INTRODUCTION
In the changing landscape of modern medicine, the use of advanced imaging studies is constantly increasing. This trend has led to an increase in incidental findings on imaging examinations performed for unrelated causes, colloquially termed “incidentalomas.” The reported prevalence of pancreatic incidentalomas (PIs) varies greatly in different series and differs between cystic and solid lesions. In two large series, Laffan et al.[1] and de Jong et al.[2] reported that the prevalence of unsuspected pancreatic cysts discovered by multidetector computerized tomography or magnetic resonance imaging (MRI) is 2.4%–2.6%, and the prevalence increased with age. This figure is even higher (9.3%) when using high-resolution MRI.[3] As cystic lesions are common, a number of guidelines addressing their management have been issued.[4,5]
The prevalence of solid PIs is less clear. Strang et al. reported a 0.6% prevalence of pancreatic masses in healthy potential kidney donors;[6] a similar prevalence of 0.49% was reported among 2941 patients undergoing 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) for unrelated causes.[7] Of 39,785 FDG-PET scans performed for cancer screening in Japan, the prevalence of pancreatic malignancy was lower than 0.001%;[8] a figure more closely related to SEER (The Surveillance, Epidemiology, and End Results Program) reported a new pancreatic cancer incidence of 12.5/100,000.[9]
The differential diagnosis of pancreatic solid lesions is broad and includes malignancy (exocrine, endocrine, lymphoproliferative, or metastatic tumors), premalignant lesions (solid pseudopapillary tumors and low-grade neuroendocrine tumor [NET]), and focal inflammatory or infectious causes. Rarer diagnoses have also been described.[10]
SOLID PANCREATIC INCIDENTALOMA
The characteristics of these incidentalomas are derived mostly from data provided from retrospective pancreatic resection series published in recent years [Table 1]. In these series, the proportion of incidental findings varied from 6% to 61%.
Table 1.
Characteristics of pancreatic incidentalomas
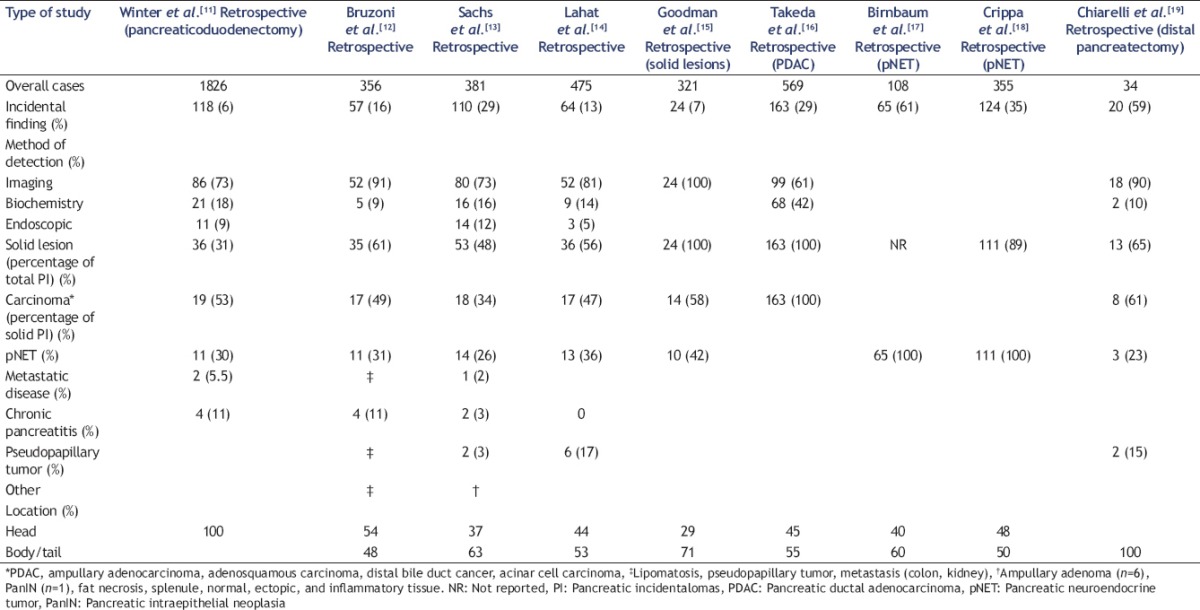
The percentage of solid lesions was 31%–65% of all lesions incidentally identified. The four most common diagnoses of these solid lesions were pancreatic carcinoma (34%–31%), pancreatic NET (pNET, 23%–42%), solid pseudopapillary tumor (3%–15%), and focal chronic pancreatitis (0%–11%).
The low prevalence of focal chronic pancreatitis is similar to that of 4.8%–6.3% in large pancreatoduodenectomy cohorts described in two historical series from the United States[20] and from Holland.[21]
In a recent multicenter Italian trial[22] on endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB), pancreatic inflammation was diagnosed in 13% of 333 biopsies taken. Pancreatic carcinoma (70%) and pNET (11%) were the other common diagnoses in that trial.
PANCREATIC CARCINOMA
The most common cause of an incidentally identified solid lesion is pancreatic ductal adenocarcinoma (PDAC). In a review of 475 pancreatic resections, Lahat et al.[14] showed that the tumor was smaller in incidental compared to symptomatic lesions (2.5 cm vs. 3.5 cm) and was more likely to be well differentiated (37.5% vs. 14.8%). Interestingly, the rate of lymph node (LN) involvement was similar between the two groups, and median survival was not significantly different (22 months vs. 19 months).
Takeda et al.[16] reviewed 569 PDAC patients; 250 were resectable. Overall tumor resectability (64% vs. 36%) and median survival (16 months vs. 10 months) were higher in patients with incidental compared with symptomatic lesions. In patients who underwent surgery, LN involvement was similar between the groups (68% vs. 77%) and a trend of increased median survival was observed (31 months vs. 20 months).
In a review of 1826 pancreatoduodenectomies, Winter et al.[11] found that the likelihood of diagnosis in Stage I was higher (34% vs. 10%) in incidentally identified PDAC, and 5-year survival rates were 50% vs. 14%.
Agarwal et al.[23] showed that patients with a smaller tumor size had a higher proportion of resectable tumors and a better median survival. Tumors smaller than 2 cm had a median survival time of 17.2 months compared to 7.6 months in tumors over 3 cm. Takeda et al.[16] showed that in the rare cases of tumors smaller than 1.5 cm, LN involvement was only 14%, compared to 76% when the tumor was over 2 cm. R0 resection was achieved in all cases when the tumor was smaller than 2 cm, compared to 80% for larger tumors.
PANCREATIC NEUROENDOCRINE TUMORS
The second most common cause of a solid PI is a pNET. Birnbaum et al.[17] presented the prognostic significance of incidental discovery of pNET in a series of 108 patients. Tumors discovered incidentally were more likely to be smaller than 2 cm (65% vs. 42%) and with G1 differentiation (66% vs. 33%). Patients undergoing surgery had a higher rate of pancreatic-sparing resections (62% vs. 30%), but there was no change in the rate of perioperative morbidity and mortality. Five-year disease-free survival was higher for incidental pNET (92% vs. 82%).
In the series presented by Crippa et al.,[18] the pNET incidence in 355 patients was 30%. The proportion of incidentally discovered tumors increased from 9% to 40% over the two decades of data collection, most likely reflecting the increasing use of imaging modalities in medical practice over that period.
Incidentally discovered tumors were smaller than 35 mm (65% vs. 45%) and more likely to have G1 differentiation (73% vs. 42%). Surgical intervention with curative intent was more frequent (85% vs. 49%), and higher R0 margins were achieved (82% vs. 46%). Five-year disease-free survival was higher (95% vs. 65%).
DISCUSSION
The majority of incidentally discovered solid lesions of the pancreas are malignant or premalignant. Incidentally discovered PDAC are smaller, are discovered at an earlier stage, and have a higher resectability rate. Survival data are conflicting, but it seems that incidental discovery has better survival. This is specifically true for tumors smaller than 2 cm.[16]
The rate of incidental pNET discovery is increasing.[18,24] Smaller incidentally discovered pNETs are at lower stages, are more likely to be resected using pancreatic-sparing resection such as enucleation, and generally have much more favorable survival.
The role of EUS in the assessment of pNET has taken center stage as treatment options are guided by the tumor grade and the Ki67 index, which can be assessed only by histology.[25,26] Recently, EUS-guided radiofrequency ablation for pNET[27,28,29] has become an option, increasing the role of the endosonographer in the multidisciplinary management team.
Focal inflammatory lesions such as focal chronic pancreatitis constitute approximately 5%–13% of solid pancreatic lesions. Although these lesions are benign and normally do not require surgical treatment, they are notoriously difficult to differentiate from pancreatic cancer by imaging alone. Even when biopsies are negative for malignancy, many still advocate resection of suspected lesions due to fear of sampling errors.
In recently published data, the utility of contrast-enhanced EUS to differentiate a hypervascular chronic pancreatitis from a relatively hypovascular pancreatic cancer has been demonstrated, with a sensitivity and specificity of over 90%.[30]
SUMMARY
Pancreatic solid incidentalomas present a unique opportunity to the clinician as early diagnosis may increase treatment options and lead to higher cure rates. The role of EUS in the evaluation of pancreatic lesions is pivotal, with a higher diagnostic yield than that of other imaging modalities. This is especially true for smaller lesions, which may be more amenable to early treatment. The role of tissue acquisition in diagnosis and guiding therapy is also of paramount importance. Moreover, as new technological advances evolve, EUS will undoubtedly play an important role in the treatment of these lesions. An algorithm for the investigation of pancreatic solid incidentalomas is presented in Figure 1.
Figure 1.
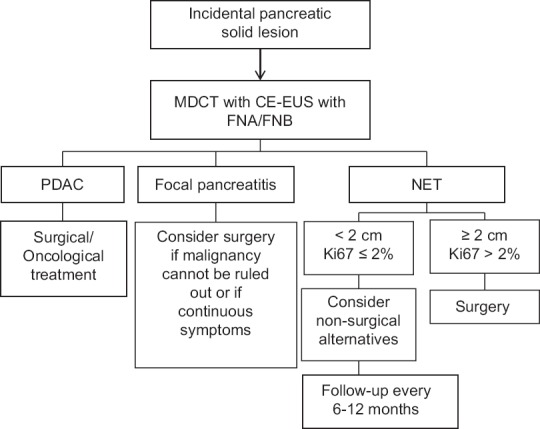
Management of solid pancreatic incidentalomas. MDCT: Multi-dimensional computed tomography, CE-EUS: Contrastenhanced endoscopic ultrasound, PDAC: Pancreatic ductal adenocarcinoma, NET: Neuroendocrine tumor
REFERENCES
- 1.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–7. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–11. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 3.de Oliveira PB, Puchnick A, Szejnfeld J, et al. Prevalence of incidental pancreatic cysts on 3 tesla magnetic resonance. PLoS One. 2015;10:e0121317. doi: 10.1371/journal.pone.0121317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka M, Fernández-del Castillo C, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819–22. doi: 10.1053/j.gastro.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Strang AM, Lockhart ME, Kenney PJ, et al. Computerized tomographic angiography for renal donor evaluation leads to a higher exclusion rate. J Urol. 2007;177:1826–9. doi: 10.1016/j.juro.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Pitts A, Nissen NN, Waxman A, et al. Unsuspected fluorodeoxyglucose positron emission tomography (FDG-PET)-positive pancreatic lesions: Prevalence and significance. Pancreas. 2013;42:1191–3. doi: 10.1097/MPA.0b013e318287d06e. [DOI] [PubMed] [Google Scholar]
- 8.Weckesser M, Schober O. Is whole-body FDG-PET valuable for health screening? Eur J Nucl Med Mol Imaging. 2005;32:342–3. doi: 10.1007/s00259-005-1775-2. [DOI] [PubMed] [Google Scholar]
- 9. [Last accessed on 2017 Jun 09]. Available from: https://www.seer.cancer.gov/statfacts/html/pancreas.html .
- 10.Herrera MF, Pantoja JP, Salazar MS, et al. London: Springer; 2009. Pancreatic incidentaloma: Endocrine surgery. [Google Scholar]
- 11.Winter JM, Cameron JL, Lillemoe KD, et al. Periampullary and pancreatic incidentaloma: A single institution's experience with an increasingly common diagnosis. Ann Surg. 2006;243:673–80. doi: 10.1097/01.sla.0000216763.27673.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruzoni M, Johnston E, Sasson AR. Pancreatic incidentalomas: Clinical and pathologic spectrum. Am J Surg. 2008;195:329–32. doi: 10.1016/j.amjsurg.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Sachs T, Pratt WB, Callery MP, et al. The incidental asymptomatic pancreatic lesion: Nuisance or threat? J Gastrointest Surg. 2009;13:405–15. doi: 10.1007/s11605-008-0788-0. [DOI] [PubMed] [Google Scholar]
- 14.Lahat G, Ben Haim M, Nachmany I, et al. Pancreatic incidentalomas: High rate of potentially malignant tumors. J Am Coll Surg. 2009;209:313–9. doi: 10.1016/j.jamcollsurg.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Goodman M, Willmann JK, Jeffrey RB. Incidentally discovered solid pancreatic masses: Imaging and clinical observations. Abdom Imaging. 2012;37:91–7. doi: 10.1007/s00261-011-9720-2. [DOI] [PubMed] [Google Scholar]
- 16.Takeda Y, Saiura A, Takahashi Y, et al. Asymptomatic pancreatic cancer: Does incidental detection impact long-term outcomes? J Gastrointest Surg. 2017;21:1287–95. doi: 10.1007/s11605-017-3421-2. [DOI] [PubMed] [Google Scholar]
- 17.Birnbaum DJ, Gaujoux S, Cherif R, et al. Sporadic nonfunctioning pancreatic neuroendocrine tumors: Prognostic significance of incidental diagnosis. Surgery. 2014;155:13–21. doi: 10.1016/j.surg.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Crippa S, Partelli S, Zamboni G, et al. Incidental diagnosis as prognostic factor in different tumor stages of nonfunctioning pancreatic endocrine tumors. Surgery. 2014;155:145–53. doi: 10.1016/j.surg.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Chiarelli M, Gerosa M, Tagliabue F, et al. Left-sided pancreatic incidentalomas treated with laparoscopic approach: A report of 20 cases. World J Surg Oncol. 2016;14:204. doi: 10.1186/s12957-016-0949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CD, Behrns KE, van Heerden JA, et al. Radical pancreatoduodenectomy for misdiagnosed pancreatic mass. Br J Surg. 1994;81:585–9. doi: 10.1002/bjs.1800810435. [DOI] [PubMed] [Google Scholar]
- 21.van Gulik TM, Reeders JW, Bosma A, et al. Incidence and clinical findings of benign, inflammatory disease in patients resected for presumed pancreatic head cancer. Gastrointest Endosc. 1997;46:417–23. doi: 10.1016/s0016-5107(97)70034-8. [DOI] [PubMed] [Google Scholar]
- 22.Fabbri C, Fuccio L, Fornelli A, et al. The presence of rapid on-site evaluation did not increase the adequacy and diagnostic accuracy of endoscopic ultrasound-guided tissue acquisition of solid pancreatic lesions with core needle. Surg Endosc. 2017;31:225–30. doi: 10.1007/s00464-016-4960-4. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal B, Correa AM, Ho L. Survival in pancreatic carcinoma based on tumor size. Pancreas. 2008;36:e15–20. doi: 10.1097/mpa.0b013e31814de421. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald TL, Hickner ZJ, Schmitz M, et al. Changing incidence of pancreatic neoplasms: A 16-year review of statewide tumor registry. Pancreas. 2008;37:134–8. doi: 10.1097/MPA.0b013e318163a329. [DOI] [PubMed] [Google Scholar]
- 25.Falconi M, Eriksson B, Kaltsas G, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103:153–71. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrera MF, Škerström G, Angelos P, et al. AACE/ACE disease state clinical review: Pancreatic neuroendocrine incidentalomas. Endocr Pract. 2015;21:546–53. doi: 10.4158/EP14465.DSC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pai M, Habib N, Senturk H, et al. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52–9. doi: 10.4240/wjgs.v7.i4.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi S, Viera FT, Ghittoni G, et al. Radiofrequency ablation of pancreatic neuroendocrine tumors: A pilot study of feasibility, efficacy, and safety. Pancreas. 2014;43:938–45. doi: 10.1097/MPA.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 29.Lakhtakia S, Ramchandani M, Galasso D, et al. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos) Gastrointest Endosc. 2016;83:234–9. doi: 10.1016/j.gie.2015.08.085. [DOI] [PubMed] [Google Scholar]
- 30.Hocke M, Dietrich CF. Vascularisation pattern of chronic pancreatitis compared with pancreatic carcinoma: Results from contrast-enhanced endoscopic ultrasound. Int J Inflam. 2012;2012:420787. doi: 10.1155/2012/420787. [DOI] [PMC free article] [PubMed] [Google Scholar]


