Abstract
Objective
We sought to determine the effect of acute electrolyte and osmolar shifts on brain volume and neurologic function in patients with liver failure and severe hepatic encephalopathy (HE).
Design
Retrospective analysis of brain computed tomography (CT) scans and clinical data.
Setting
Tertiary care hospital ICUs.
Patients
Patients with acute or acute-on-chronic liver failure and severe HE.
Interventions
Clinically indicated CT scans and serum laboratory studies.
Measurements and main results
Change in intracranial cerebrospinal fluid (CSF) volume between sequential CT scans was measured as a biomarker of acute brain volume change. Corresponding changes in serum osmolality, chemistry measurements and Glasgow Coma Scale (GCS) were determined. Associations with CSF volume change and GCS change for initial volume change assessments were identified by Spearman’s correlations (rs) and regression models. Consistency of associations with repeated assessments was evaluated using generalized estimating equations. Forty patients were included. Median baseline osmolality was elevated (310 [296–321] mOsm/Kg) while sodium was normal (137 [134–142] mEq/L). Median initial osmolality change was 9 (5–17) mOsm/kg. Neuroimaging consistent with increased brain volume occurred in 27 (68%) initial assessments. CSF volume change was more strongly correlated with osmolality (r=0.70, p=4×10−7) than sodium (r=0.28, p=0.08) change. Osmolality change was independently associated with GCS change (p=1×10−5) and CSF volume change (p=2.7×10−5) in initial assessments and in generalized estimating equations using all 103 available assessments.
Conclusion
Acute decline in osmolality was associated with brain swelling and neurologic deterioration in severe HE. Minimizing osmolality decline may avoid neurologic deterioration.
Keywords: hepatic encephalopathy, liver failure, coma, cerebral edema, neuroimaging, osmolality, renal replacement therapy
Hepatic encephalopathy (HE)—liver disease-related brain dysfunction—is a leading reason for intensive care unit (ICU) admission and contributes to morbidity and mortality in liver disease.(1–4) During liver failure, severe HE is associated with life-threatening cerebral edema.(2, 5) While HE is associated with hyperammonemia and the resulting compromise of astrocytes’ homeostatic functions, there are likely other contributors.(2, 6)
Hyponatremia is associated with increased HE occurrence and influences mortality after acute liver dysfunction.(7–9) Some hypothesize that the osmotic mechanism by which astrocytes regulate brain volume is disturbed in HE and hyponatremia strains this system’s functional reserve.(2, 10, 11) Acute reduction in serum osmotically active substances (osmolytes) is a proposed mechanism underlying instances of cerebral edema and neurologic deterioration in metabolic disease states including dialysis disequilibrium syndrome, diabetic ketoacidosis, hyperosmolar hyperglycemic state, exercise-associated hyponatremia, and rebound cerebral edema after mannitol therapy.(12–18) As osmolality is rarely monitored in acute liver disease, it is possible that unrecognized osmolality changes might contribute to cerebral edema and neurologic deterioration in these patients.
Our aim was to investigate if changes in brain volume and neurologic function are associated with acute changes in serum osmolality in patients with severe HE from acute (ALF) or acute-on-chronic (ACLF) liver failure. Secondarily, we examined the effects of renal replacement therapy (RRT) on serum osmolality and brain volume to illustrate an application of attention to osmolality change. We compared RRT administered concurrently with hypertonic saline (HTS)—a therapy used in the ICU to increase serum osmolality—to RRT administered without HTS.
Methods
We abstracted electronic record data from our neurologic critical care service to retrospectively identify patients ≥18 years old admitted to an ICU between July 2012 and June 2017 with ALF or ACLF and severe HE. Given similar phenotypic presentations and our intention to explore pathophysiologic mechanisms, we included patients with either ALF and ACLF.(19, 20) Neurologic critical care consultation is routine for patients with liver failure and West Haven grade 2 (lethargy) or worse HE at our institution.(21) The diagnosis of liver failure was identified from attending intensivist documentation and confirmed according to published definitions.(21, 22) Severe HE was defined as West Haven grade 3 (somnolent) or 4 (coma) as documented by an attending neurologist.(21) Study inclusion required (i) ≥2 head computed tomography (CT) scans acquired within 48 hours of each other, (ii) the first CT obtained within 48 hours of admission, (iii) availability of serially measured serum osmolality and chemistries, and (iv) serially measured Glasgow Coma Scale (GCS) scores, a neurologic examination scale ranging from 3 (deepest coma) to 15 (normal, alert and oriented). The GCS has good inter-rater reliability and is recommended for use in HE.(20, 23–25). We excluded patients with craniectomy or drain placement for CSF diversion and acute focal brain lesions (e.g., stroke).
Consistent with several members of the US Acute Liver Failure Study Group, we infrequently use invasive intracranial pressure (ICP) monitors to manage HE.(26, 27) In this population, we use hourly neurologic examinations and repeat head CT scans as previously described.(28, 29) Neuroimaging frequency is guided by degree of neurologic impairment, prior imaging, and clinical trajectory. All HE patients in our ICUs receive hourly neurologic assessments, including GCS, performed by trained ICU nurses who electronically record assessments as they are performed.(28, 29)
We collected demographic and clinical data including GCS, measured serum osmolality, and serum chemistry and laboratory data from the electronic medical record for each patient at the time of admission and each CT. We collected Richmond Agitation and Sedation Scores (RASS, −5 [un-arousable] to 0 [alert and calm] to +4 [combative]) as a secondary neurologic examination. We collected doses of analgesics and sedatives administered within both a 2-hour and 6-hour period prior to each neurologic assessment and determined whether sedation increased, decreased, or remained the same between assessments. No patient was exposed to paralytics during these time periods. We calculated model for end-stage liver disease (MELD-Na) and Acute Physiology Scores (APS) as measures of overall illness severity.(30) Demographic, neurologic exam, laboratory, medication, and neuroimaging data were collected as separate blinded queries.
Intervals for Assessing Volume, Osmolality, and GCS Change
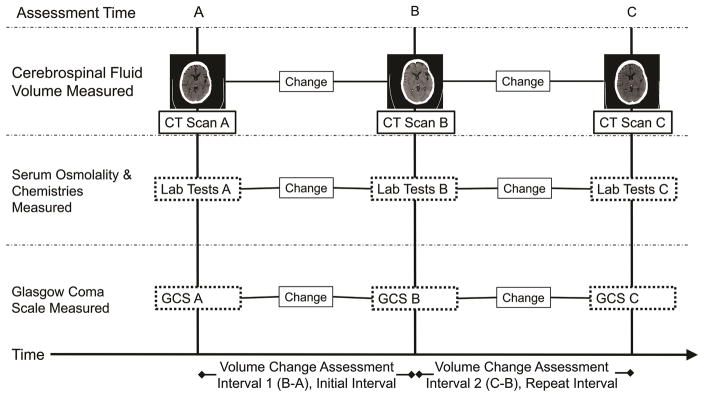
We used the acquisition times of two sequential head CT scans acquired within 48 hours of one another to define intervals over which changes in brain volume and corresponding changes in GCS, individual osmolytes, and total serum osmolality were assessed. Figure 1 illustrates how these assessment intervals were defined. We used patients’ initial assessment intervals for our primary analysis but also employed repeated measures statistical techniques to analyze all available assessment intervals. In these patients, we routinely monitor serum osmolality and chemistry panels at least every 6 hours and calculate serum osmolar gaps.(31)
Figure 1. Defining volume change assessment intervals.
Volume change assessment intervals were defined for each patient as the time between subsequent computed tomography (CT) scans. Cerebrospinal fluid volume was measured on each CT scan and the change in volume was calculated. The corresponding changes in serum osmolality, serum chemistries, and Glasgow Coma Scale (GCS) neurologic examinations for each assessment interval were determined using values collected nearest the acquisition time of the CT scans. For GCS, we used the hourly neurologic assessment immediately prior to CT scan. Only the initial assessment intervals were used for Spearman correlations and linear and ordinal regression models. All assessment intervals were used for generalized estimating equation models.
Renal Replacement Therapy and Hypertonic Saline
We identified the use and administration time of RRT and HTS for each patient. RRT was administered following current methods for liver failure and brain injury.(21, 32)
For all patients with severe HE receiving RRT, our neurocritical care consultants recommend administering concurrent HTS to avoid serum osmolality reductions greater than 10 (grade 4 HE) to 20 (grade 3 HE) mOsm/Kg per day.(32, 33) This includes 3% infusion or 23.4% bolus HTS when RRT is initiated and monitoring osmolality panels to guide HTS dosing adjustments. We do not use mannitol in liver failure patients, which was confirmed by record review. The use of HTS during RRT is ultimately at the discretion of the primary ICU service. As such, we identified assessment intervals in which patients underwent RRT either with or without concurrent HTS.
Quantification of Volume Change
We measured volumes on 5-mm thick contiguous slice digital imaging and communications in medicine (DICOM) CT scans using semi-automated computer software (Analyze Direct 11.0, Overland Park, KS) and the pixel intensity threshold technique. We previously demonstrated that CSF volume is a reliable biomarker of brain volume change and more indicative of changes than measuring whole brain volume on CT scans.(28, 34)
Statistical Analysis and Approvals
Initial assessments
We used only initial assessments for calculation of Spearman correlations and both linear and ordinal regression models. We determined correlation coefficients (rs) for associations between initial changes in CSF volume, osmolality and chemistry variables, and GCS. We sought to determine if acute serum osmolality change is independently associated with CSF volume change (linear regression) and GCS change (ordinal regression) and to quantify these associations with predictive models examining the impact of multiple covariates. We constructed models stepwise starting with osmolality change and, for GCS models, included sedation change between neurologic assessments. We treated analgesia and sedation categorically as increase, decrease, or no change between assessments because the response to a given dose of sedative varies between patients. Subsequent demographic and clinical variables (see Table 3) were individually assessed for model inclusion. Variables qualified for inclusion by: 1) inclusion changed the model’s Beta coefficients by greater than the coefficient’s standard error, suggesting modification effect, or 2) the variable demonstrated trend towards significance (p<0.2). For ordinal regression, we confirmed the proportional odds assumption using the test of parallel lines.
Table 3.
Regression models for associations with CSF volume change and GCS change.
| Linear Regression of CSF Volume Change (mL), initial assessment intervals | |||
|---|---|---|---|
| Variables included | βa coefficient | 95% CI | P-value |
| Unadjusted | |||
| Osmolality Change (mOsm/kg) | 1.17 | 0.70 – 1.64 | 1×10−5 |
|
| |||
| Adjusted | |||
| Osmolality Change (mOsm/kg) | 1.54 | 0.90 – 2.19 | 2.7×10−5 |
| Admission Serum Ammonia | 0.05 | −0.02 – 0.11 | 0.16 |
| BUN Change (mg/dL) | −0.29 | −0.72 – 0.14 | 0.19 |
| Admission APS Score | 0.11 | −0.12 – 0.34 | 0.35 |
|
| |||
| GEE Linear Regression of CSF Volume Change (mL), all assessment intervals | |||
| Variables included | βa coefficient | 95% CI | P-value |
|
| |||
| Osmolality Change (mOsm/kg) | 1.34 | 0.92 – 1.75 | 3×10−10 |
| Admission Serum Ammonia | 0.03 | 0.002 – 0.06 | 0.04 |
| BUN Change (mg/dL) | −0.15 | −0.43 – 0.13 | 0.29 |
| Admission APS Score | 0.13 | 0.02 – 0.24 | 0.02 |
|
| |||
| Ordinal Regression of GCS Change, initial assessment intervals | |||
| Variables included | Odds Ratiob | 95% CI | P-value |
|
| |||
| Unadjusted | |||
| Osmolality Change (mOsm/kg) | 1.06 | 1.03 – 1.09 | 0.0004 |
|
| |||
| Adjusted | |||
| Osmolality Change (mOsm/kg) | 1.08 | 1.05 – 1.12 | 1×10−5 |
| Ammonia Change (μg/dL) | 0.994 | 0.989 – 0.998 | 0.011 |
| Admission APS score | 1.018 | 1.003 – 1.033 | 0.015 |
| Temperature Change (°C) | 0.945 | 0.285 – 3.13 | 0.95 |
| Sedation Changec | |||
| No Change | Reference | -- | -- |
| Decrease | 0.12 | 0.008 – 1.90 | 0.13 |
| Increase | 0.73 | 0.17 – 3.26 | 0.68 |
|
| |||
| GEE Ordinal Regression of GCS Change, all assessment intervals | |||
| Variables included | Odds Ratiob | 95% CI | P-value |
|
| |||
| Osmolality Change (mOsm/kg) | 1.07 | 1.05 – 1.10 | 1×10−8 |
| Ammonia Change (μg/dL) | 0.997 | 0.994 – 1.000 | 0.04 |
| Admission APS score | 1.02 | 1.01 – 1.02 | 0.0001 |
| Temperature Change (°C) | 1.56 | 0.94 – 2.61 | 0.09 |
| Sedation Changec | |||
| No Change | Reference | -- | -- |
| Decrease | 0.78 | 0.38 – 1.60 | 0.50 |
| Increase | 1.41 | 0.71 – 2.80 | 0.32 |
Variables assessed for model inclusion included: serum osmolality change, age, sex, acute versus acute-on-chronic liver failure, APS score, number of admission SIRS criteria met, MELD-NA, renal replacement therapy exposure during assessment interval, mean temperature during assessment interval, temperature change across the assessment interval, serum ammonia change, serum sodium change, serum BUN change, serum glucose change, admission serum ammonia, time delay between serum osmolality measurement and CT scan, and (for GCS models) analgesia/sedation dosage change across the assessment interval.
Beta (β) coefficients represent the expected change in CSF volume (mL) for a 1-unit increase in the predictor variable.
Odds ratios represent the expected odds of GCS improvement (≥1 GCS point increase) with a 1-unit increase in the predictor variable.
Results using the 6-hour time period for analgesia/sedation assessment, as described under methods, are presented. The 2-hour time period yielded similar results.
APS=acute physiology score, BUN=blood urea nitrogen, CI=confidence interval, CSF=cerebrospinal fluid, GCS=Glasgow Coma Scale, MELD-Na=model for end-stage liver disease—sodium
Repeated assessments
We also wanted to determine if associations identified in the initial assessment intervals were present in patients’ subsequent assessments. Generalized estimating equations (GEE) allowed us to analyze all available volume change assessments while accounting for some patients having more than one assessment. The variables in the GEE models were those used for the corresponding initial assessment models. We used linear modeling with identity link function for CSF volume change, ordinal modeling with cumulative logit link function for GCS change, and exchangeable correlation matrices.
We compared continuous and ordinal variables between RRT groups using Mann-Whitney U test. We considered two-tailed p value of ≤0.05 significant. Standard statistical software was used (SPSS v.23, IBM, Armonk, NY). The study was approved by our institutional review board with waiver of consent for retrospective study.
Results
There were 140 patients with liver failure and at least grade 2 HE. Reasons for exclusion included: structural brain lesions (30), HE grades <3 (36), and no qualifying neuroimaging (34). Forty patients (29%) with ALF or ACLF and severe HE were included and contributed 103 assessment intervals (median 2/patient). Median initial assessment interval duration was 22.8 (10.5–31.2) hours and for all intervals was 13.0 (8.6–24.7) hours. Median time between CT scan acquisition and corresponding laboratory measurements was 1.5 (0.8–2.6) hours. No patient had invasive ICP monitoring during the study.
Demographic and clinical data for the entire cohort are summarized in Table 1. Median initial serum osmolality was elevated (310 [296–321] mOsm/Kg) while initial serum sodium was normal (137 [134–142] mEq/L). Median magnitude of osmolality change in the initial assessment intervals was 9 (5–17) mOsm/kg. Reduction in CSF volume commensurate with increased brain volume occurred in 27 (68%) initial assessment intervals. We found ALF patients were younger, had higher admission serum sodium, and lower admission serum blood urea nitrogen than ACLF patients but similar disease severity scores and ammonia levels (Supplemental 1). Supplemental 1 also summarizes data for all 103 assessment intervals.
Table 1.
Cohort demographics and initial clinical and radiographic characteristics
| Demographic and clinical characteristics, N=40 patients | Result, n (%) or Median (Interquartile Range) | ||
|---|---|---|---|
|
| |||
| Age (years) | 47.5 (36–58) | ||
|
| |||
| Female | 26 (65%) | ||
|
| |||
| Acute Liver Failure | 22 (55%) | ||
|
| |||
| Etiology of Liver Failure | |||
| Acetaminophen | 12 (30%) | ||
| Autoimmune | 5 (13%) | ||
| Alcohol | 4 (10%) | ||
| Non-alcoholic steatohepatitis | 3 (8%) | ||
| Viral | 7 (18%) | ||
| Wilson’s Disease | 2 (5%) | ||
| Other | 7 (18%) | ||
|
| |||
| MELD-Na Score | 33.5 (29–41) | ||
|
| |||
| APS Score | 84 (60–113) | ||
|
| |||
| Admission Systemic Inflammatory Response Syndrome Criteria | |||
| Number of Criteria Met (out of 4) | 2.5 (1–3) | ||
| Heart Rate (/min) | 112 (93–132) | ||
| Respiratory Rate (/min) | 23 (18–30) | ||
| Body Temperature (°C) | 36.8 (36.4–37.2) | ||
| White Blood Cell Count (103/μL) | 13.9 (7.9–19.0) | ||
|
| |||
| Admission Glasgow Coma Scale Score | 9 (5–13) | ||
| Intubated | 28 (70%) | ||
|
| |||
| Admission Richmond Agitation and Sedation Scale1 | −3 (−4 - −2) | ||
|
| |||
| Admission Intracranial CSF Volume (mL) | 90.7 (50.0–128.4) | ||
|
| |||
| Admission Serum Osmolality (mOsm/Kg) | 310 (296–321) | ||
|
| |||
| Admission Serum Sodium (mEq/L) | 137 (134–142) | ||
|
| |||
| Admission Serum Blood Urea Nitrogen (mg/dL) | 38 (16–74) | ||
|
| |||
| Admission Serum Glucose (mg/dL) | 144 (108–169) | ||
|
| |||
| Admission Gap Osmoles (mOsm/L) | 9 (5–16) | ||
|
| |||
| Admission Serum Ammonia (μg/dL) | 193 (106–295) | ||
|
| |||
| Lactulose During Initial Assessment Interval | 33 (83%) | ||
|
| |||
| Rifaximin During Initial Assessment Interval | 28 (70%) | ||
|
| |||
| Initial Assessment Interval Radiographic or Clinical Variable | Median Magnitude of Change | Range of Change (min., max.) | |
|
| |||
| Intracranial CSF volume (mL) | 18.7 (8.3–31.3) | −84.5, 100.3 | |
|
| |||
| Serum Osmolality (mOsm/Kg) | 9 (5–17) | −47, 35 | |
|
| |||
| Serum Sodium (mEq/L) | 4 (2–7) | −8, 17 | |
|
| |||
| Serum Blood Urea Nitrogen (mg/dL) | 7 (2–18) | −123, 9 | |
|
| |||
| Serum Glucose (mg/dL) | 27 (11–58) | −178, 73 | |
|
| |||
| Serum Gap Osmoles (mOsm/L) | 6 (2–10) | −27, 11 | |
|
| |||
| Serum Ammonia (μg/dL) | 57 (24–153) | −408, 255 | |
|
| |||
| Body Temperature (°C) | 0.4 (0.2–0.6) | −2.4, 1.3 | |
|
| |||
| Glasgow Coma Scale Score | 2 (1–3) | −7, 4 | |
|
| |||
| Richmond Agitation and Sedation Scale | 1 (0–1) | −3, 2 | |
|
| |||
| Analgesia/Sedation Corresponding to Initial Assessment Intervals2 | Number of Intervals, n(%) | Median Dose | |
|
| |||
| Propofol (infusion, μg/kg/min) | 16 (40%) | 18 (5.6–33) | |
| Fentanyl (total dose IV, μg) | 16 (40%) | 120 (69–238) | |
| Hydromorphone (IV, mg) | 1 (3%) | 0.2, twice | |
| None | 15 (38%) | ---- | |
|
| |||
| Change in Analgesia/Sedation Dosage During Initial Assessment Interval2 | Median Glasgow Coma Scale Change During Corresponding Interval | ||
|
| |||
| Increased | 13 (33%) | 0 (−2 to 2) | |
| Decreased | 7 (18%) | −2 (−5 to 0) | |
| No Change | 20 (50%) | 0 (−2 to 1) | |
APS=acute physiology score, CSF=cerebrospinal fluid, IV=intravenous MELD-Na=model for end-stage liver disease
All Richmond Agitation and Sedation scores in the cohort were between 0 and −5.
Determined from 6-hour time period before neurologic assessments, as described in methods section. 2-hour time periods yielded similar results.
Table 2 summarizes Spearman correlation coefficients between changes in each osmolyte and changes in CSF volume and GCS score using only the initial volume change assessment from each patient. CSF volume change was associated with osmolality change (rs=0.70, p=4×10−7). Change in CSF volume was associated with change in neurologic function measured by GCS (rs=0.75, p=4×10−8). Graphical illustrations of CSF volume, GCS, and osmolality change are provided in Supplemental 2. Additional correlations between admission clinical variables and CSF volume and GCS change are provided in Supplemental 3.
Table 2.
Spearman correlation coefficients for associations between osmolyte, CSF volume, and Glasgow Coma Scale score changes during initial assessment intervals
| Change in: | CSF volume | Osmolality | Sodium | Blood Urea Nitrogen | Glucose | Gap Osmoles | Ammonia | Temperature (°C) | Glasgow Coma Scale |
|---|---|---|---|---|---|---|---|---|---|
| CSF volume | 1 | 0.70 P=4×10−7 |
0.28 P=0.08 |
0.46 P=0.003 |
0.28 P=0.09 |
0.41 P=0.01 |
0.08 P=0.62 |
0.09 P=0.58 |
0.75 P=4×10−8 |
| Osmolality | 1 | 0.51 P=8×10−4 |
0.64 P=7×10−6 |
0.26 P=0.11 |
0.45 P=0.003 |
0.23 P=0.15 |
0.12 P=0.46 |
0.54 P=3×10−4 |
|
| Sodium | 1 | 0.10 P=0.54 |
0.02 P=0.92 |
−0.33 P=0.035 |
−0.18 P=0.28 |
−0.16 P=0.33 |
0.24 P=0.14 |
||
| Blood Urea Nitrogen | 1 | 0.00 P=0.99 |
0.23 P=0.15 |
P=0.30 P=0.06 |
0.19 P=0.25 |
0.31 P=0.05 |
|||
| Glucose | 1 | 0.10 P=0.54 |
0.03 P=0.84 |
0.19 P=.25 |
0.24 P=0.15 |
||||
| Gap Osmoles | 1 | 0.41 P=0.008 |
0.21 P=0.19 |
0.29 P=0.07 |
|||||
| Ammonia | 1 | −0.05 P=0.74 |
−0.12 P=0.45 |
||||||
| Temper-ature (°C) | 1 | 0.22 P=0.18 |
|||||||
| Glasgow Coma Scale | 1 |
CSF=cerebrospinal fluid
Table 3 summarizes linear, ordinal, and GEE regression models for CSF volume change and GCS change. In a linear regression model, serum osmolality change was independently associated with CSF volume change (β=1.54, p=2.7×10−5). In an ordinal regression model, serum osmolality change (odds ratio=1.08, p=1×10−5), serum ammonia change (odds ratio 0.994, p=0.011), and admission APS score (odds ratio 1.018, p=0.015) were independently associated with GCS change. Serum osmolality change associations were similar using RASS examinations (Supplemental 4).
The linear GEE model using all 103 assessments also showed an independent association between serum osmolality change and CSF volume change (β=1.34, p=3×10−10). The ordinal GEE model using all 103 assessments showed an independent association between serum osmolality change and GCS change (odds ratio=1.07, p=1×10−8).
Of note, ALF versus ACLF did not qualify as a variable for inclusion in any of the above models. Moreover, separate GEE models demonstrated significant independent associations between CSF volume, GCS, and serum osmolality change in both ALF and ACLF patients (Supplemental 5).
Twenty-nine (73%) patients had an assessment interval that occurred with RRT. RRT was initiated with concurrent HTS (indicating management with attention to osmolality) in 14 (48%) and without HTS in 15 (52%) patients. These groups did not differ in MELD-Na, APS, initial GCS score, initial serum ammonia, osmolality, sodium, BUN, glucose, gap osmoles, or method of RRT (intermittent versus continuous). Table 4 compares the volume change intervals where RRT was initiated with concurrent HTS versus without HTS. Osmolality increased for RRT with HTS relative to RRT without HTS. The clinical sequelae were that CSF volume decreased, commensurate with increased cerebral edema, and GCS worsened in those who initiated RRT without HTS.
Table 4.
Characteristics of volume change intervals corresponding to initiation of renal replacement therapy either with or without concurrent hypertonic saline infusion.
| Variable | RRT with HTS infusion (14 assessments) | RRT without HTS infusion (15 assessments) | P value |
|---|---|---|---|
| Change in Cerebrospinal Fluid Volume (mL) | −0.2 (−16.7 to 17.0) | −18.3 (−36.0 to −10.0) | 0.012 |
| Change in Glasgow Coma Scale Score | 0.5 (0 to 2) | −2 (−3 to 0) | 0.008 |
| Change in Serum Osmolality (mOsm/Kg) | 3 (−11 to 11) | −14 (−31 to −5) | 0.002 |
| Change in Serum Sodium (mEq/L) | 7 (−1 to 10) | 1 (−4 to 3) | 0.012 |
| Change in Serum Blood Urea Nitrogen (mg/dL) | −2 (−24 to 2) | −16 (−48 to −4) | 0.070 |
| Change in Serum Glucose (mg/dL) | −9 (−38 to 15) | −28 (−51 to −19) | 0.35 |
| Change in Serum Gap Osmoles (mOsm/L) | −6 (−11 to 2) | −4 (−9 to 3) | 0.91 |
| Change in Serum Ammonia (μg/dL) | −38 (−85 to 5) | −119 (−162 to −9) | 0.077 |
| Mean Body Temperature During Interval (°C) | 36.7 (36.4 to 37.2) | 36.7 (36.2 to 36.8) | 0.25 |
| Change in Body Temperature (°C) | −0.5 (−1.4 to 0.3) | −0.1 (−0.5 to 0.3) | 0.35 |
| Continuous renal replacement therapy, n(%) | 14 (100%) | 13 (87%) | 0.48 |
HTS=hypertonic saline, RRT=renal replacement therapy, median (interquartile range)
Thirteen (33%) patients died during hospitalization. While those who died tended to experience lower nadir GCS scores during their initial assessment interval (median 6[3–7] vs. 7[4–12], p=0.08), we did not appreciate a pattern of CSF volume or osmolality change that predicted death.
Discussion
In our cohort of patients with severe HE, decreases in serum osmolality were significantly associated with worsened cerebral edema. Decreasing serum osmolality and increased edema were associated with neurologic worsening. We found evidence that serum osmolality changes affected cerebral edema and neurologic examination in both ALF and ACLF patients, suggesting both groups are subject to the underlying mechanism. Despite bivariate associations between individual osmolytes and CSF volume change, only the aggregate change in osmolality was independently associated with CSF volume change.
While most of our cohort initially had low to normal serum sodium, as expected in liver disease,(7–9) the majority nevertheless had elevated serum osmolality. Furthermore, this elevated osmolality included a gap not accounted for by directly measured osmolytes. Gap osmole levels acutely changed in some patients and contributed to total osmolality change. These gap osmoles might represent unrecognized osmotically active molecules that accumulate in liver failure. The contribution of gap osmolytes to acute osmolality change requires that osmolality be directly measured to accurately trend dynamic changes.
Therapeutically induced increases in osmolality, called “osmotherapy”, is a widely used treatment for cerebral edema in neurologic injuries.(18) One may suspect that reductions in serum osmolality, potentially accelerated by BUN and gap osmolyte clearance during RRT, could have the inverse effect of worsening cerebral edema. In a trial of 30 patients with acute liver failure, gradual HTS infusion targeting moderate hypernatremia was associated with reduced incidence of intracranial hypertension compared to controls.(33) While those authors did not measure osmolality or demonstrate a potential physiologic mechanism, they proposed osmolyte changes as an explanation.(33) Our findings suggest that the gradual infusion of HTS may have buffered changes in non-sodium osmolytes, thereby lessening cerebral edema.
RRT is of interest given its widespread use in liver failure and its mechanism in rapidly clearing small molecules from circulation. We found that patients who received RRT with concurrent HTS infusion were less likely to experience a decrease in osmolality or radiographic evidence of brain swelling compared to those who received RRT without HTS. These observations highlight a potential implication of the association between osmolality, neuroimaging findings, and neurologic examination. Even with modern techniques and continuous methods of RRT, critically ill patients—particularly those with brain pathology—may be at risk of neurologic injury or death from cerebral edema associated with RRT.(13, 34, 35) While some have suggested prophylactic adjustments to dialysate or infusion of hyperosmolar substances during RRT, most suggestions are based on limited human data and are largely untested.(32) Our data suggest that minimizing decline in serum osmolality during RRT may be associated with favorable clinical endpoints. However, unavailability of data on dialysis settings such as blood flow rate and fluid removal volume is a study limitation.
Dialysis disequilibrium syndrome—a syndrome of acute neurologic symptoms and cerebral edema after RRT—is the clinical entity in which the effect of acute osmolality change has been most studied. It has been theorized that rapid serum BUN reduction accompanying RRT creates an osmolar gradient between the brain and serum. Since the brain is not freely permeable to BUN, water flows down the osmolar gradient in to the brain.(12, 13) Uremic animals demonstrate post-dialysis urea gradients consistent with observed degrees of cerebral edema.(36, 37) Furthermore, uremic rats experience reduced brain expression of urea transporters and increased aquaporin-4 water channel expression while aquaporin-4 expression also increases during liver failure.(38–40) These molecular changes facilitate cerebral edema formation in the presence of a urea gradient. The associations seen in our cohort could be due to a similar mechanism of acute osmolar gradient generation, delayed osmolyte equilibration, and facilitated water movement in to the brain.
There are limitations to our study. Retrospective methods limit our ability to account for confounders. While we assessed for an effect of timing delay between CT and osmolality collection, a prospective investigation with contemporaneous CT and biochemical sample acquisition would be required to eliminate the potential bias of timing delays. The single center design may limit generalizability. Our attention to osmolality likely mitigated the potential for severe osmolality changes that could be observed at centers where osmolality monitoring is not routine. Our preference to avoid ICP monitors prevents us from commenting on associations with intracranial pressure.(27) While our data are consistent with a mechanistic effect of osmolality in both ALF and ACLF, the reader should be aware of additional unique contributors to HE, such as inflammation, and the possibility that the magnitude of effect from specific mechanisms may differ between these groups.(2, 6) Clinical trials based on mechanistic research studies in HE should investigate therapeutic effects in ALF and ACLF separately.
Conclusion
Acute changes in serum osmolality are independently associated with changes in intracranial CSF volume—a biomarker of brain volume change due to cerebral edema—and neurologic function in patients with severe HE. Measuring serum osmolality in severe HE may be beneficial since a hyperosmolar state may exist despite low or normal serum sodium. Efforts to avoid acute reductions in serum osmolality may minimize cerebral edema and neurologic deterioration in patients with severe HE.
Supplementary Material
Supplemental 1a. Comparison of demographic and clinical variables between ALF and ACLF patients
Supplemental 1b. Absolute magnitude and range of change for imaging and laboratory variables in the 103 assessment intervals.
APS=acute physiology score, CSF=cerebrospinal fluid, IQR=interquartile range, MELD-Na=model of end-stage liver disease--sodium
Acute liver failure (n=22) patients contributed 62 (60%) assessment intervals while acute-on-chronic liver failure (n=18) patients contributed 41 (40%) assessment intervals.
Supplemental 2a. Scatterplot of cerebrospinal fluid volume change versus osmolality change for the forty initial volume change assessment intervals
Supplemental 2b. Scatterplot of cerebrospinal fluid volume change versus Glasgow Coma Scale change for the forty initial volume change assessment intervals
Supplemental 2c. Change in Glasgow Coma Scale between the beginning and end of the initial assessment intervals
Supplemental 3. Spearman correlation coefficients for associations between change in CSF volume and Glasgow Coma Scale score during the forty initial assessment intervals and admission clinical variables
CSF=cerebrospinal fluid, APS=Acute Physiology Score, MELD-NA=model of end-stage liver disease—sodium score, SIRS=systemic inflammatory response syndrome, GCS=Glasgow Coma Scale, RASS=Richmond Agitation and Sedation Scale
Supplemental 4. Ordinal regression model for associations with RASS change.
aOdds ratios represent the expected odds of GCS improvement (≥1 GCS point increase) with a 1-unit increase in the predictor variable.
Variables assessed for model inclusion included: serum osmolality change, age, sex, acute versus acute-on-chronic liver failure, APS score, number of admission SIRS criteria meet, MELD-NA, renal replacement therapy exposure during assessment interval, mean temperature during assessment interval, temperature change across the assessment interval, serum ammonia change, serum sodium change, serum BUN change, serum glucose change, admission serum ammonia, time delay between serum osmolality measurement and CT scan, and (for GCS models) analgesia/sedation dosage change across the assessment interval.
APS=acute physiology score, BUN=blood urea nitrogen, CI=confidence interval, CSF=cerebrospinal fluid, MELD-Na=model for end-stage liver disease—sodium, RASS=Richmond Agitation and Sedation Scale
Supplemental 5a. Generalized estimating equation models for associations with CSF volume change and GCS change in acute liver failure patients (n=22).
Supplemental 5b. Generalized estimating equation models for associations with CSF volume change and GCS change in acute-on-chronic liver failure patients (n=18).
aBeta (β) coefficients represent the expected change in CSF volume (mL) for a 1-unit increase in the predictor variable.
bOdds ratios represent the expected odds of GCS improvement (≥1 GCS point increase) with a 1-unit increase in the predictor variable.
APS=acute physiology score, BUN=blood urea nitrogen, CI=confidence interval, CSF=cerebrospinal fluid, GCS=Glasgow Coma Scale, MELD-Na=model for end-stage liver disease—sodium
Acknowledgments
Financial Support
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number KL2TR001424, and the National Institutes of Health, Grant Number L30 NS098427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This study was supported, in part, by departmental funding from the Ken & Ruth Davee Department of Neurology in the Northwestern University Feinberg School of Medicine.
Dr. Liotta is supported by a KL2 career development grant awarded through the Northwestern University Clinical and Translational Sciences Institute (NUCATS) and funded by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number KL2TR001424. He is also supported by National Institutes of Health, Grant Number L30 NS098427.
List of Abbreviations
- HE
hepatic encephalopathy
- CT
computed tomography scan
- CSF
cerebrospinal fluid
- GCS
Glasgow Coma Scale
- RASS
Richmond Agitation and Sedation Scale
- RRT
renal replacement therapy
- HTS
hypertonic saline
- mOsm
milliosmole
- Kg
kilogram
- rs
Spearman correlation coefficient
- ICU
intensive care unit
- BUN
blood urea nitrogen
- MELD-Na
model for end-stage liver disease score (2016 version with sodium correction)
- APS
acute physiology score
- L
liter
- Mg
milligram
- dL
deciliter
- DICOM
digital imaging and communications in medicine
- mEq
milliequivalent
- mL
milliliter
Conflict of Interest Statement
The authors report no conflicts of interest related to this manuscript
Author contributions
Eric M. Liotta: Dr. Liotta contributed to study conception and design; data acquisition, analysis, and interpretation; drafted the manuscript and revised it critically for important intellectual content; approved the final manuscript draft; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Dr. Liotta had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Liotta takes responsibility for the integrity of the work as a whole.
Anna L. Romanova: Dr. Romanova contributed to data acquisition and analysis, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Bryan D. Lizza: Dr. Lizza contributed to data acquisition, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Laura J. Rasmussen-Torvik: Dr. Rasmussen-Torvik contributed to study design; data analysis and interpretation; revised the manuscript critically for important intellectual content; approved the final manuscript draft; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Dr. Rasmussen-Torvik also provided statistical consultation and review.
Minjee Kim: Dr. Kim contributed to data interpretation, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Brandon Francis: Dr. Francis contributed to data acquisition, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rajbeer Singh Sangha: Dr. Sangha contributed to data acquisition, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Timothy J. Carroll: Dr. Carroll contributed to study design, data interpretation, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Daniel Ganger: Dr. Ganger contributed to study conception, data interpretation, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Daniela P. Ladner: Dr. Ladner contributed to study conception, data interpretation, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Andrew M. Naidech: Dr. Naidech contributed to study conception and design, data interpretation, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
James J. Paparello: Dr. Paparello contributed to data interpretation, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Shyam Prabhakaran: Dr. Prabhakaran contributed to study conception, data interpretation, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Farzaneh A. Sorond: Dr. Sorond contributed to study conception, data interpretation, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Matthew B. Maas: Dr. Maas contributed to study conception and design, data interpretation, critically revised the manuscript for important intellectual content, approved the final manuscript draft, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Copyright form disclosure: Drs. Liotta, Rasmussen-Torvik, Sorond, and Maas received support for article research from the National Institutes of Health (NIH). Dr. Liotta’s institution received funding from NIH’s National Center for Advancing Translational Sciences and the NIH’s National Center for Advancing Translational Sciences, Grant Number KL2TR001424; he received funding from the NIH, Northwestern University, and NIH Grant Number L30 NS098427; and he disclosed that his institution received grant support from Placement of AoRTic TraNscathetER Valves II (PARTNER II) Trial (Edwards Lifesciences), TranScatheter Aortic Valve RepLacement System US Feasibility Trial (Direct Flow Medical Inc), and SAGE-547 Clinical Trial (SAGE therapeutics) for his work on those clinical trials. Dr. Ganger received funding from Abbvie, Gilead, and Merck. Dr. Naidech’s institution received funding from AHRQ, K18 HS023437. Dr. Maas’ institution received funding from the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Stepanova M, Mishra A, Venkatesan C, et al. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10(9):1034–1041. e1031. doi: 10.1016/j.cgh.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Romero-Gomez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62(2):437–447. doi: 10.1016/j.jhep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Cordoba J, Ventura-Cots M, Simon-Talero M, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF) J Hepatol. 2014;60(2):275–281. doi: 10.1016/j.jhep.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Martinez R, Rovira A, Alonso J, et al. Hepatic encephalopathy is associated with posttransplant cognitive function and brain volume. Liver Transpl. 2011;17(1):38–46. doi: 10.1002/lt.22197. [DOI] [PubMed] [Google Scholar]
- 5.Clemmesen JOLF, Kondrup J, Hansen BA, Ott P. Cerebral Herniation in Patients With Acute Liver Failure Is Correlated With Arterial Ammonia Concentration. Hepatology. 1999;29(3):648–653. doi: 10.1002/hep.510290309. [DOI] [PubMed] [Google Scholar]
- 6.Bemeur C, Butterworth RF. Liver-brain proinflammatory signalling in acute liver failure: role in the pathogenesis of hepatic encephalopathy and brain edema. Metab Brain Dis. 2013;28(2):145–150. doi: 10.1007/s11011-012-9361-3. [DOI] [PubMed] [Google Scholar]
- 7.Guevara M, Baccaro ME, Torre A, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol. 2009;104(6):1382–1389. doi: 10.1038/ajg.2009.293. [DOI] [PubMed] [Google Scholar]
- 8.Guevara M, Baccaro ME, Rios J, et al. Risk factors for hepatic encephalopathy in patients with cirrhosis and refractory ascites: relevance of serum sodium concentration. Liver Int. 2010;30(8):1137–1142. doi: 10.1111/j.1478-3231.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 9.Cardenas A, Sola E, Rodriguez E, et al. Hyponatremia influences the outcome of patients with acute-on-chronic liver failure: an analysis of the CANONIC study. Crit Care. 2014;18(6):700. doi: 10.1186/s13054-014-0700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gines P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48(3):1002–1010. doi: 10.1002/hep.22418. [DOI] [PubMed] [Google Scholar]
- 11.Haussinger D, Laubenberger J, vom Dahl S, et al. Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology. 1994;107(5):1475–1480. doi: 10.1016/0016-5085(94)90552-5. [DOI] [PubMed] [Google Scholar]
- 12.Walters RJLFN, Crum WR, Taube D, Thomas DJ. Haemodialysis and Cerebral Oedema. Nephron. 2001;87(2):143–147. doi: 10.1159/000045903. [DOI] [PubMed] [Google Scholar]
- 13.Osgood M, Compton R, Carandang R, et al. Rapid unexpected brain herniation in association with renal replacement therapy in acute brain injury: caution in the neurocritical care unit. Neurocrit Care. 2015;22(2):176–183. doi: 10.1007/s12028-014-0064-y. [DOI] [PubMed] [Google Scholar]
- 14.Hoorn EJ, Carlotti AP, Costa LA, et al. Preventing a drop in effective plasma osmolality to minimize the likelihood of cerebral edema during treatment of children with diabetic ketoacidosis. J Pediatr. 2007;150(5):467–473. doi: 10.1016/j.jpeds.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 15.Troy P, Clark R, Kakarala S, et al. Cerebral Edema During Treatment of Diabetic Ketoacidosis in an Adult With New Onset Diabetes. Neurocrit Care. 2005;2(1):55–58. doi: 10.1385/NCC:2:1:055. [DOI] [PubMed] [Google Scholar]
- 16.Scott AR Joint British Diabetes Societies for Inpatient C group Jhhg. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med. 2015;32(6):714–724. doi: 10.1111/dme.12757. [DOI] [PubMed] [Google Scholar]
- 17.Ayus JC, Varon J, Arieff AI. Hyponatremia, Cerebral Edema, and Noncardiogenic Pulmonary Edema in Marathon Runners. Annals of Internal Medicine. 2000;132(9):711–714. doi: 10.7326/0003-4819-132-9-200005020-00005. [DOI] [PubMed] [Google Scholar]
- 18.Ropper AH. Hyperosmolar Therapy for Raised Intracranial Pressure. N Engl J Med. 2012;367(8):746–752. doi: 10.1056/NEJMct1206321. [DOI] [PubMed] [Google Scholar]
- 19.Wright G, Sharifi Y, Jover-Cobos M, et al. The brain in acute on chronic liver failure. Metab Brain Dis. 2014;29(4):965–973. doi: 10.1007/s11011-014-9553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajaj JS, Cordoba J, Mullen KD, et al. Review article: the design of clinical trials in hepatic encephalopathy--an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33(7):739–747. doi: 10.1111/j.1365-2036.2011.04590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stravitz RT, Kramer AH, Davern T, et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007;35(11):2498–2508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 22.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–1437. 1437 e1421–1429. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 23.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 24.Mouri S, Tripon S, Rudler M, et al. FOUR score, a reliable score for assessing overt hepatic encephalopathy in cirrhotic patients. Neurocrit Care. 2015;22(2):251–257. doi: 10.1007/s12028-014-0078-5. [DOI] [PubMed] [Google Scholar]
- 25.Fischer M, Ruegg S, Czaplinski A, et al. Inter-rater reliability of the Full Outline of UnResponsiveness score and the Glasgow Coma Scale in critically ill patients: a prospective observational study. Crit Care. 2010;14(2):R64. doi: 10.1186/cc8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaquero J, Fontana RJ, Larson AM, et al. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11(12):1581–1589. doi: 10.1002/lt.20625. [DOI] [PubMed] [Google Scholar]
- 27.Karvellas CJ, Fix OK, Battenhouse H, et al. Outcomes and complications of intracranial pressure monitoring in acute liver failure: a retrospective cohort study. Crit Care Med. 2014;42(5):1157–1167. doi: 10.1097/CCM.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liotta EM, Lizza BD, Romanova AL, et al. 23.4% Saline Decreases Brain Tissue Volume in Severe Hepatic Encephalopathy as Assessed by a Quantitative CT Marker. Crit Care Med. 2016;44(1):171–179. doi: 10.1097/CCM.0000000000001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maas M, Rosenberg N, Kosteva A, et al. Surveillance neuroimaging and neurologic examinations affect care for intracrebral hemorrhage. Neurology. 2013;81:107–112. doi: 10.1212/WNL.0b013e31829a33e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmerman J, Kramer A, McNair D, et al. Acute Physiology and Chronic Health Evaluation (APACHE) IV: Hospital mortality assessment for today’s critically ill patients. Critical Care Medicine. 2006;34(5):1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 31.Worthley L, Guerin M, Pain R. For calculating osmolality, the simplest formula is the best. Anaesth Intensive Care. 1987;15(2) doi: 10.1177/0310057X8701500214. [DOI] [PubMed] [Google Scholar]
- 32.Davenport A. Practical guidance for dialyzing a hemodialysis patient following acute brain injury. Hemodialysis International. 2008;12(3):307–312. doi: 10.1111/j.1542-4758.2008.00271.x. [DOI] [PubMed] [Google Scholar]
- 33.Murphy N, Auzinger G, Bernel W, et al. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology. 2004;39(2):464–470. doi: 10.1002/hep.20056. [DOI] [PubMed] [Google Scholar]
- 34.Liotta EM, Bauer RM, Berman MD, et al. Acute changes in ventricular volume during treatment for hepatic and renal failure. Neurol Clin Pract. 2014;4(6):478–481. doi: 10.1212/CPJ.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuchman S, Khademian ZP, Mistry K. Dialysis disequilibrium syndrome occurring during continuous renal replacement therapy. Clin Kidney J. 2013;6(5):526–529. doi: 10.1093/ckj/sft087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silver S, DeSimone JAJ, Smith D, et al. Dialysis disequilibrium syndrime (DDS) in the rate: Role of the “reverse urea effect”. Kidney International. 1992;42(1):161–166. doi: 10.1038/ki.1992.273. [DOI] [PubMed] [Google Scholar]
- 37.Silver SM. Cerebral Edema After Rapid Dialysis Is Not Caused by an Increase in Brain Organic Osmolytes. J Am Soc Nephrol. 1995;6(6):1600–1606. doi: 10.1681/ASN.V661600. [DOI] [PubMed] [Google Scholar]
- 38.Trinh-Trang-Tan MM, Cartron JP, Bankir L. Molecular basis for the dialysis disequilibrium syndrome: altered aquaporin and urea transporter expression in the brain. Nephrol Dial Transplant. 2005;20(9):1984–1988. doi: 10.1093/ndt/gfh877. [DOI] [PubMed] [Google Scholar]
- 39.Rama Rao KV, Jayakumar AR, Tong X, et al. Brain aquaporin-4 in experimental acute liver failure. J Neuropathol Exp Neurol. 2010;69(9):869–879. doi: 10.1097/NEN.0b013e3181ebe581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rama Rao KV, Verkman AS, Curtis KM, et al. Aquaporin-4 deletion in mice reduces encephalopathy and brain edema in experimental acute liver failure. Neurobiol Dis. 2014;63:222–228. doi: 10.1016/j.nbd.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental 1a. Comparison of demographic and clinical variables between ALF and ACLF patients
Supplemental 1b. Absolute magnitude and range of change for imaging and laboratory variables in the 103 assessment intervals.
APS=acute physiology score, CSF=cerebrospinal fluid, IQR=interquartile range, MELD-Na=model of end-stage liver disease--sodium
Acute liver failure (n=22) patients contributed 62 (60%) assessment intervals while acute-on-chronic liver failure (n=18) patients contributed 41 (40%) assessment intervals.
Supplemental 2a. Scatterplot of cerebrospinal fluid volume change versus osmolality change for the forty initial volume change assessment intervals
Supplemental 2b. Scatterplot of cerebrospinal fluid volume change versus Glasgow Coma Scale change for the forty initial volume change assessment intervals
Supplemental 2c. Change in Glasgow Coma Scale between the beginning and end of the initial assessment intervals
Supplemental 3. Spearman correlation coefficients for associations between change in CSF volume and Glasgow Coma Scale score during the forty initial assessment intervals and admission clinical variables
CSF=cerebrospinal fluid, APS=Acute Physiology Score, MELD-NA=model of end-stage liver disease—sodium score, SIRS=systemic inflammatory response syndrome, GCS=Glasgow Coma Scale, RASS=Richmond Agitation and Sedation Scale
Supplemental 4. Ordinal regression model for associations with RASS change.
aOdds ratios represent the expected odds of GCS improvement (≥1 GCS point increase) with a 1-unit increase in the predictor variable.
Variables assessed for model inclusion included: serum osmolality change, age, sex, acute versus acute-on-chronic liver failure, APS score, number of admission SIRS criteria meet, MELD-NA, renal replacement therapy exposure during assessment interval, mean temperature during assessment interval, temperature change across the assessment interval, serum ammonia change, serum sodium change, serum BUN change, serum glucose change, admission serum ammonia, time delay between serum osmolality measurement and CT scan, and (for GCS models) analgesia/sedation dosage change across the assessment interval.
APS=acute physiology score, BUN=blood urea nitrogen, CI=confidence interval, CSF=cerebrospinal fluid, MELD-Na=model for end-stage liver disease—sodium, RASS=Richmond Agitation and Sedation Scale
Supplemental 5a. Generalized estimating equation models for associations with CSF volume change and GCS change in acute liver failure patients (n=22).
Supplemental 5b. Generalized estimating equation models for associations with CSF volume change and GCS change in acute-on-chronic liver failure patients (n=18).
aBeta (β) coefficients represent the expected change in CSF volume (mL) for a 1-unit increase in the predictor variable.
bOdds ratios represent the expected odds of GCS improvement (≥1 GCS point increase) with a 1-unit increase in the predictor variable.
APS=acute physiology score, BUN=blood urea nitrogen, CI=confidence interval, CSF=cerebrospinal fluid, GCS=Glasgow Coma Scale, MELD-Na=model for end-stage liver disease—sodium