INTRODUCTION
Multiple pathological processes can induce alterations of tissue stiffness.[1] For example, malignant tumors are often stiffer than the surrounding tissue. Manual palpation provides very useful information about the elasticity of tissues. However, the organs located deep in the body cannot be evaluated by manual palpation. Nowadays, it is possible to perform a virtual palpation of the internal organs by applying elastography, which is an emerging set of imaging modalities used to reproduce tissue elasticity.[2]
Endoscopic ultrasound (EUS) elastography, described for the first time in 2005, remains an appropriate method for the assessment of pancreaticobiliary diseases.[3,4,5,6,7,8]
THE ELASTICITY OF INTERNAL ORGANS
Elasticity refers to the physical properties of a material to resume its original size and shape after being subjected to a deforming force or stress.[9] Elasticity varies in different types of materials. For example, fluids possess only volume elasticity while solids possess rigidity or shear elasticity, as well as volume elasticity. The change in size or shape is known as the strain, which is expressed as a ratio.[9] An organ is a complex structure that contains at least two types of tissues while the elasticity varies in different types of tissues. Thus, the elasticity varies in the same organ, especially when it is affected by pathological conditions.[8,9,10]
PRINCIPLES OF ENDOSCOPIC ULTRASOUND ELASTOGRAPHY EXAMINATION
Discovered nearly 30 years ago,[11] elastography represents a noninvasive method where the relative stiffness of tissues can be imaged as a color map.[12]
Resuming the general principle of elastography
A compressive force is applied to the tissue, causing a tissue deformation (strain), which is then calculated by comparing the echo sets before and after the compression. Finally, the resultant information can be displayed as images, either directly of the spatial distributions of strains or shear waves or of elastic moduli or tissue stiffnesses.[9,11,13] For EUS, only strain elastography is currently available. Furthermore, two types of elastography are used currently: qualitative elastography and semiquantitative elastography.[12,14]
QUALITATIVE ELASTOGRAPHY
Strain elastography is a qualitative method based on tissues grade compression to a generated force, considering that the tissue deformation is smaller in hard tissue as compared to soft tissue.[8,14,15] This technique allows the direct visualization of information reflecting strain superimposed on usual conventional gray-scale B-mode EUS image as a strain distribution map (elastogram), which, for visualization purposes, is color-coded and displayed next to the conventional B-mode image on the screen [Figure 1].[9,13,16]
Figure 1.
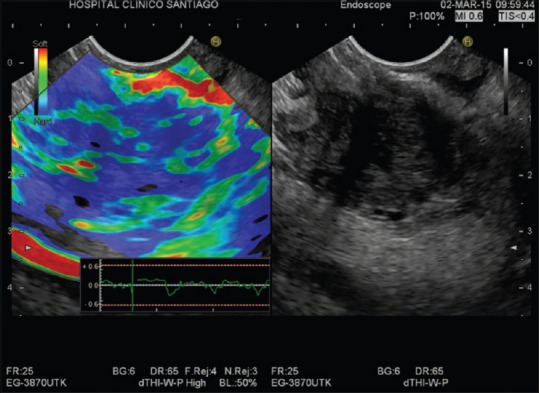
Qualitative real-time elastography: Malignant pancreatic mass-markedly hard area with soft spots (blue heterogenous color)
A region of interest (ROI) is used to define the area of interest in a similar manner to that used for a color Doppler examination. The ROI for the elastographic evaluation is manually selected and should include both the entire lesion (when possible) and also normal surrounding tissue, as a reference. To reduce operator bias, the ROI should be sufficiently large.[14,16] Different colors are used to demonstrate differences between stiffness of the ROI included tissues[14] while the degree of relative deformation between the tissues is displayed on a scale of 1–255. Each value is assigned a different shade from a hue color spectrum for further visual recognition. Thus, the hard tissue is colored in blue, red indicates soft tissues, and yellow or green indicates intermediate tissues.[13,17]
The classification of color patterns, which is qualitative, may be subjected to perception errors of the human eye to characterize all color hues and thus, elastography may be limited by its subjectivity, which could lead to differences in interpretation between endosonographers.[16,18] To reduce possible interpretation errors, an elastic score has been proposed for the pancreas to assign the elastographic images, based on the combination of the predominant color pattern inside the lesion (blue or green), and the homogeneity of color map (homogeneous or heterogeneous).[14] Thus, homogeneous area and soft color are observed in normal pancreas whilst markedly hard area with soft spots (blue color and heterogeneous) is observed in pancreatic ductal adenocarcinoma [Figure 1], and a mixture of various colors is observed in chronic pancreatitis.[4,19]
Clinical applications
From its development, elastography has been used to evaluate several organs including the breast, thyroid, prostate, cervix, liver, muscles, and others.[8] Described for the first time in 2005,[3] EUS elastography is a very important technique for the examination of organs near the gastrointestinal tract, such as the pancreas and lymph nodes. Published studies showed that EUS elastography represents a promising, noninvasive method for the differential diagnosis of benign and malignant lymph nodes, with good sensitivity (88%) and specificity (85%).[18] EUS elastography is also an excellent method for characterization of solid pancreatic masses. Most studies demonstrated that qualitative EUS elastography represents an useful tool for differential diagnosis of solid pancreatic masses with very high sensitivity (95%–98%) and relatively low specificity (42%–76%).[20,21,22]
SEMIQUANTITATIVE ELASTOGRAPHY
To improve the accuracy and reproducibility of the elastography and to reduce the human bias, recently, second-generation devices allow for semiquantitative analysis of tissue stiffness.[1,16] There are two semi-quantitative elastography methods: strain histogram (SH) and strain ratio (SR).[14]
Strain histogram
SH realizes a conversion of the elastography images of elemental areas inside a ROI in a graph. A gray scale is used to mark the color tone of the elastography image.[14] The mean histogram value corresponds to the global hardness of the lesion expressed on the color scale from softest (0) to hardest (255).[1] There are also software applications where the color scale is inversely numbered (the value “0” represents the hardest tissue – blue and the softest tissue – red corresponds to the value “255”). For both color scales, the principle remains the same: red color represents the softest tissue and blue color represents the hardest tissue [Figure 2]. The numerical values from the SH can be fed into a neural network analysis, thus the arbitrary analysis can be avoided. However, this method is not prevalent among other investigators.[4,23]
Figure 2.
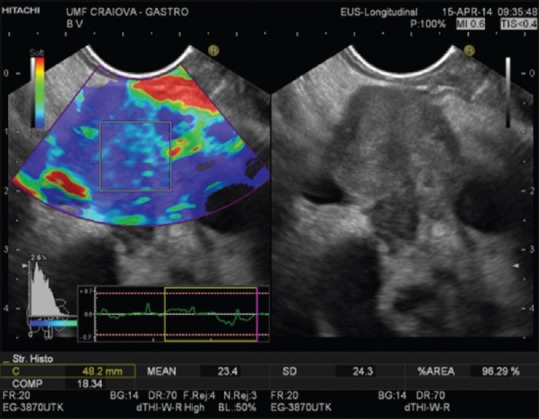
Quantitative real-time elastography (strain histogram) malignant pancreatic mass (mean strain histogram = 23.4)
Strain ratio
By SR method, the elasticity of the target tissue is expressed as a relative ratio compared to a reference value.[8,14] Initially, the operator selects, from the ROI, two different areas for quantitative elastographic analysis: the lesion (area A) and the reference zone (area B). Area A includes the entire lesion (when possible) while area B includes the peripancreatic reference area outside the tumor (fat-soft tissue) or nontumoros area inside pancreatic parenchyma.[24] The ratio B/A is considered as the measure of the elastographic evaluation [Figure 3].[16] To limit the selection bias of areas A and B, three measures for each patient are considered appropriate.[15,16] The optimal cutoff and reference SR value of semiquantitative EUS-elastography for differential diagnosis in patients with pancreatic disease remains unclear. The optimal cutoff values of SR quite differ from report to report, this value being affected by the position of the reference area.[4,25] Recently, it was demonstrated that the mean SR for normal pancreas is 3.78, for chronic pancreatitis is 8.21, and 21.80 for pancreatic cancer [Figure 3].[24]
Figure 3.
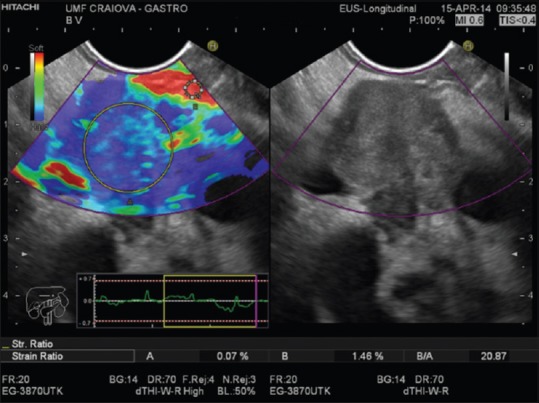
Quantitative real-time elastography (strain ratio) in malignant pancreatic mass: area A – pancreatic mass (blue); area B – fat tissue (red); strain ratio = 20.87
Clinical applications
Semiquantitative EUS elastography method has also excellent sensitivity and quite low specificity in distinguishing between malignant and benign pancreatic masses. Using the quantitative SH method, the studies showed sensitivity between 85% and 96% and specificity between 64% and 76%.[1,4,18,20,23] Using SR method, the published studies demonstrated that EUS elastography has sensitivity between 93.4% and 100% and specificity between 45% and 96%.[1,4,23,24]
We can thus conclude that EUS elastography cannot replace EUS-FNA in the diagnosis and characterization of lymph node and pancreatic masses although it represents an excellent complementary method for the differential diagnosis between malignant and benign pancreatic masses.[4,20] The type of EUS elastography method that should be used (semiquantitative or qualitative?) is still in dispute. The sensitivity for the differential diagnosis of pancreatic masses is excellent for both methods although the specificity is quite low. Most studies demonstrated that there are no significant differences concerning the specificity between the qualitative and semiquantitative method.[17]
Financial support and sponsorship
This work was supported by a grant of Ministery of Reserch and Innovation, CNCS - UEFISCDI, project number PN-III-P4-ID-PCE-2016-0561, within PNCDI III.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Saftoiu A, Vilman P. Endoscopic ultrasound elastography – A new imaging technique for the visualization of tissue elasticity distribution. J Gastrointestin Liver Dis. 2006;15:161–5. [PubMed] [Google Scholar]
- 2.Dewall RJ. Ultrasound elastography: Principles, techniques, and clinical applications. Crit Rev Biomed Eng. 2013;41:1–19. doi: 10.1615/critrevbiomedeng.2013006991. [DOI] [PubMed] [Google Scholar]
- 3.Uchida H, Hirooka Y, Ito A, et al. Utility of elastography in the diagnosis of pancreaic diseases using transabdominal ultrasonography. Gastroenterology. 2005;128:A536. [Google Scholar]
- 4.Kawada N, Tanaka S. Elastography for the pancreas: Current status and future perspective. World J Gastroenterol. 2016;22:3712–24. doi: 10.3748/wjg.v22.i14.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Xu W, Shi J, et al. Endoscopic ultrasound elastography for differentiating between pancreatic adenocarcinoma and inflammatory masses: A meta-analysis. World J Gastroenterol. 2013;19:6284–91. doi: 10.3748/wjg.v19.i37.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich CF, Săftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS), a comprehensive review. Eur J Radiol. 2014;83:405–14. doi: 10.1016/j.ejrad.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Săftoiu A, Vilmann P, Gorunescu F, et al. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: A multicenter study. Endoscopy. 2011;43:596–603. doi: 10.1055/s-0030-1256314. [DOI] [PubMed] [Google Scholar]
- 8.Meng FS, Zhang ZH, Ji F. New endoscopic ultrasound techniques for digestive tract diseases: A comprehensive review. World J Gastroenterol. 2015;21:4809–16. doi: 10.3748/wjg.v21.i16.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells PN, Liang HD. Medical ultrasound: Imaging of soft tissue strain and elasticity. J R Soc Interface. 2011;8:1521–49. doi: 10.1098/rsif.2011.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krouskop TA, Wheeler TM, Kallel F, et al. Elastic moduli of breast and prostate tissues under compression. Ultrason Imaging. 1998;20:260–74. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 11.Doyley MM, Parker KJ. Elastography: General principles and clincial applications. Ultrasound Clin. 2014;9:1–11. doi: 10.1016/j.cult.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietrich CF, Jenssen C, Herth FJ. Endobronchial ultrasound elastography. Endosc Ultrasound. 2016;5:233–8. doi: 10.4103/2303-9027.187866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamata K, Kitano M, Omoto S, et al. New endoscopic ultrasonography techniques for pancreaticobiliary diseases. Ultrasonography. 2016;35:169–79. doi: 10.14366/usg.15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui XW, Chang JM, Kan QC, et al. Endoscopic ultrasound elastography: Current status and future perspectives. World J Gastroenterol. 2015;21:13212–24. doi: 10.3748/wjg.v21.i47.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popescu A, Săftoiu A. Can elastography replace fine needle aspiration? Endosc Ultrasound. 2014;3:109–17. doi: 10.4103/2303-9027.123009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TH, Cha SW, Cho YD. EUS elastography: Advances in diagnostic EUS of the pancreas. Korean J Radiol. 2012;13(Suppl 1):S12–6. doi: 10.3348/kjr.2012.13.S1.S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Chen L, Li C, et al. Diagnostic utility of endoscopic ultrasonography-elastography in the evaluation of solid pancreatic masses: A meta-analysis and systematic review. Med Ultrason. 2017;19:150–8. doi: 10.11152/mu-987. [DOI] [PubMed] [Google Scholar]
- 18.Xu W, Shi J, Zeng X, et al. EUS elastography for the differentiation of benign and malignant lymph nodes: A meta-analysis. Gastrointest Endosc. 2011;74:1001–9. doi: 10.1016/j.gie.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101–8. doi: 10.1016/j.gie.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Ying L, Lin X, Xie ZL, et al. Clinical utility of endoscopic ultrasound elastography for identification of malignant pancreatic masses: A meta-analysis. J Gastroenterol Hepatol. 2013;28:1434–43. doi: 10.1111/jgh.12292. [DOI] [PubMed] [Google Scholar]
- 21.Hu DM, Gong TT, Zhu Q. Endoscopic ultrasound elastography for differential diagnosis of pancreatic masses: A meta-analysis. Dig Dis Sci. 2013;58:1125–31. doi: 10.1007/s10620-012-2428-5. [DOI] [PubMed] [Google Scholar]
- 22.Mei M, Ni J, Liu D, et al. EUS elastography for diagnosis of solid pancreatic masses: A meta-analysis. Gastrointest Endosc. 2013;77:578–89. doi: 10.1016/j.gie.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 23.Săftoiu A, Vilmann P. Differential diagnosis of focal pancreatic masses by semiquantitative EUS elastography: Between strain ratios and strain histograms. Gastrointest Endosc. 2013;78:188–9. doi: 10.1016/j.gie.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Kim SY, Cho JH, Kim YJ, et al. Diagnostic efficacy of quantitative endoscopic ultrasound elastography for differentiating pancreatic disease. J Gastroenterol Hepatol. 2017;32:1115–22. doi: 10.1111/jgh.13649. [DOI] [PubMed] [Google Scholar]
- 25.Itokawa F, Itoi T, Sofuni A, et al. EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. J Gastroenterol. 2011;46:843–53. doi: 10.1007/s00535-011-0399-5. [DOI] [PubMed] [Google Scholar]


