Abstract
This review provides an in-depth description of the preclinical and clinical studies demonstrating the effectiveness and limitations of IL-15 and IL-15 analogs given as an exogenous immuno-oncology agent. IL-15 is a cytokine that primarily stimulates the proliferation and cytotoxic functions of CD8 T cells and NK cells leading to enhanced anti-tumor responses. While initially showing promise as a cancer therapeutic, the efficacy of IL-15 was limited by its short in vivo half-life. More recently, various approaches have been developed to improve the in vivo half-life and efficacy of IL-15, largely by generating IL-15/IL-15Rα conjugates. These new IL-15 based agents renew the prospect of IL-15 as a cancer immunotherapeutic agent. While having some efficacy in inducing tumor regression as a monotherapy, IL-15 agents also show great potential in being used in combination with other immuno-oncological therapies. Indeed, IL-15 used in combination therapy yields even better anti-tumor responses and prolongs survival than IL-15 treatment alone in numerous murine cancer models. The promising results from these preclinical studies have led to the implementation of several clinical trials to test the safety and efficacy of IL-15-based agents as a stand-alone treatment or in conjunction with other therapies to treat both advanced solid tumors and hematological malignancies.
Keywords: IL-15, Cytotoxic lymphocytes, Immunotherapy, Clinical trials, ALT-803, Soluble IL-15 complexes
1. Introduction
Over the past decade, the use of immunotherapy has emerged as a promising approach to treat a broad range of human cancers. Cancer immunotherapy attempts to harness the power of the immune system to kill malignant tumors while leaving healthy tissues intact. The immune system has an inherent ability to find and eliminate malignancies; however, tumors have developed mechanisms to escape immune surveillance by interfering with the development and function of anti-tumor immune responses. The challenge of immunotherapy is to develop strategies that effectively and safely augment anti-tumor responses. Several current cancer immunotherapies that have been investigated for their efficacy include cytokine therapy, adoptive cell transfer, cancer vaccines, and monoclonal antibodies (mAbs). The most promising of these approaches are those stimulating tumor-specific T cell responses that are highly-specific and possess the potential to develop long-lived tumor immunity.
Therapy with recombinant IL-2, a T cell and NK cell growth factor, was one of the earliest forays in immunotherapy that yielded some successes [1–3]. Subsequently, IL-2 was also used in conjunction with adoptive T cell therapy, the combination significantly improved the treatment efficacy [4–6]. While impressive tumor regression was observed with IL-2 therapy and adoptive T cell therapy, these responses are limited to small percentages of patients and carry with it a high level of toxicity. Nonetheless, these successes validated the concept that stimulating T cell and NK cell responses could yield effective and durable responses. IL-15 is a cytokine that has many overlapping functions as IL-2 including the ability to promote anti-tumor responses but with distinct advantages over IL-2. These attributes made IL-15 attractive enough to be ranked first as having the greatest potential for use in cancer immunotherapy by US National Cancer Institute in 2008 [7]. Since that time, the successes of monoclonal antibodies targeting checkpoint blockade molecules have revolutionized the field of immuno-oncology, not just for their ability to induce effective tumor regression but also induce long-term durable responses in a subset of patients [8–10]. Now the focus lies in increasing the response rate of checkpoint blockade and extending their utility to all cancer types. While in the shadows of checkpoint blockade, research on IL-15 as a component of immunotherapy has grown and new approaches have been realized that keep it in the game as a tool to enhance anti-tumor responses. In this review, we will provide an in-depth description of the preclinical and clinical studies that have demonstrated the effectiveness and limitations of IL-15 as an immuno-oncology agent, the different approaches taken to improve the efficacy of IL-15, and the potential of IL-15 therapies to be used as part of a combination therapy with other immuno-oncological treatments. Herein, we will focus on the utility of IL-15 given as an exogenous agent, even though its application can extend beyond this.
2. Mechanisms mediating IL-15 responses
IL-15 is a four-α-helix protein belonging to a family of cytokines consisting of interleukins IL-2, IL-4, IL-7, IL-9, and IL-21. IL-15 signals through a receptor complex composed of the IL-2/IL-15 receptor β (IL-15Rβ) (CD122) subunit, which is shared with IL-2 and the common gamma chain (γC) (CD132) receptor subunit, which is also utilized by all of the additional family members. While it does not have a crucial direct role in IL-15 signaling per se, IL-15Rα is a critical component of the IL-15 cytokine-receptor complex. IL-15Rα is a transmembrane protein with very high affinity for IL-15 that facilitates IL-15 trafficking from the endoplasmic reticulum (ER) through the cytoplasm and presentation of IL-15/IL-15Rα complexes on the cell surface. In addition to remaining associated throughout cytoplasmic and cell surface expression, IL-15/IL-15Rα can also be cleaved as a complex into the extracellular space. These peculiarities of IL-15 and IL-15R subunits lend itself to unique mechanisms of cytokine delivery.
The expression of IL-2/15Rβ and γC is believed to be the major attribute conferring IL-15 responsiveness. These receptors are present on many hematopoietic cells; however, the IL-2/15Rβ expression is highest on CD8 T cells and NK cells. Upon ligand binding, the IL-2/15Rβ and γC subunits stimulate Janus kinase (Jak)1, Jak3, and signal transducer and activator of transcription (STAT)-5 pathway. After phosphorylation, STAT5 homo-dimerizes, translocates to the nucleus, and promotes the transcription of target genes. Additionally, IL-15 stimulates both the PI3K-AKT and RAS-MAPK pathways [11]. Altogether, IL-15 signaling stimulates an array of downstream pathways leading to increased cellular growth, decreased apoptosis, and enhanced immune cell activation and migration. At a low static level, these responses have a crucial role in the development, function, and survival of CD8 T cells, NK cells, NKT cells and intestinal intraepithelial lymphocytes; this is reflected by the major deficiency of these populations in both IL-15−/− and IL-15Rα−/− mice [12,13]. The similar phenotype between these knockout strains shows the reliance of IL-15Rα for IL-15-mediated effects. While endogenously-expressed IL-15 mediates lymphocyte homeostasis, IL-15 delivered exogenously at supra-physiological levels induces the selective activation and proliferation in CD8 T cells and NK cells, the very cell types most amenable to mediating anti-tumor responses. The cellular specificity of IL-15 is one attribute that makes it so attractive for immunotherapy.
In contrast to the selective expression of the signaling subunits for IL-15, the cytokine itself and IL-15Rα are widely expressed by most cell types, including both hematopoietic and non-hematopoietic cells but are highest among myeloid cells. Once expressed on the cell surface, IL-15/IL-15Rα complexes can stimulate neighboring or opposing cells in trans through the IL-15Rβ/γC. This type of cytokine stimulation requires cell–cell contact and is referred to as transpresentation. Under steady-state conditions, trans-presentation is believed to be the primary mode of action for IL-15-mediated development and homeostasis of CD8 T cells, NK cells, NKT cells and intraepithelial lymphocytes [14,15]. Trans-presentation offers a tighter regulation than that of a secreted cytokine by providing cell-directed delivery of IL-15 to specific cell types. Nonetheless, soluble (s) IL-15/IL-15Rα complexes are also cleaved from the cell surface in response to a variety of inflammatory signals, such as TLR ligation, type I Interferons, and CD40 ligation [14,16,17]. This production of sIL-15 complexes is generally transient in nature and may provide a short-lived, but strong burst of IL-15 activity to support early immune responses. In both of these circumstances, IL-15Rα binding of IL-15 is not only a platform for IL-15 delivery but also increases the half-life of IL-15 and may also increase the affinity of IL-15 for IL-15Rβ/γC. Indeed, sIL-15 complexes have been found to be superior in their ability to stimulate IL-15 responses compared to unassociated rIL-15 [18,19]. This sparked a surge in the generation of multiple formulations of sIL-15 complexes that are now being examined for therapeutic efficacy and have reignited the interest in IL-15. In the literature, stimulation using sIL-15 complexes is sometimes referred to as a method of mediating trans-presentation. However, since “trans” by definition is a prefix defined as “across, opposite sides, or on the other side”, we suggest the terminology of trans-presentation be reserved for situations involving a cell–cell contact, while sIL-15Rα/IL-15 complexes and their variants be referred to as IL-15 agonists or superagonists.
As research on IL-15 has grown in recent years, our perspective of IL-15 has evolved from one that was originally thought of simply as a secreted soluble factor to a more complicated scenario with multiple modes of actions. Some consider IL-15Rα a chaperone protein rather than a receptor while others argue that the physiological cytokine is actually a heterodimeric protein composed of both IL-15 and IL-15Rα [20]. While both concepts are accurate, IL-15Rα may also serve other functions that are yet to be identified. In particular, IL-15Rα is expressed by T cells and NK cells but its expression by those cells is not required for stimulating IL-15 responses [21–23]. On the contrary, in vivo responses to IL-15 are completely dependent on the expression of IL-15Rα by cell types other than the cells that ultimately respond to IL-15, thus providing evidence that trans-presentation is an important mechanism utilized [21,24]. The diverse roles of IL-15Rα are a testament to the unique biology of IL-15, which has not yet been fully revealed.
3. rIL-15 as a monotherapy
As mentioned, IL-2 was an early candidate for the immunotherapy of cancer and was approved for use in 1992 by the US Food and Drug Administration to treat metastatic renal cell carcinoma and malignant melanoma [1–3,25,26]. Unfortunately, high-dose IL-2 therapy was associated with life-threatening toxicities in patients, which included arrhythmias, heart failure and capillary leak syndrome [27,28]. In addition, IL-2 displayed not only immune-enhancing but also immune-suppressive activities through the induction of activation-induced cell death of T cells and the expansion of immunosuppressive regulatory T cells (Tregs) [29]. While IL-15 has similar immune enhancing properties as IL-2, it does not share the immune-suppressive activities. Specifically, treatment with IL-15 induces the proliferation and survival of T cells, promotes the proliferation and differentiation of NK cells, and induces the generation of cytotoxic lymphocytes [30,31,21]. In contrast to IL-2, IL-15 actually suppresses activation-induced cell death, has no marked effects on Treg cells, and does not cause capillary leak syndrome in mice or nonhuman primates (NHPs) [32,33]. Preclinical studies showed that IL-15 also induced prolonged expansion and activation of NK cells and CD8 memory T cells [34,33]. These observations led to the study of IL-15 as a better option for therapeutic use in the treatment of cancer.
The shared use of IL-2/15Rβ and γC by IL-15 and IL-2 explains their similar activities whereas their divergent activities are principally due to differences in the mechanisms utilized and cellular responsiveness of these two cytokines. In large part, the responsiveness of IL-15 and IL-2 is dictated by the differential expression profile and affinity of their respective α chain receptor subunits. IL-15Rα has a much higher affinity for IL-15 than that of the IL-2/15Rβ/γC complex and the IL-15Rα is nearly ubiquitously expressed [35–37]. As such, upon treatment with rIL-15, free rIL-15 likely binds cell surface bound IL-15Rα or soluble IL-15Rα that subsequently stimulates IL-15-responsive cells. The extent to which rIL-15 is transpresented or delivered by extracellularly-formed sIL-15 complexes is not clear. In mice, the cells stimulated by rIL-15 are primarily effector and memory CD8 T cells and NK cells, as these cell types express the highest levels of the IL-2/15β/γC receptor complex [38]. However, in NHP and humans, both CD8 and CD4 T cells are responsive to rIL-15 [39,40,34,41]. Among T cell subsets, effector memory and tissue resident memory cells are more responsive to rIL-15 than naïve T cells and central memory T cells [42]. In contrast to IL-15, high affinity binding of IL-2 requires the heterotrimer composed of the IL-2Rα, IL-2/15Rβ, and γC receptor subunits [43]. Therefore, responses mediated by rIL-2 are primarily geared towards cells that express IL-2Rα, which is transiently expressed by activated T cells and NK cells and constitutively expressed by Tregs. Even among the same cell types, IL-15 and IL-2 can generate different responses as the intensity/duration of these signals can be different even though each cytokine stimulates similar intracellular signaling pathways. Altogether, rIL-2 binds pre-formed high-affinity heterotrimeric receptors at the surface of activated cells, while rIL-15 likely binds membrane bound IL-15Rα that is transpresented to NK cells and memory T cells. Hence, there is overlap in the type of responses stimulated by these two factors but the lack of stimulation of Tregs by IL-15 makes it a more attractive candidate for cancer immunotherapy than IL-2. Despite these advantages, there are drawbacks to treating with rIL-15 including its short in vivo half-life and possibly its potential reliance on trans-presentation by other cell types; however, the use of IL-15/IL-15Rα complexes overcomes these problems, which will be discussed in the next section.
The ability of IL-15, as well as IL-2, to stimulate CD8 T cells and NK cells is the main mechanism attributing to its ability to promote anti-tumor responses. Indeed, the anti-tumor functions of IL-15 have been demonstrated in model systems involving systemic treatment with IL-15 as well as cell specific engineering of IL-15. Several studies have demonstrated systemic treatment with rIL-15 can promote tumor regression, decrease metastasis, and increase survival in transplantable experimental murine tumor models, such as LA795 lung adenocarcinoma, melanoma (B16, B78-H1), MC38 colon carcinoma, and lymphoma [44–47]. The increased anti-tumor activity observed in mice was associated with enhanced CD8 T cell/NK cell cytotoxicity, which was found to be more significant in IL-15-treated mice than in IL-2-treated mice. In some IL-15-treated mice, the tumors were eradicated and the mice remained tumor-free after re-challenge, indicating long-lasting anti-tumor immunity had developed.
Potential toxicity of using therapeutic levels of IL-15 are a concern because of its overlapping activities with IL-2, its association with autoimmune diseases, and the findings that transgenic overexpression of IL-15 leads to leukemia [48,49]. Safety of rIL-15 in primates has been examined using both macaque and human rIL-15, which are 97% identical in amino acid sequence and exhibit similar binding affinities to IL-15 receptors [50]. Most of studies examining toxicities in NHP have used rIL-15 derived from E. coli, which lacks the glycosylation present on mammalian-derived rIL-15. Although not compared side by side, glycosylated rIL-15 was effective at low doses suggesting it may be potent than the unglycosylated version (Table 1). Among these studies, significant expansion of CD8 T cells, NK cells, and in some circumstances CD4 T cells could be observed with all doses and routes of IL-15 treatment; however, while higher doses and continuous delivery of rIL-15 could yield more impressive responses, clinical toxicities, such as reduced appetite, diarrhea, weight loss with transient grade 3–4 neutropenia became apparent (Table 1). Despite the transient toxicities, none of the animals died during the study and there was no evidence of autoimmune diseases or infections. Overall treatment with rIL-15 was well tolerated when given intermittently or given subcutaneously (Table 1). So based on the observations from studies in murine tumor models and NHP, the use of IL-15 was supported as an alternative treatment in patients with metastatic malignancies.
Table 1.
Effects of rIL-15 on lymphocyte responses and toxicities in NHP.
| Animal | rIL-15 | Dose | Route | Frequency | Immunological Effect | Toxicity | Reference |
|---|---|---|---|---|---|---|---|
| rhesus | E. coli derived Rhesus, | 10 μg/kg | S.Q. | Daily, 1×/wk for 6 weeks | Increased CD4 T cells | No adverse events | [90] |
| cyno | E. coli derived Rhesus, | 10, 100 μg/kg | S.Q. | 2×/wk for 4 weeks | Increase CD8 and CD4 Tem, NK cells | None reported | [91] |
| rhesus | E. coli derived Rhesus, | 10 μg/kg | Not stated | Day 1,2,or day 1,3,7 | Increase CD8 CD4 Tem | None reported | [41] |
| rhesus | E. coli derived human | 20 μg/kg/day | Continuous I.V. | 10 days | 100× CD8 Tem 10× NK |
Moderate Increase AST/ALT, neutropenia | [39] |
| 20–40 μg/kg | S.Q. | Daily | 10× CD8 Tem 10× NK |
Mild Increase AST/ALT, | |||
| rhesus | E. coli derived human | 10, 20,50 μg/kg | I.V. | Daily for 12 days | 4× CD8 Tcm, 6× CD8 Tem, 2.5× CD4 T cells, 6× NK cells | Neutropenia, weight loss, decrease appetite, diarrhea, | [33,40] |
| rhesus | Mammalian-derived human | 15 μg/kg | S.Q. | Daily for 14 days | Increase CD8 and CD4 Tm, NK cells | Severe neutropenia, anemia, weight loss, rash | [34] |
| 2.5–10 μg/kg | S.Q. | Every 3 days for 24 days | Increase CD8 and CD4 Tm, NK cells | Well tolerated |
The first in-human phase I clinical trial of daily intravenous infusions of E coli-produced rhIL-15 in adults with metastatic malignant melanoma and metastatic renal cell cancer was recently completed (Table 2). Similar to studies in murine models, a dramatic efflux of NK and memory CD8 T cells in the circulating blood was observed 30 min after IL-15 administration in all four patients sampled [42]. Changes in T cell subset proportions were more evident in patients receiving 3 μg/kg of rhIL-15 than for those receiving the lower doses of 1 and 0.3 μg/kg/per day. More than 3 days after infusion of rhIL-15, hyper-proliferation (Ki-67) and increases in circulating NK cells occurred. Absolute NK cell counts increased > 10-fold with the high dose (3 μg/kg per day) but only two-to-three-fold with the lowest dose of rhIL-15. Significant increases in the number of CD8 T cells and γδ T cells were also observed after rhIL-15 administration. Pharmacokinetic studies revealed the half-life for rhIL-15 of about 2.5 h. The administration of rhIL-15 resulted in a clearance of lung lesions in patients with malignant melanoma but there were also significant IL-15 mediated toxicities at the higher doses. There were marked elevations in the pro-inflammatory cytokines IL-6, IL-8, IFN-γ, tumor necrosis factor α (TNF-α), and IL-1β, which coincided with acute clinical toxicities such as fever, chills, and blood pressure changes. Ultimately, it was concluded that to reduce toxicity, alternative dosing strategies such as continuous intravenous infusions or subcutaneous administration of IL-15 should be considered in future studies to reduce toxicity.
Table 2.
Clinical Trials Involving IL-15-based Therapies.
| Clinical ID | Phase | Aim | Status | Study Period | Tumor Type | No of patients | Treatment | IL-15 Dose | Principle Investigator |
Responsible Party |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT01021059 | I | Safety | Completed | 2009–2016 | Metastatic Melanoma and Metastatic Renal Cell Carcinoma | 18 | rhIL-15 (IV) | 0.3–25 mcg/kg/day for 12 days | T.A. Waldmann | NCI, MD |
| NCT01369888 | I | Safety/Efficacy | Terminated (autoimmune toxicity) | 2011–2014 | Metastatic Melanoma | 3 | CY + Fludarabine + TILs + rhIL-15 (IV) | 0.25–0.55 mcg/kg/day for 10 days | S.A. Rosenberg | NIH, MD |
| NCT01385423 | I | Safety/Efficacy | Completed | 2011–2015 | AML | 26 | Fludarabine + CY+ Haploidentical NK cell + rhIL-15 (IV) | 0.25–3 mcg/kg/day for 12 days | J.S. Miller | Masonic Cancer Center, MN |
| NCT01572493 | I | Safety | Recruiting | 2012–2018 | Lymphoma, Carcinoma | Estimated 52 | rhIL-15 (IV) | 0.1–8 mcg/kg/day for 10 days | K. C. Conlon | NCI, MD |
| NCT01727076 | I | Safety | Ongoing, | 2013–2016 | Advanced Melanoma, Renal Cell Cancer, Non-Small Cell Lung Cancer, Head and Neck Squamous Cell Cancer | Estimated 30 | rhIL-15 (SC) | NA | J. S. Miller | NCI, MD |
| NCT01875601 | I | Safety | Completed | 2013–2015 | Solid Tumors, Brain Tumors, Sarcoma, Pediatric Cancers, Neuroblastoma | 16 | Autologous NK cell infusion + rhIL-15 (IV) | 0.25–0.75 mcg/kg/day for 10 days | M. S. Merchant | NCI, MD |
| NCT01885897 | I/II | Safety/Efficacy | Recruiting | 2013–2018 | AML, ALL, MDS, Lymphoma, Myeloma, CLL and CML | Estimated 61 | ALT-803 (IV) | 1–30 mcg/kg once a week for 4 weeks | J.S. Miller | Masonic Cancer Center, MN |
| NCT01946789 | I | Safety/Efficacy | Recruiting | 2014–2018 | Melanoma, Renal Cell, Non-Small Cell Lung Cancer, Squamous Cell Head and Neck Cancer | Estimated 20 | ALT-803 (IV) | NA | K. Margolin | Altor Bioscience, FL |
| NCT02138734 | I/II | Safety/Efficacy | Recruiting | 2014–2018 | Non-muscle Invasive Bladder Cancer | Estimated 81 | Intravesical BCG + ALT-803 (Intravesical) | NA | H. C. Wong | Altor Bioscience, FL |
| NCT02099539 | I/II | Safety/Efficacy | Recruiting | 2014–2020 | Relapsed or Refractory Multiple Myeloma | Estimated 57 | ALT-803 (IV) | NA | H. C. Wong | Altor Bioscience, FL |
| NCT02452268 | I | Safety/Efficacy | Recruiting | 2015–2018 | Skin Melanoma, Renal Cell Cancer, Non-Small Cell Lung Cancer and Head and Neck Squamous Cell Carcinoma | Estimated 42 | hetIL-15 (IL-15/sIL-15Rα) (SC) | NA | Not Provided | Novartis, Switzerland |
| NCT02395822 | II | Efficacy | Suspended Recruiting | 2015–2019 | AML | Estimated 24 | Fludarabine + CY + Haploidentical NK cells activated with 10 ng/mL of IL–15 + rhIL-15 (SC) | 2 mcg/kg once a day for 5 days, 2 day rest, 5 days on | J. S. Miller | Masonic Cancer Center, MN |
| NCT02384954 | I/II | Safety/Efficacy | Recruiting | 2015–2023 | B Cell Non-Hodgkin Lymphoma (NHL) | Estimated 86 | Rituximab + ALT-803 (IV) | NA | H. C. Wong | Altor Bioscience, FL |
| NCT02465957 | II | Safety/Efficacy | Recruiting | 2015–2017 | Merkel Cell Carcinoma (MCC) | Estimated 24 | Activated NK-92 Natural Killer (aNK) Cell Infusions + ALT-803 (SC) | 10 μg/kg on the first day of every aNK fusion | Not Provided | NantKwest, Inc., CA |
| NCT02523469 | I/II | Safety | Recruiting | 2016–2019 | Advanced or Metastatic Non-Small Cell Lung Cancer | Estimated 91 | ALT–803 + Nivolumab | 6–15 μg/kg | J. Wrangle | Medical University of South Carolina, SC/Altor Bioscience, FL |
| NCT02559674 | I/II | Safety/Efficacy | Recruiting | 2016–2022 | Advanced Pancreatic Cancer | Estimated 66 | Alt-803 (IV) + Gemcitabine (SC) + Nab-Paclitaxel (SC) | NA | H. C. Wong | Altor Bioscience, FL |
| NCT02689453 | I | Safety/Efficacy | Recruiting | 2016–2022 | Refractory or Relapsed Chronic and Acute ATL | Estimated 30 | Alemtuzumab + rhIL-15 (SC) | 1–3 mcg/kg/dose for 10 doses over two weeks | T.A. Waldmann | NCI, MD |
| NCT02752243 | I/II | Safety/Efficacy | Recruiting | 2016–2020 | Relapsed Acute Leukemia, MDS after SCT | Estimated 40 | IL-15 Activated Cytokine Induced Killer (CIK) Cells (IV) | NA | P. Bader | Johann Wolfgang Goethe University Hospital, Germany |
| NCT02989844 | II | Efficacy | Not yet recruiting | 2017–2022 | Prevent Relapse of AML and MDS after alloHCT | Estimated 60 | ALT-803 (SC) | 6 mcg/kg once a week (4 weeks on/4 weeks off) | C. Ustun | Masonic Cancer Center, MN |
| NCT03003728 | II | Efficacy | Not yet recruiting | 2017–2018 | High-Risk Multiple Myeloma Post-Autologous Stem Cell Transplant (ASCT) | Estimated 10 | Elotuzumab + Melphalan + Expanded NK Cells + ALT-803 (SC) | 10 μg/kg on days 1, 8, 15, and 22 | F. van Rhee | University of Arkansas, AR |
| NCT02890758 | I | Safety/Efficacy | Not yet recruiting | 2017–2019 | AML, MDS, ALL, CML, CLL, NHL, Myeloproliferative Syndromes, Plasma Cell Myeloma, Colon/Rectal Carcinoma and Soft Tissue Sarcomas | Estimated 54 | NK Cells (IV) + ALT-803 (IV) | 6 mcg/kg weekly for 4 weeks | D. Wald | Case Comprehensive Cancer Center, OH |
| NCT03022825 | II/III | Safety/Efficacy | Not yet recruiting | 2017–2020 | Unresponsive High Grade Non-muscle Invasive Bladder Cancer | Estimated 80 | Intravesical BCG + ALT-803 | NA | K. Chamie | Altor Bioscience, FL |
| NCT03054909 | I | Safety/Efficacy | Not yet recruiting | 2017–2020 | Ovarian Cancer, Fallopian Tube Cancer, Primary Peritoneal Cancer | Estimated 28 | ALT-803 (SC and IP) | 10 mcg/kg weekly (4 weeks on/4 weeks off)-4 courses | M. Geller | Masonic Cancer Center, MN |
With its anti-tumor immune capabilities, rIL-15 is a promising immunotherapeutic agent that has several limitations that need to be addressed to make it applicable in clinical settings. These include its short half-life and the high doses needed to achieve functional responses in vivo, which result in clinical toxicities and limited anti-tumor responses in patients. To address these problems, new formulations of IL-15 have been developed that possess enhanced half-life, increased bioavailability in vivo, and limited toxicity. In the next section, these various IL-15 formulations and their functional activities will be described, which show promise for the utility of IL-15 in the immunotherapy of cancer.
4. IL-15 super-agonists
As previously described, the efficacy of rIL-15 as a treatment is limited by the availability of IL-15Rα, which plays an integral part in stabilizing and increasing the biological activity of IL-15. Since unassociated IL-15 isn’t found naturally in vivo [52], IL-15 bound to IL-15Rα resembles the physiological form of IL-15 and has a higher affinity for IL-15Rβ/γC than free IL-15 [51]. As a soluble version, IL-15/IL-15Rα complexes have the advantage of not being dependent on trans-presentation or a cell–cell interaction. Naturally-produced sIL-15 complexes are referred to as heterodimeric IL-15 by G.N. Pavlakis’ group [20,53,54] (Fig. 1). Their in vitro production system [47] has been licensed to Novartis for commercial production and heterodimeric human IL-15 is being tested in clinical trials (Table 2). Several other approaches have been used to generate sIL-15/IL-15Rα complexes that employ different strategies to further enhance IL-15 responses, which will be discussed below and are depicted in Fig. 1. Overall, in multiple models systems, regardless of formation, the sIL-15/IL-15Rα complexes have been shown to be significantly more potent than native IL-15 in both in vitro and in vivo studies [18,55,19,54]. Furthermore, these IL-15 agonists can also be applied to ex vivo stimulation of tumor-specific lymphocytes (TILs) and NK cells [67] or be engineered for cellular expression and viral vector expression but are not discussed herein for space consideration.
Fig. 1.
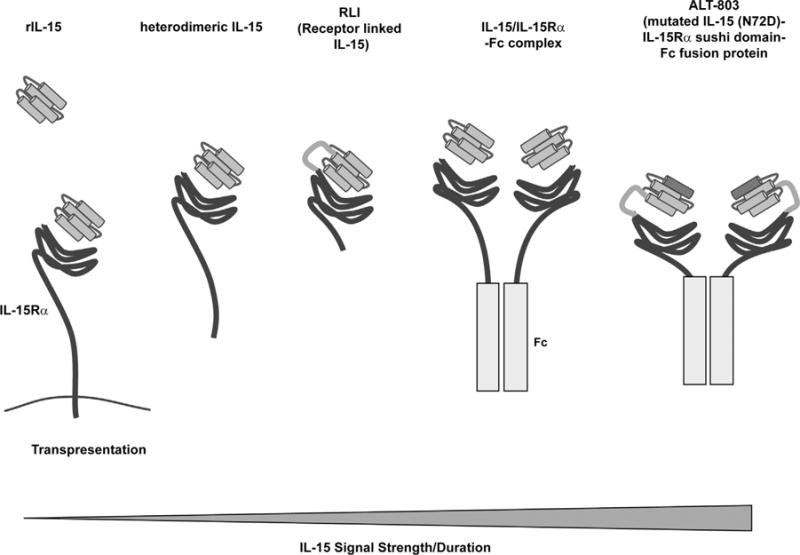
IL-15 and IL-15 agonists used in immunotherapy. Cartoon depicts the multiple IL-15-based agents that are used to stimulate cytotoxic T cell and NK cell responses. rIL-15 was the first form of IL-15 to be examined in vivo. When administered, rIL-15 is believed to predominantly bind to cell surface IL-15Rα where it is transpresented to IL-15 responsive cells. Heterodimeric IL-15 is natural form of IL-15 that is cleaved from cells and can stimulate responses independent of cell interactions. Heterodimeric hIL-15 is being produced as a therapeutic by Novartis. RLI (Cytune Pharmaceuticals) is a fusion protein consisting of IL-15 linked to the sushi (cytokine-binding) domain of the IL-15Rα that can act as a soluble IL-15 agonist. IL-15/IL-15Rα-Fc complexes are generated by mixing commercially available IL-15Rα-Fc chimeric fusion protein with rIL-15 and have been used extensively in preclinical studies. ALT-803 (Altor Pharmaceutical) is an IL-15 superagonist consisting of mutated IL-15 (asparagine replaced with an aspartic residue), which has an increased affinity for CD122-expressing immune cells, linked to the sushi domain of the IL-15Rα that is fused to an Fc fragment. The IL-15 signals mediated by these IL-15 agonists is speculated to range in strength and duration as depicted by the bottom triangle. Signal strength and duration increases with increased abundance of agonist, increased in vivo half-life (imparted by IL-15 binding to IL-15Rα and presence of an Fc fragment), dimerization of the agonists, and increased affinity for the IL-2Rβ/γC complex (imparted by IL-15 binding to the sushi domain and the mutation present in ALT-803).
One strategy of generating sIL-15 complexes combines rIL-15 with recombinant soluble murine IL-15Rα (sIL-15Rα) linked to the Fc portion of the human IgG1 antibody (IL-15/IL-15Rα-Fc complex). The fusion of the Fc domain of IgG has been widely used to enhance the plasma half-life of many proteins [56]. Whereas half-life of rIL-15 alone was ∼1 h, the half- life of sIL-15/IL-15Rα-Fc complexes ∼20 h. In addition to possessing an increased half-life, this complexed version of IL-15 is also more potent. Administration of sIL-15/IL-15Rα-Fc complexes in vivo induces a strong expansion of memory CD8 T cells, NK cells, and NK T cells that is enhanced ∼50 fold compared to rIL-15 alone[18,19,57,58]. Surprisingly, IL-15/IL-15Rα-Fc also induced T cell activation and effector function in naïve CD8 T cells. CD4 T cells demonstrated a milder proliferative response along with increased CD44 expression, indicative of T cell activation. Importantly, the level of activity obtained by the receptor-complexed rIL-15 could not be achieved by a higher dose of rIL-15 alone [19]. We suspect that the upper limit of rIL-15 activity is restricted by the amount of endogenous IL-15Rα available. More importantly, IL-15/IL-15Rα-Fc complexes can promote anti-tumor responses [19,58,55]. In mice injected systemically with B16 melanoma cells, treatment with IL-15/15Rα-Fc complexes prevented the formation of tumors whereas rIL-15 alone had no effect on tumor engraftment compared to untreated controls [19]. In another study, systemic injection of IL-15/IL-15Rα-Fc complexes led to CD8 T cell-mediated regression of solid tumors in RIP1-Tag2 pancreatic and B16F10 melanoma murine tumor models without significant toxicities [58].
A second strategy set out to further enhance the potency of IL-15 and simplify production by developing a fusion protein, consisting of the NH2-terminal (amino acids 1–77, sushi+) cytokine-binding domain of human IL-15Rα coupled to human IL-15 via a 20-amino acid flexible linker. This fusion protein, referred to as protein receptor-linker-IL-15 (RLI) acts as an IL-15 superagonists that has an increased serum half-life and biological activity similar to complexed IL-15/IL-15Rα-Fc [51]. Additionally, RLI demonstrated a strong anti-tumor effect in two different tumor models. In the B16 melanoma murine tumor model, RLI exhibited a higher efficiency in reducing lung and liver metastasis and enhancing survival than rhIL-15 or rhIL-2 [59]. Moreover, RLI reduced growth and metastasis of human colon carcinoma cells in an orthotopic nude mouse model [59], providing evidence that even in the absence of T cells, IL-15 stimulation can mediate anti-tumor effects likely through its effects on NK cells. Presently, RLI is being produced and tested by Cytune Pharmaceuticals.
Another IL-15 fusion protein, ALT-803 has been developed by Altor Biosciences to address the limitations of IL-15-based therapies. ALT-803 was engineered by combining a mutated version of IL-15-(N72D), which has superagonistic properties with the sushi domain of IL-15Rα fused to the Fc portion of human IgG1 [60]. In the IL-15 N72D superagonist (IL-15SA), replacing the asparagine with an aspartic residue enhances the affinity for CD122-expressing immune cells and promotes stronger cytoplasmic signals for activation and proliferation of NK cells and CD8 T cells at lower doses. Compared with native IL-15, ALT-803 exhibits a longer serum half-life and retention in lymphoid organs, which was consistent with its more potent immunostimulatory and anti-tumor activities in vivo [60]. Similar to the other sIL-15 complexes, ALT-803 induces expansion of effector NK cells (CD11b+, CD127hi) and memory (CD122+, CD44+) CD8 T cells. While marked elevations of the cytokines IFN-γ, TNF-α, and IL-10 were observed with ALT-803 treatment in mice, no toxicities were seen [61]. ALT-803 significantly reduced tumor burden and prolonged survival in an impressive number of animal models of cancer, namely, murine multiple myeloma [60], glioblastoma [62], 4T1 breast cancer [61], B16 melanoma [61], CT26 colon carcinoma [63] rat bladder cancer [64], ovarian cancer [65], murine mastocytoma, and B cell lymphoma[66]. In side-by-side comparison with rhIL-15, ALT-803 induced superior anti-tumor responses in the B16F10 melanoma and CT26 colon carcinoma models [61,63]. Interestingly, ALT-803 treatment increased the percentage of CD8 T cells in the tumor microenvironment in the glioblastoma and rat bladder models [62,64] suggesting IL-15 stimulation may also promote lymphocyte infiltration.
The safety, pharmokinetics, and immunological effects of ALT-803 has been assessed in nonhuman primates. Healthy cynomolgus monkeys were administered a multidose i.v. treatment of the fusion protein at 0.1 and 0.03 mg/kg. The pharmokinetic analysis estimated the half-life of ALT-803 between 7.2–8 h. Treatment with ALT-803 induced dose-dependent increases in peripheral blood lymphocytes, primary NK, CD8 and CD4 memory T cells, as well as lymphocytic infiltration in the liver, kidneys, and lungs. No significant treatment-dependent effects were seen with other blood cell types. These results contrast with previous studies of rhIL-15 administration in rhesus macaques where the major toxicity reported was grade 3/4 transient neutropenia [33,34]. More importantly, the administration of ALT-803 did not stimulate the release of the cytokines TNF-α, IL-4, or IL-10, suggesting that ALT-803 might not trigger a cytokine storm in clinical settings. The results from murine and nonhuman primate studies of ALT-803 supported the phase I clinical evaluation of weekly dosing of ALT-803, which has been initiated in patients for the treatment of advanced solid tumors, multiple myeloma, and relapsed hematologic malignancy following allogenic stem cell transplant (Table 2).
Overall, ALT-803 and the other IL-15 superagonists show superior immunostimulatory and anti-tumor activity compared to treatment with rIL-15 alone in preclinical models. Presently, it is not clear how different these various IL-15 agonists will act in the clinical setting. Elucidating differences may be a challenge as commercial agencies will not have access to the other IL-15 agonists to perform side-by side comparisons. In general, there is the assumption that all the IL-15 agonists mediate the same basic responses but with different strengths and potencies. With increased strength of signal may come preferential stimulation by specific lymphocytes subsets or extend the actions of IL-15 to cell types that typically are only minimally stimulated by IL-15 (ie. Naïve T cells). Furthermore, more intense IL-15 signaling may lead to the lymphocyte exhaustion or increased toxicities. Fig. 1 depicts the speculated differences in the strength of IL-15 signal mediated by each agent. Overall, while increased strength of IL-15 signal and potency is thought to translate into increased clinical efficacy, this assumption needs to be tested.
5. Using IL-15 in combination
The hope of finding a single agent therapy effective in mediating widespread complete tumor regression and durable anti-tumor responses is challenging because of the multiple immunological barriers present in tumors. As such, the field is focusing on the promise of targeting multiple mechanisms to enhance the immune responses to tumors through the use of combination therapies. Identifying the ideal combination therapy for all the various types of cancers is the current challenge at hand. With its well-established abilities to promote anti-tumor responses, IL-15-based agents can be included among the arsenal. The goal is that combining IL-15 agonists with other immunotherapies that stimulate distinct pathways or deplete immunesuppressing cells may work synergistically to activate anti-tumor immunity and generate improved clinical outcomes. Combinatorial approaches with IL-15 or IL-15 agonists that further enhance immune and anti-tumor responses have already been examined. These approaches involve: 1) combining IL-15 with chemotherapy agents, 2) combining IL-15 with immune checkpoint blockades, 3) augmenting IL-15Rα expression with agonistic anti-CD40 Abs, or 4) fusing IL-15 or IL-15/IL-15Rα complexes to proteins or antibodies that increase both immunostimulatory and antibody cytotoxicity functions.
The first approach combines IL-15 or IL-15 agonists with chemotherapy. Chemotherapy can enhance anti-tumor responses by reducing tumor burden, inducing release of tumor antigens upon tumor cell death, and reducing immunosuppressive populations. In multiple murine tumor models, the combination of IL-15 stimulation plus chemotherapy results in increased tumor regression, prolonged survival, and protection against tumor recurrence as compared to chemotherapy alone [68–70]. Cyclophosphamide (CY) treatment can induce remission in mice given 76-9 rhabdomyosarcoma cells locally. Treatment with rIL-15 prolonged this CY-induced remission, which was associated with increased NK cell activity [68]. In addition, CY + IL-15 enhanced the anti-tumor activity of adoptively transferred NK cells. Using this same tumor line in a pulmonary metastasis model, complete eradication of experimental pulmonary metastases could only be achieved by treatment with the combination of CY + IL-15, but not with either agent alone [69]. In both model systems, NK and CD8 T cells were required for successful combination therapy. Since IL-15 has been shown to induce the production of TNF-α and IFN-γ from T cells and NK cells [71,72], the administration of IL-15 after CY injection may further promote the production of Th1-related cytokines induced by CY. In addition to CY, IL-15 has also been shown to potentiate the anti-tumor activity of other chemotherapeutic agents, such as 5-fluorouracil, leucovorin, and gemcitabine [73,70]. Since gemcitabine induces a dramatic reduction in the number of MDSCs [74,75], this may be an example of how IL-15 stimulation can be more effective when immune suppression is removed. Taken together, these studies suggest that IL-15 signaling potentiates the anti-tumor action of chemotherapy or vice versa that could be an effective approach against tumor recurrence and metastasis.
A second approach that has demonstrated success in enhancing anti-tumor responses combines IL-15 or IL-15/IL-15Rα with checkpoint blocking antibodies targeting either PD-L1 or CTLA-4. Two studies have demonstrated that blocking PD-L1 and CTLA-4 increases the efficacy of rIL-15 in enhancing anti-tumor T cell responses in metastatic colon cancer and prostate cancer models [76,47]. In a murine metastatic colon carcinoma model, treatment with rIL-15 alone (daily treatment for 3 weeks) extended the survival of mice to 44 days compared to 19 days with PBS treatment [47]. Addition of αPD-L1 and αCTLA-4 mAbs further increased survival (median survival 74 days) while combination with either αPD-L1 or αCTLA-1 mAbs did not. IL-15 treatment also dramatically reduced the number of tumor lung nodules (∼20 nodules in IL-15 treated mice/∼ 130 nodules in control group), which was further reduced with IL-15 plus αPD-L1/αCTLA-4 (< 5 nodules) treatment. Whereas IL-15 alone increases IFN-γ production and PD-1 expression by CD8 T cells, the addition of IL-15 plus αPD-L1 decreased PD-1 expression and further increased IFN-γ secretion [47]. Combination therapy with IL-15 superagonists (RLI and ALT-803) and blocking antibodies against PD-L1 and CTLA-4 have also been shown to increase survival and reduce tumor growth in colon and glioblastoma murine tumor models, as compared to one of the treatments alone [67,61,62]. While αPD-L1 mAb alone was able to delay growth of colon carcinomas and rarely induced remission, combined treatment with αPD-L1 mAb plus RLI resulted in reduced tumor growth, longer survival, and tumor regression in 40% of mice [67,61,62]. Among the combination-treated tumor-free mice, all mice remained tumor free after second inoculation demonstrating the induction of an efficient adaptive memory immune response. IL-15 superagonist ALT-803 also has an additive effect in combination with αPD-L1 mAb on survival of glioblastoma tumor-bearing mice [62]. In the pulmonary metastasis model of CT26 colon carcinoma cells, administration of ALT-803 plus αCTLA-4, but not αPD-L1, synergistically increased the survival of CT26-bearing mice [67,61,62]. However, αPD-L1 in conjunction with both ALT-803 and αCTLA-4 further improved the survival of CT26 mice. These preclinical findings have shown that checkpoint blockade therapy in combination with IL-15 or IL-15/IL-15Rα complexes is a promising strategy to induce anti-tumor immune responses in vivo.
The third approach utilizes IL-15 in combination with CD40 stimulation. CD40 ligation leads to the stimulation of DCs resulting in a cascade of events including IL-12 production as well as increased IL-15Rα expression [77]. Co-administration of murine rIL-15 with the anti-CD40 mAb (clone FGK4.5) resulted in reduced tumor burden and prolonged survival in established TRAMP-C2 prostate cancer and two colon cancer models, compared to treatment with either agent alone [78,79]. In the TRAMP-C2 model, the combination regimen increased the numbers of tumor-specific CD8 T cells and induced protection from tumor development upon challenge with tumor cells. NK cells also showed stronger cytolytic activity in the presence of anti-CD40 mAb plus IL-15 than in the presence of either alone. Furthermore, less efficacy was observed by the combination therapy in tumor-bearing IL-15Rα−/− mice, compared to wild-type mice suggesting anti-CD40 mAb-induced expression of IL-15Rα by DCs was critical for the synergistic effect of the combination regimen on NK cell activation. However, other CD40-mediated signals may also contribute to the synergistic effects of this combination treatment. CD40 stimulation also increases production of endogenous sIL-15/IL-15Rα complexes [16].
The last approach involves fusing IL-15 or IL-15 complexes to proteins or antibodies that specifically directs IL-15 to the tumor site and/or mediates an additional immunostimulatory function or cytotoxicity function. For example, a triple fusion protein was constructed by Ochoa and colleagues that combines apolipoprotein A–I (Apo A–I), IL-15 and IL-15Rα’s sushi domain [80]. Apo A-I is the main protein component of the high-density lipoproteins (HDL) that collects cholesterol from tissues and brings it to the liver. The combination treatment delivered to the liver by hydrodynamic transfer was shown to dramatically increase the numbers of NK and memory CD8 T cells in the liver, spleen, and lungs of control wild-type mice. In addition, administration of this fusion protein resulted in the successful treatment of B16OVA melanoma lung and MC38 colon cancer liver metastatic tumors [80]. In a second approach, the IL-15 agonist, RLI is fused to the heavy chain of an anti-GD2 ganglioside antibody, which targets several tumors of neuroectodermal origin and is itself a promising cancer treatment [81]. This anti-GD2-RLI immunocytokine retains both the cytotoxic effector functions of the antibody as well as the biological activity of the cytokine. This reagent inhibited tumor development and increased survival in two murine models of syngeneic cancers, namely, subcutaneous EL4 lymphomas and metastatic NXS2 neuroblastomas [81]. In addition to a two protein fusion, a three protein fusion has been generated comprised of a tumor-specific antibody, IL-15/IL-15Rα, and the co-stimulatory ligand 4-1BBL (also known as CD137). This trifunctional fusion was effective in reducing metastasis in a melanoma (B16-FAP) tumor mouse model, which was mediated by an increase in proliferation and activation of tumor-specific CD8 memory T cells [82]. Since rhIL-15 increased B leukemic cell depletion in the presence of anti-CD20 antibodies [83], a rationale emerged to fuse IL-15 to CD20 targeting proteins. The fusion product of the IL-15 agonist, RLI to Rituximab (anti-CD20 Ab), which targets B cell lymphomas, was found to be more effective in inducing long term survival of lymphoma-bearing SCID mice than either RLI or Rituximab alone [84]. Additionally, a fusion of IL-15 agonist, ALT-803 with single chain antibody chains of Rituximab also induced more robust anti-lymphoma responses in mice and mediated depletion of B cells in cynomolgus monkeys [85,86]. Altogether, these studies demonstrate that fusing IL-15 analogs to various scaffolds/antibodies can retain IL-15 activity as well as mediate more specific cell targeting.
Overall, combining IL-15 stimulation with other immune-based therapies can drive superior anti-tumor immune responses, compared to the individual treatments alone, thus making this a worthwhile approach to continue exploring in clinical settings.
6. IL-15 clinical trials
The demonstration that IL-15 or IL-15 analogs mediates effective anti-tumor responses in preclinical studies has led to the implementation of IL-15-based therapies in several registered clinical trials. These involve the administration of rhIL-15 alone or in combination with NK transfer or chemotherapy in patients with solid tumors and hematological malignancies (Table 2). The most active initiatives being evaluated comprise the IL-15 superagonists, ALT-803 and heterodimeric IL-15 in Phase I/II and III studies involving solid and hematological cancers (Table 2). The primary goal of these studies is to evaluate the safety and to determine both the maximum tolerated dose and the minimum effective dose. The secondary objectives include determining the immunogenicity and pharmacokinetic profiles of these IL-15 agonists. A recent study has described a case report of a patient with BCG-unresponsive non-muscle invasive bladder cancer treated with intravesical ALT-803 and BCG weekly for 6 weeks [87]. During treatment, only minor fatigue was experienced and 19 months later, the patient is tumor free [87]. These findings exemplify the potential for ALT-803 to be used locally to promote anti-tumor responses, its ability to be used in combination with other therapeutics, and induce durable responses. Whereas recent endeavors have addressed the problems of short in vivo half-life of IL-15 and are close to identifying the optimal dose and route, the future challenges of developing successful IL-15-based therapies will be in determining the most effective combination therapy and the type of tumors that will be most responsive to treatment. Lastly, the question lies in whether these endeavors utilizing IL-15 agents will be more effective as a cancer immunotherapy than the numerous other combinational approaches that currently being explored.
7. Conclusions
IL-15 has evolved to become one of the most promising molecules for cancer immunotherapy, due to its ability to stimulate the very immune effector cells that are so well-equipped to target cancer cells, i.e. cytotoxic T cells and NK cells. Not only does IL-15 increase these effector cells but also enhances their cytotoxic functions. The IL-15-mediated increases in numbers and functional abilities translates to enhanced anti-tumor responses in several experimental animal models of cancers. Despite the promising preclinical responses of rIL-15, success in the clinical setting was limited by its short in vivo half-life until more recently. To date, three main modifications of IL-15 have been made to generate more potent soluble IL-15 agonists (Fig. 1): 1) combining IL-15 with a soluble IL-15Rα or the sushi domain of IL-15Rα; 2) fusing sIL-15Rα to the Fc portion of human IgG1; 3) mutating IL-15 to increase its affinity to IL-2Rβ/γC complexes. All formulations of IL-15 agonists have demonstrated increased strength and duration of IL-15 receptor signaling and subsequent enhanced anti-tumor immunity. Therefore, these IL-15 based agents are currently undergoing clinical trials in studies of numerous cancer types, therapeutic applications, and in combination with other immunotherapy. The current challenges of developing successful IL-15-based immune therapies for cancer are identifying the optimal dose and route that achieves a maximize response with limited toxicity and distinguishing functional differences between the various formulations. Uncontrolled responses to IL-15 could exert potentially harmful effects by increasing the production of proinflammatory cytokines or promoting autoimmune-like responses. Indeed, IL-15 expression is increased in individuals with rheumatoid arthritis (RA), inflammatory bowel disease (IBD), systemic lupus erythematosus (SLE), and inflammatory synovitis [48]. Similarly, certain lymphoid malignancies are associated with aberrant IL-15 expression [88,89]. Additional challenges will be in identifying the type of tumors that will be most responsive to treatment and the tumor attributes that dictate this.
Acknowledgments
The authors thank Scott M. Anthony for critically reading the manuscript.
Funding sources
Funding support is received from Cancer Prevention Research Institute of Texas (to K.S.S.), National Institutes of Health (to K.S.S.), and Nektar Therapeutics (to K.S.S.)
Funding sources were not involved in the content of this article.
Abbreviations
- IL-15
Interleukin 15
- NK cells
natural killer cells
- NHP
non-human primates
- Tregs
regulatory T cells
- RLI
receptor-linker-IL-15
- CY
cyclophosphamide
References
- 1.Rosenberg SA, Yang JC, White DE, et al. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–319. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 4.Smith FO, Downey SG, Klapper JA, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14:5610–5618. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, et al. Safety activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali AK, Nandagopal N, Lee SH. IL-15-PI3K-AKT-mTOR: a critical pathway in the life journey of natural killer cells. Front Immunol. 2015;6:355. doi: 10.3389/fimmu.2015.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodolce JP, Boone DL, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 14.Stonier SW, Ma LJ, Castillo EF, et al. Dendritic cells drive memory CD8 T cell homeostasis via IL-15 trans-presentation. Blood. 2008:4546–4554. doi: 10.1182/blood-2008-05-156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortier E, Advincula R, Kim L, et al. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8(+) T cell subsets. Immunity. 2009;31:811–822. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Anthony SM, Howard ME, Hailemichael Y, et al. Soluble interleukin-15 complexes are generated in vivo by type I interferon dependent and independent pathways. PLoS One. 2015;10:e0120274. doi: 10.1371/journal.pone.0120274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anthony SM, Rivas SC, Colpitts SL, et al. Inflammatory signals regulate IL-15 in response to lymphodepletion. J Immunol. 2016;196:4544–4552. doi: 10.4049/jimmunol.1600219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubinstein MP, Kovar M, Purton JF, et al. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergamaschi C, Rosati M, Jalah R, et al. Intracellular interaction of interleukin-15 with its receptor {alpha} during production leads to mutual stabilization and increased bioactivity. J Biol Chem. 2008;283:4189–4199. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- 21.Schluns KS, Klonowski KD, Lefrancois L. Transregulation of memory CD8 T-cell proliferation by IL-15R alpha(+) bone marrow-derived cells. Blood. 2004;103:988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 22.Burkett PR, Koka R, Chien M, et al. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proc Natl Acad Sci U S A. 2003;100:4724–4729. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koka R, Burkett PR, Chien M, et al. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burkett PR, Koka R, Chien M, et al. Coordinate expression and trans presentation of interleukin (IL)-15R{alpha} and IL-15 supports natural killer cell and memory CD8+ t cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fyfe GA, Fisher RI, Rosenberg SA, et al. Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1996;14:2410–2411. doi: 10.1200/JCO.1996.14.8.2410. [DOI] [PubMed] [Google Scholar]
- 26.Klapper JA, Downey SG, Smith FO, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenstein M, Ettinghausen SE, Rosenberg SA. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986;137:1735–1742. [PubMed] [Google Scholar]
- 28.Anderson TD, Hayes TJ, Gately MK, et al. Toxicity of human recombinant interleukin-2 in the mouse is mediated by interleukin-activated lymphocytes. Separation of efficacy and toxicity by selective lymphocyte subset depletion. Lab Invest. 1988;59:598–612. [PubMed] [Google Scholar]
- 29.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 31.Berard M, Brandt K, Bulfone-Paus S, et al. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018–5026. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 32.Marks-Konczalik J, Dubois S, Losi JM, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waldmann TA, Lugli E, Roederer M, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117:4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger C, Berger M, Hackman RC, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson DM, Kumaki S, Ahdieh M, et al. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15RA and IL2RA genes. J Biol Chem. 1995;270:29862–29869. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 36.Giri JG, Kumaki S, Ahdieh M, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo SR, Fuertes MB, Corrales L, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Sun S, Hwang I, et al. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 39.Sneller MC, Kopp WC, Engelke KJ, et al. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood. 2011;118:6845–6848. doi: 10.1182/blood-2011-09-377804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lugli E, Goldman CK, Perera LP, et al. Transient and persistent effects of IL-15 on lymphocyte homeostasis in nonhuman primates. Blood. 2010;116:3238–3248. doi: 10.1182/blood-2010-03-275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picker LJ, Reed-Inderbitzin EF, Hagen SI, et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conlon KC, Lugli E, Welles HC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33:74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeshita T, Asao H, Ohtani K, et al. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992;257:379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- 44.Tang F, Zhao LT, Jiang Y, et al. Activity of recombinant human interleukin-15 against tumor recurrence and metastasis in mice. Cell Mol Immunol. 2008;5:189–196. doi: 10.1038/cmi.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi H, Dubois S, Sato N, et al. The role of trans-cellular IL-15-presentation in the activation of NK-mediated killing, which leads to enhanced tumor immunesurveillance. Blood. 2005;105:721–727. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 47.Yu P, Steel JC, Zhang M, et al. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010;16:6019–6028. doi: 10.1158/1078-0432.CCR-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anthony SM, Schluns KS. Emerging roles for IL-15 in the activation and function of T cells during immune stimulation. Res Rep Biol. 2015;6:25–37. [Google Scholar]
- 49.Fehniger TA, Suzuki K, Ponnappan A, et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193:219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eisenman J, Ahdieh M, Beers C, et al. Interleukin-15 interactions with interleukin-15 receptor complexes: characterization and species specificity. Cytokine. 2002;20:121–129. doi: 10.1006/cyto.2002.1989. [DOI] [PubMed] [Google Scholar]
- 51.Mortier E, Quemener A, Vusio P, et al. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 × IL-15R alpha fusion proteins. J Biol Chem. 2006;281:1612–1619. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 52.Bergamaschi C, Bear J, Rosati M, et al. Circulating interleukin-15 (IL-15) exists as heterodimeric complex with soluble IL-15 receptor alpha (IL-15Ralpha) in human serum. Blood. 2012;120:1–8. doi: 10.1182/blood-2011-10-384362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chertova E, Bergamaschi C, Chertov O, et al. Characterization and favorable in vivo properties of heterodimeric soluble IL-15. IL-15Ralpha cytokine compared to IL-15 monomer. J Biol Chem. 2013;288:18093–18103. doi: 10.1074/jbc.M113.461756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watson DC, Bayik D, Srivatsan A, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. doi: 10.1016/j.biomaterials.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubois S, Patel HJ, Zhang M, et al. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 56.Czajkowsky DM, Hu J, Shao Z, et al. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med. 2012;4:1015–1028. doi: 10.1002/emmm.201201379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elpek KG, Rubinstein MP, Bellemare-Pelletier A, et al. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proc Natl Acad Sci U S A. 2010;107:21647–21652. doi: 10.1073/pnas.1012128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Epardaud M, Elpek KG, Rubinstein MP, et al. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 59.Bessard A, Sole V, Bouchaud G, et al. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol Cancer Ther. 2009;8:2736–2745. doi: 10.1158/1535-7163.MCT-09-0275. [DOI] [PubMed] [Google Scholar]
- 60.Xu W, Jones M, Liu B, et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res. 2013;73:3075–3086. doi: 10.1158/0008-5472.CAN-12-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim PS, Kwilas AR, Xu W, et al. IL-15 superagonist/IL-15RalphaSushi-Fc fusion complex (IL-15SA/IL-15RalphaSu-Fc; ALT-803) markedly enhances specific sub-populations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget. 2016;7:16130–16145. doi: 10.18632/oncotarget.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mathios D, Park CK, Marcus WD, et al. Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int J Cancer. 2016;138:187–194. doi: 10.1002/ijc.29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhode PR, Egan JO, Xu W, et al. Comparison of the superagonist complex ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol Res. 2016;4:49–60. doi: 10.1158/2326-6066.CIR-15-0093-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomes-Giacoia E, Miyake M, Goodison S, et al. Intravesical ALT-803 and BCG treatment reduces tumor burden in a carcinogen induced bladder cancer rat model; a role for cytokine production and NK cell expansion. PLoS One. 2014;9:e96705. doi: 10.1371/journal.pone.0096705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Felices M, Chu S, Kodal B, et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol. 2007;145:453–461. doi: 10.1016/j.ygyno.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bailey CP, Budak-Alpdogan T, Sauter CT, et al. New interleukin-15 superagonist (IL-15SA) significantly enhances graft-versus-tumor activity. Oncotarget. 2017;8:44366–44378. doi: 10.18632/oncotarget.17875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Desbois M, Le VP, Coutzac C, et al. IL-15 trans-Signaling with the superagonist RLI promotes Effector/Memory CD8+ t cell responses and enhances antitumor activity of PD-1 antagonists. J Immunol. 2016;197:168–178. doi: 10.4049/jimmunol.1600019. [DOI] [PubMed] [Google Scholar]
- 68.Evans R, Fuller JA, Christianson G, et al. IL-15 mediates anti-tumor effects after cyclophosphamide injection of tumor-bearing mice and enhances adoptive immunotherapy: the potential role of NK cell subpopulations. Cell Immunol. 1997;179:66–73. doi: 10.1006/cimm.1997.1132. [DOI] [PubMed] [Google Scholar]
- 69.Chapoval AI, Fuller JA, Kremlev SG, et al. Combination chemotherapy and IL-15 administration induce permanent tumor regression in a mouse lung tumor model: NK and T cell-mediated effects antagonized by B cells. J Immunol. 1998;161:6977–6984. [PubMed] [Google Scholar]
- 70.Sun H, Liu D. IL-15/sIL-15Ralpha gene transfer suppresses Lewis lung cancer growth in the lungs, liver and kidneys. Cancer Gene Ther. 2016;23:54–60. doi: 10.1038/cgt.2015.67. [DOI] [PubMed] [Google Scholar]
- 71.McInnes IB, Leung BP, Sturrock RD, et al. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 72.Carson WE, Ross ME, Baiocchi RA, et al. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J Clin Invest. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao S, Troutt AB, Rustum YM. Interleukin 15 protects against toxicity and potentiates antitumor activity of 5-fluorouracil alone and in combination with leucovorin in rats bearing colorectal cancer. Cancer Res. 1998;58:1695–1699. [PubMed] [Google Scholar]
- 74.Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 75.Ko HJ, Kim YJ, Kim YS, et al. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 76.Yu P, Steel JC, Zhang M, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci U S A. 2012;109:6187–6192. doi: 10.1073/pnas.1203479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dubois SP, Waldmann TA, Muller JR. Survival adjustment of mature dendritic cells by IL-15. Proc Natl Acad Sci U S A. 2005;102:8662–8667. doi: 10.1073/pnas.0503360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang M, Yao Z, Dubois S, et al. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci U S A. 2009;106:7513–7518. doi: 10.1073/pnas.0902637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang M, Ju W, Yao Z, et al. Augmented IL-15Ralpha expression by CD40 activation is critical in synergistic CD8 T cell-mediated antitumor activity of anti-CD40 antibody with IL-15 in TRAMP-C2 tumors in mice. J Immunol. 2012;188:6156–6164. doi: 10.4049/jimmunol.1102604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ochoa MC, Fioravanti J, Rodriguez I, et al. Antitumor immunotherapeutic and toxic properties of an HDL-conjugated chimeric IL-15 fusion protein. Cancer Res. 2013;73:139–149. doi: 10.1158/0008-5472.CAN-12-2660. [DOI] [PubMed] [Google Scholar]
- 81.Vincent M, Bessard A, Cochonneau D, et al. Tumor targeting of the IL-15 superagonist RLI by an anti-GD2 antibody strongly enhances its antitumor potency. Int J Cancer. 2013;133:757–765. doi: 10.1002/ijc.28059. [DOI] [PubMed] [Google Scholar]
- 82.Kermer V, Hornig N, Harder M, et al. Combining antibody-directed presentation of IL-15 and 4-1BBL in a trifunctional fusion protein for cancer immunotherapy. Mol Cancer Ther. 2014;13:112–121. doi: 10.1158/1535-7163.MCT-13-0282. [DOI] [PubMed] [Google Scholar]
- 83.Laprevotte E, Voisin G, Ysebaert L, et al. Recombinant human IL-15 trans-presentation by B leukemic cells from chronic lymphocytic leukemia induces autologous NK cell proliferation leading to improved anti-CD20 immunotherapy. J Immunol. 2013;191:3634–3640. doi: 10.4049/jimmunol.1300187. [DOI] [PubMed] [Google Scholar]
- 84.Vincent M, Teppaz G, Lajoie L, et al. Highly potent anti-CD20-RLI immunocytokine targeting established human B lymphoma in SCID mouse. MAbs. 2014;6:1026–1037. doi: 10.4161/mabs.28699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu B, Kong L, Han K, et al. A novel fusion of ALT-803 (Interleukin (IL)-15 superagonist) with an antibody demonstrates antigen-specific antitumor responses. J Biol Chem. 2016;291:23869–23881. doi: 10.1074/jbc.M116.733600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosario M, Liu B, Kong L, et al. The IL-15-Based ALT-803 complex enhances fcgammaRIIIa-Triggered NK cell responses and In vivo clearance of B cell lymphomas. Clin Cancer Res. 2016;22:596–608. doi: 10.1158/1078-0432.CCR-15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang J, Schisler J, Wong HC, et al. Intravesical ALT-803 for BCG-unresponsive bladder cancer – a case report, Urol. Case Rep. 2017;14:15–17. doi: 10.1016/j.eucr.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bamford RN, Battiata AP, Burton JD, et al. Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region/IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. Proc Natl Acad Sci U S A. 1996;93:2897–2902. doi: 10.1073/pnas.93.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dobbeling U, Dummer R, Laine E, et al. Interleukin-15 is an autocrine/paracrine viability factor for cutaneous T-cell lymphoma cells. Blood. 1998;92:252–258. [PubMed] [Google Scholar]
- 90.Villinger F, Miller R, Mori K, et al. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 2004;22:3510–3521. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 91.Mueller YM, Petrovas C, Bojczuk PM, et al. Interleukin-15 increases effector memory CD8+ t cells and NK Cells in simian immunodeficiency virus-infected macaques. J Virol. 2005;79:4877–4885. doi: 10.1128/JVI.79.8.4877-4885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


