Abstract
Background
The real-world experience of women receiving extended-cycle combined oral contraception (COC) versus monthly-cycle COC has not been reported.
Methods
Data were from the United States 2013 National Health and Wellness Survey. Eligible women (18–50 years old, premenopausal, without hysterectomy) currently using extended-cycle COC (3 months between periods) were compared with women using monthly-cycle COC. Treatment satisfaction (1 “extremely dissatisfied” to 7 “extremely satisfied”), adherence (8-item Morisky Medication Adherence Scale©), menstrual cycle-related symptoms, health-related quality of life (HRQOL) and health state utilities (Medical Outcomes Short Form Survey-36v2®), depression (9-item Patient Health Questionnaire), sleep difficulties, Work Productivity and Activity Impairment-General Health, and healthcare resource use were assessed using one-way analyses of variance, chi-square tests, and generalized linear models (adjusted for covariates).
Results
Participants included 260 (6.7%) women using extended-cycle and 3616 (93.3%) using monthly-cycle COC. Women using extended-cycle COC reported significantly higher treatment satisfaction (P = 0.001) and adherence (P = 0.04) and reduced heavy menstrual bleeding (P = 0.029). A non-significant tendency toward reduced menstrual pain (39.5% versus 47.3%) and menstrual cycle-related symptoms (40.0% versus 48.7%) was found in women using extended-cycle versus monthly-cycle COC. Significantly more women using extended-cycle COC reported health-related diagnoses, indicating preferential prescription for extended-cycle COC among women reporting more health problems. Consistent with this poorer health, more women using extended-cycle COC reported fatigue, headache, and activity impairment (P values < 0.05). There were no other significant differences between groups.
Conclusions
This real-world observational study supports extended-cycle COC as a valuable treatment option with high satisfaction, high adherence, and reduced heavy menstrual bleeding.
Keywords: Real-world, Extended-regimen, Heavy menstrual bleeding, Menstrual cycle, Satisfaction, Adherence, Women’s health, Hormonal contraception
Background
Women in the United States (US) and Europe who use hormonal contraception most commonly select combined oral contraception (COC) [1]. Standard 21/7 COC, including 21 days of active pills followed by a 7-day hormone-free interval, was developed to induce monthly bleeds that mimic the natural menstrual cycle; however, no health benefits of induced monthly bleeding have been demonstrated [2]. In contrast, negative effects associated with monthly bleeding, including disruption of daily life due to menstrual cycle-related discomfort and/or inconvenience, have been shown [3, 4]. Additionally, surveys of women in the US and Europe have shown a majority report a preference for reduced frequency of menstrual bleeding to every 3 months or contraceptive-induced amenorrhea [4–7].
Extended-cycle COC regimens, which include >28 days of active pills and reduce scheduled bleeding episodes to 4 per year, are effective in pregnancy prevention and their safety profile is similar to 28-day cyclical regimens [8, 9]. Due to the reduced frequency of scheduled bleeding compared with monthly-cycle COC, extended-cycle COC may provide greater improvement in menstrual cycle-related medical conditions and symptoms, such as dysmenorrhea, premenstrual syndrome (PMS), menstrually related headaches, endometriosis, heavy menstrual bleeding, iron deficiency, and anemia [10, 11]. Additionally, extended-cycle COC may reduce menstrual cycle-related inconvenience and interference with daily activities, work/school attendance, personal social events, and sports. Altogether, these positive benefits of extended-cycle COC may lead to greater treatment satisfaction and adherence, improved health-related quality of life (HRQOL), and economic benefits related to reduced expenses for feminine-hygiene products, pain medication, and medical consultations [10, 11].
Extended-cycle COC has been available in the US since 2003 [12], with more recent availability in Europe; however, the real-world experience of women receiving extended-cycle COC has not yet been described. The current study examined the real-world experience of women in the US receiving extended-cycle COC versus monthly-cycle COC and characterized treatment satisfaction, adherence, and menstrual cycle-related symptoms, as well as HRQOL, health-related work and activity impairment, and healthcare resource use.
Methods
Study design
Study data were from the 2013 National Health and Wellness Survey (NHWS), a nationally representative, self-administered, internet-based survey of adults. The NHWS is a large scale general population survey in the healthcare industry. The annual survey is collected in the US, Europe, Japan, China, Brazil, and Russia. NHWS respondents are recruited from an internet panel using a random stratified sampling framework to ensure the demographic composition (ie, age, gender, and ethnicity for the US respondents) is representative of the adult population of the country. The current study examined the US data from the 2013 NHWS. Electronic consent was obtained from all participants in the NHWS. The survey was granted exemption by the Essex Institutional Review Board (Lebanon, NJ).
Eligible women met the study inclusion criteria of 18 to 50 years old, premenopausal, without hysterectomy, and self-reported current use of COC. Women who reported using extended-cycle COC with 3 months between periods were compared with women who reported using monthly-cycle COC with 3 to 4 weeks between periods.
Assessments
Demographic and health characteristics were reported, including comorbid health-related diagnoses and comorbid disease burden using the Charlson Comorbidity Index (CCI) [13]. The weighted presence of 18 conditions is used to create a CCI score that ranges from 0 to 37, with higher scores indicating greater comorbid disease burden [14].
Primary treatment-related outcomes included satisfaction with current COC, adherence to the COC regimen, and menstrual cycle-related symptoms. Treatment satisfaction was rated from 1 (“extremely dissatisfied”) to 7 (“extremely satisfied”). Adherence was assessed using the 8-item Morisky Medication Adherence Scale© (MMAS-8; used under license and with permission) [15], a validated self-report measure of adherence to prescribed medications. Lower scores represent lower adherence (range from 0 to 8). Assessment of menstrual cycle-related symptoms included the presence in the past month of heavy menstrual bleeding, menstrual pain, dysmenorrhea, and 15 symptoms experienced as a result of PMS/premenstrual dysphoric disorder (PMDD).
Secondary health outcomes included HRQOL, depression, and sleep difficulties, and economic outcomes examined work productivity and activity impairment and healthcare resource use. HRQOL was assessed using the physical component summary (PCS) and mental component summary (MCS) scores from the Medical Outcomes Study 36-item Short Form Survey version 2® (SF-36v2) [16]. PCS and MCS scores are normed to a mean of 50 ± 10, with higher scores indicating better health status. The SF-36v2 was also used to generate the health state utilities Short Form-6 Dimensions (SF-6D) index score, using the United Kingdom general population health state preference weights [17]. The SF-6D utilities index scale anchors range from 0 (health equivalent to death) to 1 (health equivalent to full health) and calculated index scores range from 0.29 to 1.0 [18]. Higher scores indicate better health status; the general population mean is 0.78 [19]. Depression was examined using the 9-item Patient Health Questionnaire (PHQ)-9 [20]. Depression severity was assessed as no to minimal depression (score 0 to 4), mild (score 5 to 9), moderate (score 10 to 14), moderately severe (score 15 to 19), and severe (score 20 to 27). Sleep difficulties were assessed as the presence of 12 sleep-related problems.
The Work Productivity and Activity Impairment-General Health (WPAI-GH) questionnaire, a 6-item validated assessment [21], examined outcomes due to one’s health in the past 7 days. Absenteeism (percentage of work time missed due to one’s health problems), presenteeism (percentage of impairment experienced while at work due to one’s health problems), overall work impairment (overall work productivity loss calculated from the combined absenteeism and presenteeism scores) and activity impairment (percentage of impairment in daily activities due to one’s health problems) were examined. Women who were full-time or part-time employed provided responses for absenteeism, presenteeism, and overall work impairment. All women provided a response for activity impairment. Higher percentages indicate greater impairment and less productivity. Healthcare resource use (past 6 months, all-cause) included the number of any traditional healthcare provider visits, general practitioner visits, emergency room visits, and hospitalizations.
Data analysis
Unadjusted one-way analysis of variance (ANOVA) or chi-square tests were completed for demographic characteristics and all outcomes. Generalized linear models that adjusted for covariates were used to examine treatment satisfaction, adherence, heavy menstrual bleeding, and menstrual cycle pain. Covariates included demographics (age, insurance coverage of contraception), health characteristics (body mass index [BMI]), and comorbidities (CCI, migraine/headaches, depression, anxiety, and sleep difficulties). Adjusted means with 95% confidence intervals (CIs) are reported. Analyses were completed using SPSS version 23.0 (Chicago, IL) and P < 0.05 (2-tailed) was considered statistically significant.
Results
Participant characteristics
The 2013 NHWS US sample included 75,000 participants. Eligible women included in the current study totaled 3876. Use of extended-cycle COC was reported by 6.7% (260/3876) of women and monthly-cycle COC by 93.3% (3616/3876). Women using monthly-cycle COC reported longer duration of COC use (58.9 ± 62.8 months) compared with women using extended-cycle COC (46.8 ± 36.8 months; P = 0.002).
Women using extended-cycle COC were slightly older (31.6 years vs 30.3 years, P = 0.011) and more frequently reported health insurance coverage of contraception (78.5% vs 70.9%, P = 0.01; Table 1). There was no significant difference between women in the extended-cycle and monthly-cycle COC groups in mean CCI score; however, a significantly greater percentage of women receiving extended-cycle COC reported diagnoses of migraines (27.3% vs 15.9%), headaches (21.2% vs 15.7%), sleep difficulties (15.8% vs 10.1%), heartburn (15.8% vs 10.8%), hypertension (10.0% vs 6.3%), and irritable bowel syndrome (9.6% vs 5.8%), indicating preferential prescription of extended-cycle COC among women with greater health problems.
Table 1.
Demographic characteristics of women using extended-cycle or monthly-cycle COC
| Extended-cycle COC n = 260 | Monthly-cycle COC n = 3616 | |
|---|---|---|
| Age, mean ± SDa | 31.6 ± 7.7 | 30.3 ± 7.6 |
| CCI, mean ± SD | 0.1 ± 0.4 | 0.1 ± 0.5 |
| Race/Ethnicity, n (%) | ||
| Non-Hispanic White | 205 (78.8) | 2720 (75.2) |
| Non-Hispanic Black | 18 (6.9) | 303 (8.4) |
| Hispanic | 17 (6.5) | 296 (8.2) |
| Other ethnicity | 20 (7.7) | 297 (8.2) |
| Education, n (%) | ||
| < 4-year college degree | 107 (41.2) | 1583 (43.8) |
| ≥ 4-year college degree | 153 (58.8) | 2033 (56.2) |
| BMI, n (%)a | ||
| Underweight | 7 (2.7) | 150 (4.1) |
| Normal weight | 141 (54.2) | 1822 (50.4) |
| Overweight | 42 (16.2) | 838 (23.2) |
| Obese | 61 (23.5) | 723 (20.0) |
| Unknown | 9 (3.5) | 83 (2.3) |
| Marital status, n (%) | ||
| Single/divorced/separated/widowed | 127 (48.8) | 1737 (48.0) |
| Married/living with partner | 133 (51.2) | 1879 (52.0) |
| Have health insurance, n (%) Yes | 237 (91.2) | 3199 (88.5) |
| Health insurance covers COC, n (%) Yesa | 204 (78.5) | 2565 (70.9) |
| Current smoker, n (%) Yes | 22 (8.5) | 362 (10.0) |
| Exercise ≥ 20 min ≥ 1 time past month, n (%) Yes | 207 (79.6) | 2827 (78.2) |
BMI, body mass index; CCI, Charlson Comorbidity Index; COC, combined oral contraception
aP < 0.05
Satisfaction, adherence, and menstrual cycle symptoms
Women using extended-cycle COC reported significantly higher treatment satisfaction (adjusted mean 6.1 [95% CI: 5.9, 6.2]) versus women on monthly-cycle COC (adjusted mean 5.8 [95% CI: 5.8, 5.9], P = 0.001; Table 2). Women using extended-cycle COC reported significantly greater adherence (adjusted mean 6.9 [95% CI: 6.7, 7.1]) versus women using monthly-cycle COC (adjusted mean 6.7 [95% CI: 6.7, 6.8], P = 0.04).
Table 2.
Treatment satisfaction, heavy menstrual bleeding, and menstrual pain
| Extended-cycle COC | Monthly-cycle COC | |
|---|---|---|
| Adjusted Mean (95% CI) | Adjusted Mean (95% CI) | |
| Treatment satisfactiona | 6.1 (5.9, 6.2) | 5.8 (5.8, 5.9) |
| Treatment adherence (MMAS-8)a | 6.9 (6.7, 7.1) | 6.7 (6.7, 6.8) |
| Heavy menstrual bleeding (% women)a | 8.6 (5.8, 12.4) | 13.0 (11.9, 14.2) |
| Menstrual pain in past month (% women) | 39.5 (29.1, 50.9) | 47.3 (44.2, 50.5) |
CI, confidence interval; COC, combined oral contraception; MMAS-8, Morisky Medication Adherence Scale
aP values < 0.05 in generalized linear models adjusted for covariates
Significantly fewer women using extended-cycle COC reported heavy menstrual bleeding (adjusted mean percentage 8.6% [95% CI: 5.8%, 12.4%] vs monthly-cycle COC 13.0% [95% CI: 11.9%, 14.2%], P = 0.029; Table 2). Fewer women receiving extended-cycle COC appeared to report menstrual pain in the past month (adjusted mean percentage 39.5% [95% CI: 29.1%, 50.9%]) versus monthly-cycle COC (47.3% [95% CI: 44.2%, 50.5%]); however, the difference was not statistically significant (Table 2).
PMS/PMDD symptoms were reported by 40.0% (104/260) of women using extended-cycle COC and 48.7% (1760/3616) of women using monthly-cycle COC (Fig. 1). Among these women, the most frequently reported symptoms were abdominal pain (74.0% of women using extended-cycle COC; 71.0% of women using monthly-cycle COC), bloating/fluid retention (72.1%; 73.4%), fatigue (77.9%; 67.7%), and irritability (77.9%; 70.3%). Fatigue and headache were reported by a significantly greater percentage of women using extended-cycle COC versus monthly-cycle COC (P values < 0.05).
Fig. 1.
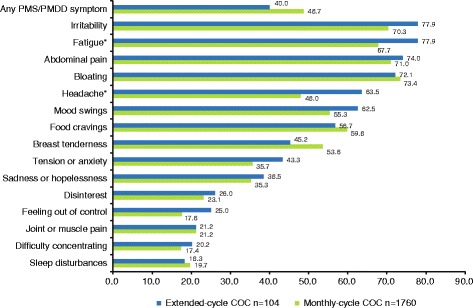
Percentage of women reporting PMS/PMDD symptoms COC, combined oral contraception; PMS/PMDD, premenstrual syndrome/premenstrual dysphoric disorder. Percentage of women reporting any PMS/PMDD symptom includes all extended-cycle (n = 260) and monthly-cycle (n = 3616) women. Percentage of women reporting specific PMS/PMDD symptoms includes only those women who reported any PMS/PMDD symptom (extended-cycle, n = 104; monthly-cycle, n = 1760). *P < 0.05, chi-square tests. Symptoms of fatigue and headache were reported by significantly more women receiving extended-cycle versus monthly-cycle COC
HRQOL, depression, and sleep difficulties
There were no significant differences between women using extended-cycle or monthly-cycle COC in PCS (unadjusted mean 54.4 ± 7.3 vs 54.4 ± 7.0, respectively), MCS (unadjusted mean 46.0 ± 11.1 vs 46.7 ± 10.2, respectively), or the SF-6D health utilities index score (unadjusted mean 0.7 ± 0.1 vs 0.8 ± 0.1, respectively) (Table 3). Depression did not significantly differ between women using extended-cycle (unadjusted mean 5.2 ± 5.5) or monthly-cycle (unadjusted mean 4.8 ± 5.3) COC and most women experienced no depression to mild depression (Table 3). Among the 12 assessed sleep difficulties (Fig. 2), significantly more women receiving extended-cycle COC versus women using monthly-cycle COC reported difficulty falling asleep (43.8% vs 36.4%, P = 0.016), pain (9.2% vs 5.9%, P = 0.03), and waking up too early (28.5% vs 21.9%, P = 0.014).
Table 3.
HRQOL, health state utilities, depression, and healthcare resource use
| Extended-cycle COC | Monthly-cycle COC | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| HRQOL | ||
| Physical Component Summary | 54.4 ± 7.3 | 54.4 ± 7.0 |
| Mental Component Summary | 46.0 ± 11.1 | 46.7 ± 10.2 |
| Health state utilities | ||
| SF-6D index score | 0.7 ± 0.1 | 0.8 ± 0.1 |
| Depression | ||
| PHQ-9 score | 5.2 ± 5.5 | 4.8 ± 5.3 |
| Healthcare Resource Use | ||
| Any traditional healthcare provider visits | 4.3 ± 6.6 | 3.7 ± 5.9 |
| General practitioner visits | 1.1 ± 1.6 | 0.9 ± 1.8 |
| Emergency room visits | 0.2 ± 0.5 | 0.2 ± 1.2 |
| Hospitalizations | 0.1 ± 0.3 | 0.1 ± 0.4 |
COC, combined oral contraception; HRQOL, health related quality of life; PHQ-9, Patient Health Questionnaire-9; SD, standard deviation; SF-6D, Short Form-6 Dimensions
All P values > 0.05, unadjusted one-way ANOVA analyses
Fig. 2.
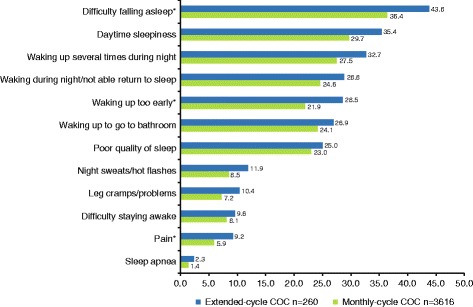
Percentage of women reporting sleep difficulties COC, combined oral contraception. *P < 0.05, chi-square tests. Women reported the presence versus absence of 12 sleep difficulties. Difficulty falling asleep, sleep difficulty associated with pain, and waking up too early were reported by significantly more women receiving extended-cycle COC compared with women receiving monthly-cycle COC
Work and activity impairment and healthcare resource use
Examination of work productivity and activity impairment due to health (Fig. 3) found no significant differences between women using extended-cycle or monthly-cycle COC in percentage of work time missed (absenteeism unadjusted means 2.6 ± 10.0% vs 2.3 ± 10.3%), percentage of impairment experienced at work (presenteeism unadjusted means 15.4 ± 24.1% vs 12.3 ± 20.8%), or overall work productivity loss (overall work impairment unadjusted means 16.2 ± 25.8% vs 13.7 ± 22.8%). Women using extended-cycle versus monthly-cycle COC reported a higher percentage of impairment in daily activities due to health (unadjusted mean 19.9 ± 26.4% vs 16.4 ± 24.0%, P = 0.025). Healthcare resource use (Table 3) did not significantly differ between women receiving extended-cycle versus monthly-cycle COC for the total number of visits to any traditional healthcare provider (unadjusted means 4.3 ± 6.6 vs 3.7 ± 5.9), general practitioner visits (unadjusted means 1.1 ± 1.6 vs 0.9 ± 1.8), emergency room visits (unadjusted means 0.2 ± 0.5 vs 0.2 ± 1.2), or hospitalizations (unadjusted means 0.1 ± 0.3 vs 0.1 ± 0.4).
Fig. 3.

Work productivity and activity impairment due to health in the past 7 days COC, combined oral contraception; SD, standard deviation; WPAI-GH, Work Productivity and Activity Impairment-General Health. *P < 0.05, unadjusted one-way ANOVA analyses. Work productivity and activity impairment were assessed using the WPAI-GH. Women who were employed full or part-time (n = 173 extended-cycle COC; n = 2504 monthly-cycle COC) responded to work productivity questions (with the exception that n = 2493 women receiving monthly-cycle COC responded to the presenteeism question). All women responded to activity impairment questions. Women receiving extended-cycle COC reported a significantly greater mean percentage of impairment due to health in their daily activities over the past 7 days compared with women receiving monthly-cycle COC
Discussion
COC has evolved to include extended-cycle COC regimens with 4 scheduled bleeding episodes per year. Many women in the US and Europe prefer less frequent menstrual cycles [4–7], and acceptance of extended-cycle COC continues to increase [8, 9, 12]. In line with reduced menstrual cycle-related symptoms in women using extended-cycle hormonal contraception [10, 11], common reasons for prescribing extended-cycle COC include relief from menstrual symptoms and convenience of menstrual suppression [12, 22–24].
The availability of the first 21/7 COC in 1960 revolutionized reproductive choice for women [25]. The development of extended-cycle COC allows women to reduce the discomfort and/or inconvenience associated with monthly menses, resulting in fewer menstrual cycle-related disruptions in social, sexual, and sport activities, concerns previously expressed by women [3–5]. Potential negative personal and societal economic effects include reduced academic/work force participation and increased costs associated with management of menstrual cycle disorders and symptoms and related healthcare resource use. Extended-cycle regimens significantly reduce use of menstrual-hygiene products and drug products to treat menstrual symptoms and may produce cost savings related to reduced work/school absenteeism and reduced doctor visits [26]. The potential benefit of extended-cycle regimens on sexual behavior and satisfaction requires further study but improvement has been suggested with a 72/4 COC regimen [27].
The current study examined the real-world experience of US women using extended-cycle versus monthly-cycle COC within the NHWS. Key findings included significantly higher treatment satisfaction and adherence and reduced heavy menstrual bleeding in women using extended-cycle COC. Additionally, fewer women using extended-cycle COC appeared to report menstrual pain in the past month or PMS/PMDD symptoms. The lack of statistical significance in these outcomes may be related to the finding of preferential prescription of extended-cycle COC in women reporting health problems. There was a greater frequency of health-related diagnoses reported by women using extended-cycle COC, including diagnoses for headaches, sleep difficulties, and health problems, and significantly more women using extended-cycle COC reported fatigue, headaches, sleep difficulties, and daily activity impairment due to general health. Poorer health among women using extended-cycle COC may have obscured group differences in non-contraceptive benefits, such as in well-being, work productivity, and healthcare resource use. In this real-world study, it is possible that women using extended-cycle COC were prescribed their COC, at least in part, because they already had worse functioning in these areas, possibly related to or exacerbated by menses. This possibility, and potential improvements in these outcomes following initiation of extended-cycle COC, could not be evaluated in the current study because the cross-sectional survey design does not allow an analysis of change from pre- (ie, no COC or monthly-cycle COC) to post-initiation of extended-cycle COC. Similarly, whether the poorer health in women using extended-cycle COC began prior to or following initiation of extended-cycle COC is not known. Prospective, controlled studies are needed to address these questions.
Study strengths include real-world data, drawn from the NHWS, which depict the real-life experience of women using extended-cycle COC and complement clinical trials. A large number of women and a broad range of health-related and economic-related measures were included. In the examination of work productivity and activity impairment, future studies may consider modifying the WPAI-GH 7-day reporting period and/or the focus of the health disruption to menstrual cycle-related, as has been done in women with endometriosis [28, 29] and heavy menstrual bleeding [30, 31]. Study limitations include the cross-sectional survey design, the consequent lack of information about menstrual- and health-related symptoms prior to using extended-cycle COC, and the smaller sample of women reporting extended-cycle versus monthly-cycle COC. Additionally, different formulations of extended-cycle COC, such as those with and without hormone-free intervals, of monthly-cycle COC, and type of progestin were not evaluated. And, as a real-world observational analysis, a sample bias may exist.
Given the small percentage of women using extended-cycle COC in the current study, there is clearly a continued need for improved awareness of the availability and utility of extended-cycle COC [12]. Almost half of the women using monthly-cycle COC reported PMS/PMDD symptoms, indicating the potential benefit of switching to COC with reduced frequency of menses [10, 11]. Specifically, the presence of any menstrual symptoms has been associated with significantly lower HRQOL [32], suggesting a benefit of reduced frequency of menses. Contraceptive counselling should evaluate bleeding preferences, which may vary with cultural background, and determine the potential fit of extended-cycle COC [2, 5]. Counseling should include identifying any misconceptions, educating patients that monthly bleeding is not necessary and is not an indication of health when using COC, and improve patient understanding of the safety and potential non-contraceptive benefits of extended-cycle COC [2].
Conclusions
Extended-cycle COC with reduced frequency of menstrual cycles may help women better manage discomfort, inconvenience, and disruption of daily activities associated with monthly menses. The real-world experience of women using extended-cycle COC supports high treatment satisfaction and adherence and reduced heavy menstrual bleeding. Preferential prescription of extended-cycle COC was found among women reporting health problems, and poorer health may have obscured significant differences in broader non-contraceptive benefits when compared with monthly-cycle COC users. Although there are some limitations, this analysis provides information regarding patient experiences in a real-world setting that are not available from clinical trials, which may be useful for health care providers and patients in clinical practice. Further research examining extended-cycle COC and patient-reported outcomes of satisfaction, menstrual symptoms, HRQOL, and the economic impact of improved management of menstrual cycle-related symptoms is warranted.
Acknowledgments
The authors wish to thank Lynanne McGuire, PhD, of MedVal Scientific Information Services, LLC, for providing medical writing and editorial assistance. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP3 Guidelines.” Kantar health holds a license to use the Morisky Medication Adherence Scale.
Funding
This work was supported by Teva Branded Pharmaceutical Products R & D, Inc. (Frazer, PA). Teva provided a full review of the article. MCM and BT, employees of Teva Pharmaceuticals, are authors of this manuscript and as such were involved in the interpretation of the data and in the drafting and revision of the manuscript. Medical writing assistance provided by MedVal Scientific Information Services, LLC (Princeton, NJ) was supported by Teva Branded Pharmaceutical Products R & D, Inc.
Availability of data and materials
The data that support the findings of this study are available from Kantar Health but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Kantar Health.
Abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- CCI
Charlson Comorbidity Index
- CI
confidence interval
- COC
combined oral contraception
- HRQOL
health-related quality of life
- MCS
mental component summary
- MMAS
Morisky Medication Adherence Scale
- NHWS
National Health and Wellness Survey
- PCS
physical component summary
- PHQ-9
9-item Patient Health Questionnaire
- PMDD
premenstrual dysphoric disorder
- PMS
premenstrual syndrome
- SF-36v2
36-item Short Form Survey version 2
- SF-6D
Short Form-6 Dimensions
- US
United States
- WPAI-GH
Work Productivity and Activity Impairment-General Health
Authors’ contributions
LKL and NMF contributed to study design, data analysis/interpretation and in drafting or critically revising the manuscript. RN, IL, MCM, BT were involved in study design, results interpretation, and in drafting or critically revising the manuscript. All authors reviewed the final manuscript and gave approval for submission. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Electronic consent was obtained from all participants in the NHWS. The survey was granted exemption by the Essex Institutional Review Board (Lebanon, NJ). Essex Institutional Review Board, Lebanon, NJ, USA reviewed and approved the US NHWS 2013 survey
Consent for publication
Not applicable.
Competing interests
REN is a consultant and/or speaker for Bayer Pharma, Eli Lilly, Gedeon Richter, HRA Pharma, Merck Sharp & Dohme, Novo Nordisk, Pfizer, Shionogi, Teva, and Zambon SpA; IL is a consultant and/or speaker for HRA Pharma, Merck Sharp & Dohme, Nordic, and Teva; LKL is a full-time employee of Kantar Health, which received funding to conduct this study; NMF was a full-time employee of Kantar Health at the time this study was conducted; MCM is a consultant for Teva Europe Medical Affairs; BT is an employee of Teva Pharmaceuticals.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Prior Presentation
Lee LK, Flores NM, Micheletti MC, Leibert R, Weiss, H, Tang B. Comparing treatment satisfaction, adherence, and menstrual symptoms between women using extended-cycle and monthly-cycle birth control pills. Poster presented at the European Board and College of Obstetrics and Gynaecology Meeting (EBCOG 2016). May 19–21, 2016.
Contributor Information
Rossella E. Nappi, Phone: +39 0382-526215, Email: renappi@tin.it
Iñaki Lete, Email: Luisignacio.Letelasa@osakidetza.eus.
Lulu K. Lee, Email: Lulu.Lee@Kantarhealth.com
Natalia M. Flores, Email: NMFlores82@gmail.com
Marie-Christine Micheletti, Email: Marie.Christine.Micheletti@tevaeu.com.
Boxiong Tang, Email: Boxiong.Tang@tevapharm.com.
References
- 1.Hooper DJ. Attitudes, awareness, compliance and preferences among hormonal contraception users: a global, cross-sectional, self-administered, online survey. Clin Drug Invest. 2010;30:749–763. doi: 10.2165/11538900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Nelson AL. Communicating with patients about extended-cycle and continuous use of oral contraceptives. J Womens Health (Larchmont) 2007;16:463–470. doi: 10.1089/jwh.2006.0206. [DOI] [PubMed] [Google Scholar]
- 3.Szarewski A, von Stenglin A, Rybowski S. Women's attitudes towards monthly bleeding: results of a global population-based survey. Eur J Contracept Reprod Health Care. 2012;17:270–283. doi: 10.3109/13625187.2012.684811. [DOI] [PubMed] [Google Scholar]
- 4.Ferrero S, Abbamonte LH, Giordano M, Alessandri F, Anserini P, Remorgida V, Ragni N. What is the desired menstrual frequency of women without menstruation-related symptoms? Contraception. 2006;73:537–541. doi: 10.1016/j.contraception.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Nappi RE, Fiala C, Chabbert-Buffet N, Hausler G, Jamin C, Lete I, Lukasiewic M, Pintiaux A, Lobo P. Women's preferences for menstrual bleeding frequency: results of the inconvenience due to Women's monthly bleeding (ISY) survey. Eur J Contracept Reprod Health Care. 2016;21:242–50. [DOI] [PubMed]
- 6.Fruzzetti F, Paoletti AM, Lombardo M, Carmignani A, Genazzani AR. Attitudes of Italian women concerning suppression of menstruation with oral contraceptives. Eur J Contracept Reprod Health Care. 2008;13:153–157. doi: 10.1080/13625180701800672. [DOI] [PubMed] [Google Scholar]
- 7.Edelman A, Lew R, Cwiak C, Nichols M, Jensen J. Acceptability of contraceptive-induced amenorrhea in a racially diverse group of US women. Contraception. 2007;75:450–453. doi: 10.1016/j.contraception.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Nappi RE, Kaunitz AM, Bitzer J. Extended regimen combined oral contraception: a review of evolving concepts and acceptance by women and clinicians. Eur J Contracept Reprod Health Care. 2016;21:106–115. doi: 10.3109/13625187.2015.1107894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panicker S, Mann S, Shawe J, Stephenson J. Evolution of extended use of the combined oral contraceptive pill. J Fam Plann Reprod Health Care. 2014;40:133–141. doi: 10.1136/jfprhc-2013-100600. [DOI] [PubMed] [Google Scholar]
- 10.Edelman A, Micks E, Gallo MF, Jensen JT. Grimes DA. Continuous or extended cycle vs cyclic use of combined hormonal contraceptives for contraception Cochrane Database Syst Rev. 2014;7:CD004695. doi: 10.1002/14651858.CD004695.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendoza N, Lobo P, Lertxundi R, Correa M, Gonzalez E, Salamanca A, Sanchez-Borrego R. Extended regimens of combined hormonal contraception to reduce symptoms related to withdrawal bleeding and the hormone-free interval: a systematic review of randomised and observational studies. Eur J Contracept Reprod Health Care. 2014:19:321–39. [DOI] [PubMed]
- 12.Seval DL, Buckley T, Kuehl TJ, Sulak PJ. Attitudes and prescribing patterns of extended-cycle oral contraceptives. Contraception. 2011;84:71–75. doi: 10.1016/j.contraception.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.McGregor JC, Kim PW, Perencevich EN, Bradham DD, Furuno JP, Kaye KS, Fink JC, Langenberg P, Roghmann MC, Harris AD. Utility of the chronic disease score and Charlson Comorbidity index as comorbidity measures for use in epidemiologic studies of antibiotic-resistant organisms. Am J Epidemiol. 2005;161:483–493. doi: 10.1093/aje/kwi068. [DOI] [PubMed] [Google Scholar]
- 15.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Mariush MEEd . User's manual for the SF-36v2® health survey. 3. Quality Metric Incorporated: Lincoln, RI; 2011. [Google Scholar]
- 17.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21:271–292. doi: 10.1016/S0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 18.Huppertz-Hauss G, Aas E, Lie Hoivik M, Langholz E, Odes S, Smastuen M, Stockbrugger R, Hoff G, Moum B, Bernklev T. Comparison of the multiattribute utility instruments EQ-5D and SF-6D in a Europe-wide population-based cohort of patients with inflammatory bowel disease 10 years after diagnosis. Gastroenterol Res Pract. 2016;2016:5023973. doi: 10.1155/2016/5023973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayiner M, Stepanova M, Pham H, Noor B, Walters M, Younossi ZM. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106. doi: 10.1136/bmjgast-2016-000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 22.Pompei LM, Fernandes CE, Steiner ML, Strufaldi R, Melo NR. Attitudes, knowledge and prescribing habits of Brazilian gynecologists regarding extended-cycle oral contraceptives. Gynecol Endocrinol. 2013;29:1071–1074. doi: 10.3109/09513590.2013.831833. [DOI] [PubMed] [Google Scholar]
- 23.Sauer U, Mann S, Brima N, Stephenson J. Offering extended use of the combined contraceptive pill: a survey of specialist family planning services. Int J Womens Health. 2013;5:613–617. doi: 10.2147/IJWH.S51329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiegratz I, Galilaer K, Sanger N, Rody A, Kuhl H, Schleussner E. Prescribing preferences and personal experience of female gynaecologists in Germany and Austria regarding use of extended-cycle oral contraceptives. Eur J Contracept Reprod Health Care. 2010;15:405–412. doi: 10.3109/13625187.2010.518708. [DOI] [PubMed] [Google Scholar]
- 25.Burkman R, Bell C, Serfaty D. The evolution of combined oral contraception: improving the risk-to-benefit ratio. Contraception. 2011;84:19–34. doi: 10.1016/j.contraception.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Guilbert E, Boroditsky R, Black A, Kives S, Leboeuf M, Mirosh M, Senikas V, Wagner MS, Weir E, York-Lowry J, Reid R, Trussell J. Canadian consensus guideline on continuous and extended hormonal contraception, 2007. J Obstet Gynaecol Can. 2007;29:S1–S32. doi: 10.1016/S1701-2163(16)32526-9. [DOI] [PubMed] [Google Scholar]
- 27.Caruso S, Malandrino C, Cicero C, Ciancio F, Cariola M, Cianci A. Quality of sexual life of women on oral contraceptive continued-regimen: pilot study. J Sex Med. 2013;10:460–466. doi: 10.1111/j.1743-6109.2012.03004.x. [DOI] [PubMed] [Google Scholar]
- 28.Fourquet J, Baez L, Figueroa M, Iriarte RI, Flores I. Quantification of the impact of endometriosis symptoms on health-related quality of life and work productivity. Fertil Steril. 2011;96:107–112. doi: 10.1016/j.fertnstert.2011.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nnoaham KE, Hummelshoj L, Webster P, D'Hooghe T, de Cicco NF, de Cicco NC, Jenkinson C, Kennedy SH, Zondervan KT. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366–373. doi: 10.1016/j.fertnstert.2011.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasiak R, Filonenko A, Vanness DJ, Law A, Jeddi M, Wittrup-Jensen KU, Stull DE, Siak S, Jensen JT. Impact of estradiol valerate/dienogest on work productivity and activities of daily living in women with heavy menstrual bleeding. J Women's Health. 2013;22:378–384. doi: 10.1089/jwh.2012.3633. [DOI] [PubMed] [Google Scholar]
- 31.Wasiak R, Filonenko A, Vanness DJ, Wittrup-Jensen KU, Stull DE, Siak S, Fraser I. Impact of estradiol-valerate/dienogest on work productivity and activities of daily living in European and Australian women with heavy menstrual bleeding. Int J Womens Health. 2012;4:271–278. doi: 10.2147/IJWH.S31740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnard K, Frayne SM, Skinner KM, Sullivan LM. Health status among women with menstrual symptoms. J Women's Health. 2003;12:911–919. doi: 10.1089/154099903770948140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Kantar Health but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Kantar Health.


