Abstract
Background
Mature spermatozoa contain numerous epididymal and seminal plasma proteins, which full identification through high-throughput technologies may allow for a better understanding of the sperm biology. Therefore, we conducted a global proteomic analysis of boar spermatozoa through shotgun and gel-based methodologies.
Results
The total proteins were extracted from mature spermatozoa and subjecsted to proteome analyses. Functional analyses of gene ontology representations and pathway enrichments were conducted on the shotgun dataset, followed by immunology and gene expression validations. Shotgun and gel-based approaches allowed the detection of 2728 proteins and 2123 spots, respectively. Approximately 38% and 59% of total proteins were respectively fully and partially annotated, and 3% were unknown. Gene ontology analysis indicated high proportions of proteins associated with intracellular and cytoplasm localizations, protein and nucleic acid binding, hydrolase and transferase activities, and cellular, metabolic, and regulation of biological processes. Proteins associated with phosphorylation processes and mitochondrial membranes, nucleic acid binding, and phosphate and phosphorous metabolics represented 77% of the dataset. Pathways associated with oxidative phosphorylation, citrate cycle, and extra-cellular matrix-receptor interaction were significantly enriched. Protein complex, intracellular organelle, cytoskeletal parts, fertilization and reproduction, and gap junction pathway were significantly enriched within the top 116 highly abundant proteins. Nine randomly selected protein candidates were confirmed with gel-based identification, immunofluorescence detection, and mRNA expression.
Conclusions
This study offers an in-depth proteomic mapping of mature boar spermatozoa that will enable comparative and discovery research for the improvement of male fertility.
Electronic supplementary material
The online version of this article (10.1186/s12864-018-4442-2) contains supplementary material, which is available to authorized users.
Keywords: Fertility, Fertilization, Semen, Spermadhesins, Swine
Background
Spermatozoa are haploid cells produced in the testis with a specific shape allowing them to carry and deliver paternal materials to the oocyte. Their biology has a multifaceted array of genetic, proteomic and metabolic differences, which unpredictable interactions with one another could influence the sperm function and consequently, male fertility. Numerous studies have focused on both liquid and solid phases of semen ejaculates to investigate the multifactorial causes of male subfertility or infertility [1–4]. With regard to spermatozoa, various methods are being used to shed light on every aspect of their structure and functionality. Motility and motion kinematics of spermatozoa are routinely assessed with computerized systems for objective, highly accurate and repeatable evaluations [5–7], while viability parameters such as the integrity of the plasma, acrosome, and mitochondrial membranes are evaluated with vital fluorescent dyes [8, 9]. Still, these critical assessments do not always translate into a consistent prediction of semen quality and fertility.
Unpredictable interactions of spermatozoa with secretions found in the male reproductive tract make their biology more complex. Indeed, spermatozoa are released within the seminiferous tubules upon their production and maintain continuous interactions with surrounding secretions of the genital tract (testes and epididymis) throughout their longitudinal migration within the epididymis lumen and accessory sex glands (prostate, cowper’s gland and seminal vesicles). These protein-based interactions contribute to shaping the sperm structure and influence their fertility [10–14]. The quantity and quality of these proteins may vary significantly with the physiological and health status of the male, and only a global investigation of spermatozoa’s total proteins and their interactions can help understand and address problems associated with sperm manipulation and fertility [15].
This past decade has been marked by the rapid development of high-throughput technologies allowing fast discoveries of the wealth of sperm molecules (i.e., RNA transcripts and proteins). The use of high-throughput techniques for either profiling or comparative studies of spermatozoa has been successful in various species such as the bull [4, 16–19], mice, rat [20, 21], men [15, 22], and the worm Caenorhabditis elegans [23]. Various proteomic platforms exist and a wide range of protein numbers have been detected in spermatozoa across and within species. To date, the best estimate figures are found to be between 2500 and 3000 proteins [15, 24–26] using either shotgun or gel-based analytical methodologies.
Each methodology has advantages and disadvantages [24–27]. The shotgun methodology is a bottom-up approach requiring an initial digestion of the crude protein extract into peptides to allow the identification of a greater number of proteins, but information concerning their likely post-translational modifications are lost. In contrast, the gel-based approach can afford a means for discovery, but several critical variables such as the protein extraction methods, the relative amount of proteins within extracts, the magnitude of the pH gradient in the first dimension, the molecular weight range in the second dimension, the means by which proteins will be stained, and repeatability remain and limit the amount of detectable proteins in samples [24, 28, 29].
So far, the rationale of selecting one technical approach over another has been driven by the objective of the study [30], and both methodologies have been used independently or in combination in many species for either profiling or discovery studies [25, 26]. Contrary to many other species [22, 31], gel-based proteomics have been the preferred methods in boar spermatozoa studies [32–34]. In spite of the usefulness of the gel-based approach, only limited numbers of proteins have been identified and the full profiling of boar spermatozoa is necessary for an in-depth comprehension of their biology to speed biomarker discoveries in an effort to improve fertility outcomes in swine. A similar approach has been recently undertaken to boost the proteome of boar seminal plasma from less than 100 [10, 35], to several hundred (536) identified proteins [36].
Here, we employed both shotgun (nanoLC-MS/MS) and gel-based (2-DE) proteomic approaches, followed by functional bioinformatics (functional annotations, enrichment, and biological pathways) analyses to provide a panoramic overview of the proteome of mature boar spermatozoa. Validations were conducted with in situ immunofluorescence, westernblotting, and mRNA gene expression. This study confirms the power of the shotgun proteomic at generating large amounts of proteins compared to the gel-based method, while providing the first and most comprehensive proteome of mature boar spermatozoa. Additionally, the bioinformatic analyses indicate the likely importance of oxidative phosphorylation, citrate (TCA) cycle, and ECM-receptor interaction and gap junction pathways on sperm function. Generated dataset will be useful to better understand the biology of boar spermatozoa that is needed to further improve fertility diagnosis and prognosis of males and develop appropriate strategies for efficient post-collection handling of semen, particularly in swine.
Methods
Semen collection and sperm purification
Twenty-four semen ejaculates (3 per boar) of eight fertile Landrace boars (Table 1) of approximately 1.5 years old were purchased (International Boar Semen; Eldora, IA). Each semen ejaculate was extended with the Beltsville Thawing Solution (BTS: Minitube of America – MOFA; Verona, WI) at the boar stud facilities and overnight-shipped to our laboratory, in three different occasions (one ejaculate/shipping). Twenty-four extended semen doses of sperm motility averaging 85 ± 2.7% (mean ± sem) at collection, were used in this study. Sperm motility were evaluated upon reception in our laboratory (75 ± 3.8%) and spermatozoa were purified through a discontinuous percoll gradient as previously reported [37]. After several washes with cold phosphate buffered-saline (PBS), sperm samples devoid of contamination (e.g. extender components and somatic cells such as leukocytes and testicular cells) were stored in aliquots of 100 × 106 spermatozoa per ejaculate and per boar at − 80 °C. Samples were arranged for proteomics (three different ejaculate aliquots per boar; Additional file 1: Figure S1), immunodetection (immunofluorescence and westernblotting), and gene expression (RT-PCR) analyses.
Table 1.
Boar reproductive characteristics
| Expected Progeny Difference (EPD) class | Values (mean ± sem) |
|---|---|
| Number of offspring Born Alive (NBA) | 12.9 ± 1.1 |
| Total Litter weight (LWT; pound) | 184.2 ± 4.2 |
| Days at 250 pounds (D/250) | 151.5 ± 0.5 |
| Sow Productivity Index (SPI) | 110.5 ± 4.4 |
Shotgun proteomic
For each boar (n = 8), spermatozoa of three different ejaculates were pooled (3 × 100 × 106 spermatozoa) and eight independent pools were used for analyses at the Institute for Genomics, Biocomputing and Biotechnology, Mississippi State University.
Sample cleanup method
Total protein of each boar (3 × 100 × 106 = 300 × 106 spermatozoa/boar) was extracted using the complete radioimmunoprecipitation assay (RIPA; Thermo Fisher Scientific Inc., Waltham, MA) buffer [38] and quantified (Bradford assay kit). Isolated protein samples were digested with trypsin and resulting peptide soup were desalted using a peptide macrotrap (TR1/25108/52; Michrom BioResources, Auburn, CA). After removal of the digestion buffer (2% and 90% acetonitrile, 0.1% formic acid), samples were cleaned using a strong cation exchange (SCX) trap (Michrom TR1/25108/53) to remove any detergents or other polymers that can interfere with MS/MS analysis. After further cleaning procedures through the SCX trap, the dried matrix of salt crystals and peptides were resuspended in 20 μl of 5% acetonitrile, 0.1% formic acid and transferred to a low retention autosampler vial for deconvolution via reverse phase, high-pressure liquid chromatography.
Nanospray LC/MS method
Each sample was loaded on a BioBasic C18 reversed phase column (Thermo 72,105-100,266) and flushed for 20 min with 5% acetonitrile (ACN), 0.1% formic acid to remove salts. Peptide separation was achieved using a Thermo Surveyor MS pump with a 655-min nano-HPLC method consisting of a gradient from 5% ACN to 50% ACN in 620 min, followed by a 20 min wash with 95% ACN and equilibration with 5% ACN for 15 min (all solvents contained 0.1% formic acid as a proton source). Ionization of peptides was achieved via nanospray ionization using a Thermo Finnigan nanospray source type I operated at 1.85 kV with 8 μm internal diameter silica tips (New Objective FS360-75-8-N-20-C12). High voltage was applied using a t-connector with a gold electrode in contact with the HPLC solvent. A Thermo LCQ DECA XP Plus ion trap mass spectrometer was used to collect data over the 655 min duration of each HPLC run. Precursor mass scans were performed using repetitive MS scans, each immediately followed by three MS/MS scans of the three most intense MS peaks. Dynamic exclusion was enabled with duration of 2 min and repeat counts. Once a mass is measured twice, it is added to a list to be excluded from further analysis for a predetermined amount of time, which was 2 min. This allowed the MS to collect data on different masses, while in the meantime, the mass will have eluted from the column. Dynamic exclusion allows for a more efficient and deeper sample coverage [39].
Gel-based proteomic
For each boar (n = 8), spermatozoa of 3 different ejaculates were pooled (300 × 106 spermatozoa) and 8 independent pools were submitted to Applied Biomics (Hayward, CA, USA) for analyses.
Protein lysate preparation
Total protein was extracted as described above (300 × 106 spermatozoa/boar) and resuspended with 200 μl 2D lysis buffer [2 M thiourea, 7 M urea, 4% (w/v) CHAPS, 30 mM Tris-HCl (pH 8.8)]. Samples were sonicated, centrifuged at high speed, and supernatants were collected, and protein quantified and normalized to 5 mg/ml with the 2-D cell lysis buffer.
Isoelectric focusing (IEF) and SDS-PAGE
Protein preparations (5 mg/ml) were mixed with 2× concentrated 2-D Sample buffer [8 M urea, 4% CHAPS, 20 mg/ml dithiothreitol or DTT, 2% pharmalytes and trace amount of bromophenol blue - 0.002% (w/v) -], followed by an addition of 100 μl Destreak solution and Rehydration buffer (7 M urea, 2 M thiourea, 4% CHAPS, 20 mg/ml DTT, 1% pharmalytes and trace amount of bromophenol blue). Protein samples (350 μl = 300 μg) were placed into a strip holder for protein loading in 18 cm IPG strips. The IEF was run as recommended by Amersham BioSciences, under dark at 20 °C. Thereafter, IPG strips (pH 3-10) were incubated in a fresh made equilibration buffer 1 (50 mM Tris-HCl, pH 8.8, containing 6 M urea, 30% glycerol, 2% SDS, trace amount of bromophenol blue and 10 mg/ml DTT) for 15 min with slow shaking. The strips were transferred to a freshly made equilibration buffer 2 (50 mM Tris-HCl, pH 8.8, containing 6 M urea, 30% glycerol, 2% SDS, trace amount of bromophenol blue and 45 mg/ml Iodoacetamide) and incubated 10 min with slow shaking. Afterwards, the IPG strips were rinsed once in the SDS-gel running buffer and placed onto 12% SDS-Gel electrophorese gels and sealed with 0.5% (w/v) agarose solution (in SDS-gel running buffer). The SDS-gels were run at 15 °C and stopped until the front dye runs out of the gels.
All gels (n = 8) were fixed and stained with the Bio-Safe colloidal Coomassie Blue G-250 solution (Bio-Rad Laboratories, Hercules, CA).
Image scan and data analysis
Image scans were carried out immediately following the SDS-PAGE using Typhoon TRIO (GE Healthcare) following the manufacture’s protocol. Scanned images were analyzed by Image QuantTL software (GE-Healthcare) and subjected to in-gel analysis and cross-gel analysis using DeCyder software version 6.5 (GE-Healthcare). Spots with either protein score or total Ion (C.I. %) greater than 95 were considered significant and protein candidates were randomly selected for spot picking.
Spot picking, in-gel trypsin digestion, and MS
The spots of interest were picked up by Ettan Spot Picker (GE Healthcare) based on the in-gel analysis and spot picking design by DeCyder software. Gel spots were washed few times and digested in-gel with modified porcine trypsin protease (Trypsin Gold; Promega, Madison, WI). Digested tryptic peptides were desalted by Zip-tip C18 (Millipore) and eluted from the Zip-tip with 0.5 μl of matrix solution (α-cyano-4-hydroxycinnamic acid, 5 mg/ml in 50% acetonitrile, 0.1% trifluoroacetic acid, 25 mM ammonium bicarbonate) and spotted on the MALDI plate. Mass spectrometer (MALDI-TOF) and TOF/TOF (tandem MS/MS) were performed (5800 mass spectrometer; AB Sciex), and MALDI-TOF mass spectra were acquired in reflectron positive ion mode, averaging 2000 laser shots per spectrum. TOF/TOF tandem MS fragmentation spectra were acquired for each sample, averaging 2000 laser shots per fragmentation spectrum on each of the 10 most abundant ions present in each sample (excluding trypsin autolytic peptides and other known background ions).
Protein identification and bioinformatics
Database searches were performed using the SEQUEST algorithm in Bioworks 3.3 [40] and GPS Explorer 3.5 equipped with search engine (Matrix science) to search the Sus scrofa databases (NCBI-nr and ENSEMBL) for resulting peptide masses and matching peptide spectra. Proteins identified with shotgun were functionally annotated (Gene ontology or GO, Enrichment, and KEGG pathway) using online tools of Agbase (www.agbase.msstate.edu; [41]) and DAVID (Database for Annotation, Visualization and Integrated Discovery; DAVID Bioinformatics Resources 6.7; https://david.ncifcrf.gov/; [42]).
Immunodetection of selected proteins
Immunofluorescence for flow cytometry and microscopy
Immediately after sperm purification, aliquots of purified spermatozoa were fixed with 4% paraformaldehyde and submitted for immunofluorescence detection using standard protocol, as previously reported [43]. Briefly, suspended and fixed spermatozoa were successively permeabilized in PBS, containing 1% triton-X100 (30 min), blocked in PBS containing 0.1% Teween-20 and 0.5% BSA (30 min), incubated overnight at 4 °C with commercial primary antibodies (Santa Cruz Biotechnology, Inc., Santa Clara, CA) diluted 100× with PBS, and finally incubated 60 min with FITC-conjugated secondary antibody (1/200 dilution). Between steps, samples were washed twice by centrifugation (800×g – 5 min) and immunolabeled spermatozoa were submitted for flow cytometry evaluation (excitation of 488 nm). Sperm cells incubated without any antibodies or only the secondary antibody served as negative controls to set up the flow cytometer (FACSCalibur; Becton Dickinson; Franklin Lakes, NJ). Sperm cells were excited with a 488-nm laser source and the FITC and the Propidium iodide (PI) dyes were detected with the FL-1 (530/30 nm) and FL-2 (585/42 nm), respectively. A total of 10,000 events was evaluated for data acquisition. In parallel, aliquots of immunolabeled spermatozoa were smeared and mounted onto histology slides with DAPI containing medium. Slides were visualized under a Laser Scanning Confocal Microscope 710 (Zeiss) with a plan-apochromat 63×/1.40 Oil DIC M27 objective and images were analyzed with the ZEN 2012 SP1 (black edition) software.
Westernblotting
Total protein was extracted from three different ejaculates per boar (300 × 106 spermatozoa) using the complete RIPA lysis buffer. After protein quantification, equal amounts of extracted proteins per each boar were pooled according to the ejaculate (n = 3 pools) and total of 30 μg/pool was loaded onto wells of SDS-PAGE (4-12.5% NuPAGE) gels for resolution at room temperature. In-gel proteins were transferred onto PVDF membranes and the immunoblotting procedure was performed according to the anti-rabbit WesternBreeze™ Chromogenic Detection kits (Thermo Fisher Scientific Inc.). Membranes were incubated 60 min with selected primary antibodies (1/500 dilution).
In both immunological techniques, primary antibodies raised against human aquaporin 1 (AQP-1) and 7 (AQP-7), sodium/glucose co-transporter 5 (GLUT-5), plasminogen (PLG), protamine-1 (PRM-1), and thioredoxin reductase 1 (TRXR-1) purchased at Santa Cruz Biotechnology, Inc. were used. Otherwise indicated, all procedures took place at room temperature.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA were extracted from boar sperm samples using the RNeasy Mini kit (Qiagen, Carlsbad, CA) as previously described [37], with an in-column DNase digestion. Isolated RNA was reverse-transcribed into the complementary DNA (cDNA; Quantitech RT kit, Qiagen) and 0.25 μg of cDNA was used for polymerase chain reaction (PCR) with primers specifically designed to amplify water aquaporin (AQP-1, − 5, and − 11), aquaglyceroporin (AQP-3, − 7, − 9, and − 10) sodium/glucose co-transporter (GLUT-3 and GLUT-5) and spermadhesins (SPMI, AWN, AQN-1, PSP-I, and PSP-II) transcripts (Table 2). Samples were amplified in a total of 25-μl reaction volume using SYBR Green kit (Qiagen) within the Rotor-gene Q Thermal Cycler (Qiagen). The PCR conditions consisted of a hot start (95 °C-5 min), 40 cycles of amplification (95 °C-15 s and 60 °C-30 s), and a final extension (72 °C-5 min). Water was used as a template for negative control, while GAPDH was used as the housekeeping gene. All PCR products were subjected to electrophoresis on a 1.5% agarose gel and product images were documented.
Table 2.
Primer characteristics
| Gene names | NCBI Accession # | Primer sequences (5’➔3′) | Product size(bp) |
|---|---|---|---|
| Water aquaporins | |||
| AQP-1 | NM_214454 | FP: CCGGCAACTCCCTTGGCCTG RP: GGGTTAATGCCGCAGCCGGT |
220 |
| AQP-5 | NM_001110424 | FP: TGGGCTGGCACCTGGCAATG RP: CGACTGCGGGGCCGAAAGAG |
266 |
| AQP-11 | NM_001112682 | FP: TGCGTGGGTGCCTTGTGGAG RP: GTGCAGCAAAGCGCTGTGGAA |
150 |
| Aquaglyceroporins | |||
| AQP-3 | NM_001110172 | FP: TCGTGTGCGTGCTGGCCATT RP: ACCGGCGATGGAACCCAGGA |
257 |
| AQP-7 | NM_001113438 | FP: AGTGACCGGTCCCACAGCCA RP: CTGTGTGCCCCAGCCAGCAA |
295 |
| AQP-9 | NM_001112684 | FP: GGGCTGCAACCCTCTTTGGCA RP: TGGGCTGTAGGCCTCTGGGGA |
234 |
| AQP-10 | NM_001128454 | FP: GGGTCTGTGGCCCAGGCAGTA RP: TGGCCAGGGAGAAGGCTGGA |
148 |
| Spermadhesins | |||
| AQN-1 | NM_001025210 | FP: CAGGCTGCTGAGATGAAGCTGGGC RP: CGCAGGCGAGGTTGAGATACGG |
235 |
| AWN | NM_213829 | FP: GTCCTCAGAGACCCTCCTGGGAA RP: GGAAGGGAGAGGCACGCTGG |
277 |
| PSP-I | NM_213837 | FP: GCGGTTCTTCAGGCATGACGGT RP: GTGGCCAGGAGATGGCGCTC |
203 |
| PSP-II | NM_213836 | FP: ACTGCCATCCCCTGGGCCTT RP: GCCGTAGACAAAAAGACAATCGCTG |
305 |
| SPMI | NM_001031776 | FP: TCCGAGACCAACGGGCAGGAC RP: TGGCTCCTTGTGGGATGCCGT |
165 |
| Glucose transporters | |||
| GLUT-3 | FJ_209733 | FP: GGGGGCCTTTGGCACTCTCA RP: AAGCCCAAGAGCAGGGGCCA |
117 |
| GLUT-5 | EU_012359 | FP: TCGGCTCCCTCATGGTCGGC RP: TGGGCGACTTTGCTGCATCCC |
118 |
Statistical analyses
Sample collection was designed to minimize inter-ejaculate and inter-individual variations, with spermatozoa harvested from three independent semen collections of eight fertile boars during spring (April and May). Proteomics were run on mixed aliquots of three ejaculates, per boar and for each boar. Only the shotgun proteome datasets providing higher number of proteins were used for statistical analyses according to Pendarvis et al. [44]. Peptide search results were filtered using a decoy based statistical method in which a probability of being a false positive match was assigned to each peptide. Proteins containing at least three peptides with a P value (Benjamini-Hochberg correction) of 0.05 or less were retained as indicative of the confidence in protein identification and relative expression [45]. The enrichment of GO terms and KEGG pathways was identified through the Fischer’s Exact Test with Multiple Testing Correction of FDR (Benjamini-Hochberg) used to calculate the probability that the association of proteins with given biological function or pathway was not due to random chance. Cutoffs were set for P ≤ 0.05 and FDR ≤ 10%.
Results
Total protein identification using shotgun
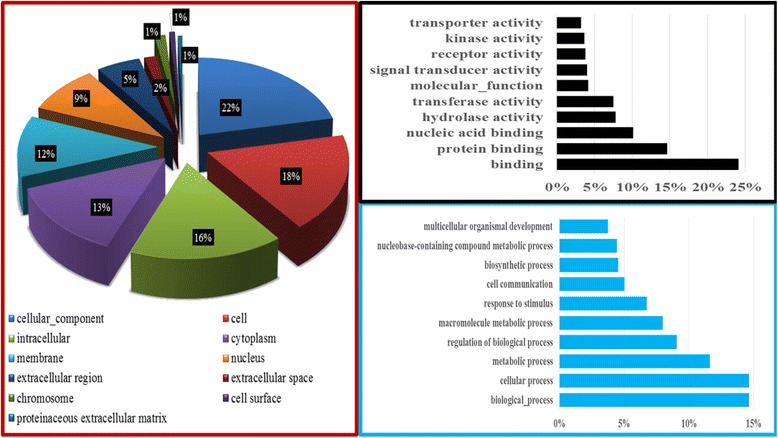
Nearly 90% concordance was found amongst individual proteome dataset (n = 8). A total of 2728 unique and commonly shared proteins were identified with confidence among samples (P ≤ 0.05; see Additional file 2 Total proteins). Total of 2406 detected proteins were annotated with NCBI-nr databases, corresponding to 781 (29%) full and 1625 (59%) partial annotations. Residual proteins showed 238 (9%) annotations with ENSEMBL and 84 (3%) that remained unknown (Fig. 1). With an arbitrary cut-off set at P ≤ 10− 99, 116 proteins were delimited and considered highly abundant (Additional file 2 Abundant proteins). Of these abundant proteins, 61 were fully annotated (NCBI-nr) with many of them deriving from testes (i.e., fascin-3 and epididymal sperm-binding protein 1) and sex glands (i.e., seminal plasma protein pB1, plasma sperm motility inhibitor, and angiotensin I converting enzyme). Number of abundant proteins appeared associated with sperm structure (i.e., protamine-1 or PRM-1, several tubulin family members, F-actin capping protein β1 and 2, and outer dense fiber protein or ODF1) and sperm-egg interactions (i.e., sperm acrosome membrane-associated protein 1 or SPACA1, sperm equatorial segment protein 1 or SPESP1, sperm adhesion molecule 1 or SPAM1, and spermadhesins (AWN, PSP-I/II, and AQN-3 or SPMI).
Fig. 1.
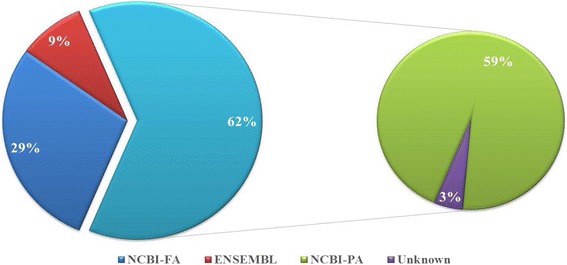
Summary of the boar sperm proteome. Total of 2728 proteins were identified using shotgun method, with 781 fully (NCBI-FA) and 1625 partially (NCBI-PA) annotated through NCBI-nr, 238 annotated with ENSEMBL and 84 that remained unknowns
Total protein identification using 2-DE
A total of 2123 spots were shared among all boar samples (n = 8). Gel electrophoreses (Additional file 3: Figure S2) usually displaying likely bimodal protein distributions based upon their isoelectric points (from ~ 5.0 to 7.5 and from ~ 8.0 to 10) and their molecular weights (from ~ 27 to 80 kDa and from ~ 21-10 kDa). Eight randomly selected spots were identified through MS/MS as albumin (69 kDa - pH 5.8), PSP-I (12 kDa - pH 7.8), ODF1 (29 kDa - pH 8.4), acrosin inhibitor (11 kDa - pH 8.9), F-Actin capping protein subunit beta (31 kDa - pH 5.5), AWN (17 kDa - pH 9.3), lactadherin precursor (48 kDa – pH 6.3), and triosephosphate isomerase (27 kDa - pH 6.5).
Shotgun protein validation through immunotechniques
Aquaporins (AQP-1 and AQP-5), aquaglyceroporins (AQP-7), transferrin (TF), plasminogen (PLG), fibronectin-1 (FN-1), and thioredoxin reductase 1 (TRXR-1) were immunodetected in sperm protein lysates (Fig. 2). With comparable protein loads into electrophoresis wells, signal bands of AQPs appeared weaker than that of TF, FN-1 and TRXR-1. These proteins together with GLUT-5 and PRM-1 were targeted using in situ immunofluorescence, followed by confocal microscope imaging (Fig. 2); thus differential fluorescence intensities and localizations of proteins on spermatozoa were revealed. Representative indications of these differences are shown in Fig. 2, with TRXR-1 exhibiting a strong signal located in the apical (acrosome) region of the sperm head. In parallel, the PLG signal was mainly detected on the head and mid-piece regions of the spermatozoon. Additionally, flow cytometry revealed high proportions (≈70-90%) of spermatozoa immunoreacting with anti-AQP-1, -AQP-7, -GLUT-5, -PLG, -PRM-1, and -TRXR-1 antibodies (Fig. 3a) and fluorescence intensities significantly above the thresholds obtained with control spermatozoa incubated without antibodies (No) or with the FITC-conjugated secondary antibody only (Fig. 3b). The fluorescence intensities of sperm alone (non-stained or control; No) and sperm incubated with the secondary antibody only (FITC) can be seen in Fig. 3.
Fig. 2.
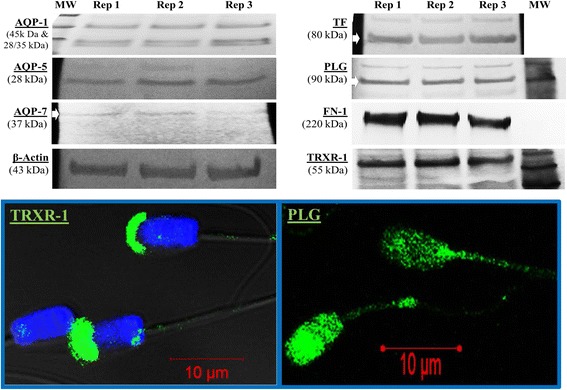
Representative westernblots and in situ immunofluorescence of boar spermatozoa. Selected proteins were targeted with antibodies raised against human AQP-1 (45 kDa & 28/35 kDa), AQP-5 (28 kDa), AQP-7 (37 kDa), β-actin (43 kDa, the internal loading control), TF (80 kDa), PLG (90 kDa), FN-1 (220 kDa), TRXR-1 (55 kDa). In situ immunofluorescence micrographs of TRXT-1 and PLG are also shown. The FITC green fluorescence indicates the sub-apical (acrosome membrane) localization of TRXR-1 on the sperm head and the localization of PLG on the head and mid-piece of spermatozoon. Nuclei are counterstained in blue with DAPI
Fig. 3.
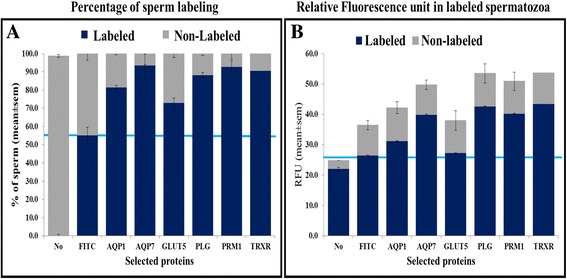
Immunofluorescence quantification through flow cytometry. Columns labeled as “No” or “FITC” correspond to spermatozoa incubated without any antibodies or with FITC-conjugated secondary antibody only, respectively. a Percentage of immunopositive sperm cells and b Relative fluorescence intensity of labeled spermatozoa. Specific targeting is revealed above the blue line, suggesting for example for GLUT-5 detection, a lower proportion of cells being targeted (a) and which cells also show lowest or no specific detection of GLUT-5 (b)
Validation through gene expression
Aquaporin (AQP-1, AQP-5, and AQP-11), aquaglyceroporins (AQP-3, AQP-7, AQP-9, and AQP-10), glucose transporters (GLUT-3 and GLUT-5), and spermadhesins (AQN-1, AWN1, PSP-I, PSP-II, SPMI) were targeted in this study. Data summarized in Fig. 4a indicate the detection of mRNA transcripts (Yes) in spermatozoa. Although similar amounts of cDNA were used for PCRs, the amplicon bands of each gene appeared at variable intensities as seen on the gel electrophoresis allowing for a relative classification, from weak (+/−) to strong (+++) for each gene target. In the current study, all spermadhesin family members and AQP-11 signal bands were the strongest, and Fig. 4b shows a representative agarose gel electrophoresis of AQP-11 and SPMI amplification products. Fig. 4a also indicates the status of protein detection of each targeted gene in boar spermatozoa by either westernblotting (W) or immunofluorescence (IF).
Fig. 4.
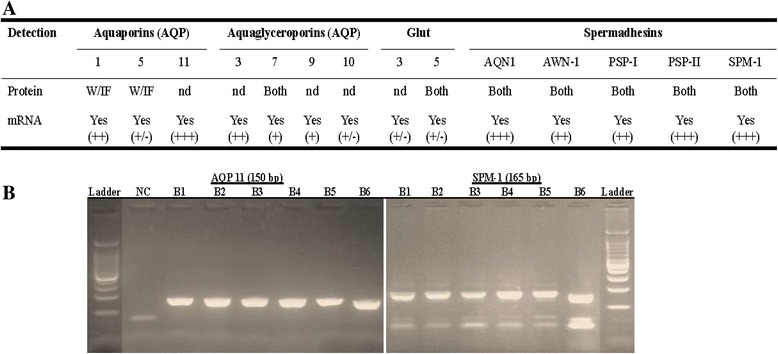
Protein and mRNA transcript comparison for selected proteins. a Table summarizes: protein detection with westernblotting (WB), immunofluorescence (IF), or altogether with shotgun approach (Both) and relative intensities of PCR amplicon bands classified as strong (+++), mid (++), weak (+), and low (+/−). b Representative gel electrophoreses of AQP11 and SPMI (AQN-3) of six boars (B1-B6) are shown. NC = Negative control and Ladder with band size starting from 100 bp (100 bp increment). On the SPMI gel, the arrow-head indicates the position of the expected amplicon size while the second band correspond to primer dimers
Functional analyses
Total proteins
Analyses were performed on the shotgun dataset only and data with P (Benjamini-Hocheberg) ≤ 0.05 and FDR ≤ 10% were retained for interpretations. A systematic search for compatible pig protein identifiers resulted in 2236 successful conversions (using GORetriever from Agbase), corresponding to 82% of the total sperm proteome being used for functional classification of all GO terms (Fig. 5). Detailed distributions of the biological functions are categorized per cellular compartmentalization (CC, with 2070 annotations), molecular function (MF, with 5659 annotations), and biological processes (BP, with 15,402 annotations).
Fig. 5.
Functional annotation of the boar sperm proteome. The distribution of total protein per gene ontology (GO) terms in the Cellular Component is shown in the red frame. The top 10 GO terms in the Molecular Function and Biological Process categories are shown in the black and the blue frames, respectively
For enrichment studies, the dataset with pig identifiers yielded only 39% conversions for DAVID analyses. However, the optimal conversion of 77% (2108 proteins) was reached by combining the residual unconverted pig identifiers with their human homologs, representing 67 Sus scrofa plus 2041 Homo sapiens proteins that were considered for analyses.
Totals of 67, 38, and 92 GO terms were found significantly enriched (P < 0.05) within the CC, MF, and BP categories, of which 34, 20, and 24 had a FDR ≤ 10%, respectively (Additional file 2 Total protein_FA). In comparison to GO term classifications by Agbase, terms associating mitochondria, extracellular matrix, and cytoskeleton in total proteins were significantly enriched was enriched (1.4× - 2.5×) in the CC category. Likewise, most GO terms (i.e., catabolic, transferase, and hydrolase activities and nucleic acid, protein binding and binding) highly classified in Agbase (Fig. 5) were significantly enriched through DAVID, in the MF category. Finally, GO terms associating response to chemical stimulus and biological, cellular, and metabolic processes in BP were significantly enriched (≈ 1.1× – 1.5×), and those associated with reproduction (i.e., sexual reproduction and reproduction) and oxygen consumption (i.e., oxidative phosphorylation, cellular respiration, and aerobic respiration) appeared amongst the highly and significantly enriched (> 1.4× – 3.6×) in the proteome dataset.
Abundant proteins
A total of 62% of proteins (71) were successfully converted to DAVID for analyses. Proteins associated with protein complex, cytoskeleton/cytoskeletal parts, and organelle GO terms were significantly expressed (3-9×) in CC category. Enrichments (> 12×, P < 0.05) were found in favor of fertilization and reproduction GO terms in the BP category and proteins such as seminal plasma protein pB1, milk fat globule EGF factor 8 protein, epididymal sperm binding protein1, and sperm associated AWN protein, PSP-I/II, sperm adhesion molecule 1, zonadhesin, and zona pellucida binding protein are among the fertilization GO term (Additional file 2 Abundant protein_FA).
Pathway analyses
The KEGG pathway analysis of the total proteome revealed 21 hits, of which only oxidative phosphorylation, citrate cycle (TCA cycle), and ECM-receptor interaction pathways were significantly enriched, with respectively 35, 13, and 23 protein counts for each (Additional file 2 Total protein_FA). Enrichment folds varied from 2.0 to 3.2×, with Benjamini P values ranging from 0.004 to 0.032 and corresponding FDR from 0.05 to 1.09. Only the gap junction pathway was significantly overrepresented in the abundant protein list, with a fold enrichment of 11.8× and Benjamini P and FDR values of 0.0045 and 0.087, respectively. This pathway comprised six proteins that included tubulin α1a, tubulin β1, and tubulin α3 (Additional file 2 Abundant protein_FA).
Discussion
The full characterization of the global sperm proteome has the potential to provide new clues to better understand the genesis and maturation of spermatozoa and to lead to concrete improvement of reproduction outcomes. Here we used two common high-throughput techniques that have overlapping strengths and weaknesses [24, 27] for a comprehensive proteome profiling of boar spermatozoa to provide a unique dataset for further in-depth investigation and improvement of post-collection boar semen handling.
The use of the shotgun technique in this study provides the largest pool of boar sperm proteome (2728 total proteins) that falls into the current range reported in spermatozoa (~ 1000 to ~ 4000) of various species such as cow, horse, human, mice, and rat [25, 26, 46]. The study confirms the power of the shotgun (LC-MS/MS) over the 2-DE (2D-MS/MS) method to generating greater number of proteins. Nonetheless, our 2-DE method yielded more protein spots (2123) than the unique reported number (1577) in boar spermatozoa [47].
Proteomic investigations are continuously and consistently showing discrepancies in the numbers of detected proteins between shotgun and gel-based proteomics [24, 27]. This situation can be caused by sample preparation procedures, cut-offs in data acquisition and processing, as well as other factors known to limit the capacity of the 2-DE gels. For example, the presence of abundant proteins on the 2-DE gel compete for space and mask or prevent the detection of neighboring protein spots, while low expressed or less represented protein spots, as seen here with proteins within the 27-80 kDa range (Additional file 3: Figure S2), may not be considered for analyses. Nonetheless, the gel-based proteomic (e.g., 2D-DIGE-LC/2MS) remains the main technique used for comparative studies performed with boar spermatozoa [33, 47–49], while the speed of discovery could be accelerated by the shotgun approach providing a panoramic proteome profile to allow for more complete comparative studies.
With 97% total protein being partially annotated, the current dataset provides a vast reservoir of proteins for further investigation and characterization of boar spermatozoa. Still, the shotgun proteomic is a bottom-up holistic approach that needs further validation of detected proteins. Both immunotechniques and gene expression led to some conflicting findings, particularly with the immunodetection of proteins that are not found in the generated proteome dataset (e.g., PRM-1, AQP-1, and AQP-5) or already reported elsewhere by other authors. For example, previous studies have detected the epididymis secretory glutathione peroxidase or GPX5 [50] and AQP-11 [51] in their small-scale studies using westernblotting in pig spermatozoa. These two proteins are not present in our dataset, where conflicting findings may be due to the stringency of peptide number cut-off for the calling of proteins. In the present study, reported proteins are from peaks of at least three peptides that were shared among the MS/MS runs of all individual samples (n = 8). It is therefore, likely that many proteins may have been filtered out in the current study, resulting in fewer observations as suggested in a previous report [27]. Furthermore, the gene expression study confirmed the presence of selected RNA transcripts in spermatozoa, including those of proteins not found in our study (e.g., AQP-1 and AQP-5). In sum, these findings highlight one of the disadvantages or limits of the shotgun proteomics, mainly for biomarker discovery [51, 52].
On the other hand, many known proteins such as spermadhesins and triosephosphate isomerase that are already reported in the literature were found in the current dataset [47, 53]. Therefore, with 29% (791) of the total protein being fully annotated, the current dataset constitutes an important platform for future annotation updates that may lead to even greater numbers of proteins that will likely provide new research opportunities to enhance our comprehension of the biology of boar spermatozoa.
Meanwhile, the investigation of highly abundant proteins has the potential to boost the knowledge needed to make further progress in assisted reproduction. Numbers of these proteins are known to play crucial roles in 1) protecting spermatozoa from the acidic pH and antimicrobial immune responses of the vagina, 2) assisting spermatozoa during the cervical mucus transit [54, 55] or 3) maintaining the function of spermatozoa until interactions with the oocyte [11]. Especially, spermadhesins have been reported as the major proteins of boar seminal plasma. Their expression has been detected in vesicular glands, epididymis and rete testis of boars while their secretions are found in the male genital tract and seminal plasma [11, 12, 52, 56, 57]. The presence of spermadhesins on sperm membrane and possible effects on sperm function, from motility to interaction with the oocyte, have been reported [11, 58, 59]. In addition to the current knowledge, our study brings further support to the small number of studies identifying the presence of heparin (AQN-1, AQN-3, and AWN-1) and non-heparin (PSP-I and PSP-II) spermadhesin mRNA transcripts in mature boar spermatozoa [52]. In the current dataset, AQN-3 was identified as sperm motility inhibitor or SPMI, which is also known to bind plasma membrane of boar spermatozoa and act as sperm receptor of the oocyte zona pellucida protein [60]. More interestingly, three major proteins associated with acrosome vesicle (acrosin, zona pellucida binding protein, and acrosin binding protein) and having important roles during sperm-egg interactions [61–63] are among the abundant proteins. The roles of many abundant proteins on sperm function are yet to be determined, and the functional analysis is giving direction into which individual or group of proteins may deserve in-depth investigation of their roles.
We found that numerous proteins were overrepresented (> 1.1× – 3.6×) in GO terms such as response to chemical stimulus and biological, cellular, and metabolic processes, oxidative phosphorylation, cellular respiration, an aerobic respiration, thus supporting a concerted effort of various pathways to regulate sperm function, especially motility. In this study, oxidative phosphorylation (vs. glycolysis) is coming up as the key or highly enriched metabolic pathway supplying the high demands for ATP, necessary to achieve progressive motility and more. However, experimental studies have shown the glycolytic pathway as the primary source of energy for sperm motility in many species, including pigs [64–67]. The likely contribution of oxidative phosphorylation to sperm motility may not be ruled out, as the detection of enzymes such as adenylate kinase and phosphoglycerate kinase that shuttle ATP away from the mitochondrion to the flagellum are suggesting the possible role of oxidative phosphorylation.
We also found that the gap junction pathway was highly and significantly represented within the in abundant proteins dataset. Gap junctions are intercellular channels relying on a family of protein membranes known as connexins. These molecules couple the cytoplasms of interacting cells to allow passage of ions and other small molecules [68]. Here, the gap junction enrichment together with the aforementioned “response to chemical stimulus” should not be a surprise given the variety of sperm interactions with their surroundings (i.e., fluids and uterine and oviduct cells). Indeed, interactions with chemical stimuli such as K+, Na+, Ca2+, and pH can leads to physiological events such as metabolic transport and signaling, capable to induce contractions in sperm assemblies resulting in motility. Studies have reported the capability of connexins 36 and 43 to anchor microtubules consisting of alpha and beta tubulin proteins dimerization [69]. Therefore, the abundance of tubulin proteins and their classification as part of the gap junction could be seen as a good indicator of their role in sperm motility.
Additionally, significant and high enrichments (> 12×) in favor of fertilization and reproduction GO terms were observed among abundant proteins; however, the full roles of associated proteins in sperm function remain to be determined [1].
Practical implications
Overall, the present study gives a panoramic list of proteins for further identification of biomarkers associated with various physiological traits of the male, which will be of interest for agricultural and translational purposes [1, 12]. The current dataset confirms the presence of proteins (e.g., acrosin-binding protein, fibronectin 1, and triosephosphate isomerase) that have been proposed as potential predictors of semen freezability in previous studies [12, 32, 47, 49, 70]. Various glucose transporters, and aquaporin family members playing crucial roles during cell membrane permeability to water (aquaporins) and cryoprotectants (aquaglyceroporins) were detected. These findings contribute to the growing investigation of their roles in sperm function [51, 53, 71, 72], while the apparent weaker detection levels of aquaglyceroporins may be consequential to boar sperm freezability [73].
Conclusions
The proposed shotgun proteomics method is the first employed in boar spermatozoa and has allowed the identification of over 2 thousand proteins for a comprehensive bioinformatic analysis of their biological functions. Findings confirmed previous reports using different investigative methods and provide a large reservoir for further search of valuable biomarkers to improve assisted reproductive technology outcomes. Yet, the generated dataset is subjected to continuous update with future annotations to determine the functional significance of detected proteins for more insights into the male (in) fertility.
Additional files
Sperm preparation and proteomics. (PDF 153 kb)
Shotgun proteome data set analyses. File contains the total and abundant proteins, with associated functional analyses (Total protein_FA and abundant protein_FA). (XLSX 417 kb)
Representative two-dimensional gel electrophoresis of the boar sperm proteome. (PDF 84 kb)
Acknowledgements
We acknowledge Ken Pendarvis (University of Arizona) for proteomic analysis, Dr. Buza Teresia (Penn State University) and Dr. Fiona McCarthy (University of Arizona) for their assistance in proteomic analyses. We thank John Stokes and Wei Tan (College of Veterinary Medicine at Mississippi State University) for their assistance in flow cytometry analyses. We are grateful to the staff members of the Institute for Imaging and Analytical Technologies of Mississippi State University (I2AT), especially Amanda Lawrence for their assistance during laser confocal microscope imaging. We thank Christy Steadman for her technical assistance.
Funding
This work was funded by the United States Department of Agriculture – Agricultural Research Service (USDA-ARS grant # 8-6402-3-018).
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Abbreviations
- 2D-MS/MS
Two-Dimensional coupled with tandem Mass Spectrometry (MS/MS)
- ARTs
Assisted reproductive technologies
- BP
Biological process
- CC
Cellular component
- ECM
Extracellular matrix
- GO
Gene ontology
- KEGG
Kyoto enclyclopedia of genes and genomes
- MF
Molecular function
- mRNA
Messenger ribonucleic acid
- NCBI–nr
National Center for Biotechnology Information non-redundant
- PBS
Phosphate-buffered solution
- SDS-PAGE
Sodium Dodecyl Sulfate -PolyAcrylamide Gel Electrophoresis
- TCA
The Citric Acid or TriCarboxylic Acid cycle
Authors’ contributions
JMF conceived and designed the study, assisted by STW and PLR; JMF executed all experiments, analyzed data, performed bioinformatics analyses, and wrote the manuscript; SFL assisted in the execution of experiments and data analyses. All authors contributed to data interpretation, manuscript development. All authors read and approved the final manuscript.
Ethics approval
This study involved the use of animal cell (spermatozoa) that were purchased at a commercial farm and no ethical approval was needed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12864-018-4442-2) contains supplementary material, which is available to authorized users.
Contributor Information
Jean M. Feugang, Phone: +1662 325-7567, Email: jn181@ads.msstate.edu
Shengfa F. Liao, Email: s.liao@ads.msstate.edu
Scott T. Willard, Email: swillard@cals.msstate.edu
Peter L. Ryan, Email: ryan@provost.msstate.edu
References
- 1.Rodríguez-Martínez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ. Seminal plasma proteins: what role do they play? Am J Reprod Immunol. 2011;66:11–22. doi: 10.1111/j.1600-0897.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 2.López Rodríguez A, Rijsselaere T, Beek J, Vyt P, Van Soom A, Maes D. Boar seminal plasma components and their relation with semen quality. Syst Biol Reprod Med. 2013;0(0):1–8. doi: 10.3109/19396368.2012.725120. [DOI] [PubMed] [Google Scholar]
- 3.Feugang JM, Pendarvis K, Crenshaw M, Willard ST, Ryan PL. High-throughput proteomic assessment of frozen-thawed boar spermatozoa. Reprod Fertil Dev. 2010;23(1):194. doi: 10.1071/RDv23n1Ab185. [DOI] [Google Scholar]
- 4.Peddinti D, Nanduri B, Kaya A, Feugang JM, Burgess SC, Memili E. Comprehensive proteomic analysis of bovine spermatozoa of varying fertility rates and identification of biomarkers associated with fertility. BMC Syst Biol. 2008;2:19. doi: 10.1186/1752-0509-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verstegen J, Iguer-Ouada M, Onclin K. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology. 2002;57(1):149–179. doi: 10.1016/S0093-691X(01)00664-1. [DOI] [PubMed] [Google Scholar]
- 6.Amann RP, Waberski D. Computer-assisted sperm analysis (CASA): capabilities and potential developments. Theriogenology. 2014;81(1):5–17.e13. doi: 10.1016/j.theriogenology.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Didion BA. Computer-assisted semen analysis and its utility for profiling boar semen samples. Theriogenology. 2008;70(8):1374–1376. doi: 10.1016/j.theriogenology.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Niżański W, Partyka A, Prochowska S. Evaluation of spermatozoal function—useful tools or just science. Reprod Domest Anim. 2015;51:37–45. doi: 10.1111/rda.12786. [DOI] [PubMed] [Google Scholar]
- 9.Sutovsky P. New approaches to boar semen evaluation, processing and improvement. Reprod Domest Anim. 2015;50:11–19. doi: 10.1111/rda.12554. [DOI] [PubMed] [Google Scholar]
- 10.Druart X, Rickard JP, Mactier S, Kohnke PL, Kershaw-Young CM, Bathgate R, Gibb Z, Crossett B, Tsikis G, Labas V, et al. Proteomic characterization and cross species comparison of mammalian seminal plasma. J Proteome. 2013;91(0):13–22. doi: 10.1016/j.jprot.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Caballero I, Vazquez J, Garcia E, Parrilla I, Roca J, Calvete J, Sanz L, Martinez E. Major proteins of boar seminal plasma as a tool for biotechnological preservation of spermatozoa. Theriogenology. 2008;70(8):1352–1355. doi: 10.1016/j.theriogenology.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Caballero I, Parrilla I, Almiñana C, del Olmo D, Roca J, Martínez EA, Vázquez JM. Seminal plasma proteins as modulators of the sperm function and their application in sperm biotechnologies. Reprod Domest Anim. 2012;47:12–21. doi: 10.1111/j.1439-0531.2012.02028.x. [DOI] [PubMed] [Google Scholar]
- 13.Leahy T, Gadella BM. Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction. 2011;142(6):759–778. doi: 10.1530/REP-11-0310. [DOI] [PubMed] [Google Scholar]
- 14.Dacheux J-L, Gatti JL, Dacheux F. Contribution of epididymal secretory proteins for spermatozoa maturation. Microsc Res Tech. 2003;61(1):7–17. doi: 10.1002/jemt.10312. [DOI] [PubMed] [Google Scholar]
- 15.Sharma R, Agarwal A, Mohanty G, Hamada AJ, Gopalan B, Willard B, Yadav S, Du Plessis S. Proteomic analysis of human spermatozoa proteins with oxidative stress. Reprod Biol Endocrinol. 2013;11(1):48. doi: 10.1186/1477-7827-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soggiu A, Piras C, Hussein HA, De Canio M, Gaviraghi A, Galli A, Urbani A, Bonizzi L, Roncada P. Unravelling the bull fertility proteome. Mol BioSyst. 2013;9(6):1188–1195. doi: 10.1039/c3mb25494a. [DOI] [PubMed] [Google Scholar]
- 17.De Canio M, Soggiu A, Piras C, Bonizzi L, Galli A, Urbani A, Roncada P. Differential protein profile in sexed bovine semen: shotgun proteomics investigation. Mol BioSyst. 2014;10(6):1264–1271. doi: 10.1039/C3MB70306A. [DOI] [PubMed] [Google Scholar]
- 18.Feugang JM, Rozanas C, Kaya A, Topper E, Memili E. Proteome of bull spermatozoa. Reprod Fertil Dev. 2008;20(1):130. doi: 10.1071/RDv20n1Ab100. [DOI] [Google Scholar]
- 19.D'Amours O, Frenette G, Fortier MN, Leclerc P, Sullivan R. Proteomic comparison of detergent-extracted sperm proteins from bulls with different fertility indexes. Reproduction. 2010;139(3):545–556. doi: 10.1530/REP-09-0375. [DOI] [PubMed] [Google Scholar]
- 20.Baker MA, Hetherington L, Reeves G, Müller J, Aitken RJ. The rat sperm proteome characterized via IPG strip prefractionation and LC-MS/MS identification. Proteomics. 2008;8(11):2312–2321. doi: 10.1002/pmic.200700876. [DOI] [PubMed] [Google Scholar]
- 21.Baker MA, Hetherington L, Reeves GM, Aitken RJ. The mouse sperm proteome characterized via IPG strip prefractionation and LC-MS/MS identification. Proteomics. 2008;8(8):1720–1730. doi: 10.1002/pmic.200701020. [DOI] [PubMed] [Google Scholar]
- 22.Johnston DS, Wooters JOE, Kopf GS, Qiu Y, Roberts KP. Analysis of the human sperm proteome. Annals of the New ork Academy of Sciences. 2005;1061(1):190–202. doi: 10.1196/annals.1336.021. [DOI] [PubMed] [Google Scholar]
- 23.Chu DS, Liu H, Nix P, Wu TF, Ralston EJ, Yates Iii JR, Meyer BJ. Sperm chromatin proteomics identifies evolutionarily conserved fertility factors. Nature. 2006;443(7107):101–105. doi: 10.1038/nature05050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliva R, De Mateo S, Castillo J, Azpiazu R, Oriola J, Ballescà JL. Methodological advances in sperm proteomics. Hum Fertil. 2010;13(4):263–267. doi: 10.3109/14647273.2010.516877. [DOI] [PubMed] [Google Scholar]
- 25.Baker MA, Aitken RJ. Proteomic insights into spermatozoa: critiques, comments and concerns. Expert review of proteomics. 2009;6(6):691–705. doi: 10.1586/epr.09.76. [DOI] [PubMed] [Google Scholar]
- 26.Baker MA. The ‘omics revolution and our understanding of sperm cell biology. Asian j androl. 2011;13(1):6–10. doi: 10.1038/aja.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Fonslow BR, Shan B, Baek M-C, Yates JR., III Protein analysis by shotgun/bottom-up proteomics. Chem Rev. 2013;113(4):2343–2394. doi: 10.1021/cr3003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marouga R, David S, Hawkins E. The development of the DIGE system: 2D fluorescence difference gel analysis technology. Anal Bioanal Chem. 2005;382(3):669–678. doi: 10.1007/s00216-005-3126-3. [DOI] [PubMed] [Google Scholar]
- 29.Westermeier R, Naven T. Expression proteomics. Proteomics in Practice: A Laboratory Manual of Proteome Analysis. 2002;16(5):11–160. doi: 10.1002/3527600175.ch2. [DOI] [Google Scholar]
- 30.Holland A, Ohlendieck K. Comparative profiling of the sperm proteome. Proteomics. 2015;15(4):632–648. doi: 10.1002/pmic.201400032. [DOI] [PubMed] [Google Scholar]
- 31.Byrne K, Leahy T, McCulloch R, Colgrave ML, Holland MK. Comprehensive mapping of the bull sperm surface proteome. Proteomics. 2012;12(23-24):3559–3579. doi: 10.1002/pmic.201200133. [DOI] [PubMed] [Google Scholar]
- 32.Vilagran I, Yeste M, Sancho S, Castillo J, Oliva R, Bonet S. Comparative analysis of boar seminal plasma proteome from different freezability ejaculates and identification of Fibronectin 1 as sperm freezability marker. Andrology. 2015;3(2):345–356. doi: 10.1111/andr.12009. [DOI] [PubMed] [Google Scholar]
- 33.Kwon WS, Rahman MS, Lee JS, Kim J, Yoon SJ, Park YJ, You YA, Hwang S, Pang MG. A comprehensive proteomic approach to identifying capacitation related proteins in boar spermatozoa. BMC Genomics. 2014;15:897. doi: 10.1186/1471-2164-15-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Zhu H, Hu C, Hao H, Zhang J, Li K, Zhao X, Qin T, Zhao K, Zhu H, et al. Identification of differentially expressed proteins in fresh and frozen-thawed boar spermatozoa by iTRAQ-coupled 2D LC-MS/MS. Reproduction. 2014;147(3):321–330. doi: 10.1530/REP-13-0313. [DOI] [PubMed] [Google Scholar]
- 35.González-Cadavid V, Martins JA, Moreno FB, Andrade TS, Santos AC, Monteiro-Moreira ACO, Moreira RA, Moura AA. Seminal plasma proteins of adult boars and correlations with sperm parameters. Theriogenology. 2014;82(5):697–707. doi: 10.1016/j.theriogenology.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Patiño C, Barranco I, Parrilla I, Valero ML, Martinez EA, Rodriguez-Martinez H, Roca J. Characterization of the porcine seminal plasma proteome comparing ejaculate portions. J Proteome. 2016;142(Supplement C):15–23. doi: 10.1016/j.jprot.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Feugang JM, Rodriguez-Munoz JC, Willard ST, Bathgate RA, Ryan PL. Examination of relaxin and its receptors expression in pig gametes and embryos. Reprod Biol Endocrinol. 2011;9:10. doi: 10.1186/1477-7827-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feugang JM, Greene JM, Sanchez-Rodriguez HL, Stokes JV, Crenshaw MA, Willard ST, Ryan PL. Profiling of relaxin and its receptor proteins in boar reproductive tissues and spermatozoa. Reprod Biol Endocrinol. 2015;13(1):46. doi: 10.1186/s12958-015-0043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Ma J, Dou L, Wu S, Qian X, Xie H, Zhu Y, He F. Mass measurement errors of Fourier-transform mass spectrometry (FTMS): distribution, recalibration, and application. J Proteome Res. 2009;8(2):849–859. doi: 10.1021/pr8005588. [DOI] [PubMed] [Google Scholar]
- 40.Yates JR, 3rd, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal Chem. 1995;67(8):1426–1436. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy FM, Wang N, Magee GB, Nanduri B, Lawrence ML, Camon EB, Barrell DG, Hill DP, Dolan ME, Williams WP. AgBase: a functional genomics resource for agriculture. BMC Genomics. 2006;7(1):1. doi: 10.1186/1471-2164-7-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 43.Feugang JM, Youngblood RC, Greene JM, Willard ST, Ryan PL. Self-illuminating quantum dots for non-invasive bioluminescence imaging of mammalian gametes. J Nanobiotechnology. 2015;13(1):38. doi: 10.1186/s12951-015-0097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pendarvis K, Kumar R, Burgess SC, Nanduri B. An automated proteomic data analysis workflow for mass spectrometry. BMC Bioinformatics. 2009;10(Suppl 11):S17. doi: 10.1186/1471-2105-10-S11-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundgren DH, Hwang S-I, Wu L, Han DK. Role of spectral counting in quantitative proteomics. Expert review of proteomics. 2010;7(1):39–53. doi: 10.1586/epr.09.69. [DOI] [PubMed] [Google Scholar]
- 46.Gilany K, Lakpour N, Vafakhah M, Sadeghi MR. The profile of human sperm proteome; a mini-review. Journal of reproduction & infertility. 2011;12(3):193–199. [PMC free article] [PubMed] [Google Scholar]
- 47.Vilagran I, Castillo J, Bonet S, Sancho S, Yeste M, Estanyol JM, Oliva R. Acrosin-binding protein (ACRBP) and triosephosphate isomerase (TPI) are good markers to predict boar sperm freezing capacity. Theriogenology. 2013;80(5):443–450. doi: 10.1016/j.theriogenology.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Calvete JJ, Mann K, Schafer W, Raida M, Sanz L, Topfer-Petersen E. Boar spermadhesin PSP-II: location of posttranslational modifications, heterodimer formation with PSP-I glycoforms and effect of dimerization on the ligand-binding capabilities of the subunits. FEBS Lett. 1995;365(2-3):179–182. doi: 10.1016/0014-5793(95)00452-F. [DOI] [PubMed] [Google Scholar]
- 49.Yeste M. Recent advances in boar sperm cryopreservation: state of the art and current perspectives. Reprod Domest Anim. 2015;50:71–79. doi: 10.1111/rda.12569. [DOI] [PubMed] [Google Scholar]
- 50.Vilagran I, Castillo-Martín M, Prieto-Martínez N, Bonet S, Yeste M. Triosephosphate isomerase (TPI) and epididymal secretory glutathione peroxidase (GPX5) are markers for boar sperm quality. Anim Reprod Sci. 2016;165:22–30. doi: 10.1016/j.anireprosci.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Prieto-Martínez N, Vilagran I, Morató R, Rodríguez-Gil JE, Yeste M, Bonet S. Aquaporins 7 and 11 in boar spermatozoa: detection, localisation and relationship with sperm quality. Reprod Fertil Dev. 2016;28(6):663–672. doi: 10.1071/RD14237. [DOI] [PubMed] [Google Scholar]
- 52.Song CY, Gao B, Wu H, Wang XY, Chen GH, Mao J. Spatial and temporal expression of spermadhesin genes in reproductive tracts of male and female pigs and ejaculated sperm. Theriogenology. 2010;73(5):551–559. doi: 10.1016/j.theriogenology.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 53.Prieto-Martínez N, Morató R, Vilagran I, Rodríguez-Gil JE, Bonet S, Yeste M. Aquaporins in boar spermatozoa. Part II: detection and localisation of aquaglyceroporin 3. Reprod Fertil Dev. 2015;18(1):1. doi: 10.1071/RD15164. [DOI] [PubMed] [Google Scholar]
- 54.Suarez SS, Oliphant G. Interaction of rabbit spermatozoa and serum complement components. Biol Reprod. 1982;27(2):473–483. doi: 10.1095/biolreprod27.2.473. [DOI] [PubMed] [Google Scholar]
- 55.Dostal J, Veselský L, Marounek M, Železná B, Jonakova V. Inhibition of bacterial and boar epididymal sperm immunogenicity by boar seminal immunosuppressive component in mice. J Reprod Fertil. 1997;111(1):135–141. doi: 10.1530/jrf.0.1110135. [DOI] [PubMed] [Google Scholar]
- 56.Töpfer-Petersen E, Ekhlasi-Hundrieser M, Kirchhoff C, Leeb T, Sieme H. The role of stallion seminal proteins in fertilisation. Anim Reprod Sci. 2005;89(1):159–170. doi: 10.1016/j.anireprosci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 57.Iwamoto T, Hiroaki H, Furuichi Y, Wada K, Satoh M, Satoh M, Osada T, Gagnon C. Cloning of boar SPMI gene which is expressed specifically in seminal vesicle and codes for a sperm motility inhibitor protein. FEBS Lett. 1995;368(3):420–424. doi: 10.1016/0014-5793(95)00701-A. [DOI] [PubMed] [Google Scholar]
- 58.Manaskova P, Jonakova V. Localization of porcine seminal plasma (PSP) proteins in the boar reproductive tract and spermatozoa. J Reprod Immunol. 2008;78(1):40–48. doi: 10.1016/j.jri.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Ekhlasi-Hundrieser M, Gohr K, Wagner A, Tsolova M, Petrunkina A, Töpfer-Petersen E. Spermadhesin AQN1 is a candidate receptor molecule involved in the formation of the oviductal sperm reservoir in the pig. Biol Reprod. 2005;73(3):536–545. doi: 10.1095/biolreprod.105.040824. [DOI] [PubMed] [Google Scholar]
- 60.van Gestel RA, Brewis IA, Ashton PR, Brouwers JF, Gadella BM. Multiple proteins present in purified porcine sperm apical plasma membranes interact with the zona pellucida of the oocyte. Mol Hum Reprod. 2007;13(7):445–454. doi: 10.1093/molehr/gam030. [DOI] [PubMed] [Google Scholar]
- 61.Castellani-Ceresa L, Berruti G, Colombo R. Immunocytochemical localization of acrosin in boar spermatozoa. J Exp Zool. 1983;227(2):297–304. doi: 10.1002/jez.1402270213. [DOI] [PubMed] [Google Scholar]
- 62.Barros C, Melendez J, Valdivia M, Rios M, Yunes R. Sperm passage through the egg coats. Biol Res. 1993;26:417. [Google Scholar]
- 63.Töpfer-Petersen E. Molecular mechanism of fertilization in the pig. Reprod Domest Anim. 1995;31(1):93–100. doi: 10.1111/j.1439-0531.1995.tb00010.x. [DOI] [Google Scholar]
- 64.Nascimento JM, Shi LZ, Chandsawangbhuwana C, Tam J, Durrant B, Botvinick EL, Berns MW: Use of laser tweezers to analyze sperm motility and mitochondrial membrane potential. J Biomed Opt 2008, 13(1):014002. [DOI] [PMC free article] [PubMed]
- 65.Ford W. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update. 2006;12(3):269–274. doi: 10.1093/humupd/dmi053. [DOI] [PubMed] [Google Scholar]
- 66.Ruiz-Pesini E, Díez-Sánchez C, López-Pérez MJ, Enriquez JA. The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr Top Dev Biol. 2007;77:3–19. doi: 10.1016/S0070-2153(06)77001-6. [DOI] [PubMed] [Google Scholar]
- 67.Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71(2):540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- 68.Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397(1):1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giepmans BNG, Verlaan I, Hengeveld T, Janssen H, Calafat J, Falk MM, Moolenaar WH. Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol. 2001;11(17):1364–1368. doi: 10.1016/S0960-9822(01)00424-9. [DOI] [PubMed] [Google Scholar]
- 70.Pinart E, Yeste M, Bonet S. Acrosin activity is a good predictor of boar sperm freezability. Theriogenology. 2015;83(9):1525–1533. doi: 10.1016/j.theriogenology.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Moretti E, Terzuoli G, Mazzi L, Iacoponi F, Collodel G. Immunolocalization of aquaporin 7 in human sperm and its relationship with semen parameters. Syst Biol Reprod Med. 2012;58(3):129–135. doi: 10.3109/19396368.2011.644385. [DOI] [PubMed] [Google Scholar]
- 72.Chen Q, Peng H, Lei L, Zhang Y, Kuang H, Cao Y, Shi Q-X, Ma T, Duan E. Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2011;21(6):922–933. doi: 10.1038/cr.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sales AD, Lobo CH, Carvalho AA, Moura AA, Rodrigues AP. Structure, function, and localization of aquaporins: their possible implications on gamete cryopreservation. Genet Mol Res. 2013;12(4):6718–6732. doi: 10.4238/2013.December.13.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sperm preparation and proteomics. (PDF 153 kb)
Shotgun proteome data set analyses. File contains the total and abundant proteins, with associated functional analyses (Total protein_FA and abundant protein_FA). (XLSX 417 kb)
Representative two-dimensional gel electrophoresis of the boar sperm proteome. (PDF 84 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].


