Abstract
In 2016, the Immunization Technical Advisory Group of the South-East Asia Region (SEAR) endorsed a regional goal to achieve ≤1% prevalence of hepatitis B surface antigen (HBsAg) among 5-year-old children by 2020. Chronic hepatitis B virus (HBV) infection is largely preventable with a birth dose of hepatitis B vaccine (HepB-BD) followed by two to three additional doses. We reviewed the progress towards hepatitis B control through vaccination in SEAR during 1992–2015. We summarized hepatitis B vaccination data and reviewed the literature to determine the prevalence of chronic HBV infection pre- and post-vaccine introduction. We used a mathematical model to determine post-vaccine prevalence of HBsAg among 5 year olds in countries lacking national serosurvey data and estimated the impact of vaccination on disease burden. Regional coverage with three doses of hepatitis B vaccine (HepB3) increased from 56% in 2011 to 87% in 2015. By 2016, 7 of 11 countries had introduced universal HepB-BD. Regional HepB-BD coverage increased from 9% in 2011 to 34% in 2015. In 2015, estimated HBsAg among 5 year olds was 1.1% with variability among countries. Myanmar (3.8%), Timor-Leste (2.7%), Indonesia (1.8%), and India (1%) had the highest prevalence of HBsAg. During 1992–2015, vaccination prevented approximately 16 million chronic HBV infections and 2.6 million related deaths. In 2015, around 197,640 perinatal HBV infections occurred in SEAR with majority occurring in India (62%), Bangladesh (24%), and Myanmar (8%). Myanmar had the highest rate of perinatal chronic HBV infections at 16 per 1000 live births. Despite significant progress in the control of HBV, SEAR needs to secure political commitment for elimination and consider additional strategies, such as promoting health facility births, universal birth dose administration, developing strong coordination between health sectors, and using alternative vaccine delivery methods, to improve HepB-BD coverage and subsequently achieve HBV control and elimination.
Keywords: Hepatitis B virus, Hepatitis B vaccine, Immunisation, Southeastern Asia
1. Introduction
Globally, approximately 257 million persons are chronically infected with hepatitis B virus (HBV), and 686,000 die every year as a result of HBV-related liver cirrhosis and hepatocellular carcinoma (HCC) [1,2]. Chronic HBV infection develops in 90% of infants infected before 1 year of age, 25–50% of children infected during 1–5 years of age, and 5–10% of persons infected after 5 years of age [3]. The World Health Organization (WHO) Strategic Advisory Groups of Experts (SAGE) recommends infants receive hepatitis B vaccine (HepB) at birth, ideally within 24 h, but administration up to 7 days after birth, followed by two or three additional doses can still be effective [3,4]. The Global Health Sector Strategy on Viral Hepatitis (GHSSVH) calls for a 30% reduction in new cases of chronic HBV infections (equivalent to 1% hepatitis B surface antigen [HBsAg] prevalence among children aged 5 years) by 2020 [5]. GHSSVH also calls for eliminating viral hepatitis by 2030 (equivalent to 0.1% HBsAg prevalence among children aged 5 years) [5].
In the WHO South-East Asia Region (SEAR), approximately 39 million persons are living with chronic HBV infection [1]. In 2016, the SEAR Immunization Technical Advisory Group (ITAG) endorsed a regional hepatitis B control goal to achieve a HBsAg prevalence of ≤1% among 5-year-old children by 2020 [6].
There has not been a comprehensive assessment of the current situation of hepatitis B vaccination and infection in the SEAR. We review the status of and progress towards hepatitis B control through vaccination in the SEAR, and we suggest strategies that would help the region reach the hepatitis B control goal by 2020 and HBV elimination by 2030.
2. Methods
For each country in SEAR, we compiled data on year of HepB introduction, HepB schedule, coverage with HepB-BD and with three doses of HepB (HepB3) [7,8]. HepB-BD coverage reported to WHO does not currently differentiate timely HepB-BD, defined as HepB-BD given within 24 h of birth, from birth dose given after 24 h. Therefore, we were not able to distinguish between timely and total HepB-BD coverage. We abstracted data on the proportion of women who attended at least one antenatal care visit, the institutional delivery rates, and the proportion of births attended by skilled birth attendant (SBA) [9]. We collected data on the number of live births in 2015 [10] and the number of surviving infants during 1992–2015 [11].
To evaluate the impact of HepB in SEAR, we conducted a comprehensive search of published literature and compiled available unpublished reports on prevalence of chronic HBV infection (i.e., percent of persons HBsAg positive) before and after vaccine introduction. To estimate pre-vaccine prevalence of HBsAg in each country, we included studies published from January 1995 to August 2016 with a sample size >100. We focused on studies reporting chronic HBV infection among children and young adults. However, in countries lacking these studies, adult populations were included. We excluded studies including populations with a known lower or higher prevalence of chronic HBV infection than the general population. We calculated the pre-vaccine regional prevalence of HBsAg among 5 year old children as a weighted average of HBsAg prevalence in each country adjusted by the number of surviving infants in 2000 from the World Population Prospects [11].
To estimate post-vaccine prevalence of HBsAg in each country, we included nationally representative serosurveys among children born after nationwide HepB introduction. For countries lacking nationally representative serosurveys, we used the hepatitis B mathematical model developed by Goldstein and colleagues to estimate the prevalence of HBsAg among 5-year-old children in 2015 [12], in accordance with the SEAR and GHSSVH indicators to measure cumulative incidence of chronic HBV infection at 5 years of age [5,6]. The Goldstein model requires country specific inputs, including number of surviving infants, vaccination coverage for HepB3 and HepB-BD, prevalence of HBsAg and hepatitis B e antigen (HBeAg) among women of childbearing age (WCBA), and prevalence of anti-hepatitis B core antibody (anti-HBc) at 5 years-of-age and ≥30 years. More details on the model are published elsewhere [12]. We determined post-vaccine regional prevalence of HBsAg among 5-year-old children in 2015 by calculating a weighted average of HBsAg prevalence in each country adjusted by the number of surviving infants in 2015 [11]. Based on vaccination coverage data during 2011–2015, reported literature findings on chronic hepatitis B prevalence in pregnant women and children, and the estimated post-vaccination hepatitis B prevalence in children, we provided recommendations and suggested strategies needed in the next 2–3 years to help each country reach the regional hepatitis B control and elimination goals.
We used the Goldstein model to estimate the total number of chronic HBV infections during the perinatal period and for all ages, and the number of chronic HBV infections averted and lives saved for each country from the year HepB was introduced to 2015. We compiled inputs of HepB-BD and HepB3 coverage [7], and the number of surviving infants [11] for each country from the year of HepB introduction to 2015. We used seroprevalence inputs from the most recent surveys and presumed they remained constant. When no data were available, we used the estimates published by Goldstein and colleagues [12]. Seroprevalence inputs used in the Goldstein model are summarized in the supplementary table. Using the Goldstein model, we calculated the number and rate of perinatal chronic HBV infections per 1000 live births in each country in 2015.
3. Results
3.1. Status of hepatitis B vaccination in the South-East Asia Region
Thailand was the first country in the region to introduce HepB in two provinces in 1988, and nationwide in 1992 (Table 1) [13]. Bhutan, Indonesia, and Maldives introduced HepB during the 1990s, while the remaining countries introduced it during the 2000s [7]. In Indonesia, HepB was introduced nationwide in 1997 following the Lombok Hepatitis B Immunization Project (1987–1991) [14]. HepB was introduced in a phased manner in Bangladesh (2003–2005) and Nepal (2002–2004) [15]. In India, the vaccine was initially introduced in 14 metropolitan cities in 2002, and nationally during 2011–2012 [16,17]. The majority of countries provide HepB as combined pentavalent vaccine (diphtheria, tetanus, pertussis, Haemophilus influenza type B, and hepatitis B vaccines) at 6, 10, and 14 weeks of age with one exception (Table 1) [7]. The overall regional HepB3 coverage increased from 56% in 2011 to 87% in 2015 [8]. In 2015, seven countries achieved >90% HepB3 coverage (Bangladesh, Bhutan, DPR Korea, Maldives, Nepal, Sri Lanka, Thailand) while Myanmar and Timor-Leste had <80% coverage (Table 1) [7].
Table 1.
Hepatitis B vaccine (HepB) schedule and coverage with three doses of hepatitis B vaccine (HepB3)—World Health Organization South-East Asia Region, 2011–2015.
| Country | HepB schedulea | Introduction of HepB (year)b | HepB3 coverage per WUENIC (%) [7] | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 2011 | 2012 | 2013 | 2014 | 2015 | |||
| Bangladesh | 6, 10, 14 weeks | 2005c | 96 | 94 | 94 | 94 | 94 |
| Bhutan | Birth, 6, 10, 14 weeks | 1996 [7] | 95 | 97 | 97 | 99 | 99 |
| DPR Korea | Birth, 6, 10, 14 weeks | 2003 [7] | 94 | 96 | 93 | 93 | 96 |
| India | Birth, 6, 10, 14 weeks | 2012 [16,17] | 44 | 73 | 70 | 79 | 87 |
| Indonesia | Birth, 2, 3, 4, 18 months | 1997 [14] | 81 | 83 | 85 | 81 | 81 |
| Maldives | Birth, 2, 4, 6 months | 1993 [7] | 96 | 99 | 99 | 99 | 99 |
| Myanmar | 2, 4, 6 months | 2003 [7] | 40 | 58 | 75 | 75 | 75 |
| Nepal | 6, 10, 14 weeks | 2004 [15] | 92 | 90 | 92 | 92 | 91 |
| Sri Lanka | 2, 4, 6 months | 2003 [7] | 99 | 99 | 99 | 99 | 99 |
| Thailand | Birth, 2, 4, 6 months | 1992 [13] | 98 | 98 | 99 | 99 | 99 |
| Timor-Leste | Birth, 6, 10, 14 weeks | 2008 [7] | 67 | 83 | 82 | 77 | 76 |
| SEAR [8] | 56 | 77 | 76 | 81 | 87 | ||
HepB: hepatitis B vaccine; HepB3: 3-dose series of Hepatitis B vaccine; SEAR: South-East Asia Region; WUENIC: WHO-UNICEF National Immunization Coverage estimates.
Hepatitis B monovalent vaccine is given at birth. All countries use pentavalent vaccine (diphtheria, tetanus, pertussis, Haemophilus influenza type B, and hepatitis B antigens [DTwPHibHepB]) for the 3 dose series except Thailand which uses DTwPHepB (diphtheria, tetanus, pertussis, and hepatitis B antigens).
Refers to year of nationwide introduction. Bangladesh, India, Indonesia, Nepal and Thailand had a phased introduction prior to nationwide introduction.
Unpublished report.
By 2015, six countries (Bhutan, DPR Korea, India, Indonesia, Maldives, and Thailand) had introduced universal HepB-BD vaccination (Table 2). In February 2016, Timor-Leste introduced universal HepB-BD. In September 2016, Myanmar introduced HepB-BD for institutional deliveries at hospitals with cold chain, and plans to expand to all deliveries in upcoming years. Myanmar first introduced HepB-BD during 2003–2005 when Gavi, the Vaccine Alliance, funded monovalent HepB introduction, however routine administration of HepB-BD stopped in 2009 after Gavi discontinued support for monovalent HepB [18]. All countries recommend HepB-BD within 24 h of birth except Indonesia which recommends HepB-BD within 7 days after birth [7]. In 2015, three of the six countries that provided universal birth dose achieved >90% coverage, while Indonesia (82%), Bhutan (78%), and India (44%) reported lower coverage (Table 2) [7]. The overall regional HepB-BD coverage increased from 9% in 2011 to 34% in 2015 (Table 2) [8].
Table 2.
Live births, antenatal and delivery care, and use of hepatitis B vaccine birth dose (HepB-BD)—World Health Organization South-East Asia Region, 2011–2015.
| Country | Live births (2015) [10] |
Women who received ≥ 1 antenatal care visit (%) [9] |
Institutional deliveries (%) [9] |
Births attended by skilled birth attendant (%) [9] |
Introduction of Hepatitis B vaccine birth dose (year) |
HepB-BD coverage per WUENIC (%) [7] | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 2011 | 2012 | 2013 | 2014 | 2015 | ||||||
| Universal birth dose | ||||||||||
| Bhutan | 12,860 | 98 | 74 | 75 | 2011 [7] | 29 | 60 | 64 | 78 | 78 |
| DPR Korea | 344,435 | 100 | 95 | 100 | 2003 [7] | 99 | 99 | 99 | 99 | 97 |
| India | 27,420,000 | 74 | 79 | 52 | 2012 [16,17] | 8 | 23 | 37 | 41 | 44 |
| Indonesia | 4,893,435 | 95 | 70 | 87 | 2002 [14] | 80a | 80a | 84a | 86a | 82a |
| Maldives | 7233 | 99 | 95 | 96 | 2000 [7] | 98 | 99 | 99 | 99 | 99 |
| Thailand | 675,530 | 98 | 100 | 100 | 1992 [13] | 99 | 99 | 99 | 99 | 99 |
| Timor-Leste | 44,854 | 84 | 22 | 29 | 2016 | – | – | – | – | – |
| Selective birth dose | ||||||||||
| Myanmar | 1,023,892 | 83 | 36 | 71 | 2016 | – | – | – | – | – |
| No birth dose | ||||||||||
| Bangladesh | 3,228,362 | 64 | 37 | 42 | – | – | – | – | – | – |
| Nepal | 614,666 | 68 | 55 | 56 | – | – | – | – | – | – |
| Sri Lanka | 334,821 | 99 | 98 | 99 | – | – | – | – | – | – |
| SEAR | 38,600,088 | 50 | 59b | 9 [8] | 19 [8] | 29 [8] | 32 [8] | 34 [8] | ||
HepB-BD: hepatitis B vaccine birth dose; SEAR: South-East Asia Region; WUENIC: WHO-UNICEF National Immunization Coverage estimates.
Coverage data for Indonesia comes from official country estimates http://apps.who.int/immunization_monitoring/globalsummary/.
World Health Organization. Health Service data by region. Available at: http://apps.who.int/gho/data/view.main.1610?lang=en [accessed 23 March 2017].
India, Indonesia, Bangladesh, and Myanmar account for 95% of live births in the region (Table 2). In most of the countries, >70% of deliveries occurred in health facilities. Only 22%, 36%, 37%, and 55% of births occurred in health facilities in Timor-Leste, Myanmar, Bangladesh, and Nepal, respectively, from which Timor-Leste is the only country that provides universal birth dose (Table 2) [9]. In Myanmar, however, 71% of home births are assisted by a SBA [9]. Births attended by SBA were lowest in Timor-Leste, Bangladesh, India, and Nepal (Table 2) [9].
In countries with high rates of home births, strategies have been developed to improve HepB-BD coverage. In Indonesia, informal notification of births using community members improved coordination with health professionals who provided the HepB-BD. Training of community health care workers and communication with mothers addressed cultural sensitivities around vaccination within the first week of life [14]. To mitigate hesitancy among healthcare workers (HCW) over opening vaccine vials for only 1 or 2 doses, India adopted an open vial policy reducing wastage by nearly 10% for monovalent HepB and pentavalent vaccines [16,19].
Indonesia and Timor-Leste use a compact pre-filled auto-disable injection device (CPAD) to deliver the HepB-BD. Use of HepB-BD CPAD was more cost-effective than use of multidose vials, because it led to lower rates of vaccine wastage, [20] and it was easy to use by health workers with minimal training [14,21]. With proper training, use of CPAD in Indonesia was highly accepted by vaccinators, midwives, and mothers; 67% of midwives stated that CPAD was more practical and efficient to use than syringes [14,21]. In addition, Indonesia has been using HepB-BD outside of the cold chain (OCC) in hard to reach areas and has found no difference in seroconversion rates among CPAD stored OCC, CPAD stored in cold chain, and vials stored in cold chain [22]. HepB-BD coverage within 7 days post-birth increased from 35% in 2003 to 82% in 2015, following implementation of national policy to use HepB-BD CPAD stored OCC in Indonesia [7].
Despite these strategies, countries reported challenges in providing timely HepB-BD. In a 2009 HepB-BD assessment covering five states in India, vaccine stock-outs were reported and only a small proportion of infants were vaccinated within 24 h of birth [16]. In addition, incomplete recording and poor reporting of timely HepB-BD administration were found in both Indonesia and India [14,16].
3.2. Prevalence of chronic hepatitis B virus infection pre- and posthepatitis B vaccine introduction
Nationally representative pre- and post-hepatitis B vaccine introduction impact serosurveys were conducted in Bangladesh, Nepal, and Thailand. A decrease in HBsAg prevalence post-vaccine introduction was found in all three countries (Table 3). In Bangladesh, a study conducted in 2012 reported HBsAg prevalence of 1.3% among children born before (10 years old) and 0.05% among those born after (5 years old) HepB introduction [unpublished report]. In Nepal, in 2012, HBsAg prevalence was 0.28% among 10–12 year old children born before vaccine introduction and 0.13% among 5–6 year old children born after vaccine introduction [15]. HepB has been available in Thailand for over 25 years, and the most recent survey conducted in 2014 found a 0.3% prevalence of HBsAg among 5–10 year old children [13].
Table 3.
Prevalence of chronic hepatitis B virus (HBV) infection among persons born before and after hepatitis B vaccine (HepB) introduction, and number of lives saved and total chronic HBV infections averted starting from year of vaccine introduction up to 2015 in each country—World Health Organization South-East Asia Region, 1992–2015
| Country | Pre-vaccination | Post-vaccination | Recommendations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Year of study |
Sample size | Sampling | Age group (years) |
% HBsAg positive |
Age group (years) |
% HBsAg positive in 2015 |
No of lives saved |
Total no of chronic HBV infections averted |
||
| Post-vaccination representative serosurvey conducted | ||||||||||
| Bangladesh | 2011/12 | 2100 pre- and 2100 post-HepB | HH survey in 105 randomly selected mouzas | 10 | 1.3a | 5–6 | 0.05a (1.5b) | 147,402 | 815,878 | Validate seroprevalence to assess whether there is need for HepB-BD |
| Nepal | 2012 | 1186 pre-and 2144 post-HepB | Stratified cluster HH survey | 10–12 | 0.28 [15] | 5–6 | 0.13[15] (0.6b) | 43,296 | 270,168 | Consider partial HepB-BD introduction in certain areas if supported by serosurvey data |
| Thailand | 2014 | 2805 pre- and 3159 post-HepB | HH survey in four regions | >22–24 | 4.5 [13] | 5–10 | 0.3 [13] (0.05b) | 84,727 | 493,742 | Maintain good coverage |
| Nationally representative post-vaccination survey not conducted; prevalence estimated by modeling | ||||||||||
| Bhutan | 1995/96 | 251 | Hospital based survey | 1–12 | 5.2 [23] | 5–6 | 0.4b | 1949 | 11,941 | Evaluate vaccine impact (serosurvey) |
| DPR Korea | ND | 5–6 | 0.8b | 86,947 | 510,506 | Evaluate vaccine impact (serosurvey) | ||||
| India | 2015 | 944 | Community-based study in a village in rural Maharashtra state | 11–30 | 1.2 [24] | 5–6 | 1.0b | 931,491 | 5,905,423 | Improve coverage, conduct national serosurvey among children after higher coverage achieved |
| 2001/02 | 1838 | Community-based survey in West Bengal | <10–19 | 2.6 [25] | ||||||
| 1998 | 1856 | Community-based representative study in Tamil Nadu | 15–45 | 5.7 [26] | ||||||
| Indonesia | 2012 | 195 | Convenience sample from universities in Banjarmasin | 18–41 | 4.6 [27] | 5–6 | 1.8b | 1,054,394 | 6,731,634 | Improve coverage, evaluate vaccine impact nationwide (serosurvey) |
| 2010 | 376 | Convenience sample of high school and college students in Ternate | 17–25 | 15.7 [28] | ||||||
| 2005 | 327 | Convenience sample in Tahuna Archipelo | 11–30 | 12.2 [29] | ||||||
| Maldives | ND | 5–6 | 0.2b | 1300 | 7050 | Document vaccine impact (serosurvey) | ||||
| Myanmar | 2015 | 5547 | Cross-sectional general population survey in 18 townshipsa | 15–80 | 6.5a | 5–6 | 3.8b | 135,975 | 819,170 | Improve HepB3 coverage, consider nationwide HepB-BD introduction to reach births outside health facilities |
| Sri Lanka | 1995 | 245 | Community-based survey in Gampaha district | 5–9 | 4.9 [30] | 5–6 | 0.8b | 72,579 | 410,105 | Document vaccine impact (serosurvey) |
| Timor-Leste | ND | 5–6 | 2.7b | 3813 | 24,359 | Increase HepB3 and HepB-BD coverage, evaluate HepB-BD introduction | ||||
| SEAR | 3.9c | 5–6 yrs | 1.1d | 2,563,873 | 15,999,976 | |||||
HBV: hepatitis B virus; HepB: hepatitis B vaccine; HBsAg: hepatitis B surface antigen; HH: household; HepB-BD: hepatitis B vaccine birth dose; HepB3: 3-dose series of hepatitis B vaccine; SEAR: South-East Asia Region; ND: no data.
Unpublished report.
Estimates of HBsAg from mathematical modeling calculated for each country among 5 year olds in 2015.
Regional pre-vaccine prevalence estimated as a weighted average of HBsAg prevalence in each country adjusted to surviving infant cohort size in 2000. For countries with multiple studies, a median estimate was calculated.
Regional post-vaccine prevalence among children aged 5 years estimated as weighted average of HBsAg prevalence in each country adjusted to surviving infant cohort size in 2015. Goldstein model HBsAg prevalence estimates were used unless the country had a nationally representative post-vaccine impact serosurvey.
For five countries in SEAR (Bhutan, India, Indonesia, Myanmar and Sri Lanka), estimates of HBsAg seroprevalence were compiled from national or subnational serosurveys conducted before HepB introduction [23–30] (Table 3). There were no available data on HBsAg prevalence pre-vaccine introduction in DPR Korea, Maldives, and Timor-Leste. For these eight countries which lacked national post-vaccine impact serosurveys, we estimated prevalence of HBsAg among 5–6 year old children in 2015 using the Goldstein model.
In Bhutan, HBsAg prevalence decreased from 5.2% in mid-1990s among 1–12 year old children [23] to 0.4% in 2015 among 5-year-old children. Of note, Bhutan is conducting a national serosurvey in the general population that will evaluate the impact of HepB among children [1]. Based on non-representative serosurveys conducted in a few states, pre-vaccine HBsAg prevalence ranged from 1.2% to 5.7% in India [24–26] and 4.6% to 15.7% in Indonesia [27–29] among children and young adult populations (Table 3). We estimated post-vaccine introduction prevalence of HBsAg among children aged 5 years in 2015 at 1.0% and 1.8% in India and Indonesia, respectively. In Myanmar and Sri Lanka, model estimated post-vaccine HBsAg prevalence among 5 year olds was 3.8% and 0.8%, respectively (Table 3). We estimated the post-vaccine introduction prevalence of HBsAg among 5 year olds in DPR Korea, Maldives and Timor-Leste at 0.8%, 0.2% and 2.7%, respectively (Table 3).
The overall estimated regional prevalence of chronic HBV infection among 5-year-old children decreased from 3.9% in the prevaccine era to 1.1% in 2015, a 72% reduction.
3.3. Impact of hepatitis B vaccination on total number of chronic HBV infections and associated deaths
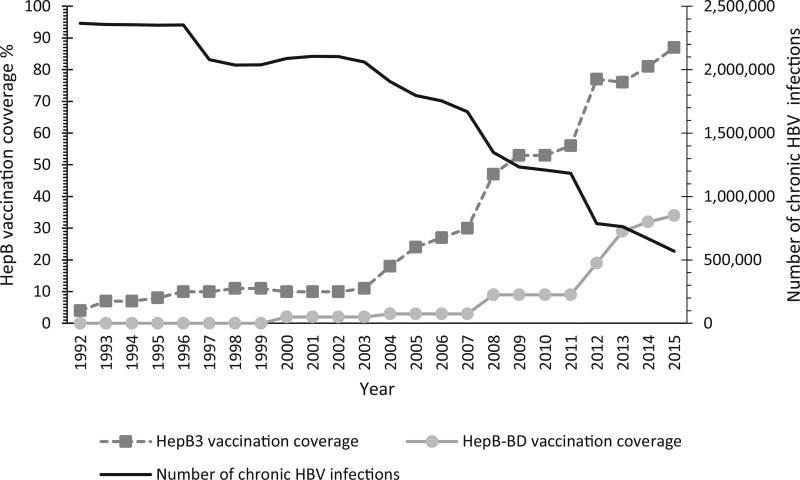
During 1992–2015, the total annual number of chronic HBV infections in the SEAR decreased significantly, as HepB3 and HepB-BD coverage increased (Fig. 1). In the region, chronic HBV infections declined from 2,366,129 in 1992 to fewer than 568,896 in 2015, a 76% reduction. Similarly, the number of HBV-related deaths decreased by 76% during this period, from nearly 376,000 deaths in 1992 to approximately 89,000 deaths in 2015. During 1992–2015, 16 million cases of chronic HBV infection were prevented through immunization, thereby averting 2.6 million hepatitis B related deaths in the region (Table 3). The most populous countries in the region, India and Indonesia, have averted nearly 13 million chronic infections and 2 million deaths combined since vaccine introduction.
Fig. 1.
Hepatitis B vaccination coverage and annual number of chronic hepatitis B virus (HBV) infections – World Health Organization (WHO) South-East Asia Region, 1992–2015. HBV: hepatitis B virus; HepB: hepatitis B vaccine; HepB3: 3-dose series of Hepatitis B vaccine; HepB-BD: Hepatitis B vaccine birth dose.
3.4. Number of perinatal chronic hepatitis B infections
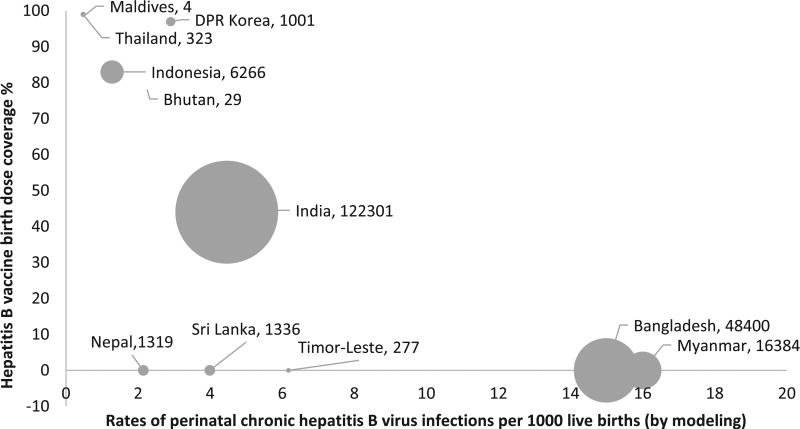
In 2015, based on model estimates, a total of 197,640 perinatal chronic HBV infections occurred in SEAR. Of these infections, 122,301 (62%) occurred in India, 48,400 (24%) in Bangladesh, 16,384 (8%) in Myanmar, and 6266 (3%) in Indonesia (Fig. 2). The highest rates of perinatal chronic HBV infections occurred in Myanmar (16 per 1000 live births) and Bangladesh (15 per 1000 live births). India had approximately 5 cases and Indonesia had 1 case of perinatal chronic HBV infection per 1000 live births. Thailand had the lowest rate (0.48 per 1000 live births) followed by the Maldives (0.55 per 1000 live births) (Fig. 2).
Fig. 2.
Estimated number* and rates of perinatal chronic hepatitis B virus (HBV) infections by hepatitis B vaccine birth dose coverage – World Health Organization (WHO) South-East Asia Region, 2015. *Numbers of perinatal chronic HBV infections are shown next to the name of the country in the figure.
4. Discussion
This paper documents the remarkable achievements in the SEAR in controlling chronic HBV infection. We estimated the 2015 regional prevalence of HBsAg among 5-year-old children at 1.1%, which highlights SEAR is on the way to meeting to the regional hepatitis B control goal of ≤1% by 2020. This estimate is within the 95% confidence interval (CI) of recently reported modeling data that estimated HBsAg prevalence at 0.7% (95% CI: 0.5–1.6%) among children aged <5 years in SEAR [1]. We chose to estimate prevalence among children aged 5 years because it is the indicator for the SEAR hepatitis B control goal and the GHSSVH. In addition, 5-year-old children have passed through the highest risk period for acquiring chronic HBV infection.
Despite the significant progress, additional efforts are required to increase HepB3 coverage and targeted strategies, are needed to increase HepB-BD coverage to ultimately achieve HBV control by 2020 and elimination by 2030 [5]. Based on the findings in this review, we highlight the specific strategies needed to reach the hepatitis B regional and global goals depending on the country’s situation in the following paragraphs and in Table 3.
Bangladesh, Nepal, and Sri Lanka are the remaining countries in SEAR without HepB-BD in their immunization schedule. These countries should assess if HepB-BD introduction is a relevant strategy considering their epidemiological situation and the global goals of HBV elimination by 2030 [5]. In Sri Lanka, the prevalence of HBsAg among children is <1%; antenatal and delivery care are nearly universal, thus high coverage with HepB-BD could be achieved. In Nepal, the prevalence of HBsAg among children is also <1%. However, subnational studies showed geographic variability in HBsAg prevalence ranging from 6.6% in the Surkhet Valley to 7.3% in the Manang region [31]. Targeted HepB-BD introduction in certain geographical areas might be needed. In Bangladesh, our model seroprevalence estimates differed from HBsAg prevalence reported in the 2012 national serosurvey. The inputs for the model were from the data reported in the national serosurvey, which limits the bias of modelled estimates. Further, a recent review found most HBV infections in Bangladesh occur in childhood with an estimated 4.2% HBsAg prevalence among children in 2011 [32]. Based on model estimates, Bangladesh had a high number of perinatal infections per year (48,400) and a rate of 15 perinatal infections per 1000 live births (Fig. 2). Bangladesh needs more evidence to validate HBsAg prevalence among children and might need to consider introducing HepB-BD.
Timely HepB-BD administration should be easy to achieve in countries with high rates of health facility (HF) births. However, India, which had ≥70% of births in HF, reported HepB-BD coverage <50%. SEAR countries should consider adopting strategies proven successful in the Western Pacific Region (WPR) to improve timely HepB-BD coverage. These include adding HepB-BD to routine immunization registers and cards, training and supervision of HCWs on reporting HepB-BD administration, and verifying vaccine stocks in the delivery wards. Furthermore, instituting standing orders assigning the responsibility of HepB-BD administration to the person delivering the infant would avoid confusion among HCWs, increase opportunities for infant vaccination, and increased timely HepB-BD coverage [33–35].
In countries with low rates of HF births, such as Bangladesh, Myanmar, Nepal, and Timor-Leste, several approaches could be implemented to improve timely HepB-BD coverage. Promoting institutional deliveries through provision of financial incentives and encouraging parents to deliver in HF led to an increase in timely HepB-BD coverage in Cambodia and China [33,34]. ANC visits could be used as an opportunity to promote HF births and educate mothers on timely HepB-BD. This strategy relies on strong coordination and collaboration between MCH and immunization services [33,34,36]. In addition, availability of SBA in HF is vital to ensure adequate care and timely HepB-BD coverage. In India, while 79% of deliveries are institutional, skilled personnel attend only 52% of deliveries, which did not improve care [37]. Therefore, India needs to establish a cadre of SBA to improve maternal and neonatal health and timely HepB-BD coverage.
Countries with a low proportion of HF births should improve timely HepB-BD coverage among home births and births outside HF. Currently, Myanmar provides HepB-BD for HF births, which account for 36% of total births. This strategy will not be sufficient to reduce chronic HBV infection given the high number and rate of perinatal infections, and an estimated HBsAg prevalence of >3% among 5-year-old children. Therefore, Myanmar should expand HepB-BD administration outside HF. This should be feasible since 70% of births are attended by SBA [9], and midwives are responsible for routine immunization services. Timor-Leste has low HF delivery and SBA rates, which could affect timely HepB-BD administration. Educating parents on the importance of timely HepB-BD during ANC visits and through community health volunteers were successful in China to improve HepB-BD coverage among home births [34]. In Lao PDR, providing cell phones to village health volunteers to notify health workers of a labor or a recent delivery increased HF deliveries by 13% and improved HepB-BD coverage by a median of 57% [38]. In Viet Nam, timely HepB-BD was higher (90–97%) in areas with community volunteers tracking and notifying HCWs of births compared to other areas (52%) [39]. Preparing combined microplans for routine immunization and MCH activities contributed to an increase in HepB-BD coverage in China [34]. Hence, countries in SEAR should apply these strategies to make sure all children are protected against HBV, irrespective of birth location.
Because HepB is the only vaccine that should be given within 24 h of birth, lack of cold chain can limit access to and provision of the vaccine. Use of HepB-BD outside the cold chain (OCC) is another strategy used by countries to reach home births or births occurring in facilities without proper cold chain storage. WHO SAGE supports countries that choose to pursue an OCC policy with monovalent hepatitis B vaccine, however if a policy is adopted, the WHO Immunization in Practices Advisory Committee (IPAC) recommendations for OCC and controlled temperature chain use of vaccines should be followed [3,4]. HepB from several manufacturers has been shown to be heat stable and able to withstand exposure to temperatures as high as 37 °C up to one month [4]. In Lao PDR, HepB-BD coverage improved by a median of 27% in districts with an OCC policy compared to no change in districts without an OCC policy [40]. In Viet Nam, coverage of HepB-BD administered within 3 days of birth improved from 45% to 89% when HepB was stored OCC, and seroconversion rates were similar to vaccines stored in the cold chain [41]. In Indonesia, use of HepB CPAD OCC contributed to significant increase in coverage and ability to reach home births especially in areas that lacked SBA [14,21]. Use of HepB CPAD OCC improved timely HepB-BD coverage and was widely accepted in pilot studies conducted in China and Papua New Guinea [33,36]. For countries with high proportion of home births or suboptimal cold chain, use of HepB-BD OCC in monovalent single dose vials or in CPAD might need to be considered to improve timely HepB-BD and promote the introduction of HepB-BD.
For some countries, another challenge to introduction and use of HepB-BD is the cost of a separate monovalent HepB. Monovalent HepB was funded by Gavi, the Vaccine Alliance, starting in 2001 but in 2005, Gavi discontinued monovalent HepB support and supported only combination vaccines [42]. Among the remaining four countries in SEAR without universal HepB-BD, all currently or previously have received Gavi support. In a recent WHO survey, vaccine cost was cited as a major obstacle to HepB-BD introduction although the cost of a single dose vial of HepB is only $0.20 [43,44]. Moving forward, strong political commitment and advocacy for government budget allocation will be necessary. In the WPR, countries continued to administer HepB-BD by identifying national funds or donor support after Gavi funding for monovalent HepB vaccine discontinued [33].
Our review has shown that several SEAR countries have not conducted nationally representative hepatitis B serosurveys among children. In India and Indonesia, a number of studies focused on one area or state. DPR Korea, Timor Leste, and Maldives have no serosurvey data. Countries must now document the seroprevalence of chronic HBV infection by conducting nationally representative serosurveys among children at least 5 years of age [45], and monitor vaccination coverage as the region works towards reaching the hepatitis B control target by 2020 and ultimately elimination by 2030.
A major limitation was the lack of nationally representative data in several countries and the inability to assess timeliness of HepB-BD. We used a mathematical model, which relies heavily on the quality of input data, to estimate the current seroprevalence of HBsAg among 5-year-old children. The Goldstein model does not consider the effects of herd immunity, immunity from incomplete vaccination, or delayed HepB-BD vaccination. Given the inability of current WHO/UNICEF coverage estimates to report timely HepB-BD, the model assumed HepB-BD coverage to be timely. The effectiveness of HepB-BD in prevention of perinatal transmission is highest if given within 24 h of birth and decreases gradually afterwards [3]. Given this limitation, we might have underestimated HBsAg prevalence if countries reported untimely HepB-BD. For Bangladesh, Nepal, and Thailand, we were able to compare post-vaccination estimates generated from the model to the estimates from national serosurveys. In Thailand, the model estimated a lower post-vaccination prevalence of HBsAg (0.05%) than the estimates from the most recent serosurvey (0.3%), which does not align with the conservative nature of the model. These results demonstrate the variability of model generated data, and reemphasize the need for countries to conduct well-designed nationally representative serosurveys.
Despite these limitations, this paper fills the gap in reporting the status of and progress towards hepatitis B control in the SEAR, and it provides baseline achievements that can be tracked. The region has made considerable progress towards chronic HBV prevention through vaccination; however, additional work is needed. Strong political commitment and secured vaccine financing in the region and among member states will be essential, in order to prioritize HBV prevention, reach the control goal by 2020, and subsequently elimination by 2030 [5]. Various strategies such as promoting health facility births, strong MCH and EPI coordination, and using alternative vaccine delivery methods could be used to improve HepB-BD and HepB3 coverage. Clear policies and guidelines communicated to all sectors involved in immunization will reduce missed opportunities. Assessing vaccine impact and estimating disease burden among children aged 5 years is needed as countries move towards verification of the achievement of the hepatitis B control goal.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Eric Wiesen, Dr. Sergey Diorditsa, and Dr. Xi Li for providing an outline to a great review assessing the progress of hepatitis B control through vaccination in the Western Pacific Region. We would like to specifically thank Dr. Xi Li, Consultant to WHO WPRO Philippines, for providing guidance on the use of the mathematical model.
Funding
None.
Footnotes
Disclaimer
The findings and conclusions in this study are those of the authors and do not necessarily reflect the position of the Centers for Disease Control and Prevention and the World Health Organization.
Conflicts of interest
None.
Author contributions
Ms. Lana Childs designed the study, reviewed the literature, analyzed and summarized the data, and wrote the manuscript; Dr. Sigrun Roesel reviewed the manuscript; Dr. Rania A Tohme designed the study, reviewed the literature, and reviewed the manuscript.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2017.11.027.
References
- 1.World Health Organization. Global hepatitis report; 2017. [accessed 09.05.17]; Available at: < http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?ua=1>.
- 2.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec. 2017;92:369–92. [Google Scholar]
- 4.World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, October 2016 – conclusions and recommendations; 2016. Wkly Epidemiol Rec. 2016;91(48):561–84. [PubMed] [Google Scholar]
- 5.World Health Organization. Global health sector strategy on viral hepatitis 2016–2021: towards ending viral hepatitis; 2016. [accessed 16.02.17]; Available at: < http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1>.
- 6.World Health Organization. Regional Office for South-East Asia. Regional Immunization Technical Advisory Group. Report of the seventh meeting SEARITAG; 2016. [accessed 10.02.17]; Available at: < http://www.searo.who.int/entity/immunization/documents/sear_itag_2016.pdf?ua=1>.
- 7.World Health Organization. WHO/UNICEF joint reporting form on immunization: WHO vaccine preventable diseases: monitoring system; 2016. [accessed 03.03.17]; Available at: < http://apps.who.int/immunization_monitoring/globalsummary>.
- 8.World Health Organization. WHO/UNICEF coverage estimates for 1980–2015: Regional global coverage; 2016. [accessed 02.03.17]; Available at: < http://www.who.int/entity/immunization/monitoring_surveillance/data/coverage_estimates_series.xls>.
- 9.The United Nations Children’s Fund. UNICEF data: monitoring the situation of children and women. [accessed 25.01.17]; (Updated February 2016). Available at: < https://data.unicef.org/>.
- 10.World Health Organization. Regional Office for South-East Asia. EPI fact sheet; 2016. [accessed 22.03.17]; Available at: < http://www.searo.who.int/entity/immunization/data/sear.pdf?ua=1>.
- 11.United Nations. World population prospects the 2015 revision; 2015. [accessed 07.11.16]; Available at: < https://esa.un.org/unpd/wpp/Download/Standard/Interpolated/>.
- 12.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34(6):1329–39. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 13.Posuwan N, Wanlapakorn N, Sa-Nguanmoo P, Wasitthankasem R, Vichaiwattana P, Klinfueng S, et al. The success of a universal hepatitis B immunization program as part of Thailands’s EPI after 22 years’ implementation. PLoS One. 2016 doi: 10.1371/journal.pone.0150499. https://doi.org/10.1371/journal.pone.0150499. [DOI] [PMC free article] [PubMed]
- 14.Creati M, Saleh A, Ruff TA, Stewart T, Otto B, Sutanto A, et al. Implementing the birth dose of hepatitis B vaccine in rural Indonesia. Vaccine. 2007;25(32):5985–93. doi: 10.1016/j.vaccine.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 15.Upreti SR, Gurung S, Patel M, Dixit SM, Krause LK, Shakya G, et al. Prevalence of chronic hepatitis B virus infection before and after implementation of a hepatitis B vaccination program among children in Nepal. Vaccine. 2014;32(34):4304–9. doi: 10.1016/j.vaccine.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahariya C, Subramanya BP, Sosler S. An assessment of hepatitis B vaccine introduction in India: lessons for roll out and scale up of new vaccines in immunization programs. Indian J Public Health. 2013;57(1):8–14. doi: 10.4103/0019-557X.111357. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal R, Babu JJ, Hemalatha R, Reddy AV, Sharma D, Kumar T. Effect of inclusion of hepatitis B vaccine in childhood immunization program in India: a retrospective cohort study. Indian Pediatr. 2014;51(11):875–89. doi: 10.1007/s13312-014-0520-y. [DOI] [PubMed] [Google Scholar]
- 18.Gavi, the Vaccine Alliance. Myanmar country hub. [accessed 27.03.17]; Available at: < http://www.gavi.org/country/myanmar/>.
- 19.Patel PB, Rana JJ, Jangid SG, Bavarva NR, Patel MJ, Bansal RK. Vaccine wastage assessment after introduction of open vial policy in Surat municipal corporation area of India. Int J Health Policy Manag. 2015;5(4):233–6. doi: 10.15171/ijhpm.2015.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin CE, Nelson CM, Widjaya A, Moniaga V, Anwar C. The costs of home delivery of a birth dose of hepatitis B vaccine in a prefilled syringe in Indonesia. Bull World Health Organ. 2005;83(6):456–61. [PMC free article] [PubMed] [Google Scholar]
- 21.Sutanto A, Suarnawa IM, Nelson CM, Stewart T, Soewarso TI. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull World Health Organ. 1999;77(2):119–26. [PMC free article] [PubMed] [Google Scholar]
- 22.Otto BF, Suarnawa IM, Stewart T, Nelson C, Ruff TA, Widjaya A, et al. At-birth immunisation against hepatitis B using a novel pre-filled immunisation device stored outside the cold chain. Vaccine. 1999;18:498–502. doi: 10.1016/s0264-410x(99)00242-x. [DOI] [PubMed] [Google Scholar]
- 23.Da Villa G, Andjaparidze A, Cauletti M, Franco E, Roggendorf M, Sepe A, et al. Viral hepatitis in the Bhutanese population: preliminary results of a seroepidemiological investigation. Res Virol. 1997;148(2):115–7. doi: 10.1016/s0923-2516(97)89894-9. [DOI] [PubMed] [Google Scholar]
- 24.Bhate P, Saraf N, Parikh P, Ingle M, Phadke A, Sawant P. Cross sectional study of prevalence and risk factors of hepatitis B and hepatitis C infection in a rural village of India. Arg Gastroenterol. 2015;52(4):321–4. doi: 10.1590/S0004-28032015000400013. [DOI] [PubMed] [Google Scholar]
- 25.Chowdhury A, Santra A, Chakravorty R, Banerji A, Pal S, Dhali GK, et al. Community-based epidemiology of hepatitis B virus infection in West Bengal, India: prevalence of hepatitis B e antigen-negative infection and associated viral variants. J Gastroenterol Hepatol. 2005;20(11):1712–20. doi: 10.1111/j.1440-1746.2005.04070.x. [DOI] [PubMed] [Google Scholar]
- 26.Kurien T, Thyagarajan SP, Jeyaseelan L, Peedicayil A, Rajendran P, Sivaram S, et al. Community prevalence of hepatitis B infection and modes of transmission in Tamil Nadu, India. Indian J Med Res. 2005;121(5):670–5. [PubMed] [Google Scholar]
- 27.Darmawan E, Turyadi, El-Khobar KE, Nursanty NK, Thedja MD, Muljono DH. Seroepidemiology and occult hepatitis B virus infection in young adults in Banjarmasin, Indonesia. J Med Virol. 2015;87(2):199–207. doi: 10.1002/jmv.24045. [DOI] [PubMed] [Google Scholar]
- 28.Ie SI, Turyadi Sidarta E, Sadhewa A, Purnomo GA, Soedarmono YS, et al. High prevalence of hepatitis B virus infection in young adults in Ternate, Indonesia. Am J Trop Med Hyg. 2015;93(6):1349–55. doi: 10.4269/ajtmh.15-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Achwan WA, Muttagin Z, Zakaria E, Depamede SA, Mulyanto Sumoharjo S, et al. Epidemiology of hepatitis B, C, and E viruses and human immunodeficiency virus infections in Tahuna, Sangihe-Talaud Archipelago, Indonesia. Intervirology. 2007;50(6):408–11. doi: 10.1159/000112915. [DOI] [PubMed] [Google Scholar]
- 30.Padmasiri E, Rajapaksa L, Jayakuru WS, Withana N. The prevalence of hepatitis B surface antigen in the Gampaha district. Ceylon Med J. 1995;40(1):10–3. [PubMed] [Google Scholar]
- 31.Shrestha SM, Shrestha S. Chronic hepatitis B in Nepal: an Asian country with low prevalence of HBV infection. Trop Gastroenterol. 2012;33(2):95–101. doi: 10.7869/tg.2012.24. [DOI] [PubMed] [Google Scholar]
- 32.Alam S, Azam G, Mustafa G, Alam M, Ahmad N. Past, present, and future of hepatitis B and fatty liver in Bangladesh. Gastroenterol Hepatol Open Access. 2017 < https://doi.org/10.15406/ghoa.2017.06.00197>.
- 33.Hennessey K, Mendoza-Aldana J, Bayutas B, Lorenzo-Mariano KM, Diorditsa S. Hepatitis B control in the World Health Organization’s Western Pacific Region: targets, strategies, status. Vaccine. 2013;31(S9):J85–92. doi: 10.1016/j.vaccine.2012.10.082. https://doi.org/10.1016/j.vaccine.2012.10.082. [DOI] [PubMed] [Google Scholar]
- 34.Hutin Y, Hennessey K, Cairns L, Zhang Y, Li H, Zhao L, et al. Improving hepatitis B vaccine timely birth dose coverage: lessons from five demonstration projects in China, 2005–2009. Vaccine. 2013;31(S9):J49–55. doi: 10.1016/j.vaccine.2013.03.025. https://doi.org/10.1016/j.vaccine.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Sobel HL, Mantaring JB, 3rd, Cuevas F, Ducusin JV, Thorley M, Hennessey KA, et al. Implementing a national policy for hepatitis B birth dose vaccination in Philippines: lessons for improved delivery. Vaccine. 2011;29(5):941–5. doi: 10.1016/j.vaccine.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Li J, Chen H, Li F, Armstrong GL, Nelson C, et al. Hepatitis B vaccination of newborn infants in rural China: evaluation of village-based, out-of-coldchain delivery strategy. Bull World Health Organ. 2007;85(9):688–94. doi: 10.2471/BLT.06.037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaturvedi S, De Costa A, Raven J. Does the Janani Suraksha Yojana cash transfer programme to promote facility births in India ensure skilled birth attendance? A qualitative study of intrapartum care in Madhya Pradesh. Glob Health Action. 2015;8:27427. doi: 10.3402/gha.v8.27427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xeuatvongsa A, Datta SS, Moturi E, Wannemuehler K, Philakong P, Vongxay V, et al. Improving hepatitis B birth dose in rural Lao People’s Democratic Republic through the use of mobile phones to facilitate communication. Vaccine. 2016;34(47):5777–84. doi: 10.1016/j.vaccine.2016.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murakami H, Van Cuong N, Huynh L, Hipgrave DB. Implementation of and costs associated with providing a birth-dose of hepatitis B vaccine in Viet Nam. Vaccine. 2008;26(11):1411–9. doi: 10.1016/j.vaccine.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Kolwaite AR, Xeuatvongsa A, Ramirez-Gonzalez A, Wannemuehler K, Vongxay V, Vilayvone V, et al. Hepatitis B vaccine stored outside the cold chain setting: a pilot study in rural Lao PDR. Vaccine. 2016;34(28):3324–30. doi: 10.1016/j.vaccine.2016.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hipgrave DB, Tran TN, Huong VM, Dat DT, Nga NT, Long HT, et al. Immunogenicity of a locally produced hepatitis B vaccine with the birth dose stored outside the cold chain in rural Vietnam. Am J Trop Med Hyg. 2006;74(2):255–60. [PubMed] [Google Scholar]
- 42.Gavi, The Vaccine Alliance. Hepatitis B vaccine support. [accessed 27.03.17]; Available at: < http://www.gavi.org/support/nvs/hepb/>.
- 43.World Health Organization. Immunization, vaccines, and biologicals. SAGE meeting of October 2016. Review of the barriers to implement the birth dose of hepatitis B; 2016. [accessed 11.01.17]; Available at: < http://who.int/immunization/sage/meetings/2016/october/7_Review_of_the_barriers_to_implement_the_birth_dose_of_hepb.pdf?ua=1>.
- 44.The United Nations Children’s Fund. Vaccine price data. [accessed 25.03.17]; Available at: < https://www.unicef.org/supply/files/HepB.pdf>.
- 45.World Health Organization. Documenting the impact of hepatitis B immunization: best practices for conducting a serosurvey; 2011. Available at: < http://whqlibdoc.who.int/hq/2011/WHO_IVB_11.08_eng.pdf>.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.