SUMMARY
Staphylococcus aureus colonization contributes to skin inflammation in diseases such as atopic dermatitis, but the signaling pathways involved are unclear. Herein, epicutaneous S. aureus exposure to mouse skin promoted MyD88-dependent skin inflammation initiated by IL-36 but not IL-1α/β, IL-18 or IL-33. By contrast, an intradermal S. aureus challenge promoted MyD88-dependent host defense initiated by IL-1β rather than IL-36, suggesting that different IL-1 cytokines trigger MyD88-signaling depending on the anatomical depth of S. aureus cutaneous exposure. The bacterial virulence factor PSMα but not α-toxin or δ-toxin contributed to the skin inflammation, which was driven by IL-17-producing γδ and CD4+ T cells via direct IL-36R-signaling in the T cells. Finally, adoptive transfer of IL-36R-expressing T cells to IL-36R-deficient mice was sufficient for mediating S. aureus-induced skin inflammation. Together, this study defines a previously unknown pathway by which S. aureus epicutaneous exposure promotes skin inflammation involving IL-36R/MyD88-dependent IL-17 T cell responses.
In Brief
Staphylococcus aureus colonization during atopic dermatitis contributes to skin inflammation, but the underlying mechanisms are unclear. Liu et al. demonstrate that epicutaneous S. aureus exposure drives skin inflammation, which is mediated by the bacterial PSMα and host IL-36R/MyD88-induced production of IL-17 by T cells.

INTRODUCTION
Staphylococcus aureus is a gram-positive extracellular bacterium that is the most common cause of skin infections in humans, resulting in 11–14 million outpatient visits and nearly 500,000 hospitalizations per year in the U.S. (Hersh et al., 2008; McCaig et al., 2006). In addition, community-acquired methicillin-resistant S. aureus (CA-MRSA) strains are causing severe skin infections in healthy people outside of hospitals, and also are responsible for more invasive and life-threatening infections such as cellulitis, pneumonia, bacteremia, endocarditis, osteomyelitis and septic shock (DeLeo et al., 2010; Tong et al., 2015).
A major risk factor for S. aureus infections is skin or mucosal colonization (Brown et al., 2014). Persistent nasal colonization is found in approximately 20–30% of individuals and is transient in up to 80% of individuals in the population (Brown et al., 2014). Interestingly, S. aureus skin colonization approaches 90% in the lesional skin of atopic dermatitis (AD) (Breuer et al., 2002), an inflammatory skin disease that affects 15–30% of children and 5% of adults. Although the precise etiology is not clear, AD is associated with a barrier defect in the skin, a dermal infiltration of T helper 2 (Th2) cells and a systemic Th2 cytokine response with elevated serum IgE and eosinophilia (Weidinger and Novak, 2016). S. aureus is linked to AD disease severity and flares (Kong et al., 2012) that have been attributed to S. aureus secreted toxins and superantigens (Ong and Leung, 2016; Spaulding et al., 2013). However, the immune signaling pathways that are activated during S. aureus epicutaneous exposure that contribute to skin inflammation are unclear.
During an S. aureus intradermal infection of the skin, a key signaling pathway involves MyD88-dependent neutrophil recruitment and abscess formation. In this context, MyD88-signaling is initiated by Nlrp3/ASC-induced inflammasome-dependent production of IL-1β and subsequent activation of IL-1R/MyD88-signaling (Miller et al., 2006; Miller et al., 2007; Munoz-Planillo et al., 2009). This IL-1β-dependent response was a more important determinant for host defense than other MyD88-dependent signals such as IL-1α (Miller et al., 2007), another activating ligand for the IL-1R, or TLR2, which recognizes S. aureus lipopeptides and lipoteichoic acid (Miller et al., 2006). Furthermore, IL-1β produced during S. aureus intradermal infection in mice also promoted IL-17A/F responses by γδ T cells, which was required for effective neutrophil recruitment and bacterial clearance (Chan et al., 2015; Cho et al., 2010; Myles et al., 2013). Nonetheless, it is unknown whether IL-1β or potentially other IL-1 family members that signal via My88 (e.g., IL-1α, IL-18, IL-33 and IL-36 isoforms) contribute to skin inflammation in response to epicutaneous S. aureus exposure.
In human AD skin, IL-1α, IL-1β, IL-18 and IL-33 have all been found to have increased expression in epidermal keratinocytes (Inoue et al., 2011; Kezic et al., 2012; Savinko et al., 2012). Regarding IL-36, loss-of-function mutations in IL-36RN, which encodes the IL-36 receptor antagonist (IL-36Ra) in humans, result in generalized pustular psoriasis, an autoimmune inflammatory skin disease with marked collections of neutrophils in the epidermis (Marrakchi et al., 2011). Subsequent studies found that IL-36 isoforms are highly expressed in human skin in pustular psoriasis and other forms of psoriasis (Johnston et al., 2016). Recently, the IL-36 isoforms, IL-36α and IL-36γ, were also found to be expressed in human AD skin (Suarez-Farinas et al., 2015).
In addition to these findings in human skin, IL-1 family members can be induced in vitro in cells exposed to S. aureus or in in vivo mouse models of S. aureus skin infections and AD-like skin inflammation. S. aureus exposure to keratinocytes in vitro resulted in IL-1α production and subsequent autocrine production of neutrophil-attracting chemokines (Olaru and Jensen, 2010). IL-1β produced during S. aureus intradermal infection in mice promoted IL-17A/F responses by γδ T cells, which was dependent on IL-1R/MyD88-signaling and was required for effective neutrophil recruitment and bacterial clearance (Chan et al., 2015; Cho et al., 2010; Myles et al., 2013). In mice, IL-18 contributed to host defense against an S. aureus burn wound infection (Kinoshita et al., 2011) and promoted skin inflammation induced by exposure to specific S. aureus virulence factors such as protein A and phenol soluble modulins (Syed et al., 2015; Terada et al., 2006). Also in mice, IL-33 contributed to host defense against an S. aureus skin infection (Li et al., 2014a; Yin et al., 2013) and induced Th2 and ILC2 responses to promote AD-like skin inflammation (Imai et al., 2013; Oyoshi et al., 2016; Salimi et al., 2013). Finally, in the imiquimod mouse model of psoriasiform dermatitis, IL-36R-signaling promoted Th17/IL-17 responses (Milora et al., 2015; Tortola et al., 2012) but the mechanism by which IL-36 might contribute to S. aureus skin infections or AD pathogenesis has yet to be determined.
Taken together, although studies from humans and mice suggest a role for IL-1 family members in S. aureus-induced skin infections or skin inflammation, the relative contribution of the IL-1 family members in promoting skin inflammation in the context of S. aureus colonization has not been evaluated. Therefore, we chose to investigate MyD88-dependent immune mechanisms and the role of the different IL-1 family members during skin inflammation using an in vivo mouse model of epicutaneous S. aureus exposure.
RESULTS
MyD88-deficient mice develop decreased skin inflammation after epicutaneous S. aureus exposure
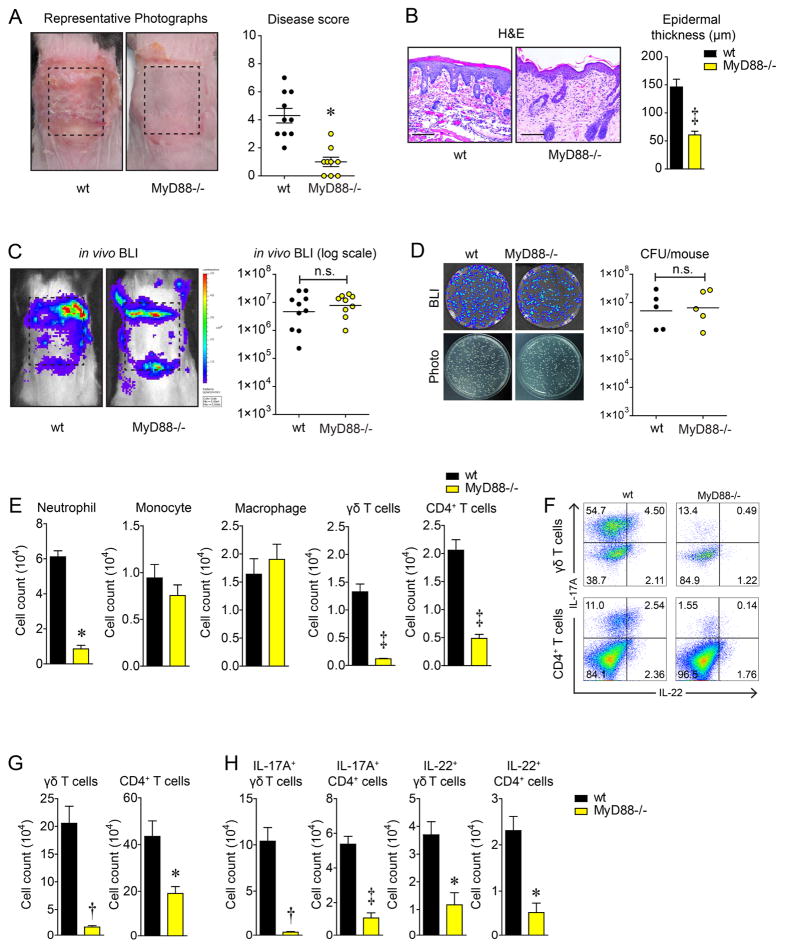
To evaluate a role for MyD88-signaling in response to S. aureus epicutaneous exposure, a gauze pad soaked with a bioluminescent community-acquired methicillin-resistant S. aureus strain (1×108 CFU/100 μL) was secured on the dorsal skin of wt and MyD88−/− mice for 7 days as previously described (Nakamura et al., 2013). After the gauze pad was removed, wt mice had severely inflamed erythematous scaly skin, whereas MyD88−/− mice had markedly less skin inflammation and lower skin disease scores (Fig. 1A). By histology, the skin of wt mice exhibited parakeratosis, increased epidermal thickness with spongiosis and a dermal inflammatory infiltrate, which were all substantially reduced in MyD88−/− mice (Fig. 1B). To measure bacterial burden on day 7 after epicutaneous S. aureus exposure, the skin was scraped with metal forceps to remove non-adherent bacteria and in vivo whole animal bioluminescence imaging (BLI) was performed, which detects light emitted only from live and actively metabolizing S. aureus bacteria. In addition, the entire affected back skin was homogenized and CFU were counted after overnight culture. Although both techniques cannot distinguish between adherent bacteria on the skin surface versus bacteria invading into the skin, there was no significant differences with in vivo BLI signals or ex vivo CFU between MyD88−/− and wt mice (Fig. 1C,D), indicating that decreased skin inflammation in MyD88−/− mice was not due to any difference in the degree of S. aureus bacterial burden.
Figure 1.
MyD88−/− mice develop decreased skin inflammation after epicutaneous S. aureus exposure. Wt and MyD88−/− mice were epicutaneously challenged with S. aureus (1×108 CFU) on the dorsal skin for 7 days. (A) Representative skin photographs and mean disease score ± s.e.m. (B) Representative histology (hematoxylin and eosin [H&E] stain, 200× magnification) and mean epidermal thickness ± s.e.m. (C) Representative in vivo bioluminescent imaging (BLI) signals and total flux (photons/s) (log10 scale). (D) Representative bacterial culture plates after overnight culture ± BLI and CFU (log10 scale). (E) Mean cell number ± s.e.m. from the skin as measured by FACS. (F) Representative flow plots and (G,H) mean cell number ± s.e.m. of total and IL-17A- and IL-22-producing T cells from dLNs as measured by FACS. *P<0.05, †P<0.01, ‡ P<0.001 (MyD88−/− mice versus wt mice) as calculated by two-tailed Student’s t-test. n.s. = not significant. Results are representative of at least 2 independent experiments.
Next, to ascertain whether wt and MyD88−/− mice exposed to epicutaneous S. aureus had differences in recruitment of myeloid cells and T cells to the skin (which are increased in AD patients (Weidinger and Novak, 2016)), FACS was performed on cells from the inflamed skin (Fig. 1E). Wt mice had significantly higher numbers of neutrophils, γδ T cells and CD4+ T cells in the skin than MyD88−/− mice while the numbers of monocytes and macrophages were similar. FACS analysis of draining lymph nodes (dLNs) revealed that wt mice had markedly increased numbers of γδ T cells and CD4+ T cells (Fig. 1F,G) and especially IL-17A- and IL-22-producing γδ T cells and CD4+ T cells, which were greatly diminished in MyD88−/− mice (Fig. 1H). Taken together, although the bacterial burden on the skin was similar, MyD88−/− mice had substantially decreased skin inflammation and fewer T cells (especially IL-17A- and IL-22-producing γδ and CD4+ T cells) compared with wt mice.
Skin inflammation is mediated by MyD88-signaling in T cells but not in keratinocytes or myeloid cells
To identify the cell types responsible for mediating the MyD88-dependent skin inflammation, S. aureus epicutaneous exposure was performed on cre/lox mice with deletion of MyD88 in keratinocytes (K14-creERT2×MyD88fl/fl mice), myeloid cells (LysM-cre×MyD88fl/fl mice) or T cells (Lck-cre×MyD88fl/fl mice). Notably, only the Lck-cre×MyD88fl/fl mice had decreased skin inflammation, disease scores and epidermal thickness compared with wt mice (Fig. 2A–C), indicating an essential role for MyD88-signaling in T cells but not in keratinocytes or myeloid cells in contributing to skin inflammation. FACS analysis of the lesional skin showed that Lck-cre×MyD88fl/fl mice had decreased recruitment of neutrophils, monocytes, and CD4+ T cells, while no differences in macrophages or γδ T cells compared with wt mice (Fig. S1A). In the dLNs, Lck-cre×MyD88fl/fl mice had decreased numbers of both total and especially IL-17A- and IL-22-producing γδ T cells and CD4+ T cells (Fig. S1B,C), similar to that seen in MyD88−/− mice (Fig. 1G,H). Collectively, these data suggest epicutaneous S. aureus exposure induced skin inflammation by initiating MyD88-signaling in T cells.
Figure 2.
IL-36R/MyD88 signaling by T cells mediates S. aureus-induced skin inflammation. Mice were epicutaneously challenged with S. aureus (1×108 CFU) on the dorsal skin for 7 days. (A) Representative skin photographs for wt versus K14CreER×MyD88fl/fl (K14-MyD88−/−), LysMCre×MyD88fl/fl (LysM-MyD88−/−), LckCre×MyD88fl/fl (Lck-MyD88−/−) mice, and MyD88−/− mice. (B) Mean disease score ± s.e.m. (C) Mean epidermal thickness ± s.e.m. (D,E) Wt C57BL/6 versus IL-1α−/−, IL-β−/−, IL-18R1−/−, and IL-36R−/− mice mean disease score ± s.e.m. and mean epidermal thickness ± s.e.m. (F,G) Wt Balb/c versus IL-33−/− mice mean disease score ± s.e.m. and mean epidermal thickness ± s.e.m. *P<0.05, †P<0.01, ‡ P<0.001 as calculated by two-tailed Student’s t-test. Results are representative of at least 2 independent experiments. See also Figure S1 and S2.
IL-36R is the primary MyD88-dependent signal that promotes skin inflammation after S. aureus epicutaneous exposure
To determine which MyD88-dependent signal that was responsible for mediating the skin inflammation, S. aureus epicutaneous exposure was performed on IL-1α−/−, IL-1β−/−, IL-18R1−/−, IL-33−/− and IL-36R−/− mice. IL-1α−/−, IL-1β−/−, and IL-18R1−/− mice had disease scores and epidermal thickness similar to wt C57BL/6 control mice (Fig. 2D,E). IL-33−/− mice (which were in a Balb/c background) also had similar disease scores and epidermal thickness as wt Balb/c control mice (Fig. 2F,G), although higher skin disease scores were observed in Balb/c than C57BL/6 mice (Fig. 2D,F). In contrast, IL-36R−/− mice had markedly reduced skin inflammation, with significantly decreased disease scores and epidermal thickness compared with wt C57BL/6 mice (Fig. 2D,E), similar to that seen in MyD88−/− mice (Fig. 1A,B). These results suggest that IL-36 was the predominant MyD88-dependent cytokine that promoted skin inflammation in response to S. aureus epicutaneous exposure. Since IL-36R/MyD88-signaling by T cells was required for skin inflammation, Q-PCR was performed to determine whether there were any differences in mRNA levels of IL-36α, IL-36R or MyD88 from the inflamed skin or from CD3+ T cells isolated from day 7 dLNs from wt, Lck-cre×MyD88fl/fl and IL-36R−/− mice (Fig. S2A,B). IL-36R−/− mice had no differences in the expression of IL-36α or MyD88 in skin or T cells compared with wt mice and there were no differences in MyD88 expression in the skin among all three mouse strains (and as expected decreased MyD88 expression on T cells in Lck-cre×MyD88fl/fl mice). IL-36α was not expressed in the T cells and as expected IL-36R was not expressed in IL-36R−/− mice. Interestingly, although Lck-cre×MyD88fl/fl mice had decreased skin inflammation, they had markedly increased expression of IL-36α and IL-36R in the inflamed skin compared with wt mice. Taken together, these results suggest that decreased skin inflammation in Lck-cre×MyD88fl/fl mice or IL-36R−/− mice was a result of T cell-intrinsic IL-36R/MyD88-signaling rather than through the activity of other cells expressing IL-36R or MyD88.
PSMα contributes to skin inflammation during S. aureus epicutaneous exposure
Next, a PBS-soaked sterile gauze pad was compared with a S. aureus-soaked gauze pad to determine whether the skin inflammation was due to the skin occlusion in the absence of bacteria or skin occlusion in the presence of bacteria. The PBS-soaked sterile gauze resulted in virtually no skin inflammation with a negligible disease score and epidermal thickness compared with the epicutaneous S. aureus exposure (Fig. 3A–C). To identify whether S. aureus secreted toxins contributed to the skin inflammation, wt mice were epicutaneously challenged with different toxin mutant S. aureus (USA300 LAC) strains, including Δδ-toxin, Δα-toxin, and Δphenol-soluble modulin α (PSMα) mutants. Only ΔPSMα had significantly decreased disease scores and epidermal thickness compared with the S. aureus parent strain (Fig. 3D–F), suggesting that PSMα contributed to skin inflammation in response to S. aureus epicutaneous exposure. In addition, we evaluated whether the skin inflammation could be induced by S. epidermidis by applying a S. epidermidis-soaked gauze pad at the same inoculum as the S. aureus (1×108 CFU). S. epidermidis resulted in significantly reduced skin inflammation compared with S. aureus (Fig. 3G–I). This result is similar to human AD patients in which a predominance of skin colonization with either S. aureus or S. epidermidis correlated with more or less disease severity, respectively (Byrd et al., 2017).
Figure 3.
PSMα plays major role in mediating skin inflammation during S. aureus epicutaneous exposure. Wt C57/BL6 mice were epicutaneously challenged with PBS, S. aureus USA300 LAC parent, δ-toxin, α-toxin, PSMα mutant (Δ) (1×108 CFU) strains, and S. epidermidis (1×108 CFU) on dorsal skin for 7 days. (A,D,G) Representative skin photographs. (B,E,H) Mean disease score ± s.e.m. (C,F,I). Representative histology (hematoxylin and eosin [H&E] stain, 200× magnification) and mean epidermal thickness ± s.e.m. *P<0.05, ‡ P<0.001 as calculated by two-tailed Student’s t-test. Results are representative of at least 2 independent experiments.
Differential triggering of MyD88-signaling is dependent upon the depth of exposure to S. aureus in the skin
It was previously reported that in response to an S. aureus intradermal infection, IL-1β−/− mice developed larger skin lesions with increased bacterial burden (Miller et al., 2007). Thus, to evaluate whether IL-36R−/− mice also had impaired host defense against a S. aureus intradermal (i.d.) infection, IL-1β−/−, IL-36R−/−, and wt mice were inoculated via i.d. injection with a bioluminescent community-acquired methicillin-resistant S. aureus strain (USA300 LAC::lux). The skin lesion sizes (Fig. 4A,B) and in vivo BLI signals (Fig. 4C,D) were monitored for 14 days. As expected, IL-1β−/− mice developed larger lesions with higher in vivo BLI signals than wt mice. By contrast, IL-36R−/− mice had lesion sizes and in vivo BLI signals that closely resembled wt mice.
Figure 4.
The trigger for MyD88-signaling is different dependent upon the depth of exposure to S. aureus in the skin. Mice were infected with S. aureus (3×107 CFU) through i.d. injection on dorsal skin, and the skin lesion and bacterial burden were monitored for 14 days in wt versus IL-β−/− and IL-36R−/− mice. (A,B) Representative skin photographs and quantitative total lesion area (cm2) ± s.e.m. (C,D) Representative in vivo bioluminescent imaging (BLI) signals and mean total flux (photons/s) ± s.e.m. (log10 scale). ‡ P<0.001 (IL-β−/− mice versus wt mice) as calculated by two-way ANOVA. (E,F) Mean levels of mRNA in arbitrary units (A.U.) ± s.e.m. of IL-1β and IL-36α from skin tissue homogenates at 0, 3, 7 days after S. aureus epicutaneous exposure (1×108 CFU) or intradermal infection (3×107 CFU). *P<0.05, ‡ P<0.001 as calculated by two-tailed Student’s t-test. Results are representative of at least 2 independent experiments.
To determine whether the compartmentalization of IL-36 and IL-1β responses in epicutaneous and i.d. models, respectively, were due to differential cytokine expression, mRNA levels of IL-36α and IL-1β were compared. In the i.d. model, IL-1β was induced more than 10-fold higher than epicutaneous model during days 3 and 7 (Fig. 4E). IL-36α was induced at similar levels on day 3 in both models whereas IL-36α levels increased in the i.d. infection and decreased to baseline in epicutaneous model on day 7 (Fig. 4F). Collectively, the greatly increased expression of IL-1β provides an explanation for the more predominant role for IL-1β in the i.d. infection model. In contrast, the predominant role for IL-36α in the epicutaneous model might be due to the anatomical and functional activity of IL-36α rather than its overall expression in the skin.
IL-36R-deficient mice have impaired IL-17 and IL-22 responses
Since IL-36R-signaling was found to mediate skin inflammation following S. aureus epicutaneous exposure, induction of mRNA levels for IL-36α, IL-36β and IL-36γ, the ligands to IL-36R, were evaluated in inflamed skin after S. aureus epicutaneous exposure (Fig. 5A). Of note, mRNA levels of IL-36α had the highest induction in both IL-36R−/− and wt mice, whereas there was very little induction of IL-36β and IL-36γ, suggesting that IL-36α was the primary IL-36R ligand that mediated the IL-36R/MyD88-dependent skin inflammation. To determine the cellular source for IL-36α production, immunofluorescence labeling of day 7 wt mouse skin was performed (Fig. 5B). IL-36α was localized to the cytoplasm of the keratinocytes in the epidermis, suggesting keratinocytes are the major cellular source. There was also baseline IL-36α expression in the epidermis of naïve mouse skin (day 0), suggesting there might be some constitutive IL-36α expression in keratinocytes. In contrast, during a S. aureus i.d. infection, we previously reported that a predominant cellular source of IL-1β were the recruited neutrophils, which was required to amplify the neutrophil recruitment response (Cho et al., 2012). Taken together, our current and prior results indicate that there is anatomical compartmentalization of IL-36α and IL-1β activity, depending on whether the S. aureus challenge was epicutaneous or intradermal, respectively.
Figure 5.
IL-36R−/− mice have impaired IL-17A and IL-22 T cell responses. Wt and IL-36R−/− mice were epicutaneously challenged with S. aureus (1×108 CFU) on the dorsal skin for 7 days. (A) mRNA levels of IL-36α, IL-36β, and IL-36γ relative to β-actin, on day 3 and 7 after S. aureus challenge, measured by RT-PCR ± s.e.m. (B) Representative immunofluorescence of skin sections labeled with anti-IL-36α (green, upper panels) and merged with DAPI (blue, lower panels), 400× magnification. Dashed line = dermoepidermal junction. (C–E) Protein array analysis of IL-17A, IL-22, IFN-γ, IL-13, and IL-33 protein concentration ± s.e.m. in wt and IL-36R−/− mice lesional skin. (F–I) Representative flow plots and mean cell number ± s.e.m. of total and IL-17A-, IL-22-producing T cells from dLNs as measured by FACS. *P<0.05, †P<0.01, ‡ P<0.001 (IL-36R−/− mice versus wt mice) as calculated by two-tailed Student’s t-test. n.s. = not significant. Results are representative of at least 2 independent experiments.
Since MyD88-signaling in T cells was found to be critical for mediating skin inflammation, protein levels of Th17, Th1 and Th2 cytokines were determined in the skin of IL-36R−/− and wt mice on day 7 after of S. aureus epicutaneous exposure (Fig. 5C–E). IL-36R−/− mice had significantly decreased IL-17A, IL-22 and IFN-γ levels but no differences in IL-13 or IL-33 compared with wt mice (IL-4 protein levels were undetectable, data not shown). In the dLNs, IL-36R−/− mice had decreased total numbers of γδ T cells but not CD4+ T cells and substantial decreases in the numbers of IL-17A-producing γδ T cells and CD4+ T cells compared with wt mice (Fig. 5F–I), similar to MyD88−/− mice (Fig. 1F–H) and Lck-cre×MyD88fl/fl mice (Fig. S1B,C). Unlike MyD88−/− mice and Lck-cre×MyD88fl/fl mice, IL-22-producing γδ T cells and CD4+ T cells were not decreased in IL-36R−/− mice compared with wt mice. Thus, IL-36R/MyD88 signaling was essential for primarily inducing IL-17A in inflamed skin and for the generation of IL-17A-producing T cells in the dLNs.
IL-17A/F-deficient mice develop decreased skin inflammation after epicutaneous S. aureus exposure
To determine whether IL-17 and/or IL-22 had any impact on skin inflammation, S. aureus epicutaneous exposure was performed on IL-17A/F double knockout mice (IL-17A/F−/− mice) and IL-22−/− mice. IL-17A/F−/− mice but not IL-22−/− mice had decreased skin inflammation as measured by disease scores and epidermal thickness compared with wt mice (Fig. 6A,B), which were nearly identical to MyD88−/− mice, Lck-cre×MyD88fl/fl mice and IL-36R−/− mice. To determine the cellular sources for IL-17A, intracellular FACS analysis was performed on cells isolated from the inflamed skin and dLNs of wt mice on day 7 after epicutaneous S. aureus exposure. The predominant IL-17A-producing cells were γδ and CD4+ T cells compared with the very low production of IL-17A in CD8+ T cells, ILC3s, B cells, NK cells and CD11b+ myeloid cells (Fig. 6C,D), indicating that γδ and CD4+ T cells were the major sources of IL-17A.
Figure 6.
IL-17A/F−/− mice have decreased skin inflammation after epicutaneous S. aureus exposure. Mice were epicutaneously challenged with S. aureus (1×108 CFU) on the dorsal skin for 7 days in wt versus IL-17A/F−/− and IL-22−/− mice. (A) Mean disease score ± s.e.m. (B) Mean epidermal thickness ± s.e.m. (C,D) Mean cell number ± s.e.m. of IL-17A-producing cells including γδ T cells, CD4+ T cells, CD8+ T cells, ILC3s (CD45+ CD127+ RORγt+, Lin−), B cells (B220+), NK cells (NK1.1+), and myeloid cells (CD11b+) from skin and dLNs as measured by FACS. *P<0.05 (IL-17A/F−/− mice versus wt mice) as calculated by two-tailed Student’s t-test. Results are representative of at least 2 independent experiments.
IL-36α directly induces T cell production of IL-17A
Previous studies have found that IL-36R-signaling can influence T cell responses by either directly activating T cells (Gresnigt et al., 2013; Vigne et al., 2011; Vigne et al., 2012) or indirectly by activating dendritic cells (DCs) (Foster et al., 2014; Tortola et al., 2012; Vigne et al., 2011). To evaluate the mechanism by which IL-36R-signaling promoted IL-17 T cell responses, DCs from naïve IL-36R−/− or wt mice were pulsed with heat-killed S. aureus and then cultured with total CD3+ T cells from dLNs of naïve IL-36R−/− or wt mice ± exogenous recombinant murine IL-36α (rIL-36α). Cultures were continued for 3 days, PMN/ionomycin was added for the last 4 hours, and intracellular FACS was performed. Significantly increased total numbers of γδ and CD4+ T cells and IL-17A-producing γδ T cells were seen in cultures containing T cells from wt mice compared with IL-36R−/− mice, and the presence of rIL-36α further increased the numbers of these T cells from wt mice but not from IL-36R−/− mice (Fig. 7A,B). This effect of rIL-36α even occurred in the absence of heat-killed S. aureus but to a lesser extent (Fig. S4). Whether the cultures contained DCs from IL-36R−/− or wt mice had little or no impact on the numbers of T cells (Fig. 7A,B). Combined, these data indicate that IL-36R-signaling had a direct effect on the T cells rather than DCs to induce IL-17A production.
Figure 7.
IL-36R-signaling by T cells directly promotes IL-17A production and skin inflammation. Naïve CD3+ T cells purified from wt or IL-36R−/− mice skin dLNs were co-cultured in vitro for 72h with CD11c+ DCs from naïve wt or IL-36R−/− mice pulsed with heat-killed S. aureus ± exogenous IL-36α. (A,B) Representative flow plots and mean cell number ± s.e.m. of total and IL-17A-, IL-22-producing T cells in the in vitro culture as measured by FACS. (C) 3×106 naïve CD3+ T cells purified from wt or IL-36R−/− mice skin dLNs were adoptively transferred into wt or IL-36R−/− mice; after 7 days of rest, mice were colonized with S. aureus (1×108 CFU) for 7 days. Mean disease score ± s.e.m., mean epidermal thickness ± s.e.m. and lesional skin IL-17A protein concentration ± s.e.m. *P<0.05, †P<0.01, ‡ P<0.001 as calculated by two-tailed Student’s t-test. n.s. = not significant. Results are representative of at least 2 independent experiments. See also Figure S3 and S4.
Adoptively-transferred wt T cells restore skin inflammation in IL-36R-deficient mice
To determine whether the T cell-intrinsic IL-36R-signaling was relevant in vivo, total lymph node-derived naïve T cells (3×106 cells) from IL-36R−/− and wt mice (which had similar percentages of γδ and CD4+ T cells) (Fig. S3) were adoptively transferred to IL-36R−/− mice 1 week prior to S. aureus epicutaneous exposure. T cells transferred from wt but not IL-36R−/− mice led to a partial restoration of S. aureus-induced skin inflammation as measured by skin disease scores, epidermal thickness and skin IL-17A levels (Fig. 7C). These results suggest that cell-intrinsic IL-36R-signaling in T cells was sufficient to mediate skin inflammation in vivo in response to epicutaneous S. aureus exposure.
DISCUSSION
S. aureus colonization and impetiginization of the skin contribute to the exacerbation of skin inflammation in diseases such as AD, which has previously been attributed to the activity of S. aureus toxins and superantigens (Ong and Leung, 2016; Spaulding et al., 2013). In addition, studies in humans and mice have suggested that IL-1 family members (i.e., IL-1α, IL-18, IL-33 and IL-36), which all signal via MyD88, have increased expression in the skin of individuals with AD (Inoue et al., 2011; Kezic et al., 2012; Savinko et al., 2012; Suarez-Farinas et al., 2015) or contribute to host defense against S. aureus skin infections (Kinoshita et al., 2011; Li et al., 2014a; Miller et al., 2007; Yin et al., 2013). However, a link between IL-1 family members and S. aureus colonization in contributing to skin inflammation has not been demonstrated. In this study, we report that IL-36R/MyD88-signaling and subsequent IL-17 T cell responses were required for promoting skin inflammation after S. aureus epicutaneous challenge, whereas IL-1α/β, IL-18 and IL-33 were not involved. T cells but not keratinocytes or myeloid cells were the primary cell type that utilized MyD88-signaling to induce the skin inflammation. The skin inflammation was in part due to the activity of the S. aureus toxin PSMα but not α-toxin or δ-toxin. Furthermore, the increased skin inflammation was dependent upon IL-17 production by γδ and CD4+ T cells. Finally, production of IL-17 was due to direct IL-36R/MyD88-signaling by the T cells rather than indirectly through DCs. Importantly, the direct role of IL-36R-signaling on T cells was confirmed in vivo, as skin inflammation was restored after adoptive transfer of IL-36R-expressing T cells into IL-36R-deficient mice. Taken together, our results suggest a model by which epicutaneous S. aureus exposure promoted IL-36α production by keratinocytes (in part via the activity of PSMα), which triggered IL-36R/MyD88-signaling on T cells to produce IL-17A/F that mediated skin inflammation.
The finding that epicutaneous S. aureus exposure induces skin inflammation by triggering IL-36R/MyD88-signaling and responses defines a mechanism by which bacteria on the surface of the skin can promote effector T cell responses. Our findings provide an explanation and functional role for the increased IL-36α and IL-36γ transcripts in human skin of AD patients (Suarez-Farinas et al., 2015) and for the increased numbers of Th17 cells and IL-17 cytokine expression in the skin and blood from human patients with acute AD (Koga et al., 2008; Toda et al., 2003), especially compared with chronic AD, which has smaller increases in Th17 cells (Gittler et al., 2012; Nograles et al., 2009). The role of IL-36 could also be relevant beyond AD. For example, increased S. aureus colonization is found in lesional skin of individuals with psoriasis (Ng et al., 2017), a disease in which IL-36 contributes to IL-17 responses (Tortola et al., 2012). Finally, IL-36 could have similar activity as IL-1α in inducing IL-17 T cell responses during homeostasis, as was previously reported to occur after exposure of germ-free mice to epicutaneous S. epidermidis (Naik et al., 2012).
Another interesting finding was that different IL-1 cytokines triggered MyD88-signaling depending on the anatomical depth of S. aureus cutaneous exposure. IL-36 mediated skin inflammation in response to an epicutaneous S. aureus exposure, whereas IL-1β mediated host defense in response to an i.d. S. aureus infection. These differences are likely due to the predominant cells that recognized and responded to S. aureus. In epicutaneous S. aureus exposure, keratinocytes were most likely activated and they are a known source of IL-36 isoforms (Carrier et al., 2011; Johnston et al., 2016; Li et al., 2014b; Milora et al., 2015; Tortola et al., 2012). In contrast, during intradermal S. aureus exposure, IL-1β was primarily induced by myeloid cells, especially recruited neutrophils, which amplified the neutrophil recruitment response and host defense (Cho et al., 2012). Furthermore, the IL-1 cytokines have different functional activity in the skin. For example, in the imiquimod mouse model of psoriasiform dermatitis, IL-36 contributed to acanthosis, and IL-1R activation mainly contributed to neutrophil recruitment (Tortola et al., 2012). Finally, prior work found that IL-36 induced IL-1α to contribute to skin inflammation in the imiquimod mouse model of psoriasiform dermatitis (Milora et al., 2015), whereas IL-1α did not have a major role following S. aureus epicutaneous exposure (Fig. 2D,E) or i.d. infection (Miller et al., 2007), suggesting that there might be differences in the role of IL-1α, IL-36 and likely other IL-1 family members depending on the stimulus.
Prior work has found the IL-36 isoforms can activate keratinocytes (Carrier et al., 2011; Li et al., 2014b; Milora et al., 2015), DCs (Foster et al., 2014; Tortola et al., 2012; Vigne et al., 2011) and T cells (Gresnigt et al., 2013; Vigne et al., 2011; Vigne et al., 2012). However, we found that IL-36R/MyD88-signaling by T cells was critical for inducing skin inflammation whereas IL-36R/MyD88-signaling by keratinocytes and DCs was dispensable. These findings do not rule out a contribution for IL-36R-signaling on keratinocytes, DCs or other cells but IL-36R/MyD88-signaling by T cells was both necessary and sufficient to mediate the skin inflammation induced by S. aureus epicutaneous exposure.
It should be mentioned that we did not find that the Δδ-toxin S. aureus mutant strain resulted in decreased skin inflammation as was previously reported in a similar model of epicutaneous S. aureus exposure (Nakamura et al., 2013). A potential explanation for this difference is that the prior study performed tape-stripping of the skin before applying the S. aureus-soaked gauze pad. This likely caused a greater skin barrier defect that allowed the δ-toxin to penetrate into the dermis to cause the degranulation of mast cells that contributed to the skin inflammation (Nakamura et al., 2013). In contrast, in the present study, tape stripping was not performed prior to applying the S. aureus-soaked gauze pad.
There are also some limitations. First, there is no mouse model that fully recapitulates all the features of human AD. In our epicutaneous S. aureus exposure model, IL-4 protein expression was not detected in the inflamed skin. However, there was increased IL-4 mRNA in dLNs and increased serum IgE levels (data not shown), which was similar to the previously published model of S. aureus epicutaneous exposure (Nakamura et al., 2013). It is also plausible that the IL-36/MyD88-signaling pathway could be a more general mechanism for skin inflammation following epicutaneous exposure to pathogenic bacteria. Second, mice possess a population of epidermal population of Vγ5high dendritic epidermal T cells (DETCs) that are not present in human skin. However, DETCs do not produce IL-17 (Cai et al., 2011; Gray et al., 2011; Sumaria et al., 2011) and it is more likely that circulating Vγ4+ γδ T cells contributed to the skin inflammation because they are rapidly recruited to sites of inflammation and infection and can produce IL-17 (Cai et al., 2011; Gray et al., 2011; Mabuchi et al., 2011; Pantelyushin et al., 2012; Ramirez-Valle et al., 2015; Sumaria et al., 2011). In addition, adoptive transfer T cells from lymph nodes rescued the skin inflammation in IL-36R−/− mice. Our findings with γδ T cells might better translate to humans because circulating Vγ9/Vδ2 and Vδ1+ γδ T cell populations in human blood have been increasingly recognized to participate in the immune response to pathogens (Davey et al., 2017; Ravens et al., 2017). Finally, in response to a deeper subcutaneous S. aureus challenge in mice, adipocytes produced the antimicrobial peptide CRAMP to promote bacterial clearance, but a role for IL-36 or other IL-1 family members was not evaluated (Zhang et al., 2015). This will be the subject of our future research.
In conclusion, IL-36R/MyD88-dependent IL-17 T cell responses were identified as a key pathway in contributing to skin inflammation in response to epicutaneous S. aureus exposure. These findings provide a previously unknown mechanism by which bacterial colonization of the skin surface promotes cutaneous immune responses, which is likely relevant to inflammatory skin diseases such as AD and psoriasis and potentially during homeostatic conditions. Finally, IL-36 could serve as a therapeutic target to reduce skin inflammation in AD and other inflammatory skin diseases associated with increased S. aureus skin colonization.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Lloyd Miller (lloydmiller@jhmi.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Bacterial strains
The bioluminescent Staphylococcus aureus LAC4303 strain (also designated SAP430) was used in all experiments with the epicutaneous S. aureus exposure model and the bioluminescent USA300 LAC::lux strain was used in all experiments with the intradermal S. aureus infection. LAC4303 had brighter bioluminescent signals than USA300 LAC::lux and had improved detection in the epicutaneous S. aureus exposure model. Both strains were derived from the parent S. aureus strain USA300 LAC, a well-described community-acquired methicillin-resistant S. aureus (CA-MRSA) clinical isolate that was obtained from an S. aureus skin and soft tissue infection outbreak in the Los Angeles County (LAC) Jail. Both LAC4303 and USA300 LAC::lux possess a modified luxABCDE operon from the bacterial insect pathogen, Photorhabdus luminescens (Plaut et al., 2013; Thurlow et al., 2011). USA300 LAC::lux was previously generated (Thurlow et al., 2011). LAC4303 was generated from S. aureus strain JE2 (BEI Resources), which is the LAC strain but cured of its native plasmids (Fey et al., 2013), by integrating plasmid pRP1195 into the chromosome a previously described (Plaut et al., 2013). S. aureus PSMα and δ-toxin mutant strains (engineered from LAC) were kindly provided by Dr. Michael Otto (NIH/NIAID). The S. aureus α-toxin mutant strain was derived by transposon mutagenesis of JE2 and was obtained from BEI Resources. The bioluminescent Staphylococcus epidermidis Xen43 strain (PerkinElmer, Hopkinton, MA) used in the epicutaneous S. epidermidis exposure model was previously derived from S. epidermidis 1457, a clinical isolate from an infected central venous catheter that has established biofilm-producing activity as previously described (Vuong et al., 2008).
Bacterial preparation
S. aureus and S. epidermidis bacteria were streaked onto a tryptic soy agar (TSA) plate (tryptic soy broth [TSB] plus 1.5% bacto agar (BD Biosciences) and grown overnight at 37°C in a bacterial incubator. Single colonies were picked and cultured in TSB at 37°C in a shaking incubator (240 rpm) overnight (18 h), followed by a 1:50 subculture at 37°C for 2 h to obtain mid-logarithmic phase bacteria. The bacteria were pelleted, washed 3 times, and resuspended in sterile PBS at a concentration of 1×108 CFU/100 μL. The absorbance (A600) was measured to estimate the number of CFU, which was verified after overnight culture on TSA plates.
Mice
Age- and gender-matched male and female 6–8 week-old mice on a C57BL/6 background or on a Balb/c background (i.e., IL-33-deficient mice and wt Balb/c mice) were used in all experiments. The mice were co-housed with 3–5 mice per cage in all experiments. K14-creERT2, LysM-cre and Lck-cre mice were crossed with the MyD88fl/fl to obtain cell-specific deletion of MyD88 in keratinocytes, myeloid cells and T cells, respectively. The K14-creERT2×MyD88fl/fl mice were treated systemically with tamoxifen (1 mg/mL in 100 μl of sunflower oil i.p.) for 5 consecutive days and rested for 2 weeks prior to use. Deletion of MyD88 was confirmed by performing QPCR on skin tissue from the K14-creERT2×MyD88fl/fl mice ± tamoxifen with specific primers for the constitutive MyD88 floxed gene and the cre/lox recombined MyD88 gene (Transnetyx). All mouse strains were bred and maintained under the same specific pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care (AAALAC)-accredited animal facility at Johns Hopkins University and housed according to procedures described in the Guide for the Care and Use of Laboratory Animals. All animal experiments were approved by the Johns Hopkins University Animal Care and Use Committee. The IL-36R−/− mice (Amgen, Inc.) and IL-33−/− mice (MedImmune, LLC) were obtained via an MTA between Johns Hopkins University School of Medicine and the respective entities.
Mouse model of epicutaneous bacteria exposure
A previously described mouse model of epicutaneous S. aureus exposure was used (Nakamura et al., 2013). Briefly, the dorsal skin of anesthetized mice (2% isoflurane) were shaved and depilated (Nair cream). A 100 μL volume of PBS containing 1×108 CFU of USA300 LAC4303 or S. epidermidis Xen 43 was placed on a sterile gauze pad (1×1cm) and attached to the shaved skin with transparent bio-occlusive dressing (Tegaderm; 3M), and secured with 2 layers of adhesive bandages (BAND-AID, Johnson and Johnson) for 7 days. The severity of skin inflammation was assessed from digital photographs by a blinded observer and was quantified using a total disease score, which is the sum of the individual grades for erythema, edema, erosion and scaling, where each graded as: 0 (none), 1 (mild), 2 (moderate) or 3 (severe).
Mouse model of S. aureus intradermal infection
A previously described mouse model of S. aureus intradermal (i.d.) infection was used (Cho et al., 2012). Briefly, the dorsal skin of anesthetized mice (2% isoflurane) was shaved and a 100 μL volume of 3×107 CFU of USA300 LAC::lux was injected intradermally. Digital photographs of the backs of anesthetized mice (2% isoflurane) and total lesion area (cm2) measurements were analyzed using the image analysis software program Image J (http://imagej.nih.gov/ij/) with a millimeter ruler as a reference.
METHOD DETAILS
In vivo bioluminescent imaging (BLI)
In vivo bioluminescent imaging was performed on anesthetized mice (2% isoflurane) using a Lumina III IVIS (PerkinElmer) and total flux (photons/s) was measured within a 1×103 pixel square region of interest using Living Image software (PerkinElmer; limit of detection: 2×104 photons/s). Prior to imaging, the skin was scraped with metal forceps to remove non-adherent bacteria. The bioluminescent signals detected from infected skin using S. aureus strains LAC4303 (epicutaneous exposure) and USA300 LAC::lux (i.d. infection) closely approximated the actual bacterial burden measured by ex vivo CFU counting (Fig. 1C,D) and as previously described (R2 = 0.9996) (Guo et al., 2013).
Ex vivo CFU enumeration
Skin homogenates were obtained by performing cutting a 2×2 cm skin biopsy specimen that included the area where BLI signals were visualized and bacteria were isolated after homogenization (Pro200 Series homogenizer; Pro Scientific) in PBS. Ex vivo CFU were counted after plating serially diluted skin tissue homogenates overnight on TSA plates.
Measurement of cytokine levels
Skin homogenates were obtained by performing a 10-millimeter lesional skin punch biopsy and homogenizing each specimen (Pro200 Series homogenizer; Pro Scientific) in the Reporter Lysis buffer (Promega) containing protease inhibitor cocktail tablets (Roche Life Sciences) at 4°C. The concentrations of multiple cytokines were determined by a Bio-Plex Pro Mouse Cytokine 23-plex assay and Bio-Plex Pro Mouse Th17 Panel (Bio-Rad), according to the manufacturer’s recommendations. Specific cytokine ELISAs were performed to confirm the protein array data.
Histology
Skin punch biopsy specimens (10-millimeter) were collected, fixed in formalin (10%) and embedded in paraffin. Sections (4 μm) were mounted onto glass slides and stained with hematoxylin and eosin (H&E) by the Johns Hopkins Reference Histology Laboratory, according to guidelines for clinical samples. To measure epidermal thickness, at least 10 epidermal thickness measurements per mouse were averaged from images taken at 200× magnification (Leica, DFC495) using ImageJ software.
Immunofluorescence and fluorescence microscopy
Immunofluorescence and fluorescence microscopy for mouse IL-36α was performed on de-paraffinized histologic sections after heat mediated antigen-retrieval in Trilogy buffer (Cell Marque). Sections were blocked for 1 h at room temperature in PBS with 10% goat serum (blocking buffer), and then incubated at 4°C overnight with 10 μg/mL rabbit anti-mIL-36α (Abcam) diluted in blocking buffer. The next day, sections were incubated for 1 h at room temperature with 2 μg/mL AlexaFluor-488 goat anti-rabbit IgG (Invitrogen) diluted in blocking buffer and subsequently mounted in Vectashield with DAPI (Vector Labs). Fluorescent images were taken at 400× magnification (Leica, DFC365FX).
RNA isolation and quantitative real-time PCR
Skin punch biopsy specimens (10-millimeter) were snap frozen in liquid nitrogen and homogenized in TRIzol reagent (Thermo Fisher Scientific). RNA isolation was performed with the Direct-zol RNA Mini Prep kit according to the manufacturer’s instructions (Zymo Research). cDNA was synthesized using a High Capacity RNA-to-cDNA Kit according to the manufacturer’s instructions (Applied Biosystems). Commercially available primers and probes for mouse IL 36α, IL-36β, IL-36γ, IL-1β, MyD88, and the Taqman Gene Expression Master Mix were used (Applied Biosystems), and quantitative real-time PCR (QPCR) was performed using a CFX96 Real-time PCR system (Bio-Rad). The relative quantities of mRNA per sample were determined using the ΔΔC(T) formula.
Flow cytometry
A single-cell suspension of skin tissue was obtained after digestion using RPMI containing Liberase TL 1.67 Wunsch unites/mL (ROCHE) and 10% DNAse (SIGMA) at 37°C for 1h, shaking at 240 rpm, then manually pushed through a cell separation filter (40 μm), resuspended in FACS buffer (PBS containing 1% BSA) and proceeded with surface staining. Neutrophils, monocytes, and macrophages were stained using antibodies against Ly6C (clone AL-21), Ly6G (clone 1A8), CD11b (clone M1/70), and F4/80 (clone REA126) (BD Biosciences). A single-cell suspension of lymph nodes was obtained after manually pushing draining lymph nodes through a cell separation filter (40 μm) and was resuspended in RPMI containing FBS (10%), penicillin (100 U/mL) and streptomycin (100 μg/ml). 1×106 cells per well were plated in 96-well cell culture plates in the presence of a GolgiStop (eBioscience) ± a cell activation cocktail of PMA (20 ng/mL) and ionomycin (1 μg/mL) (eBioscience) and incubated at 37°C for 4 h. Cells were washed before staining for viability (Viability Fixable Dye, Miltenyi Biotec) and surface markers using antibodies against TCRγδ (clone GL3) and CD4 (clone GK1.5) (Miltenyi Biotec) for 30 min at 4°C. Cells were washed before being fixed using FACSFix (BD Biosciences) for 30 min. For intracellular flow cytometry, cells were washed three times, permeabilized by incubating for 10 minutes in Perm/Wash (BD Biosciences) and incubated with antibodies against IL-17A (clone TC11-18H10) (BD Biosciences) and IL-22 (clone IL22JOP) (eBioscience) for 30 minutes before being washed and resuspended. Cell acquisition was performed on a MACSQuant flow cytometer (Miltenyi Biotec) and data analyzed using FlowJo software (Treestar).
Lymphocyte isolation and adoptive transfer to mice
A single-cell suspension of lymph nodes from naïve IL-36R−/− and wt mice was obtained after manually pushing draining lymph nodes through a cell separation filter (40 μm). Cells were counted and resuspended in MACS Separation Buffer (degassed PBS containing BSA [0.5%] and EDTA [2 mM]) (Miltenyi Biotec). Total CD3+ T cells were obtained using the Pan T cell Isolation Kit for mouse, according to the manufacturer’s instructions (Miltenyi Biotec). Briefly, non-T cells were depleted using biotin-antibody cocktail of CD11b, CD11c, CD45R, CD19, CD49b, CD105, MHCII and Ter-119, labeling with anti-biotin microbeads. The remaining T cells were collected via flow through on the MACS column. 3×106 CD3+ T cells were transferred to mice i.v. (via retro-orbital vein) at 1 week prior to epicutaneous S. aureus exposure. The adoptively transferred cells were 0.6–0.8% GL3+ γδ T cells and 55–57% CD4+ T cells (Fig. S3).
In vitro stimulation of lymph node T cells
Spleens from naïve wt and IL-36R−/− mice were obtained. After manually pushing them through a cell separation filter (40 μm), CD11c+ dendritic cells (DCs) were isolated using CD11c MicroBeads (Miltenyi Biotec). In 96 well plates, 2×104 DCs were stimulated with heat-killed S. aureus (log-phase bacteria incubated at 70°C for 1 h) (MOI 10) at 37°C for 16 h. CD3+ T cells were isolated from naïve and IL-36R−/− draining lymph nodes using Pan T cell isolation kit (Miltenyi Biotec). Heat-killed S. aureus were washed off, and DCs were further co-cultured for 72 h with purified CD3+ T cells (1×105) in complete RPMI 1640 containing extra L-Glutamine and 2-mercaptoethanol ± the exogenous recombinant murine IL-36α 1 μg/mL (R&D systems). During the last 4 h of co-culture, cells were restimulated with PMA (20 ng/mL), ionomycin (1 μg/mL) and GolgiStop (BD Biosciences), and intracellular cytokines were analyzed by flow cytometry.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data from single comparisons were compared using a two-tailed Student’s t-test and data for multiple comparisons were compared using a two-way ANOVA as specifically indicated in the figure legends. All statistical analysis was performed using Prism software (GraphPad). Data are presented as mean ± standard error of the mean (s.e.m.) and values of *P < 0.05 were considered statistically significant.
Supplementary Material
Highlights.
Epicutaneous S. aureus exposure drives MyD88- and IL-36R-dependent skin inflammation
IL-36R/MyD88 signaling by T cells mediates S. aureus-induced skin inflammation
S. aureus virulence factor PSMα, but not α- or δ-toxin, contributes to skin inflammation
IL-36α directly induces T cell production of IL-17A, which is required for inflammation
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases (contract/grant number: UM2AI117870/The Atopic Dermatitis Research Network U19AI117673-01 to R.S.G. and L.S.M.) and the National Institutes of Arthritis and Musculoskeletal and Skin Diseases (grant number: R01AR069502 to L.S.M.) from the U.S. National Institutes of Health, Department of Health and Human Services, and by the ASPIRE Rheumatology, Dermatology and Inflammatory Bowel Disease Research Award program from Pfizer, Inc. (grant number WI214880 to L.S.M.). We thank Amgen, Inc. for generously providing the IL-36R−/− mice, MedImmune, LLC. for generously providing the IL-33−/− mice and Genentech, Inc., for generously providing the IL-22−/− mice. We thank Michael Otto (NIH/NIAID) for providing the USA300 LAC parent, ΔPSMα and Δδ-toxin strains, Tammy Kielian (University of Nebraska) for providing USA300 LAC::lux strain and Ambrose Cheung and Niles Donegan (Dartmouth Medical School) for confirming deletion of α-toxin in the Δα-toxin strain from BEI Resources.
Footnotes
AUTHOR CONTRIBUTIONS
H.L., N.K.A., C.A.D., Y.W, A.G.A, R.V.O., T.K., S.K.L., S.S.C., R.J.M., M.C.M., E.Z., D.P.R., R.D.P., S.S. performed experiments and analyzed data. H.L., R.S.G. and L.S.M. conceived the study, designed experiments, interpreted data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Breuer K, SHA, Kapp A, Werfel T. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. The British journal of dermatology. 2002;147:55–61. doi: 10.1046/j.1365-2133.2002.04872.x. [DOI] [PubMed] [Google Scholar]
- Brown AF, Leech JM, Rogers TR, McLoughlin RM. Staphylococcus aureus Colonization: Modulation of Host Immune Response and Impact on Human Vaccine Design. Frontiers in immunology. 2014;4:507. doi: 10.3389/fimmu.2013.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AL, Deming C, Cassidy SKB, Harrison OJ, Ng WI, Conlan S, Program NCS, Belkaid Y, Segre JA, Kong HH. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Science translational medicine. 2017:9. doi: 10.1126/scitranslmed.aal4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier Y, Ma HL, Ramon HE, Napierata L, Small C, O’Toole M, Young DA, Fouser LA, Nickerson-Nutter C, Collins M, et al. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. The Journal of investigative dermatology. 2011;131:2428–2437. doi: 10.1038/jid.2011.234. [DOI] [PubMed] [Google Scholar]
- Chan LC, Chaili S, Filler SG, Barr K, Wang H, Kupferwasser D, Edwards JE, Jr, Xiong YQ, Ibrahim AS, Miller LS, et al. Nonredundant Roles of Interleukin-17A (IL-17A) and IL-22 in Murine Host Defense against Cutaneous and Hematogenous Infection Due to Methicillin-Resistant Staphylococcus aureus. Infection and immunity. 2015;83:4427–4437. doi: 10.1128/IAI.01061-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, Granick JL, Matsushima H, Takashima A, Iwakura Y, et al. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS pathogens. 2012;8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. The Journal of clinical investigation. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MS, Willcox CR, Joyce SP, Ladell K, Kasatskaya SA, McLaren JE, Hunter S, Salim M, Mohammed F, Price DA, et al. Clonal selection in the human Vdelta1 T cell repertoire indicates gammadelta TCR-dependent adaptive immune surveillance. Nature communications. 2017;8:14760. doi: 10.1038/ncomms14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster AM, Baliwag J, Chen CS, Guzman AM, Stoll SW, Gudjonsson JE, Ward NL, Johnston A. IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. Journal of immunology. 2014;192:6053–6061. doi: 10.4049/jimmunol.1301481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, Mitsui H, Cardinale I, de Guzman Strong C, Krueger JG, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. The Journal of allergy and clinical immunology. 2012;130:1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. Journal of immunology. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresnigt MS, Rosler B, Jacobs CW, Becker KL, Joosten LA, van der Meer JW, Netea MG, Dinarello CA, van de Veerdonk FL. The IL-36 receptor pathway regulates Aspergillus fumigatus-induced Th1 and Th17 responses. European journal of immunology. 2013;43:416–426. doi: 10.1002/eji.201242711. [DOI] [PubMed] [Google Scholar]
- Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Archives of internal medicine. 2008;168:1585–1591. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, Nakanishi K, Yamanishi K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Aihara M, Kirino M, Harada I, Komori-Yamaguchi J, Yamaguchi Y, Nagashima Y, Ikezawa Z. Interleukin-18 is elevated in the horny layer in patients with atopic dermatitis and is associated with Staphylococcus aureus colonization. The British journal of dermatology. 2011;164:560–567. doi: 10.1111/j.1365-2133.2010.10145.x. [DOI] [PubMed] [Google Scholar]
- Johnston A, Xing X, Wolterink L, Barnes DH, Yin Z, Reingold L, Kahlenberg JM, Harms PW, Gudjonsson JE. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. The Journal of allergy and clinical immunology. 2016 doi: 10.1016/j.jaci.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezic S, O’Regan GM, Lutter R, Jakasa I, Koster ES, Saunders S, Caspers P, Kemperman PM, Puppels GJ, Sandilands A, et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. The Journal of allergy and clinical immunology. 2012;129:1031–1039. e1031. doi: 10.1016/j.jaci.2011.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Miyazaki H, Ono S, Inatsu A, Nakashima H, Tsujimoto H, Shinomiya N, Saitoh D, Seki S. Enhancement of neutrophil function by interleukin-18 therapy protects burn-injured mice from methicillin-resistant Staphylococcus aureus. Infection and immunity. 2011;79:2670–2680. doi: 10.1128/IAI.01298-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. The Journal of investigative dermatology. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, Program NCS, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome research. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Li H, Jiang Z, Zhang T, Wang Y, Li Z, Wu Y, Ji S, Xiao S, Ryffel B, et al. Interleukin-33 increases antibacterial defense by activation of inducible nitric oxide synthase in skin. PLoS pathogens. 2014a;10:e1003918. doi: 10.1371/journal.ppat.1003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Yamasaki K, Saito R, Fukushi-Takahashi S, Shimada-Omori R, Asano M, Aiba S. Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36gamma induction in human epidermal keratinocytes. Journal of immunology. 2014b;193:5140–5148. doi: 10.4049/jimmunol.1302574. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ gammadelta T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. Journal of immunology. 2011;187:5026–5031. doi: 10.4049/jimmunol.1101817. [DOI] [PubMed] [Google Scholar]
- Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, Zribi J, Bal E, Cluzeau C, Chrabieh M, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. The New England journal of medicine. 2011;365:620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- McCaig LF, McDonald LC, Mandal S, Jernigan DB. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerging infectious diseases. 2006;12:1715–1723. doi: 10.3201/eid1211.060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O’Connell RM, Iwakura Y, Cheung AL, Cheng G, et al. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. Journal of immunology. 2007;179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- Milora KA, Fu H, Dubaz O, Jensen LE. Unprocessed Interleukin-36alpha Regulates Psoriasis-Like Skin Inflammation in Cooperation With Interleukin-1. The Journal of investigative dermatology. 2015;135:2992–3000. doi: 10.1038/jid.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Planillo R, Franchi L, Miller LS, Nunez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. Journal of immunology. 2009;183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, Ouyang W, Datta SK. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1beta and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nature immunology. 2013;14:804–811. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, Villaruz AE, Cheung GY, McGavin MJ, Travers JB, et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503:397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CY, Huang YH, Chu CF, Wu TC, Liu SH. Risks for Staphylococcus aureus Colonization in Psoriasis Patients: A Systematic Review and Meta-Analysis. The British journal of dermatology. 2017 doi: 10.1111/bjd.15366. [DOI] [PubMed] [Google Scholar]
- Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG, Guttman-Yassky E. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. The Journal of allergy and clinical immunology. 2009;123:1244–1252. e1242. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaru F, Jensen LE. Staphylococcus aureus stimulates neutrophil targeting chemokine expression in keratinocytes through an autocrine IL-1alpha signaling loop. The Journal of investigative dermatology. 2010;130:1866–1876. doi: 10.1038/jid.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong PY, Leung DY. Bacterial and Viral Infections in Atopic Dermatitis: a Comprehensive Review. Clinical reviews in allergy & immunology. 2016;51:329–337. doi: 10.1007/s12016-016-8548-5. [DOI] [PubMed] [Google Scholar]
- Oyoshi MK, Venturelli N, Geha RS. Thymic stromal lymphopoietin and IL-33 promote skin inflammation and vaccinia virus replication in a mouse model of atopic dermatitis. The Journal of allergy and clinical immunology. 2016;138:283–286. doi: 10.1016/j.jaci.2015.12.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, Becher B. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. The Journal of clinical investigation. 2012;122:2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut RD, Mocca CP, Prabhakara R, Merkel TJ, Stibitz S. Stably luminescent Staphylococcus aureus clinical strains for use in bioluminescent imaging. PloS one. 2013;8:e59232. doi: 10.1371/journal.pone.0059232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vgamma4+ gammadeltaT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8046–8051. doi: 10.1073/pnas.1508990112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravens S, Schultze-Florey C, Raha S, Sandrock I, Drenker M, Oberdorfer L, Reinhardt A, Ravens I, Beck M, Geffers R, et al. Human gammadelta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nature immunology. 2017;18:393–401. doi: 10.1038/ni.3686. [DOI] [PubMed] [Google Scholar]
- Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. The Journal of experimental medicine. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimaki S, Karisola P, Reunala T, Wolff H, Lauerma A, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. The Journal of investigative dermatology. 2012;132:1392–1400. doi: 10.1038/jid.2011.446. [DOI] [PubMed] [Google Scholar]
- Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. Staphylococcal and streptococcal superantigen exotoxins. Clinical microbiology reviews. 2013;26:422–447. doi: 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M, Ungar B, Correa da Rosa J, Ewald DA, Rozenblit M, Gonzalez J, Xu H, Zheng X, Peng X, Estrada YD, et al. RNA sequencing atopic dermatitis transcriptome profiling provides insights into novel disease mechanisms with potential therapeutic implications. The Journal of allergy and clinical immunology. 2015;135:1218–1227. doi: 10.1016/j.jaci.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, Shklovskaya E, Fazekas de St Groth B, Triccas JA, Weninger W. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. The Journal of experimental medicine. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed AK, Reed TJ, Clark KL, Boles BR, Kahlenberg JM. Staphlyococcus aureus phenol-soluble modulins stimulate the release of proinflammatory cytokines from keratinocytes and are required for induction of skin inflammation. Infection and immunity. 2015;83:3428–3437. doi: 10.1128/IAI.00401-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M, Tsutsui H, Imai Y, Yasuda K, Mizutani H, Yamanishi K, Kubo M, Matsui K, Sano H, Nakanishi K. Contribution of IL-18 to atopic-dermatitis-like skin inflammation induced by Staphylococcus aureus product in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8816–8821. doi: 10.1073/pnas.0602900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. Journal of immunology. 2011;186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda M, Leung DY, Molet S, Boguniewicz M, Taha R, Christodoulopoulos P, Fukuda T, Elias JA, Hamid QA. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. The Journal of allergy and clinical immunology. 2003;111:875–881. doi: 10.1067/mai.2003.1414. [DOI] [PubMed] [Google Scholar]
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clinical microbiology reviews. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortola L, Rosenwald E, Abel B, Blumberg H, Schafer M, Coyle AJ, Renauld JC, Werner S, Kisielow J, Kopf M. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. The Journal of clinical investigation. 2012;122:3965–3976. doi: 10.1172/JCI63451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne S, Palmer G, Lamacchia C, Martin P, Talabot-Ayer D, Rodriguez E, Ronchi F, Sallusto F, Dinh H, Sims JE, et al. IL-36R ligands are potent regulators of dendritic and T cells. Blood. 2011;118:5813–5823. doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- Vigne S, Palmer G, Martin P, Lamacchia C, Strebel D, Rodriguez E, Olleros ML, Vesin D, Garcia I, Ronchi F, et al. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood. 2012;120:3478–3487. doi: 10.1182/blood-2012-06-439026. [DOI] [PubMed] [Google Scholar]
- Vuong C, Kocianova S, Yu J, Kadurugamuwa JL, Otto M. Development of real-time in vivo imaging of device-related Staphylococcus epidermidis infection in mice and influence of animal immune status on susceptibility to infection. The Journal of infectious diseases. 2008;198:258–261. doi: 10.1086/589307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- Yin H, Li X, Hu S, Liu T, Yuan B, Ni Q, Lan F, Luo X, Gu H, Zheng F. IL-33 promotes Staphylococcus aureus-infected wound healing in mice. International immunopharmacology. 2013;17:432–438. doi: 10.1016/j.intimp.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, Gallo RL. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.