Abstract
The atrophy and hypofunction of the adrenal cortex following long-term pharmacologic glucocorticoid therapy is a major health problem necessitating chronic glucocorticoid replacement that often prolongs the ultimate return of endogenous adrenocortical function. Underlying this functional recovery is anatomic regeneration, the cellular and molecular mechanisms of which are poorly understood. Investigating the lineage contribution of cortical Sonic hedgehog (Shh)+ progenitor cells and the SHH–responsive capsular Gli1+ cells to the regenerating adrenal cortex, we observed a spatially and temporally bimodal contribution of both cell types to adrenocortical regeneration following cessation of glucocorticoid treatment. First, an early repopulation of the cortex is defined by a marked delamination and expansion of capsular Gli1+ cells, recapitulating the establishment of the capsular-cortical homeostatic niche during embryonic development. This rapid repopulation is promptly cleared from the cortical compartment only to be supplanted by repopulating cortical cells derived from the resident long-term-retained zona glomerulosa Shh+ progenitors. Pharmacologic and genetic dissection of SHH signaling further defines an SHH-dependent activation of WNT signaling that supports regeneration of the cortex following long-term glucocorticoid therapy. We define the signaling and lineage relationships that underlie the regeneration process.
This study shows the contribution of capsular and cortical progenitor lineages to adrenal cortex regeneration following dexamethasone-induced atrophy.
The adrenal cortex is an endocrine organ divided into three concentric zones: the outermost zona glomerulosa (zG), middle zona fasciculata (zF), and inner zona reticularis (zR), each of which is responsible for the secretion of different steroid hormones: mineralocorticoids (zG), glucocorticoids (zF), and sex hormone (zR) (1).
The anti-inflammatory properties of glucocorticoids are frequently exploited for therapeutic benefit in many patients having a variety of disorders that manifest with activation of inflammatory responses. However, such pharmacologic dosing of glucocorticoids poses a substantial clinical challenge due to the resultant glucocorticoid-mediated inhibition of adrenocorticotropic hormone (ACTH) secretion that leads to adrenal atrophy and clinical adrenal failure (2, 3). Adrenal function only returns upon removal of the exogenous glucocorticoids and the subsequent repopulation of the zF and differentiation in response to reactivated ACTH. Binding to its receptor, ACTH triggers a series of molecular processes leading to activation of cyclic adenosine monophosphate (cAMP)–dependent protein kinase A (PKA) and transcription of steroidogenic enzymes in the zF (1, 4–6).
The adrenal cortex is dynamically maintained through continual centripetal displacement and differentiation of cortical cells from the most peripheral cortex to the cortico-medullary junction, where cells ultimately undergo apoptosis (7–10). Cell fate mapping studies have identified a population of undifferentiated subcapsular Sonic hedgehog (Shh)–expressing cells embedded within the outer zG that signal to nonsteroidogenic glioma-associated oncogene homolog 1 (Gli1)–expressing cells located in the adrenal capsule [for review see Finco et al. (11)]. Capsular Gli1+ cells facilitate the establishment of the capsular/cortical unit during development by giving rise to the emerging underlying cortex, including the Shh+ cells. Postnatally, the Gli1+ cell contribution to cortical lineage is markedly diminished. Instead, the Shh+ cells serve as self-renewing progenitors for the differentiated descendants in the zG and zF throughout life (12). Moreover, differentiated zG cells expressing the marker gene Cyp11b2 give rise to the underlying cells of the zF that express Cyp11b1, responsible for glucocorticoid production in response to ACTH (13). Although the mechanisms by which SHH is engaged to activate capsular GLI1-mediated transcription in the adrenal is unclear, emerging evidence implicates the canonical WNT signaling as an additional paracrine signaling pathway essential for controlling renewal vs. differentiation of the adrenocortical progenitors (14, 15). Like Shh expression, canonical WNT pathway activation is restricted to the zG. Moreover, while most of Shh+ cells are WNT-responsive, the WNT-responsive population also includes zG cells that express Cyp11b2 (and not Shh) (14–16). Further experiments detail a reciprocal inhibition of the canonical WNT signaling pathway by cAMP/PKA, the primary signaling pathway mediating the effects of ACTH (17).
The interdependence of SHH and WNT signaling pathways in adrenocortical cell maintenance is slowly emerging. Capsular deletion of R-spondin 3 (Rspo3), a ligand involved in the activation of WNT signaling (18), causes decreased expression of Shh with loss of zG markers and a reduced adrenocortical volume (19). These data together suggest that paracrine and endocrine signals jointly regulate the complex process of progenitor cell renewal and conversion to hormone-responsive, differentiated cortical cells that is central to the homeostatic maintenance of the adrenal cortex.
In our study, we posited that adrenocortical regeneration requires increased paracrine signaling normally involved in homeostasis and that we could exploit these pathways experimentally to deconstruct the roles of both SHH and WNT signaling in the capsular/cortical niche. We used adrenal atrophy induced by the synthetic glucocorticoid dexamethasone to interrogate the histological regrowth and functional recovery of the zF. We observed a spatially and temporally bimodal contribution of both the capsular Gli1+ cells and the cortical Shh+ cells to adrenocortical regeneration. Further pharmacologic and genetic dissection of paracrine SHH signaling indicated an additional contribution of canonical WNT signaling in mediating cortical regeneration following long-term glucocorticoid therapy.
Materials and Methods
Mouse models
All experiments were performed in accordance with institutionally approved protocols under the auspice of the University Committee on Use and Care of Animals at the University of Michigan. Gli1CreERT2 (20) and Gt(ROSA)26Sortm1(Smo/EYFP)Amc/J (21) (referred as SmoM2) (both strains kindly provided by A. Dlugosz) and B6.129S-Shhtm2(cre/ESR1)Cjt/J (22) (referred as ShhCreERT2 mice) (kindly provided by S. Wong) were kept in a mixed genetic background. B6.129-Ctnnb1tm2Kem/KnwJ (23) (kindly provided by E. Fearon) and B6.129(Cg)-Axin2tm1(cre/ERT2)Rnu/J (24) were kept on a pure C57Bl6 background. Reporter strains used were the following: R26REGFP B6.Cg-Gt(ROSA)26Sortm6(CAG-ZsGreen1)Hze/J (25), R26RmTomatom/mEGFP (26), TCF/Lef: H2B-GFP mice (27) (referred to as WntGFP mice), Shh-LacZ (28), and Gli1-LacZ (29) (kindly provided by A. Dlugosz). Mice were obtained from the Jackson Laboratory (Bar Harbor, ME), unless otherwise stated. Only male mice were studied, and for each experiment 4 to 10 animals were evaluated at each time point. Female mice were excluded from this study because of the presence of an additional histological zone (X-zone), which would potentially add variability.
Tamoxifen induction
Tissue lineage analyses were conducted by evaluating Gli1CreERT2 mice carrying the R26REGFPreporter. Three-week-old mice were fed with a tamoxifen-supplemented chow diet (TD.130859, Envigo) for 3 weeks to achieve a complete and consistent activation of the Cre recombinase. Three-week-old ShhCreERT2 mice carrying the R26REGFPreporter, Axin2CreERT2 mice carrying a R26RmTomatom/mEGFP on a mixed 129/C57Bl6 background, and Gli1CreERT-R26REGFP for lineage tracing during SHH pathway inhibition were administered tamoxifen (50 mg/kg body weight) via intraperitoneal (IP) injection for 2 consecutive days. For Axin2CreERT2-bcatfl/fl kept in a pure C57Bl6 background, Cre-mediated recombination was achieved via daily IP injections of tamoxifen (100 mg/kg body weight) for 3 consecutive days. Tamoxifen (Sigma-Alridch) was dissolved in 10% ethanol and 90% corn oil (Sigma-Aldrich) to a final concentration of 20 mg/mL. Adrenal glands harvested at all time points were evaluated by immunohistochemistry. No Cre activity (as determined by R26R reporter activity) was detected following inductions when only oil was administered to Cre-expressing mice or when tamoxifen was administered to mice not expressing Cre.
Analysis of mouse adrenal gland histology and immunohistochemistry
Adrenal glands were collected at the indicated time points, fixed in 4% paraformaldehyde for 2 hours at 4°C, and dehydrated in graded ethanol solutions if paraffin-embedded, or embedded in optimal-cutting-temperature compound (Sakura Finetek USA Inc.). Five-micrometer tissue sections from paraffin blocks were treated with boiling 10 mM citric acid (pH, 2) for 10 minutes or 10 mM sodium citrate (pH, 6) for 20 minutes, followed by 20 minutes' cooling for antigen retrieval. Slides were then washed 3 × 5 minutes in phosphate-buffered saline (PBS), and nonspecific staining was blocked by incubating the sections with 2% nonfat dry milk in PBS with 5% goat serum (Thermo Fisher Scientific) for at least 1 hour, followed by primary antibody (Table 1) incubation at 4°C overnight. The next day, slides were washed with PBS and incubated with secondary antibodies (Table 2) for 1 hour at room temperature, followed by nuclear counterstaining with 4′,6-diamidino-2-phenylindole (DAPI), and mounted by using ProLong Gold Antifade Mountant (Thermo Fisher Scientific). Tissue sections from frozen samples were rehydrated in PBS for 15 minutes and tissue was permeabilized with PBS + 0.1% Triton-X 100 for 10 minutes. When needed, antigen retrieval was performed by incubating 5-μm tissue sections with 0.1 mg/mL proteinase K, 50 mM Tris (pH, 8), and 5 mM EDTA (pH 8) in PBS for 10 minutes at room temperature and washed with PBS 3 × 5 minutes. Blocking and antibody staining were performed as described above. Antibodies used are listed below. Endogenous fluorescence of the R26REGFP and R26RtdTomato reporters were visualized in frozen section. Sections were analyzed by fluorescence microscopy conducted on a Zeiss ApoTome using its structured illumination to provide thigh-resolution images for each sample, and images were captured with an AxioCam MRm (Zeiss). High-resolution colocalization analyses were conducted with Olympus Fluoview 500 confocal microscope. 3,3′-Diaminobenzidine (DAB) staining was performed for processing of adrenal glands as described above. After antigen retrieval, antibody staining was carried out by using the VECTASTAIN ABC kit (Vector Laboratories) according to the manufacturer’s protocol.
Table 1.
Primary Antibodies
| Antigen | Source Species | Dilution | Manufacturer; Catalog No.; and/or Name of Individual Providing the Antibody; RRID |
|---|---|---|---|
| SF1 | Rabbit | 1:1000 | Ab Proteintech Group (PTGlabs) (custom made); AB_2716716 |
| CYP11B1 | Mouse | 1:20 | Gift from Dr. C. Gomez-Sanchez; AB_2716718 |
| CYP11B2 | Rabbit | 1:200 | Gift from Dr. C. Gomez-Sanchez; AB_2716717 |
| β-Catenin | Mouse | 1:500 | BD Transduction Laboratories; 610153; AB_397554 |
| SCC | Rabbit | 1:400 | EMD Millipore; AB1244; AB_90525 |
| PCNA | Mouse | 1:500 | Cell Signaling; 13110; AB_2160343 |
| KI67 | Mouse | 1:500 | BD Pharmingen; 550609; AB_393778 |
| Cleaved caspase 3 | Rabbit | 1:200 | Cell Signaling; 9669; AB_2069869 |
| GFP | Chicken | 1:1000 | Abcam; ab13970; AB_300798 |
Abbreviation: SCC, side chain cleavage enzyme.
Table 2.
Secondary Antibodies
| Antigen | Source Species | Dilution | Fluor | Manufacturer; Catalog No.; and/or Name of Individual Providing the Antibody; RRID |
|---|---|---|---|---|
| Mouse IgG | Goat | 1:800 | Alexa-488 | Jackson ImmunoResearch; 115-545-003; AB_2338840 |
| Rabbit IgG | Goat | 1:800 | Alexa-488 | Jackson ImmunoResearch; 111-545-003; AB_2338046 |
| Mouse IgG | Goat | 1:800 | Dylight 549 | Jackson ImmunoResearch; 115-505-146; AB_2341133 |
| Rabbit IgG | Goat | 1:800 | Dylight 549 | Jackson ImmunoResearch; 111-505-003; AB_2493180 |
| Chicken IgG | Donkey | 1:800 | Dylight 549 | Jackson ImmunoResearch; 003-500-003; AB_2336975 |
Pharmacological treatments
Water-soluble dexamethasone (Sigma-Aldrich) was reconstituted in autoclaved deionized water at the concentration of 0.0167 mg/mL. GANT61 (Tocris), 50 mg/kg body weight, dissolved in 4:1 corn oil:ethanol was administered via IP. NVP-LDE225 (Sonidegib; Selleckchem), 40 mg/kg body weight, prepared in a solution of 0.5% methyl cellulose/0.5% Tween80 in water was given by oral gavage once a day.
RNA extraction and real-time quantitative polymerase chain reaction
Harvested adrenals were cleaned from adherent fat and homogenized, and RNA was extracted by using the RNeasy Mini Plus Kit (Qiagen) following the manufacturer's instructions. One microgram of RNA was converted into complementary DNA (cDNA) by using the iScript Supermix Reverse transcription Kit (Bio-Rad) as recommended. Quantitative real-time polymerase chain reaction (PCR) was performed by using 10 ng cDNA, 1 μM of specific primers (Table 3), and Power SYBR Green reagent (Applied Biosystems) on an ABI 7300 thermocycler (Applied Biosystems). Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was used as an internal control, and data are expressed by using the 2-ddCt method.
Table 3.
Primers Used for qPCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Gapdh | TGTCCGTCGTGGATCTGAC | CCTGCTTCACCACCTTCTTG |
| Sf1 | CGCTGTCCCTTCTGCGGCTT | AGCACGCACAGCTTCCAGGC |
| Cyp11b1 | AAGTATGGCCCCATTTACAGG | CATTACCAAGGGGGTTGATG |
| Shh | CCAATTACAACCCCGACATC | GCATTTAACTTGTCTTTGCACCT |
| Gli1 | CAGGGAAGAGAGCAGACTGAC | CGCTGCTGCAAGAGGACT |
| Ctnnb1 | GCAGCAGCAGTTTGTGGA | TGTGGAGAGCTCCAGTACACC |
| Axin2 | GCAGGAGCCTCACCCTTC | TGCCAGTTTCTTTGGCTCTT |
| Lef1 | CTGAAATCCCCACCTTCTACC | TGGGATAAACAGGCTGACCT |
| Wnt4 | CTGGACTCCCTCCCTGTCTT | ATGCCCTTGTCACTGCAAA |
| Rspo3 | TCAAAGGGAGAGCGAGGA | CAGAGGAGGAGCTTGTTTCC |
RT2 profiler PCR array
A mouse-specific RT2 profiler PCR array, which provides gene expression profiling focused on canonical WNT signaling pathway using real-time quantitative PCR (qPCR) (catalog no. PAMM-043A; Qiagen) was carried out following the recommended directions provided by the manufacturer. Briefly, high-quality RNA was obtained from adrenals using the QIAzol/TRIzol protocol and subjected to clean up with the RNeasy Mini kit (Qiagen). RNA concentration and purity were assessed by NanoDrop (Thermo Fisher Scientific). RT2 First Strand Kit (SABiosciences) was used to eliminate potential genomic DNA contaminations and to convert 1 μg total RNA into cDNA. The resulting product was loaded in a 96-well RT2 profiler PCR array according to the provided protocol, and PCR detection was carried out on an ABI 7300 thermocycler. Data analysis was performed with the manufacturer’s software (SABiosciences).
Microarray analysis
Microarray analysis was carried by using a Mouse Gene ST 2.1 Array Plate (Affymetrix). For each analyzed time point [before dexamethasone administration (D0), after dexamethasone administration (R0), 3 days after cessation of dexamethasone (R3), and 7 days after cessation of dexamethasone (R7)], quality of RNA extracted from adrenals of four 6-week-old male C57BL/6 mice was assessed with Agilent 2100 Bioanalyzer (Agilent Technologies). Microarray data analysis was performed by using R software (R Core Team). Raw data were read and normalized by using the R-Bioconductor package “oligo” (30). Quality control assessment of microarray data was carried out by principal component analysis and by means of the normalized unscaled standard errors and relative log expression value plots using the R-Bioconductor package “affyPLM” (31). One sample failed the quality control standards and was excluded from the analysis. Normalization was performed by using the RMA algorithm (32). Independent component analysis was executed using the R-Bioconductor “MineICA” package (33). Annotation was performed with the R-Bioconductor package “biomaRt” (34). The array data have been deposited in the Gene Expression Omnibus (GEO) of the National Center for Biotechnology Information and are accessible through GEO Series accession number GSE107715.
Corticosterone measurement
Blood samples obtained within 45 seconds of handling via decapitation were collected between 10:00 and 11:00 in BD Vacutainer 3K EDTA tubes (Thermo Fisher Scientific) and centrifuged at 2000g for 15 minutes at 4°C. Plasma samples were transferred to polypropylene tubes and stored at −80°C until use. The corticosterone content was determined by using a mouse and rat corticosterone enzyme-linked immunosorbent assay kit (ALPCO Diagnostics). Assays were conducted following the manufacturer’s instructions.
Quantification and statistical analysis
For lineage and cell density analyses, cells were counted in at least three sections per adrenal gland. Adrenal glands were weighed after removal of adjacent fat, and the average of the two adrenal weights was normalized to body weight. A two-tailed Student t test was carried out for normally distributed data. For nonparametric distributions (data shown as the median), a two-tailed Mann-Whitney test was performed. For the multiple comparison test, analysis of variance with the Tukey multiple comparisons test was used. Statistical analyses were carried out using Prism (GraphPad).
Results
Shh-expressing cells contribute to zF regeneration in adrenal atrophy model
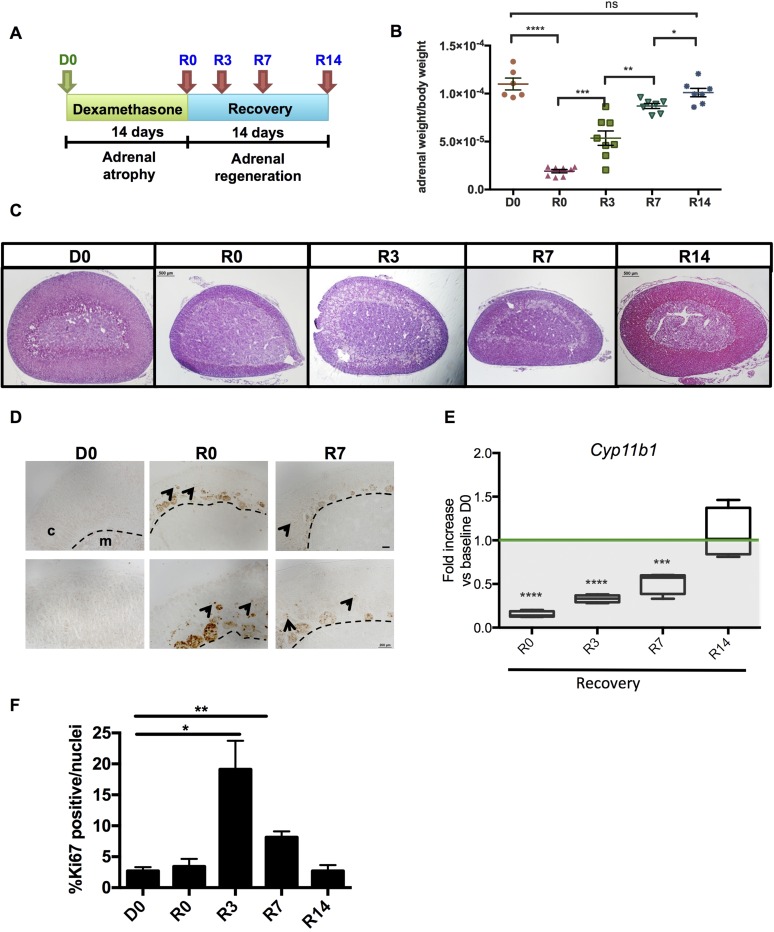
The effects of iatrogenic glucocorticoid excess on the adrenocortical growth and function are well documented to consist of a suppressed plasma ACTH level that results in decreased expression of enzymes involved in cortisol/corticosterone production, concomitant atrophy of the zF, and ultimate cell death (35–38). Although glucocorticoid-induced adrenocortical atrophy is a relatively well-characterized phenomenon, little is known about the mechanisms responsible for adrenal regeneration following suspension of such therapies. Therefore, we set out to determine whether and how the Shh-expressing cells, believed to serve as adrenal cortex progenitors, contribute to cells of the replenished zF. First, we created a model of zF atrophy by administering the synthetic glucocorticoid dexamethasone to wild-type male mice for 14 days in drinking water, followed by 14 additional days of recovery to allow for functional regeneration [defined as the return of Cyp11b1 messenger RNA (mRNA) levels to the homeostatic levels measured before dexamethasone treatment (D0)]. Adrenals were collected before and after dexamethasone treatment (D0 and R0, respectively) and at 3, 7, and 14 days after cessation of the treatment (R3, R7, R14) (Fig. 1A).
Figure 1.
Long-term administration of dexamethasone causes atrophy of the zF. (A) Experimental scheme of dexamethasone treatment and recovery. (B) Adrenal weights normalized to body weight. (C) Hematoxylin and eosin staining of adrenals shows atrophy of the zF after dexamethasone treatment (R0) and its regrowth following withdrawal. Scale bars: 500 μm. (D) Cleaved caspase 3 staining. Arrowheads point to nuclear staining. Dashed line marks the cortico-medullary border. Scale bars: top panels 100 μm, bottom panels 200 μm. (E) mRNA expression of the zF enzyme Cyp11b1 normalized to D0. (F) Proliferation quantification (Ki67+ cells/nuclei per high-powered field). Biological replicates for each time point: D0, n = 5; R0, n = 7; R3, n = 8; R7, n = 7; R14, n = 7. Error bars represent the standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.001. c = cortex; m = medulla; ns, not significant.
Significant reductions in adrenal weights were observed after 14 days of dexamethasone treatment beginning at R0 and persisting through R3 and R7 compared with nontreated mice (Fig. 1B). At R0, the adrenal cortex appeared to be mostly composed of zG cells, immune cells, and vacuoles (data not shown). A specific dramatic loss of zF cells was confirmed by histologic evaluation (Fig. 1C). Consistent with previously published studies (39), the histologic atrophy of the zF was accompanied by apoptosis, as reflected in an increase in Cleaved Caspase 3 in the dexamethasone-treated adrenal cortices (Fig. 1D). Additionally, as revealed by qPCR, a marked decrease (85%) in Cyp11b1 mRNA levels was also observed in the R0 adrenal tissue when compared with baseline D0 levels.
After dexamethasone withdrawal, Cyp11b1 expression gradually increased and reached homeostatic levels by R14, indicating a functional recovery of the zF (Fig. 1E). The changes in adrenal weight paralleled these functional changes. Additionally, apoptosis and immune cell infiltration decreased during functional recovery, whereas proliferation (determined by KI67 staining) increased commensurate with the increase in adrenal weights (Fig. 1F), indicating a coordinated anatomic and functional recovery of the atrophied zF.
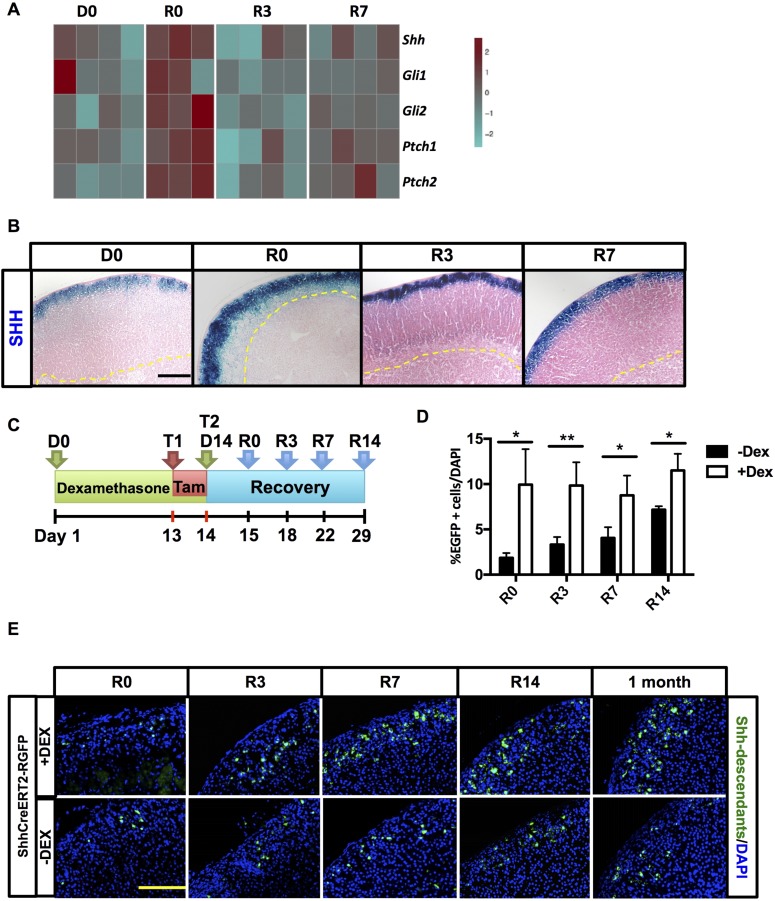
To interrogate the transcriptome of the adrenals of dexamethasone-treated mice and identify dominant coregulatory gene networks during adrenal regeneration after dexamethasone suspension, we used cDNA expression microarrays. An independent component analysis (33) identified a gene signature characterized by the presence of Shh and several downstream components of the hedgehog (HH) pathway, suggesting its involvement in the cortical regeneration process. The expression of these genes peaked on R0 (Fig. 2A). To address whether this augmented expression was due to an increase of mRNA per cell and/or to an expansion of Shh-expressing cell population, Shh-LacZ reporter mice were first treated with the dexamethasone strategy detailed above, followed by histochemical analysis. An expanded zone of β-galactosidase activity, consistent with an increase in the number of cells expressing Shh (marked in blue in Fig. 2B), was observed during the course of dexamethasone treatment (Supplemental Fig. 1 (9.9MB, pdf) ), after the suspension of the treatment (R0) and during early recovery (R3 and R7) (Fig. 2B).
Figure 2.
ZF atrophy triggers Shh overexpression and increases the number of Shh-descendant cells. (A) Heatmap showing selected genes of the HH signature. For each time point, n = 4. (B) β-Galactosidase expression (in blue) in Shh-LacZ reporter mice. An increase of cell Shh+ is evident following dexamethasone treatment and recovery. For each time point, n = 5. (C) Schematic of treatment protocol for ShhCreERT2-R26REGFP mice. Mice were treated with vehicle (control mice) or dexamethasone for 14 days and injected with tamoxifen both on the last day of dexamethasone treatment and on the following day. Adrenals were harvested 1 day later at R0 and at R3, R7, and R14. (D) Quantification of EGFP+ cells per high-powered field normalized to number of nuclei. Error bars represent standard error of the mean. *P < 0.05; **P < 0.01; and ***P < 0.001. For each time point, n = 5. (E) Immunofluorescent staining of adrenals from ShhCreERT2-R26REGFP mice. EGFP+ cells (green) indicate Shh lineage. Nuclei stained with DAPI (in blue). For each time point, n = 5. Scale bars: 100 μm. Dex, dexamethasone; Tam, tamoxifen.
On the basis of these findings, we set out to determine the contribution of Shh+ progenitor cells to the regenerating cortex by lineage tracing. We crossed the inducible ShhCreERT2 mouse line with the R26REGFP reporter mouse line, which activates expression of cre-recombinase in Shh-expressing cells after administration of tamoxifen, resulting in the permanent expression of the enhanced green fluorescent protein (EGFP) reporter gene in Shh+ cells and in their descendants. We chose to perform a low-dose tamoxifen pulse to achieve low-efficiency recombination and therefore label only a subset of Shh+ clones to better identify their progeny. ShhCreERT2-R26REGFP mice were treated according to the protocol detailed in Fig. 2C. After dexamethasone treatment, a quantifiable increase in Shh-expressing cells and their descendants (EGFP+ cells) was observed (Fig. 2D), resulting in the presence of clonal cords of EGFP+ cells as early as R7 and R14, which were not observed at these time points in control mice that did not receive dexamethasone (Fig. 2E).
As expected, 1 month following dexamethasone withdrawal, at a time when regeneration is complete and the normal homeostatic renewal of adrenocortical cells is engaged in both the dexamethasone and vehicle-treated groups, we observed persistent Shh-derived clusters in the zF in the dexamethasone-treated mice (Fig. 2E). These data are consistent with the process of regeneration, in part reflecting an enhancement of the known homeostatic replenishment of adrenocortical cells through engagement of long-term retained Shh+ progenitor cells of the zG (12).
Capsular Gli1+ cells participate in the regrowth process
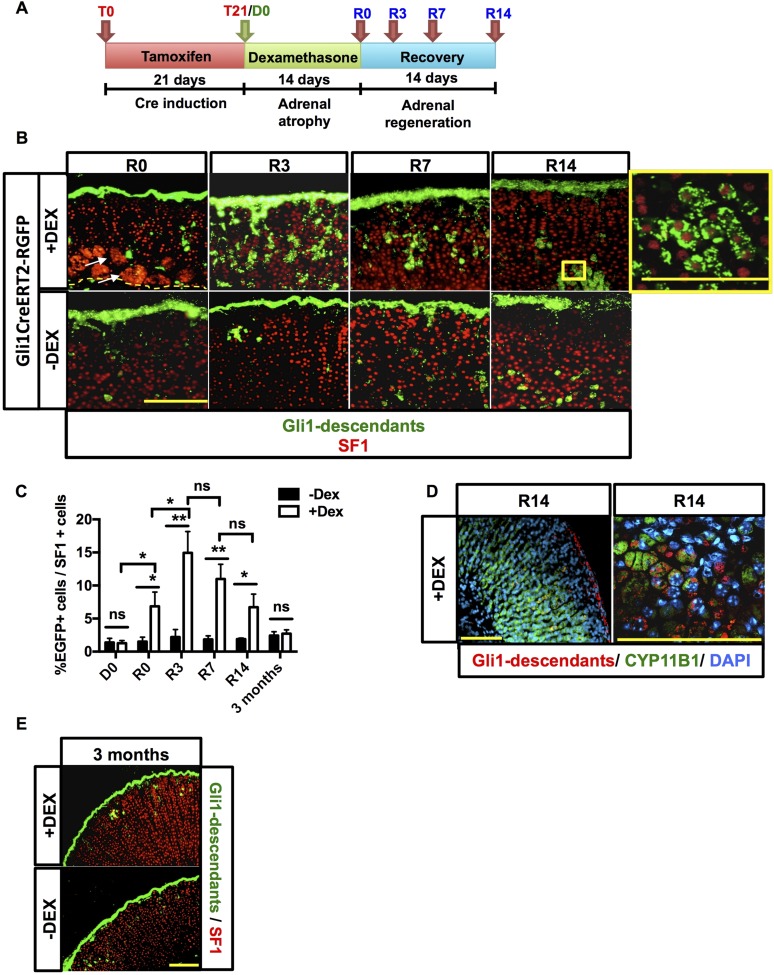
GLI1 is a transcription factor activated by HH signaling (40), and its expression is restricted to a subset of cells embedded within the adrenal capsule (12, 16). Given the contribution of Shh+ cells to homeostatic adrenal replenishment and the regeneration following dexamethasone treatment, we wanted to determine whether increased Shh expression following dexamethasone served to activate SHH signaling in capsular Gli1+ cells, as suggested by the increased expression of downstream components of the SHH signaling pathway in our array study (Fig. 2A). Mice expressing a tamoxifen-inducible cre-recombinase under control of the Gli1 promoter (Gli1CreERT2) were mated with the R26REGFP reporter mouse line and Gli1CreERT2-R26REGFP mice were treated according to the protocol detailed in Fig. 3A.
Figure 3.
Gli1 progeny participates in recovery of the adrenal gland. (A) Experimental scheme for treatment of Gli1CreERT2-R26REGFP mice. Following a tamoxifen chow diet for 21 days and administration of either dexamethasone or vehicle for 2 weeks, mice were euthanized at R0, R3, R7, and R14. (B) Immunofluorescence showing nuclear SF1 staining in red. Endogenous green EGFP marks Gli1+ cells and descendants. An increase in delamination of capsular Gli1+ cells into the adrenal cortex (green) that migrate centripetally toward the cortex-medulla boundary is present after dexamethasone (DEX) treatment. Capsular Gli1+ cells are SF1−, but their cortical progeny is SF1+, as shown in the enlargement box at the far top right. Scale bars: 100 μm. (C) Quantification of EGFP+ cells normalized to cortical Sf1+ cells in the cohorts of dexamethasone- and vehicle-treated mice. Error bars represent the standard error of the mean. *P < 0.05; **P < 0.01; and ***P < 0.001. (D) Gli1 descendant cells can differentiate into zF Cyp11b1+ cells. Scale bars: 100 μm. (E) Three-month chase of Gli1 descendants. For each time point, n = 5. Scale bars: 100 μm. ns, not significant.
Visualization of EGFP+ cells (capsular Gli1+ cells and descendants) at R0 did not significantly differ between dexamethasone-treated mice and controls (Fig. 3B and 3C), with strong capsular labeling in both groups. However, 3 days into the regeneration process, cords of capsule-derived EGFP+ cells were observed in the replenishing cortex of dexamethasone-treated adrenals, whereas in the control group only a few clusters or EGFP+ cells were found (Fig. 3B). Cortical EGFP+ cells expressed SF1 and cholesterol side chain cleavage enzyme (SCC) (Supplemental Fig. 2 (9.9MB, pdf) ), confirming the capability of capsular Gli1+ cells to give rise to cortical cells with steroidogenic features (12). Moreover, these steroidogenic cells also stained for CYP11B1, the steroidogenic enzyme responsible for corticosterone synthesis in the zF (Fig. 3D), supporting the conclusion that Gli1 descendants contribute to cortical replenishment. Because cortical clusters and occasional radial cords of EGFP+ cells were still observed 7 and 14 days after dexamethasone withdrawal, and because Gli1 descendants have been demonstrated to give rise to long-term retained Shh+ cells (12), we questioned whether delaminated descendants of the capsular Gli1+ cells (after dexamethasone treatment) became new long-term retained cortical cells that were capable of continued homeostatic repopulation of the cortex. We performed a 3-month chase in dexamethasone-treated Gli1CreERT2-R26REGFP mice, allowing for turnover of the entire adrenal cortex following regeneration (13). At this late time point, no significant differences were observed in the number or location of EGFP+ descendants of the treated vs. the control group. Minimal EGFP+ cells were evident in the cortex of both cohorts (Fig. 3E), indicating the lack of contribution of capsular Gli1+ cells to long-term retained Shh+ cortical progenitor cells engaged in zF regeneration following dexamethasone treatment. Instead, the data support a regeneration model whereby the capsular Gli1+ descendants give rise to early, transient functioning new cortical cells of the regenerating zF.
Inhibition of paracrine SHH signaling impairs regeneration
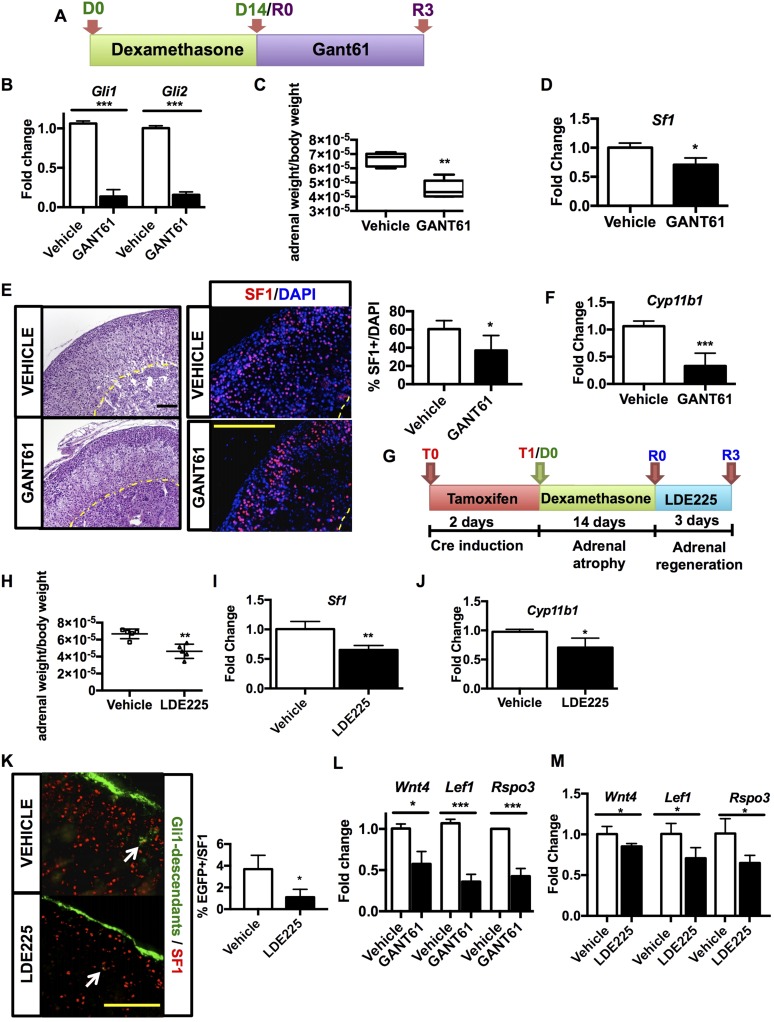
After identifying the involvement of both a transient capsular Gli1+ cell lineage and a sustained cortical Shh+ cell lineage in the regeneration process, we next examined the specific contribution of capsular SHH signaling to cellular regeneration in the cortex. To inhibit SHH-activated GLI1-mediated transcription in the capsular Gli1+ cells, we treated mice with GANT61, a specific inhibitor of GLI1 and GLI2 DNA binding and subsequent transcriptional activation (41). To verify the efficacy of GANT61 in inhibiting GLI1-mediated transcription in our mouse model, Gli1-LacZ mice, which exhibit robust capsular β-galactosidase activity, were treated with GANT61 for 3 days. β-Galactosidase activity decreased in capsular cells compared with vehicle-treated mice, indicating drug efficacy (Supplemental Fig. 3A (9.9MB, pdf) ). We then applied GANT61 treatment in the context of our regeneration protocol (Fig. 4A). Similar to the observed decrease in β-galactosidase activity in the adrenal capsule of GANT61-treated Gli1-LacZ mice, both Gli1 and Gli2 expression were decreased by 80% in the adrenals of mice following dexamethasone and subsequent 3 days of GANT61 treatment during regeneration (compared with vehicle-treated mice), further indicating a substantial inhibition of SHH-mediated GLI1-dependent transcription in the capsule following GANT61 treatment (Fig. 4B).
Figure 4.
Inhibition of SHH signaling impairs zF regrowth. (A) Experimental design of GANT61 treatment. Three-week-old wild-type male mice were treated with dexamethasone for 14 days. Starting on the day of dexamethasone withdrawal, mice were injected with GANT61 or vehicle for 3 days followed by organ harvest. (B) Relative mRNA quantification of Gli1 and Gli2 after GANT61 treatment normalized to vehicle. (C) Adrenal weight of GANT61- and vehicle-treated mice. (D) mRNA expression of Sf1 following GANT61 treatment normalized to vehicle. (E) Hematoxylin and eosin staining of adrenals treated with GANT61 or vehicle (left) shows altered adrenal morphology. Immunofluorescence staining (right) of cortical SF1+ cells (red) and nuclei (DAPI, blue) displays fewer steroidogenic cells in the cortex, as confirmed by quantification of SF1+ cell (far right). (F) mRNA levels of Cyp11b1 in GANT61-treated mice normalized to vehicle. (G) Experimental design of LDE225 treatment: After tamoxifen pulse to activate cre-recombinase, Gli1CreERT2-R26REGFP mice were administered dexamethasone for 14 days, followed by LDE225 for 3 consecutive days, and were euthanized on the next day. (H) Adrenal weight of vehicle- and LDE225-treated mice. (I) mRNA expression of Sf1 following LDE225 treatment normalized to vehicle. (J) Relative mRNA quantification of Cyp11b1 after LDE225 treatment normalized to vehicle. (K) Immunofluorescent staining showing cortical SF1+ nuclei (in red) and Gli1-expressing cells and their descendant (green) in mice treated with LDE225 (bottom) or vehicle (top). Arrows point at cortical Gli1 descendants that coexpress SF1. A decreased delamination into the cortex of Gli1 descendants is appreciable in LDE225-treated mice compared with vehicle-treated animals. Quantification of EGFP+; SF1+ cells is shown in the histogram (right). (L) Relative mRNA quantification of Wnt4, Lef1, and Rspo3 of GANT61-treated mice compared with vehicle-treated mice. (M) Relative mRNA quantification of Wnt4, Lef1, and Rspo3 of LDE225-treated mice compared with vehicle-treated mice. For statistical analyses, n = 5 animals per treatment. Error bars represent the standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bar: 100 μm.
Macroscopically, adrenals from GANT61-treated dexamethasone-suppressed mice were characterized by a lower weight compared with controls (Fig. 4C), which was histologically correlated with numerous vacuolizations, a more compact zG, and a 20% reduction in steroidogenic cortical cells as determined by quantification of Sf1 expression (Fig. 4D) and SF1+ cell number (Fig. 4E). Analysis of steroidogenic gene expression in GANT61-treated adrenal glands compared with vehicle-treated mice revealed a >50% decrease in zF Cyp11b1 expression, indicating a slowed functional cortical recovery following inhibition of capsular GLI signaling (Fig. 4F). The corticosterone content in the plasma of GANT61-treated mice mirrored Cyp11b1 expression with an approximate 50% decrease compared with vehicle-treated (Supplemental Fig. 3B (9.9MB, pdf) ). Altogether, these data indicate that GANT61 treatment attenuates GLI1-mediated capsular transcription, leading to a diminished repopulation of the zF and a consequent slower functional recovery.
Further verification of the involvement of paracrine SHH signaling was observed after treating mice with LDE225, an antagonist of Smoothened (Smo) receptor, which mediates HH signaling (42). After tamoxifen pulse and 14 days of dexamethasone administration, Gli1CreERT2-R26REGFP were treated for 3 days with LDE225 and euthanized the following day (Fig. 4G). LDE225 treatment during adrenal gland regeneration impaired adrenal weight (Fig. 4H) and led to decreased mRNA expression of Sf1 (Fig. 4I), Cyp11b1 (Fig. 4J), and, as expected, Gli1 and Shh (Supplemental Fig. 3C (9.9MB, pdf) ). Moreover, corticosterone levels displayed a slower recover compared with controls (Supplemental Fig. 3D (9.9MB, pdf) ). Lineage tracing of Gli1+ cells revealed that inhibition of SHH signaling led to a decreased delamination of Gli1 descendant into the adrenal cortex, as shown by the lower number cells that coexpressed EGFP+ and SF1 (Fig. 4K).
To explore whether inhibition of GLI signaling resulted in a commensurate downregulation of WNT signaling pathway (19), we focused on three critical mediators of canonical WNT signaling during adrenocortical homeostasis and zonation: Wnt4, Lef1, and Rspo3 (14, 15, 17, 19, 43). Following GANT61 treatment after dexamethasone-induced atrophy, we observed a 50% decrease in expression of adrenal Wnt4, a 50% decrease in the capsular-restricted Rspo3, and a 60% decrease in cortical-restricted WNT effector Lef1 compared with vehicle-treated regenerating adrenals (Fig. 4L). Paralleling these observations, canonical WNT pathway genes Wnt4, Lef1, and Rspo3 also exhibited diminished expression following LDE225 treatment (Fig. 4M).
Altogether, these data support a model whereby paracrine SHH signaling is important in zF regeneration and its inhibition leads to both a decrease in early delamination of capsular Gli1+ cells and a decrease in cortical WNT signaling.
Constitutive activation of GLI1+ signaling improves cortical regeneration
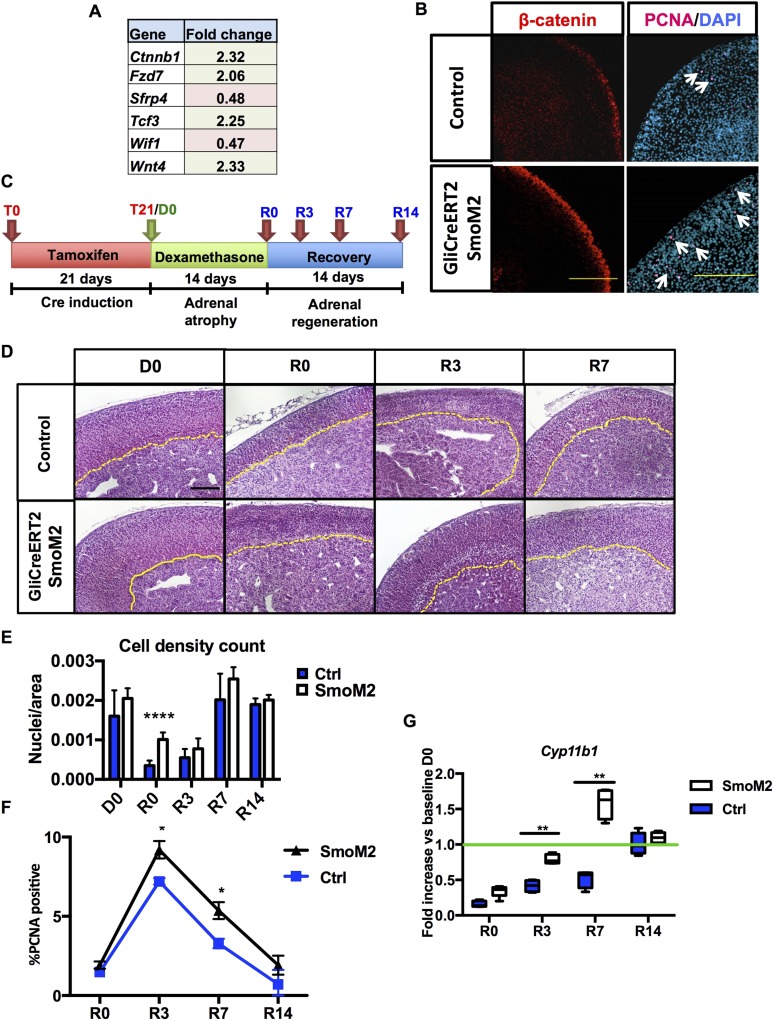
After observing that inhibition of transcriptional activity of capsular GLI1 impairs WNT signaling and regrowth of the functioning cortical zF, we tested whether constitutive capsular Gli1 activation can enhance WNT signaling, cortical regeneration, and functional zF recovery following dexamethasone-induced atrophy. We took advantage of the SmoM2 mouse, characterized by a mutant allele carrying a constitutively activating W539L point mutation, and crossed with Gli1CreERT2 mice to induce constitutive SHH activity only in capsular Gli1+ cells. Three-week-old male Gli1CreERT2-SmoM2 mice and SmoM2 controls were fed a tamoxifen diet for 3 weeks to efficiently activate the cre-recombinase. Before applying the dexamethasone protocol, we characterized these mice for evidence of histologic and functional changes in homeostasis together with changes in Shh and Wnt activation at baseline. Increased gene expression profiling for canonical WNT signaling pathway identified a 2.32- and 2.33-fold increase in Ctnnb1 and Wnt4 expression, respectively (Fig. 5A), whereas immunofluorescence analyses revealed enhanced peripheral β-catenin staining and proliferation (Fig. 5B). These data are consistent with a model whereby SHH activation in Gli1+ capsular cells enhances WNT responsiveness in the underlying cortex.
Figure 5.
Constitutively active HH signaling in capsular Gli cells improves adrenal recovery. Analysis of Gli1CreERT2-SmoM2 mice: (A) qPCR array for canonical WNT pathway components detected differential expression of β-catenin (Ctnnb1), Fzd7, Sfrp4, Tcf3, Wif1, and Wnt4. (B) Immunofluorescence staining on 10-week-old male mice shows increased proliferation, as indicated by nuclear proliferating cell nuclear antigen (PCNA) in magenta (right panel, white arrows pointing at the PCNA-positive cells) and β-catenin expression in red (left panel). (C) Schematic of the experimental design. (D) Hematoxylin and eosin staining of control adrenals and adrenals expressing constitutively active smoothened (SmoM2 mice). Cortical medullary border marked in yellow. Scale bars: 100 μm. (E) Cell density count reveals higher cell density in SmoM2 cortices after dexamethasone treatment. (F) Quantification of proliferation shown as percentage of PCNA+ cells per field. (G) Relative quantification of Cyp11b1. For statistical analyses, all time points n = 5 animals, except R0, R3, R7, and R14 control groups (n = 6). Error bars represent the standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
We next explored whether constitutive capsular Gli1 activation and resultant enhanced cortical WNT signaling was commensurate with augmented adrenal regeneration in Gli1CreERT2-SmoM2 mice after dexamethasone. Following a 3-week-long tamoxifen-containing diet, we administered dexamethasone for 14 days and collected tissues at the designated time points (Fig. 5C). Gli1CreERT2-SmoM2 mice showed no significant difference in adrenal weight at baseline compared with SmoM2 controls (Supplemental Fig. 4 (9.9MB, pdf) ); however, histological analysis of Gli1CreERT2-SmoM2 adrenals (Fig. 5D) revealed a cortex characterized by a higher cell density (Fig. 5E), coincident with a significant increase in cortical proliferating cell nuclear antigen staining (Fig. 5F) during regeneration (R0, R3), indicating an increase in proliferation. In addition, a significant relative increase in Cyp11b1 mRNA in the Gli1CreERT2-SmoM2 mice was observed during early regeneration at R3, consistent with a more rapid recovery of steroidogenesis (Fig. 5G). At R14, Cyp11b1 levels in Gli1CreERT2-SmoM2 mice and controls did not differ from the baseline consistent with return to homeostatic steroidogenesis, commensurate with completion of regeneration and return to homeostatic baseline.
WNT-responsive cells participate in adrenal regrowth
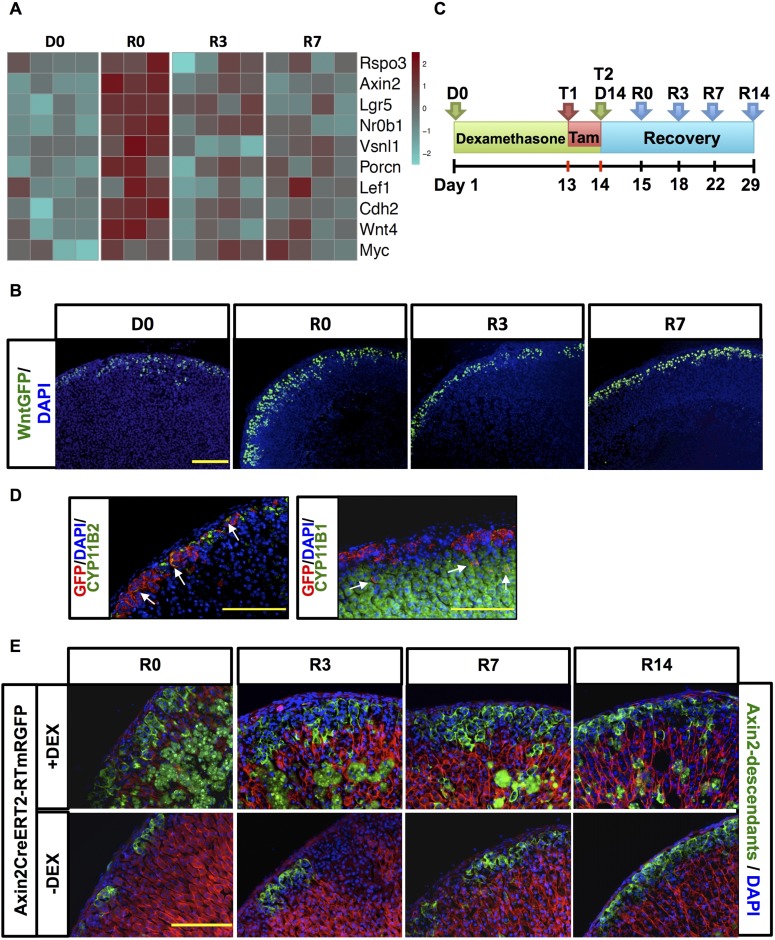
Although our previous experiments indicate that canonical WNT signaling in cortical cells is influenced by capsular SHH/GLI signaling during adrenal regeneration, we undertook experiments to more clearly define a role for canonical WNT signaling during adrenocortical recovery. Gene expression obtained with our microarray analysis showed increased expression of genes affiliated with canonical WNT signaling during adrenocortical recovery following dexamethasone treatment (Fig. 6A).
Figure 6.
Canonical WNT signaling pathway is actively engaged in adrenal cortex regeneration. (A) Heatmap showing selected genes of the canonical WNT signaling pathway signature during regeneration. For each time point, n = 4. (B) Immunofluorescence staining of adrenals from WntGFP mice. After dexamethasone there is an increase in WNT-responsive cells (green nuclear staining). Nuclei in blue (DAPI). For each time point, n = 5 (C) Scheme of experimental design for lineage tracing of AxinCreERT2- R26RmTomato/mEGFP mice. (D) Adrenals of Axin2CreERT2- R26RmTomato/mEGFP mice injected with tamoxifen (Tam) after 3 days of chase show that Axin2 descendants (in red) give rise to CYP11B2+ zG cells (left) and to CYP11B1+ zF cells (right). White arrows indicate labeled cells that coexpress CYP11B2 and GFP (left) and Cyp11B1 and GFP (right). Scale bar: 200 μm. (E) Lineage tracing of Axin2 after dexamethasone treatment. Axin2+ and descendants cells are marked in green, nuclei in blue. Dexamethasone-treated adrenals display a higher number of EGFP+ cells after treatment and during recovery. Nonspecific EGFP+ staining occurring in the inner zF following dexamethasone treatment is consistent with the vacuolization and immune infiltrate observed during glucocorticoid-induced atrophy as previously mentioned (refer to Fig. 1). For each time point n = 5. Scale bars: 200 μm. GFP, green fluorescent protein.
To identify the behavior of WNT-responsive cortical cells during recovery, 3-week-old WntGFP mice were treated according to our dexamethasone protocol (Fig. 1A). As expected, at R0, R3, and R7, green fluorescent protein–tagged WNT-responsive cortical cells increased markedly, suggesting that increased canonical WNT signaling part results from increasing WNT-responsive cell number during adrenal recovery. The number of WNT-responsive cells increases over the time course of dexamethasone treatment, as observed for Shh expression (Supplemental Fig. 5 (9.9MB, pdf) ).
To further study the contribution of WNT-responsive cells to cortical regeneration, we performed a lineage tracing study by crossing Axin2CreERT2 mice into the reporter line R26RmTomato/mEGFP. Three-week-old Axin2CreERT2-R26RmTomato/mEGFP mice were then treated according to the dexamethasone protocol previously described after tamoxifen pulse (Fig. 6C). These mice ubiquitously express a Tomato reporter until recombination occurs in Axin2+ cells, which results in permanent labeling of these cells and their descendants with EGFP.
Lineage tracing analysis revealed that WNT-responsive Axin2+ cells give rise to cells of both the zG and the zF (Fig. 6D and 6E). After dexamethasone withdrawal, an extended concentric layer of EGFP+ cells in the zG of an approximate depth of four-cell layers was present, whereas in control mice vehicle-treated EGFP tagging occurred only in clusters embedded within the histological zG. During regeneration, stream and clusters of EGFP+ cell clones penetrated deeper into the cortex, consistent with the contribution of both labeled and unlabeled WNT-responsive Axin2+ cells (which interpose between each other during regeneration) to the repopulating cortex. This process was not noticeable in control mice, in which an expansion of EGFP-tagged cells was visible but started to encompass the whole zG only at later time points, with a negligible number of cells observed as clusters deep into the cortex. These data confirmed our hypothesis that WNT-responsive cells actively contribute to the process of adrenal regeneration.
Genetic ablation of canonical WNT signaling leads to reduced adrenal recovery
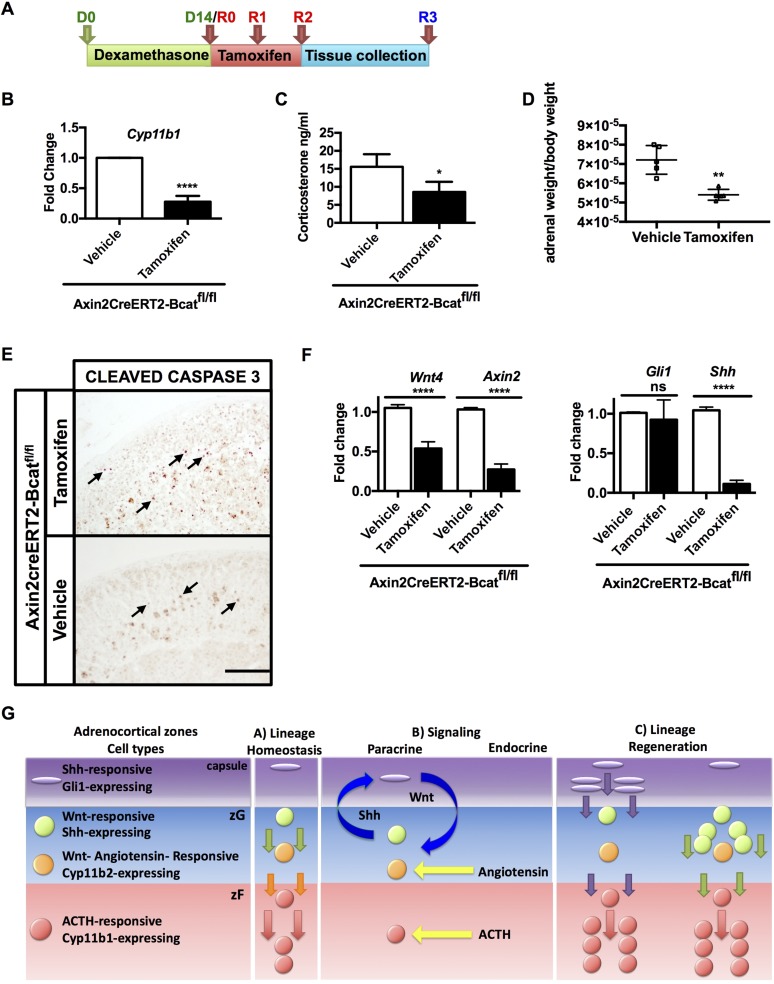
To test the hypothesis that canonical WNT signaling is necessary for adrenal regeneration, we genetically ablated Ctnnb1 in cells that express Axin2, by crossing Axin2CreERT2 mice with β-cateninfl/fl mice (referred as Bcatfl/fl mice) (Supplemental Fig. 6A (9.9MB, pdf) ). AxinCreERT2-bcatfl/fl male mice (n = 5) receiving regular drinking water and subjected to tamoxifen treatment served as homeostatic (non–dexamethasone-treated) controls. In this context, the acute loss of β-catenin for 3 days did not affect gross adrenal morphology or Sf1, Cyp11b2, Cyp11b1, Shh, and Gli1 gene expression (data not shown). We then compared adrenals from mice treated according to our regeneration protocol (Fig. 7A). Tamoxifen-induced Ctnnb1 excision was confirmed by PCR (Supplemental Fig. 6B (9.9MB, pdf) ). Control AxinCreERT2-Bcatfl/fl male mice (n = 5) were subjected to the same protocol described above, but instead of tamoxifen they were injected with vehicle, which did not cause Ctnnb1 excision. Both groups were subject to the dexamethasone-mediated zF atrophy protocol.
Figure 7.
Genetic ablation of WNT signaling pathway leads to impaired adrenal recovery. (A) Experimental scheme for treatment of AxinCreERT2-Bcatfl/f mice. Following 14 days of dexamethasone, mice were injected with tamoxifen for 3 consecutive days (days 14, 15, and 16) starting on the last day of dexamethasone treatment to achieve β-catenin excision, and harvested on day 17. (B) Tamoxifen-treated Ctnnb1-deleted mice display lower Cyp11b1 transcript during recovery compared with vehicle-treated mice, (C) lower corticosterone levels, and (D) lower adrenal weights. (E) Immunohistochemistry for cleaved caspase-3 in adrenals from mice with deleted Ctnnb1 (upper panel) and controls (lower panel). Increased apoptosis in Ctnnb1-deleted mice occurs in cells in both zG and zF (labeled nuclei indicated by black arrows). (F) Left: Relative quantification of transcripts for Wnt4 and Axin2. Right: relative quantification of Gli1 and Shh transcripts. Error bars represent the standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001 (Student t test). Scale bars: 200μm. For each treatment group, n = 5. (G) Model describing the lineage relationship between Shh- and Wnt-expressing cells and SHH- and WNT-responding cells during adrenal homeostasis (panel A) and regeneration (panel C). Arrows facing down indicate the cell centripetal migration; yellow arrows indicate the endocrine signaling mediated by angiotensin II and ACTH; blue arrows show paracrine SHH- and WNT-mediated signaling.
Following recovery, mice with loss of β-catenin exhibited decreased Cyp11b1 expression, diminished corticosterone production, and significantly lower adrenal weights compared with controls (Fig. 7B–7D). An increase in apoptosis, occurring in cells of both the zG and zF, was also observed (Fig. 7E). Following β-catenin ablation, we found significantly decreased Wnt4 and Axin2 expression (Fig. 7F, left), together with decreased Shh expression, consistent with the known WNT-responsiveness of the Shh-expressing progenitor cells. Whereas capsular Gli1 expression did not change (Fig. 7F, right), a longer time point could be necessary to observe a decrease in the expression of the SHH target, Gli1. These data support the hypothesis that activation of cortical WNT signaling is necessary for complete adrenal regeneration mediated by WNT-responsive Shh+ progenitor cells.
Discussion
To sustain endocrine homeostasis, the adrenal cortex must be capable of adapting to acute and chronic hormonal demands. The studies described in this report aimed to investigate the regenerative processes occurring in the adrenal cortex after atrophy induced by long-term administration of glucocorticoids. Long-term exogenous glucocorticoid administration is currently used for treating several medical conditions and has been long known to cause zF atrophy. However, little is known about the mechanisms involved in the cortical replenishment subsequent to atrophy (2,44).
Our studies use a model of adrenal atrophy induced by long-term administration of synthetic glucocorticoid dexamethasone, which is characterized by suppression of Cyp11b1 expression and corticosterone production, long assumed to be solely dependent on the suppression of the adrenal tropic factor ACTH, necessary for endocrine maintenance of adrenocortical glucocorticoid homeostasis. The adrenal cortex is characterized by cellular turnover that maintains adrenal homeostasis (45, 46). The current model of centripetal unidirectional differentiation involves Shh+ cells as progenitors giving rise to differentiated zG cells expressing Cyp11b2, from which the zF cells expressing Cyp11b1 descend (12, 13). Although recent studies have also revealed Shh (12, 16), Wnt4, Ctnnb1, and Rspo3 as required for proper adrenal homeostasis (14, 15, 17, 19, 43), the integrated role of these pathways (and the associated “paracrine factor–producing” cells and “paracrine factor–responsive” cells) in adrenal homeostasis remains unknown. Our data support a model whereby endocrine hormones that stimulate the adrenocortical cell (ACTH) and/or are secreted by adrenocortical cells (glucocorticoids) communicate with the intrinsic paracrine signaling of the gland to coordinate integration of growth (presence of zF) and differentiated function (synthesis of glucocorticoids) of the gland (13).
SHH signaling activates Gli family transcription factors in cells located in the adrenal capsule. During adrenal organogenesis, Gli1+ cells give rise to cortical Sf1+ cells, including Shh+ progenitor cells located in the outermost zG (12, 16, 47). Whereas prior studies using mice with genetic loss of Shh have observed hypoplastic adrenal glands with a thinner capsule, decreased corticosterone secretion, and increase in ACTH secretion (12, 16, 47), it has remained unclear whether the adrenal hypoplasia reflected a loss of signaling to the Gli1+ cells of the capsule and/or a primary defect in the Shh+ progenitor. It is important to note that Gli1+ cells and their progeny mildly contribute to the adult homeostasis and adrenocortical renewal (12). However, during regeneration in our paradigm, inhibition of capsular SHH/GLI1 signaling prevented, whereas constitutive activation augmented, regeneration and functional recovery. The current study examined the roles of both (1) SHH signaling between Shh+ cells and the SHH-responsive Gli1+ cells of the capsule and (2) the role of Gli-expressing cell lineage and Shh-expressing cell lineage in the regeneration process.
Gli1-lineage tracing experiments in our atrophy model reveal a surge of cells deriving from Gli1+ cells that delaminate from the capsule into the adult cortex during recovery/regeneration. These descendants possess steroidogenic potential and acquire expression of zonation markers relative to their position. The delaminated cells are not retained long term, presumably reflecting a vestigial developmental response or simply a mechanism for an early rapid recovery of cortical function before engagement of cortical Shh+ progenitor cells. Indeed, activation of Shh expression, engagement of long-termed retained Shh+ progenitors with lineage conversion to differentiated zF cells is a dominant feature in the regenerating cortex.
Importantly, canonical WNT pathway activation was also observed in the regenerating cortex. Because Shh+ progenitor cells are a subset of the Wnt-responsive cells of the peripheral zG (15), this observation is not unexpected. Indeed, the loss of β-catenin and SHH in the adrenal cortex during development causes variable adrenal hypofunction and hypoplasia (12, 14). A similar engagement of both SHH and canonical WNT signaling pathway has been described in a model of bladder injury, wherein basal Shh+ stem cells produces increased stromal expression of WNT signals; this, in turn, stimulates the proliferation of both urothelial and stromal cells to restore functionality of the organ. Impairment of GLI1 signaling negatively affected bladder regeneration (48), similar to our observations. In our current mouse model of adrenal regeneration, inhibition of capsular GLI1 results in diminished activation of cortical WNT signaling (Lef1) that is presumably mediated through a decrease in secreted WNT4 and capsular RSPO3 (19), whereas constitutive activation of SHH signaling in the capsular Gli1+ cell (SmoM2) induces an increased expression of canonical WNT pathway genes, commensurate enhanced adrenal proliferation, and a more rapid recovery of zF function. Although the SmoM2 mutation was first observed in a sample of human basal cell carcinomas (49), we did not observe hyperplasia or tumor formation in our mouse model.
To formally explore the contribution of WNT-responsive cells to adrenal regeneration, we examined the activation of Axin2-expressing cells and their downstream lineage. Our tracing experiments in non–dexamethasone-treated mice found that Axin2+ cells in the zG give rise to differentiated Cyp11b2+ zG cells, which subsequently migrate centripetally to contribute to Cyp11b1+ cells of the zF during normal homeostasis. After dexamethasone-mediated atrophy, increased expression of Axin2 (consistent with canonical WNT activation) was accompanied by an expansion of Axin2+ cells and their descendants with increased contribution of Axin2+ lineage to the regenerating cortex, confirming engagement of the WNT responsive cells to the repopulation of the atrophied zF.
To define the dependency of the regeneration process on active canonical WNT signaling in the WNT-responsive Axin2+ cells, we genetically ablated Ctnnb1 in Axin2+ cells (and their progeny) and observed profoundly impaired regeneration characterized by a persistent histological disorganization following dexamethasone treatment and increased apoptosis in both the zF and zG. Importantly, controls that did not undergo dexamethasone treatment did not present with a histological phenotype or with impairment of Cyp11b1 expression despite genetic loss of β-catenin. Although this may simply reflect slower WNT-dependent homeostatic renewal of the zF compared with the regeneration paradigm, the data support a critical role of active WNT signaling in the Axin2+ cells that serve to give rise to descendent cells that populate the regeneration zF. The observed decrease in Shh expression is consistent with specific loss of the WNT-responsive Shh+ cell, as predicted.
Our data outline the signaling and lineage relationship between Shh- and Wnt-expressing cells and SHH- and WNT-responding cells during adrenal homeostasis (Fig. 7G). SHH secreted by the WNT-responsive SHH-producing progenitor cells in the zG activates SHH signaling in the Gli1+ capsular cells. These cells engage in a rapid expansion, delamination, and differentiation to steroidogenic cells of the regenerating gland. In addition, Gli1 activation results in an increased expression of upstream Wnt ligands (i.e., Rspo3 and Wnt4). RSPO3 expressed in the capsule amplifies ligand-dependent WNT signaling and induces β-catenin activity (19). Secreted canonical WNT ligands and RSPO3 signal to the WNT-responsive (including the WNT-responsive SHH-producing cells) that serve as long-term retained progenitors that repopulate the atrophic gland (Fig. 7G).
Both WNT and SHH signaling have been reported to be inhibited by PKA (the primary downstream effector of ACTH) (17, 50). In other cell/organ systems, PKA can limit the activity of HH signaling (51), and repression of PKA can induce a noncanonical SHH-independent activation of Gli (52). In the adrenal cortex, WNT and PKA signaling have a reciprocal relationship with WNT signaling, inhibiting ACTH/PKA-mediated steroidogenesis in vitro (15) and in vivo, where Wnt4 expression is restricted to the zG, whereas PKA activity is restricted to the zF, where it promotes zF differentiation (17). Whether dexamethasone treatment, which inhibits pituitary ACTH and results in lowered PKA activation in the zF, accounts for the activation of WNT-responsive Shh+ cells is unknown. Although glucocorticoids can also influence steroidogenesis by upregulating the WNT-responsive corepressor Dax-1 (53) in the peripheral adrenal cortex (54), the role of direct glucocorticoid action in adrenal regeneration remains unclear.
Acknowledgments
We thank Dr. A. Dlugosz, Dr. S. Wong, and Dr. E. Fearon (University of Michigan, Ann Arbor, MI) and Dr. C. Gomez-Sanchez (University of Mississippi Medical Center, Jackson, MS) for providing reagents. We also thank the Microscopy and Image Analysis Core at the University of Michigan for assistance with our imaging and histological techniques. We are grateful to Dr. S. Wong for critical reading of the manuscript.
Financial Support: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health Research Grant 2R01-DK062027 (to G.D.H).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACTH
- adrenocorticotropic hormone
- cAMP
- cyclic adenosine monophosphate
- cDNA
- complementary DNA
- D0
- before dexamethasone
- DAPI
- 4′,6-diamidino-2-phenylindole
- EGFP
- enhanced green fluorescent protein
- GLI1
- glioma-associated oncogene homolog 1
- HH
- hedgehog
- IP
- intraperitoneal
- mRNA
- messenger RNA
- PBS
- phosphate-buffered saline
- PCR
- polymerase chain reaction
- PKA
- protein kinase A
- qPCR
- quantitative polymerase chain reaction
- R0
- after dexamethasone administration
- R7
- 7 days after cessation of dexamethasone
- R14
- 14 days after cessation of dexamethasone
- Rspo3
- R-spondin 3
- SHH
- Sonic hedgehog
- Smo
- smoothened
- zF
- zona fasciculata
- zG
- zona glomerulosa
- zR
- zona reticularis.
References
- 1.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaltsas G, Alexandraki KI Adrenal suppression. In De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, eds. Endotext; 2011. Available at http://www.endotext.org/. Accessed 12 December 2017. [Google Scholar]
- 3.Bayman E, Drake AJ. Adrenal suppression with glucocorticoid therapy: still a problem after all these years? Arch Dis Child. 2017;102(4):338–339. [DOI] [PubMed] [Google Scholar]
- 4.Aumo L, Rusten M, Mellgren G, Bakke M, Lewis AE. Functional roles of protein kinase A (PKA) and exchange protein directly activated by 3′,5′-cyclic adenosine 5′-monophosphate (cAMP) 2 (EPAC2) in cAMP-mediated actions in adrenocortical cells. Endocrinology. 2010;151(5):2151–2161. [DOI] [PubMed] [Google Scholar]
- 5.Waterman MR, Bischof LJ. Mechanisms of ACTH(cAMP)-dependent transcription of adrenal steroid hydroxylases. Endocr Res. 1996;22(4):615–620. [DOI] [PubMed] [Google Scholar]
- 6.Ruggiero C, Lalli E. Impact of ACTH signaling on transcriptional regulation of steroidogenic genes. Front Endocrinol (Lausanne). 2016;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nickerson PA, Brownie AC, Skelton FR. An electron microscopic study of the regenerating adrenal gland during the development of adrenal regeneration hypertension. Am J Pathol. 1969;57(2):335–364. [PMC free article] [PubMed] [Google Scholar]
- 8.Perrone RD, Bengele HH, Alexander EA. Sodium retention after adrenal enucleation. Am J Physiol. 1986;250(1 Pt 1):E1–E12. [DOI] [PubMed] [Google Scholar]
- 9.Skelton FR. Adrenal regeneration and adrenal-regeneration hypertension. Physiol Rev. 1959;39(1):162–182. [DOI] [PubMed] [Google Scholar]
- 10.Beuschlein F, Mutch C, Bavers DL, Ulrich-Lai YM, Engeland WC, Keegan C, Hammer GD. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology. 2002;143(8):3122–3135. [DOI] [PubMed] [Google Scholar]
- 11.Finco I, LaPensee CR, Krill KT, Hammer GD. Hedgehog signaling and steroidogenesis. Annu Rev Physiol. 2015;77(1):105–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci USA. 2009;106(50):21185–21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman BD, Kempna PB, Carlone DL, Shah M, Guagliardo NA, Barrett PQ, Gomez-Sanchez CE, Majzoub JA, Breault DT. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell. 2013;26(6):666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, Lavery GG, Parker KL, Hammer GD. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135(15):2593–2602. [DOI] [PubMed] [Google Scholar]
- 15.Walczak EM, Kuick R, Finco I, Bohin N, Hrycaj SM, Wellik DM, Hammer GD. Wnt signaling inhibits adrenal steroidogenesis by cell-autonomous and non-cell-autonomous mechanisms. Mol Endocrinol. 2014;28(9):1471–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang CC, Miyagawa S, Matsumaru D, Parker KL, Yao HH. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010;151(3):1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drelon C, Berthon A, Sahut-Barnola I, Mathieu M, Dumontet T, Rodriguez S, Batisse-Lignier M, Tabbal H, Tauveron I, Lefrançois-Martinez AM, Pointud JC, Gomez-Sanchez CE, Vainio S, Shan J, Sacco S, Schedl A, Stratakis CA, Martinez A, Val P. PKA inhibits WNT signalling in adrenal cortex zonation and prevents malignant tumour development. Nat Commun. 2016;7:12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Lau W, Peng WC, Gros P, Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28(4):305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidal V, Sacco S, Rocha AS, da Silva F, Panzolini C, Dumontet T, Doan TM, Shan J, Rak-Raszewska A, Bird T, Vainio S, Martinez A, Schedl A. The adrenal capsule is a signaling center controlling cell renewal and zonation through Rspo3. Genes Dev. 2016;30(12):1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118(4):505–516. [DOI] [PubMed] [Google Scholar]
- 21.Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18(8):937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118(4):517–528. [DOI] [PubMed] [Google Scholar]
- 23.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128(8):1253–1264. [DOI] [PubMed] [Google Scholar]
- 24.van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11(3):387–400. [DOI] [PubMed] [Google Scholar]
- 25.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. [DOI] [PubMed] [Google Scholar]
- 27.Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis AK. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ß-catenin signaling in the mouse. BMC Dev Biol. 2010;10(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol. 2004;270(2):393–410. [DOI] [PubMed] [Google Scholar]
- 29.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129(20):4753–4761. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26(19):2363–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolstad BM, Collin F, Brettschneider J, Simpson K, Cope L, Irizarry RA, Speed TP. Quality Assessment of Affymetrix GeneChip Data In: Gentleman R, Carey VJ, Huber W, Irizarry RA, and Dudoit S, eds. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York, NY: Springer New York; 2005:33–47. [Google Scholar]
- 32.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31(4):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biton A, Bernard-Pierrot I, Lou Y, Krucker C, Chapeaublanc E, Rubio-Pérez C, López-Bigas N, Kamoun A, Neuzillet Y, Gestraud P, Grieco L, Rebouissou S, de Reyniès A, Benhamou S, Lebret T, Southgate J, Barillot E, Allory Y, Zinovyev A, Radvanyi F. Independent component analysis uncovers the landscape of the bladder tumor transcriptome and reveals insights into luminal and basal subtypes. Cell Reports. 2014;9(4):1235–1245. [DOI] [PubMed] [Google Scholar]
- 34.Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc. 2009;4(8):1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheys JO, Heaton JH, Hammer GD. Evidence of adrenal failure in aging Dax1-deficient mice. Endocrinology. 2011;152(9):3430–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas M, Keramidas M, Monchaux E, Feige JJ. Dual hormonal regulation of endocrine tissue mass and vasculature by adrenocorticotropin in the adrenal cortex. Endocrinology. 2004;145(9):4320–4329. [DOI] [PubMed] [Google Scholar]
- 37.Illera JC, Peña L, Martínez-Mateos MM, Camacho L, Blass A, Garcia-Partida P, Illera MJ, Silván G. The effect of long-term exposure to combinations of growth promoters in Long Evans rats: part 2. Adrenal morphology (histopathology and immunochemical studies). Anal Chim Acta. 2007;586(1-2):252–258. [DOI] [PubMed] [Google Scholar]
- 38.Greenstein S, Ghias K, Krett NL, Rosen ST. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin Cancer Res. 2002;8(6):1681–1694. [PubMed] [Google Scholar]
- 39.Kiess W, Gallaher B. Hormonal control of programmed cell death/apoptosis. Eur J Endocrinol. 1998;138(5):482–491. [DOI] [PubMed] [Google Scholar]
- 40.Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124(13):2537–2552. [DOI] [PubMed] [Google Scholar]
- 41.Mazumdar T, Devecchio J, Agyeman A, Shi T, Houghton JA. Blocking Hedgehog survival signaling at the level of the GLI genes induces DNA damage and extensive cell death in human colon carcinoma cells. Cancer Res. 2011;71(17):5904–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan S, Wu X, Jiang J, Gao W, Wan Y, Cheng D, Han D, Liu J, Englund NP, Wang Y, Peukert S, Miller-Moslin K, Yuan J, Guo R, Matsumoto M, Vattay A, Jiang Y, Tsao J, Sun F, Pferdekamper AC, Dodd S, Tuntland T, Maniara W, Kelleher JF III, Yao YM, Warmuth M, Williams J, Dorsch M. Discovery of NVP-LDE225, a potent and selective smoothened antagonist. ACS Med Chem Lett. 2010;1(3):130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heikkilä M, Peltoketo H, Leppäluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143(11):4358–4365. [DOI] [PubMed] [Google Scholar]
- 44.Leśniewska B, Nowak KW, Malendowicz LK. Dexamethasone-induced adrenal cortex atrophy and recovery of the gland from partial, steroid-induced atrophy. Exp Clin Endocrinol. 1992;100(3):133–139. [DOI] [PubMed] [Google Scholar]
- 45.Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y. Development of functional zonation in the rat adrenal cortex. Endocrinology. 1999;140(7):3342–3353. [DOI] [PubMed] [Google Scholar]
- 46.Chang SP, Morrison HD, Nilsson F, Kenyon CJ, West JD, Morley SD. Cell proliferation, movement and differentiation during maintenance of the adult mouse adrenal cortex. PLoS One. 2013;8(12):e81865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ching S, Vilain E. Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. Genesis. 2009;47(9):628–637. [DOI] [PubMed] [Google Scholar]
- 48.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472(7341):110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH Jr, de Sauvage FJ. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391(6662):90–92. [DOI] [PubMed] [Google Scholar]
- 50.Marks SA, Kalderon D. Regulation of mammalian Gli proteins by Costal 2 and PKA in Drosophila reveals Hedgehog pathway conservation. Development. 2011;138(12):2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Sci Signal. 2012;5(246):re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iglesias-Bartolome R, Torres D, Marone R, Feng X, Martin D, Simaan M, Chen M, Weinstein LS, Taylor SS, Molinolo AA, Gutkind JS. Inactivation of a Gα(s)-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol. 2015;17(6):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizusaki H, Kawabe K, Mukai T, Ariyoshi E, Kasahara M, Yoshioka H, Swain A, Morohashi K. Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1) gene transcription is regulated by wnt4 in the female developing gonad. Mol Endocrinol. 2003;17(4):507–519. [DOI] [PubMed] [Google Scholar]
- 54.Gummow BM, Scheys JO, Cancelli VR, Hammer GD. Reciprocal regulation of a glucocorticoid receptor-steroidogenic factor-1 transcription complex on the Dax-1 promoter by glucocorticoids and adrenocorticotropic hormone in the adrenal cortex. Mol Endocrinol. 2006;20(11):2711–2723. [DOI] [PubMed] [Google Scholar]