Abstract
Background
Widespread use of the HPV vaccine has the potential to reduce incidence from HPV-associated cancers. However, vaccine uptake among adolescents remains well below the Healthy People 2020 targets. The Centers for Disease Control and Prevention (CDC)’s National Comprehensive Cancer Control Program awardees (NCCCP) are well positioned to work with immunization programs to increase vaccine uptake.
Methods
CDC’s chronic disease management information system was queried for objectives and activities associated with HPV vaccine that were reported by NCCCP awardees from 2013 – 2016 as part of program reporting requirements. A content analysis was conducted on the query results to categorize interventions according to strategies outlined in The Guide to Community Preventive Services and the 2014 President’s Cancer Panel report.
Results
Sixty-two percent of NCCCP awardees had planned or implemented at least one activity since 2013 to address low HPV vaccination coverage in their jurisdictions. Most NCCCP awardees (86%) reported community education activities, while 65% reported activities associated with provider education. Systems-based strategies such as client reminders or provider assessment and feedback were each reported by less than 25% of NCCCP awardees.
Conclusion
Many NCCCP awardees report planning or implementing activities to address low HPV vaccination coverage, often in conjunction with state immunization programs. NCCCP awardees can play a role in increasing HPV vaccination coverage through their cancer prevention and control expertise and access to partners in the health care community.
Keywords: human papillomavirus vaccine, Public Health Practice, neoplasms/prevention and control, cancer control, Papillomavirus Vaccines/organization and administration
Introduction
Over 10 years have passed since the first human papilloma virus (HPV) vaccine was licensed and recommended by the Advisory Council for Immunization Practices (ACIP) in the United States to prevent cervical cancer and other HPV-related outcomes.1 Vaccine uptake for adolescent girls and boys aged 13 – 17 years lags behind those for other adolescent vaccines such as vaccines for tetanus, diphtheria, and pertussis; and meningococcal meningitis.2 In 2015, only about 42% of adolescent girls and 28% of adolescent boys had received all three HPV vaccine doses recommended, while coverage with one or more vaccine doses was 63% for adolescent girls and nearly 50% for adolescent boys.2 Coverage for adolescent boys lags behind girls because ACIP did not recommend routine vaccination of boys until 2011.3
Nearly 31,000 newly diagnosed cancer cases each year may be attributable to any HPV type.4 Fewer cases are diagnosed in men, but they experience a greater burden of oropharyngeal cancers each year than women.4 Among women, cervical cancer is the leading HPV-attributable cancer, followed by anal, vulvar, oropharyngeal, vaginal, and rectal cancers.4 Overall, nearly 12,000 women are diagnosed each year with cervical cancer, and over 4,000 women die of the disease.5 Although screening for cervical cancer using the Papanicolaou (Pap) test has dramatically reduced the cervical cancer burden in the US over the past several decades, over half of cervical cancer cases are among women who have never or rarely been screened.6–8 Cervical cancer rates also vary by race/ethnicity and other demographic factors, and tend to be highest for Hispanic and African American women and women living in the southern US.4, 5 Widespread use of the HPV vaccine has the potential to reduce incidence and mortality from HPV-associated cancers, and narrow absolute differences in HPV-associated cancer incidence rates among Hispanic and African American women compared to non-Hispanic white women.9
In recent years, the Centers for Disease Control and Prevention (CDC) has worked to raise awareness of persistently low HPV vaccine uptake and address some of the issues that research has identified as contributing to low vaccine uptake, such as the lack of a strong recommendation by many health care providers or a risk-based approach toward vaccination; lack of awareness by parents and concerns about vaccine safety, or a desire to delay vaccination until older adolescence; and missed opportunities to administer the vaccine.10–16 The 2014 President’s Cancer Panel Report17 highlighted similar barriers to HPV vaccine uptake, and provided several key goals and objectives to focus intervention efforts as a way to accelerate vaccine uptake. These initiatives have brought about prioritization of HPV vaccine interventions and new opportunities for collaboration across CDC and among other government agencies and organizations. One example includes collaboration between CDC’s National Comprehensive Cancer Control Program (NCCCP) and the National Center for Immunization and Respiratory Diseases (NCIRD).
CDC’s NCCCP funds all 50 states, the District of Columbia, seven tribes/tribal organizations, and 7 Pacific Island Jurisdictions and territories for comprehensive cancer control (CCC) activities (http://www.cdc.gov/cancer/ncccp/index.htm). Priorities of the NCCCP include primary prevention, secondary prevention and screening, and cancer survivorship, along with cross-cutting areas that include health equity, data and evaluation, and policy, systems, and environmental change approaches (http://www.cdc.gov/cancer/ncccp/about.htm). The CCC approach is built on the premise that bringing together cancer control stakeholders to collectively address the cancer burden in a jurisdiction creates synergy and allows for the leveraging of resources.18 NCCCP awardees create and implement data-informed cancer plans in collaboration with their CCC coalition or partnership. CCC coalition partners often include academic and medical partners, health systems, community-based organizations, businesses, other governmental organizations, and other health department divisions, such as NCIRD awardees. NCIRD provides funding to all 50 states, the District of Columbia, eight Pacific Island Jurisdictions and other territories, and selected city health departments (http://www.cdc.gov/vaccines/imz-managers/awardee-imz-websites.html) to administer the Vaccines for Children program, section 317 immunization grants, support immunization information systems, provide training and education to health professionals, and improve the uptake and delivery of vaccines.
Some awardees of both the NCIRD and NCCCP have worked together to add an objective to jurisdictional cancer plans about implementing interventions to promote HPV vaccine uptake. The purposes of this report are to identify which NCCCP awardees have recently been involved in interventions to promote HPV vaccine uptake and to describe the types of interventions they have implemented. This knowledge will help identify which intervention areas NCCCP awardees can be most effective in implementing and identify areas of the US in which collaboration between NCIRD and NCCCP awardees could be enhanced to address the burden of HPV-associated cancers.
Materials and Methods
Data source and search strategy
We queried CDC’s Web-based chronic disease management information system (CDMIS) for NCCCP awardee action plans containing objectives, interventions, or activities to promote uptake of HPV vaccine (http://www.cdc.gov/cdmis/index.html). CDMIS is a secure government system that meets all federal requirements and approvals for public data collection (OMB #0920-0841). CDMIS collects detailed information on priority objectives and interventions included in an action plan section. The action plan is a requirement of the cooperative agreement, and describes program implementation within a given budget year. All NCCCP awardees complete action plans each year that contain the long-term (i.e. five year) objectives in their cancer plans, annual objectives to achieve the five-year ones, and activities to achieve the annual objectives. NCCCP awardees are also required to report progress on objectives as a condition of funding. The terms “HPV and vaccination” or “HPV and vaccine” were used to conduct key word searches. We also searched for the terms “vaccination: HPV” or “immunization,” which are associated with long-term objectives. To coincide with the most recent funding period (2012 – 2017) and to capture more current HPV vaccine activities, we limited our queries to the time period covering 2013 – 2016, which represents the full programmatic implementation phase.
Data analysis
We downloaded the query results into Microsoft Excel for further qualitative content analyses. We removed content from the analysis that did not pertain to planning or implementation of activities or interventions for promoting HPV vaccine use. We developed a codebook to assist with coding HPV vaccine content according to the Guide for Community Preventive Services (The Community Guide) strategies for increasing vaccination coverage, and other categories associated with common public health activities in the NCCCP (e.g. planning interventions or convening partners). Content was also coded according to priority areas identified in the 2014 President’s Cancer Panel Report, and we developed code definitions based on the strategies outlined within the priority areas. We reviewed each objective and associated text fields describing the public health impact of the objective, as well as narrative descriptions of progress toward meeting the objective and the types of activities or partners involved. We coded multi-component interventions to multiple categories, and many programs conducted intervention planning prior to implementation over the time period. In some instances, objectives required more information in order to properly categorize the specific strategy or intervention. Therefore, we reviewed products (e.g., PowerPoint presentations, fact sheets, toolkits) uploaded to CDMIS, or appearing either on the NCCCP awardee’s or their coalition’s website. SAS version 9 (SAS Institute Inc., Cary, NC) was used to calculate frequency counts and percentages for each coded category.
Data visualization
In order to provide local context for initiatives undertaken by NCCCP awardees, HPV vaccination coverage and the burden of cervical cancer (most are attributed to HPV) was downloaded from the National Immunization Survey (NIS)-Teen and the United States Cancer Statistics (USCS), respectively. Vaccine coverage with one or more doses among adolescent females aged 13 – 17 in 2015,2 and cervical cancer incidence rates among women of all ages from 2009 – 2013 were downloaded from these sources.19 We created maps in ArcGIS software (Esri, Redlands, CA) to show 1) adolescent female HPV vaccination coverage and 2) cervical cancer incidence rates among all women; both were layered with information about NCCCP awardees implementing HPV-related interventions.
Results
Overall, 62% of all NCCCP awardees had planned or implemented at least one activity since 2013 to address low HPV vaccination coverage (Table 1). NCCCP awardees in the Midwest more frequently reported HPV vaccine activities and interventions (28%), followed by those in the South (23%, Table 1). Although most activities were reported by states, eight tribal organizations and Pacific Island Jurisdictions/territories also engaged in HPV-related activities (Table 1).
Table 1.
Characteristics of Comprehensive Cancer Control Programs with HPV vaccine objectives or interventions in their action plans, 2013 – 2016
| Characteristic | Number of Programs (%) |
|---|---|
| One or more objectives or interventions to promote HPV vaccination | 43 (62.3) |
| Jurisdictional setting | |
| State | 35 (81.4) |
| Territory/Pacific Island Jurisdiction | 5 (11.6) |
| Tribe/Tribal Organization | 3 (7.0) |
| Census region | |
| Northeast | 7 (16.3) |
| Midwest | 12 (27.9) |
| South | 10 (23.3) |
| West | 9 (20.9) |
| Territory/Pacific Island Jurisdiction | 5 (11.6) |
| Prevention and Public Health Funding to the immunization program | 16 (37.2) |
| Collaboration with immunization program | 35 (81.4) |
| Intervention typea | |
| Provider education | 28 (65.1) |
| Provider reminders | 3 (7.0) |
| Provider assessment and feedback/quality improvement | 10 (23.3) |
| Client reminders | 8 (18.6) |
| Community education | 37 (86.0) |
| School-based vaccination programs | 5 (11.6) |
| Partnership building | 10 (23.3) |
| Planning | 19 (44.2) |
| Other | 9 (20.9) |
| Multicomponent strategyb | 29 (67.4) |
Some CCC programs reported more than one intervention type, or conducted planning activities before implementing interventions over the time period. Therefore, categories are not mutually-exclusive.
Two or more intervention types were used (e.g. community education along with client reminders)
The majority of NCCCP awardees (86%) reported engaging in activities to educate the community, particularly parents, about the need for HPV vaccine (Table 1). Sixty-five percent of NCCCP awardees reported activities associated with provider education. Intervention planning before implementing interventions was reported by 44% of NCCCP awardees at some point during the time period. Relatively fewer awardees reported interventions that addressed provider reminders (7%), provider assessment and feedback or quality assurance (23%), client reminders (19%), or school-based vaccination (12%). Most NCCCP awardees used multiple strategies to conduct HPV interventions.
Nearly all NCCCP awardees engaged in HPV-related work reported interventions or activities that aligned with increasing vaccine acceptance (86%, Table 2). Over half of those described activities to address missed opportunities for vaccination (56%), and few reported interventions to maximize access to vaccination (23%). Examples of activities to increase vaccine acceptance included comprehensive media or communications campaigns, and training school nurses to provide education to parents or students about the vaccine. Interventions focused on reducing missed opportunities to vaccinate included provider education on making a strong vaccine recommendation to parents, and minimizing other missed opportunities to provide the vaccine by co-administering it with other adolescent vaccines, and taking advantage of other clinical encounters, such as sports physicals or back-to-school checkups. Some NCCCP awardees described efforts to implement client reminder systems within partner health systems or insurance companies, or through the state immunization information system. Provider reminder systems were another intervention to reduce missed opportunities. Examples of interventions to maximize access to vaccination services included school-based vaccination clinics, and educating college students about the HPV vaccine and its availability in the college health clinic.
Table 2.
HPV vaccine goal areas along with examples and associated objectives from Comprehensive Cancer Control (CCC) action plans, 2013 – 2016, n=43
| Goals from President’s Cancer Panel Report |
Number of Programs, (%) |
Example Intervention/activity |
Associated Objective from CCC Action Plan |
|---|---|---|---|
| Address missed opportunities for vaccination | 24 (55.8) | Client reminders sent to patients seen at primary care clinics | “Increase the number of client reminders distributed to female and male adolescents ages 11–26, who are eligible patients at the seven primary care partner health clinics and are eligible for HPV vaccination…” |
| Peer-to-peer provider education on effective communication strategies to make strong vaccine recommendations and co administration of HPV vaccine with other adolescent vaccines | “Increase the number of providers completing the HPV peer-to-peer training curriculum…” | ||
| Maximize access to vaccination services | 10 (23.3) | Special school-based immunization clinics combined with HPV vaccine education at the local level | “Increase the number of cumulative HPV interventions implemented…” |
| HPV vaccine education and catch-up vaccination on college campuses through health clinics | “Increase the percent of vaccinations administered by an in state university's student health center…” | ||
| Increase vaccine acceptance | 37 (86.0) | Multi-year, ongoing communications campaign aimed at providers and parents | “Maintain the number of communications campaigns, aimed to increase awareness of HPV vaccination among parents and providers…” |
| School nurses trained in the prevention of cervical cancer and HPV vaccine | “Increase the number of educational workshops to promote human papillomavirus (HPV) vaccinations…” |
Engagement in HPV-related activities was not entirely dependent on vaccination coverage (Figure 1) or cervical cancer incidence rates (Figure 2). Several NCCCP awardees within states that have relatively high HPV vaccination coverage for the first dose among adolescent females and those with the lowest coverage engaged in HPV-related activities (Figure 1). Although a number of NCCCP awardees in states with comparatively higher cervical cancer incidence rates reported activities to increase HPV vaccination coverage, some states with similarly high rates did not have any HPV-related activities (Figure 2).
Figure 1.
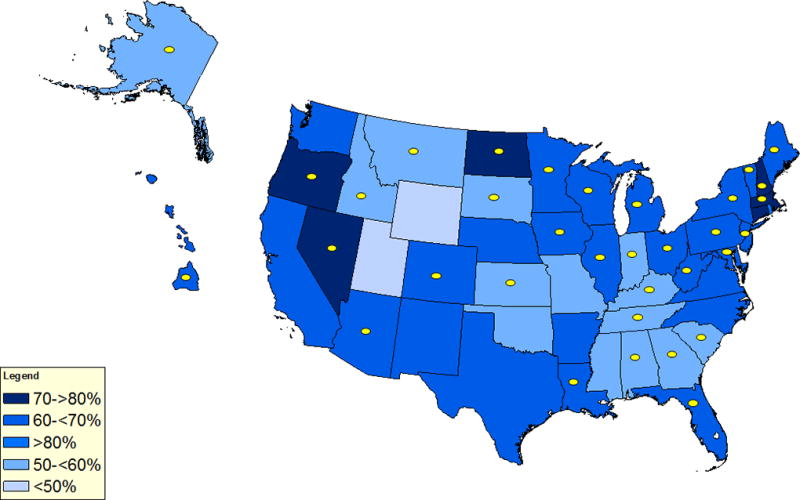
Vaccination coverage with 1 or more doses of HPV vaccine among adolescent females aged 13 – 17 years and state Comprehensive Cancer Control Programs implementing activities to promote HPV vaccination. Note: HPV vaccination coverage data are from the 2015 National Immunization Survey – Teen. State National Comprehensive Cancer Control Program (NCCCP) awardees reporting activities to promote HPV vaccination are noted with yellow dots. Not shown on the map are five United States-affiliated Pacific Island Jurisdictions and other territories and three Tribal Nations and organizations who reported activities. Activities were reported by 43 out of 69 (62%) of NCCCP awardees.
Figure 2.

Cervical cancer incidence rates among women of all ages from 2009 – 2013 and state Comprehensive Cancer Control Programs implementing activities to promote HPV vaccination. Note: Cervical cancer incidence rates are per 100,000 population and age-adjusted to the 2000 US standard population (19 age groups: <1, 1 – 4, 5 – 9,…, 80 – 84, 85+). Data source: CDC's National Program of Cancer Registries Cancer Surveillance System (NPCR-CSS) November 2015 data submission and the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program November 2015 submission as published in United States Cancer Statistics. Data are available at https://www.statecancerprofiles.cancer.gov/index.html.
State National Comprehensive Cancer Control Program (NCCCP) awardees reporting activities to promote HPV vaccination are noted with yellow dots. Not shown on the map are five United States-affiliated Pacific Island Jurisdictions and other territories and three Tribal Nations and organizations who reported activities. Activities were reported by 43 out of 69 (62%) of NCCCP awardees.
Discussion
Objectives and activities to increase HPV vaccine uptake were common in NCCCP awardees’ action plans over the 2013 – 2016 time period. These activities coincided with a large national effort to increase HPV vaccine uptake, as well as new funding opportunities to promote HPV vaccine, such as through Prevention and Public Health Funding (PPHF) for select immunization programs (https://pphf.hhs.gov/jsp/about.jsp). NCCCP awardees in states with PPHF-funded immunization programs more frequently reported multicomponent strategies, such as both public and provider education, and strategies directed at systems change (e.g. routine use of client reminders). However, some NCCCP awardees relied on either community education or provider education alone as a strategy, neither of which are recommended by The Community Guide as single intervention strategies due to insufficient evidence of effectiveness.20 Although large scale media campaigns can result in a temporary increase in vaccination coverage rates, they may not result in sustained progress.21 However, some recent studies have found patient education implemented alone in community settings to be effective at increasing coverage for HPV vaccine and series completion.22–24
NCCCP awardees frequently reported planning interventions in collaboration with immunization programs. Increasingly, partnerships are critical in public health to successfully implement and sustain interventions. In the Six Components Necessary for Effective Public Health Program Implementation, partnerships are identified as a key component of implementation.25 When partnership building is successful and done through a mutually beneficial approach with shared vision and goals and collaborative decision-making, then synergy can arise for sustainable interventions.26–28 Practically speaking, the right partners can bring expertise, resources (e.g. financial, donated staff time), and connections to other partners and sectors. In the case of efforts to increase HPV vaccine uptake, NCCCP awardees are logical partners for immunization programs not only because of the cancer prevention focus of the vaccine, but because they have connections to cancer coalitions that includes additional partners in the cancer community who can bring expertise and new avenues for interventions.
These findings reveal that some NCCCP awardees in states with historically low HPV vaccination coverage, such as Alaska, Kansas, New Jersey, and Tennessee, are implementing activities such as community or provider education.29 In some instances, states with low HPV vaccination coverage are also states with high cervical cancer incidence and mortality, so directing public health efforts toward this area may be a strategic way to address the burden of cervical cancer and other HPV-associated cancers. For example, HPV vaccination coverage is typically lower in the South while cervical cancer incidence and death rates are higher in this geographic area compared to other US regions; therefore, encouraging NCCCP awardees in southern states to implement interventions may be a promising strategy to lower incidence and death rates.29, 30 However, some states with high cervical cancer burden reported no interventions to increase HPV vaccine uptake, representing a missed opportunity to further reduce disparities for a preventable disease.31 Rates of HPV vaccine initiation do tend to be higher among Hispanic and African American adolescent girls, and adolescents of lower socioeconomic status, so ensuring these adolescents receive timely reminders to complete the vaccination series may reduce existing cervical cancer disparities.2 Thus, sustained, multicomponent interventions utilizing immunization and cancer control partners may have the most impact over time.20, 22
In recent years, NCIRD has developed numerous resources to promote HPV vaccine that are directed to both providers and parents of adolescents (http://www.cdc.gov/hpv/). Provider resources include You are the Key to HPV Cancer Prevention continuing education series, factsheets, posters, and other resources (e.g. radio PSAs and video content). Resources were also developed to engage with HPV vaccination partners, including an HPV Vaccination Partner Toolkit that includes print materials, video and audio content, information on quality improvement and evidence-based interventions, and ideas for developing partner networks and engaging local partners.
Resources from other national efforts have supported NCCCP awardees to implement interventions along with their immunization partners. The CCC National Partners (http://www.cccnationalpartners.org/), which also include the National Cancer Institute (NCI) and the American Cancer Society (ACS), have prioritized promoting HPV vaccine. NCI has funded comprehensive cancer centers to conduct an environmental scan of HPV vaccine activities in their states, and to enhance linkages with coalitions and immunization and cancer control programs in their areas (http://healthcaredelivery.cancer.gov/hpvuptake/). CDC’s Division of Cancer Prevention and Control (DCPC) and the Immunization Services Division funded the ACS to develop and administer the National HPV Vaccination Roundtable (http://www.cancer.org/healthy/informationforhealthcareprofessionals/nationalhpvvaccinationroundtable/), a coalition of public, private, and voluntary organizations with expertise relevant to increasing HPV vaccination coverage in the US, as a way to reduce illness and death from HPV-associated cancers, through coordinated leadership, strategic planning, and advocacy. Finally, CDC’s DCPC has funded and administered a national educational awareness campaign since 2009, Inside Knowledge: Get the Facts About Gynecologic Cancer, which includes specific messages on HPV vaccination as prevention for cervical, vaginal, and vulvar cancers.32
Our study has limitations. We relied on data reported by the program director or an individual or individuals to complete the tasks. NCCCP awardees vary in providing details that describe their activities and interventions; therefore, some interventions may not have been accurately classified because of data limitations. Additionally, not all NCCCP awardees with HPV vaccine-related interventions reported them for each year in our study period, and most systems-based interventions or interventions to maximize access to HPV vaccine were limited to a few local areas or provider practices and were not implemented population wide. In terms of the data search strategy, we focused on the most common terms that would yield relevant content; this could have limited our system’s search capabilities to capture all related interventions. Also, the use of one reviewer to review and code content may have been a limitation; however, the straightforward nature of this analysis has allowed for successful coding with one reviewer in previous similar studies using this data source.33, 34 One strength of our study is that CDMIS allows for current monitoring of activities and objectives which are captured in a fairly standardized manner.
Conclusion
It is advantageous for immunization programs and NCCCP awardees to collaborate on the goal of increasing HPV vaccine uptake. NCCCP awardees can leverage their network of partners and coalition members to plan activities, disseminate information, and implement interventions. It may be useful to define specific roles for NCCCP awardees, particularly in implementing multicomponent interventions, to avoid duplication of effort. While working in the vaccine arena, NCCCP awardees may have challenges, such as parental concerns about vaccine safety or early initiation of sexual activity among their children if they get vaccinated, or resistance by management or stakeholders who may not see the value in promoting HPV vaccine. Regular training by national organizations and ongoing dialogue with immunization partners about how to best address emerging issues may be helpful in these areas. NCCCP awardees in areas with a high cervical cancer burden may want to consider implementing multicomponent HPV vaccine interventions that include client reminders or provider assessment and feedback, and evaluate promising practices such as offering catchup HPV vaccination or systems changes that include adding the HPV vaccine to lists of recommended adolescent vaccines that may be provided by school nurses, athletic departments or camps. The goal is to raise HPV vaccination coverage to the level of other adolescent vaccines, and normalize HPV vaccine as a routine adolescent vaccine. NCCCP awardees can play a role through their cancer prevention and control expertise and access to partners in the health care community.
Acknowledgments
The authors would like to acknowledge Dr. Mary Puckett for her development of the cervical cancer and HPV vaccine maps. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 2.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years - United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016;65:850–858. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- 3.Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb. Mortal. Wkly. Rep. 2011;60:1705–1708. [PubMed] [Google Scholar]
- 4.Viens LJ, Henley SJ, Watson M, et al. Human Papillomavirus-Associated Cancers - United States, 2008–2012. MMWR Morb. Mortal. Wkly. Rep. 2016;65:661–666. doi: 10.15585/mmwr.mm6526a1. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed 08/05/2016];U.S. Cancer Statistics Working Group: United States Cancer Statistics: 1999–2013 Incidence and Mortality Web-based Report. 2016 www.cdc.gov/uscs.
- 6.Leyden WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J. Natl. Cancer Inst. 2005;97:675–683. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- 7.Moyer VA the U. S. Preventive Services Task Force: Screening for cervical cancer. U. S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2012;156:880–891. W312. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 8.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger EA, Lee K, Saraiya M, et al. Racial and ethnic disparities in human papillomavirus-associated cancer burden with first-generation and second-generation human papillomavirus vaccines. Cancer. 2016;122:2057–2066. doi: 10.1002/cncr.30007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168:76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allison MA, Hurley LP, Markowitz L, et al. Primary Care Physicians' Perspectives About HPV Vaccine. Pediatrics. 2016;137:e20152488. doi: 10.1542/peds.2015-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moss JL, Reiter PL, Brewer NT. Concomitant Adolescent Vaccination in the U.S., 2007–2012. Am. J. Prev. Med. 2016 doi: 10.1016/j.amepre.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilkey MB, Malo TL, Shah PD, Hall ME, Brewer NT. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol. Biomarkers Prev. 2015;24:1673–1679. doi: 10.1158/1055-9965.EPI-15-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrer HB, Trotter C, Hickman M, Audrey S. Barriers and facilitators to HPV vaccination of young women in high-income countries: a qualitative systematic review and evidence synthesis. BMC Public Health. 2014;14:700. doi: 10.1186/1471-2458-14-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendry M, Lewis R, Clements A, Damery S, Wilkinson C. "HPV? Never heard of it!": a systematic review of girls' and parents' information needs, views and preferences about human papillomavirus vaccination. Vaccine. 2013;31:5152–5167. doi: 10.1016/j.vaccine.2013.08.091. [DOI] [PubMed] [Google Scholar]
- 16.Galbraith KV, Lechuga J, Jenerette CM, Moore LA, Palmer MH, Hamilton JB. Parental acceptance and uptake of the HPV vaccine among African-Americans and Latinos in the United States: A literature review. Soc. Sci. Med. 2016;159:116–126. doi: 10.1016/j.socscimed.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Accelerating HPV Vaccine Uptake: Urgency for Action to Prevent Cancer. A Report to the President of the United States from the President’s Cancer Panel. Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]
- 18.Abed J, Reilley B, Butler MO, Kean T, Wong F, Hohman K. Developing a framework for comprehensive cancer prevention and control in the United States: an initiative of the Centers for Disease Control and Prevention. J. Public Health Manag. Pract. 2000;6:67–78. doi: 10.1097/00124784-200006020-00011. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention and the National Cancer Institute. [Accessed 05/02/2016];State Cancer Profiles. 2016 http://www.statecancerprofiles.cancer.gov/index.html.
- 20.Guide to Community Preventive Services. [Accessed 08/17/2016];Increasing appropriate vaccination. 2016 2016; www.thecommunityguide.org/vaccines/index.html.
- 21.Walling EB, Benzoni N, Dornfeld J, et al. Interventions to Improve HPV Vaccine Uptake: A Systematic Review. Pediatrics. 2016;138 doi: 10.1542/peds.2015-3863. [DOI] [PubMed] [Google Scholar]
- 22.Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: A systematic review. Hum. Vaccin. Immunother. 2016;12:1566–1588. doi: 10.1080/21645515.2015.1125055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanderpool RC, Cohen E, Crosby RA, et al. "1-2-3 Pap" Intervention Improves HPV Vaccine Series Completion among Appalachian Women. J. Commun. 2013;63:95–115. doi: 10.1111/jcom.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopfer S. Effects of a narrative HPV vaccination intervention aimed at reaching college women: a randomized controlled trial. Prev. Sci. 2012;13:173–182. doi: 10.1007/s11121-011-0254-1. [DOI] [PubMed] [Google Scholar]
- 25.Frieden TR. Six components necessary for effective public health program implementation. Am. J. Public Health. 2014;104:17–22. doi: 10.2105/AJPH.2013.301608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woulfe J, Oliver TR, Zahner SJ, Siemering KQ. Multisector partnerships in population health improvement. Prev. Chronic Dis. 2010;7:A119. [PMC free article] [PubMed] [Google Scholar]
- 27.Zakocs RC, Edwards EM. What explains community coalition effectiveness? A review of the literature. Am. J. Prev. Med. 2006;30:351–361. doi: 10.1016/j.amepre.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Schell SF, Luke DA, Schooley MW, et al. Public health program capacity for sustainability: a new framework. Implement Sci. 2013;8:15. doi: 10.1186/1748-5908-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years--United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2015;64:784–792. doi: 10.15585/mmwr.mm6429a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benard VB, Thomas CC, King J, Massetti GM, Doria-Rose VP, Saraiya M. Vital signs: cervical cancer incidence, mortality, and screening - United States, 2007–2012. MMWR Morb. Mortal. Wkly. Rep. 2014;63:1004–1009. [PMC free article] [PubMed] [Google Scholar]
- 31.Moss JL, Reiter PL, Brewer NT. Correlates of human papillomavirus vaccine coverage: a state-level analysis. Sex. Transm. Dis. 2015;42:71–75. doi: 10.1097/OLQ.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rim SH, Polonec L, Stewart SL, Gelb CA. A national initiative for women and healthcare providers: CDC's Inside Knowledge: Get the Facts About Gynecologic Cancer campaign. J Womens Health (Larchmt) 2011;20:1579–1585. doi: 10.1089/jwh.2011.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neri A, Stewart SL, Angell W. Radon control activities for lung cancer prevention in national comprehensive cancer control program plans, 2005–2011. Prev. Chronic Dis. 2013;10:E132. doi: 10.5888/pcd10.120337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Momin B, Richardson L. An analysis of content in comprehensive cancer control plans that address chronic hepatitis B and C virus infections as major risk factors for liver cancer. J. Community Health. 2012;37:912–916. doi: 10.1007/s10900-011-9507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


