modification for carbon nanomaterials and provides a highly reactive surface with chemical resistance and coating properties for MWCNT incorporation into multiple applications (Vohrer et al., 2007). Plasma coating of surfaces relies on using free radicals to bind unique polymers onto surfaces. Inductive coupled plasma effectively grafts several different functional group types to CNT surfaces with scaling up potential to industrial scales (Felten et al., 2005). Many negative side effects, such as carbon matrix damage, lengthy process, and average shorter tube lengths are avoided (Naseh et al., 2010). The highly reactive surface without stable covalent bonds, however, also renders the surface highly susceptible to atmospheric oxidation (Khelifa et al., 2016).
Here, our FTIR time course study of plasma surface functionalization found that both MW-COOH and MW-NHx experienced significant atmospheric oxidation in laboratory storage conditions. Studies investigating aging of plasma-deposited polymers reported radical-induced autoxidation, which can occur over several weeks to months resulting in substantial addition of random carbon-oxygen functionalities. Whittle et al. (2000) reported that the loss of oxygen-associated peaks in different spectra analyses was due to the loss of low molecular weight oligomers. In addition, carbon–nitrogen bonds were highly susceptible to these reactions resulting in amides and/or rapid loss of nitrogen altogether (Gengenbach et al., 1996; Whittle et al., 2000). Autoxidation during the first few weeks results in degradation or transformation of the plasma-coated surface to hydroperoxides and, over long time scales, random and uncontrolled oxidation (Khelifa et al., 2016; Whittle et al., 2000). These authors suggested that small quantities of surface radicals on plasma treated surfaces may persist over longer time frames (months). Similar to our observation, Chang et al. (2016) showed that drastically different forms of sp2 carbon allotropes with unique surface properties converged over time with atmospheric aging resulting in similar surface properties. Thus, based on the inherent instability of the side wall functional groups, time associated with material characterization, and the long-term exposure experimental design, this study was not able to compare and assess neoplastic potential of freshly synthesized plasma fMWCNTs. We did observe a loss of C–H signatures within the FTIR spectra over time. This also coincided with an initial loss (0 vs. 2 month) and then a gain of C–O signature indicating that MWCNTs experienced biphasic autoxidation as the plasma functionalization became unstable. pMWCNTs also exhibited this phenomenon suggesting that argon plasma-synthesized MWCNTs are susceptible to oxidation over time (Vohrer et al., 2007).
Limited studies have evaluated the effects of atmospheric aging on toxicity of fCNTs. Liu et al. (2015) conducted controlled O3 and OH radical exposure studies over 12 days to evaluate atmospheric aging impacts on SWCNT cytotoxicity. Aged SWCNTs were found to experience random oxidation/reduction reactions, causing increase in surface carboxylic acids and esters with little change in redox or cytotoxic potential. Surprisingly, ambient urban air reduced toxicity through binding of organic vapors. Other recent atmospheric aging studies on CNTs, including MWCNTs indicated that differences in MWCNT diameter and surface morphology influence oxidation reaction kinetics during aging and may influence redox potential (Tsuruoka et al., 2015a,b).
Here, aged fMWCNTs did not vastly differ from aged pMWCNT in SAEC transformation ability since both fMWCNT lost a substantial amount of their functionalization during aging. All aged MWCNT exhibited similar short, thin, poly-dispersed fibers that coincided with a less potent transformation, which largely differed from our findings with long, thick, and more dispersed MWCNT-7 (Stueckle et al., 2017; Wang et al., 2014). This finding matches the established paradigm that increased length and dispersion of high aspect ratio (HAR) fibers increases their toxicity, cell transformation, and tumorigenic potential (Donaldson et al., 2013). Zeta potential differences among MWCNT particles with intrinsic negatively charged surfaces were minimized once in SAGM, suggesting that residual functionalization was minimized by protein corona effect and probably did not contribute to observed transformation differences. Similar dispersion characteristics for all tested fMWCNTs matched those previously reported (Jackson et al., 2015; Poulsen et al., 2016). In addition, both publications reported that their –OH and –COOH fMWCNTs possessed less oxygen than the pristine MWCNTs, indicating loss of surface functionalization. Of note, even though our particle stock solutions contained water instead of saline (Wang et al., 2014), the small difference in salt concentration (<0.3%) would be negligible over the course of an 8-week exposure given the presence of Survanta lung surfactant in both particle-free and exposed treatment groups. All fMWCNT stimulated cell proliferation rate, which partially correlated with increased mitochondrial metabolism and matches numerous reports that a low MWCNT dose stimulates epithelial cell proliferation associated with either wound healing or hyperplasia, an early indicator of neoplasia (Donaldson et al., 2013). UFCB- and pMWCNT-exposed cells exhibited increased mitochondrial activity, while all fMWCNTs possessed elevated proliferation rates. Similarly, these fMWCNTs, except MW-NHx, caused transient, particle- dependent invasion ability. These results coincide with published literature concerning low dose carbonaceous ENM stimulation of cell proliferation and invasion processes associated with wound healing via epithelial-mesenchymal transition. UFCB at low doses is known to stimulate cell signaling pathways, epithelial hyperplasia, ROS damage, inflammation, and is a suspected lung carcinogen (IARC, 2010; Kolling et al., 2011). Ozone oxidation is known to increase the redox activity of black soot and may increase its reactivity once exposed to biological systems (Li Q et al., 2013). Black carbon possesses higher amount of disorganized carbon, quinones, and metals, which possess high reactivity compared to graphene carbon (Li R et al., 2013). ASB-exposed cells exhibited reduced cell proliferation and no observable changes in cell mobility or soft agar colony formation ability. These results are consistent given that low-dose asbestos exposures result in DNA damage, cell cycle delay, and low soft agar colony formation in human SAECs over short exposure time frames (Kisin et al., 2011; Wang et al., 2014). Based on these studies, in vitro SAEC transformation does not predict asbestos-induced carcinogenesis. The decline in cell proliferation for all HAR fibers after 12 weeks exposure suggested potential accumulated genetic damage, primary cell senescence, and matched our previous findings with MWCNT-7s (Stueckle et al., 2017).
Conversely, MW-NHx-exposed cells showed significantly greater attachment-independent growth ability in soft agar, greater number of Type II foci, but reduced cell mobility, indicating a moderate cell transformation effect over short exposure time frame. Early in vivo lung neoplasia studies exhibit hyperplasia, defined borders, altered cell survival/death signaling, but minimal ability to invade neighboring tissue. Invasion ability is not a defining trait for neoplastic transformation, but an indicator of malignant transformation (Hanahan & Weinberg, 2011). A sister study conducted in parallel (Stueckle et al., 2017) found that long-term MWCNT-7 exposure on pSAEC resulted ~4-fold increase in soft agar colonies (~120 colonies per well) compared to MW-NHx-exposed cells. This indicates that MWCNT-7 are much more potent at cell transformation than all MWCNTs tested here. Since MWCNT-7 are well-dispersed in our dispersion preparation (Wang et al., 2014), MW-NHx agglomeration and reduced length may potentially reduce the ability to induce a strong neoplastic-like phenotype. Regardless, the ability of polydisperse MW-NHx to elevate cell proliferation, mitochondrial metabolism, Type II foci, and soft agar colony formation compared to UFCB, but similar to MWCNT-7, indicates that aged MW-NHx with low oxygen content possess the ability to induce human pSAEC transformation.
The mechanism by which aged MW-NHx caused increased cell transformation is currently unknown. It is possible that reduced oxygen content, compared to aged pMWCNT and MW-COOH, resulted in a more exposed carbon surface resulting in increased surface reactivity with biomolecules. This hypothesis is supported by UFCB and MWCNT-7 possessing relatively low oxygen content and induction of soft agar colony in pSAEC (Stueckle et al., 2017). Secondly, amine plasma coated surfaces are known to covalently bind and immobilize numerous biological molecules, including DNA (Khelifa et al., 2016), however similar fMWCNTs previously showed relatively little DNA breakage (Jackson et al., 2015). Enhanced lung surfactant and protein-binding in SAGM indicates that MW-NHx harbored greater ability to interact with biological macromolecules. Although a significant amount of aging occurred during the first two months of storage, our analysis indicates that each fMWCNT was still susceptible to atmospheric aging during the long term exposure. This suggests that aged MW-NHx harbored some residual surface reactivity that caused increased protein and biomolecule binding resulting in moderate cell transformation. Since no detectable differences were observed with dispersion/agglomeration or co-localization with pSAECs, enhanced proteinbinding ability may have had an impact once MW-NHx were internalized. In vivo pulmonary exposure to similar fMWCNT found that autoxidized amine-fMWCNTs elicited a stronger and persistent neutrophil response (Poulsen et al., 2016). These results suggest that aged MW-NHx harbor elevated pulmonary damage potential.
Conclusion
Laboratory aging of three different plasma fMWCNT resulted in autoxidation of surface functional groups resulting in a convergence of physicochemical properties over time. Eight week long term in vitro exposure of pSAECs to aged MW-NHx, with less surface oxygen resulted in a moderate cell transformation compared to pMWCNT and MW-COOH. Based on these results, further studies into how ENMs with similar surface modifications transform during their life cycle and how they impact biological response following exposure is important and warranted. As carbon ENMs move through their life cycle, incorporation of in vitro screening studies using primary human cells paired with physicochemical characterization can assist in identifying those materials with neoplastic transformation potential.
Supplementary Material
Acknowledgments
We kindly thank Dr. Afshin Tarat and Perpetuus Carbon Technologies for independent validation of MWCNT characterization. Vamsi Kodali kindly assisted with DTT surface reactivity determination.
Disclosure statement
The findings and conclusions in this article are those of the authors and do not necessarily represent the view of the National Institute for Occupational Safety and Health.
Funding
This study was supported by funds from the National Institute for Occupational Safety and Health/Nanotechnology Research Center, National Institutes of Health [R01-ES022968 and R01-EB018857], and National Science Foundation [CBET-1434503].
Footnotes
Supplemental data for this article can be accessed here.
References
- Alam A, Puri N, Saxena RK. Uptake of poly-dispersed single-walled carbon nanotubes and decline of functions in mouse NK cells undergoing activation. J Immunotox. 2016;13:758–65. doi: 10.1080/1547691X.2016.1191562. [DOI] [PubMed] [Google Scholar]
- Allegri M, Perivoliotisb DK, Bianchic MG, Chiua M, Pagliaroa A, Koklioti MA, et al. Toxicity determinants of multi-walled carbon nanotubes: the relationship between functionalization and agglomeration. Toxicol Rep. 2016;3:230–43. doi: 10.1016/j.toxrep.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H, Herzberg F, Schulte A, Kolossa-Gehring M. The carcinogenic potential of nanomaterials, their release from products and options for regulating them. Int J Hyg Environ Health. 2011;214:231–8. doi: 10.1016/j.ijheh.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Bishop LM, Orandle M, Cena L, Kodali V, Dahm M, Erdely, et al. The occupational life cycle of MWCNT: toxicity evaluation from as-produced to post-production modifications and composites. Toxicologist. 2016;3488:584. [Google Scholar]
- Chang YH, Olukan T, Lai CY, Marbou K, Apostoleris HN, Ghaferi AA, et al. Divergent surface properties of multidimensional sp (2) carbon allotropes: the effect of aging phenomena. Nanotechnology. 2016;27:295701. doi: 10.1088/0957-4484/27/29/295701. [DOI] [PubMed] [Google Scholar]
- Chidawanyika W, Nyokong T. Characterization of amine-functionalized single walled carbon nanotube-low symmetry phthalocyanine conjugates. Carbon. 2010;48:2831–8. [Google Scholar]
- Coccini T, Manzo L, Roda E. Safety evaluation of engineered nanomaterials for health risk assessment: an experimental tiered testing approach using pristine and functionalized carbon nanotubes. ISRN Toxicol. 2013;2013:825427. doi: 10.1155/2013/825427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creton S, Aardema MJ, Carmichael PL, Harvey JS, Martin FL, et al. Cell transformation assays for prediction of carcinogenic potential: state of the science and future research needs. Mutagenesis. 2012;27:93–101. doi: 10.1093/mutage/ger053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm MM, Evans DE, Schubauer-Berigan MK, Newbold RF, O'Donovan MR, Pant K, et al. Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers: mobile direct-reading sampling. Ann Occup Hyg. 2012;57:328–44. doi: 10.1093/annhyg/mes079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandley EC, Taylor AJ, Duke KS, Ihrie MD, Shipkowski KA, Parsons GN, et al. Atomic layer deposition coating of carbon nanotubes with zinc oxide causes acute phase immune responses in human monocytes in vitro and in mice after pulmonary exposure. Part Fibre Toxicol. 2016;13:29. doi: 10.1186/s12989-016-0141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsyuk V, Kalva M, Papagelis K, Parthenios J, Tasis D, Siokou A, et al. Chemical oxidation of multiwalled carbon nanotubes. Carbon. 2008;46:833–40. [Google Scholar]
- Donaldson K, Poland CA, Murphy FA, MacFarlane M, Chernova T, Schinwald A. Pulmonary toxicity of carbon nanotubes and asbestos – similarities and differences. Adv Drug Deliv Rev. 2013;65:2078–86. doi: 10.1016/j.addr.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Felten A, Bittencourt C, Pireaux JJ, Van Lier G, Charlier JC. Radio-frequency plasma functionalization of carbon nanotubes surface O2, NH3, and CF4 treatments. J Appl Phys. 2005;98:074308. [Google Scholar]
- Gengenbach TR, Chatelier RC, Griesser HJ. Characterization of the ageing of plasma-deposited polymer films: global analysis of X-ray photoelectron spectroscopy data. Surf Interface Anal. 1996;24:271–81. [Google Scholar]
- Grosse Y, Loomis D, Guyton KZ, Lauby-Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol. 2014;15:1427–8. doi: 10.1016/S1470-2045(14)71109-X. [DOI] [PubMed] [Google Scholar]
- Hamilton RF, Wu Z, Mitra S, Shaw PK, Holian A. Effect of MWCNT size, carboxylation, and purification on in vitro and in vivo toxicity, inflammation and lung pathology. Part Fibre Toxciol. 2013a;10:57. doi: 10.1186/1743-8977-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RF, Xiang CC, Li M, Ka I, Yang F, Ma D, et al. Purification and sidewall functionalization of multiwalled carbon nanotubes and resulting bioactivity in two macrophage models. Inhal Toxicol. 2013b;25:199–210. doi: 10.3109/08958378.2013.775197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hedmer M, Isaxon C, Nilsson PT, Ludvigsson L, Messing ME, Genberg J, et al. Exposure and emission measurements during production, purification, and functionalization of arc-discharge-produced multi-walled carbon nanotubes. Ann Occup Hyg. 2014;58:355–79. doi: 10.1093/annhyg/met072. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) Carbon Black. IARC Monographs. 2010;93:1–149. [Google Scholar]
- Jackson P, Kling K, Jensen KA, Clausen PA, Madsen AM, Wallin H, Vogel U. Characterization of genotoxic response to 15 multiwalled carbon nanotubes with variable physicochemical properties including surface functionalizations in the FE1-Muta(TM) mouse lung epithelial cell line. Environ Mol Mutagen. 2015;56:183–203. doi: 10.1002/em.21922. [DOI] [PubMed] [Google Scholar]
- Jain S, Thakare VS, Das M, Godugu C, Jain AK, Mathur R, et al. Toxicity of multiwalled carbon nanotubes with end defects critically depends on their functionalization density. Chem Res Toxicol. 2011;24:2028–39. doi: 10.1021/tx2003728. [DOI] [PubMed] [Google Scholar]
- Kasai T, Umeda Y, Ohnishi M, Mine T, Kondo H, Takeuchi T, et al. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part Fibre Toxicol. 2016;13:53. doi: 10.1186/s12989-016-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelifa F, Ershov S, Habibi Y, Snyders R, Dubois P. Free-radical-induced grafting from plasma polymer surfaces. Chem Rev. 2016;116:3975–4005. doi: 10.1021/acs.chemrev.5b00634. [DOI] [PubMed] [Google Scholar]
- Kim J. Carbon Nanotubes for Polymer Reinforcement. Boca Raton, FL: CRC Press; 2011. Functionalization of Carbon Nanotubes. [Google Scholar]
- Kisin ER, Murray AR, Sargent L, Lowry D, Chirila M, Siegrist KJ, et al. Genotoxicity of carbon nanofibers: are they potentially more or less dangerous than carbon nanotubes or asbestos? Toxicol Appl Pharmacol. 2011;252:1–10. doi: 10.1016/j.taap.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling A, Ernst H, Rittinghausen S, Heinrich U. Relationship of pulmonary toxicity and carcinogenicity of fine and ultrafine granular dusts in a rat bioassay. Inhal Toxicol. 2011;23:544–54. doi: 10.3109/08958378.2011.594458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel ED, Jaurand MC, Møller P, Morimoto Y, Kobayashi N, Pinkerton KE, et al. Evaluating the mechanistic evidence and key data gaps in assessing the potential carcinogenicity of carbon nanotubes and nanofibers in humans. Crit Rev Toxicol. 2017;47:1–58. doi: 10.1080/10408444.2016.1206061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Cushing SK, Zhou JX, Guo SW, Wu NQ. Fingerprinting photoluminescence of functional groups in graphene oxide. J Mater Chem. 2012;22:23374–9. [Google Scholar]
- Li Q, Shang J, Zhu T. Physicochemical characteristics and toxic effects of ozone-oxidized black carbon particles. Atmos Environ. 2013;81:68–75. [Google Scholar]
- Li R, Wang X, Ji Z, Sun B, Zhang H, Chang CH, et al. Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano. 2013;7:2352–68. doi: 10.1021/nn305567s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liggio J, Li SM, Breznan D, Vincent R, Thomson EM, et al. Chemical and toxicological evolution of carbon nanotubes during atmospherically relevant aging processes. Environ Sci Technol. 2015;49:2804–14. doi: 10.1021/es505298d. [DOI] [PubMed] [Google Scholar]
- Lowry GV, Gregory KB, Apte SC, Lead JR. Transformations of nanomaterials in the environment. Environ Sci Technol. 2012;46:6893–9. doi: 10.1021/es300839e. [DOI] [PubMed] [Google Scholar]
- Mackevica A, Hansen SF. Release of nanomaterials from solid nanocomposites and exposure assessment – a foreward-looking review. Nanotoxicology. 2016;10:641–53. doi: 10.3109/17435390.2015.1132346. [DOI] [PubMed] [Google Scholar]
- Mishra A, Rojanasakul Y, Chen BT, Castranova V, Mercer RR, Wang L. Assessment of pulmonary fibrogenic potential of multiwalled carbon nanotubes in human lung cells. J Nanomater. 2012;2012:930931. [Google Scholar]
- Mitrano DM, Motellier S, Clavagueara S, Nowack B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ Int. 2015;77:132–47. doi: 10.1016/j.envint.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Mrakovcic M, Meindl C, Leitinger G, Roblegg E, Frohlich E. Carboxylated short single-walled carbon nanotubes but not plain and multi-walled short carbon nanotubes show in vitro genotoxicity. Toxicol Sci. 2015;144:114–27. doi: 10.1093/toxsci/kfu260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseh MV, Khodadadi AA, Mortazavi Y, Pourfayaz F, Alizadeh O, Maghrebi M. Fast and clean functionalization of carbon nanotubes by dielectric barrier discharge plasma in air compared to acid treatment. Carbon. 2010;48:1369–79. [Google Scholar]
- NCL NIEHS. NCL-NIEHS201211A. Frederick, MD: Nanotechnology Characterization Laboratory, Frederick National Laboratory for Cancer Research; 2012. Characterization Data for Multi-Walled Carbon Nanotubes. [Google Scholar]
- OECD. Series on Testing and Assessment, No. 31. OECD. Paris, France: OECD Environment, Health and Safety Publications; 2007. Detailed Review Paper on Cell Transformation Assays for Detection of Chemical Carcinogens; pp. 1–164. [Google Scholar]
- Patlolla AK, Hussain SM, Schlager JJ, Patolla S, Tchounwou PB. Comparative study of the clastogenicity of functionalized and non-functionalized multi-walled carbon nanotubes in bone marrow cells of Swiss-Webster mice. Environ Toxicol. 2010;25:608–21. doi: 10.1002/tox.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen EJ, Zhang L, Mattison NT, O’Carroll DM, Whelton AJ, O'Carroll DM, et al. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ Sci Technol. 2011;45:9837–56. doi: 10.1021/es201579y. [DOI] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Chen BT, McKinney W, Mercer RR, Wolfarth MG, et al. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes. Nanotoxicology. 2013;7:1179–94. doi: 10.3109/17435390.2012.719649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen SS, Jackson P, Kling K, Knudsen KB, Skaug V, Kyjovska ZO, et al. Multi-walled carbon nanotube physicochemical properties predict pulmonary inflammation and genotoxicity. Nanotoxicology. 2016;10:1263–75. doi: 10.1080/17435390.2016.1202351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JR, Mercer RR, Stefaniak AB, Seehra MS, Geddam UK, Chaudhuri IS, et al. Evaluation of pulmonary and systemic toxicity following lung exposure to graphite nanoplates: a member of the graphene-based nanomaterial family. Part Fibre Toxicol. 2016;13:34. doi: 10.1186/s12989-016-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roda E, Coccini T, Acerbi D, Barni S, Vaccarone R, Manzo L. Comparative pulmonary toxicity assessment of pristine and functionalized multi-walled carbon nanotubes intratracheally instilled in rats: Morphohistochemical evaluations. Histol Histopath. 2011;26:357–67. doi: 10.14670/HH-26.357. [DOI] [PubMed] [Google Scholar]
- Sager TM, Wolfarth MW, Andrew M, Hubbs A, Friend S, Chen TH, et al. Effect of multi-walled carbon nanotube surface modification on bioactivity in the C57BL/6 mouse model. Nanotoxicology. 2014;8:317–27. doi: 10.3109/17435390.2013.779757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent LM, Porter DW, Staska LM, Hubbs A, Lowry D, Battelli L, et al. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 2014;11:3. doi: 10.1186/1743-8977-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena RK, Williams W, Mcgee JK, Daniels MJ, Boykin E, Gilmour I. Enhanced in vitro and in vivo toxicity of poly-dispersed acid-functionalized single-wall carbon nanotubes. Nanotoxicology. 2007;4:291–300. [Google Scholar]
- Sayes CM, Liang F, Hudson JL, Mendez J, Guo W, Beach JM, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett. 2006;161:135–42. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Siegrist KJ, Reynolds SH, Kashon ML, Lowry DT, Dong C, Hubbs AF, et al. Genotoxicity of multi-walled carbon nanotubes at occupationally relevant doses. Part Fibre Toxicol. 2014;11:6. doi: 10.1186/1743-8977-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueckle TA, Davidson DC, Derk R, Kornberg TG, Schwegler-Berry D, Pirela SV, et al. Evaluation of tumorigenic potential of CeO2 and Fe2O3 engineered nanoparticles by a human cell in vitro screening model. NanoImpact. 2017;6:39–54. doi: 10.1016/j.impact.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruoka S, Matsumoto H, Koyama K, Akiba E, Yanagisawa T, Cassee FR, et al. Radical scavenging reaction kinetics with multiwalled carbon nanotubes. Carbon NY. 2015a;83:232–9. doi: 10.1016/j.carbon.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruoka S, Matsumoto H, Castranova V, Porter DW, Yanagisawa T, Saito N, et al. Differentiation of chemical reaction activity of various carbon nanotubes using redox potential: classification by physical and chemical structures. Carbon NY. 2015b;95:302–8. doi: 10.1016/j.carbon.2015.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini CL, Cavallo D, Fresegna AM, Ciervo A, Maiello R, Buresti G, et al. Comparative cyto-genotoxicity assessment of functionalized and pristine multiwalled carbon nanotubes on human lung epithelial cells. Toxicol In Vitro. 2012;26:831–40. doi: 10.1016/j.tiv.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Ursini CL, Maiello R, Ciervo A, Fresegna AM, Buresti G, Casciardi S, et al. Evaluation of uptake, cytotoxicity and inflammatory effects in respiratory cells exposed to pristine and –OH and –COOH functionalized multi-wall carbon nanotubes. J Appl Toxicol. 2016;36:374–403. doi: 10.1002/jat.3228. [DOI] [PubMed] [Google Scholar]
- Vohrer U, Zschoerper NP, Koehne Y, Langowski S, Oehr C. Plasma modification of carbon nanotubes and bucky papers. Plasma Process Polym. 2007;4:S871–S877. [Google Scholar]
- Wang L, Stueckle TA, Mishra A, Meighan T, Castranova V, Rojanasakul Y. Neoplastic-like transformation effect of single-walled and multi-walled carbon nanotubes vs. asbestos in human small airway epithelial cells. Nanotoxicology. 2014;8:485–507. doi: 10.3109/17435390.2013.801089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle JD, Short RD, Douglas CWI, Davies J. Differences in the aging of allyl alcohol, acrylic acid, allyamine, and octa-1,7-diene plasma polymers as studied by X-ray photoelectron spectroscopy. Chem Mater. 2000;12:2664–71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.