Abstract
Cannabis-related impairments to cognitive function may represent novel therapeutic targets for cannabis-use disorder, although the nature, persistence, and reversibility of such deficits remain unclear. Adult male rhesus monkeys (N = 6) responded in the morning on tasks designed to assess different cognitive domains using the Cambridge Neuropsychological Test Automated Battery (CANTAB) touchscreens followed by responding maintained under a fixed-ratio (FR) 10 schedule of food presentation in different operant chambers. First, the acute effects of Δ9-tetrahydrocannabinol (THC; 0.01–0.56 mg/kg, i.v.) on cognitive performance, FR responding, and body temperature were determined. Next, THC (1.0–2.0 mg/kg, s.c.) was administered daily after FR 10 sessions for 12 weeks, during which the residual effects of THC (i.e., 22 hours after administration) on cognition were examined and the acute effects of THC were redetermined. In a subgroup of monkeys, dopamine D2/D3 receptor availability was assessed after 4 weeks of chronic THC exposure and compared with drug-naive controls using positron emission tomography and [11C]-raclopride (N = 4/group). Acute THC pretreatments dose-dependently decreased FR responding and body temperature, and impairment to cognitive performance was task specific. During chronic treatment, THC produced persistent residual impairment only to working memory; tolerance differentially developed to acute cognitive impairments. There was recovery from residual cognitive impairments to working memory within 2 weeks of abstinence. Compared with controls, D2/D3 receptor availability was not altered during chronic THC treatment. In conclusion, THC-induced disruptions in cognition were task-specific, as was tolerance development, and not related to changes in D2/D3 receptor availability. Intervention strategies for cannabis-use disorder that enhance working memory performance may facilitate positive treatment outcomes.
Introduction
Marijuana is the most widely abused illicit substance in the United States (Center for Behavioral Health Statistics and Quality, 2016); however, effective treatment options are limited (Copeland and Pokorski et al., 2016). One factor in particular associated with poorer treatment outcome and relapse is drug-induced disruption to cognitive function (Aharonovich et al., 2006, 2008; Carroll et al., 2011). Therefore, a better understanding of the mechanisms underlying Δ9-tetrahydrocannabinol (THC)-induced cognitive impairment may identify targets for cognitive enhancement as an adjunct treatment strategy for cannabis use disorder (Sofuoglu et al., 2013; Jentsch and Pennington, 2014; Rezapour et al., 2016).
Studies in humans have shown that the acute effects of cannabis consistently impair such executive cognitive functions as attention (Hart et al., 2001; D’Souza et al., 2008; Anderson et al., 2010), working memory (Heishman et al., 1997; Hart et al., 2001, 2010), and inhibition/impulsivity (McDonald et al., 2003; Ramaekers et al., 2009). However, the residual effects of THC (i.e., after the intoxicating effects have subsided) that are relevant for treatments that target cognitive functioning are equivocal for most cognitive domains (Crean et al., 2011; Crane et al., 2013). Some of these disparate findings may be the result of experimental factors that are difficult to control in studies with human subjects, such as time since last cannabis use, amount and duration of cannabis use, previous/current drug histories, social variables, and innate cognitive differences prior to initiating cannabis use. Therefore, it is useful to employ longitudinal, within-subject studies in preclinical animal models that can control for these variables in a systematic manner.
The present study used a within-subjects study design in monkeys to examine the acute and residual effects of chronic THC administration on multiple cognitive domains, including 1) attention and working memory via delayed match-to-sample (DMS) performance, 2) associative learning via simple/compound discrimination (SD, CD), 3) inhibitory/impulse control via compound discrimination reversal learning (CDR), and 4) cognitive flexibility via attentional set-shifting [intradimensional (ID)/extradimensional (ED)]. Intact functioning within these particular cognitive domains is important for positive treatment response, as they underlie the skills necessary for behavioral change and for reducing the likelihood of relapse during treatment (Rezapour et al., 2016). The effects of THC administration were also assessed on another behavioral baseline, fixed-ratio (FR) schedule of food reinforcement, and the effects of THC on body temperature were examined. We hypothesized that the effects of chronic THC administration would be different on cognition compared with simple operant responding under the FR schedule and on changes in body temperature. Moreover, we hypothesized that working memory would be most vulnerable to THC-induced impairment on the basis of previous research in nonhuman primates (Taffe, 2012; Wright et al., 2013; Kangas et al., 2016).
Prior work also suggests that the acute effects of THC on behavior may differ as a function of THC history (Tanda and Goldberg, 2003). As it relates to cognitive performance, studies in humans have demonstrated equivocal findings for the acute effects of cannabis in occasional versus frequent cannabis users (Kirk and de Wit, 1999; D’Souza et al., 2008; Ramaekers et al., 2009, 2016), which may have been owing to differences in baseline performance and extent of cannabis use history. The use of within-subject experimental designs in animal models can address these confounds, which was the major goal of the present study.
Regarding neurobiological substrates mediating cognition and executive function, dopamine D2/D3 receptors (D2/D3R) are particularly important (Tomasi and Volkow, 2013). For instance, D2/D3R in the dorsal striatum have been shown to be associated with activation of prefrontal brain regions implicated in executive function (Volkow et al., 1993, 1998) and with frontostriatal circuitry implicated in inhibitory control (Ghahremani et al., 2012). Moreover, chronic drug abuse in humans has been associated with reductions in D2/D3R availability compared with healthy control subjects for a variety of substances (Volkow et al., 2012). Taken together, cognitive impairment resulting from chronic drug use may be related to low striatal D2/D3R availability. Indeed, cognitive impairment in long-term marijuana users has been associated with reduced activity in frontal cortical regions (Block et al., 2002; Eldreth et al., 2004; Bolla et al., 2005; Gruber and Yurgelun-Todd, 2005). However, findings for D2/D3R availability in long-term marijuana users have been inconsistent (Stokes et al., 2012; Albrecht et al., 2013). Thus, a final aim of our study was to directly examine the association between chronic THC exposure, cognitive performance, and D2/D3R availability by using positron emission tomography (PET) and the D2/D3R selective radiotracer [11C]-raclopride.
Materials and Methods
Subjects
For Experiments 1 and 2, six individually housed adult male rhesus monkeys (Macaca mulatta), with extensive drug histories and indwelling intravenous catheters (John et al., 2015a,b, 2017) served as subjects. Each monkey was also implanted subcutaneously with a transponder (Model IPTT-300; Bio Medic Data Systems, Inc., Seaford, DE) to noninvasively measure body temperature. Two of these monkeys were used in PET imaging studies as part of Experiment 3, prior to any drug history and again after chronic THC administration; two additional drug-naïve monkeys were used in Experiment 3. Therefore, a total of eight monkeys was used in these studies. Each monkey was fitted with an aluminum collar (Model B008; Primate Products, Redwood City, CA) and trained to sit in a primate restraint chair (Primate Products). Monkeys were housed in stainless-steel cages with visual and auditory contact with each other, ad libitum access to water in their home cage and were fed sufficient standard laboratory chow (5045; Purina LabDiet, St. Louis, MO) to maintain healthy body weights as determined by the veterinary staff. Environmental enrichment was provided as outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and was approved by the Animal Care and Use Committee of Wake Forest University.
Overview of Behavioral Procedures.
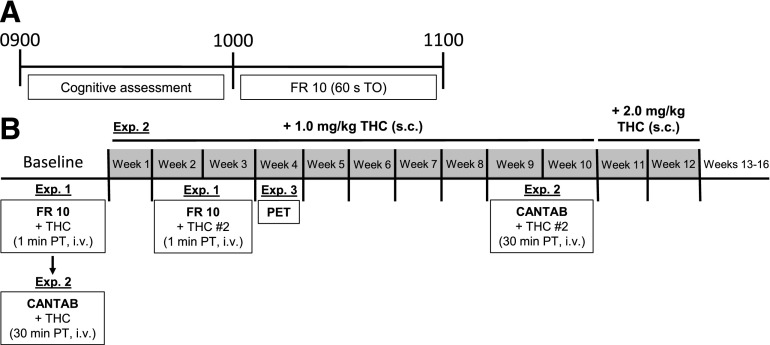
Monkeys in Experiments 1 and 2 underwent two behavioral sessions each day (Fig. 1). At ∼0900 cognitive performance was assessed using the Cambridge Neuropsychological Test Automated Battery (CANTAB) apparatus; sessions typically lasted 1 hour. Immediately following, monkeys were transported to a different room and operant sessions were conducted in separate experimental chambers where monkeys responded under a fixed-ratio 10 schedule of food (1.0-g banana-flavored pellets) presentation with a 60-second timeout (TO) following each reinforcer presentation. FR sessions were approximately 1 hour in duration.
Fig. 1.
Experimental timeline. (A) Sequence of daily experimental sessions: Monkeys performed two operant behavioral sessions each day, which included cognitive testing immediately followed by responding under an FR 10 schedule of food presentation. (B) Sequence of treatments across weeks: Following stable performance, the acute effects of THC (0.003–0.3 mg/kg, i.v.) were first determined on FR responding (Exp. 1) and then on cognition (Exp. 2). THC (1.0–2.0 mg/kg, s.c.) was next administered chronically for 12 weeks (shaded segments), during which residual effects on cognitive performance and FR responding were assessed (i.e., ∼22 and ∼23 hours after daily administration, respectively) (Exp. 2). During chronic treatment, dopamine D2/D3R availability was assessed using PET and [11C]-raclopride at Week 4 (Exp. 3) and the acute effects of THC were redetermined on FR responding (Exp. 1) and cognition (Exp. 2) during Weeks 2–3 and 9–10, respectively. Exp., experiment; + THC #2, redetermination of acute effects of THC.
Apparatus
The apparatus for food-maintained responding was a ventilated, sound-attenuating chamber (1.5 × 0.74 × 0.76 m; Med Associates, East Fairfield, VT) designed to accommodate a primate chair. Two photo-optic switches (5-cm wide) were located on one side of the chamber with a horizontal row of three stimulus lights 14 cm above each switch and a food receptacle between the switches. The food receptacle was connected with Tygon tubing to a pellet dispenser (Gerbrands Corp., Arlington, MA) located on the top of the chamber for delivery of 1.0-g banana-flavored food pellets (Bio-Serv, Frenchtown, NJ). An infusion pump (Cole-Palmer, Inc., Chicago, IL) was located on the top of the chamber.
The apparatus for cognitive testing was a separate sound-attenuating, ventilated chamber (0.8 × 0.8 × 1.32 m) consisting of a CANTAB panel (0.38 × 0.56 × 0.31 m) that included a touch-sensitive screen (0.3 × 0.23 m), a stimulus light, a nonretractable response lever, and a pellet receptacle located on the right side. For each task, responding was maintained by presentation of 190-mg sucrose pellets.
Surgery
Monkeys included in behavioral experiments were surgically prepared with a chronic indwelling venous catheter (femoral, internal, or external jugular) and subcutaneous vascular port (Access Technologies, Skokie, IL) using aseptic surgical procedures, as described in detail elsewhere (John et al., 2017). These monkeys were also implanted subcutaneously with a transponder (Model IPTT-300; Bio Medic Data Systems, Inc.) to noninvasively measure body temperature.
General Procedures.
Food-Maintained Responding.
Sessions began with illumination of a white stimulus light above one of two photo-optic switches in the chamber, counterbalanced between monkeys (N = 6). Ten consecutive responses (FR 10) emitted on the appropriate switch produced the delivery of a food pellet accompanied by extinction of the white light and illumination of a red stimulus light above the food receptacle for 3 seconds. Responses emitted on the alternate photo-optic switch had no programmed consequence. Sessions lasted 60 minutes or until 30 reinforcers were obtained, whichever occurred first. Stable performance was defined as ±20% of the mean rate of responding for three consecutive sessions, with no trends as used previously (John et al., 2017).
Delayed Match-to-Sample.
The delayed match-to-sample (DMS) task is a measure of working memory. Each trial began with the appearance of a “target” stimulus in the center of the screen (sample phase). A response on this stimulus was followed by a delay and then the presentation of a stimulus matching the previous image and at least two distractor stimuli that do not match (match phase). Responding on the match resulted in delivery of a sucrose pellet followed by a 10-second TO, whereas responding on the nonmatch resulted in trial termination and the 10-second TO. If a response was not emitted within 10-second during either phase, the trial was terminated. Three delays were randomly distributed throughout a total of 60 trials per session (20 trials/delay). Delays and number of distractor stimuli were individually determined (Table 1) to generate delay-effect curves that met the following criteria: short delay (80–100% accuracy), medium delay (60–79% accuracy), long delay (<60% accuracy). The stimuli used were unique abstract patterns preprogrammed in CANTAB software, consisting of four rectangular quadrants of different colors and shapes. Each session lasted for a maximum of 100 minutes or after 60 trials were completed, whichever occurred first. Baseline performance was obtained prior to drug testing and was defined as seven consecutive sessions with percent accuracy for each delay ±25% of the mean rate for those sessions with no trends.
TABLE 1 .
Individual parameters used throughout study that produced delay-dependent effects on DMS performance
| Subject | Delays (s) |
# Dist. | ||
|---|---|---|---|---|
| Short | Mid | Long | ||
| R-1567 | 1 | 30 | 60 | 2 |
| R-1682 | 0 | 30 | 90 | 4 |
| R-1710 | 0 | 10 | 30 | 2 |
| R-1711 | 0 | 30 | 100 | 4 |
| R-1713 | 0 | 30 | 60 | 3 |
| R-1756 | 1 | 30 | 60 | 3 |
Dist., distractor stimuli.
Stimulus Discrimination, Reversal Learning, and Intradimensional/Extradimensional Set-Shifting (Attentional Set-Shifting).
This task consisted of five distinct stages that tested attention, rule learning and reversal, and executive function. Stages advanced within-session following acquisition of performance criteria, defined as 16 of 20 consecutive trials correct. Stage 1, simple discrimination (SD): Two stimuli (e.g., shapes) were presented and monkeys were required to discriminate between the two stimuli in which one resulted in reinforcement (S+) and the other resulted in trial termination (S−). Stage 2, compound discrimination: The same stimuli as in the previous stage remained present; however, an additional dimension (e.g., lines) was superimposed to construct compound stimuli that consisted of shape and line dimensions. The same stimulus dimension that was associated with reinforcement in the first stage (e.g., shapes) remained relevant for reinforcement in this stage, whereas the other dimension (e.g., lines) was irrelevant. Stage 3, compound reversal (CDR): Stage 2 rules were reversed such that the previously nonreinforced stimulus within the dimension was now reinforced. Stage 4, intradimensional shift: A new pair of compound stimuli consisting of shape and line dimensions was presented; however, despite new stimuli, the same dimension of the stimuli as in Stages 1–3 (e.g., shapes) remained relevant for reinforcement. Stage 5, extradimensional shift: Another novel set of compound stimuli was introduced; however, in this stage the previous irrelevant stimulus dimension (e.g., lines) became relevant for reinforcement. Each session lasted for a maximum of 100 minutes or after criterion was met for each stage, whichever occurred first. Baseline performance was obtained prior to drug testing and was defined as four consecutive sessions over 2 weeks with percent accuracy for each stage ±25% of the mean rate for those sessions with no trends.
Procedures.
Experiment 1: Effects of Acute and Chronic THC Administration on Food-Maintained Responding.
Once FR responding was stable, a noncontingent injection of vehicle or THC (0.003–0.3 mg/kg, i.v.) was administered 1 minute prior to operant sessions, typically on Tuesdays and Fridays; all doses were tested two to three times in random order for each monkey. Body temperature was taken noninvasively using the implanted telemetry device immediately prior to THC administration and again 60 minutes after the start of the session. After completion of the THC dose-response curve and prechronic THC dose-response curve for Experiment 2 (see below), THC (1.0 mg/kg, s.c.) was administered daily approximately 5 minutes after the session for 10 weeks, followed by 2 weeks of 2.0 mg/kg (s.c.) THC for a total of 12 weeks of treatment. Hence, FR responding occurred ∼23 hours after the daily THC administration. Doses were selected on the basis of preliminary findings and other studies in rhesus monkeys showing these doses produced similar THC blood levels as when humans smoke cannabis in controlled laboratory studies (Ginsburg et al., 2014). Following 1 week of chronic THC (1.0 mg/kg, s.c.) administration, the effects of acutely administered THC on FR responding were redetermined over the course of 2 weeks (Weeks 2–3), during which monkeys were still treated daily with 1.0 mg/kg THC postsession. After 12 weeks of chronic treatment, responding was studied for 4–6 weeks after discontinuation of daily THC administration. The daily dose was not raised to 2.0 mg/kg in R-1710 owing to marked behavioral disruption from 1.0 mg/kg THC, to which tolerance did not develop.
Experiment 2: Effects of THC on Cognitive Performance.
Each week, DMS performance was assessed Monday to Wednesday, and SD, CD, CDR, ID, and ED set-shifting performances were assessed on Thursday and Friday. Following stable performance, the acute effects of vehicle and THC (0.003–0.3 mg/kg, i.v.) were determined on each task prior to chronic THC treatment. Each dose was administered 30 minutes prior to the start of the session, the pretreatment time having been selected on the basis of the time course of cognitive impairment and subjective/euphoric effects following i.v. THC administration in humans and nonhuman primates (Lindgren et al., 1981; D’Souza et al., 2004; Verrico et al., 2012). All doses were tested two to three times for each monkey, and at least two intervening sessions were conducted in which no drug or vehicle was administered.
After acute dose-response curves were determined, the residual effects (i.e., 22 hours postadministration) of daily THC (1.0 mg/kg, s.c.) on cognition were assessed for 8 weeks. After 8 weeks of THC treatment, the acute effects of THC on cognitive performance were redetermined over the course of 2 weeks while monkeys still received 1.0 mg/kg THC at the end of FR sessions. During this period, acute effects were assessed approximately every other day, as long as performance was deemed stable in the preceding session. Following the redetermination of the acute effects, the dose of daily-administered THC was increased to 2.0 mg/kg (s.c.) for 2 weeks, in an effort to overcome tolerance, after which treatment was discontinued and performance during 4–6 weeks of abstinence was examined.
Experiment 3: Effects Of Chronic THC on D2/D3R Availability.
PET studies with [11C]-raclopride were used to assess D2/D3R availability (Farde et al., 1985). Two groups of monkeys underwent [11C]-raclopride PET scans; one group (N = 4) was studied after 4 weeks of daily THC administration, and another group (N = 4) was drug-naive and served as control subjects. Two subjects (R-1710, R-1711) were part of both groups. Approximately 30 minutes prior to the PET scan, the monkey was anesthetized with ketamine (10 mg/kg, i.m.) and transported to the PET Center. Anesthesia was maintained during the scan by inhaled isoflurane (1.5%). The monkey was placed in a GE Advance NXi PET scanner with 4-mm resolution and a catheter was inserted into an external vein for tracer injection and fluid replacement throughout the study. Body temperature was maintained at 40°C and vital signs (heart rate, blood pressure, respiration rate, and temperature) were monitored throughout the scanning procedure. A 5-minute transmission scan was acquired in 3D mode. Next, the monkey received a bolus dose of [11C]-raclopride (6.5–8.0 mCi), and a 90-minute dynamic acquisition scan was acquired consisting of 18 frames over 90 minutes (18 × 5 minutes) in 3D mode (i.e., septa retracted). Image reconstruction of 3D data was done using the 3D-reprojection method (Kinahan and Rogers, 1989) with full quantitative corrections. Once scanning was complete, the transmission scan data were smoothed transaxially using a 4-mm Gaussian filter and segmented (Bettinardi et al., 1999). Emission data were corrected for attenuation and reconstructed into 128 × 128 matrices using a Hanning filter with a 4-mm cutoff transaxially and a ramp filter with an 8.5-mm cutoff axially.
Data Analysis
Experiment 1.
The primary dependent variable for food-maintained responding was response rate (responses/second). Drug effects were expressed as a percentage of baseline responding, which was designated as three consecutive sessions of stable responding prior to the start of the study. Change in body temperature (°C) following administration of either vehicle or THC was determined by subtracting the temperature recorded prior to the start of the session from the temperature recorded at the end of the 60-minute session. The potency of THC to decrease food-maintained responding was estimated by calculating the median effective dose (ED50) using the linear portion of the curve that crossed 50% reduction in baseline response rate. Potencies were considered statistically significant when 95% confidence intervals of ED50 values did not overlap. Subject R-1710 was excluded from analyses owing to a significant reduction in response rates during chronic THC treatment that precluded a determination of ED50 values.
Experiment 2.
The primary dependent variable for DMS and the SD, CD, CDR, ID and ED tasks was percent accuracy. Changes in percent accuracy from baseline were examined by two-way repeated-measures analysis of variance (ANOVA) using delay (short, middle, long), stage (SD, CD, CDR, ID, ED) and treatment condition (baseline, chronic, abstinence) as factors. Omitted trials were excluded from all analyses. Sessions during the chronic THC treatment phase in which the total number of omissions exceeded 50% of the total trials were excluded from analyses. As a result, two subjects (R-1567, R-1710) were excluded from all DMS analyses; both subjects were also excluded from analyses of acute THC effects on ID/ED and R-1710 was excluded from analysis of chronic THC effects on ID/ED performance. One-way repeated-measures ANOVAs were conducted to compare number of omissions, response latency, and pellet retrieval latency between baseline and test conditions. Significant main effects were followed by posthoc Fisher least significant difference tests. For all analyses, P < 0.05 was considered statistically significant.
Experiment 3.
PET data were coregistered to individual T1-weighted magnetic resonance images (acquired using a 3.0-T Siemens SKYRA scanner), which were used to anatomically define regions of interest (ROI), including the caudate nucleus, putamen, ventral striatum, and cerebellum. PMOD Biomedical Image Quantification Software (version 3.1; PMOD Technologies, Zurich, Switzerland) was used for image registration and to calculate distribution volume ratios (DVRs) for each ROI by implementing the Logan method of analysis (Logan et al., 1996) using the cerebellum as the reference region. DVRs for each region were not different between left and right sides, therefore data from each monkey was expressed as a mean of both sides. To compare DVRs between control and THC-treated subjects, a two-way ANOVA was conducted using region and group as factors.
Drugs
Δ9-tetrahydrocannabinol was provided by the National Institute on Drug Abuse (Rockville, MD) as 20–100 mg/ml ampules dissolved in 95% EtOH. THC was diluted in a vehicle containing one part Tween 80, one part ethanol, and 18 parts sterile water. THC was administered i.v., through the vascular access port, and was followed by a 3-ml saline injection to ensure the entire dose was delivered.
Results
Experiment 1: Effects of Acute and Chronic THC on Food-Maintained Responding
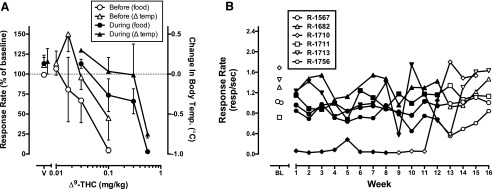
Acute THC administration resulted in dose-dependent decreases in food-maintained responding (Fig. 2A, open circles) and body temperature (Fig. 2A, open triangles). The ED50 (±95% confidence limits) for THC to reduce response rates and body temperature was 0.04 (0.01–0.06) and 0.07 (0.02–0.19) mg/kg, respectively. There were no residual effects of chronic THC administration on food-maintained responding in any subject, except for one (R-1710) in which tolerance to rate-decreasing effects did not develop (Fig. 2B). After 1 week of daily THC (1.0 mg/kg, s.c.) treatment, the ED50 (95% confidence limit) for THC to reduce food-maintained responding and body temperature was 0.34 (0.16–0.51) and 0.46 (0.23–0.90) mg/kg, respectively (Fig. 2A, closed circles/triangles). The potency of THC to reduce food-maintained responding and body temperature was significantly reduced by approximately 9.7- and 6.6-fold, respectively, after 1 week of daily THC (1.0 mg/kg, s.c.) treatment.
Fig. 2.
(A) Mean ± S.E.M. (N = 5) effects of acute THC administration on FR food-maintained responding (circles) and body temperature (triangles) before (open symbols) and during (closed symbols) chronic THC (1.0 mg/kg, s.c.) treatment (Weeks 2–3). Abscissae: Dose of THC in milligram per kilogram. Left ordinate: Mean response rate expressed as a percentage of baseline. Right ordinate: Mean change in body temperature expressed in degrees centigrade. (B) Mean (±S.D.) FR food-reinforced response rates for sessions before (BL, baseline), during (i.e., 23 hours after daily administration), and after chronic THC treatment of individual monkeys (N = 6). Abscissae: consecutive weeks before, during, and after chronic THC treatment. Ordinate: response rate (responses/second). Filled symbols indicate weeks during chronic THC treatment. Open symbols indicate weeks where THC was not administered. All monkeys were treated with 1.0 mg/kg THC during Weeks 1–10 and 2.0 mg/kg THC during Weeks 11 to 12 except for one monkey (R-1710) who was only treated with 1.0 mg/kg THC during Weeks 1–8.
Experiment 2: Effects of THC on Cognitive Performance
DMS Performance.
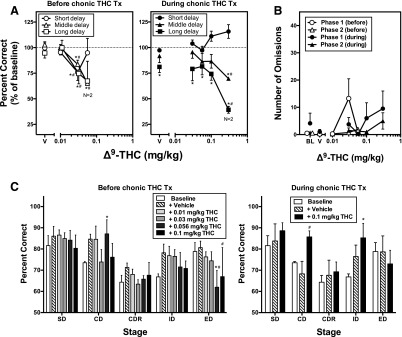
For each monkey, three delays (short, mid, long) and number of distractor stimuli were chosen (Table 1) that resulted in similar delay-effect curves between monkeys (Table 2). There was a main effect of dose [F(4, 39) = 11.66, P < 0.0001] for the acute effects of THC on DMS performance prior to chronic treatment; posthoc analyses revealed that percent accuracy was significantly reduced by 0.03 mg/kg THC at the short delay and by 0.03 and 0.056 mg/kg THC at the middle and long delays compared with baseline (Fig. 3A, open symbols). When the THC dose-response curve was redetermined during chronic THC treatment, there was a main effect of delay [F(2, 48) = 31.63, P < 0.0001], dose [F(5, 48) = 4.07, P < 0.01], and a significant interaction [F(10, 48) = 2.59; P < 0.01). Posthoc analyses indicated that tolerance developed to the performance-disrupting effects of THC such that 0.03 and 0.056 mg/kg THC did not alter percent accuracy from baseline at the short and middle delays (Fig. 3A, closed symbols). During chronic treatment, accuracy at the long delay was significantly lower than baseline following vehicle pretreatment, indicative of sustained residual impairment. The number of omissions following acutely administered THC before or during chronic treatment was not significantly different from baseline or vehicle during the Sample or the Match phases (Fig. 3B).
TABLE 2 .
Baseline percent accuracy (±S.D.) at each delay for individual monkeys responding under the DMS task
| Subject | Delays |
||
|---|---|---|---|
| Short | Mid | Long | |
| R-1567 | 78.8 (7.2) | 46.5 (17.2) | 38.3 (9.9) |
| R-1682 | 81.7 (5.9) | 70.9 (9.8) | 58.7 (9.8) |
| R-1710 | 75.8 (4.0) | 52.9 (11.4) | 41.7 (13.6) |
| R-1711 | 82.2 (10.2) | 72.0 (15.5) | 58.1 (8.7) |
| R-1713 | 87.1 (5.7) | 56.5 (10.2) | 49.3 (14.0) |
| R-1756 | 72.7 (11.2) | 52.0 (11.0) | 48.5 (5.1) |
Fig. 3.
Mean ± S.E.M. (N = 4) effects of acutely administered THC on cognitive performance before and during chronic THC (1.0 mg/kg, s.c.) treatment (Weeks 9 to 10). THC (0.01–0.3 mg/kg, i.v.) was administered 30 minutes before experimental sessions. (A) DMS performance at short-, mid-, and long-delays before (left panel, open symbols) and during (right panel, closed symbols) chronic THC treatment. (B) Acute effects of THC on DMS phase 1 (circles) and phase 2 (triangles) omissions before (open symbols) and during (closed symbols) chronic THC treatment. Data point for 0.056 mg/kg THC before chronic treatment and for 0.3 mg/kg THC during chronic treatment represents N = 2. (C) Acute effects of THC on SD, CD, CDR, ID, and ED set-shifting performance before (left panel) and during (right panel) chronic THC treatment. Data point for 0.056 and 0.1 mg/kg THC before chronic treatment represents N = 3. *P < 0.05, significant difference from baseline; #significant difference from vehicle. BL, baseline; SD, stimulus discrimination; CD, compound discrimination; CDR, reversal of compound discrimination; ID intradimensional shift; ED, extradimensional shift.
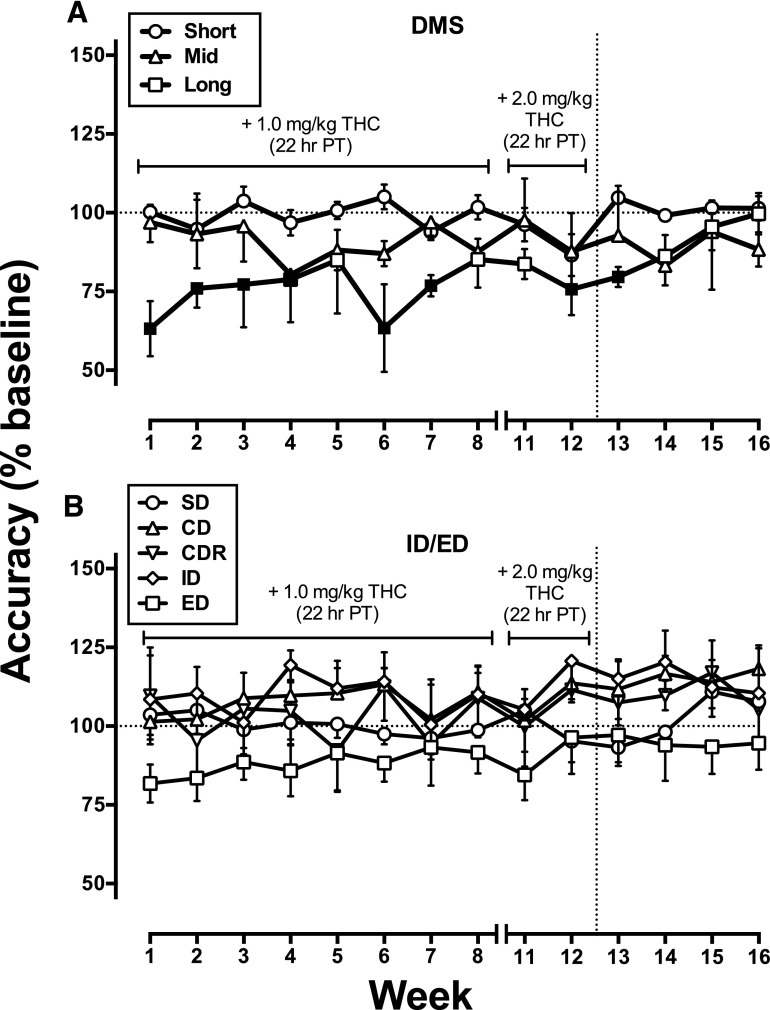
The residual effects of THC (1.0–2.0 mg/kg, s.c., 22 hours after treatment) on DMS performance over the course of 12 weeks of treatment and 4 weeks of abstinence (Fig. 4A) showed a significant main effect of study week (baseline, THC treatment, or abstinence) on percent change in accuracy from baseline [F(14, 42) = 2.29; P < 0.05). Significant impairments were detected at the middle delay during Week 4 of treatment and at the long delay during Weeks 1–4, 6, and 7. After the dose of daily administered THC was increased to 2.0 mg/kg, percent accuracy from baseline at the longest delay was significantly decreased again at Week 12, which persisted until Week 2 of abstinence (Fig. 4A). There were no residual effects of chronic THC treatment on omissions during DMS performance.
Fig. 4.
Residual effects (i.e., 22 hours after administration) of chronic THC treatment on delayed match-to-sample performance, N = 4 (A), and discrimination, reversal learning, and attentional set-shifting performance, N = 5 (B). Data are expressed as percent of baseline performance. Filled symbols indicate a significant difference from baseline (P < 0.05). Break in x-axis indicates 2-week period (Weeks 9–10), when acute effects of THC on cognitive performance were reassessed during THC treatment. Vertical dashed lines indicate discontinuation of chronic THC treatment, after which effects during abstinence were assessed. DMS, delayed match-to-sample; all other symbols as described in Fig. 3
SD, CD, CDR, ID, and ED Performance.
There was a main effect of stage [F(4,64) = 13.96, P < 0.0001] and dose [F(4, 98) = 3.49, P < 0.05] for the acute effects of THC on attentional set-shifting performance prior to chronic THC treatment. There was a significant increase in percent accuracy of the CD stage after 0.056 mg/kg THC compared with baseline (P < 0.05). Posthoc analysis also revealed a significant decrease in percent accuracy of ED at 0.056 (P < 0.001) and 0.1 mg/kg THC (P < 0.05) compared with baseline (Fig. 3C). During chronic treatment, the acute effects of only 0.1 mg/kg THC were re-examined because it was the highest dose that produced impairments without producing a significant number of omissions in the initial assessment prior to treatment. Repeated-measures two-way ANOVA demonstrated a main effect of stage [F(4, 36) = 3.83, P < 0.05]. During THC treatment, percent accuracy of ED after administration of 0.1 mg/kg THC was not significantly different from vehicle administration nor baseline prior to chronic treatment (Fig. 3C). Acute THC administration did not have a significant effect on omissions compared with baseline or vehicle for any stage.
The residual effects of THC on SD, CD, CDR, ID, and ED set-shifting performance (Fig. 4B) showed a significant main effect of stage [F(4, 232) = 14.46; P < 0.0001] although posthoc analysis revealed no significant decreases in percent accuracy from baseline during treatment or abstinence. There were no residual effects of THC on number of omissions for any stage. An overall summary of the acute and residual effects of THC on all cognitive tasks is presented in Table 3.
TABLE 3 .
Summary of THC-induced effects on cognitive performance
Effects were dose-dependent; refer to figures for details. Upward arrow, significant decrease from baseline; downward arrow, significant increase from baseline; —, no significant change from baseline.
| Task |
Acute- 30-min PT (Prechronic Tx) | Residual- 23 hr PT (During Chronic Tx) | Acute- 30-min PT (During Chronic Tx) | Abstinence |
|---|---|---|---|---|
| DMS | ||||
| Short delay | ↓ | — | — | — |
| Middle delay | ↓ | — | — | — |
| Long delay | ↓ | ↓ | ↓ | ↓ |
| SD | — | — | — | — |
| CD | ↓ | — | — | — |
| CDR | — | — | — | — |
| IDS | — | — | ↑ | — |
| EDS | ↓ | — | — | — |
IDS, intradimensional set-shifting; SD, stimulus discrimination.
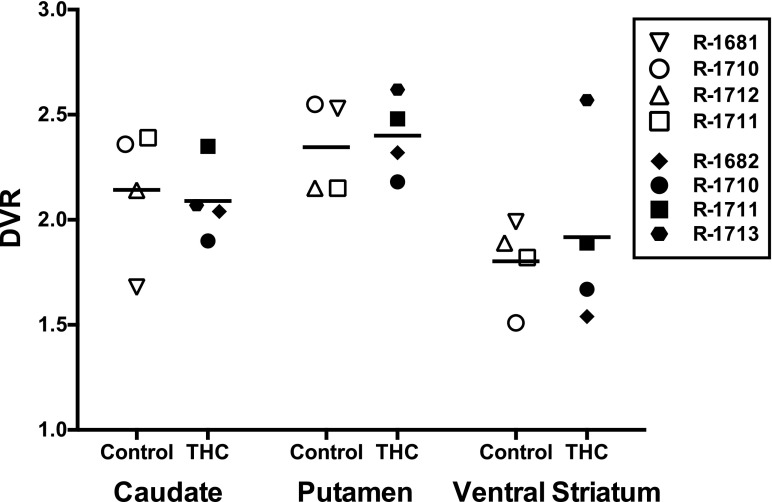
Experiment 3: Effects of Chronic THC on D2/D3R Availability
In control monkeys, the distribution volume ratio for [11C]-raclopride was highest in the putamen, followed by the caudate nucleus and ventral striatum (Table 4). There were no significant differences in D2/D3R availability in the caudate nucleus, putamen, and ventral striatum between control and chronic THC-treated monkeys (Fig. 5; Table 4). Individual differences were noted between the two monkeys that participated in scans both before and during THC treatment such that DVRs in the putamen were decreased by 14.2% during THC treatment in one monkey (R-1710) and increased by 15.3% in the other (R-1711).
TABLE 4 .
Individual and mean (±S.E.M.) [11C]-raclopride DVRs in control and THC-treated monkeys
| Control | Caudate | Putamen | Ventral Striatum |
|---|---|---|---|
| R-1710 | 2.36 | 2.55 | 1.51 |
| R-1712 | 2.14 | 2.15 | 1.89 |
| R-1711 | 2.39 | 2.15 | 1.82 |
| R-1681 | 1.68 | 2.53 | 1.99 |
| Mean | 2.14 | 2.35 | 1.80 |
| ±S.E.M. | 0.16 | 0.11 | 0.10 |
| Δ9-THC | |||
| R-1710 | 1.90 | 2.18 | 1.67 |
| R-1711 | 2.35 | 2.48 | 1.89 |
| R-1713 | 2.07 | 2.62 | 2.57 |
| R-1682 | 2.04 | 2.32 | 1.54 |
| Mean | 2.09 | 2.40 | 1.92 |
| ±S.E.M. | 0.09 | 0.10 | 0.23 |
| Difference | −2.3% | +2.1% | +6.7% |
Fig. 5.
PET imaging data using [11C]-raclopride to measure dopamine D2/D3R distribution volume ratios in control monkeys (open symbols) and in monkeys treated daily (after 4 weeks) with 1.0 mg/kg (s.c.) THC (closed symbols). Different shaped symbols represent individual subjects. Two monkeys (R-1710, R-1711) served as both control and THC-treated subjects.
Discussion
The main goal of the present study was to examine the effects of THC on multiple cognitive domains in nonhuman primates and to examine tolerance development following chronic THC administration as shown by multiple behavioral baselines, and to examine THC-induced changes in body temperature. Acutely administered THC decreased FR response rates and body temperature dose-dependently, and tolerance developed to both effects during chronic THC administration. With regard to cognition, acute THC dose dependently decreased working memory (i.e., DMS) and ED set-shifting performance. During chronic treatment, tolerance developed to acute impairments in ED set-shifting performance but not DMS working memory when the cognitive demand was high (i.e., at long delays using complex stimuli). The residual effects of chronically administered THC were specific to impairments in working memory at the long delay. Tolerance developed transiently to the residual deficits in working memory during chronic treatment; an increase in THC dose resulted in impairments to working memory at the long delay. Working memory deficits recovered within 2 weeks of abstinence. Overall, the present findings show that tolerance differentially developed to THC-induced effects on cognitive performance relative to food-maintained operant responding and body temperature. In an effort to examine potential mechanisms mediating the effects of chronic THC, dopamine D2/D3R availability was examined and found not to be different from that of control monkeys.
Tolerance to the acute effects of cannabinoids following a period of chronic cannabinoid exposure can differ depending on the behavioral outcomes measured (Lichtman and Martin, 2005). As such, studies in humans comparing groups of frequent versus infrequent cannabis users suggest that chronic THC exposure can produce differential tolerance to the acute effects of THC on cognition. For instance, compared with infrequent users, some studies report that frequent users were less impaired by acutely administered THC on measures of attention (divided attention task) and memory (DMS, spatial working memory) (D’Souza et al., 2008; Ramaekers et al., 2009), whereas other studies indicate a lack of tolerance (Theunissen et al., 2012; Ramaekers et al., 2016). However, the latter studies depended on the use of a group design, so the extent to which the drug history was actually a factor is unclear without data on the effects of acute THC on cognitive performance prior to the initiation of frequent use. By using a within-subjects design, the present study showed THC history was a causal factor for the development of tolerance to THC-induced cognitive impairment within similar domains. These findings have implications for assessing THC-induced driving impairment, to the extent that the studied cognitive domains map onto driving performance, as well as impairment in other behavioral tasks, and suggest that cannabis use history should be considered in addition to measuring THC plasma concentrations.
Prior to chronic treatment, acutely administered THC was more potent in decreasing cognitive performance (i.e., ED set-shifting and working memory) than rates of FR responding and body temperature. However, the dose was similar (i.e., 0.3 mg/kg) to decrease each behavioral measure compared with vehicle pretreatment during chronic THC treatment. Previous studies in rhesus monkeys demonstrated that the cannabinoid receptor type 1 (CB1) antagonist SR 141716A did not fully attenuate THC-induced rate-decreasing and hypothermic effects, implicating a role for non-CB1 mechanisms in mediating these behavioral effects (McMahon and France, 2005; McMahon, 2011). Therefore, the present results suggest a relatively greater role for CB1 receptors in THC-induced cognitive impairment. However, a limitation of the present study was the different points during chronic treatment that acute effects were assessed on the various behavioral and physiologic measures, which should be addressed in future studies.
With regard to the residual effects of chronic THC exposure on cognition, results from studies in humans have also shown task-specific deficits, although not all have been consistent with the present findings in monkeys. For instance, in contrast to the present results, several studies in recently abstinent (6–36 hours) adult cannabis users showed no differences in working memory abilities compared with infrequent or non-users (Pope and Yurgelun-Todd, 1996; Solowij et al., 2002; Kanayama et al., 2004; Whitlow et al., 2004; Fisk and Montgomery, 2008). Human studies assessing the residual effects of cannabis use on cognitive flexibility are more mixed, with some studies showing impairments (Pope and Yurgulen-Todd, 1996; Solowij et al., 2002) and others showing no deficits (Whitlow et al., 2004; Gruber and Yurgelun-Todd, 2005; Hermann et al., 2007). Consistent with the present findings, associative learning in recently abstinent adolescent and adult users has been shown to be unaffected (Harvey et al., 2007; Fisk and Montgomery, 2008; Jager et al., 2010).
To our knowledge, only one preclinical study in nonhuman primates has examined the residual effects of chronic THC exposure (Verrico et al., 2014). In that study, adolescent rhesus monkeys were treated with THC (120 or 240 μg/kg, i.v.), 5 days/week for approximately 6 months during which working memory performance was assessed 23 hours after drug administration throughout the study. In contrast to our findings, there were no residual effects of chronic THC administration on object-working memory (i.e., DMS). However, spatial-working memory was impaired; tolerance or sensitization to these effects did not develop.
One explanation for discrepant findings among human studies may be owing to the daily amount of THC consumed across studies. Although participants in the previously mentioned studies used marijuana on nearly a daily basis, the number of marijuana cigarettes smoked per day is often not reported, which makes it difficult to discern THC-induced effects across studies. It is also possible that cognitive deficits emerge after longer periods of chronic THC exposure than what was presently studied in monkeys. For instance, Solowij et al. (2002) found that the severity of cognitive deficits after at least 12 hours of abstinence in frequent cannabis users was correlated with years of use. Other factors, such as cannabis strain, method of administration, and other smoking behaviors, also may have contributed to inconsistencies in the literature. Furthermore, inconsistent results between studies could be a reflection of different cognitive demand across the tasks employed. For example, the previous study in nonhuman primates showing no effect of chronic THC treatment on DMS performance (Verrico et al., 2014) employed relatively short delay lengths and a less complex set of sample stimuli than our study. In line with these observations, previous studies of abstinent adolescent cannabis users demonstrated poorer working memory performance relative to control subjects but only during tasks that required greater memory load (Jacobsen et al., 2007; Hanson et al., 2010; Jager et al., 2010). Thus, the working memory task parameters employed in the present study may have had the highest cognitive demand of the other tasks making it most sensitive to impairment during chronic THC treatment.
Another explanation for the task-specific impairment of cognitive performance by chronic THC in the present study may be the regional differences in brain CB1-receptor function related to the domain of executive function. Electrophysiological and lesion studies demonstrate that working memory is preferentially activated by the ventrolateral prefrontal cortex (Mishkin and Manning, 1978; Wilson et al., 1993), whereas discrimination reversal learning and set-shifting are preferentially associated with activation of the orbitofrontal cortex and dorsolateral prefrontal cortex, respectively (Dias et al., 1996a,b). Thus, it is possible that working memory impairments during chronic THC treatment were a reflection of greater dysregulation of CB1 receptor dynamics (e.g., downregulation and/or densensitization) or signaling in regions that mediate working memory.
Previous studies in rhesus monkeys have observed physical signs of withdrawal (i.e., yawning, anorexia, piloerection, irritability, and headshaking) and the onset of cannabinoid receptor antagonist discriminative stimulus effects within 24–48 hours of discontinuation of chronic THC treatment (Deneau and Kaymakcalan, 1971; Beardsley et al., 1986; Stewart and McMahon, 2010), thus raising the possibility that the cognitive deficits noted in the present study were related to withdrawal as opposed to sustained agonist effects of THC on brain function. Although cognitive assessments were made at time frames similar to those used to detect withdrawal symptoms in previous studies, no overt physical signs of withdrawal were noted throughout the present study. This was most likely the result of the once-a-day administration of a lower daily dose of THC compared with continuous infusion of higher daily doses used in previous studies documenting physical withdrawal symptoms [0.05–0.17 mg/kg per hour (Beardsley et al., 1986), 1.0 mg/kg per 12 hours (Stewart and McMahon, 2010)]. Nevertheless, a systematic procedure for monitoring overt physical signs of withdrawal was not included in the present study design, which precludes more conclusive evidence. However, notwithstanding this limitation, rates of food-maintained responding and numbers of omissions were not altered during the course of chronic treatment or after discontinuation, which suggests that cognitive impairment was not the result of a nonselective disruption in operant responding.
The present study did not find differences in striatal D2/D3R availability measured by [11C]-raclopride in monkeys chronically treated with THC compared with control subjects, even though deficits in working memory performance at the same time point were apparent. These findings are consistent with studies in humans also showing no differences in baseline D2/D3R availability between marijuana abusers and healthy controls (Stokes et al., 2012; Urban et al., 2012; Albrecht et al., 2013; Volkow et al., 2014). However, recent PET studies have shown reduced striatal dopamine release (after amphetamine challenge) in cannabis abusers (Volkow et al., 2014; van de Giessen et al., 2017). Taken together, striatal dysfunction appears to be a component of cannabis abuse, albeit to a lesser extent perhaps than with other drugs of abuse.
Acknowledgments
The authors thank Michael Coller, Jillian Odom, Phillip Epperly, and Stephanie Danner for technical assistance.
Abbreviations
- ANOVA
analysis of variance
- CANTAB
Cambridge Neuropsychological Test Automated Battery
- CB1
cannabinoid receptor type 1
- CD
compound discrimination
- CDR
reversal of compound discrimination
- D2/D3R
dopamine D2/D3 receptors
- DMS
delayed match-to-sample
- DVR
distribution volume ratio
- ED
extradimensional shift
- ED50
median effective dose
- FR
fixed-ratio
- ID
intradimensional shift
- PET
positron emission tomography
- THC
Δ9-tetrahydrocannabinol
Authorship Contributions
Participated in research design: John, Martin, S. H. Nader, M. A. Nader.
Conducted experiments: John, Solingapuram Sai.
Performed data analysis: John, Solingapuram Sai, S. H. Nader, Gage, Mintz.
Wrote or contributed to the writing of the manuscript: John, Martin, Solingapuram Sai, S. H. Nader, Gage, Mintz, M. A. Nader.
Footnotes
This work was supported by the National Institutes of Health Grants R37 DA10584 (M.A.N.), P50 DA06634 (M.A.N.), F31 DA041825 (W.S.J.), and T32 AA-007565 (W.S.J.).
References
- Aharonovich E, Brooks AC, Nunes EV, Hasin DS. (2008) Cognitive deficits in marijuana users: effects on motivational enhancement therapy plus cognitive behavioral therapy treatment outcome. Drug Alcohol Depend 95:279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. (2006) Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend 81:313–322. [DOI] [PubMed] [Google Scholar]
- Albrecht DS, Skosnik PD, Vollmer JM, Brumbaugh MS, Perry KM, Mock BH, Zheng QH, Federici LA, Patton EA, Herring CM, et al. (2013) Striatal D(2)/D(3) receptor availability is inversely correlated with cannabis consumption in chronic marijuana users. Drug Alcohol Depend 128:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BM, Rizzo M, Block RI, Pearlson GD, O’Leary DS. (2010) Sex, drugs, and cognition: effects of marijuana. J Psychoactive Drugs 42:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. (1986) Dependence on tetrahydrocannabinol in rhesus monkeys. J Pharmacol Exp Ther 239:311–319. [PubMed] [Google Scholar]
- Bettinardi V, Pagani E, Gilardi MC, Landoni C, Riddell C, Rizzo G, Castiglioni I, Belluzzo D, Lucignani G, Schubert S, et al. (1999) An automatic classification technique for attenuation correction in positron emission tomography. Eur J Nucl Med 26:447–458. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, Arndt S, Hurtig RR, Watkins GL, Hall JA, et al. (2002) Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol Biochem Behav 72:237–250. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. (2005) Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage 26:480–492. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, Babuscio TA, Brewer JA, Potenza MN, Ball SA, Martino S, Rounsaville BJ, Lejuez CW. (2011) Cognitive function and treatment response in a randomized clinical trial of computer-based training in cognitive-behavioral therapy. Subst Use Misuse 46:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality (CBHSQ) (2016) 2015 National Survey on Drug Use and Health: Detailed Tables, pp 2, Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Copeland J, Pokorski I. (2016) Progress toward pharmacotherapies for cannabis-use disorder: an evidence-based review. Subst Abuse Rehabil 7:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R. (2013) Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev 23:117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. (2011) An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med 5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneau GA, Kaymakcalan S. (1971) Physiological and psychological dependence to synthetic delta-9-tetrahydocannabinol (THC) in rhesus monkeys. Pharmacologist 13:246. [Google Scholar]
- Dias R, Robbins TW, Roberts AC. (1996a) Dissociation in prefrontal cortex of affective and attentional shifts. Nature 380:69–72. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. (1996b) Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci 110:872–886. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman B, Ranganathan M, Bhakta S, Zimolo Z, Cooper T, et al. (2008) Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 198:587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. (2004) The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 29:1558–1572. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. (2004) Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage 23:914–920. [DOI] [PubMed] [Google Scholar]
- Farde L, Ehrin E, Eriksson L, Greitz T, Hall H, Hedström CG, Litton JE, Sedvall G. (1985) Substituted benzamides as ligands for visualization of dopamine receptor binding in the human brain by positron emission tomography. Proc Natl Acad Sci USA 82:3863–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk JE, Montgomery C. (2008) Real-world memory and executive processes in cannabis users and non-users. J Psychopharmacol 22:727–736. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, et al. (2012) Striatal dopamine D2/D3 receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci 32:7316–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Hruba L, Zaki A, Javors MA, McMahon LR. (2014) Blood levels do not predict behavioral or physiological effects of Δ9-tetrahydrocannabinol in rhesus monkeys with different patterns of exposure. Drug Alcohol Depend 139:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. (2005) Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res 23:107–118. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. (2010) Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav 35:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Ilan AB, Gevins A, Gunderson EW, Role K, Colley J, Foltin RW. (2010) Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacol Biochem Behav 96:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW. (2001) Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology 25:757–765. [DOI] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, Frampton CM. (2007) The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev 26:309–319. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Arasteh K, Stitzer ML. (1997) Comparative effects of alcohol and marijuana on mood, memory, and performance. Pharmacol Biochem Behav 58:93–101. [DOI] [PubMed] [Google Scholar]
- Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G, Mann K. (2007) Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry 61:1281–1289. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. (2007) Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry 61:31–40. [DOI] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. (2010) Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry 49:561–572, 572.e1–572.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Pennington ZT. (2014) Reward, interrupted: inhibitory control and its relevance to addictions. Neuropharmacology 76 (Pt B):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Banala AK, Newman AH, Nader MA. (2015a) Effects of buspirone and the dopamine D3 receptor compound PG619 on cocaine and methamphetamine self-administration in rhesus monkeys using a food-drug choice paradigm. Psychopharmacology (Berl) 232:1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Martin TJ, Nader MA. (2017) Behavioral determinants of cannabinoid self-administration in old world monkeys. Neuropsychopharmacology 42:1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Newman AH, Nader MA. (2015b) Differential effects of the dopamine D3 receptor antagonist PG01037 on cocaine and methamphetamine self-administration in rhesus monkeys. Neuropharmacology 92:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. (2004) Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 176:239–247. [DOI] [PubMed] [Google Scholar]
- Kangas BD, Leonard MZ, Shukla VG, Alapafuja SO, Nikas SP, Makriyannis A, Bergman J. (2016) Comparisons of Δ9-tetrahydrocannabinol and anandamide on a battery of cognition-related behavior in nonhuman primates. J Pharmacol Exp Ther 357:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinahan PE, Rogers JG. (1989) Analytic 3-D image reconstruction using all detected events. IEEE Trans Nucl Sci 36:964–968. [Google Scholar]
- Kirk JM, de Wit H. (1999) Responses to oral delta9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacol Biochem Behav 63:137–142. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. (2005) Cannabinoid tolerance and dependence. Handb Exp Pharmacol 168:691–717. [DOI] [PubMed] [Google Scholar]
- Lindgren JE, Ohlsson A, Agurell S, Hollister L, Gillespie H. (1981) Clinical effects and plasma levels of delta 9-tetrahydrocannabinol (delta 9-THC) in heavy and light users of cannabis. Psychopharmacology (Berl) 74:208–212. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834–840. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. (2003) Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology 28:1356–1365. [DOI] [PubMed] [Google Scholar]
- McMahon LR. (2011) Chronic Δ9-tetrahydrocannabinol treatment in rhesus monkeys: differential tolerance and cross-tolerance among cannabinoids. Br J Pharmacol 162:1060–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, France CP. (2003) Discriminative stimulus effects of the cannabinoid antagonist, SR 141716A, in delta -sup-9-tetrahydrocannabinol-treated rhesus monkeys. Exp Clin Psychopharmacol 11:286–293. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Manning FJ. (1978) Non-spatial memory after selective prefrontal lesions in monkeys. Brain Res 143:313–323. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Yurgelun-Todd D. (1996) The residual cognitive effects of heavy marijuana use in college students. JAMA 275:521–527. [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. (2009) Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol 23:266–277. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, van Wel JH, Spronk DB, Toennes SW, Kuypers KP, Theunissen EL, Verkes RJ. (2016) Cannabis and tolerance: acute drug impairment as a function of cannabis use history. Sci Rep 6:26843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezapour T, DeVito EE, Sofuoglu M, Ekhtiari H. (2016) Perspectives on neurocognitive rehabilitation as an adjunct treatment for addictive disorders: from cognitive improvement to relapse prevention. Prog Brain Res 224:345–369. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. (2013) Cognitive enhancement as a treatment for drug addictions. Neuropharmacology 64:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J, Marijuana Treatment Project Research Group (2002) Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 287:1123–1131. [DOI] [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. (2010) Rimonabant-induced Δ9-THC withdrawal in rhesus monkeys: discriminative stimulus effects and other withdrawal signs. J Pharmacol Exp Ther 334:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes PR, Egerton A, Watson B, Reid A, Lappin J, Howes OD, Nutt DJ, Lingford-Hughes AR. (2012) History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. J Psychopharmacol 26:144–149. [DOI] [PubMed] [Google Scholar]
- Taffe MA. (2012) Δ9Tetrahydrocannabinol impairs visuo-spatial associative learning and spatial working memory in rhesus macaques. J Psychopharmacol 26:1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. (2003) Cannabinoids: reward, dependence, and underlying neurochemical mechanisms--a review of recent preclinical data. Psychopharmacology (Berl) 169:115–34. [DOI] [PubMed] [Google Scholar]
- Theunissen EL, Kauert GF, Toennes SW, Moeller MR, Sambeth A, Blanchard MM, Ramaekers JG. (2012) Neurophysiological functioning of occasional and heavy cannabis users during THC intoxication. Psychopharmacology (Berl) 220:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. (2013) Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Crit Rev Biochem Mol Biol 48:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, Haney M, Abi-Dargham A. (2012) Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biol Psychiatry 71:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Giessen E, Weinstein JJ, Cassidy CM, Haney M, Dong Z, Ghazzaoui R, Ojeil N, Kegeles LS, Xu X, Vadhan NP, et al. (2017) Deficits in striatal dopamine release in cannabis dependence. Mol Psychiatry 22:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Gu H, Peterson ML, Sampson AR, Lewis DA. (2014) Repeated Δ9-tetrahydrocannabinol exposure in adolescent monkeys: persistent effects selective for spatial working memory. Am J Psychiatry 171:416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Liu S, Bitler EJ, Gu H, Sampson AR, Bradberry CW, Lewis DA. (2012) Delay- and dose-dependent effects of Δ9-tetrahydrocannabinol administration on spatial and object working memory tasks in adolescent rhesus monkeys. Neuropsychopharmacology 37:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. (1993) Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14:169–177. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. (1998) Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 155:344–349. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. (2012) Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol 52:321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Alexoff D, Logan J, Jayne M, Wong C, Tomasi D. (2014) Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc Natl Acad Sci USA 111:E3149–E3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Laurienti PJ, Porrino LJ. (2004) Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend 76:107–111. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. (1993) Dissociation of object and spatial processing domains in primate prefrontal cortex. Science 260:1955–1958. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Vandewater SA, Parsons LH, Taffe MA. (2013) Δ(9)Tetrahydrocannabinol impairs reversal learning but not extra-dimensional shifts in rhesus macaques. Neuroscience 235:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]