Abstract
Background
This study aimed to investigate the predictive value of multislice spiral computed tomography (MSCT) perfusion imaging for the efficacy of preoperative concurrent chemoradiotherapy (CCRT) in middle-aged and elderly patients with locally advanced gastric cancer (LAGC).
Material/Methods
One-hundred twenty-six middle-aged and elderly patients with LAGC were selected. MSCT was performed before and after CCRT to obtain perfusion parameters: blood flow volume (BF), blood volume (BV), mean transit time (MTT), and permeability surface (PS). After CCRT, according to Response Evaluation Criteria in Solid Tumors (RECIST), patients were categorized into the effective group and the ineffective group. Overall survival rate was measured by Kaplan-Meier analysis. ROC curve was applied to evaluate the predictive value of perfusion parameters. Multiple logistic regression analysis was applied to analyze the association of perfusion parameters with the efficacy of preoperative treatment.
Results
Tumor volume reduction rates of the effective and ineffective groups were 59.23±8.53% and 10.41±3.36%. BF, BV, and PS values in the effective group were significantly decreased after CCRT. ROC curves indicated high sensitivities and specificities of BF value (79.00%, 73.44%), BV value (71.00%, 75.00%), and PS value (82.30%, 90.63%). The incidence rate of weakness and anorexia in the effective group was much higher than that in the ineffective group. Patients with low BF, BV, and PS values (less their optimal cutoff values) had longer survival times than these with high BF, BV, and PS values.
Conclusions
MSCT might have predictive values for the efficacy of preoperative CCRT in the treatment of LAGC.
MeSH Keywords: Chemoradiotherapy, Multidetector Computed Tomography, Perfusion Imaging, Self Efficacy, Stomach Neoplasms
Background
Gastric cancer (GC) is one of the most common malignant tumors in the world, and in 2008, its mortality rate ranked second among all malignant tumors [1,2]. Due to a lack of routine screening, the diagnosis rate of early GC is low, and most patients are diagnosed in advanced stages [3]. Surgical resection is the preferred treatment for locally advanced GC (LAGC). Although it can be widely used to achieve lymph node clearance, the recurrence rate is as high as 60% [4]. Previous studies have shown that in 50% to 60% of LAGC patients, cancer relapse occurred within five years after surgery [4,5]. In order to improve the long-term survival of GC, adjuvant radiotherapy and chemotherapy are being used as new treatment for LAGC [6]. Concurrent chemoradiotherapy (CCRT) has been used as a neoadjuvant treatment option for GC because of its applicable dose radiation delivery, selective sparing of critical structures (the kidneys or liver), and more complete coverage of the target to full dose. The reduction of acute toxicity and late sequelae caused by image-guided preoperative CCRT may be more feasible for treating LAGC [7,8]. Recently, preoperative CCRT achieved excellent target coverage and pathologic response to localized gastric adenocarcinoma [9]. It may be necessary to explore the efficacy of preoperative CCRT, so as to provide appropriate treatment planning for LAGC.
In recent years, with the development of cloud computing and an increase in Z-axis detectors and data acquisition channels, multislice computed tomography (MSCT) has greatly enhanced its image resolution, scanning speed, and the thickness of acquisition layer. These advantages of MSCT contribute to a better imaging determination of LNM in GC and high performance of preoperative assessment in clinical applications [10]. Computed tomography (CT) perfusion imaging is a new imaging technology that can display the concentration change of contrast agents in tissues and organs, so as to reflect their perfusion characteristics [11]. Perfusion parameters before and after treatment provide a better understanding of blood supply and vascular permeability in GC, which is conducive to predict the therapeutic efficacy of the treatment [11]. A previous study indicated that the correlation between breast cancer MSCT perfusion parameters and VEGF and MMP-2 expression might be useful for detection of breast lesions, qualitative diagnosis of breast cancer, and evaluation of breast cancer treatment [12]. However, there have been few studies in the predictive value of MSCT perfusion imaging for the efficacy of CCRT in patients with GC. In this study, a variety of rigorous data processing methods have been used to assess the predictive value of MSCT perfusion imaging for the efficacy of preoperative CCRT in middle-aged and elderly patients with LAGC.
Material and Methods
Ethnic statement
This study was approved by the ethics committee of Dongying People’s Hospital and all patients receiving chemotherapy before surgery signed written informed consents.
Study patients
One-hundred twenty-six patients pathologically diagnosed with LAGC were selected from the Department of Radiology at Dongying People’s Hospital between January 2010 and September 2012. These patients were 55 to 80 years old with a mean age of 64.27±7.77 years [13], including 95 male patients and 31 female patients. On the basis of tumor location, 60 cases were diagnosed with tumors located in the gastric cardia, 27 cases with tumors located in the gastric corpus, 28 cases with tumors located in the gastric antrum, and 11 cases with tumors located in the entire stomach. The pathological grade of 56 cases was identified as grade II and that of 70 cases was identified as grade III. In accordance with Tumor Node Metastasis (TNM) classification for malignant tumors published in 1997 [14], there were two cases with stage II LAGC, 56 cases with stage III LAGC, and 68 cases with stage IV LAGC. As for histological classification, there were 72 cases with papillary-tubular adenocarcinoma, 50 cases with low-grade adenocarcinoma, signet-ring cell carcinoma (SRCC), two cases with squamous cell carcinoma, and two cases of carcinoids. Inclusion criteria were as follows: patients with Karnofsky performance scale (KPS) scores >80 points; patients without distant metastasis; and patients without any contraindication to chemotherapy or radiotherapy. Exclusion criteria was as follows: patients with allergy or contraindication to iodine; patients with severe heart, liver, or kidney dysfunctions; patients with thyrotoxicosis; unclear images of the lesions due to a poor image quality or gastric filling defect after computed tomography (CT) scan; and patients with a history of hormonotherapy or other therapy.
MSCT
All patients were required to undergo at least eight hours of food and water fasting before the MSCT scan, and they were injected with 25 mg of anisodamine (National Medicine Permit No. H33021706, Hangzhou Minsheng Pharmaceutical Group Co., Ltd., Zhejiang, China.) 15 minutes before the scan. Before treatment, MSCT was performed using a GE Lightspeed QX/iScanner (Philips Brilliance, USA). During the MSCT scan, patients received routine abdominal CT scan in the supine position (pitch, 1.35; scanning speed, 0.8 second per rotation; interval spacing, 5 mm; slice thickness, 5 mm). The slice with the largest tumor size was selected as a target slice, followed by scanning for continuous four slices above and four below the target slice with 45 times per slice. Then, all patients were intravenously injected with 45 mL of contrast agent Ultravist (National Medicine Permit No. H33021706, Guangzhou Schering Pharmaceutical Ltd., Guangzhou, China) at a speed of 4 mL/second. The scan restarted at the eighth second of injection, and continued for 150 seconds at a speed of 1.5 seconds per scan. In addition, during the scan, patients who could not hold their breath for a long time were requested to breathe slowly. And the other patients were requested to hold their breath for about 50 seconds with a band tied on their abdomen. After scanning, all images were uploaded to an Advantage Workstation (AW) 4.4 (GE Health Care, USA), and were processed by the GE Medical systems CT Pe-sion software package. Perfusion maps and time-density curves of the abdominal aorta and gastric lesions were analyzed to obtain the perfusion parameters, including the blood flow volume (BF), blood volume (BV), mean transit time (MTT), and permeability surface (PS).
CCRT
Before surgery, CCRT was performed using a Varian CadPlan treatment planning system with a varian 23EX linear accelerator (Varian Inc., Walnut Creek, CA, USA). All patients were treated with a 6-MV x-ray CCRT with a total dose of 45 Gy (1.8 Gy/day) before the surgery. Patients were requested to fast for four hours before positioning, and were treated with oral contrast medium (200~400 mL) 10 minutes before positioning. Fixed on dorsal decubitus position, patients held in their hands and put them on their foreheads. Laser light was positioned and molding of thermoplastic materials was used to fix patients’ body. Siemens Plus4 CT (Siemens, Germany) was performed for enhancement scanning from 5 cm above diaphragm to the level of umbilicus (if it was cardiac cancer, the upper bound of scanning would be on the level of sternal angle). The interlayer spacing was 5 mm. The result of CT scan was transferred to TPS treatment planning system. Clinical target volume (CTV) was sketched layer-by-layer on CT images, including tumor bed, stoma, and lymph-node region. If the tumors occurred in cardia or gastric fundus, the lower esophagus (5 cm) and splenoportal lymph-node region was included; if the tumors occurred in the gastric antrum, the cardia and splenoportal lymph-node region was not be included. Planning target volume (PTV) was sketched at external 0.5~0.7 cm on the basis of CTV, and at external 1 cm in cephalo-caudal direction. Inverse planning design was adopted to optimize treatment plan using multi-leaf collimator. Five to seven radiation fields were set. Prescription dose of all plans was 45 Gy, and all plans were optimized to assure that PTV (≥95%) was received 42.75 Gy. The times of segmentation was 25, 1.8 Gy/time, one time/day, five times/week (the total period, five weeks). The renal volume that receiving 15~18 Gy was less than 50% (V15–18 ≤50%), and the normal liver volume that receiving 30 Gy was less than 30% (V30 ≤30%), and the dose of spinal cord was less than 40 Gy. In IMRT plan, the highest dose of any region within PTV was less than 49.50 Gy (110% prescription dose). The applied dose-dose-volume histogram (DVH) was performed to evaluate the target area of conformal radiation therapy and normal organs and tissues. Patients were requested fasting and were treated with the equal dose of plain boiled water to positioning. During radiotherapy, patients were given antacids to protect gastric mucosa. The procedure was immediately halted if the white blood cells <3.0×109/L. All patients were treated with subcutaneous injection of granulocyte colony-stimulating factor (G-CSF). Patients were also received concurrent chemotherapy since the first day of radiotherapy. Carboplatin (AUC 2) was administered intravenously once a week. On the first, eighth, fifteenth, twenty-second, and twenty-ninth day of radiotherapy, patients were intravenously injected with paclitaxel (50 mg/m2).
Evaluation of efficacy and adverse reaction
After one month of CCRT, tumor response was assessed using MSCT, and perfusion parameters (BF, BV, MTT, and PS) were obtained. According to World Health Organization (WHO) Response Evaluation Criteria in Solid Tumors (RECIST), the clinical response was evaluated as following standards: complete remission (CR): all visible lesions have been completely vanished for at least four weeks; partial remission (PR): the tumor volume (maximum horizontal diameter × maximum vertical diameter) has been reduced by more than 50% for at least four weeks; stable disease (SD): the tumor volume has been reduced or enlarged by less than 25%, and no new lesion was generated; progressive disease (PD): the tumor volume has been reduced or enlarged by more than 25%, or new lesions were generated. Based on the tumor response, patients were categorized into the effective group (CR + PR) and the ineffective group (SD + PD).
Maximum horizontal diameter and maximum vertical diameter of the tumor were measured before and one month after CCRT. The tumor volume was obtained by multiplying maximum horizontal diameter by maximum vertical diameter. Tumor volume reduction rate [15] (%)=(preoperative tumor volume–postoperative tumor volume)/preoperative tumor volume ×100%.
The criteria for evaluation of toxicity and side effects were in accordance with classification standard on toxicity and side effects of chemotherapy drugs provided by the WHO. The routine blood test was detected twice a week, and liver and renal function and electrocardiogram was evaluated before and after chemotherapy.
Surgical method
Four to six weeks after CCRT, gastrointestinal radiography, gastroscopy, upper abdominal enhanced CT, and transabdominal color Doppler ultrasound were performed to evaluate the respectability of the tumor. All patients underwent radical total gastrectomy. Complete resection of the lesion (R0 resection) was confirmed if no residual tumor was observed at resection margin by naked eyes or a negative microscopic resection margin was defined. Next, D2 dissection was performed for removing perigastric lymph nodes; dissection was undertaken during the surgery. Except when judged by the naked eye, the frozen sections during surgery were used to determine whether LNM had or had not occurred. When LNM had occurred, it served as a parameter of D3 dissection. Postoperative adjuvant chemotherapy was determined by chemotherapists.
Follow-up
The follow-up was conducted for all patients through outpatient clinic visits and telephone follow-up. It started from the day when the patient was discharged from hospital, and ended at the date of death, loss to follow-up, or the last visit. Three-year relapse-free survival rates were calculated. The last follow-up ended in January 2016. Clinicopathological features were obtained, and the diagnosis method of relapse, the time of verification, and the location of relapse were recorded during the follow-up. The overall survival rate was analyzed by Kaplan-Meier life table method.
Statistical analysis
SPSS 19.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. Measurement data were presented as mean ± standard deviations (SD). Comparisons of measurement data between two independent groups were analyzed using the paired t-test; Comparisons among multiple groups were detected by an independent-samples t-test. Enumeration data were expressed as a percentage or ratio and comparisons of enumeration data were assessed with chi-square test. Overall survival rate was measured by Kaplan-Meier analysis. The receiver operating characteristic (ROC) curve was applied to evaluate the predictive value of MSCT perfusion parameters. Multiple logistic regression analysis was applied to analyze the association of MSCT perfusion parameters with the efficacy of preoperative CCRT. A p value less than 0.05 was considered to be statistically significant.
Results
Perfusion images of tumor lesions in middle-aged and elderly patients with LAGC
Among 126 LAGC patients, perfusion images showed that mass-like enhancement and rough serous were observed in 81 patients, linear enhancement and fuzzy fat intervals nearby lesions were observed in 63 patients, and heterogeneous enhancement and nodular hyperplasia were observed in 12 patients (Figure 1).
Figure 1.
MSCT perfusion images of patients with different degrees of enhancement of locally advanced gastric cancer after preoperative CCRT. (A) The perfusion image for a 63-year-old male patient with mass-like enhancement and rough serous. (B) The perfusion image for a 58-year-old female patient with linear enhancement and fuzzy fat intervals nearby lesions. (C) The perfusion image for a 71-year-old male patient with heterogeneous enhancement and nodular hyperplasia. MSCT – multislice computed tomography; CCRT – concurrent chemoradiotherapy.
Comparisons of clinicopathological features of LAGC patients between the effective and ineffective groups
On the basis of tumor response, there were 25 patients with CR, 39 patients with PR, and 62 patients with SD. Thus, there were 64 patients in the effective group and 62 patients in the ineffective group. The tumor volume reduction rate of the effective group was 59.23±8.53%, and that of the ineffective group was 10.41±3.36%. No significant difference was observed between the effective group and the ineffective group in terms of gender, age, preoperative tumor size, tumor location, LNM, tumor differentiation, and histological type (all p>0.05) (Table 1).
Table 1.
Comparisons of clinicopathological features of LAGC patients between the effective group and the ineffective group.
| Clinicopathological feature | Effective group | Ineffective group | χ2 | P |
|---|---|---|---|---|
| CR + PR (n=64) | PD + SD (n=62) | |||
| Gender | 0.522 | 0.470 | ||
| Male | 50 | 45 | ||
| Female | 14 | 17 | ||
| Age (years) | 3.811 | 0.051 | ||
| <60 | 24 | 34 | ||
| ≥60 | 40 | 28 | ||
| Preoperative tumor size (cm) | 1.526 | 0.217 | ||
| <6 | 41 | 33 | ||
| ≥6 | 23 | 29 | ||
| Tumor location | 3.872 | 0.276 | ||
| In the gastric cardia | 33 | 27 | ||
| In the gastric corpus | 16 | 11 | ||
| In the gastric antrum | 10 | 18 | ||
| In the entire stomach | 5 | 6 | ||
| Lymph node metastasis | 0.658 | 0.417 | ||
| No | 24 | 19 | ||
| Yes | 40 | 43 | ||
| Tumor differentiation | 2.710 | 0.100 | ||
| Moderate/high differentiation | 32 | 22 | ||
| Low differentiation | 32 | 40 | ||
| Histological type | 4.409 | 0.256 | ||
| Low-grade adenocarcinoma/SRCC | 24 | 26 | ||
| Papillary-tubular adenocarcinoma | 36 | 36 | ||
| Squamous cell carcinoma | 2 | 0 | ||
| Carcinoid | 2 | 0 |
LAGC – locally advanced gastric cancer; CR – complete remission; PR – partial remission; SD – stable disease; PD – progressive disease; SRCC – signet-ring cell carcinoma.
Associations of perfusion parameters derived from MSCT before CCRT with clinicopathological features of LAGC patients between the effective and ineffective groups
The results indicated that perfusion parameters (BF, BV, MTT, and PS) before CCRT were not related to gender, tumor size, and tumor location of patients with LAGC (all p>0.05). And BF, BV, and MTT values also were not associated with LNM, tumor differentiation, and histological type of LAGC patients (all p>0.05). Higher PS values were observed in LAGC patients with LNM, in patients with moderate/high differentiation of LAGC, and in patients with low-grade adenocarcinoma/SRCC than those without LNM, those with low differentiation of LAGC, and those with papillary-tubular adenocarcinoma (all p<0.05), as shown in Table 2.
Table 2.
Associations of preoperative perfusion parameters derived from MSCT with clinicopathological features of lagc patients.
| Clinicopathological feature | BF (ml/100 g) | BV (ml/100 g) | MTT (sec) | PS (ml/100 g) |
|---|---|---|---|---|
| Gender | ||||
| Male | 78.03±15.96 | 10.68±2.52 | 8.76±1.21 | 26.72±12.32 |
| Female | 75.82±13.57 | 9.88±2.87 | 8.89±1.54 | 24.04±11.13 |
| Preoperative tumor size (cm) | ||||
| <6 cm | 76.58±15.07 | 10.52±2.72 | 8.97±1.20 | 26.82±12.81 |
| ≥6 cm | 78.77±14.16 | 10.43±2.72 | 8.53±1.39 | 24.97±10.90 |
| Tumor location | ||||
| In the gastric cardia | 77.32±14.61 | 10.69±2.92 | 8.87±1.27 | 26.01±13.01 |
| In the gastric corpus | 78.34±16.72 | 10.52±2.92 | 9.12±1.38 | 28.54±11.86 |
| In the gastric antrum | 77.65±13.74 | 10.24±2.21 | 8.28±1.15 | 24.29±10.99 |
| In the entire stomach | 75.90±13.90 | 9.88±2.39 | 8.88±1.39 | 24.75±9.85 |
| Lymph node metastasis | ||||
| No | 78.92±15.58 | 10.92±2.66 | 8.82±1.30 | 22.07±12.35 |
| Yes | 76.74±14.23 | 10.25±2.66 | 8.77±1.30 | 28.13±11.48* |
| Tumor differentiation | ||||
| Moderate/high differentiation | 77.11±14.29 | 10.82±2.33 | 8.87±1.39 | 21.19±10.88 |
| Low differentiation | 77.77±15.06 | 10.23±2.33 | 8.73±1.23 | 29.71±11.65* |
| Histological type | ||||
| Low-grade adenocarcinoma/SRCC | 76.30±13.12 | 10.42±2.59 | 8.84±1.17 | 29.42±13.84 |
| Papillary-tubular adenocarcinoma | 78.52±16.04 | 10.43±2.65 | 8.75±1.38 | 23.82±10.47* |
| Squamous cell carcinoma | 72.55±2.52 | 14.05±0.69 | 9.12±2.06 | 25.37±2.49 |
| Carcinoid | 74.70±5.11 | 10.50±1.67 | 8.54±1.33 | 23.54±4.52 |
MSCT – multislice spiral computed tomography; SD – standard deviation; LAGC – locally advanced gastric cancer; BF – blood flow volume; BV – blood volume; MTT – mean transit time; PS – permeability surface; SRCC – signet-ring cell carcinoma;
P<0.05, compared with LAGC patients without lymph node metastasis, with high/moderate/low-grade adenocarcinoma/SRCC.
Perfusion parameters derived from MSCT of LAGC patients in the effective and ineffective groups before and after CCRT
As shown in Table 3, compared with perfusion parameters before CCRT, the BF, BV, and PS, values of LAGC patients in the effective group were significantly decreased after one month of CCRT (all p<0.05). However, no significant change was observed in the PS value of LAGC patients in the effective group before and after CCRT (p>0.05). For LAGC patients in the ineffective group, there was no evident difference in perfusion parameters (BF, BV, MTT, and PS) before and after CCRT (all p>0.05). After one month of CCRT, the BF, BV, and PS values of LAGC patients in the effective group were significantly lower than these in the ineffective group (all p>0.05).
Table 3.
Comparisons of perfusion parameters derived from MSCT of LAGC patients in the effective and ineffective groups before and after CCRT.
| Perfusion parameter | Effective group (n=64) | P | Ineffective group (n=62) | P | ||
|---|---|---|---|---|---|---|
| Before IMRT | After IMRT | Before IMRT | After IMRT | |||
| BF (ml/100 g·min) | 76.50±14.47 | 54.23±11.89* | <0.001 | 78.50±14.95 | 73.36±14.95 | 0.059 |
| BV (ml/100 g) | 10.91±2.65 | 7.55±1.84* | <0.001 | 10.04±2.53 | 10.01±2.33 | 0.775 |
| MTT (sec) | 8.73±1.26 | 8.69±1.11 | 0.844 | 8.85±1.34 | 9.13±1.35 | 0.264 |
| PS (ml/100 g·min) | 25.22±13.13 | 14.22±4.10* | <0.001 | 26.92±10.85 | 25.98±7.67 | 0.584 |
MSCT – multislice spiral computed tomography; LAGC – locally advanced gastric cancer; CCRT – concurrent chemoradiotherapy; SD – standard deviation; BF – blood flow volume; BV – blood volume; MTT – mean transit time; PS – permeability surface;
P<0.05, compared with LAGC patients in the ineffective group after CCRT.
Predictive value of perfusion parameters derived from MSCT before CCRT for the efficacy of CCRT in LAGC patients
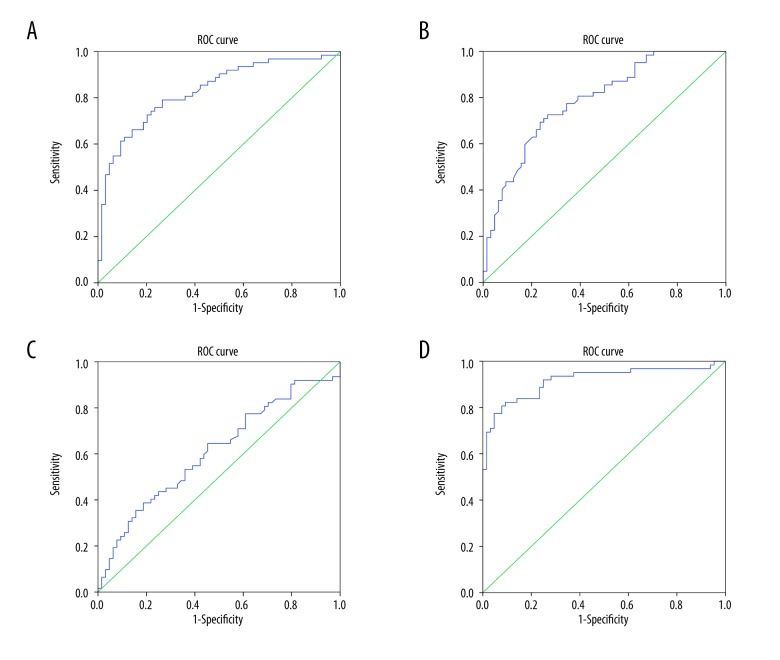
With an optimal cutoff point of 60.985 mL/100 g·min, the sensitivity and specificity of BF value for predicting the efficacy of CCRT in LAGC patients were 79.00% and 73.44%, respectively. As shown in Figure 2A, the area under the ROC curve (AUC) of BF value was 0.828. With an optimal cutoff point of 8.650 mL/100 g, the sensitivity and specificity of BV value for predicting the efficacy of CCRT in LAGC patients were 71.00% and 75.00%, respectively. As shown in Figure 2B, the AUC of BV value was 0.817. With an optimal cutoff point of 9.680 seconds, the sensitivity and specificity of MTT value for predicting the efficacy of CCRT in LAGC patients were 38.70% and 81.25%, respectively. As shown in Figure 2C, the AUC of MTT value was 0.609. With an optimal cutoff point of 18.960 mL/100 g min, the sensitivity and specificity of PS value for predicting the efficacy of CCRT in LAGC patients were 82.30% and 90.63%, respectively. As shown in Figure 2D, the AUC of PS value was 0.917. Therefore, BF, BV, and PS values might be considered as predictive factors for the efficacy of CCRT in LAGC patients.
Figure 2.
ROC curves of BF, BV, MTT, and PS values predicting the efficacy of preoperative CCRT in LAGC patients. (A) ROC curve of BF value predicting the efficacy of preoperative CCRT in LAGC patients. (B) ROC curve of BV value predicting the efficacy of preoperative CCRT in LAGC patients. (C) ROC curve of MTT value predicting the efficacy of preoperative CCRT in LAGC patients. (D) ROC curve of PS value predicting the efficacy of preoperative CCRT in LAGC patients. ROC – receiver operating characteristic; BF – blood flow volume; BV – blood volume; MTT – mean transit time; PS – permeability surface; CCRT – concurrent chemoradiotherapy; LAGC – locally advanced gastric cancer.
Toxicity and side effects of LAGC patients before CCRT
Toxicity and side effects were presented as gastrointestinal reaction: the incidence rate of anorexia in the effective group was 65.63%, and in the ineffective group was 32.26%; the incidence rate of weakness in the effective group was 75.00%, and in the ineffective group was 38.71%; the incidence rate of nausea and vomiting in the effective group was 23.44%, and in the ineffective group was 9.69%; the incidence rate of leukocytosis in the effective group was 46.88%, and in the ineffective group was 30.65%, which indicated that the incidence rate of weakness and anorexia in the effective group was much higher than that in the ineffective group. The patients without liver and renal dysfunction had no treatment-related mortality (Table 4).
Table 4.
Adverse reaction of patients before surgery.
| Adverse reaction | I~II grade | III~IV grade | Incidence rate (%) | |||
|---|---|---|---|---|---|---|
| Effective group | Ineffective group | Effective group | Ineffective group | Effective group | Ineffective group | |
| Aleukocytosis | 21 | 12 | 9 | 7 | 46.88 | 30.65 |
| Weakness | 48 | 15 | 0 | 9 | 75.00 | 38.71 |
| Anorexia | 31 | 14 | 11 | 6 | 65.63 | 32.26 |
| Nausea and vomitting | 12 | 6 | 3 | 0 | 23.44 | 9.68 |
Multiple logistic regression analysis
Multiple logistic regression analysis (Table 5) was performed for the efficacy of preoperative CCRT as the dependent variable, and LNM, tumor differentiation, histological type, BF, BV, and PS as independent variables. The results presented that BF value (OR=0.171, 95% CI=0.052–0.556), BV value (OR=0.151, 95% CI=0.045–0.506) and PS value (OR=0.033, 95% CI=0.010–0.113) were the influence factors for the efficacy of preoperative CCRT in LAGC patients (all p<0.05).
Table 5.
Multiple logistic regression analysis.
| Factor | Wald | P | OR | 95% CI |
|---|---|---|---|---|
| Histological type | 0.286 | 0.593 | 1.425 | 0.389–5.219 |
| Lymph node metastasis | 0.515 | 0.473 | 0.633 | 0.182–2.204 |
| Tumor differentiation | 0.313 | 0.576 | 1.434 | 0.409–5.077 |
| BF (ml/100 g·min) | 8.375 | 0.004 | 0.174 | 0.053–0.569 |
| BV (ml/100 g) | 9.583 | 0.002 | 0.143 | 0.042–0.490 |
| PS (ml/100 g·min) | 28.259 | <0.001 | 0.035 | 0.010–0.121 |
BF – blood flow volume; BV – blood volume; MTT – mean transit time; PS – permeability surface; OR – odds ratio; CI – confidence interval.
Associations of perfusion parameters derived from MSCT before CCRT with the survival of LAGC patients
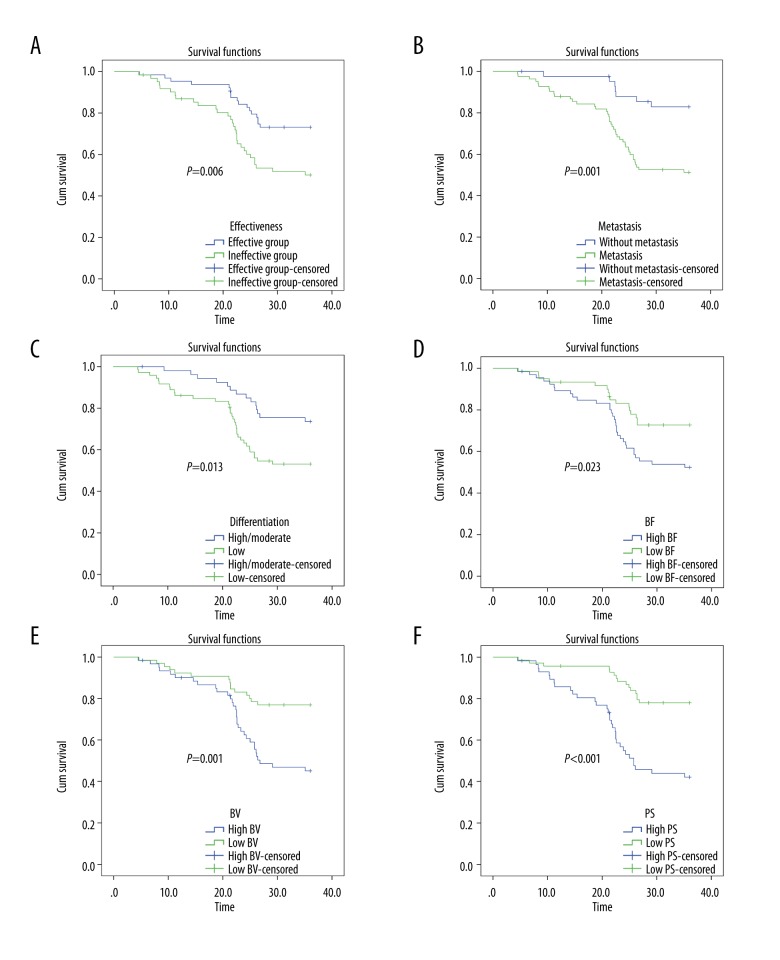
After three-year follow-up for all patients, there were 52 deaths, and five patients lost to follow-up. The overall survival rate was 62.70% with a median survival time of 29.2 months. According to Kaplan-Meier survival curves (Figure 3), the overall survival rate of patients in the effective group was 71.88%, and that of patients in the ineffective group was 53.23%. The effective group exhibited longer survival times of LAGC patients than the ineffective group (p<0.05). With LNM, tumor differentiation, histological type, and perfusion parameters before CCRT (BF, BV, and PS) as variables, univariate analysis was performed to analyze the association of these variables with the survival time of LAGC patients after CCRT. The results indicated that LNM, tumor differentiation, BF, BV, and PS were associated with the survival time of LAGC patients (all p<0.05). Survival times of LAGC patients without LNM were longer than that of LAGC patients with LNM (p<0.05). LAGC patients with moderate/high differentiation had longer survival times than patients with low differentiation (p<0.05). Besides, LAGC patients with low BF value (<60.985 mL/100 g·min), low BV (<8.650 mL/100 g) and low PS value (<18.960 mL/100 g·min) had longer survival times than patients with high BF value (>60.985 mL/100 g·min), high BV value (>8.650 mL/100 g) and high PS value (>18.960 mL/100 g·min) (all p<0.05).
Figure 3.
Kaplan-Meier survival curves of overall survival times of LAGC patients. (A) Comparison of the overall survival time of LAGC patients between the effective and ineffective groups. (B) Comparison of the overall survival time between the LAGC patients with LNM and without LNM. (C) Comparison of the overall survival time between the patients with low differentiation of LAGC and with high/moderate differentiation of LAGC. (D) Comparison of the overall survival time between the patients with low BF value and with high BF value (cutoff point=60.985 mL/100 g·min). (E) Comparison of the overall survival time between the patients with low BV value and with high BV value (cutoff point=8.650 mL/100 g). (F) Comparison of the overall survival time between the patients with low PS value and with high PS value (cutoff point=18.960 mL/100 g·min). LAGC – locally advanced gastric cancer; LNM – lymph node metastasis; BF – blood flow volume; BV – blood volume; PS – permeability surface.
Discussion
It has been reported that the application of postoperative radiotherapy in the treatment of late stage GCs significantly reduced cancer recurrence rate and improved survival [16]. In this paper, MSCT perfusion imaging was performed to predict the efficacy of preoperative CCRT in the treatment of LAGC. And ROC curves, Kaplan-Meier survival curves, and multiple logistic regression analysis were applied to evaluate the predictive value of MSCT perfusion imaging. It is hoped that this study would provide a clinical basis to evaluate the efficacy of preoperative chemo-radiotherapy in the treatment of LAGC.
In this study, perfusion parameters derived from MSCT before treatment was closely associated with LNM, tumor differentiation, and histological type in LAGC patients. MSCT perfusion imaging has been widely used in the diagnosis, staging, prognosis, and efficacy monitoring of tumor response [17]. It has been shown that MSCT perfusion imaging is applied in the assessment of tumor markers using perfusion parameters, such as BF, BV), and PS. This may be helpful to quantify physiological changes during angiogenesis, thus showing a great prospect in the quantitative and qualitative tumor research [18]. MSCT perfusion imaging may be also helpful at colonic neoplasms. A previous study has indicated that diagnosis of bowel wall thickening (BWT) by abdominal CT imaging reveals pathologies in many cases, and colonoscopies can be helpful in the differential diagnosis [19]. Moreover, inguinal hernia presently can be repaired laparoscopically [20]. Histological type is a main basis for the decision for surgical resection, and it is also an important factor affecting the prognosis of GC [21]. Histological types of GC mainly include the intestinal type of well-differentiated tubular and papillary adenocarcinoma, and the diffused type of low-differentiated adenocarcinoma and signet ring cell carcinoma [22]. It has been demonstrated that GC of different histological types responded differently to therapies and hence resulting in different outcomes [23]. The results of this study demonstrated that perfusion parameters were important indicators for LNM, tumor differentiation, and histological type in LAGC patients. In addition, intuitive images and quantitative data of perfusion parameters from MSCT could accurately evaluate the efficacy of preoperative CCRT during the treatment of LAGC. MSCT perfusion parameters were reported to be of important clinical value in the preoperative assessment of GC [24]. Perfusion parameters changed when a normal tissue turned into a cancerous tissue, and the PS and BF values were associated with the proliferation of tumor factors [25]. In addition, PS and BF values in GC tissues were significantly higher than those in normal gastric tissues [26], and the BF, BV, and PS values were different in different degrees of tumor differentiation, suggesting these perfusion parameters could serve as indicators of cancer malignancy and severity [27].
In our study, compared with perfusion parameters before CCRT, BF, BV, and PS values of LAGC patients in the effective group were significantly decreased after one month of CCRT. After MRT, BF, BV, and PS values of LAGC patients in the effective group were significantly lower than in the ineffective group. After radiotherapy, patients with high BF and low MTT values have been shown to achieve a higher survival rate, and the decrease in the BF value was correlated to the change in tumor size [28], indicating that the perfusion parameters could provide a basis for the evaluation of radiotherapy efficacy. Bellomi et al. reported that BF, BV, and PS of CT perfusion were evidently decreased after chemo-radiotherapy in patients with rectal cancer [29]. Therefore, perfusion parameters derived from MSCT might be feasible for the outcomes of CCRT. In this study, LNM, tumor differentiation, BF, BV, and PS were associated with the survival time of LAGC patients after treatment. Patients with low BF, BV, and PS values (less than their optimal cutoff values) had longer survival times. CT perfusion may predict the response to the treatment of transarterial chemo-lipiodol infusion, and BV, BF, and PS values also predict the survival of patients with unresectable colorectal cancer liver metastases [30]. The ROC curves in our study indicated that these three values had high sensitivities and specificities. Therefore, BF, BV, and PS values might be considered as predictive factors for the efficacy of CCRT in LAGC patients. Confirmed by multiple logistic regression analysis, these three parameters were influence factors for efficacy of CCRT.
Conclusions
Our study results suggest that MSCT perfusion imaging could provide an accurate assessment for the efficacy of preoperative CCRT in the treatment of LAGC. Perfusion parameters (BF, BV, and PS) might be predictors for the efficacy of preoperative CCRT with chemotherapy. However, the specific mechanism of MSCT perfusion parameters on evaluating the efficacy of preoperative CCRT with chemotherapy is not yet been entirely understood. It is necessary to further explore how these perfusion parameters function in treatment, which may be of great value for treatment and recovery of LAGC. A limitation of this study was the age range of patients. Thus, this should be further verified in a larger sample size of patients without age limitation.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Chen K, Pan Y, Cai JQ, et al. Totally laparoscopic versus laparoscopic-assisted total gastrectomy for upper and middle gastric cancer: A single-unit experience of 253 cases with meta-analysis. World J Surg Oncol. 2016;14:96. doi: 10.1186/s12957-016-0860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beeharry MK, Liu WT, Yan M, Zhu ZG. New blood markers detection technology: A leap in the diagnosis of gastric cancer. World J Gastroenterol. 2016;22:1202–12. doi: 10.3748/wjg.v22.i3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajani JA. Evolving chemotherapy for advanced gastric cancer. Oncologist. 2005;10(Suppl 3):49–58. doi: 10.1634/theoncologist.10-90003-49. [DOI] [PubMed] [Google Scholar]
- 5.Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 6.Biondi A, Lirosi MC, D’Ugo D, et al. Neo-adjuvant chemo(radio)therapy in gastric cancer: Current status and future perspectives. World J Gastrointest Oncol. 2015;7:389–400. doi: 10.4251/wjgo.v7.i12.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milano MT, Garofalo MC, Chmura SJ, et al. Intensity-modulated radiation therapy in the treatment of gastric cancer: Early clinical outcome and dosimetric comparison with conventional techniques. Br J Radiol. 2006;79:497–503. doi: 10.1259/bjr/43441736. [DOI] [PubMed] [Google Scholar]
- 8.Hu JB, Sun XN, Gu BX, et al. Effect of intensity modulated radiotherapy combined with s-1-based chemotherapy in locally advanced gastric cancer patients. Oncol Res Treat. 2014;37:11–16. doi: 10.1159/000358164. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarty T, Crane CH, Ajani JA, et al. Intensity-modulated radiation therapy with concurrent chemotherapy as preoperative treatment for localized gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 2012;83:581–86. doi: 10.1016/j.ijrobp.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 10.Dai CL, Yang ZG, Xue LP, Li YM. Application value of multi-slice spiral computed tomography for imaging determination of metastatic lymph nodes of gastric cancer. World J Gastroenterol. 2013;19:5732–37. doi: 10.3748/wjg.v19.i34.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HY, Kim N, Goo JM, et al. Perfusion parameters as potential imaging biomarkers for the early prediction of radiotherapy response in a rat tumor model. Diagn Interv Radiol. 2016;22:231–40. doi: 10.5152/dir.2015.15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu N, Lei Z, Li XL, et al. Clinical study of tumor angiogenesis and perfusion imaging using multi-slice spiral computed tomography for breast cancer. Asian Pac J Cancer Prev. 2013;14:429–33. doi: 10.7314/apjcp.2013.14.1.429. [DOI] [PubMed] [Google Scholar]
- 13.Kunisaki C, Akiyama H, Nomura M, et al. Comparison of surgical outcomes of gastric cancer in elderly and middle-aged patients. Am J Surg. 2006;191:216–24. doi: 10.1016/j.amjsurg.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–4. doi: 10.1002/(sici)1097-0142(19971101)80:9<1803::aid-cncr16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Neri E, Guidi E, Pancrazi F, et al. MRI tumor volume reduction rate vs. tumor regression grade in the pre-operative re-staging of locally advanced rectal cancer after chemo-radiotherapy. Eur J Radiol. 2015;84:2438–43. doi: 10.1016/j.ejrad.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Marrelli D, Polom K, de Manzoni G, et al. Multimodal treatment of gastric cancer in the west: Where are we going? World J Gastroenterol. 2015;21:7954–69. doi: 10.3748/wjg.v21.i26.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma SH, Le HB, Jia BH, et al. Peripheral pulmonary nodules: Relationship between multi-slice spiral CT perfusion imaging and tumor angiogenesis and VEGF expression. BMC Cancer. 2008;8:186. doi: 10.1186/1471-2407-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng G, Lei Z, Wang D, et al. The evaluation of anti-angiogenic effects of Endostar on rabbit VX2 portal vein tumor thrombus using perfusion MSCT. Cancer Imaging. 2014;14:17. doi: 10.1186/1470-7330-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isik A, Soyturk M, Suleyman S, et al. Correlation of bowel wall thickening seen using computerized tomography with colonoscopies: A preliminary study. Surg Laparosc Endosc Percutan Tech. 2017;27:154–57. doi: 10.1097/SLE.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 20.Isik A, Gursul C, Peker K, et al. Metalloproteinases and their inhibitors in patients with inguinal hernia. World J Surg. 2017;41:1259–66. doi: 10.1007/s00268-016-3858-6. [DOI] [PubMed] [Google Scholar]
- 21.Chen YC, Fang WL, Wang RF, et al. Clinicopathological variation of lauren classification in gastric cancer. Pathol Oncol Res. 2016;22:197–202. doi: 10.1007/s12253-015-9996-6. [DOI] [PubMed] [Google Scholar]
- 22.Piazuelo MB, Correa P. Gastric cancer: Overview. Colomb Med (Cali) 2013;44:192–201. [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Z, Jin X, He Q, et al. The efficacy of taxanes- and oxaliplatin-based chemotherapy in the treatment of gastric cancer after D2 Gastrectomy for different lauren types. Medicine (Baltimore) 2016;95:e2785. doi: 10.1097/MD.0000000000002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundsgaard Hansen M, Fallentin E, Lauridsen C, et al. Computed tomography (CT) perfusion as an early predictive marker for treatment response to neoadjuvant chemotherapy in gastroesophageal junction cancer and gastric cancer – a prospective study. PLoS One. 2014;9:e97605. doi: 10.1371/journal.pone.0097605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin HY, Sun H, Wang X, et al. Correlation between CT perfusion parameters and microvessel density and vascular endothelial growth factor in adrenal tumors. PLoS One. 2013;8:e79911. doi: 10.1371/journal.pone.0079911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao J, Yang ZG, Chen TW, et al. Perfusion changes in gastric adenocarcinoma: Evaluation with 64-section MDCT. Abdom Imaging. 2010;35:195–202. doi: 10.1007/s00261-009-9503-1. [DOI] [PubMed] [Google Scholar]
- 27.Sun ZQ, Cheng XF, Ge YX, et al. Role of CT perfusion imaging in patients with variously differentiated gastric adenocarcinoma. J Xray Sci Technol. 2015;23:737–44. doi: 10.3233/XST-150524. [DOI] [PubMed] [Google Scholar]
- 28.Curvo-Semedo L, Portilha MA, Ruivo C, et al. Usefulness of perfusion CT to assess response to neoadjuvant combined chemoradiotherapy in patients with locally advanced rectal cancer. Acad Radiol. 2012;19:203–13. doi: 10.1016/j.acra.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Bellomi M, Petralia G, Sonzogni A, et al. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: Initial experience. Radiology. 2007;244:486–93. doi: 10.1148/radiol.2442061189. [DOI] [PubMed] [Google Scholar]
- 30.Lv WF, Han JK, Cheng DL, et al. CT perfusion imaging can predict patients’ survival and early response to transarterial chemo-lipiodol infusion for liver metastases from colorectal cancers. Korean J Radiol. 2015;16:810–20. doi: 10.3348/kjr.2015.16.4.810. [DOI] [PMC free article] [PubMed] [Google Scholar]