Abstract
ACTH, a member of the melanocortin family of peptides, is often used in the treatment of the developmental epileptic encephalopathy spectrum disorders including, Ohtahara, West, Lennox Gastaut and Landau-Kleffner Syndromes and electrical status epilepticus of sleep. In these disorders, although ACTH is often successful in controlling the seizures and/or inter-ictal EEG abnormalities, it is unknown whether ACTH possesses other beneficial effects independent of seizure control. We tested whether ACTH can ameliorate the intrinsic impairment of hippocampal-based learning and memory in epileptic Kcna1-null (KO) mice. We found that ACTH – administered in the form of Acthar Gel given i.p four times daily at a dose of 4 IU/kg (16 IU/kg/day) for 7 days – prevented impairment of long-term potentiation (LTP) evoked with high-frequency stimulation in CA1 hippocampus and also restored spatial learning and memory on the Barnes maze test. However, with this treatment regimen, ACTH did not exert a significant effect on the frequency of spontaneous recurrent seizures. Together, our findings indicate that ACTH can ameliorate memory impairment in epileptic Kcna1-null mice separate from seizure control, and suggest that this widely used peptide may exert direct nootropic effects in the epileptic brain.
Keywords: Acthar gel (ACTH1-39), long-term potentiation, hippocampus, Kcna1-null mice, memory consolidation, epilepsy, neuroprotection, nootropic effects
Introduction
ACTH, a member of the melanocortin family of peptides, is often used in the treatment of the developmental epileptic encephalopathy spectrum disorders including, Ohtahara, West, Lennox Gastaut and Landau-Kleffner Syndromes and electrical status epilepticus of sleep [1]. In the case of West syndrome, characterized by infantile spasms, hypsarrhythmia on the electroencephalogram (EEG), and developmental arrest or regression [2], ACTH is often used as a first-line treatment and is considered by many to be superior to other therapies despite its considerable toxic side-effect profile [3]. As a whole, in the developmental epileptic encephalopathies, ACTH is given to arrest the seizures and normalize the interictal EEG with the objective of improving the poor cognitive developmental outcome associated with these disorders.
Interestingly, several short peptide sequences of ACTH have been shown to accelerate maturation of the central nervous system, enhance neuronal regeneration and to facilitate memory consolidation and retention. These results suggest that ACTH may afford beneficial cognitive neuroprotective effects apart from its ability to control seizures and interictal abnormalities [4,5]. Furthermore, one analog of ACTH (Semax) has been shown to improve cognitive function in both rodents and humans. Specifically, intranasal Semax is used in Russia to safeguard memory function following stroke and traumatic brain injury [6,7]. With respect to epilepsy, one study has shown that subcutaneous treatment with several ACTH-like fragments improved cognitive function in aged rats exposed to acute, pilocarpine-induced seizures [8]. However, given that the severity and frequency of seizures were also reduced by ACTH, it was not possible to determine in this model whether the effects of ACTH on learning and memory were independent from its anti-seizure effects. In another recent study, ACTH treatment has been shown to normalize enhanced LTP in an acute model of NMDA-driven spasms [9]. However, it is unclear how enhanced LTP contributes to memory impairment in this model. To date, there have been no additional studies exploring the ability of ACTH to protect neurocognitive function in animal models of chronic epilepsy.
Given the prior evidence indicating that ACTH may induce cognitive neuroprotective effects, we hypothesized that ACTH might provide functional neuroprotection by restoring hippocampal long-term potentiation (LTP) and memory consolidation in epileptic Kcna1-null mice. These mice have a targeted deletion of the Kv1.1 potassium channel α subunit protein, and develop severe spontaneous recurrent seizures (SRS) early in life. Although Kcna1-null mice have intractable focal-onset limbic epilepsy similar to humans with temporal lobe epilepsy, these mice do exhibit seizures starting in the third post-natal week corresponding to childhood epileptic encephalopathies in humans that are associated with significant cognitive deficits [10].
Materials and Methods
Animals
Kcna1-null mice were bred at the Barrow Neurological Institute (Phoenix, Arizona) vivarium. Mice were born and reared in a quiet, temperature-controlled room and entrained to a 12 hour light–dark cycle; lights on at zeitgeber time (ZT) 00:00. Tail clips were taken at P13–P15 and sent to Transnetyx Inc. for genotyping (Cordova, TN, U.S.A.). All protocols were in accordance with National Institutes of Health (NIH) guidelines and approved by Barrow Institutional Animal Care and Use Committee.
Pharmacokinetic study
In Acthar gel-treated wild-type mice, serum levels of ACTH were measured using High Performance Liquid Chromatography (HPLC) with UV detection at 6 time-points (15, 30, 45, 60, 90 and 120 min) following a single intraperitoneal (i.p.) injection of Acthar gel [ACTH1-39; 4 IU/kg; Questcor Pharmaceuticals] (N=6/group). Levels were assessed using a triple quadrupole tandem mass spectrometer (water/micormass Quattro LC) in negative ion mode.
Video EEG Monitoring
To determine the effects of ACTH on both seizure frequency and cognitive function in Kcna1-null mice, we conducted long-term video-EEG monitoring in treated and untreated animals (N=13/group). Seizures were recorded using Stellate video-EEG technology and Harmonie software (Stellate, Quebec, Montreal, Canada). At postnatal day P26-28, animals were anesthetized with isoflurane (5% induction, 2% maintenance) prior to transmitter implantation. A wireless, PhysioTel telemetry transmitter (Data Sciences International, St. Paul, MN, U.S.A.) was implanted in a subcutaneous pocket along the dorsal flank. Biopotential leads were implanted in the same manner, bilaterally over the dura, 2 mm lateral of the mid-sagittal suture and 1 mm caudal to bregma, with the ground implanted in the occipital bone. Following a 3-day recovery period, animals underwent continuous video-EEG monitoring for three consecutive days until they were sacrificed for the LTP experiment. The number of daily electroclinical generalized tonic-clonic seizures was manually counted. Kcna1-null mice were treated with Acthar Gel (4 IU/kg, i.p., four times daily for 7 days) beginning immediately after the post-surgical recovery period following implantation of the EEG electrodes and then changes in daily seizure frequency were compared with saline-injected KO mice using the last three days of EEG data. This dose was selected based on our HPLC data obtained in mice injected with ACTH. Additionally, low doses of ACTH are shown to be as effective as high doses in the treatment of IS [11]. At present, the minimal effective dose is unknown in humans and the rodent models. An age-matched control group of Kcna1-null mice were injected with saline under similar experimental conditions.
Barnes Maze Behavioral Testing
To test spatial learning and memory function between the control cohort and additional experimental cohorts (8 mice/group), we used the Barnes circular table maze [12]. Our Barnes maze set-up consists of a circular table that is 122 cm in diameter, stands 3-feet tall, and contains 19 circular 5 cm-wide evenly spaced faux holes around the circumference. There is a 20th hole with an open top and a slot for the escape (or drop) box. The goal of the maze is for the mouse to reach the drop box and avoid aversive stimuli provided by two powerful box fans, a 600-Watt light-bulb, and a metronome buzzer. At the third day (P28-30) of injection of either saline or Acthar gel in KO mice, each mouse underwent a one-time acquisition trial wherein the animal was allowed to explore the maze independently of stimuli. Then, all three aversive stimuli were activated. After 30 seconds, the mouse was guided to the edge of the escape hole, and aversive stimuli were discontinued once the mouse entered the drop box chamber. Following the acquisition trial, each mouse was given 4 additional trials for 5 consecutive days [13]. Each mouse had up to 3 minutes to locate the drop box via the escape hole. Our digital-video tracking system (EthoVision XT, Noldus, Leesburg, Virginia, U.S.A.) recorded all movements and tracked the latency to the animal entering the hidden chamber (at which time all aversive stimuli were terminated). Further testing involved probe trials (with the target hole blocked on day 5), which tested actual spatial learning by dissociating proximal cues that may otherwise exist [13,14]. An enzyme detergent (Tergazyme, Alconox, White Plains, NY) were used to clean the table surface between each trial to eliminate olfactory cues.
Cellular Electrophysiology
At P37-39, mice were euthanized by inhalation of anesthetic (isoflurane) following completion of Barnes Maze testing and video-EEG analysis. The whole brain was rapidly removed and submerged in ice-cold oxygenated physiological saline (composition in mM: 124 NaCl, 1.8 MgSO4, 4 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2.4 CaCl2, and 10 D-glucose; pH = 7.4). Transverse hippocampal slices (400 μm) were prepared using a standard vibratome (The Vibratome Company, St. Louis, MO, U.S.A.), and then transferred to an incubation chamber containing physiological saline bubbled with 95% O2/5% CO2 at 35°C for 1 hr. Each slice was then transferred to a submersion-type recording chamber attached to an Axioskop FS2 microscope (Zeiss Instruments) and superfused with warmed (31 ± 1°C) physiological saline at a rate of 2–3 ml/min before the start of each experiment. Upon Schaffer collateral/commissural fiber stimulation, field excitatory post-synaptic potentials (fEPSPs) were recorded at a control test frequency of 0.5 Hz (0.1 ms, 20–100 μA) from the stratum radiatum of the CA1 subfield. After input-output curves (stimulus intensity vs. EPSP amplitude) were constructed, baseline fEPSP amplitudes (> 1 mV) were set to 30–40 % of maximum responses. LTP was evoked by high-frequency stimulation (HFS; 100 HZ for 1 second) and was expressed as the percent of the mean baseline EPSP amplitude. Data were filtered at 3 kHz, sampled at 10 kHz using pClamp, and analyzed with Clampfit (Molecular Devices, Sunnyvale, CA, U.S.A.).
Results
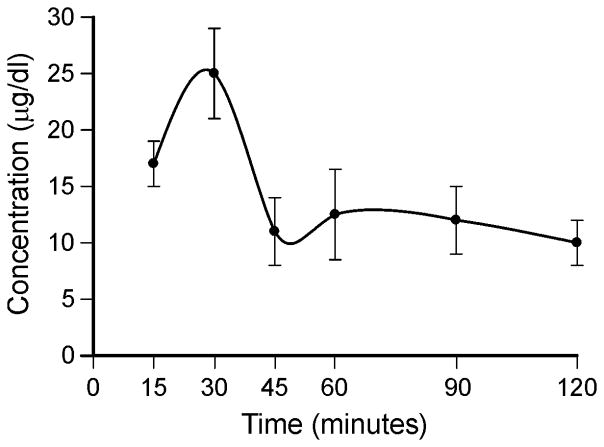
Serum levels of ACTH1-39 were measured in eight groups of mice (N=6) - administered at 6 time-points (15, 30, 45, 60, 90 and 120 min) with subcutaneous injection of 4 IU/kg Acthar gel - using solid phase extraction and HPLC. Peak ACTH levels of 25 ± 4.2 μg/dl (250 ng/ml) were observed at 30 minutes post-injection and then serum ACTH level subsequently dropped to 10 ± 2.0 μg/dl (100 ng/ml) at 2hr post-injection (Fig. 1). The increases in ACTH level in Acthar gel-treated mice were greater than physiological plasma ACTH levels in control mice; plasma ACTH values measured 65 or 194 pg/ml (0.065 or 0.194 ng/ml) at a diurnal nadir [15,16]. These data indicate that 4 IU/kg Acthar gel is sufficient to induce elevations in the blood level of ACTH level.
Figure 1.
Pharmacokinetic profiles of mice treated with Acthar gel. 4 IU/kg of Acthar gel was administered to wild-type (C3HeB/FeJ) mice. Serum was collected through a serial sampling method and the concentration of the drug determined by liquid chromatography. Values represent group means ± SEM.
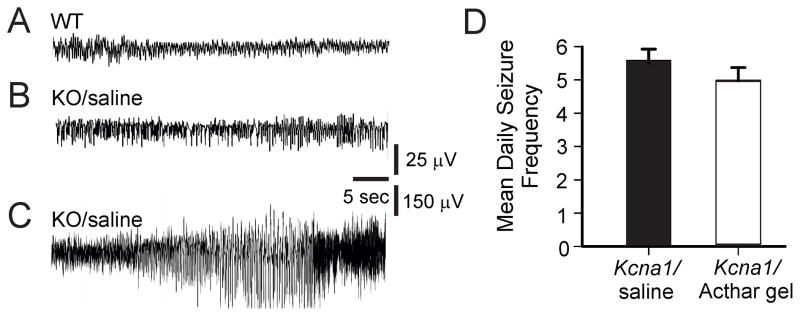
Spontaneous recurrent seizures (SRS; generalized tonic-clonic seizures) in Kcna1-null mice were observed during the multi-day continuous video-EEG recording period (Fig. 2A–C). Mice treated with Acthar gel had a slightly decreased seizure frequency (4.9 ± 0.33 seizures/day, N=13) compared to saline-injected controls (5.6 ± 0.46 seizures/day, N=13), however this finding was not statistically significant (Fig. 2D).
Figure 2.
Effects of Acthar gel on spontaneous recurrent seizures in Kcna1-null (KO) mice. (A & B) Representative interictal electroencephalographic (EEG) recording from wild-type (WT) and KO mice injected with saline. (C) Trace depicts the ictal EEG changes associated with a generalized tonic-clonic seizure in saline-injected KO mice. (D) Daily seizure counts; mean ± SEM. While 16 IU/kg/day Acthar gel slightly reduces daily tonic-clonic seizure frequency compared to saline-administrated KO mice, no significant differences were found between the two groups.
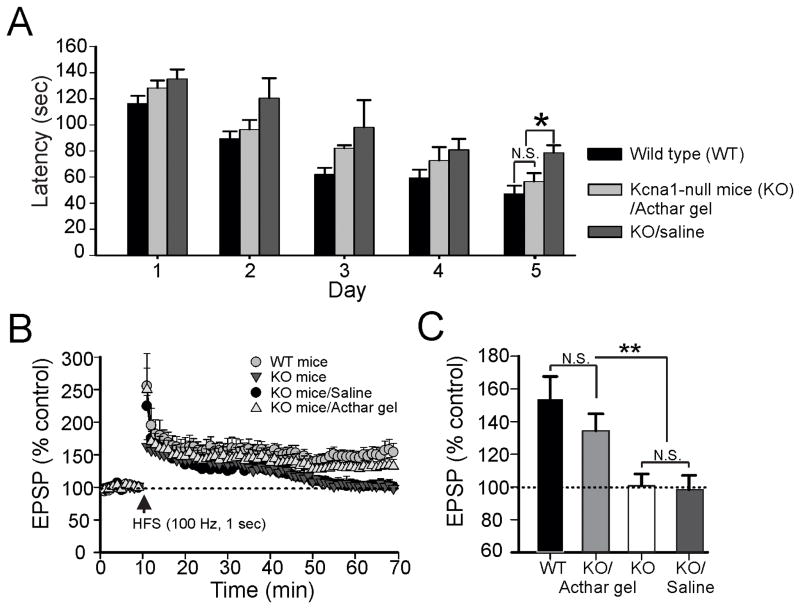
Given that we have previously observed impairment of long-term potentiation (LTP), an electrophysiological measure of learning and memory, in the hippocampus of Kcna1-null mice [10], we asked whether ACTH might provide protective effects on learning and memory function in these animals. Compared to wild type mice, saline-treated Kcna1-null mice showed a significant increase in the latencies to finding the hidden escape chamber (Fig. 3A). Administration of ACTH strongly rescued this memory deficit in Kcna1-null mice during testing compared to saline-treated Kcna1-null mice. Similar to what we observed in separate cohorts of KO mice (above), there were no significant differences in seizure frequency between treated and untreated groups (data not shown). Spatial learning and memory function in the Barnes maze test was largely mirrored by our cellular electrophysiological findings. LTP responses in hippocampal slices from wild-type (WT) mice were seen after HFS of Schaffer collaterals (Fig. 3B). The EPSP amplitude measured 153 ± 13.7 % at 60 min post-HFS (Fig. 3C). In hippocampal slices prepared from either saline-injected Kcna1-null mice or non-injected Kcna1-null animals, HFS failed to induce intact LTP responses; the EPSP amplitude measured 100 ± 7.2% and 98.3 ± 8.7 % at 60 min post-HFS, respectively (Fig. 3C). In contrast, Acthar gel-treated Kcna1-null mice displayed robust LTP following HFS. Despite the slightly decreased EPSP amplitude (133 ± 10.4 %) at 60 min post-HFS, there was no difference between the EPSP amplitude of the ACTH-treated group compared to WT controls (Fig. 3C).
Figure 3.
Acthar gel restores impairment of memory consolidation in spontaneous epileptic Kcna1-null mice. Kcna1-null (KO) mice administered Acthar gel performed as well or better than saline-injected KO mice. N=7 per group *, p <0.05. (A) Average time latencies for mice to find the escape chamber in the Barnes maze are summarized for the three groups of mice. (B) Impaired LTP induced by high-frequency stimulation (HFS; 100 Hz, 1 sec) of Schaffer collaterals in KO mice and saline-injected KO mice compared to wild-type (WT) animals (N=8/group). Acthar gel given to KO mice resulted in restoration of LTP formation. Vertical arrow indicates the time-point at HFS initiation. Dotted line denotes the average of the baseline field potential amplitudes during a 10 min recording before the initiation of HFS. (C) Summary bar graph of various groups indicating changes in EPSP amplitudes at 60 min after HFS. Each vertical bar expresses the EPSP amplitude ± SEM (obtained in 8 slices from 4). One way ANOVA followed by Tukey post-hoc analysis; **, p < 0.01, NS, not significant.
Discussion
In the Kcna1-null mouse model of severe, chronic epilepsy, we showed that a low-dose regimen of ACTH, in the form of Acthar Gel, prevented deficits in learning and memory function despite not exerting significant anti-seizure effects. To our knowledge, this is the first study demonstrating that ACTH provides functional neuroprotective effects against intrinsic impairment of hippocampal-based learning and memory in epileptic animals independent of seizure control, suggesting that this peptide may provide nootropic effects in epileptic brain.
Long-term potentiation (LTP) is widely considered the functional mechanism underlying induction and storage of memory. LTP results in an increase in synaptic efficacy lasting form hours to days following a brief tetanic stimulation of afferent pathways, and is usually recorded in the hippocampus [17]. The mechanism by which ACTH protects LTP is currently unknown. However, seizures can increase basal levels of stress hormones [18], and in turn, stress can disrupt hippocampal LTP. Stress-induced impairment of LTP is linked to corticotropin releasing factor (CRF) produced by hippocampal inhibitory interneurons. CRF binds to the CRF1 receptor localized to dendritic spines of CA1 pyramidal cells leading to acute dendritic spine loss or atrophy if the stressors are enduring [19–22]. ACTH may restore LTP by either suppressing the release of CRF in the hippocampus or by reversing the effect of seizures to cause overexpression of the CRF receptor. Such changes have been reported to occur following chronic seizures in some human and rodent models of epilepsy [23,24]. In support of this, one study has shown that a single i.p. injection of a high dose of ACTH4-10 (80 IU/Kg) – having a high affinity for melanocortin receptors - reduced CRF mRNA receptor expression in the central nucleus of the amygdala [25]. This effect was blocked by the melanocortocotropin MC4 receptor antagonist SHU919, indicating that activation of melanocortin receptors are responsible for the effect of ACTH in reducing CRF receptor expression in the amygdala [25]. Furthermore, a recent study has shown that activation of MC4 receptors located post-synaptically in the hippocampus results in enhanced LTP due to a protein kinase A-dependent up-regulation in the surface expression of GluA1-containing AMPA receptors which are involved in the morphological maturation and number of dendritic spines [26]. Interestingly, activation of MC4 receptors has been implicated in the spasms-suppressing effects of ACTH in infantile spasms, but the role of ACTH in relation to MC4 receptors and cognition in IS remains unclear [27]. Given the possibility that CRF mRNA expression may be sensitive to the dose and specific fragmentary composition of ACTH, it is unknown whether a low dose of Acthar gel can alter CRF receptor expression, leading to memory restoration in our model.
Notwithstanding the lack of current evidence for seizure control with low-dose ACTH administration in Kcna1-null mice, previous studies have demonstrated that both ACTH1-39 and ACTH1-24 (at a dose of 15–80 IU/kg) have no effect in a rodent model of NMDA-induced infantile spasms [28]. Clinical use of natural ACTH - comparing 150 IU with 20 IU – results in similar beneficial effects in spasms cessation and hypsarrhythmia suppression [29]. Although the basis of this discrepancy remains unclear, species-specific differences may exist. In contrast, at a dose range of 135–180 IU/kg, ACTH1-39 strongly reduced the frequency of spasms in a model of NMDA-induced IS [30]. We failed to observe prominent anti-seizure activity of ACTH in Kcna1-null mice and this is likely because the dose used for these studies was lower than those used in other rodent seizure models [30–32]. Future dose-response studies will be needed to determine if higher doses are necessary to control the focal-onset seizures in Kcna1-null mice. However, it is interesting to note that low doses of ACTH protected against learning and memory deficits without affecting the frequency of seizures, suggesting that ATCH may provide beneficial nootropic effects, even in patients with poorly controlled seizures.
Conclusions
Our findings support the notion that ACTH can mitigate impairment of hippocampal-based learning and memory in the epileptic brain, separate from that arising from reduced seizure activity, thus suggesting that this widely used treatment may exert direct nootropic effects. However, future studies are necessary to delineate the functional neuroprotective effects of ACTH in currently available rodent models of the developmental epileptic encephalopathies (such as IS) for which ACTH is an indicated treatment.
Highlights.
Low-dose ACTH protects hippocampal-based learning and memory in epileptic Kcna1-null mice
Low-dose of ACTH has no effect on seizure frequency in epileptic Kcna1-null mice
ACTH has beneficial nootropic effects independent of seizure control in epileptic Kcna1-null mice
Acknowledgments
The authors would like to thank Heather Milligan and Derek O’Neill for technical assistance. This study was supported by a research grant from Questcor Pharmaceuticals (JMR), the Alberta Children’s Hospital Research Institute (MHS, JMR), the Barrow Neurological Foundation (DYK), and NIH grant NS 070261 (JMR, DYK).
Abbreviations
- ACTH
Adrenocorticotropic Hormone
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CRF
corticotropin releasing factor
- EPSP
excitatory post synaptic potentials
- HFS
high frequency stimulation
- KO
knock-out
- LTP
long-term potentiation
- NMDA
N-methyl-D-aspartate
- MC4
melanocorticotropin 4
- WT
wild-type
- IS
infantile spasms
Footnotes
Conflicts of interest
The authors declare that no conflicts of interest exist.
References
- 1.McTague A, Cross JH. Treatment of epileptic encephalopathies. CNS Drugs. 2013;27:175–184. doi: 10.1007/s40263-013-0041-6. [DOI] [PubMed] [Google Scholar]
- 2.Frost JD, Jr, Hrachovy RA. Pathogenesis of infantile spasms: a model based on developmental desynchronization. J Clin Neurophysiol. 2005;22:25–36. doi: 10.1097/01.wnp.0000149893.12678.44. [DOI] [PubMed] [Google Scholar]
- 3.Harrell RM, Sibley R, Vogelzang NJ. Renal vascular lesions after chemotherapy with vinblastine, bleomycin, and cisplatin. Am J Med. 1982;73:429–433. doi: 10.1016/0002-9343(82)90748-3. [DOI] [PubMed] [Google Scholar]
- 4.Shen Y, Li R. The role of neuropeptides in learning and memory: possible mechanisms. Med Hypotheses. 1995;45:529–538. doi: 10.1016/0306-9877(95)90235-x. [DOI] [PubMed] [Google Scholar]
- 5.Strand FL, Lee SJ, Lee TS, Zuccarelli LA, Antonawich FJ, et al. Non-corticotropic ACTH peptides modulate nerve development and regeneration. Rev Neurosci. 1993;4:321–363. doi: 10.1515/revneuro.1993.4.4.321. [DOI] [PubMed] [Google Scholar]
- 6.Manchenko DM, Glazova N, Levitskaia NG, Andreeva LA, Kamenskii AA, et al. Nootropic and analgesic effects of Semax following different routes of administration. Ross Fiziol Zh Im I M Sechenova. 2010;96:1014–1023. [PubMed] [Google Scholar]
- 7.Asmarin IP, Nezavibat’ko VN, Miasoedov NF, Kamenskii AA, Grivennikov IA, et al. A nootropic adrenocorticotropin analog 4–10-semax (l5 years experience in its design and study) Zh Vyssh Nerv Deiat Im I P Pavlova. 1997;47:420–430. [PubMed] [Google Scholar]
- 8.Croiset G, De Wied D. ACTH: a structure-activity study on pilocarpine-induced epilepsy. Eur J Pharmacol. 1992;229:211–216. doi: 10.1016/0014-2999(92)90557-k. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji M, Takahashi Y, Watabe AM, Kato F. Enhanced long-term potentiation in mature rats in a model of epileptic spasms with betamethasone-priming and postnatal N-methyl-d-aspartate administration. Epilepsia. 2016;57:495–505. doi: 10.1111/epi.13315. [DOI] [PubMed] [Google Scholar]
- 10.Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, et al. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol. 2015;78:77–87. doi: 10.1002/ana.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Go CY, Mackay MT, Weiss SK, Stephens D, Adams-Webber T, et al. Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78:1974–1980. doi: 10.1212/WNL.0b013e318259e2cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 13.Sunyer B, Patil S, Frischer C, Hoger H, Selcher J, et al. Strain-dependent effects of SGS742 in the mouse. Behav Brain Res. 2007;181:64–75. doi: 10.1016/j.bbr.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP. Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem. 2006;13:809–819. doi: 10.1101/lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karanth S, Linthorst AC, Stalla GK, Barden N, Holsboer F, et al. Hypothalamic-pituitary-adrenocortical axis changes in a transgenic mouse with impaired glucocorticoid receptor function. Endocrinology. 1997;138:3476–3485. doi: 10.1210/endo.138.8.5331. [DOI] [PubMed] [Google Scholar]
- 16.Muglia LJ, Jacobson L, Luedke C, Vogt SK, Schaefer ML, et al. Corticotropin-releasing hormone links pituitary adrenocorticotropin gene expression and release during adrenal insufficiency. J Clin Invest. 2000;105:1269–1277. doi: 10.1172/JCI5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebaudo R, Melani R, Balestrino M, Izvarina N. Electrophysiological effects of sustained delivery of CRF and its receptor agonists in hippocampal slices. Brain Res. 2001;922:112–117. doi: 10.1016/s0006-8993(01)03160-2. [DOI] [PubMed] [Google Scholar]
- 18.Joels M. Stress, the hippocampus, and epilepsy. Epilepsia. 2009;50:586–597. doi: 10.1111/j.1528-1167.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Rex CS, Rice CJ, Dube CM, Gall CM, et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci U S A. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivy AS, Rex CS, Chen Y, Dube C, Maras PM, et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, et al. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, et al. Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience. 2004;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Dow KE, Fraser DD. Elevated corticotropin releasing hormone/corticotropin releasing hormone-R1 expression in postmortem brain obtained from children with generalized epilepsy. Ann Neurol. 2001;50:404–409. doi: 10.1002/ana.1138. [DOI] [PubMed] [Google Scholar]
- 24.Jinde S, Masui A, Morinobu S, Takahashi Y, Tsunashima K, et al. Elevated neuropeptide Y and corticotropin-releasing factor in the brain of a novel epileptic mutant rat: Noda epileptic rat. Brain Res. 1999;833:286–290. doi: 10.1016/s0006-8993(99)01510-3. [DOI] [PubMed] [Google Scholar]
- 25.Brunson KL, Khan N, Eghbal-Ahmadi M, Baram TZ. Corticotropin (ACTH) acts directly on amygdala neurons to down-regulate corticotropin-releasing hormone gene expression. Ann Neurol. 2001;49:304–312. [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Y, Fu WY, Cheng EY, Fu AK, Ip NY. Melanocortin-4 receptor regulates hippocampal synaptic plasticity through a protein kinase A-dependent mechanism. J Neurosci. 2013;33:464–472. doi: 10.1523/JNEUROSCI.3282-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunson KL, Eghbal-Ahmadi M, Baram TZ. How do the many etiologies of West syndrome lead to excitability and seizures? The corticotropin releasing hormone excess hypothesis. Brain Dev. 2001;23:533–538. doi: 10.1016/s0387-7604(01)00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velisek L, Jehle K, Asche S, Veliskova J. Model of infantile spasms induced by N-methyl-D-aspartic acid in prenatally impaired brain. Ann Neurol. 2007;61:109–119. doi: 10.1002/ana.21082. [DOI] [PubMed] [Google Scholar]
- 29.Yanagaki S, Oguni H, Hayashi K, Imai K, Funatuka M, et al. A comparative study of high-dose and low-dose ACTH therapy for West syndrome. Brain Dev. 1999;21:461–467. doi: 10.1016/s0387-7604(99)00053-4. [DOI] [PubMed] [Google Scholar]
- 30.Chachua T, Yum MS, Veliskova J, Velisek L. Validation of the rat model of cryptogenic infantile spasms. Epilepsia. 2011;52:1666–1677. doi: 10.1111/j.1528-1167.2011.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortez MA, Shen L, Wu Y, Aleem IS, Trepanier CH, et al. Infantile spasms and Down syndrome: a new animal model. Pediatr Res. 2009;65:499–503. doi: 10.1203/PDR.0b013e31819d9076. [DOI] [PubMed] [Google Scholar]
- 32.Scantlebury MH, Galanopoulou AS, Chudomelova L, Raffo E, Betancourth D, et al. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37:604–612. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]