Abstract
Di(2-ethylhexyl) phthalate (DEHP) is a plasticizer in many consumer products. Although DEHP is a known endocrine disruptor, little is known about the effects of DEHP exposure on female reproduction. Thus, this study tested the hypothesis that prenatal DEHP exposure affects follicle numbers, estrous cyclicity, and hormone levels in multiple generations of mice. Pregnant CD-1 mice were orally dosed with corn oil (vehicle control) or DEHP (20 and 200 µg/kg/d and 500 and 750 mg/kg/d) from gestational day 11 until birth. The F1 females were mated with untreated males to create the F2 generation, and the F2 females were mated with untreated males to create the F3 generation. At 1 year, ovaries, hormones, and estrous cycles were analyzed in each generation. Prenatal DEHP exposure altered estrous cyclicity (750 mg/kg/d), increased the presence of ovarian cysts (750 mg/kg/d), and decreased total follicle numbers (750 mg/kg/d) in the F1 generation. It also decreased anogenital distance (200 µg/kg/d) and altered follicle numbers (200 µg/kg/d and 500 mg/kg/d) in the F2 generation, and it altered estrous cyclicity (20 and 200 µg/kg/d and 500 and 750 mg/kg/d) and decreased folliculogenesis (200 µg/kg/d and 500 mg/kg/d) in the F3 generation. Further, prenatal DEHP increased estradiol levels (F1 and F3), decreased testosterone levels (F1, F2, and F3), decreased progesterone levels (F2), altered gonadotropin hormone levels (F1 and F3), and decreased inhibin B levels (F1 and F3). Collectively, these data show that prenatal exposure to DEHP has multigenerational and transgenerational effects on female reproduction and it may accelerate reproductive aging.
This study shows that prenatal exposure to di(2-ethylhexyl) phthalate causes long-term transgenerational effects on female reproduction and may accelerate reproductive aging.
Di(2-ethylhexyl) phthalate (DEHP) is used as a plasticizer in polyvinyl chloride products, including building materials, children’s toys, and medical tubing (1, 2). Because of its widespread use, >2 million tons of DEHP are produced annually in the world (3). This large production leads to humans being exposed daily to DEHP via ingestion, inhalation, and dermal contact (2). Average daily human exposure falls within 3 to 30 µg/kg/d, although occupational exposure levels have been calculated to reach up to 300 to 600 µg/kg body weight/d (4, 5). DEHP and its metabolite, mono(2-ethylhexyl) phthalate (MEHP), are found in human tissues, including urine (6–9), breast milk (6, 10), blood (8, 10), cord blood (11), and follicular fluid (12).
DEHP is a known endocrine-disrupting chemical (EDC) that affects normal ovarian function and reproduction (13). Recent studies show that DEHP exposure for 10 days during early adult life accelerates primordial follicle recruitment (14). Further, it (200 and 500 mg/kg/d) decreases total follicle numbers at 9 months after dosing (15). Accelerated folliculogenesis and decreased total follicle numbers are a concern because they can result in early depletion of the follicle pool (16, 17), leading to premature reproductive senescence. Because adult exposure to DEHP caused early depletion of the follicle pool 9 months postdosing (15), the current study was designed to determine if prenatal exposure to DEHP would have the same effects and if these effects would be transgenerational in nature. Specifically, this study determined the long-term effects of prenatal exposure to DEHP on primordial, primary, preantral, and antral follicles in the F1, F2, and F3 generations of mice.
While examining the effects of prenatal DEHP exposure on folliculogenesis, we also examined the effects of DEHP exposure on hormone levels. We focused on the effects of DEHP on sex steroids (estradiol, progesterone, and testosterone) because the levels of these hormones as well as some peptide hormones (inhibin B and anti-Müllerian hormone) usually decrease with normal aging (18–21). We also focused on gonadotropins [follicle-stimulating hormone (FSH) and luteinizing hormone (LH)] because these hormone levels typically increase with age (19, 20). Further, Hannon et al. (15) found that acute DEHP exposure for 10 days in adult mice increased progesterone, decreased the ratio of estradiol/progesterone, and decreased the levels of inhibin B at 9 months postdosing. These changes in hormones can lead to irregular cyclicity, with the length of each cycle increasing or decreasing, and eventually an animal can become acyclic (22–24). Thus, the current study was designed to examine the effects of prenatal DEHP exposure on estradiol, progesterone, testosterone, inhibin B, FSH, and LH hormone levels as well as estrous cyclicity in aging female mice.
We also examined whether prenatal DEHP exposure led to the presence of ovarian cysts. This is because as animals age, hormones become irregular, estrous cycles are lengthened (25), and the formation of cysts in the ovary is relatively common (26–28). These cysts can arise from follicles, corpora lutea, rete ovarii, surface epithelium, ovarian bursa, or embryonic remnants (25). Recently, Zhou et al. (29) showed that a mixture of phthalates, including DEHP, increased the occurrence of cystic ovaries at 13 months of age in mice (29). Similarly, in a transgenerational study, prenatal exposure to a mixture of phthalates, including DEHP, increased cystic ovaries in the F2 generation of mice (30).
In addition to very limited information on the impact of prenatal DEHP exposure on female reproductive aging, few studies have focused on the transgenerational effects of DEHP on female reproduction. In one previous study, gestational DEHP exposure reduced the primordial follicle reserve and increased preantral follicle numbers in the F1 generation of mice, and the same phenotype was observed in the F2 and F3 generations (31). Further, in another study, prenatal exposure to a mixture of phthalates, including DEHP, increased uterine weights, altered anogenital distance (AGD), increased body weight, and increased days to pregnancy at multiple ages in both the F2 and F3 generations of mice (30). Although these previous studies indicate that prenatal DEHP exposure may cause some transgenerational effects on female reproduction, they did not examine in detail whether prenatal DEHP exposure has long-term transgenerational effects on follicle numbers, cyclicity, or hormone levels. Therefore, the current study was designed to determine the effects of prenatal DEHP exposure on the F1, F2, and F3 generations of mice. Specifically, we tested the hypothesis that prenatal DEHP exposure affects ovarian follicle numbers and estrous cyclicity and alters hormone levels in multiple generations of aging mice.
Materials and Methods
Chemicals
DEHP (99% purity) was purchased from Sigma-Aldrich (St. Louis, MO). Tocopherol-stripped corn oil (MP Biomedicals, Solon, OH) was used as a vehicle to create the desired stock solutions of DEHP.
Studies using DEHP have shown that DEHP causes nonmonotonic dose-response curves (32); therefore, a wide range of doses was chosen for our study. The doses selected were 20 µg/kg/d, 200 µg/kg/d, 500 mg/kg/d, and 750 mg/kg/d. The dose of 20 µg/kg/d was chosen because it falls within the range of daily human exposure and causes effects on ovarian development in mice (7, 33). The dose of 500 mg/kg/d was chosen because it was found to impair testicular germ cell organization and decrease sperm count in mice (34). Further, several doses (20 and 200 μg/kg/d and 750 mg/kg/d) were shown to accelerate folliculogenesis after 10-day adult exposure (14).
Animals
Adult cycling female and adult male CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in the College of Veterinary Medicine Animal Facility at the University of Illinois at Urbana-Champaign (Champaign, IL). The mice were acclimated to the facility for at least 2 weeks before use. The mice were housed individually at 25°C in conventional polystyrene cages with a 12-hour light/dark cycle. Mice were provided the Teklad Rodent Diet 8604 (Envigo, Huntingdon, United Kingdom) and high-purity water (reverse-osmosis filtered) ad libitum. Animal procedures including euthanasia and tissue collections were approved by the University of Illinois Institutional Animal Care and Use Committee.
Study design and dosing
Female mice (F0) were mated with nontreated males, both 8 weeks of age, to create the F1 generation. A female was considered pregnant when a vaginal sperm plug was present. After a vaginal sperm plug was observed, females were separated from males and individually housed. From gestational day (GD) 11 until delivery of pups, the pregnant females were dosed orally by inserting a pipette tip into the cheek of the animal with the control (tocopherol-stripped corn oil) or DEHP solution. We chose to dose between GD 11 and birth because this is a critical time during ovarian development when primordial germ cells arrive at the gonad and the primordial follicle pool forms (35, 36). The dosing volume was determined by the weight of the dam. Oral dosing was used to mimic oral exposure in humans. At 3 months of age, the F1 generation was mated with nontreated males to generate the F2 generation. When the F2 generation was 3 months of age, they were mated with nontreated males to create the F3 generation. The F3 generation was the first generation that was not exposed directly to DEHP.
Tissues
At 12 months of age, mice were euthanized, and tissues were collected. Before collections, the cyclicity of the mice was observed at approximately the same time every day, and tissues were only collected if the mice were in diestrus or metestrus (these stages are similar in both cytology and hormone profiles). Body weights (in grams) and AGDs (in millimeters) were taken prior to tissue collections. Once body weight and AGD were recorded, AGD was normalized to body weight by taking the AGD measurement and dividing it by the cubic root of body weight (37). After the mice were euthanized, the ovaries, uteri, and liver were removed and cleaned properly, and the organ weights were recorded in grams. One ovary was fixed in Dietrich fixative to use for histological evaluation as described below. The other ovary was snap-frozen and stored for future studies. Blood was also obtained during collections, and serum was used for hormone assays as described below.
Estrous cyclicity
Estrous cyclicity was monitored by subjecting mice to daily vaginal lavage for 14 days prior to tissue collections in each generation. A mouse was considered to be in estrus when the majority of the vaginal cells were cornified epithelial cells. Mice were considered to be in diestrus when mostly leukocytes and some nucleated epithelial cells were present in the lavage. Mice were considered to be in proestrus when mainly nucleated epithelial cells were visible in the lavage. Lastly, a mouse was considered to be in metestrus if all three cell types were observed, usually with few nucleated epithelial cells. The percentage of days spent in different cycles of estrus was calculated by the number of days in a specific cycle divided by 14 and multiplying that value by 100. Metestrus and diestrus are similar in cytology and hormone profiles, so these stages were combined together for analysis.
Histological evaluation of ovaries
Ovaries were collected at 12 months of age in the F1, F2, and F3 generations of mice. The ovaries were fixed in Dietrich fixative, embedded in paraffin, and sectioned at 8 µm using a microtome. After sectioning, slides were stained with Weigert hematoxylin and picric acid-methyl blue. Every 10th section of the ovary was used to count all follicle types including primordial, primary, preantral, and antral follicles (38). In addition, every 10th section was used to count atretic follicles, abnormal follicles, and corpora lutea (39, 40). Follicles were considered primordial if they had an oocyte surrounded by a single layer of squamous granulosa cells; primary if they had an oocyte surrounded by a single layer of cuboidal granulosa cells; preantral if they contained an oocyte surrounded by at least two layers of cuboidal granulosa cells with a theca cell layer; and antral if they contained an oocyte surrounded by many layers of cuboidal granulosa cells with a thecal cell layer and a fluid-filled antral space called the antrum. Follicles were considered atretic if at least 10% of the follicle contained apoptotic bodies. Abnormal follicles included follicles with double oocytes and/or fragmented nuclei. Primordial and primary follicles were counted whether nuclear material was present or not, but preantral, antral, and atretic follicles were required to have nuclear material present to avoid double counting. Total follicle numbers, numbers and percent of each follicle type, percent of atretic follicles, corpora lutea per number of total sections, abnormal follicles, and presence of cysts were recorded without knowledge of treatment group. The percent of each follicle type was examined to observe the effects of DEHP exposure on the shift of the follicle pool.
Analysis of sex steroid, gonadotropin, and peptide hormone levels
At 12 months of age, mice in each generation were euthanized, and blood was obtained during the collection of tissues. The sera were sent to the University of Virginia Center for Research in Reproduction Ligand Assay & Analysis Core for measurement of estradiol, testosterone, progesterone, inhibin B, FSH, and LH by enzyme-linked immunosorbent assays (estradiol, testosterone, progesterone, and inhibin B) or radioimmunoassays (FSH and LH). The lowest limits of detection were 0.15 ng/mL for progesterone, 10 ng/dL for testosterone, 3 pg/mL for estradiol, 1.17 ng/mL for FSH, 0.04 ng/mL for LH, and 10 pg/mL for inhibin B. If the measurement was lower than the lowest limit of detection, the value was substituted with the lowest limit of detection divided by the square root of 2. The intra- and interassay coefficients of variability were <10% (https://med.virginia.edu/research-in-reproduction/wp-content/uploads/sites/311/2016/08/2016-INTRA-INTER-ASSAY-CVs__032316.pdf).
Statistical analysis
For all data analysis, the software SPSS (SPSS Inc., Chicago, IL) was used. If data were normally distributed, a one-way analysis of variance was performed, followed by a Dunnett test (two-sided) if equal variances were assumed or Games-Howell test if equal variances were not assumed. If data were not equally distributed or presented as percentages, independent sample Kruskal-Wallis H tests were used to compare each treatment group, followed by Mann-Whitney U test. To evaluate cystic ovaries, mice were assigned a 0 (indicates no cysts) or 1 (cysts observed), and Fisher exact tests were used to compare treatment groups to the control. For all comparisons, statistical significance was assigned at P ≤ 0.05. When P values were >0.05 but <0.1, data were considered to exhibit a trend toward significance.
Results
Effect of prenatal DEHP exposure on body weights, tissue weights, and AGD in F1, F2, and F3 generations in mice at 1 year of age
In the F1 generation, prenatal exposure to DEHP did not affect body weight, liver weight, or AGD; however, it decreased ovarian weight (20 µg/kg/d) and increased uterine weights (500 and 750 mg/kg/d) compared with control (Table 1; n = 3 to 7 dams per treatment). In the F2 generation, DEHP (200 µg/kg/d) decreased AGD, but did not affect body weight, ovary weight, uterine weight, or liver weight (Table 1; n = 3 to 8 dams per treatment). In the F3 generation, prenatal DEHP exposure did not affect uterine weight or AGD; however, it decreased body weight (200 µg/kg/d), decreased ovarian weight (20 and 200 µg/kg/d and 500 mg/kg/d), and decreased liver weight (20 and 200 µg/kg/d and 500 and 750 mg /kg/d) compared with control (Table 1; n = 3 to 7 dams per treatment).
Table 1.
The Effects of Prenatal Exposure to DEHP on Body and Organ Weights and AGD in 1-Year-Old Female Mice
|
Treatment |
Generation |
|||
|---|---|---|---|---|
| F1 | F2 | F3 | ||
| Body weight (g) | Control | 43.85 ± 1.91 | 42.29 ± 2.32 | 48.00 ± 1.98 |
| 20 µg/kg/d | 45.91 ± 2.67 | 39.83 ± 2.26 | 43.32 ± 0.63 | |
| 200 µg/kg/d | 44.06 ± 2.33 | 42.54 ± 2.21 | 39.42 ± 3.93a | |
| 500 mg/kg/d | 42.66 ± 1.58 | 44.93 ± 1.28 | 47.26 ± 2.03 | |
| 750 mg/kg/d | 44.03 ± 1.28 | 40.21 ± 1.32 | 44.07 ± 1.87 | |
| Ovary weight (g) | Control | 0.0215 ± 0.0014 | 0.0257 ± 0.0034 | 0.0281 ± 0.0028 |
| 20 µg/kg/d | 0.0168 ± 0.0016b | 0.0195 ± 0.0018 | 0.0187 ± 0.0009b | |
| 200 µg/kg/d | 0.0184 ± 0.0012 | 0.0254 ± 0.0032 | 0.0150 ± 0.0045a | |
| 500 mg/kg/d | 0.0185 ± 0.0036 | 0.0262 ± 0.0057 | 0.0157 ± 0.0038b | |
| 750 mg/kg/d | 0.0499 ± 0.0204 | 0.0282 ± 0.0063 | 0.0247 ± 0.0035 | |
| Uterine weight (g) | Control | 0.1982 ± 0.0184 | 0.2547 ± 0.0069 | 0.2612 ± 0.0368 |
| 20 µg/kg/d | 0.1729 ± 0.0116 | 0.2849 ± 0.0452 | 0.2661 ± 0.0487 | |
| 200 µg/kg/d | 0.1574 ± 0.0150 | 0.2815 ± 0.0415 | 0.2116 ± 0.0426 | |
| 500 mg/kg/d | 0.3788 ± 0.0412a | 0.2264 ± 0.0136 | 0.2033 ± 0.0317 | |
| 750 mg/kg/d | 0.2701 ± 0.0289b | 0.2202 ± 0.0256 | 0.2347 ± 0.0272 | |
| Liver weight (g) | Control | 2.7050 ± 0.0493 | 2.6155 ± 0.1299 | 2.9902 ± 0.1644 |
| 20 µg/kg/d | 3.0313 ± 0.3264 | 2.4707 ± 0.2109 | 2.5752 ± 0.0292a | |
| 200 µg/kg/d | 2.6209 ± 0.1089 | 2.5910 ± 0.2551 | 2.1909 ± 0.2050a | |
| 500 mg/kg/d | 2.5862 ± 0.1325 | 2.6431 ± 0.1067 | 2.5003 ± 0.0390a | |
| 750 mg/kg/d | 2.8670 ± 0.1080 | 2.5210 ± 0.2764 | 2.4496 ± 0.0980a | |
| AGD (mm)/∛body weight (g) | Control | 1.9583 ± 0.0573 | 1.5262 ± 0.0512 | 1.4180 ± 0.0749 |
| 20 µg/kg/d | 1.9674 ± 0.0205 | 1.4262 ± 0.0183 | 1.5663 ± 0.0520 | |
| 200 µg/kg/d | 1.9799 ± 0.0634 | 1.3533 ± 0.0393a | 1.5807 ± 0.1534 | |
| 500 mg/kg/d | 2.0502 ± 0.0694 | 1.4531 ± 0.0354 | 1.5184 ± 0.1181 | |
| 750 mg/kg/d | 1.9508 ± 0.0557 | 1.5348 ± 0.0550 | 1.3153 ± 0.0445 | |
Significant differences compared with the control (P ≤ 0.05).
Borderline significance compared with the control (0.05 < P < 0.1).
Effect of prenatal DEHP exposure on estrous cyclicity in F1, F2, and F3 generations in mice at 1 year of age
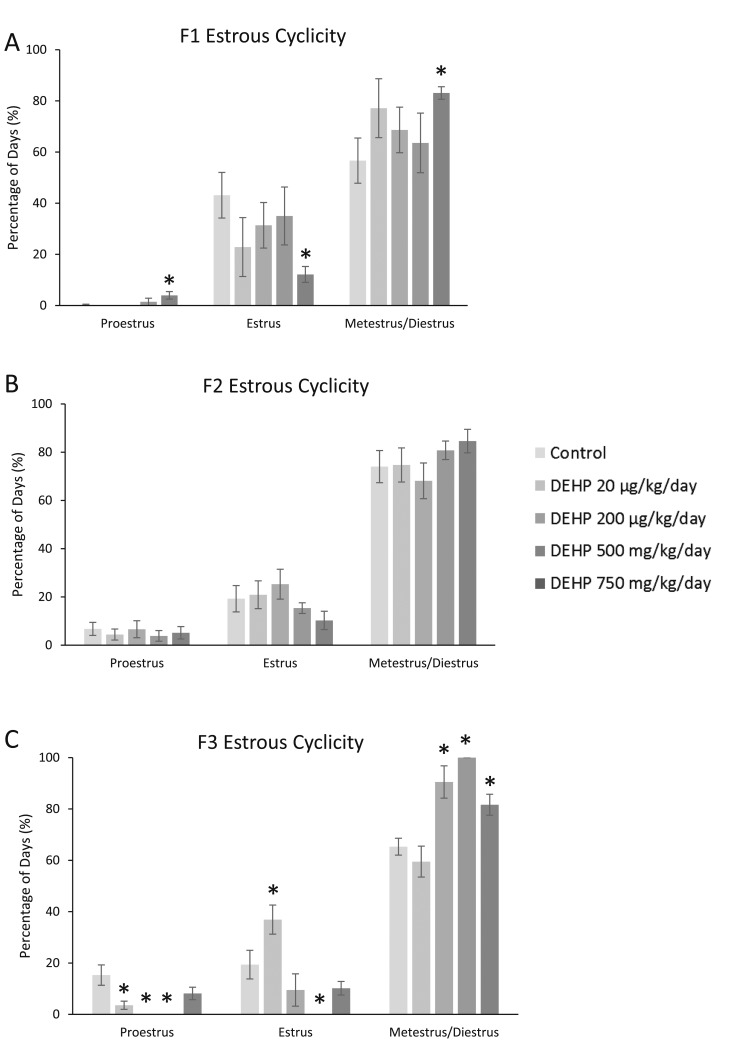
Estrous cyclicity was monitored for 14 days in each generation prior to tissue collections. In the F1 generation, prenatal DEHP exposure significantly increased the time spent in proestrus, decreased the time spent in estrus, and increased the time spent in metestrus/diestrus in the 750-mg/kg/d treatment group when compared with the control (Fig. 1A; n = 4 to 7 dams per treatment; P ≤ 0.05). In the F2 generation, prenatal exposure to DEHP did not affect estrous cyclicity (Fig. 1B). In the F3 generation, DEHP significantly decreased the time spent in proestrus (20 and 200 µg/kg/d and 500 mg/kg/d), significantly altered the time spent in estrus (increased in the 20-µg/kg/d and decreased in the 500-mg/kg/d groups), and significantly increased the time spent in metestrus/diestrus (200 µg/kg/d and 500 and 750 mg/kg/d) compared with control (Fig. 1C; n = 3 to 7 dams per treatment; P ≤ 0.05).
Figure 1.
Effect of prenatal exposure to DEHP on estrous cyclicity at 1 year of age in the F1, F2, and F3 generations of mice. Percentage of days in proestrus, estrus, and metestrus/diestrus were calculated and compared with controls in each treatment group. Graphs represent means ± standard error of the mean from four to seven females per treatment group in the F1 generation, three to eight females per treatment group in the F2 generation, and three to seven females per treatment group in the F3 generation. (A) is the F1 generation, (B) is the F2 generation, and (C) is the F3 generation. *Significant differences compared with the control (P ≤ 0.05).
Effect of prenatal DEHP exposure on folliculogenesis in F1, F2, and F3 generations in mice at 1 year of age
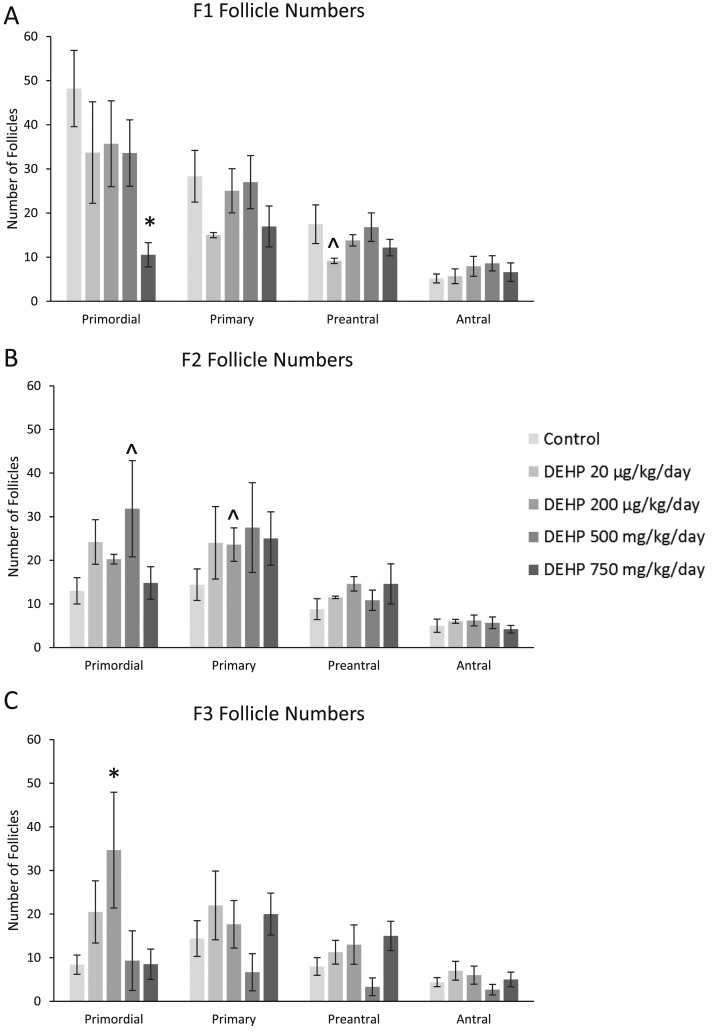
In the F1 generation, prenatal DEHP exposure significantly decreased the number of primordial follicles (750 mg/kg/d) and caused a borderline decrease in the number of preantral follicles (20 µg/kg/d) compared with controls (Fig. 2A; n = 3 to 6 dams per treatment; P ≤ 0.05; 0.05 < P < 0.1, borderline significance). In the F2 generation, DEHP caused a borderline increase in the number of primordial follicles (500 mg/kg/d) and primary follicles (200 µg/kg/d) compared with controls (Fig. 2B; n = 3 to 6 dams per treatment; 0.05 < P < 0.1, borderline significance). In the F3 generation, prenatal DEHP significantly increased the number of primordial follicles in the 200-µg/kg/d treatment group compared with controls (Fig. 2C; n = 3 to 5 dams per treatment; P ≤ 0.05).
Figure 2.
Effect of prenatal exposure to DEHP on follicle numbers. At 1 year of age, ovaries from the F1, F2, and F3 generations were subjected to histological evaluation of follicle numbers. Follicles in each generation [F1 in (A), F2 in (B), and F3 in (C)] were counted and separated by stage of development in each treatment group. Graphs represent means ± standard error of the mean from three to six females per treatment group in the F1 generation, three to five females per treatment group in the F2 generation, and three to five females per treatment group in the F3 generation. *Significant differences compared with the control (P ≤ 0.05); ^borderline significance compared with the control (0.05 < P < 0.1).
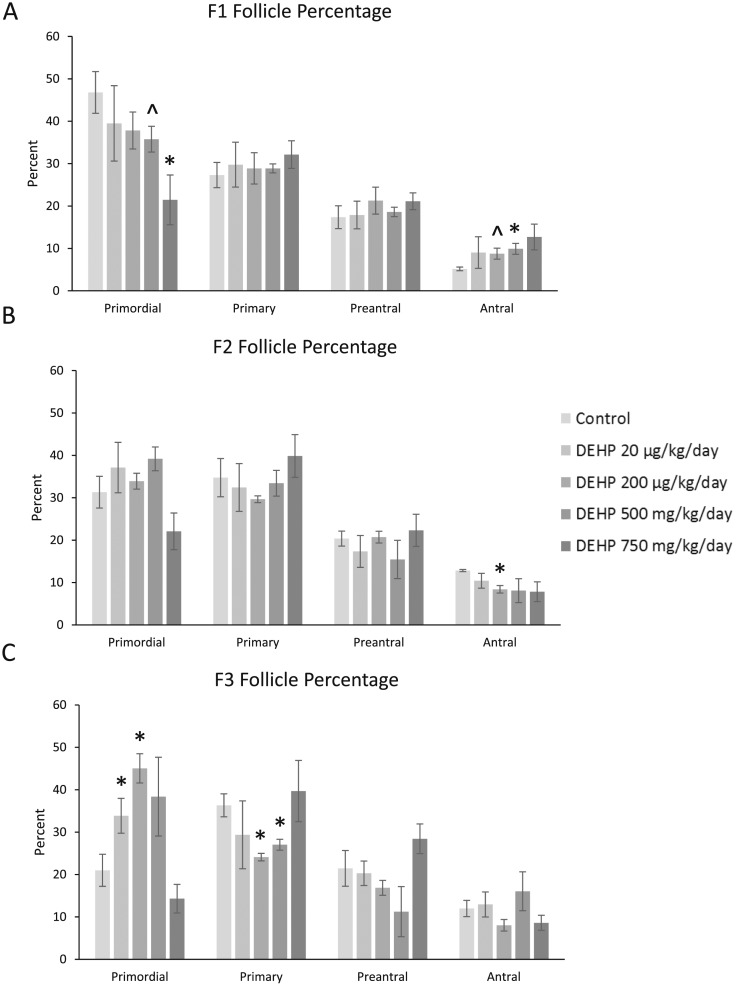
When we examined the percent of each follicle type, in the F1 generation, prenatal DEHP exposure significantly decreased the percentage of primordial follicles (750 mg/kg/d), borderline decreased percentage of primordial follicles (500 mg/kg/d), significantly increased the percentage of antral follicles (500 mg/kg/d), and borderline increased the percentage of antral follicles in the 200-µg/kg/d treatment group compared with controls (Fig. 3A; n = 4 to 6 dams per treatment; P ≤ 0.05; 0.05 < P < 0.1, borderline significance). In the F2 generation, DEHP significantly decreased the percentage of antral follicles in the 200-µg/kg/d treatment group when compared with control (Fig. 3B; n = 3 to 5 dams per treatment; P ≤ 0.05). In the F3 generation, prenatal DEHP exposure (20 and 200 µg/kg/d) significantly increased the percentage of primordial follicles, and it (200 µg/kg/d and 500 mg/kg/d) significantly decreased the percentage of primary follicles when compared with controls (Fig. 3C; n = 3 to 5 dams per treatment; P ≤ 0.05).
Figure 3.
Effect of prenatal exposure to DEHP on the percentage of follicle types per ovary. At 1 year of age, ovaries from the F1, F2, and F3 generations were subjected to histological evaluation for the percentage of each follicle type. Follicles in each generation [F1 in (A), F2 in (B), and F3 in (C)] were counted and separated by stage of development, and percentages of each follicle type were calculated for each treatment group. Graphs represent means ± standard error of the mean from four to six females per treatment group in the F1 generation, three to five females per treatment group in the F2 generation, and three to five females per treatment group in the F3 generation. *Significant differences compared with the control (P ≤ 0.05); ^borderline significance compared with the control (0.05 < P < 0.1).
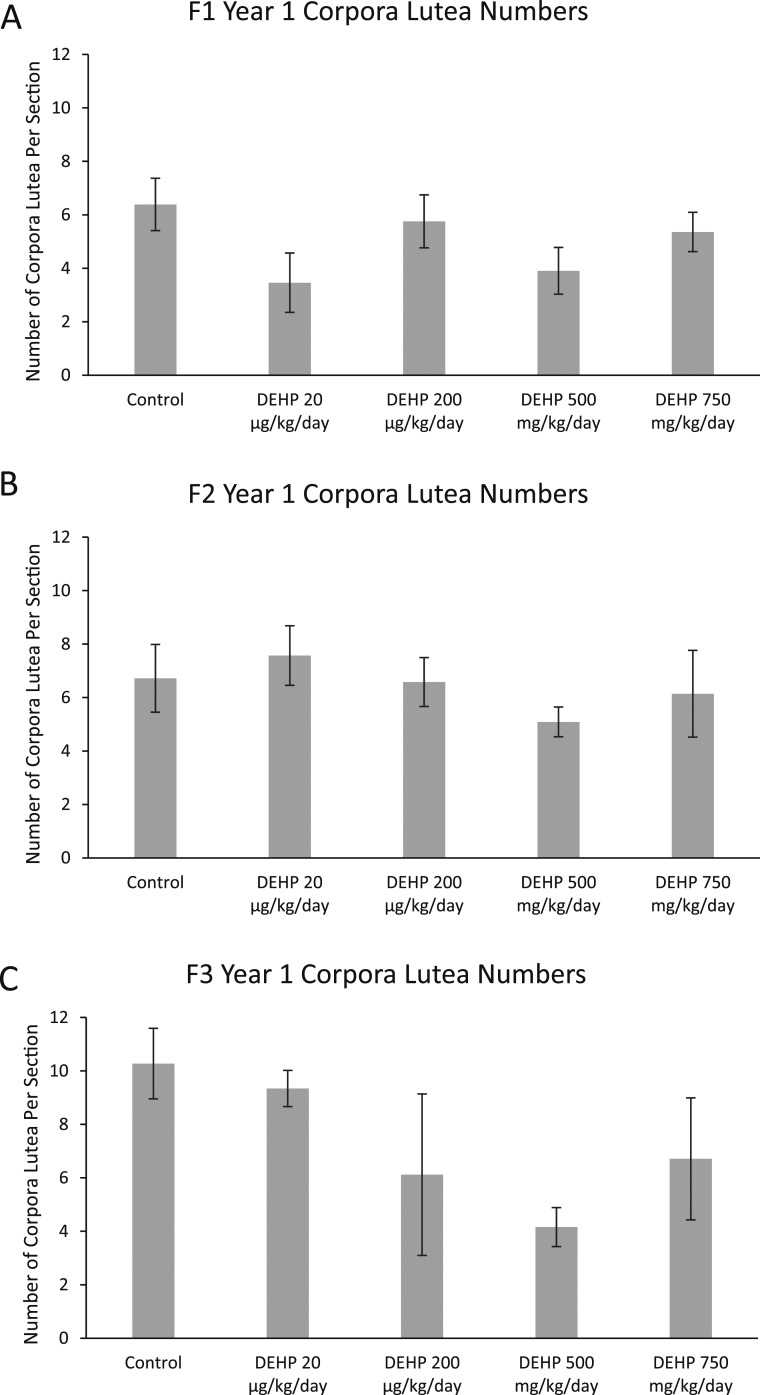
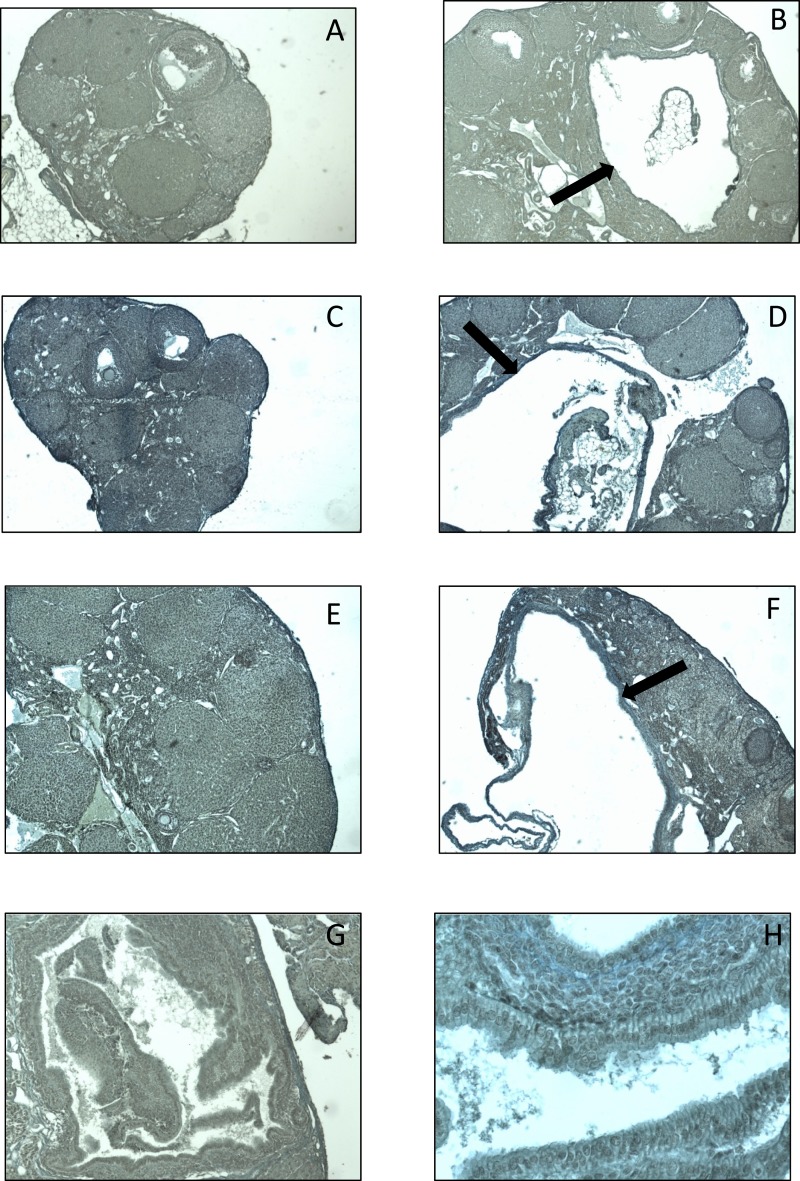
In the F1, F2, and F3 generations, DEHP exposure did not affect the number of corpora lutea compared with controls (Fig. 4A–4C). Interestingly, in the F1 generation, DEHP exposure increased the presence of ovarian cysts compared with the controls (Table 2). Specifically, in the F1 generation, DEHP exposure (750 mg/kg/d) significantly increased the presence of cysts compared with controls (Table 2 and Fig. 5A and 5B; n = 6 to 11 dams per treatment). In the F2 and F3 generations, prenatal DEHP exposure did not statistically significantly affect the presence of cysts compared with controls (Table 2). However, it is notable that in the F2 generation, 100% of the females in the 200-µg/kg/d treatment group had cysts, whereas only 40% of the females in the control group had cysts. In the F3 generation, 100% of the females in the 500-mg/kg/d treatment group had cysts, whereas only 60% of the females in the control group had cysts. Some of these cystic structures also appeared to display ovarian endosalpingiosis. Histologically, these cysts resembled cystic glandular structures lined by benign salpingeal epithelium (41) (Fig. 5G and 5H). In the F1 generation, two out of four ovaries in the 20-µg/kg/d treatment appeared to have cystic glandular structures lined by benign salpingeal epithelium. In the F2 generation, two out of five ovaries in the 20-µg/kg/d treatment group, one out of five ovaries in the 200-µg/kg/d treatment group, and one out of three ovaries in the 500-mg/kg/d treatment group resembled this structure. Lastly, in the F3 generation, one out of five control ovaries and one out of three ovaries treated with 500 mg/kg/d had cystic glandular structures lined by benign salpingeal epithelium.
Figure 4.
Effect of prenatal exposure to DEHP on numbers of corpora lutea. At 1 year of age, ovaries from the (A) F1, (B) F2, and (C) F3 generations were subjected to histological evaluation of corpora lutea numbers. Graphs represent means ± standard error of the mean from four to six females per treatment group in the F1 generation, three to five females per treatment group in the F2 generation, and three to five females per treatment group in the F3 generation.
Table 2.
The Effects of Prenatal Exposure to DEHP on the Percent of Cystic Ovaries Present in 1-Year-Old Female Mice
| Treatment |
Percent (%) Females With Cystic Ovaries |
||
|---|---|---|---|
|
Generation | |||
| F1 | F2 | F3 | |
| Control | 30.0 | 40.0 | 60.0 |
| 20 µg/kg/d | 33.3 | 40.0 | 16.7 |
| 200 µg/kg/d | 54.5 | 100.0 | 33.3 |
| 500 mg/kg/d | 66.7 | 66.7 | 100.0 |
| 750 mg/kg/d | 88.9a | 60.0 | 75.0 |
Significant differences compared with the control (P ≤ 0.05).
Figure 5.
Effect of prenatal exposure to DEHP on the presence of ovarian cysts and ovarian endosalpingiosis. The photographs show histological sections of ovaries from 1-year-old mice in the F1, F2, and F3 generations. In the F1 generation, ovaries from (A) control and (B) 750-mg/kg/d treatment groups. In the F2, ovaries from (C) control and (D) 200-µg/kg/d treatment groups. In the F3 generation, ovaries from (E) control and (F) 500-mg/kg/d treatment groups. The black arrows indicate a cyst. The endosalpingiosis from an ovary treated with 20 µg/kg/d is shown at (G) ×10 and (H) ×40 original magnification.
In the F1, F2, and F3 generations, DEHP exposure did not affect the number of atretic follicles compared with control. In the F1 generation, DEHP exposure at 750 mg/kg/d significantly decreased total follicle numbers compared with controls (n = 6 to 11 dams per treatment; P ≤ 0.05) (data not shown). In the F2 generation, prenatal exposure to DEHP at 750 mg/kg/d significantly increased the number of abnormal follicles when compared with control (n = 4 to 5 dams per treatment; P ≤ 0.05) (data not shown).
Effect of prenatal DEHP exposure on serum sex steroid, gonadotropin, and peptide hormone levels in F1, F2, and F3 generations in mice at 1 year of age
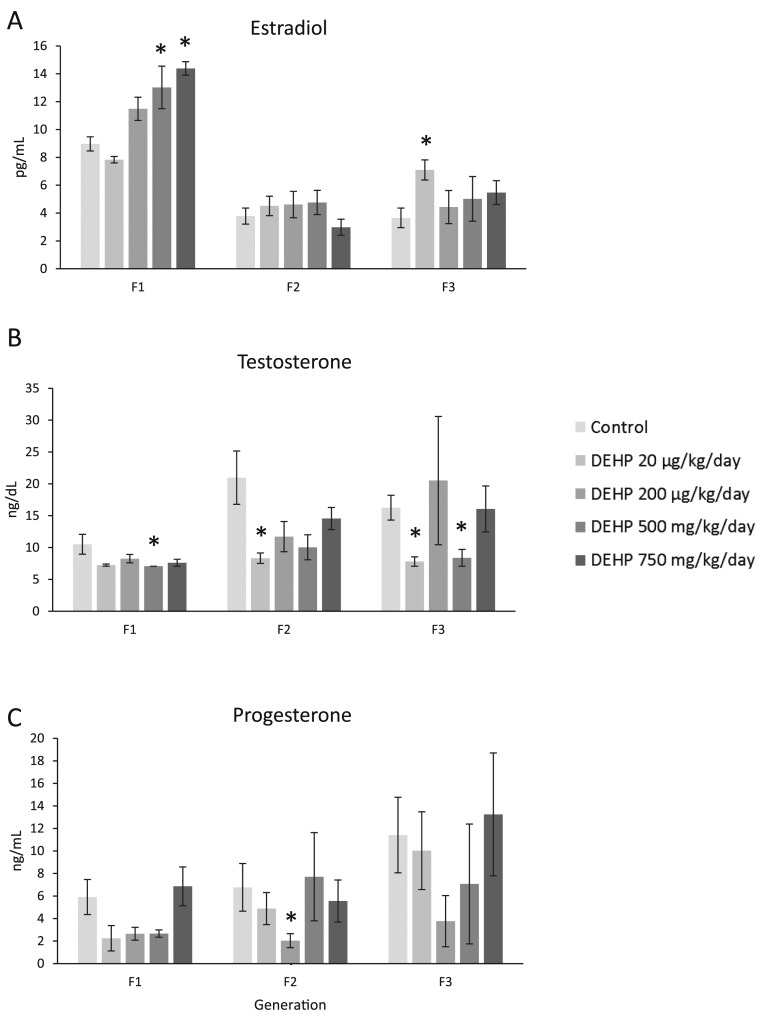
In all three generations of mice, prenatal exposure to DEHP altered the levels of estradiol, testosterone, and progesterone. In the F1 generation, prenatal DEHP exposure (500 and 750 mg/kg/d) significantly increased levels of estradiol compared with the control, and it (500 mg/kg/d) significantly decreased levels of testosterone compared with control (Fig. 6A and 6B; n = 3 to 7 dams per treatment; P ≤ 0.05). In contrast, DEHP exposure did not affect progesterone levels compared with control (Fig. 6C; n = 3 to 7 dams per treatment). In the F2 generation, DEHP exposure did not affect estradiol levels compared with the control, but it significantly decreased testosterone (20 µg/kg/d) and progesterone (200 µg/kg/d) compared with the control (Fig. 6A–6C; n = 3 to 10 dams per treatment; P ≤ 0.05). In the F3 generation, prenatal DEHP exposure (20 µg/kg/d) significantly increased levels of estradiol and it (20 µg/k/d and 500 mg/kg/d) significantly decreased levels of testosterone compared with controls (Fig. 6A and 6B; n = 3 to 7 dams per treatment; P ≤ 0.05). In contrast, prenatal DEHP exposure did not affect progesterone levels compared with controls (Fig. 6C; n = 3 to 7 dams per treatment).
Figure 6.
Effect of prenatal exposure to DEHP on serum sex steroid hormone levels at 1 year of age in the F1, F2, and F3 generations of mice. Sera were subjected to enzyme-linked immunosorbent assays for the measurements of (A) estradiol, (B) testosterone, and (C) progesterone. Graphs represent means ± standard error of the mean from 3 to 7 females per treatment group in the F1 generation, 3 to 10 females per treatment group in the F2 generation, and 3 to 7 females per treatment group in the F3 generation. *Significant differences compared with the control (P ≤ 0.05).
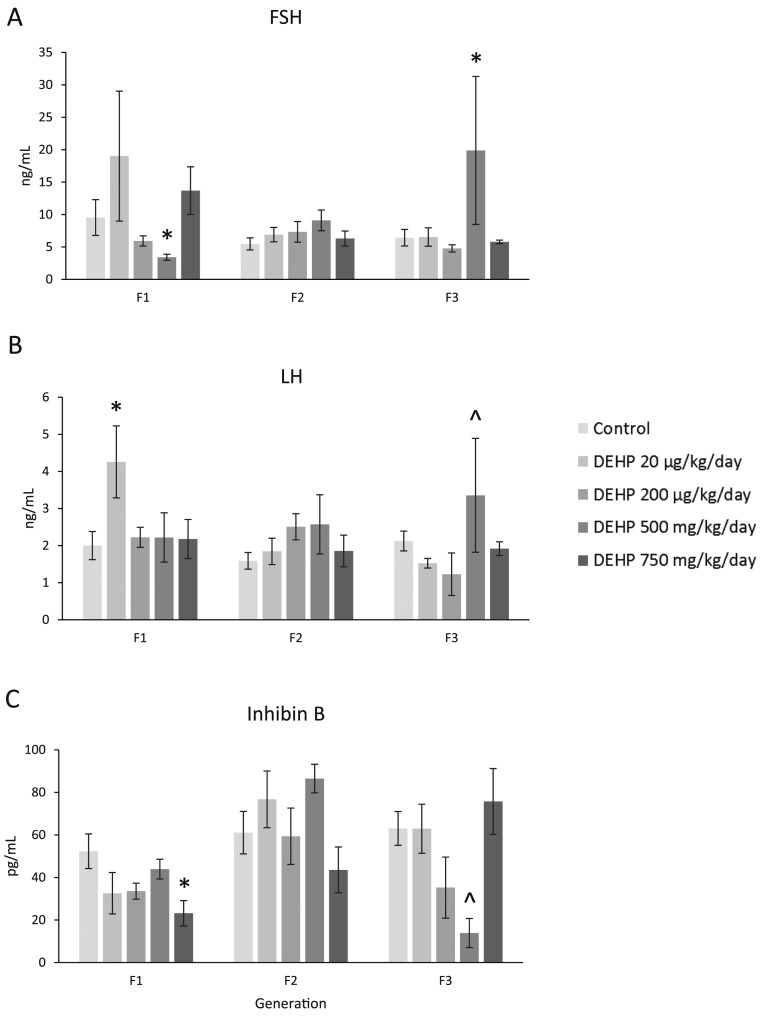
Prenatal DEHP exposure caused some changes in the levels of FSH and LH. In the F1 generation, prenatal DEHP exposure (500 mg/kg/d) significantly decreased levels of FSH, and it (20 µg/kg/d) significantly increased LH levels compared with controls (Fig. 7A and 7B; n = 3 to 7 dams per treatment; P ≤ 0.05). In the F2 generation, prenatal DEHP exposure did not affect FSH or LH levels (Fig. 7A and 7B). In the F3 generation, prenatal DEHP exposure (500 mg/kg/d) significantly increased the levels of FSH, and it borderline increased in the levels of LH (P = 0.086) compared with control (Fig. 7A and 7B; n = 3 to 7 dams per treatment, P ≤ 0.05; 0.05 < P < 0.1, borderline significance).
Figure 7.
Effect of prenatal exposure to DEHP on serum gonadotropin and peptide hormone levels at 1 year of age in the F1, F2, and F3 generations of mice. Sera were subjected to enzyme-linked immunosorbent assays or radioimmunoassays for the measurements of (A) FSH, (B) LH, and (C) inhibin B. Graphs represent means ± standard error of the mean from 3 to 7 females per treatment group in the F1 generation, 3 to 10 females per treatment group in the F2 generation, and 3 to 7 females per treatment group in the F3 generation. *Significant differences compared with the control (P ≤ 0.05); ^borderline significance compared with the control (0.05 < P < 0.1).
In the F1 generation, prenatal DEHP exposure (750 mg/kg/d) significantly decreased levels of inhibin B when compared with control (Fig. 7C; n = 3 to 7 dams per treatment; P ≤ 0.05). In the F2 generation, prenatal DEHP exposure did not change inhibin B levels compared with control (Fig. 7C). In the F3 generation, prenatal DEHP exposure (500 mg/kg/d) caused a borderline decrease in the levels of inhibin B compared with controls (P = 0.080) (Fig. 7C, n = 3 to 7 dams per treatment; 0.05 < P < 0.1, borderline significance).
Discussion
In this study, we examined the effects of prenatal DEHP exposure on multiple generations of aging female mice. This study provides information on the long-term effects of prenatal DEHP exposure during the second half of gestation on aging female mice. Further, this study examines these long-term effects of prenatal DEHP exposure in multiple generations of mice. In this study, we found that prenatal DEHP exposure affected body weights, liver weights, and AGD at 1 year in the F2 and F3 generations. It also decreased ovarian weights at 1 year in both the F1 and F3 generations, whereas it increased uterine weight at 1 year only in the F1 generation. DEHP exposure also altered estrous cyclicity at 1 year in the F1 and F3 generations of mice. Further, DEHP increased the presence of cysts and altered follicle numbers in multiple generations. Finally, DEHP exposure affected sex steroid, gonadotropin, and peptide hormone levels at 1 year in all three generations of mice.
Interestingly, the effects of DEHP exposure were not the same in each generation. This is most likely due to each generation being exposed to the phthalate differently. The F1 generation was exposed directly as the developing pup, the F2 generation was exposed as gametes within the developing pup, and the F3 generation was the first generation that did not have direct exposure to DEHP.
DEHP has a short half-life because it is quickly metabolized and excreted (42). Therefore, it is unlikely that the DEHP levels present during gestation continued into the subsequent generations. Although we did not measure metabolites in the pregnant dams or offspring, it is possible that the pregnant dams quickly metabolized DEHP to its metabolite, MEHP. One study has shown that adult exposure to DEHP significantly increased the amount of MEHP in liver tissues taken from pregnant dams, the same mice postpartum, and their male offspring (43). Because DEHP is metabolized rapidly, it is unlikely that all of the observed effects in the F1, F2, or F3 generations are due to DEHP itself. Instead, it is possible that MEHP or other DEHP metabolites caused several of the reproductive outcomes observed in this study.
Our data indicate that DEHP exposure (200 µg/kg/d) decreased body weight at 1 year in the F3 generation, but did not affect body weights at 1 year in the F1 and F2 generations. This differs from a previous study in which prenatal exposure to a phthalate mixture, including DEHP, increased body weight in the F2 generation at 13 months of age (200 µg/kg/d) (30). Similarly, Manikkam et al. (44) found that low doses of plasticizers (bisphenol A, DEHP, and dibutyl phthalate) increased obesity in the F3 generation of 1-year-old female rats. It is possible our results differ from previous studies because our study exposed mice to DEHP only, and the other studies exposed animals to mixtures consisting of multiple EDCs, including DEHP.
Our data also show that prenatal DEHP exposure decreased ovarian weights at 1 year in the F1 and F3 generations, but not the F2 generation. The reasons that DEHP decreased ovarian weight are unknown. Because ovarian weight can be dependent on follicle numbers and corpora lutea numbers, we expected to observe a decrease in follicle and/or corpora lutea numbers. However, this was not the case. In the F1 generation, DEHP (20 µg/kg/d) decreased ovarian weight, but not follicle numbers. The 750-mg/kg/d treatment of DEHP did not decrease ovarian weight, but it decreased follicle numbers. Further, in the F3 generation, the 20- and 200-µg/kg/d and 500-mg/kg/d treatments decreased ovarian weight, but they did not decrease any follicle type or total follicle numbers. In addition, DEHP did not affect corpora lutea numbers in any generation of mice, and corpora lutea contribute more to ovarian weight more than follicles.
DEHP exposure also increased uterine weights at 1 year in the F1 generation, but not in other generations. This finding correlates with our data showing that DEHP exposure increased levels of estradiol in the F1 generation. Estrogen causes hyperemia and fluid uptake in the uterus, therefore causing an increase in uterine weight (45). In contrast to our study, prenatal exposure to a phthalate mixture did not affect uterine weights at 13 months of age in mice (29). The difference in these studies could be that our study focused on only DEHP, rather than a mixture of multiple phthalates, which could be acting differently on the uterus.
Our data indicate that all doses of DEHP decreased liver weights at 1 year in the F3 generation, but not in the F1 or F2 generations. This DEHP-induced decrease in liver weight is different from what we expected because phthalates, including DEHP, have been shown to increase liver weights by acting on peroxisome proliferator–activated receptors, resulting in liver cell hypertrophy (46). In a similar study, prenatal DEHP exposure reduced liver weights in female mice at postnatal day (PND) 8 (47). In contrast, prenatal exposure to a phthalate mixture increased liver weights in the F2 generation of mice at PND 1 and PND 21, but did not alter liver weight in the F3 generation of female mice (30). It is possible our results differ due to a mixture of phthalates vs one single phthalate and the route of exposure (F2 vs F3).
Prenatal DEHP exposure decreased AGD at 1 year in the 200-µg/kg/d treatment group in the F2 generation, but not the F1 and F3 generations. AGD is determined by prenatal androgen levels, so it is possible that DEHP decreased androgen levels in the F1 generation, leading to a decrease in AGD in the F2 generation. Although our data indicate that DEHP treatment decreased testosterone levels, this decrease was not statistically noteworthy. However, it is possible that this study did not have a large enough sample size to detect statistically meaningful differences in testosterone levels.
Prenatal exposure to DEHP altered cyclicity at 1 year in both the F1 and F3 generations of mice, but it did not affect cyclicity in the F2 generation. In the F1 generation, DEHP increased the time spent in proestrus and metestrus/diestrus, whereas it decreased time in estrus. These results are very similar to a study by Hannon et al. (15), in which adult acute exposure to DEHP decreased time spent in estrus, but increased time spent in metestrus/diestrus. The increased time spent in metestrus/diestrus indicates the animals are becoming acyclic, and they are exhibiting signs of reproductive aging. It is interesting that we did not observe any changes in cyclicity in the F2 generation of mice, but this could be due to different exposure windows (exposure as fetus vs germ cells). In the F3 generation, prenatal DEHP exposure decreased the time spent in proestrus, altered the time spent in estrus, and increased the time spent in metestrus/diestrus. Rodents exhibit two stages of acyclicity, the first being a persistent state of estrus followed by a persistent state of diestrus (23). Interestingly, mice in the 500-mg/kg/d treatment group spent 100% of their time in diestrus/metestrus, clearly demonstrating persistent diestrus.
DEHP altered follicle numbers at 1 year in the F1, F2, and F3 generations of mice. In the F1 generation of mice, prenatal DEHP exposure decreased follicle numbers, whereas in the F2 and F3 generations, DEHP increased follicle numbers. In the F1 generation, DEHP exposure drastically decreased the number of primordial follicles. This drastic decline in primordial follicles, decrease in total follicle counts, and prolonged estrous cyclicity indicates that prenatal exposure to DEHP can accelerate reproductive aging. Interestingly, acute DEHP exposure (500 mg/kg/d) for 10 days decreased primordial follicle numbers and total follicle counts 9 months after dosing in mice (15). In women, this decrease in the primordial follicle pool is the final reproductive event in menopause (48). In the F2 and F3 generations, we observed increases in follicle numbers, but this could be due to different windows of exposure.
Prenatal DEHP exposure affected the percent of follicles at 1 year in multiple generations of mice. DEHP accelerated folliculogenesis in the F1 generation, whereas DEHP decreased folliculogenesis in the F3 generation. Our results in the F1 generation are similar to those of Hannon et al. (15), who observed a drastic decrease in the percent of primordial follicles 9 months after an acute exposure to DEHP. It is possible that this decrease in the percent of primordial follicles is due to an increase in atresia. However, this is unlikely because we did not observe any changes in atresia in any generation of mice (data not shown), although we may have missed the time point when we would observe an increase in atresia. The mice were not euthanized until they were 12 months of age, whereas Hannon et al. (15) observed an increase in atresia when mice were 9 months old. In the F3 generation, the decrease in folliculogenesis could be due to epigenetic modifications to the DNA, such as DNA methylation (49, 50). For example, a low-dose mixture of plastics given to the F0 generation, including DEHP, significantly increased the incidence of primordial follicle pool loss in the F3 generation of female rats, and this is thought to be caused by epigenetic transgenerational inheritance following ancestral environmental exposures (44). Our results may differ in the F3 generation, however, because we examined the effects of DEHP alone and not a mixture of plasticizers, and our exposure in the F0 ended at birth, whereas exposure to the mixture of plasticizers ended at GD 14.
Besides affecting the number of follicles in multiple generations of female mice, prenatal DEHP exposure also increased the presence of cystic ovaries at 1 year in multiple generations of mice. In the F1 generation, DEHP exposure significantly increased the presence of cysts. In the F2 and F3 generations, 100% of the DEHP-treated (200 μg/kg/d and 500 mg/kg/d) females had cystic ovaries. However, due to small numbers in each treatment group, we did not have enough statistical power for these treatment groups to be statistically different from the controls. Ovarian cysts often are a sign of reproductive aging (28). Different types of cysts can be found in the ovary, including bursal cysts, follicular cysts, luteal cysts, and rete ovarii cysts (51). In this study, we observed bursal, follicular, and luteal cysts. Similar to our results, prenatal exposure to a phthalate mixture, including DEHP, increased the incidence of cystic ovaries in both the F1 and F2 generations of female mice (29, 30). Further, gestational exposure to the EDC vinclozolin increased the percentage of ovarian cysts in the F1, F2, and F3 generations of ∼1-year-old female mice (52). The mechanism by which EDCs cause cyst formations is unknown, but studies suggest the Notch signaling pathway may be involved because transgenic mice with conditionally activated NOTCH1 developed ovarian cysts at 8 months of age (53).
While examining cysts in each generation, it appears that prenatal DEHP may be causing ovarian endosalpingiosis. Histologically, this is defined by the presence of cystic glandular structures lined by benign tubal epithelium (41). Little is known about endosalpingiosis, but it has been linked to pelvic pain in women (41). A study examining the demographics and clinical presentation of endosalpingiosis discovered that out of 110 women, 40% displaying endosalpingiosis were postmenopausal women (54). It is possible that DEHP may be accelerating reproductive aging and, as a result, causing the onset of endosalpingiosis. However, future studies should examine this possibility in more detail.
Our data also indicate that prenatal DEHP exposure altered sex steroid hormone levels at 1 year in multiple generations of mice. DEHP increased estradiol levels in the F1 and F3 generations, but did not affect estradiol levels in the F2 generation. In the F1 generation, the increases in estradiol were in the same treatment groups (500 and 750 mg/kg/d) as the increases in uterine weight. These data suggest that the DEHP-induced increase in uterine weight could be due to the DEHP-induced increase in estradiol, particularly because estradiol levels are known to increase uterine weight. DEHP also decreased the levels of testosterone in all three generations of mice. The levels of testosterone decline during the menopausal transition (55, 56). Thus, it is possible that prenatal DEHP exposure decreases the levels of testosterone, leading to early onset of reproductive aging. DEHP exposure decreased the levels of progesterone in the F2 generation, but did not affect progesterone levels in the F1 and F3 generations. This is in contrast to one study that has shown that oral exposure to DEHP for 16 weeks decreased serum progesterone levels in female mice (57). The difference in results could be due to different timing of exposure. Our study prenatally exposed mice to DEHP for ∼10 days, whereas the other study exposed mice during adulthood for 16 weeks. Further, our DEHP doses ranged from 20 µg to 750 mg, whereas in the other study (57), doses ranged from 125 to 2000 mg.
DEHP exposure altered the levels of gonadotropin and peptide hormones at 1 year in the F1 and F3 generations of mice, but not in the F2 generation. In the F1 generation in the 750-mg/kg/d treatment group and in the F3 generation in the 500-mg/kg/d treatment group, DEHP exposure increased FSH levels and decreased inhibin B levels. This finding is interesting because a decrease in inhibin B and rise in FSH levels are major characteristics of reproductive aging (58, 59). Further, the low levels of inhibin B may be affecting the theca cells in the ovary. Low levels of inhibin B have been shown to act on theca cells in the ovary, leading to decreased synthesis of dehydroepiandrosterone and causing a decrease in follicle numbers and early reproductive aging (60). However, this mechanism should be examined in future studies.
Our data showing that DEHP exposure affects gonadotropin levels suggest that DEHP may be acting on the hypothalamic-pituitary-gonadal axis. This is consistent with studies that have shown that adult exposure to DEHP significantly decreased serum FSH levels, pituitary FSH levels, and pituitary LH levels in female rats (61). Further, adult exposure to DEHP increased the levels of gonadotropin-releasing hormone, whereas it decreased the levels of FSH and LH in female rats (62).
In conclusion, our data show that prenatal exposure to DEHP causes some multigenerational and transgenerational effects on female reproduction. DEHP caused changes in body and organ weights, altered estrous cyclicity, altered folliculogenesis, increased the presence of cystic ovaries, and affected sex steroid, gonadotropin, and peptide hormone levels at 1 year across multiple generations. Collectively, these data suggest that prenatal DEHP exposure may accelerate reproductive aging in mice. It would be ideal if future studies could observe similar transgenerational effects with DEHP and other EDCs as well. Further, future studies should examine the mechanisms by which DEHP is affecting the many aspects of female reproduction.
Acknowledgments
The authors thank the members of J.A.F.’s laboratory for assistance and the University of Virginia Center for Research in Reproduction Ligand Assay & Analysis Core [supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (National Centers for Translational Research in Reproduction and Infertility) Grant P50-HD28934] for assistance with measuring serum hormone levels.
Financial Support: This work was supported by National Institutes of Health Grant P01 ES022848 (to J.A.F.), US Environmental Protection Agency Grant RD-83543401 (to J.A.F.), and National Institutes of Health Grant T32 ES007326 (to S.R.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AGD
- anogenital distance
- DEHP
- di(2-ethylhexyl) phthalate
- EDC
- endocrine-disrupting chemical
- FSH
- follicle-stimulating hormone
- GD
- gestational day
- LH
- luteinizing hormone
- MEHP
- mono(2-ethylhexyl) phthalate
- PND
- postnatal day.
References
- 1.Koniecki D, Wang R, Moody RP, Zhu J. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res. 2011;111(3):329–336. [DOI] [PubMed] [Google Scholar]
- 2.Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29(1):134–139, discussion 181–185. [DOI] [PubMed] [Google Scholar]
- 3.Latini G. Monitoring phthalate exposure in humans. Clin Chim Acta. 2005;361(1-2):20–29. [DOI] [PubMed] [Google Scholar]
- 4.Doull J, Cattley R, Elcombe C, Lake BG, Swenberg J, Wilkinson C, Williams G, van Gemert M. A cancer risk assessment of di(2-ethylhexyl)phthalate: application of the new U.S. EPA Risk Assessment Guidelines. Regul Toxicol Pharmacol. 1999;29(3):327–357. [DOI] [PubMed] [Google Scholar]
- 5.Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, Zacharewski T. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol. 2002;16(5):529–653. [DOI] [PubMed] [Google Scholar]
- 6.Arbuckle TE, Fisher M, MacPherson S, Lang C, Provencher G, LeBlanc A, Hauser R, Feeley M, Ayotte P, Neisa A, Ramsay T, Tawagi G. Maternal and early life exposure to phthalates: The Plastics and Personal-care Products use in Pregnancy (P4) study. Sci Total Environ. 2016;551-552:344–356. [DOI] [PubMed] [Google Scholar]
- 7.Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, Rosskamp E, Schlüter C, Seifert B, Ullrich D. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207(5):409–417. [DOI] [PubMed] [Google Scholar]
- 8.Genuis SJ, Beesoon S, Lobo RA, Birkholz D. Human elimination of phthalate compounds: blood, urine, and sweat (BUS) study. Sci World J. 2012;2012:615068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ Health Perspect. 2004;112(3):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Högberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, Johansson N, Appelgren M, Håkansson H. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect. 2008;116(3):334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. Exposure to Di(2-ethylhexyl)phthalate in humans during pregnancy. A preliminary report. Biol Neonate. 2003;83(1):22–24. [DOI] [PubMed] [Google Scholar]
- 12.Krotz SP, Carson SA, Tomey C, Buster JE. Phthalates and bisphenol do not accumulate in human follicular fluid. J Assist Reprod Genet. 2012;29(8):773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front Endocrinol (Lausanne). 2015;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hannon PR, Peretz J, Flaws JA. Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod. 2014;90(6):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannon PR, Niermann S, Flaws JA. Acute exposure to di(2-ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicol Sci. 2016;150(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, Focarelli R. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14(2):131–142. [DOI] [PubMed] [Google Scholar]
- 17.Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol Reprod. 1994;50(3):653–663. [DOI] [PubMed] [Google Scholar]
- 18.Nelson JF, Bergman MD, Karelus K, Felicio LS. Aging of the hypothalamo-pituitary-ovarian axis: hormonal influences and cellular mechanisms. J Steroid Biochem. 1987;27(4-6):699–705. [DOI] [PubMed] [Google Scholar]
- 19.Santoro N. The menopause transition: an update. Hum Reprod Update. 2002;8(2):155–160. [DOI] [PubMed] [Google Scholar]
- 20.Santoro N, Isaac B, Neal-Perry G, Adel T, Weingart L, Nussbaum A, Thakur S, Jinnai H, Khosla N, Barad D. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88(11):5502–5509. [DOI] [PubMed] [Google Scholar]
- 21.de Kat AC, van der Schouw YT, Eijkemans MJ, Herber-Gast GC, Visser JA, Verschuren WM, Broekmans FJ. Back to the basics of ovarian aging: a population-based study on longitudinal anti-Müllerian hormone decline. BMC Med. 2016;14(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gee DM, Flurkey K, Finch CE. Aging and the regulation of luteinizing hormone in C57BL/6J mice: impaired elevations after ovariectomy and spontaneous elevations at advanced ages. Biol Reprod. 1983;28(3):598–607. [DOI] [PubMed] [Google Scholar]
- 23.Scarbrough K, Wise PM. Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology. 1990;126(2):884–890. [DOI] [PubMed] [Google Scholar]
- 24.Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endocrinol. 2011;25(12):2157–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vidal JD, Mirsky ML, Colman K, Whitney KM, Creasy DM. Reproductive system and mammary gland. In: Sahota PS, Popp JA, Hardisty JF, Gopinath C, eds. Toxicologic Pathology. Boca Raton, FL: CRC Press; 2013:717–830. [Google Scholar]
- 26.Peluso JJ, Steger RW, Huang H, Meites J. Pattern of follicular growth and steroidogenesis in the ovary of aging cycling rats. Exp Aging Res. 1979;5(4):319–333. [DOI] [PubMed] [Google Scholar]
- 27.Bukovsky A, Ayala ME, Dominguez R, Keenan JA, Wimalasena J, McKenzie PP, Caudle MR. Postnatal androgenization induces premature aging of rat ovaries. Steroids. 2000;65(4):190–205. [DOI] [PubMed] [Google Scholar]
- 28.Creasy D. Reproduction of the rat, mouse, dog, non-human primate and minipig In: McInnes EF, ed. Background Lesions in Laboratory Animals. St. Louis, MO: W.B. Saunders; 2012:101–122. [Google Scholar]
- 29.Zhou C, Gao L, Flaws JA. Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol Appl Pharmacol. 2017;318:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou C, Gao L, Flaws JA. Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology. 2017;158(6):1739–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pocar P, Fiandanese N, Berrini A, Secchi C, Borromeo V. Maternal exposure to di(2-ethylhexyl)phthalate (DEHP) promotes the transgenerational inheritance of adult-onset reproductive dysfunctions through the female germline in mice. Toxicol Appl Pharmacol. 2017;322:113–121. [DOI] [PubMed] [Google Scholar]
- 32.Do RP, Stahlhut RW, Ponzi D, Vom Saal FS, Taylor JA. Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod Toxicol. 2012;34(4):614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson HKL, Svingen T, Fowler PA, Vinggaard AM, Boberg J. Environmental influences on ovarian dysgenesis - developmental windows sensitive to chemical exposures. Nat Rev Endocrinol. 2017;13(7):400–414. [DOI] [PubMed] [Google Scholar]
- 34.Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod. 2013;88(5):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. [DOI] [PubMed] [Google Scholar]
- 36.Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44(12):622–632. [DOI] [PubMed] [Google Scholar]
- 37.Gallavan RH Jr, Holson JF, Stump DG, Knapp JF, Reynolds VL. Interpreting the toxicologic significance of alterations in anogenital distance: potential for confounding effects of progeny body weights. Reprod Toxicol. 1999;13(5):383–390. [DOI] [PubMed] [Google Scholar]
- 38.Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol. 1994;8(6):509–514. [DOI] [PubMed] [Google Scholar]
- 39.Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicol Sci. 2002;68(2):473–478. [DOI] [PubMed] [Google Scholar]
- 40.Benedict JC, Miller KP, Lin TM, Greenfeld C, Babus JK, Peterson RE, Flaws JA. Aryl hydrocarbon receptor regulates growth, but not atresia, of mouse preantral and antral follicles. Biol Reprod. 2003;68(5):1511–1517. [DOI] [PubMed] [Google Scholar]
- 41.Bristol-Gould SK, Hutten CG, Sturgis C, Kilen SM, Mayo KE, Woodruff TK. The development of a mouse model of ovarian endosalpingiosis. Endocrinology. 2005;146(12):5228–5236. [DOI] [PubMed] [Google Scholar]
- 42.Koch HM, Bolt HM, Angerer J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol. 2004;78(3):123–130. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi Y, Ito Y, Yanagiba Y, Kamijima M, Naito H, Nakajima T. Differences in metabolite burden of di(2-ethylhexyl)phthalate in pregnant and postpartum dams and their offspring in relation to drug-metabolizing enzymes in mice. Arch Toxicol. 2012;86(4):563–569. [DOI] [PubMed] [Google Scholar]
- 44.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8(1):e55387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281(36):26683–26692. [DOI] [PubMed] [Google Scholar]
- 46.Rusyn I, Peters JM, Cunningham ML. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit Rev Toxicol. 2006;36(5):459–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niermann S, Rattan S, Brehm E, Flaws JA. Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod Toxicol. 2015;53:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8(2):141–154. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova MI, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS One. 2012;7(5):e36129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nilsson EE, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of reproductive disease. Biol Reprod. 2015;93(6):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dixon D, Alison R, Bach U, Colman K, Foley GL, Harleman JH, Haworth R, Herbert R, Heuser A, Long G, Mirsky M, Regan K, Van esch E, Westwood FR, Vidal J, Yoshida M. Nonproliferative and proliferative lesions of the rat and mouse female reproductive system. J Toxicol Pathol. 2014;27(3-4 Suppl):1s–107s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerrero-Bosagna C, Covert TR, Haque MM, Settles M, Nilsson EE, Anway MD, Skinner MK. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod Toxicol. 2012;34(4):694–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferguson L, Kaftanovskaya EM, Manresa C, Barbara AM, Poppiti RJ, Tan Y, Agoulnik AI. Constitutive notch signaling causes abnormal development of the oviducts, abnormal angiogenesis, and cyst formation in mouse female reproductive tract. Biol Reprod. 2016;94(3):67. [DOI] [PubMed] [Google Scholar]
- 54.Prentice L, Stewart A, Mohiuddin S, Johnson NP. What is endosalpingiosis? Fertil Steril. 2012;98(4):942–947. [DOI] [PubMed] [Google Scholar]
- 55.Bancroft J, Cawood EH. Androgens and the menopause; a study of 40-60-year-old women. Clin Endocrinol (Oxf). 1996;45(5):577–587. [DOI] [PubMed] [Google Scholar]
- 56.Overlie I, Moen MH, Morkrid L, Skjaeraasen JS, Holte A. The endocrine transition around menopause--a five years prospective study with profiles of gonadotropines, estrogens, androgens and SHBG among healthy women. Acta Obstet Gynecol Scand. 1999;78(7):642–647. [PubMed] [Google Scholar]
- 57.Li N, Liu T, Zhou L, He J, Ye L. Di-(2-ethylhcxyl) phthalate reduces progesterone levels and induces apoptosis of ovarian granulosa cell in adult female ICR mice. Environ Toxicol Pharmacol. 2012;34(3):869–875. [DOI] [PubMed] [Google Scholar]
- 58.Burger HG. The endocrinology of the menopause. Maturitas. 1996;23(2):129–136. [DOI] [PubMed] [Google Scholar]
- 59.Ahmed Ebbiary NA, Lenton EA, Cooke ID. Hypothalamic-pituitary ageing: progressive increase in FSH and LH concentrations throughout the reproductive life in regularly menstruating women. Clin Endocrinol (Oxf). 1994;41(2):199–206. [DOI] [PubMed] [Google Scholar]
- 60.Ford JH. Reduced quality and accelerated follicle loss with female reproductive aging - does decline in theca dehydroepiandrosterone (DHEA) underlie the problem? J Biomed Sci. 2013;20(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirosawa N, Yano K, Suzuki Y, Sakamoto Y. Endocrine disrupting effect of di-(2-ethylhexyl)phthalate on female rats and proteome analyses of their pituitaries. Proteomics. 2006;6(3):958–971. [DOI] [PubMed] [Google Scholar]
- 62.Liu T, Li N, Zhu J, Yu G, Guo K, Zhou L, Zheng D, Qu X, Huang J, Chen X, Wang S, Ye L. Effects of di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-ovarian axis in adult female rats. Reprod Toxicol. 2014;46:141–147. [DOI] [PubMed] [Google Scholar]