Abstract
Gait impairment in Multiple Sclerosis (MS) can result from muscle weakness, physical fatigue, lack of coordination, and other symptoms. Walking speed, as measured by a number of clinician-administered walking tests, is the primary measure of gait impairment used by clinical researchers, but inertial gait features from body-worn sensors have been proven to add clinical value. This paper seeks to understand and differentiate the physiological significance of four such features with proven value in MS to facilitate adoption by clinical researchers and incorporation in gait monitoring and analysis systems. In addition, this information can be used to select features which might be appropriate in other forms of disability. Two of the four features are computed using the dynamic time warping (DTW) algorithm: the “DTW Score” is based on the usual DTW distance, and the “Warp Score” is based on the warping length. The third feature, based on kernel density estimation (KDE), is the “KDE Peak” value. Finally, the “Causality Index” is based on the phase slope index between inertial signals from different body parts. Relationships between these measures and the aforementioned gait-related symptoms are determined by applying factor analysis to three common, clinical walking outcomes, then correlating the inertial measures as well as walking speed to each extracted factor. Statistically significant differences in correlation coefficients to the three extracted clinical factors support their distinct physiological meaning and suggest they may have complimentary roles in the analysis of MS-related walking disability.
Index Terms: Inertial Sensors, Multiple Sclerosis, 6-Minute Walk, Gait Impairment, Signal Processing
I. Introduction
Multiple sclerosis (MS) is a chronic progressive neurological disorder affecting about 2.5 million people globally [1]. Impaired mobility is a common symptom of MS even early in the disease course, with significant negative effects on quality of life [2]. As a result, the evaluation and monitoring of walking ability is a central component of the clinical management of persons with MS as well as an ongoing focus of clinical outcomes research.
Assessment of MS-associated walking impairment includes timed walking tests, questionnaires completed by the patient (called patient-reported outcomes, or PROs), and direct evaluation by a clinician, including neurological exam. The last category, clinician evaluation, is typically quantified using the Expanded Disability Status Scale (EDSS), a ten-point scale ranging from zero (normal neurological exam) to ten (death from MS) [3]. This physician-assessed composite score is based on walking ability and seven functional systems: pyramidal, cerebellar, brainstem, sensory, vision, bowel and bladder, and cognitive. Commonly collected PROs include the Multiple Sclerosis Walking Scale (MSWS-12) [4], a self-reported measure of the impact of MS on walking ability, and the Modified Fatigue Impact Scale (MFIS) [5], which concerns the impact of physical, cognitive, and psychosocial fatigue on daily life.
Due to the multi-factorial nature of walking impairment in MS, all of these components are clinically important, as they tend to capture distinct aspects of walking ability. While walking tests are most commonly the primary outcome for clinical trials, for example, patient-reported outcomes remain important ancillary measures [6]. In this setting, continued exploration of new, objective measures of impairment – coupled with insight into their physiological underpinnings – is needed to develop more reliable and sensitive measures to guide management, provide endpoints for clinical trials, and support research concerning the underlying pathophysiology of MS.
In this work, we explore the physiological and clinical meaning of four recently-developed objective measures with proven benefit in the analysis of MS-associated walking impairment. All four have been derived from inertial data collected during a six-minute walk (6MW) test [7], a longer walking test often favored in research settings. In contrast to shorter walk tests, the 6MW is thought to assess dynamic motor fatigue, particularly in the later minutes of the walk [7]. The first two measures, named the DTW Score and Warp Score [8], were designed to quantify progressive changes to walking patterns occurring over the course of the 6MW. The third measure – the peak of the kernel density estimate (KDE) – relates to the probability density function of the inertial data associated with the walk [9]. Finally, the Causality Index [10] is a measure of causal relationships between different components of the inertial data, including those associated with different body parts. These features have been found to be the most useful among a large pool of features upon extended study – including extensive walk testing – at the University of Virginia Department of Neurology.
To gain insight into the physiological meaning of these inertial gait features, we seek to (1) organize and analyze the results of commonly used walking assessments to facilitate comparisons to the new features; and (2) explore relationships to the existing assessments through correlational analysis. We propose to accomplish (1) through factor analysis (FA), a method of identifying latent factors that adequately predict a dataset of interest, coupled with expert (clinician) labeling of the resulting factors. We hypothesize that statistically significant differences will be oberved when when correlating inertial features of interest to the FA-derived clinical factors. If so, this would establish the distinct physiological meaning of these measures. This meaning could then be described in clinical terms according to the clinician-defined labels.
II. Related Work
A number of efforts have been made to augment walking assessment in MS with additional objective information. Chetta et al. focused on the cardiorespiratory response during the 6MW in 11 MS subjects with mild disability: 6MW distance correlated to disability score but not subjective fatigue [11]. Savci et al. evaluated factors that contribute to functional capacity in MS subjects [12]. Resting heart rate, activities of daily living, subjective fatigue, and EDSS all correlated significantly with 6MW total distance. In [7], Goldman et al. evaluated a modified 6MW in MS subjects with varying disability and controls to assess reliability. The authors also assessed 6MW distance correlation to subjective measures of fatigue, physical function and ambulation.
Other work concerning inertial gait features includes the research of Aminian et al., who performed gait analysis on patients before hip arthroplasty and again three, six and nine months afterwards. The authors observed a mean decrease of 88% of asymmetry of stance time and a mean decrease of 250% of asymmetry of double support time nine months after the operation [13]. These results show that gait analysis can be used to evaluate gait performance. Pau et al. observed that inertial gait features like stride length, gait speed, cadence, double support duration, stance, and swing duration were significantly modified in MS subjects with higher EDSS compared to healthy controls and subjects with less severe disability [14]. Claas et al. showed that accelerometer-based features detected freezing of gait episodes with accuracies above 90% with a single waist sensor [15], and Le Moyne [16] derived machine learning features from body-worn inertial sensors to distinguish gait patterns between a person with Friedreich’s ataxia and a healthy subject. All of these papers compare clinical scores with gait features, but place less emphasis on physiological meaning.
While we have focused on the collection of inertial data using wearable devices, it is important to note that nonintrusive, vision-based methods such as [17] offer an alternative approach to gait analysis, one that is less portable but does not require a device to be worn.
Our prior investigations of DTW, KDE, and the Causality Index applied to MS have yielded several results important to the current analysis. The DTW Score and Warp Score complement walking speed in the analysis of MS-associated walking impairment; for example, the Warp Score is correlated with the MSWS (rs = 0.429, p < 0.001), EDSS (rs = 0.367, p < 0.001) and MFIS (rs = 0.273, p < 0.001) [8]. Prior investigation of the KDE peak has demonstrated significant correlations to the MFIS (r = 0.437, p < 0.0001) as well as item 5 of the MSWS (r = 0.620, p < 0.0001) [9]. Finally, study of the Causality Index in MS has established significant correlations to the MSWS (r = −0.562, p < 0.001) and EDSS (r = −0.436, p < 0.001) [18].
III. Methods
A. Participants and Data Collection
The University of Virginia (UVa) Institutional Review Board approved all study procedures, and written consent was obtained from all participants. Subjects with clinically definite MS [19] were recruited from the UVa Neurology Department outpatient clinic population. All subjects were age 18–64 years and able to walk for six minutes. Those with neurological impairment from other diagnoses, orthopedic limitations, morbid obesity, or known cardiac or respiratory disease were excluded. All fatigue-related medications were withheld 48 hours prior to study procedures.
Clinical outcomes were assessed in both MS subjects and controls, who had no neurological impairment or gait pathology. A neurological exam was performed by Neurostatus-certified staff for EDSS [3] assessment prior to the 6MW. The Modified Fatigue Impact Scale (MFIS) [5] was collected in all subjects, whereas the MS Walking Scale (MSWS-12) [4] was collected only in subjects with MS, as it explicitly pertains to MS-associated symptoms. In keeping with other evaluations of the 6MW in MS, walking impairment was classified by EDSS as mild (0–2.5), moderate (3.0–4.0), or severe (≥ 4.5) [11]. However, the current analysis is dimensional rather than categorical, so disease severity and control/MS status has been used only to describe the demographics of participants.
The 6MW was completed in a 75-foot hospital corridor using the script developed by Goldman et al., which instructs subjects to walk ”as far as possible and as fast as possible” [11]. Subjects wore an ActiGraph GT3X accelerometer on their non-dominant hip and 6-axis inertial sensor nodes (3 accelerometer axes and 3 gyroscope axes per node) on each wrist, each ankle, and the sacrum. Hardware and calibration details for the 6-axis nodes have been previously described [20]. Distance was manually recorded in 1-minute epochs. Sampling rates were 30 Hz and 128 Hz for ActiGraph and our 6-axis sensor nodes, respectively, both of which are sufficient to capture walking dynamics.
B. Factor Analysis
As a first step toward comprehensive, comparative analysis of the four inertial features, a factor analysis was performed on elements of the MSWS-12, MFIS, and EDSS collectively using the psych package for R [21]. This step was designed to reduce the large number of clinical measurements related to walking into a manageable number of factors so that relationships to the four inertial measures could be meaningfully quantified and compared.
Three classical tests were performed to determine the optimal number of latent factors: parallel analysis, an optimal coordinate scree test, and an accelerator factor scree test. In the parallel analysis, factors are only retained if their eigenvalue is greater than or equal to the average of eigenvalues from multiple correlation matrices generated with random observations. Here the number of matrices was 100, and the eigenvalue threshold was placed at the 95th percentile. In the optimal coordinate scree test, eigenvalues for all factors are graphed, and the optimal number of factors is determined as the number of eigenvalues preceding the scree, defined as the point beyond which the plot behaves randomly. Finally, in the accelerator factor scree test, a similar procedure is followed, but the number of optimal factors is determined as the point where the acceleration of the curve is maximum.
To determine the relationship between the latent factors identified through this analysis and our inertial gait features, factor scores for each participant were calculated. Then, Pearson correlations between these scores and all features (described below) were calculated. Finally, derived factors were given a clinically-informative label through inspection of factor loadings by a panel of three MS clinicians not otherwise connected to this work.
C. Description of Features
This section briefly describes the four features derived from 6MW inertial data which are subsequently analyzed. The first two features, the DTW Score and Warp Score, are based on the DTW algorithm [22] and derived from acceleration data from ActiGraph. The third feature, namely the peak of the kernel density estimate [9] (KDE Peak), is derived from the squared magnitude of the gyroscope signal (3D angular velocity) from the 6-axis nodes worn at the ankle. The fourth feature, the Causality Index, is based on the phase slope index, as subsequently described, and draws upon all five of the 6-axis nodes [10].
1) Dynamic Time Warping
The DTW algorithm is a method of determining the optimal alignment between two sequences using dynamic programming. A detailed description of DTW is beyond the current scope, but may be found in [22], and its application to gait pathology is described in [23]. The approach is essentially one of template matching: test sequences are compared to baseline sequences from the same subject, so that accumulated differences between current gait cycles and the known baseline can be quantified on an ongoing basis. This algorithm has been incorporated in gait recognition [24] and more recently to evaluate gait pathology [23]. In the current work, gait cycles are aligned with DTW to generate two distinct measures: the DTW Score and the Warp Score. The DTW Score – which is based on the usual DTW distance reported elsewhere in the literature – summarizes the degree of similarity between sequences following alignment. The Warp Score, a novel measure first presented in [8], summarizes the number of “warps”, or repetitions of samples, needed to achieve this optimal alignment.
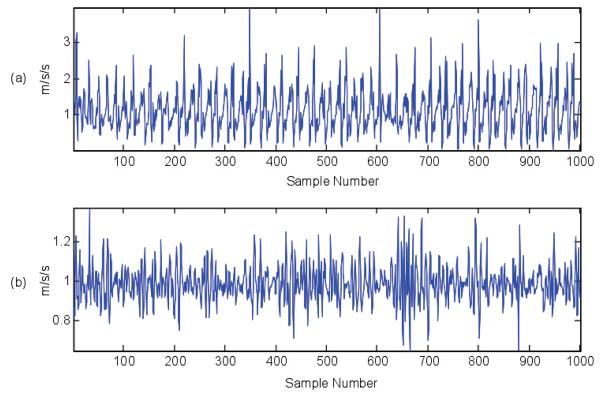
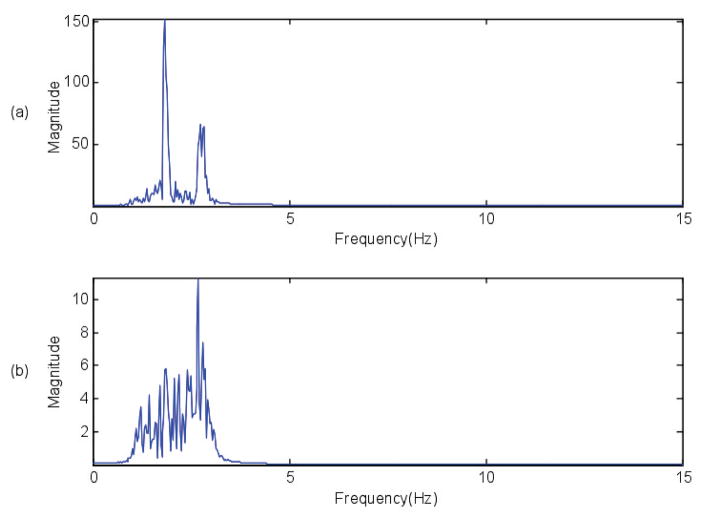
To compute DTW metrics, individual 6MW gait cycles are required. Gait patterns in MS are quite variable, as shown Figure 1, due to the heterogeneity of MS-associated gait pathology, so frequency-based cycle segmentation was required to extract individual cycles. Walking is predominantly a low frequency activity, thus a bandpass filter (1–3 Hz) was applied prior to frequency domain analysis. Figure 2 shows the frequency spectrum of the 6MW data from Figure 1 after bandpass filtering. This filtered data is used to identify gait cycles, which are resampled to a common length and passed to the DTW algorithm. Please refer to [25] for a more detailed explanation of gait extraction steps.
Fig. 1.
Six-minute walk accelerometer data. The top and bottom figures show 1000 data points from two different subjects
Fig. 2.
Frequency of 6MW after applying the bandpass filter for the data sample shown in Figure 1. The peak magnitude of frequency spectrum, which is clearly visible in both the cases, is used to obtain the gait length.
Given gait cycles P = (p1, p2, p3, …, pn) and Q = (q1, q2, q3, …, qn) of equal length (achieved through resampling), the outputs of the DTW algorithm may be described in terms of corresponding aligned, “warped” sequences PW and QW produced by our implementation of the algorithm:
| (1) |
| (2) |
where (·)k means k repetitions of a given sample. Given this representation, the DTW distance between P and Q is the Euclidean distance between PW and QW, and the warping length may simply be computed by the following:
| (3) |
For each subject, composite DTW Scores and Warp Scores were calculated as previously described in [25] based on comparison of all gait cycles collected in the second minute of the walk with all gait cycles collected in the final (sixth) minute. Briefly, the composite scores summarize DTW-based calculations between two sets of gait cycles as single values by reducing a matrix of values calculated between pairs of cycles as follows. First, each row and column is reduced to its minimum value, which represents the best match between each cycle and all cycles belonging to the other set. The median value over rows and columns is then taken, which makes the process less sensitive to outlying or unusual cycles compared to a sum or mean. Finally, the row-wise and column-wise median values are averaged.
Baseline measurements were taken from minute 2 to allow subjects to establish a consistent cadence, as earlier research has demonstrated significant reduction in speed in the first minute of the 6MW [7].
2) Kernel Density Estimation
Kernel density estimation (KDE) is a standard, non-parametric method to estimate the probability density of a random variable using a given finite data sample [26]. Let X be the underlying random variable with unknown density function, fX (x). We want to estimate fX (x) using n random observations (x1, x2, …, xn) of X. To approximate fX (x), KDE counts observations within distance h of the point of interest x, assigning weights that diminish with distance from x, where h > 0 is the so-called smoothing parameter or bandwidth. Specifically, the kernel estimator can be defined as
| (4) |
where , and K(x) is a non-negative kernel function which assigns the weights to the neighbors of x.
A normal kernel function was used to estimate the density functions at 100 equally spaced inertial gait data amplitude values. The optimal bandwidth was calculated using the standard deviation of the gait sample. The KDE peak computed for every 6MW is used for analysis in the later sections.
3) Causality Index
The causality index is designed to be a whole-body gait assessment integrating gyroscope and accelerometer data from the wrists, ankles, and sacrum. In contrast to other methods, the causality index examines causal relationships between all channels of sensor data, then creates a single index from the causality matrix that encodes all such causal relationships. Briefly, the procedure for generating the causality index from 6MW data consists in these steps: 1) coarse-level segmentation to remove turns and stops from the 6MW inertial data; 2) causal inference based on the Phase Slope Index (PSI) to estimate the causal strength of pairwise relationships between sensor data channels; 3) a pairwise causality matrix that integrates all pairs of channels and places a threshold on the PSI to generate binary pairwise causality matrix; 4) computation of the causality index as the sum of the number of significant relationships remaining after thresholding. Please refer to [10] for a more detailed explanation. The causality index computed for every 6MW is used for analysis in the later sections.
D. Statistical Analysis
Pearson (linear) correlations were used to quantify pairwise relationships between inertial features and the latent factors identified through factor analysis. 95% confidence intervals were calculated for each correlation coefficient, and relationships were considered statistically significant when this confidence interval did not include 0 (i.e. p < 0.05). Statistically significant differences between correlation coefficients have been assessed pairwise using the Fisher r-to-z transformation (one-tailed) [27].
IV. Results
A. Participants
Due of differing data requirements for the various inertial features, not all features were available in all subjects who completed the 6MW. DTW results were based on 115 participants (48 mild MS, 27 moderate MS, 11 severe MS, and 29 control); KDE results were based on 77 participants (29 mild MS, 26 moderate MS, 10 severe MS, and 12 control); and Causality Index results were based on 41 participants (17 mild MS, 11 moderate MS, and 13 control) from the total study population. Clinical outcomes for these participants have been summarized previously [9], [18], [25].
B. Factor Analysis
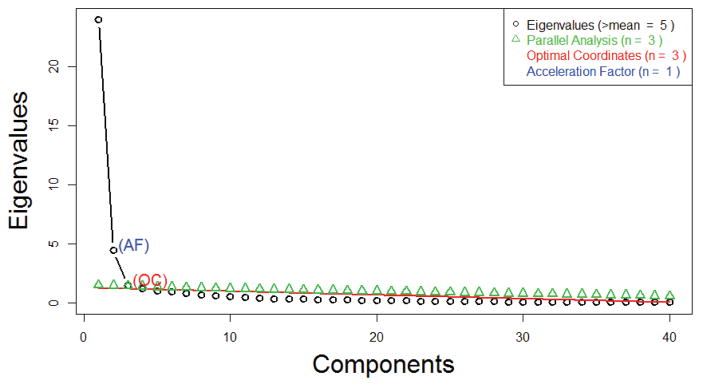
This first phase of the factor analysis, in which the optimal number of factors is determined, is summarized in the scree plot shown in Figure 3.
Fig. 3.
Scree Plot to Determine the Optimal Number of Factors
The parallel analysis and optimal coordinate scree test suggested that 3 was the optimal number of factors, whereas the accelerator factor scree test favored a 1-factor solution. Visually, Figure 3 shows that the ‘elbow’ of the curve is at 3, further supporting the parallel analysis and optimal scree test results. Thus by majority results – as well as the consensus of our clinical collaborators – a 3-factor solution was used.
Table 1 briefly summarizes the variance explained by the extracted factors. While 3 is the optimal number of latent factors in this analysis, a substantial amount of variance in the clinical data remains unexplained. The vision, brainstem, and sensory functional system subscores of the EDSS do not load onto any of the three explained factors, so any variance present in these scores is unexplained. Since these scores are not closely related to walking ability, their failure to load on Factors 1 – 3 may be viewed as divergent validity supporting our interpretation of the factors.
C. Labeling of Factors
Based on the factor loadings presented in Table 2, Factor 3 may be labeled as “Physical Fatigue”, as 6 out of 7 of the items loading on this factor belong to the MFIS physical subscale. The remaining item, MFIS 9, asks whether fatigue has “limited [your] ability to do things away from home”, and is part of the psychosocial subscale [5]. Items on the MFIS cognitive subscale load heavily on Factor 1, as does the cerebral (cognitive) component of the EDSS. The cerebellar component of the EDSS, which pertains to balance and coordination, also loads on this factor, and MSWS items with high Factor 1 loadings emphasize the effects of concentration and coordination on walking ability. For example, MSWS 12 asks whether your MS has “made you concentrate on your walking”. Consequently, our clinician panel has labeled this factor as “Walking Concentration and Coordination”. Lastly, Factor 2 is arguably the most straightforward of the three factors. All MSWS items as well as the pyramidal component of the EDSS load heavily on this factor, and the cerebellar FSS loads less heavily. Most MFIS items loading on this factor relate to physical fatigue. Considered together, these findings suggested to our panel that Factor 2 relates primarily to the strength of the lower limbs as enlisted in walking. Thus, this factor has been labeled as “Walking Strength”.
D. Interpretation of Features
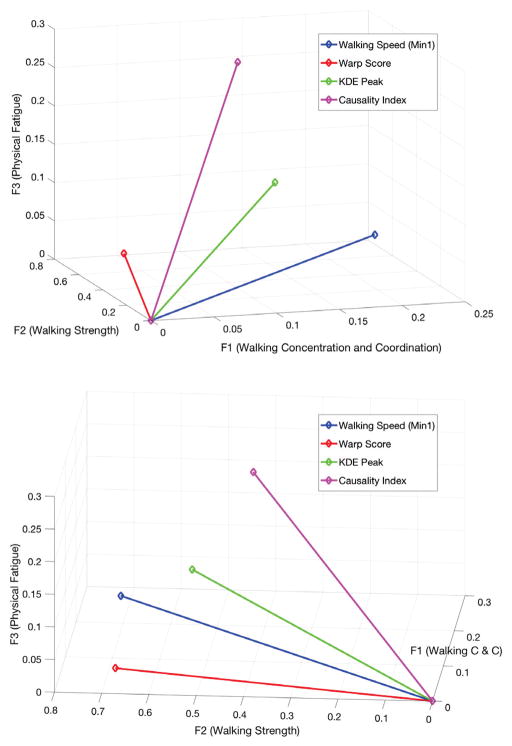
Correlations between each inertial feature and the components of MS-associated walking disability identified through factor analysis are summarized in Table 3, including 95% confidence intervals. These values may also be visualized via the two different projections shown in Figure 4, which plots the correlation coefficients for each feature as a 3-vector. Table 4 summarizes the statistically significant differences between correlation coefficients; when two inertial features differ significantly in correlation to a given factor, that factor has been written in the respective location in Table 4.
Fig. 4.
Correlations between selected inertial features and the three clinical factors plotted as 3-vectors from two perspectives
V. Discussion
As Table 3 and Figure 4 show, all of our objective measurements – including walking speed – demonstrate robust relationships to the categories of walking impairment quantified herein. Not all relationships are equally strong; for example, the Warp Score is most strongly correlated with strength-related disability (F2), whereas the Causality Index is most strongly correlated to physical fatigue. Among all of these measures, it is walking speed that shows the strongest overall relationship to walking disability, specifically F1 and F2. This is not a criticism of the inertial measures, as the relationship between walking disability and walking speed is self-evident and has been repeatedly validated. Instead, we offer yet another confirmation that walking speed, the cornerstone of clinical walking assessment, is strongly associated with several distinct types of neurological impairment affecting walking. Our inertial measures are not intended to replace walking speed as a best measure of disability, but to supplement it.
The factor analysis results themselves are a novel contribution of this work. They are intended as a first step toward more comprehensive exploration of these factors as determinants of walking impairment in MS. In particular, the loading of both MSWS items and MFIS cognitive subscale items on a single latent factor may warrant further study. Walking is known to be a complex cognitive task due to research showing that decreases in walking performance are associated with lowered cognitive functioning [28]. However, little is known concerning the direct impact of concentration and/or cognitive impairment on MS-related walking disability. In future work, inertial gait features may help to untangle a potential direct effect of cognitive factors versus an indirect association by precisely quantifying distinct facets of walking impairment.
It is important to note that while 3 factors were optimal, they do not explain 28% of the clinical data, including variation on the Sensory functional system of the EDSS. Along with other sensory modalities, this subscore measures proprioception, which helps to coordinate walking through awareness of joint position. Differences in our inertial measures’ sensitivity to the clinical information not quantified in Factors 1 – 3 have not been adequately assessed in this work.
The statistically significant differences in correlation coefficients summarized in Table 4 demonstrate that the four inertial measures do indeed have distinct physiological significance, validating our hypothesis. Most features differ significantly in their correlation to at least one factor, but none differ in more than one. In some cases, these results challenge the conclusions – though not the results – of previous work. For example, the causality index was expected to correlate most strongly with F1 (Concentration & Coordination), yet it is most notable for its correlation to F3 (Physical Fatigue). The Warp Score and walking speed were expected to differ in their correlation to F3, but in fact differ most in correlation to F1. Perhaps these results show that the clinical meaning of these features still is not fully understood; or perhaps they simply reveal a pitfall of naming latent factors and/or trusting intuition to understand inertial features.
In labeling each of the extracted factors via clinical panel, we hope to not only improve understanding of these features’ utility in MS, but also support informed selection of features that are likely to be informative in other disorders.
An important limitation of this study is the fact that participants varied between features, thus the analyses of features were not equally powered to identify correlational relationships to the latent factors. For this reason, the statistical significance of correlation coefficients should be interpreted with caution, particularly when comparing p-values between features.
While all features are based on inertial data, they differ in data collection and processing requirements as well as physiological significance. The DTW-based measures are most convenient to collect, as they require only a single waist-worn accelerometer, and were constructed to measure progressive gait deterioration. The KDE Peak is most convenient from a processing standpoint, as it distribution-based and does not require cycle segmentation. The Causality Index is demanding from a data collection standpoint but less so in terms of processing, requiring only coarse-level segmentation. This measure quantifies interrelationships between signals from different body parts not captured by the other features.
VI. Conclusion
This work demonstrates that four different inertial gait features provide information about MS-associated walking ability that compliments traditional objective measures of walking, which report distance walked and/or walking speed. Each feature captures distinct walking-related information, as demonstrated via correlation to the latent factors underlying a battery of self- and physician-reported walking outcomes. Taken together, our results emphasize the multi-dimensional nature of walking and walking impairment in MS, and suggest that sensor-based measures have an important role to play in distinguishing between these different facets of impairment. The four inertial features discussed in this work should be used together with walking speed, not instead of it, and in fact have been designed to capture information about walking that is not directly related to speed. By exploring the physiological significance of objective, sensor-based measures of walking, this work supports continued clinical and research applications of inertial features to MS and other pathologies affecting gait.
TABLE I.
Variance Explained by Extracted Factors
| F1 | F2 | F3 | |
|---|---|---|---|
| Proportion of Variance Explained | 0.34 | 0.33 | 0.05 |
| Cumulative Variance Explained | 0.34 | 0.67 | 0.72 |
TABLE II.
Factor Loadings for All Clinical Data
| Clinical Score | F1 (Concentration & Coordination) | F2 (Walking Strength) | F3 (Physical Fatigue) |
|---|---|---|---|
| MFIS 1 | 0.83 | ||
| MFIS 2 | 0.85 | ||
| MFIS 3 | 0.85 | ||
| MFIS 4 | 0.72 | 0.55 | |
| MFIS 5 | 0.81 | 0.32 | |
| MFIS 6 | 0.59 | 0.55 | 0.41 |
| MFIS 7 | 0.69 | 0.34 | 0.40 |
| MFIS 8 | 0.65 | 0.42 | |
| MFIS 9 | 0.60 | 0.61 | 0.35 |
| MFIS 10 | 0.66 | 0.52 | 0.45 |
| MFIS 11 | 0.83 | 0.31 | |
| MFIS 12 | 0.84 | ||
| MFIS 13 | 0.68 | 0.46 | |
| MFIS 14 | 0.64 | 0.36 | |
| MFIS 15 | 0.91 | ||
| MFIS 16 | 0.78 | 0.35 | |
| MFIS 17 | 0.69 | 0.47 | 0.41 |
| MFIS 18 | 0.81 | ||
| MFIS 19 | 0.90 | ||
| MFIS 20 | 0.59 | 0.55 | 0.48 |
| MFIS 21 | 0.64 | 0.41 | 0.47 |
|
| |||
| MSWS 1 | 0.88 | ||
| MSWS 2 | 0.35 | 0.73 | |
| MSWS 3 | 0.88 | ||
| MSWS 4 | 0.40 | 0.75 | |
| MSWS 5 | 0.43 | 0.82 | |
| MSWS 6 | 0.88 | ||
| MSWS 7 | 0.31 | 0.87 | |
| MSWS 8 | 0.88 | ||
| MSWS 9 | 0.90 | ||
| MSWS 10 | 0.32 | 0.90 | |
| MSWS 11 | 0.33 | 0.91 | |
| MSWS 12 | 0.40 | 0.87 | |
|
| |||
| FSS Cerebral | 0.54 | ||
| FSS Pyramidal | 0.60 | ||
| FSS Bladder | 0.58 | ||
| FSS Vision | |||
| FSS Brainstem | |||
| FSS Cerebellar | 0.35 | 0.47 | |
| FSS Sensory | |||
TABLE III.
Correlations Between Gait Features and Clinical Factors, Including 95% Confidence Intervals
| Inertial Gait Feature | F1 (Concentration & Coordination) | F2 (Walking Strength) | F3 (Physical Fatigue) |
|---|---|---|---|
| Causality Index | 0.108 [−0.11, 0.32] | 0.404 [0.19, 0.57] | 0.286 [0.06, 0.47] |
| KDE Peak | 0.151 [−0.03, 0.32] | 0.543 [0.41, 0.65] | 0.111 [−0.07, 0.28] |
| DTW Score | 0.113 [−0.14, 0.36] | 0.516 [0.29, 0.68] | −0.052 [−0.30, 0.21] |
| Warp Score | 0.044 [−0.21, 0.29] | 0.681 [0.51, 0.79] | 0.015 [−0.24, 0.27] |
| Walking Speed (Min1) | 0.247 [−0.01, 0.47] | 0.716 [0.56, 0.82] | 0.016 [−0.24, 0.27] |
| Walking Speed (Min6) | 0.270 [0.01, 0.49] | 0.734 [0.58, 0.83] | 0.026 [−0.23, 0.28] |
TABLE IV.
Statistically Significant Differences in Correlation to Latent Factors between Inertial Features
| CI | KDE | DTW | Warp | M1 | M6 | |
|---|---|---|---|---|---|---|
| Causality Index | F3 | F2 | F2 | F2 | ||
| KDE Peak | F2 | F2 | ||||
| DTW Score | F3 | F2 | F2 | F2 | ||
| Warp Score | F2 | F2 | F1 | |||
| Speed (M1) | F2 | F2 | F2 | |||
| Speed (M6) | F2 | F2 | F2 | F1 |
Acknowledgments
This work was supported in part by the National Science Foundation (IIS-1065262, IIS-1231712), the National Institutes of Health – National Institute of Neurologic Disorders and Stroke (K23NS062898), the University of Virginia Broadband Wireless Access & Applications Center (BWAC) (NSF award #1266311), and a gift from the ziMS Foundation.
References
- 1.WHO | Atlas: Multiple Sclerosis Resources in the World 2008. [Online]. Available: http://www.who.int/mental_health/neurology/atlas_multiple_sclerosis_resources_2008/en/
- 2.Zwibel HL. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Advances in Therapy. 2009 Dec;26(12):1043–1057. doi: 10.1007/s12325-009-0082-x. [DOI] [PubMed] [Google Scholar]
- 3.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983 Nov;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 4.Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12) Neurology. 2003 Jan;60(1):31–36. doi: 10.1212/wnl.60.1.31. [DOI] [PubMed] [Google Scholar]
- 5.Ritvo PG, Fischer JS, Miller DM, Andrews H, Paty D, LaRocca N. Multiple sclerosis quality of life inventory: a users manual. New York: National Multiple Sclerosis Society; 1997. pp. 1–65. [Google Scholar]
- 6.Goodman AD, Brown TR, Edwards KR, Krupp LB, Schapiro RT, Cohen R, Marinucci LN, Blight AR. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Annals of neurology. 2010;68(4):494–502. doi: 10.1002/ana.22240. [DOI] [PubMed] [Google Scholar]
- 7.Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Multiple Sclerosis (Houndmills, Basingstoke, England) 2008 Apr;14(3):383–390. doi: 10.1177/1352458507082607. [DOI] [PubMed] [Google Scholar]
- 8.Engelhard MM, Dandu SR, Patek SD, Lach JC, Goldman MD. Quantifying six-minute walk induced gait deterioration with inertial sensors in multiple sclerosis subjects. Gait & Posture. 2016 Sep;49:340–345. doi: 10.1016/j.gaitpost.2016.07.184. [Online]. Available: // www.sciencedirect.com/science/article/pii/S0966636216303228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qureshi A, Brandt-Pearce M, Engelhard MM, Goldman MD. Biomedical and Health Informatics (BHI), EMBS Int Conf. IEEE; 2017. Relationship between kernel density function estimates of gait time series and clinical data. (in press) [Google Scholar]
- 10.Gong J, Qi Y, Goldman MD, Lach J. Causality analysis of inertial body sensors for multiple sclerosis diagnostic enhancement. IEEE Journal of Biomedical and Health Informatics. 2016;20(5):1273–1280. doi: 10.1109/JBHI.2016.2589902. [DOI] [PubMed] [Google Scholar]
- 11.Chetta A, Rampello A, Marangio E, Merlini S, Dazzi F, Aiello M, Ferraro F, Foresi A, Franceschini M, Olivieri D. Cardiorespiratory response to walk in multiple sclerosis patients. Respiratory Medicine. 2004 Jun;98(6):522–529. doi: 10.1016/j.rmed.2003.11.011. [Online]. Available: // www.sciencedirect.com/science/article/pii/S0954611103004281. [DOI] [PubMed] [Google Scholar]
- 12.Savci S, Inal-Ince D, Arikan H, Guclu-Gunduz A, Cetisli-Korkmaz N, Armutlu K, Karabudak R. Six-minute walk distance as a measure of functional exercise capacity in multiple sclerosis. Disability and Rehabilitation. 2005 Nov;27(22):1365–1371. doi: 10.1080/09638280500164479. [DOI] [PubMed] [Google Scholar]
- 13.Aminian K, Rezakhanlou K, Andres ED, Fritsch C, Leyvraz P-F, Robert P. Temporal feature estimation during walking using miniature accelerometers: an analysis of gait improvement after hip arthroplasty. Medical & Biological Engineering & Computing. 1999 Nov;37(6):686–691. doi: 10.1007/BF02513368. [Online]. Available: http://link.springer.com/article/10.1007/BF02513368. [DOI] [PubMed] [Google Scholar]
- 14.Pau M, Caggiari S, Mura A, Corona F, Leban B, Coghe G, Lorefice L, Marrosu MG, Cocco E. Clinical assessment of gait in individuals with multiple sclerosis using wearable inertial sensors: Comparison with patient-based measure. Multiple Sclerosis and Related Disorders. 2016 Nov;10:187–191. doi: 10.1016/j.msard.2016.10.007. [Online]. Available: // www.sciencedirect.com/science/article/pii/S2211034816301912. [DOI] [PubMed] [Google Scholar]
- 15.Ahlrichs C, Sam A, Lawo M, Cabestany J, Rodrguez-Martn D, Prez-Lpez C, Sweeney D, Quinlan LR, Laighin G, Counihan T, Browne P, Hadas L, Vainstein G, Costa A, Annicchiarico R, Alcaine S, Mestre B, Quispe P, Bayes, Rodrguez-Molinero A. Detecting freezing of gait with a tri-axial accelerometer in Parkinsons disease patients. Medical & Biological Engineering & Computing. 2016 Jan;54(1):223–233. doi: 10.1007/s11517-015-1395-3. [Online]. Available: http://link.springer.com/article/10.1007/s11517-015-1395-3. [DOI] [PubMed] [Google Scholar]
- 16.LeMoyne R, Heerinckx F, Aranca T, Jager RD, Zesiewicz T, Saal HJ. Wearable body and wireless inertial sensors for machine learning classification of gait for people with Friedreich’s ataxia. 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN); Jun. 2016; pp. 147–151. [Google Scholar]
- 17.Kastaniotis D, Theodorakopoulos I, Theoharatos C, Economou G, Fotopoulos S. A framework for gait-based recognition using kinect. Pattern Recognition Letters. 2015;68:327–335. [Google Scholar]
- 18.Gong J, Engelhard MM, Goldman MD, Lach J. Correlations between inertial body sensor measures and clinical measures in multiple sclerosis. Proceedings of the 10th EAI International Conference on Body Area Networks; ICST (Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering); 2015. pp. 18–24. [Google Scholar]
- 19.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the mcdonald criteria. Annals of neurology. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barth AT, Hanson MA, Powell HC, Jr, Lach J. Tempo 3.1: A body area sensor network platform for continuous movement assessment. Wearable and Implantable Body Sensor Networks, 2009. BSN 2009. Sixth International Workshop on; IEEE; 2009. pp. 71–76. [Google Scholar]
- 21.Revelle W. psych: Procedures for psychological, psychometric, and personality research. Vol. 165. Northwestern University; Evanston, Illinois: 2014. [Google Scholar]
- 22.Keogh E, Ratanamahatana CA. Exact indexing of dynamic time warping. Knowledge and Information Systems. 2005 Mar;7(3):358–386. [Online]. Available: https://doi.org/10.1007/s10115-004-0154-9. [Google Scholar]
- 23.Engelhard MM, Dandu SR, Lach JC, Goldman MD, Patek SD. Toward Detection and Monitoring of Gait Pathology Using Inertial Sensors Under Rotation, Scale, and Offset Invariant Dynamic Time Warping. Proceedings of the 10th EAI International Conference on Body Area Networks, ser. BodyNets ’15; ICST, Brussels, Belgium, Belgium: ICST (Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering); 2015. pp. 269–275. [Online]. Available: http://dx.doi.org/10.4108/eai.28-9-2015.2261503. [Google Scholar]
- 24.Boulgouris NV, Plataniotis KN, Hatzinakos D. Gait recognition using dynamic time warping. Multimedia Signal Processing, 2004 IEEE 6th Workshop on; IEEE; 2004. pp. 263–266. [Google Scholar]
- 25.Dandu SR, Engelhard MM, Goldman MD, Lach J. Determining physiological significance of inertial gait features in multiple sclerosis. 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN); Jun. 2016; pp. 266–271. [Google Scholar]
- 26.The Elements of Statistical Learning - Data Mining, Inference. Trevor Hastie: Springer; [Online] Available: http://www.springer.com/us/book/9780387848570. [Google Scholar]
- 27.Meng XL, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychological bulletin. 1992;111(1):172. [Google Scholar]
- 28.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Experimental Brain Research. 2005;164(4):541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]