INTRODUCTION
Endoscopic ultrasound (EUS)-guided tissue sampling is a well-established method to accurately diagnose pancreatic lesions. The accuracy of fine-needle aspiration (FNA) and fine-needle biopsy (FNB) is influenced by several factors, including the operator's skill, target lesion characteristics, sampling equipment and technique, tissue handling and processing, and the experience of the pathologist. Importantly, EUS-guided tissue sampling practices vary substantially within the international endosonographic community, and there are no common guidelines for handling samples. A recent international survey showed that the approach to sample handling is highly variable, including tissue processing and rapid on-site pathological evaluation (ROSE).[1] As a result, the diagnostic accuracy among institutions is still highly variable; most highly experienced institutions report a sensitivity of 80% or more.[2,3,4] It must be noted that technological research efforts in this field primarily focus on improvement of the material, design, and size of sampling devices to provide material for histological rather than cytological analysis. However, cytological tissue handling procedures have recently undergone limited developments for thin preparations (liquid-based cytology [LBC]). Importantly, he cellblock techniques are proposed as an adjunct or an alternative to conventional smears. The implementation of these methods requires an additional, although limited, investment in laboratory infrastructure and specific training for cytotechnologists and pathologists for accurate interpretation. Adequate preparation of FNA and FNB samples and dedicated training of cytotechnologists and pathologists are the prerequisites for achieving optimal results.
ENDOSCOPIC ULTRASOUND-FINE-NEEDLE ASPIRATION TISSUE PREPARATION
Smear preparation and rapid on-site evaluation
In current EUS-FNA practice, pancreatic cancer diagnosis is typically accomplished through cytological smear preparation. Unfortunately, smear samples often show pronounced artifacts such as cell degeneration, obscuring material, and drying effect. More importantly, diagnostic accuracy might be hampered by the technique of making the smear if preparation of an optimal smear is not possible, even if the quantity of the material is sufficient. Therefore, if a trained cytotechnician cannot attend the endoscopy session, specific training of the endoscopy personnel responsible for properly preparing smear samples is strongly advised. Ideally, the aspirated material should be dispersed evenly onto the glass slide by an experienced operator. The next steps in the preparation of the smears are either air drying for Romanowsky or May–Grunwald–Giemsa staining, submersion of the smear in ethanol, or spraying of the smear with alcohol-based fixatives such as Cytofix®.
The purpose of ROSE is to improve the diagnostic performance of EUS-FNA.[5,6,7] This is supported by a recent meta-analysis of 34 distinct studies that included 3644 patients and showed that ROSE significantly increases the diagnostic yield of EUS-FNA.[8] However, according to the recent international survey, only 50% of endoscopists from Europe and Asia use ROSE, while it is routinely performed by 98% of US endoscopists. Reasons for omitting ROSE included “limited pathology staffing” (74%), “disbelief in its additive value” (32%), “high cost” (24%), and “additional procedure time” (24%).[1] This evidence reflects the fact that ROSE frequently refers to the amount of material but not to its diagnostic quality,[9] as in most European institutions, cytotechnicians and not pathologists are available for ROSE. The “ideal” ROSE should meet adequacy as well as diagnostic requirements: the ability of the operator to make an on-site diagnosis. Therefore, the ROSE criteria should be further standardized. Important questions for standardization are: (1) who is performing the ROSE (pathologists, cytotechnicians, endoscopists); (2) what is the optimal technique for on-site sample preparation; (3) which expertise is required for the ROSE assessment; and (4) is there a role for digital pathology?
Partitioning and preservation of the material
To increase the diagnostic yield, a portion of pancreatic samples obtained by EUS-FNA or FNB could be processed using a fixative for additional evaluation. These include mostly cellblock and thin preparations (LBC). LBC may be a good complement to smear preparation if blood contamination is profound.[10] Although cellblock preparation is not cost-effective in the short term and increases the technical workload, many pathologists prefer the evaluation of cellblock-processed samples to that of conventional smears. When experienced operators performed EUS-FNA of pancreatic lesions in the absence of an on-site cytopathologist, rinsing the entire sample in fixative demonstrated an improved diagnostic rate with adequate visualization of the sampled material.[11] In addition, direct histological processing of EUS-guided biopsies may permit more rapid molecular biomarker analysis.
In the absence of exact guidelines for material processing, each institution should thoroughly evaluate the optimal preparation method of EUS-FNA samples. Based on (cyto)pathologist's experience, the EUS-FNA material could be divided into smears (n = 2, per pass) and fixative for additional evaluation. Very tiny (<2 mm) cores should be evaluated using cellblock preparation, while larger cores (>2 mm) might be processed as histological material [Figure 1].
Figure 1.
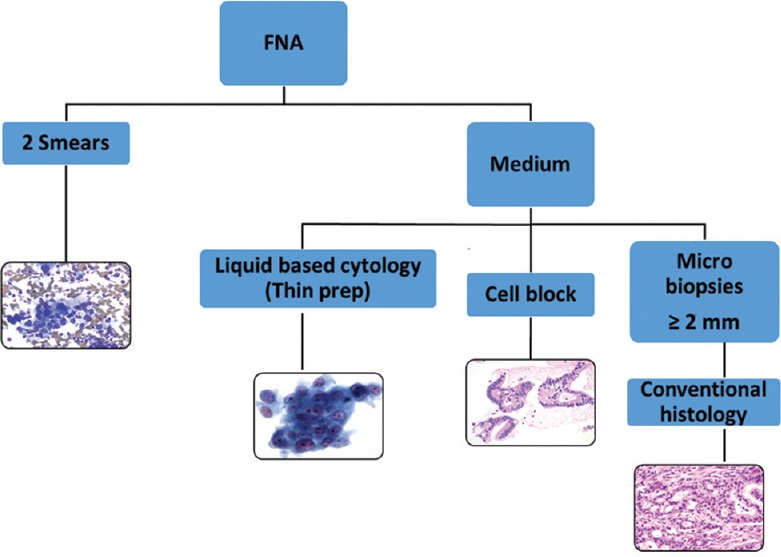
Proposed algorithm for tissue processing of endoscopic ultrasound aspirations. FNA: Fine needle aspiration
DIAGNOSIS OF ENDOSCOPIC ULTRASOUND-FINE-NEEDLE ASPIRATION SAMPLES AND ANCILLARY TECHNIQUES
Limits of EUS-FNA include sampling error and interpretation error. One difficulty of EUS-FNA sample evaluation is the lack of uniform criteria for adequacy. Cellblock preparations may allow improvements in the diagnostic accuracy of the samples. In each case, pathologists should be informed of the clinical/biochemical and radiological findings before histological examination. The reporting is preferably done using the standardized terminology proposed by the six-tiered Bethesda classification.[12]
Evaluation of smears, small tissue fragments in cellblocks, or small core biopsies often provide sufficient information for correct diagnosis [Figure 2]. Some differential diagnoses might still be difficult, such as a well-differentiated adenocarcinoma versus chronic pancreatitis; some differential diagnosis including serous cystadenoma and autoimmune pancreatitis might be extremely difficult, if not impossible, on cytological smears. In difficult cases, a second opinion from an expert cytopathologist using digital slides might be useful and could enable a rapid diagnosis. Immunohistochemical staining performed on cell blocks using additional liquid-based slides and already stained conventional slides might be helpful. The technique is especially helpful for differentiation of metastasis versus primary lesions, as well as for less frequent pancreatic tumors such as solid pseudopapillary neoplasm using beta-catenin immunostaining. If a well-differentiated adenocarcinoma is suspected, additional analysis, such as SMAD4 immunohistochemistry, might be useful. Approximately 50% of pancreatic adenocarcinomas show the loss of tumor suppressor gene SMAD4, while reactive ductal epithelium is SMAD4-positive. Mutation analysis, including TP53, CDKN2A, and SMAD4, may also prove useful for supporting diagnosis when cytology is indeterminate. KRAS mutation analysis must be interpreted with caution, as this genomic alteration is present very early in pancreatic carcinogenesis and cannot be considered indicative of a malignant process.
Figure 2.
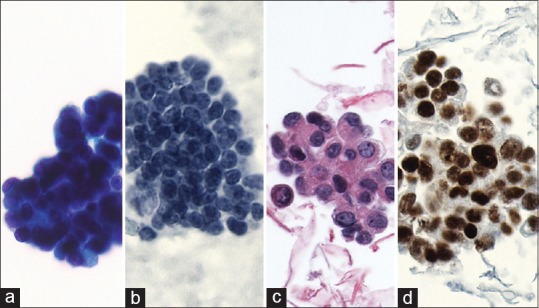
Example of endoscopic ultrasound-fine-needle aspiration (a) with subsequent smear, (b) thin preparation, and (c) cellblock preparation (H and E) staining, (d) Immunohistochemical staining
FINE-NEEDLE ASPIRATION OR FINE-NEEDLE BIOPSY: THE DEBATE CONTINUES
To increase the yield of sampling and core tissue, many endoscopists utilize needles designed to obtain material for biopsy. Expected advantages of FNB are as follows: (1) greater cellularity, and thus, improved adequacy, (2) histologic sections retaining tissue architecture that most pathologists consider more familiar, (3) more standardized specimen handling and tissue processing, (4) diminished need for ROSE, (5) fewer passes necessary to obtain sufficient material, and (6) extensive ancillary studies are possible. Currently, randomized trials comparing different FNA and FNB needles are underway, and initial results indicate that the diagnostic efficacy, technical performance, and safety profiles of FNA and FNB needles are comparable.[13,14,15] However, the use of larger needles may have some disadvantages due to increased tissue injury, a more hematic specimen, and increased difficulty for the endoscopist performing the procedure. Therefore, FNB is not yet fully established as a routine technique for sampling of solid pancreatic lesions, and further studies are needed to determine the utilization and performance of different FNB needles.
FINAL REMARKS AND FUTURE CHALLENGES
The handling procedures of pancreatic samples are highly variable within the international pathology community. They include sample processing, ROSE, and the experience of the operating cytotechnicians and pathologists. Cell block preparation and LBC are becoming common additional methods for the evaluation of pancreas EUS-FNAs. However, standardization of these procedures and clear cytological criteria for optimal categorization of samples are needed to further improve diagnostic accuracy and achieve uniformity in pathological evaluation. Optimally, each pathology laboratory should annually monitor the diagnostic accuracy of pancreatobiliary cytology. New centers beginning diagnostic EUS of pancreatic lesions are strongly advised, at least in the early phases, to use ROSE to optimize the quality and quantity of material and to establish strict cooperation between endoscopists and pathologists. The utilization of digital images and remote evaluation should be evaluated as a surrogate of ROSE when and where ROSE is not feasible for logistical or economic reasons, and also to improve the diagnostic accuracy of difficult lesions.
We must also be prepared for the next challenges in this diagnostic field; immunophenotypic and genomic characterization are becoming more relevant to select personalized therapeutic strategies.[16,17]
For molecular characterization, both the quality and quantity of the material are of key relevance. We must fill the gap between the high technology endosonography apparatus with sophisticated needles and the low/no technology sample preparation tools that pathologists use. It is of strategic relevance and includes the involvement of industrial companies, design of new devices, and development of new protocols to provide optimal diagnostic support to echoendoscopic activities.
REFERENCES
- 1.van Riet PA, Cahen DL, Poley JW, et al. Mapping international practice patterns in EUS-guided tissue sampling: Outcome of a global survey. Endosc Int Open. 2016;4:E360–70. doi: 10.1055/s-0042-101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brais RJ, Davies SE, O’Donovan M, et al. Pathologic work-up of pancreatic cyst fluid. Pancreatology. 2012;12:8–15. [Google Scholar]
- 3.Qin SY, Zhou Y, Li P, et al. Diagnostic efficacy of cell block immunohistochemistry, smear cytology, and liquid-based cytology in endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesions: A single-institution experience. PLoS One. 2014;9:e108762. doi: 10.1371/journal.pone.0108762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelsen EM, Buehler D, Soni AV, et al. Endoscopic ultrasound in the evaluation of pancreatic neoplasms-solid and cystic: A review. World J Gastrointest Endosc. 2015;7:318–27. doi: 10.4253/wjge.v7.i4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–90. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 6.Layfield LJ, Bentz JS, Gopez EV. Immediate on-site interpretation of fine-needle aspiration smears: A cost and compensation analysis. Cancer. 2001;93:319–22. doi: 10.1002/cncr.9046. [DOI] [PubMed] [Google Scholar]
- 7.LeBlanc JK, Emerson RE, Dewitt J, et al. A prospective study comparing rapid assessment of smears and ThinPrep for endoscopic ultrasound-guided fine-needle aspirates. Endoscopy. 2010;42:389–94. doi: 10.1055/s-0029-1243841. [DOI] [PubMed] [Google Scholar]
- 8.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nayar MK, Chatterjee S, Wadehra V, et al. Does on-site adequacy assessment by cytotechnologists improve results of EUS guided FNA of solid pancreaticobiliary lesions? JOP. 2013;14:44–9. doi: 10.6092/1590-8577/1277. [DOI] [PubMed] [Google Scholar]
- 10.Qin SY, Zhou Y, Li P, et al. Diagnostic efficacy of cell block immunohistochemistry, smear cytology, and liquid-based cytology in endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesions: A single-institution experience. PLoS One. 2014;9:e108762. doi: 10.1371/journal.pone.0108762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JK, Choi ER, Jang TH, et al. A prospective comparison of liquid-based cytology and traditional smear cytology in pancreatic endoscopic ultrasound-guided fine needle aspiration. Acta Cytol. 2011;55:401–7. doi: 10.1159/000330811. [DOI] [PubMed] [Google Scholar]
- 12.Pitman MB1, Layfield LJ. Guidelines for pancreaticobiliary cytology from the Papanicolaou Society of Cytopathology: A review. Cancer Cytopathol. 2014;122:399–411. doi: 10.1002/cncy.21427. [DOI] [PubMed] [Google Scholar]
- 13.Yang MJ, Yim H, Hwang JC, et al. Endoscopic ultrasound-guided sampling of solid pancreatic masses: 22-gauge aspiration versus 25-gauge biopsy needles. BMC Gastroenterol. 2015;15:122. doi: 10.1186/s12876-015-0352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bang JY, Hebert-Magee S, Trevino J, et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321–7. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jovani M, Abidi WM, Lee LS. Novel fork-tip needles versus standard needles for EUS-guided tissue acquisition from solid masses of the upper GI tract: A matched cohort study. Scand J Gastroenterol. 2017;52:784–7. doi: 10.1080/00365521.2017.1306879. [DOI] [PubMed] [Google Scholar]
- 16.Valero V, 3rd, Saunders TJ, He J, et al. Reliable detection of somatic mutations in fine needle aspirates of pancreatic cancer with next-generation sequencing: Implications for surgical management. Ann Surg. 2016;263:153–61. doi: 10.1097/SLA.0000000000001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gleeson FC, Kerr SE, Kipp BR, et al. Targeted next generation sequencing of endoscopic ultrasound acquired cytology from ampullary and pancreatic adenocarcinoma has the potential to aid patient stratification for optimal therapy selection. Oncotarget. 2016;7:54526–36. doi: 10.18632/oncotarget.9440. [DOI] [PMC free article] [PubMed] [Google Scholar]


