Abstract
OBJECTIVE:
The aim of this study was to assess the prevalence of painful diabetic peripheral neuropathy (PDPN) and its associated risk factors in patients with type 2 diabetes mellitus attending primary health care (PHC) in Saudi Arabia.
MATERIALS AND METHODS:
A multicenter, cross-sectional study evaluated 242 type 2 diabetics who attended the National Guard PHC clinics in Riyadh. Trained physicians obtained the relevant data and medical history and assessed PDPN using the Michigan Neuropathy Screening Instrument.
RESULTS:
About 35% of patients with type-2 diabetes in this study had painful diabetic peripheral neuropathy. High risk hemoglobin level and poor compliance with treatment were associated with increased odds of PDPN (Odds ratio [OR] =3.121, 95% confidence interval [CI] 1.154–8.444, OR = 3.546, 95% CI 1.531–8.214, respectively). It is noted that only in one patient with PDPN, was their PDPN recognized by physicians. Furthermore, none of our study participants was taking medication to control the pain.
CONCLUSIONS:
One-third of Saudi Arabia's Type 2 diabetes patients have PDPN. PHC physicians treating diabetes should be more aware of the importance of screening for PDPN and the treatment plan.
Keywords: Diabetic, neuropathy, painful, peripheral, Saudi
Introduction
Diabetes mellitus affects about 382 million people worldwide, and its prevalence is expected to increase to 592 million by the year 2035.[1] According to a recent World Health Organization (WHO) report, Saudi Arabia ranks as the second highest in the Middle East, and the seventh in the world for the rate of diabetes.[1] Diabetic neuropathy is one of the most common long-term complications of diabetes and is the main initiating factor for foot ulceration, Charcot neuroarthropathy, and lower-extremity amputation.[2] Diabetic neuropathy encompasses a variety of clinical and subclinical presentations. Painful diabetic peripheral neuropathy (PDPN) is a common type of diabetic neuropathy and is described as a superficial burning pain associated with other sensory symptoms affecting the feet and lower extremities.[3,4,5] The symptoms are typically characterized as prickling, burning pain like an electric shock with nocturnal exacerbation. Furthermore, PDPN not only causes pain but also associated with a high degree of functional impairment, an impairment of health-related quality of life and activities of daily living.[6,7]
The prevalence of PDPN ranges from 10% to 20% of patients with diabetes and from 40% to 50% of those with diabetic neuropathies.[8] Studies conducted in the Middle East report higher rates of PDPN, ranging from 35% to 65%.[9,10,11,12] PDPN reportedly results in significantly higher health-care costs compared with costs for age- and sex-matched diabetic patients without PDPN.[8,13] The annual health-care costs in two separate databases were 24%–38% higher even after adjusting for differences in comorbid medical conditions such as cardiovascular illness.[14]
However, unlike other diabetic vascular complications including retinopathy, nephropathy, and atherosclerosis, PDPN has not been extensively studied, and the epidemiological data on it is scarce, especially in Saudi Arabia. In addition, there have been few reports on how aware physicians are of patients’ PDPN and the pharmaceutical management of these patients. The aim of this study was to estimate the prevalence of PDPN and its associated risk factors in a primary care setting in Saudi Arabia.
Materials and Methods
The study was a multicenter, cross-sectional study carried out in Primary Health Care (PHC) clinics in the National Guard in Riyadh. Two PHC clinics were selected in each center (Khashm Alaan, Om Al-Hamam, and Iskan). Two hundred and forty-two patients were enrolled between June 2011 and January 2013. Eligible were participants aged from 25 to 70 years who had type 2 diabetes mellitus diagnosed according to the WHO criteria.[15] We excluded patients who had neurologic disorders and other pain conditions unrelated to diabetic peripheral neuropathy, history of nerve root compression, malignancy, or alcoholism. The investigators at each center who performed the screening were trained in the standard operating methods before the survey and were given CDs containing a video tutorial. The study was approved by the institutional review board of King Abdullah International Medical Research Center, Riyadh, Saudi Arabia.
After obtaining written informed consent, a questionnaire of the general characteristics, height and weight, smoking status, duration of diabetes, type of medication, and a history comorbid conditions was completed. Then, height, weight, and blood pressure were recorded, and the body mass index (BMI) was calculated as weight (kg)/height (m2). Obesity was defined using the WHO classification.[16] Venous blood was collected from each participant to determine fasting blood glucose (FBG) and glycated hemoglobin (HbA1c) level. The diagnostic criteria for hypertension were based on the Seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure guidelines,[17] and/or using the blood-pressure-lowering drugs.
In each PHC center, two trained PHC physicians conducted the PDPN screening using Michigan Neuropathy Screening Instrument (MNSI), a structured assessment of the feet to identify deformities, dry skin, calluses, infection, fissures, or ulcers, and an evaluation of ankle reflexes, vibration sensation in the great toe, and fine touch using 10-g Semmes–Weinstein Monofilament. Neuropathic painful symptoms were assessed using the MNSI questionnaire. We defined PDPN as at least one symptoms with a score >2.0 on the MNSI examination, thresholds defined by prior validation studies.[18,19] Statistical analyses were performed using Statistical Package for Social Studies (SPSS, Inc, Chicago, IL, USA) for Windows version 18.0. Continuous variables were expressed as means ± standard deviation, and Categorical variables were described using frequency distributions and presented as frequency %. Comparisons between groups were made with the Chi-square test or Student's t-test as appropriate. Multivariate logistic regression models, to identify those risk factors associated with PDPN, were performed. The P < 0.05 was considered statistically significant.
Results
General characteristics of the study population are shown in Table 1. A total of 242 diabetic patients were included in this study. Nine patients were excluded (seven had a neurological disorder, and two had a history of nerve root compression). Overall prevalence of PDPN among the study participants was 34.71%. The mean age of the PDPN patients was 56.9 ± 8.3 years, and 66.7% of the PDPN patients were female. Almost half of PDPN patients were either overweight or obese, and more than half had HbA1c >9%. Most of PDPN patients (79.7%) were treated with oral hypoglycemic agents, while 20.3% used insulin, either on its own or in combination with other oral agents.
Table 1.
Demographic and clinical characteristics of the patients with and without painful diabetic peripheral neuropathy
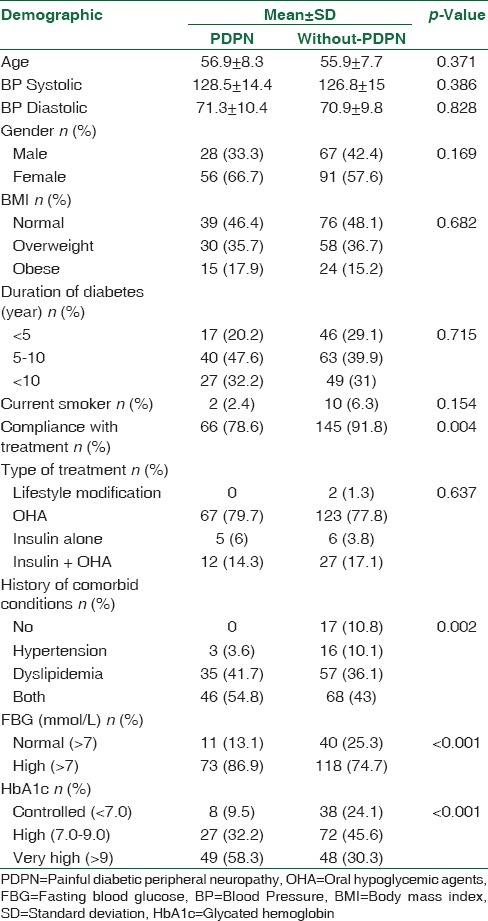
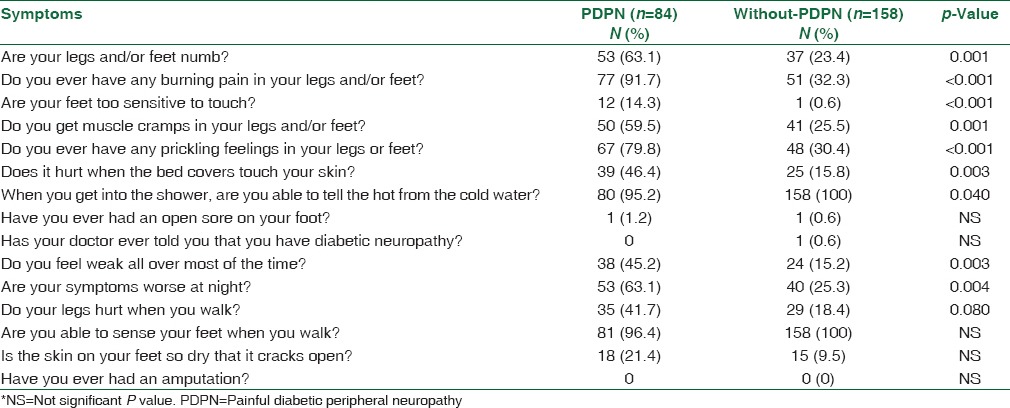
A statistical significant relationship was found between FBG, HbA1c, presence of co-morbid conditions, compliance with treatment, and PDPN (P ≤ 0.001, P ≤ 0.001, P = 0.002, P = 0.004, respectively). In terms of age, gender, blood pressure, BMI, smoking habit, and duration of diabetes, there were no statistically differences between the patients with and without PDPN [Table 1]. Common symptoms reported in the prevalence sample were “burning pain in legs and/or feet” (91.7%), “prickling feelings in legs or feet,” (79.8%) and “symptoms worse at night” (63.1%) [Table 2]. In addition, most of the questions in the MNSI questionnaire were significantly associated with PDPN. It was noted that only in one patient was PDPN recognized by the physician. Furthermore, none in the study took medication to control the pain.
Table 2.
Results from the Michigan Neuropathy Screening Instrument
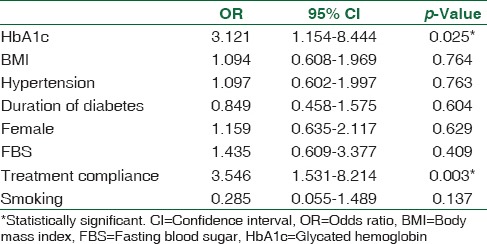
A logistic regression model was developed to predict PDPN in patients with type 2 diabetes. Sex, BMI, HbA1c, FBG, history of hypertension, smoking, duration of diabetes, and compliance with treatment were included in the model. Diabetes control and compliance with treatment emerged as significant independent predictors of PDPN. Specifically, high HbA1c level was associated with increased odds of PDPN (Odds ratio [OR] = 3.121, 95% confidence interval [CI] 1.154–8.444, P = 0.025). Poor compliance with treatment also increased the risk of PDPN (OR = 3.546, 95% CI 1.531–8.214, P = 0.003). ORs with 95% CIs are shown for each variable in the model [Table 3].
Table 3.
The logistic regression model to predict painful diabetic peripheral neuropathy in patient with type 2 diabetes
Discussion
In this study, the prevalence of PDPN in adult patients at the PHC setting in Saudi Arabia was found to be 34.7%, and the presence of comorbid conditions, noncompliance with treatment and glycemic control were associated with PDPN.
The observed prevalence of PDPN was higher than the worldwide estimated prevalence range of 15%–26%.[20,21,22] The previous studies of the prevalence of PDPN have reported different results. In a recent Saudi study of a nationally representative diabetic population, a prevalence of 65.3% was reported for PDPN.[12] However, PDPN cases in these studies were estimated by a questionnaire (Douleur Neuropathique 4, DN4) rather than a clinical evaluation. Another study reported the prevalence of PDPN in Turkey to be 14%.[23] In Japan, the prevalence of PDPN was estimated by questionnaire to be 22.1%.[24] Furthermore, in a recent community-based diabetic population study in the UK, a similar prevalence of 34% was reported.[25] The variations in prevalence rates within the country and in diverse countries may be due to the differing patient populations, study design, or diagnostic methods used to define PDPN.
The prevalence of PDPN was significantly associated with glycemic control, as we observed that a higher level of HbA1c and poor compliance with treatments was significantly associated with higher odds of PDPN. This strong association of PDPN with a high level of HbA1c and the lack of tight glycemic control confirm what is known already from previous studies and recommended in clinical guidelines.[23,26] We observed no significant association between PDPN and gender, BMI, and smoking status, which supported the results of some studies.[12,24] With regard to age and duration of diabetes, no significant correlation was found between them and PDPN, unlike some previous studies.[12]
However, despite the high prevalence of PDPN, the present study also concluded that most of Type II diabetic patients at PHC did not know they had PDPN since only one patient had previously been told he had PDPN. Furthermore, no one in the study used an FDA approved treatment for PDPN. Although there is no clear evidence, several assumptions can be made. This could be the result of the lack of knowledge of PHC physicians in this area suggesting that there is little awareness of PDPN in the PHC setting. A study in Japan showed that only 36.4% of patients with PDPN, had their condition recognized by physicians.[24] The second assumption is that patients may not mention all their painful symptoms to physicians. In the UK, Daousi reported that only 12.5% of patients with PDPN ever reported their symptoms to physicians.[20] Another reason may be that owing to the large numbers of patients visiting PHC; the physicians do not have enough time to screen and explain the chronic complications to every diabetic case. However, further studies need to be done to clarify the reasons for the lack of correlation between the high prevalence of PDPN and the lack of physicians’ awareness.
Conclusions
This study showed that PDPN affects 34.7% of Saudi type 2 diabetic patients attending PHC in Saudi Arabia. Poor glycemic control and noncompliance with treatment were significantly associated with PDPN. PHC physicians treating diabetes need to have a better grasp of the importance of PDPN screening and the treatment plan.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.International Diabetes Federation. IDF Diabetic Atlas 7th Edition. 166 Chaussee de La Hulpe, B-1170 Brussels, Belgium. [Last accessed on 2016 Sep 02]. http://www.idf.org/idf-diabetes-atlas-seventh-edition .
- 2.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–24. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 3.Chong MS, Hester J. Diabetic painful neuropathy: Current and future treatment options. Drugs. 2007;67:569–85. doi: 10.2165/00003495-200767040-00006. [DOI] [PubMed] [Google Scholar]
- 4.Sommer C. Painful neuropathies. Curr Opin Neurol. 2003;16:623–8. doi: 10.1097/01.wco.0000093106.34793.06. [DOI] [PubMed] [Google Scholar]
- 5.Kästenbauer T, Irsigler P, Sauseng S, Grimm A, Prager R. The prevalence of symptoms of sensorimotor and autonomic neuropathy in type 1 and type 2 diabetic subjects. J Diabetes Complications. 2004;18:27–31. doi: 10.1016/S1056-8727(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 6.Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: Epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47:123–8. doi: 10.1016/s0168-8227(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 7.Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26:1790–5. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- 8.Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: Epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9:660–74. doi: 10.1111/j.1526-4637.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 9.Sulimani RA, Famuyiwa OO, Mekki MO. Pattern of diabetic foot lesions in Saudi Arabia: Experience from King Khalid university hospital, Riyadh. Ann Saudi Med. 1991;11:47–50. doi: 10.5144/0256-4947.1991.47. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen JV. Peripheral neuropathy, hypertension, foot ulcers and amputations among Saudi Arabian patients with type 2 diabetes. Diabetes Res Clin Pract. 1998;41:63–9. doi: 10.1016/s0168-8227(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 11.Akbar DH, Mira SA, Zawawi TH, Malibary HM. Subclinical diabetic neuropathy: A common complication in Saudi diabetics. Saudi Med J. 2000;21:433–7. [PubMed] [Google Scholar]
- 12.Halawa MR, Karawagh A, Zeidan A, Mahmoud AE, Sakr M, Hegazy A, et al. Prevalence of painful diabetic peripheral neuropathy among patients suffering from diabetes mellitus in Saudi Arabia. Curr Med Res Opin. 2010;26:337–43. doi: 10.1185/03007990903471940. [DOI] [PubMed] [Google Scholar]
- 13.Barrett AM, Lucero MA, Le T, Robinson RL, Dworkin RH, Chappell AS, et al. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: A review. Pain Med. 2007;8(Suppl 2):S50–62. doi: 10.1111/j.1526-4637.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 14.Dworkin RH, Malone DC, Panarites CJ, Armstrong EP, Pham SV. Impact of postherpetic neuralgia and painful diabetic peripheral neuropathy on health care costs. J Pain. 2010;11:360–8. doi: 10.1016/j.jpain.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Geneva, Switzerland: WHO; 2006. WHO. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia; p. 50. [Google Scholar]
- 16.Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1-253. [PubMed] [Google Scholar]
- 17.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL., Jr Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 18.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA, et al. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–9. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 19.Factors in development of diabetic neuropathy. Baseline analysis of neuropathy in feasibility phase of diabetes control and complications trial (DCCT). The DCCT Research Group. Diabetes. 1988;37:476–81. [PubMed] [Google Scholar]
- 20.Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ, et al. Chronic painful peripheral neuropathy in an Urban community: A controlled comparison of people with and without diabetes. Diabet Med. 2004;21:976–82. doi: 10.1111/j.1464-5491.2004.01271.x. [DOI] [PubMed] [Google Scholar]
- 21.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–22. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 22.Van Acker K, Bouhassira D, De Bacquer D, Weiss S, Matthys K, Raemen H, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009;35:206–13. doi: 10.1016/j.diabet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Erbas T, Ertas M, Yucel A, Keskinaslan A, Senocak M. TURNEP Study Group. Prevalence of peripheral neuropathy and painful peripheral neuropathy in Turkish diabetic patients. J Clin Neurophysiol. 2011;28:51–5. doi: 10.1097/WNP.0b013e3182051334. [DOI] [PubMed] [Google Scholar]
- 24.Tsuji M, Yasuda T, Kaneto H, Matsuoka TA, Hirose T, Kawamori R, et al. Painful diabetic neuropathy in Japanese diabetic patients is common but underrecognized. Pain Res Treat 2013. 2013:318352. doi: 10.1155/2013/318352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34:2220–4. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care. 2005;28:956–62. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]


