Abstract
The estrogen-related receptor α (ERRα) is an orphan nuclear receptor (NR) that plays a role in energy homeostasis and controls mitochondrial oxidative respiration. Increased expression of ERRα in certain ovarian, breast, and colon cancers has a negative prognosis, indicating an important role for ERRα in cancer progression. An interaction between ERRα and peroxisome proliferator–activated receptor γ coactivator 1α (PGC-1α) has also recently been shown to regulate an enzyme in the β-oxidation of free fatty acids, thereby suggesting that ERRα plays an important role in obesity and type 2 diabetes. Therefore, it would be prudent to identify compounds that can act as activators of ERRα. In this study, we screened ∼10,000 (8311 unique) compounds, known as the Tox21 10K collection, to identify agonists of ERRα. We performed this screen using two stably transfected HEK 293 cell lines, one with the ERRα-reporter alone and the other with both ERRα-reporter and PGC-1α expression vectors. After the primary screening, we identified more than five agonist clusters based on compound structural similarity analysis (e.g., statins). By examining the activities of the confirmed ERRα modulators in other Tox21 NR assays, eliminating those with promiscuous NR activity, and performing follow-up assays (e.g., small interfering RNA knockdown), we identified compounds that might act as endocrine disrupters through effects on ERRα signaling. To our knowledge, this study is the first comprehensive analysis in discovering potential endocrine disrupters that affect the ERRα signaling pathway.
ERRα agonist compounds were identified from the Tox21 compound library using ERR and PGC/ERR reporter cells.
The estrogen-related receptor (ERR), an orphan nuclear receptor (NR), was discovered in 1988 based on the structural similarity at the DNA binding domain of the estrogen receptor α (ERα) (1). It was classified as one of the NR subfamily members (NR3B1) (2). Although their name implies a relationship with hormones, this NR does not bind natural estrogens and remains under the orphan classification. ERRα was first identified to bind an extended estrogen response element half-site (5′TCAAGGTCA3′; later named the ERRα response element ERRE) of the lactoferrin gene and to modulate ERα function (3). A recent functional genomic study displayed binding site specificities for ERRα and ERα (4) and, using an unbiased microarray approach, found that most ERRα regulated genes are unrelated to estrogen signals (5). Nonetheless, depending on the target genes, crosstalk between these two receptors does occur, thus highlighting a complicated relationship between ERRα and ERα activity (6).
ERRα activity is primarily controlled by its level of expression and interaction with coactivators; in particular, its most common interaction is with the peroxisome proliferator–activated receptor γ coactivator 1α (PGC-1α) (7). Not all ERRα agonists use PGC-1α for coactivation; other coactivators can be recruited as well. However, the PGC-1α/ERRα axis has been implicated in controlling the expression of metabolic gene networks and mitochondrial biogenesis (8, 9). Furthermore, PGC-1α and ERRα form autoregulatory feed-forward loops to promote the expression of its target genes (10). Expression of PGC-1α and ERRα is sensitive to physiological and pathological cues, which underscores the critical role these molecules play in energy homeostasis in normal and disease states.
Energy homeostasis is a complex process that needs to be balanced in order for the body to function properly. Because ERRα is highly expressed in metabolically active tissues such as the brain, kidney, heart, and muscle, its defects will have a severe impact on tissue-specific functions. For example, the ERR−/− mouse is resistant to obesity that is linked to a high-fat diet (11), and PGC-1α deficiency causes multisystem metabolic failure that includes abnormal weight control, hepatic steatosis, and defects in muscle function (12, 13). Increased ERRα gene expression in certain diseases such as breast cancer (14), prostate cancer (15), and endometrial cancer (16) carries a poor prognosis for these diseases. Therefore, compounds that modulate the activity of ERRα could play a critical role in disease progression as well as maintenance of homeostasis. Although there is as yet no endogenous ligand identified for ERRα, several synthetic antagonists have been reported (17–19). Recently, dietary products such as genistein, apigenin, resveratrol, rutacarpine, piceatanol, daidzein, flavone, and cholesterol have been reported as potential agonists (20–22).
Quantitative high-throughput screening (qHTS) has recently been used to evaluate the effect of environmental chemicals on many NRs (23–25). The Tox21 program, a collaboration among the National Institutes of Health, including the National Center for Advancing Translational Sciences and the National Toxicology Program at the National Institute of Environmental Health Sciences, along with the Environmental Protection Agency and the Food and Drug Administration, has been created to efficiently assess toxicities of environmental chemicals that may affect the human body. Previously, the ERRα and PGC-1α/ERRα assays were developed in a qHTS format and validated by screening the LOPAC library (22). Here, we identified agonists of ERRα by screening the Tox21 10K collection of environmental chemical and drug samples (∼8300 unique compounds) using these two different ERRα cell lines. From our primary and follow-up screening assays, we have compiled a list of unique structural clusters and several singletons that stimulate ERRα signaling as potential endocrine disrupting chemicals.
Materials and Methods
Tox21 chemical library
qHTS evaluated ∼10,500 compounds (8311 unique chemical substances) gathered from commercial sources by the National Toxicology Program, the National Center for Advancing Translational Sciences Chemical Genomics Center, and the Environmental Protection Agency. Included in this chemical library were a variety of chemical classes (e.g., pesticides, drugs, industrial, and food compounds). These substances were selected on the basis of multiple criteria, including possible and definite environmental hazard or exposure concerns, compounds with properties conducive to high-throughput screening (molecular weight, volatility, solubility, logP), commercial availability, and cost. There were also 88 diverse compounds selected as internal controls to assess reproducibility and determine positional plate effects as previously stated (23).
Cell culture
ERR reporter cells (22) were cultured in high-glucose Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, 4 mM l-glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, and 100 mg/mL streptomycin. PGC/ERR reporter cells (22) were cultured in the same medium as the ERR reporter cells with an additional 1 μg/mL puromycin as the selection marker for PGC-1α expression. The cells were maintained at 37°C under a humidified atmosphere and 5% CO2. All the cell culture reagents were obtained from Invitrogen (Carlsbad, CA).
ERR reporter and PGC/ERR reporter assays
ERR reporter cells or PGC/ERR reporter cells suspended in culture medium without puromycin were dispensed at 2500 cells/5 μL/well in tissue culture–treated 1536-well white assay plates (Greiner Bio-One North America, Monroe, NC) using a Thermo Scientific Multidrop Combi (Thermo Fisher Scientific, Inc., Waltham, MA). Each compound has been tested at 15 concentrations ranging from 1.2 nM to 92 μM in the primary screening. After the cells were incubated at 37°C with 5% CO2 for 6 hours, 23 nL of compounds or control, genistein, was transferred into the assay plates using a Wako Pintool station (Wako Automation, San Diego, CA). The assay plates were incubated at 37°C for 18 hours, followed by the addition of 5 μL ONE-Glo luciferase reagent (Promega, Madison, WI) using a Flying Reagent Dispenser (Aurora Discovery, Carlsbad, CA). After 30 minutes of incubation at room temperature, the luminescence intensity of the assay plates was quantified using a ViewLux plate reader (PerkinElmer, Shelton, CT).
For the follow-up luciferase assay, ERR reporter cells were suspended and dispensed in the same manner described above. Each compound, from the statin clusters, was tested at 11 concentrations ranging from 0.78 nM to 46 μM. Each of these concentrations was then cotreated with 0 μM, 5 μM, or 10 μM XCT790, an ERRα-specific inverse agonist. The cells were then assayed in the same method mentioned above.
qHTS data analysis
The qHTS data were analyzed as previously described (23). In short, each titration point was normalized relative to the positive control compound (genistein = 100%) and dimethyl sulfoxide (DMSO)–only wells (0%) according to the following equation: % Activity = [(Vcompound − VDMSO)/(Vpos − VDMSO)] × 100, where Vcompound denotes the compound well values, Vpos denotes the median value of the positive control wells, and VDMSO denotes the median values of the DMSO-only wells. The data set was then corrected using the DMSO-only compound plates at the beginning and end of the compound plate stack by applying an in-house pattern correction algorithm as seen in a study by Wang and Huang (26). The half maximum effective values (EC50) for each compound and maximum response (efficacy) values were obtained by fitting the concentration-response curves of each compound to a four-parameter Hill equation (27). Concentration-response curves were designated as class 1 to 4 according to the type of concentration-response curve observed (1.1, 1.2, 1.3, 1.4, 2.1, 2.2, 2.3, 2.4, and 3 for activators; 4 for inactive) (27–29) and then assigned a curve rank combining curve class and efficacy (29). The activity outcome of a test compound was categorized based on the average curve rank from the triplicate runs and the reproducibility calls. Data reproducibility was categorized as active match, inactive match, inconclusive, and mismatch as previously described (23). The Tox21 10K library was clustered based on structural similarity (Leadscope fingerprints; Leadscope, Inc., Columbus, OH) using the self-organizing map algorithm (30). Each cluster was evaluated for its enrichment of active agonists and significance of enrichment as determined by P values from the Fisher exact test.
In vitro small interfering RNA transfection
Small interfering RNAs (siRNAs) for ERRα (s4831) and negative control siRNA were Silencer Select siRNAs purchased from Ambion (Austin, TX). The siRNA transfection experiments were performed using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s protocol. The knockdown effects of the siRNAs were confirmed by using real-time polymerase chain reaction (PCR). For the following experiments, we used siRNA for ERRα (s4831) at a final concentration of 20 nM.
ERR siRNA reporter assay
ERR reporter cells suspended in culture medium without antibiotics were dispensed at 4.0 × 104 cells/100 μL/well, following siRNA transfection in tissue culture–treated 96-well white assay plates (Falcon, Durham, NC). After the cells were incubated at 37°C with 5% CO2 for 24 hours, they were treated with the compounds. The assay plates were incubated at 37°C for 18 hours, followed by the addition of 100 μL/well of ONE-Glo luciferase reagent (Promega). After 5 minutes of incubation at room temperature, the luminescence intensity of the assay plates was quantified using a SpectraMax M3 plate reader (Molecular Devices, Sunnyvale, CA).
RNA extraction and quantitative real-time PCR
ERR reporter cells suspended in culture medium without antibiotics were dispensed at 1.0 × 106 cells/1 mL/well, following siRNA transfection in tissue culture–treated 6-well plates. Cells were treated with compounds for 18 hours. Total RNA (1 μg) was extracted from cultured cells using an RNeasy Mini kit (QIAGEN, Germantown, MD) and then used to synthesize complementary DNA (cDNA) with SuperScript IV VILO Master Mix (Invitrogen). Quantitative real-time PCR was performed using CFX Connect Real-Time System (Bio-Rad, Hercules, CA) with PerfeCTa SYBR Green FastMix (Quantabio, Beverly, MA). The primers used in the real-time PCR are as follows: ERRα 5′-ATGGTGTGGCATCCTGTGAG-3 (forward) and 5′-TGGTGATCTCACACTCGTTGG-3′ (reverse), β-actin 5′-CACCAACTGGGACGACAT-3′ (forward) and 5′-GCACAGCCTGGATAGCAAC-3′ (reverse), COX8H 5′-AGG AGT GCG ACC CGA GAA TC-3′ (forward) and 5′-GGC TAA GAC CCA TCC TGC TGG-3′ (reverse), and IDH3A 5′-AGGACTGATTGGAGGTCTTGG-3′ (forward) and 5′-ATC ACAGCACTAAGCAGGAGG-3′ (reverse). The target gene messenger RNA (mRNA) level was normalized to β-actin expression.
Statistical analysis
For the luciferase assays cotreated with XCT790 and the siRNA data, statistical comparisons were made by one-way analysis of variance with post hoc Dunnett’s analysis. The statistical significance was set at P values of <0.05 (*), <0.01 (**), and <0.001 (***).
Results
qHTS assay performance and reproducibility
We performed a primary qHTS of the Tox21 10K compound library, using HEK293 cells incorporated with the expression of an ERR reporter construct (ERR) or expression of both ERR reporter and PGC-1α expression constructs (PGC/ERR), to identify environmental chemicals and drugs as possible ERR agonists. The positive control, genistein, showed consistent activity throughout the screen (Table 1) with an EC50 of 5.59 μM and 8.42 μM in ERR and PGC/ERR assays, respectively. The assay performance statistics include signal-to-background ratios of >2, coefficients of variance <10%, and Z′ factors between 0.5 and 1 for both assays, indicating a good quality for the primary screens.
Table 1.
Positive Control 10K Screen Statistics
| Characteristic | EC50 (μM) | S/B | CV (%) | Z′ Factor |
|---|---|---|---|---|
| ERR agonist | 5.59 ± 0.98 | 2.10 ± 0.16 | 2.96 ± 0.88 | 0.62 ± 0.15 |
| PGC/ERR agonist | 8.42 ± 2.25 | 3.17 ± 0.20 | 6.17 ± 1.48 | 0.64 ± 0.10 |
Abbreviations: CV, coefficient of variation; S/B, signal to background.
We next evaluated the reproducibility of three separate runs of the Tox21 10K screen experiments for both cell lines, as well as the Tox21-88 compound array, which was plated in duplicate on every compound plate. Each compound following the primary 10K compound screening fell into one of three categories: active, inactive, or inconclusive. Reproducibility data were then calculated based on the comparison between those categories for each compound. The ERR and PGC/ERR 10K triplicate runs produced mismatch rates of 0.28% and 0.25%, respectively (Table 2). The Tox21-88 duplicates indicated a robust assay performance as well by showing mismatch rates of <2% (Table 2).
Table 2.
Assay Performance Validation Using the 10K Triplicate Run and Tox21-88 Duplicates
| Assay Reproducibility | Active Match (%) | Inactive Match (%) | Inconclusive (%) | Mismatch (%) | EC50 Fold Change |
|---|---|---|---|---|---|
| 10K triplicate | |||||
| ERR agonist | 18.30 | 70.52 | 10.89 | 0.28 | 1.30 |
| PGC/ERR agonist | 11.49 | 79.56 | 8.69 | 0.25 | 1.49 |
| Tox21-88 duplicate | |||||
| ERR agonist | 39.96 | 43.28 | 14.87 | 1.89 | 1.29 |
| PGC/ERR agonist | 18.61 | 63.83 | 16.81 | 0.76 | 1.40 |
For each assay, the reproducibility was calculated for the 10K library (three copies) and the Tox21-88 compounds (duplicates in each plate) with compounds plated in different well locations.
Identification of ERR agonists and their clusters
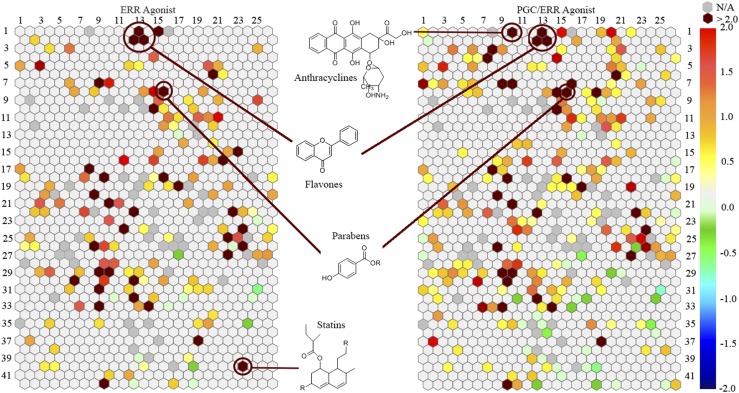
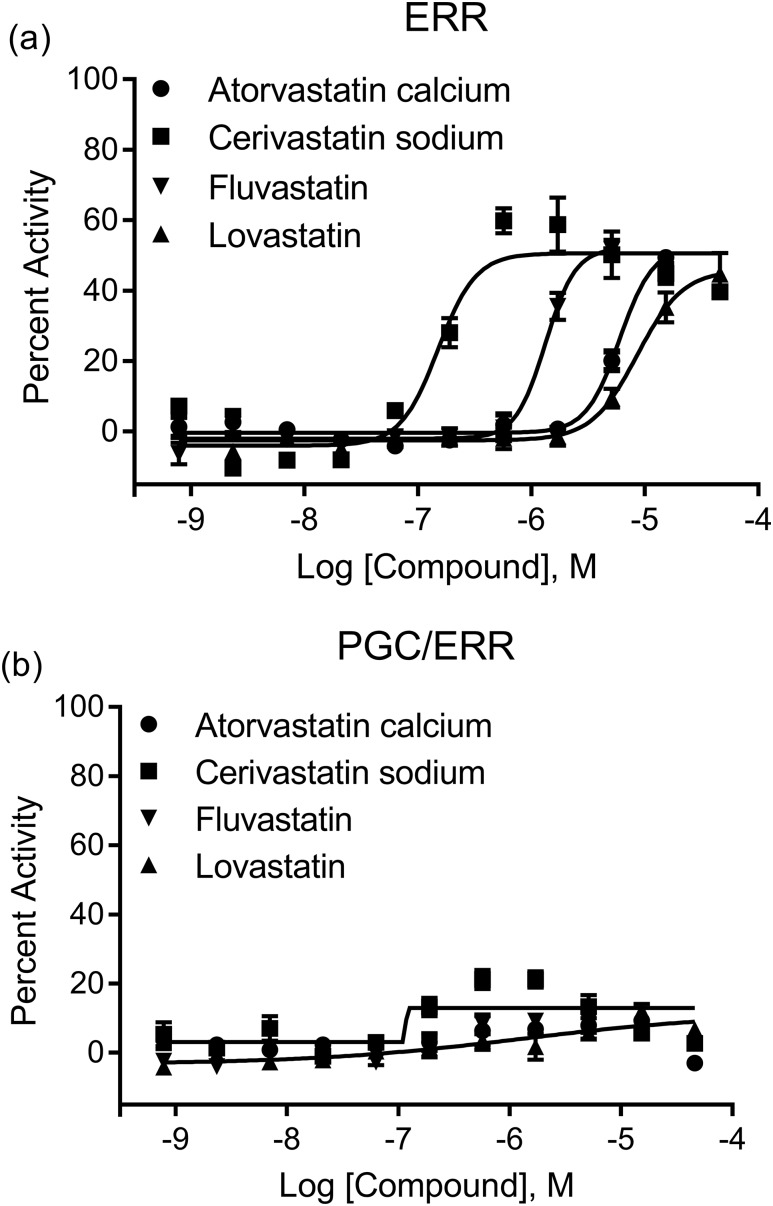
After the primary screen of the ERR reporter cell line, a self-organizing map algorithm was used to group the chemicals into 1014 clusters based on their structural similarities (30). Each cluster was then color coordinated with the significance of enrichment of ERR activators in the cluster (Fig. 1, left). Thirty-five clusters were significantly (P < 0.01) enriched with ERR agonists for this assay. Genistein, a well-known ERR agonist, was categorized into cluster 1.13. The P value of this cluster is <0.001, which indicates that this group of structurally similar flavones contains a large percentage of ERR agonists. Two other clusters, 2.12 and 2.13, also have flavone backbones and had P values of <0.01, indicating that they also are enriched with ERR agonists. Another group of compounds with an enhanced number of ERR agonists was the statin group. To our knowledge, this is a novel class of ERR agonists; these compounds were only identified in the ERR screen and were not active in the PGC/ERR screen, implying PGC-1α independence. Atorvastatin calcium, cerivastatin sodium, fluvastatin, and lovastatin were all potent agonists, with efficacies >50% and EC50 values of 1.79 μM, 0.0986 μM, 1.54 μM, and 2.78 μM, respectively (Supplemental Table 1 (836.7KB, docx) ). These statins were graphically plotted in a concentration-dependent manner using these primary screening data to demonstrate their range of potency and efficacy values [Fig. 2(a)].
Figure 1.
Structural class heat map of ERR agonist activity. Both the ERR and PGC/ERR cell lines were screened and divided into clusters. Each hexagon represents a class of structurally similar compounds. The color gradient is indicative of the enrichment of ERR actives in that specific cluster [negative logarithmic scale of the P value, −log (P value)]. Each color represents a group of chemicals with similar scaffolds to activate ERR. Clusters with multiple actives in their class are closer to a maroon color, whereas clusters with no activity are a light gray color. Empty clusters with no available (N/A) compounds in them are darker gray in color.
Figure 2.
Concentration curves of the statin structural class. The 11-point dilution of the (a) ERR and (b) PGC/ERR follow-up studies is represented in a concentration curve for four of the statins from structural classes 15.23 and 40.23. The stable cell lines were treated with each respective compound for 18 hours in 1536-well plates. ONE-Glo was then added and the luminescence intensity was calculated. The efficacies were compared with the positive control, genistein. Data are expressed as mean ± standard error of the mean using triplicate assays.
Single compounds, separate from the clusters, were also discovered using the primary ERR screen. The top nine compounds that had efficacies >100% of the positive control and potencies <10 μM are listed in Supplemental Table 2 (836.7KB, docx) . The most consistently potent compound in this group was axitinib, which had potencies of <1 μM in both the primary and follow-up screens. In addition, febuxostat and para-azoxyanisole also had potencies <1 μM in the primary ERR screen (Supplemental Table 2 (836.7KB, docx) ).
Identification of PGC/ERR agonists and their clusters
Compounds were grouped into clusters, as described above, after the PGC/ERR primary screen. For the PGC/ERR cell line, 33 clusters were enriched statistically significantly with ERR agonists, as shown in Fig. 1 (right). Many flavones in clusters 1.13, 2.12, and 2.13 were also identified as PGC/ERR agonists. Twenty-four clusters that were in common between the ERR and PGC/ERR assays were categorized as having P values <0.01. One example is the parabens (cluster 8.15) (Fig. 1). Interestingly, anthracyclines were identified as a cluster enriched with PGC/ERR agonists only. Daunorubicin, daunomycin, adriamycin, and pirarubicin all had EC50s <1 μM and efficacies >45% of the positive control (Supplemental Table 1 (836.7KB, docx) ). The statins, which were identified in the primary ERR screen as agonists of ERR, showed no activity in the PGC/ERR screen, suggesting that their ERR activation pathway is unique [Fig. 2(b)].
Of the single active compounds in the PGC/ERR primary screen, none of the compounds had an efficacy >100% and an EC50 <1 μM in the primary screen. However, two compounds had an efficacy >100% and an EC50 <10 μM. Forskolin had an EC50 of 1.73 μM and an efficacy of 277%, whereas suberoylanilide hydroxamic acid (SAHA) had an efficacy of 112% and an EC50 of 1.96 μM (Supplemental Table 2 (836.7KB, docx) ). Remarkably, these two compounds had no agonist activity in the screen with ERRα alone.
Selectivity of clusters and compounds to ERR and PGC/ERR
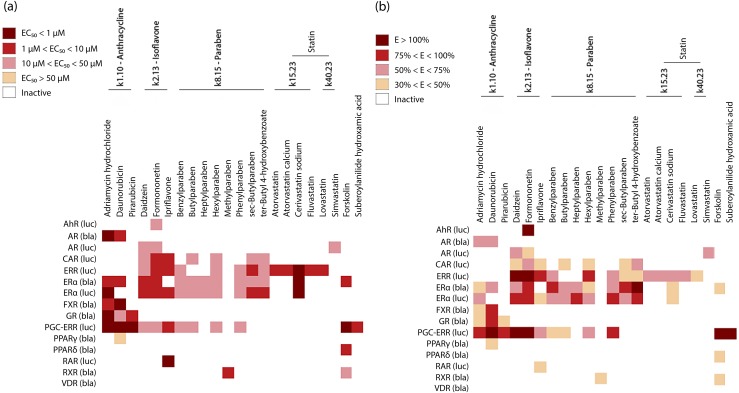
Focusing on five clusters and the three single actives mentioned above, based on compound potency [Fig. 3(a)] and efficacy [Fig. 3(b)], a heat map was generated of the agonistic activity for 23 compounds in 15 different follow-up assays (including ERR and PGC/ERR). Each of the 23 compounds had agonist activity in either the ERR or PGC/ERR assays, with the exception of simvastatin, which was not confirmed in the follow-up study (Supplemental Table 1 (836.7KB, docx) ). However, the other statins appear to be the most selective compounds toward ERR agonist activity. This group was identified only using the ERR assay and not the PGC/ERR assay, suggesting ERR agonist activity without employing the coactivator PGC-1α. Interestingly, only cerivastatin sodium shows any other agonistic activity because it was also shown to activate ERα signaling. This compound was identified as a potent (EC50 < 1 μM) but weakly efficacious (30% < efficacy < 50%) ERα agonist [Fig. 3]. Atorvastatin, atorvastatin calcium, fluvastatin, and lovastatin were all identified as potent (1 μM < EC50 < 10 μM) and weak to moderate (30% < efficacy < 75%) ERR agonists. Pirarubicin (cluster 1.10—anthracycline) had activity only in the glucocorticoid receptor agonist assay in addition to the PGC/ERR assay [Fig. 3]. Its efficacy ranged between 30% and 50% in the glucocorticoid receptor assay, which identifies pirarubicin as a somewhat selective ERR agonist that requires PGC-1α for activation. Forskolin (single active) was found to be an ERR pathway activator in the PGC/ERR assay. It has some agonistic activity (30% < efficacy < 50%) in the ERα, PPARδ, and retinoid X receptor pathways [Fig. 3]. However, forksolin’s strongest (efficacy > 100%) and most potent (EC50 < 1 μM) activity was in the PGC/ERR assay, suggesting this compound preferentially targets and is most effective against PGC/ERR signaling. SAHA is the only single active compound that shows complete selectivity for the PGC/ERR assay. It is a potent agonist (EC50 < 10 μM) and very efficacious (efficacy > 100%). None of the other identified active compounds appears to show selectivity toward ERR activity.
Figure 3.
Selectivity of structural clusters and singletons. Heat maps were generated based on the (a) EC50 and (b) efficacy of each of the chosen compounds against 15 different NR agonist assays.
Decrease in statin-induced luciferase activity employing inhibition or knockdown of ERR
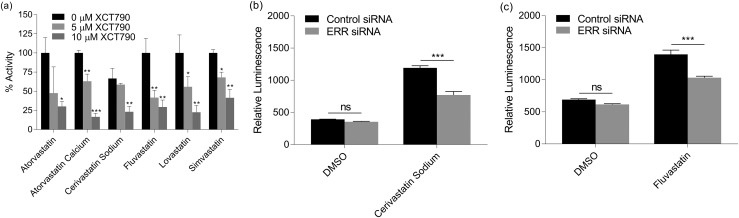
To confirm ERR activation from the statin cluster, XCT790, a known inverse agonist of ERR, was coadministered with each of the statins in a dose-dependent manner using the ERR cell line [Fig. 4(a)]. All six of the statins tested in combination with 10 μM XCT790 showed a statistically significant decrease in activity. To further confirm ERR activity in the cells, two statins—cerivastatin sodium and fluvastatin—were examined by measuring luciferase activity in the ERR cell line transfected with control or ERR siRNA [Fig. 4(b) and 4(c)]. Both compounds showed a decreased activation of the reporter in the presence of the ERR siRNA but were unaffected by control siRNA.
Figure 4.
ERR activity modulation by compounds in the statin cluster. (a) An ERR-luciferase assay was conducted by cotreating the known ERR inverse agonist, XCT790, at concentrations of 0 μM, 5 μM, or 10 μM with atorvastatin (23 μM), atorvastatin calcium (23 μM), cerivastatin sodium (23 μM), fluvastatin (23 μM), lovastatin (23 μM), or simvastatin (46 μM). The activities were compared with each respective compound without cotreatment of XCT790. (b) A knockdown ERR-luciferase assay was performed by transfecting ERR cells with either control or ERR siRNA. Cells were then treated with DMSO (0.2%), cerivastatin sodium (569 nM), or fluvastatin (15 μM). Each bar represents the mean ± standard deviation (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. ns, not significant.
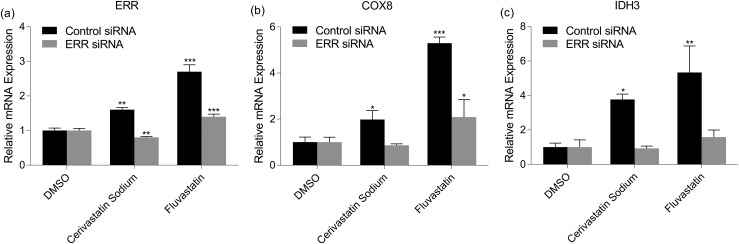
Effect of cerivastatin sodium and fluvastatin on ERR, COX8, and IDH3 mRNA expression
To further define the activity of cerivastatin sodium and fluvastatin, RNA was extracted after transfection with either control or ERR siRNA, and quantitative reverse transcription PCR was performed to quantify the levels of ERR mRNA in the cells [Fig. 5(a)]. In the cells treated with cerivastatin sodium or fluvastatin, the ERR mRNA levels in the ERR siRNA transfected cells were decreased, compared with the ERR mRNA expression in the control siRNA transfected cells. The expression of two of the ERR downstream target genes in the mitochondria, COX8 and IDH3, was also determined between the control and ERR siRNA transfected cells [Fig. 5(b) and 5(c)]. ERR siRNA treatment reduced both cerivastatin sodium- and fluvastatin-induced expression of COX8 and IDH3, providing additional evidence that these compounds are true ERR agonists.
Figure 5.
ERR, COX8, and IDH3 mRNA expression modulation by cerivastatin sodium and fluvastatin. The ERR cell line was transfected with control or ERR siRNA and treated with DMSO (0.2%), cerivastatin sodium (569 nM), or fluvastatin (15 μM). The RNA was extracted and quantitative reverse transcription PCR was performed to determine mRNA expression of (a) ERR, (b) COX8, and (c) IDH3. Each gene was expressed relative to the levels of β-actin and normalized to the levels of their respective vehicle control (DMSO). Each bar represents the mean ± standard deviation (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
ERRα signaling plays a critical role in regulating cellular bioenergetics and metabolism. ERRα regulates the transcriptional activity of metabolic genes in skeletal muscle, liver, heart, and kidney that require high energy utilization. Therapeutic and environmental chemical interference with the ERRα pathway could have adverse health effects, contributing to diseases such as type 2 diabetes, metabolic syndrome, and insulin resistance (6, 9, 31). Because metabolic dysfunction accompanies cancer development and progression, ERRα’s role has been explored in many types of cancer such as breast, prostate, ovarian, and colon (32). Increased ERRα expression in different cancer types indicates a poor prognosis (16, 32–34); therefore, identifying ERRα agonists could help in understanding exposures that might increase the risk for metabolic diseases and cancer in an otherwise healthy individual, as well as elucidate potential impacts of these diseases. Recently, eating disorder predisposition was linked to a mutation in the ERRα gene (35). This observation is consistent with results from studies using ERRα null mice (36), in which disruption of the ventral-striatal synaptic function associated with reward and motivation circuitry was observed in female mice. Our current study suggests certain compounds use a chemical interference of the ERRα pathway, which could alter homeostatic and bioenergetic processes throughout the body for long-term consequences in the development of disease. Identification of ERRα agonists can provide valuable tools to probe ERRα axis responses relevant for therapeutic development and protection of public health.
PGC-1α and ERRα form an endogenous feed-forward circuit to regulate cellular and mitochondrial metabolism (13, 20, 28). Compounds affecting either the coactivator or the receptor could modulate signaling activity. Indeed, the current study has identified compounds in the Tox21 10K library that influence this signaling axis in both ERR and PGC/ERR cell lines, as well as compounds that affect only one or the other. For example, the statins clearly enhanced the reporter activity of the ERR cell line without displaying any activity in the PGC/ERR cell line, suggesting the target for this compound might be the receptor. Similarly, forskolin and SAHA stimulated the reporter activity robustly in the PGC/ERR cell line only, suggesting the coactivator is the target in this scenario. This study was also validated by identifying previously known ERR agonists, such as flavones (37–39).
It has been reported that the expression of PGC-1α in liver and brown adipose tissue is cyclic adenosine monophosphate (cAMP) dependent (40). Forskolin, a natural activator of adenyl cyclase, can increase intracellular levels of cAMP via the cAMP/PKA pathway. Therefore, forskolin could directly or indirectly influence the activity of the reporter in the PGC/ERR cell line, whereas SAHA, a member of the histone deacetylase inhibitors (41, 42), could enhance cAMP-induced activation (43). It is likely that these two compounds stimulate PGC-1α expression and activity in the PGC/ERR cell line. The current study has also found that an entire cluster of compounds (the statins) could be ERR agonists only in the ERR screen and not the PGC/ERR screen. This finding suggests these compounds may influence the expression or activity of the ERR receptor itself. Alternatively, they may also affect a different coactivator than PGC-1α. However, both the receptor and coactivator could be subject to posttranslational modifications, such as phosphorylation (44, 45), and compounds that stimulate or repress such activity will modify the PGC and ERR signal axis. Currently, many studies have focused on the potential mechanism of the PGC/ERR action (46–48). There is still much to learn about the mechanistic linkages between the compounds and the PGC and ERR signaling axis.
In this study, multiple potential novel ERR agonists were identified. The histone deacetylase inhibitor, SAHA, was the first of its kind to have been used in the treatment of cutaneous T-cell lymphoma (49, 50). Due to its nature, SAHA promotes an accumulation of acetylated histones that in turn relaxes the chromatin structure, facilitating transcription. Therefore, normally this compound is used in combination therapy to enhance the efficacy of chemotherapeutic compounds that target DNA (42, 51). Because SAHA was only active in the PGC/ERR assay, it appears this agonistic effect is dependent on PGC-1α and possibly induces the transcription of this coactivator, allowing for an increase in ERRα activation. Interestingly, SAHA also displays selectivity toward ERR (in the PGC/ERR assay), as it did not activate any other NR assay we studied. Further studies are needed to better characterize the activity of SAHA and understand its interaction with the ERRα pathway.
One group of compounds was selective toward ERR compared with other NRs (Fig. 3), and those were the statins, with the exception of cerivastatin sodium and simvastatin. Atorvastatin, atorvastatin calcium, fluvastatin, and lovastatin were all active in the ERR assay only. Interestingly, a recent study identified lovastatin as an ERR deactivator when ERR and PGC-1α were transfected into CV-1 cells (21). However, this does not conflict with our data because they transfected the coactivator along with ERR. Our data resulted in nonactivation in the PGC/ERR cell line. Lovastatin, a cholesterol synthesis inhibitor, was active in the ERR reporter assay alone, implying it modulates activation through the receptor and not the coactivator. All six of the statin-induced luciferase activities were reduced when the known ERR inverse agonist, XCT790, was cotreated, further supporting the notion that they have true ERR agonist activity. Also, cerivastatin sodium and fluvastatin were confirmed as ERR agonists through multiple ERR knockdown studies, including an ERR-luciferase assay as well as mRNA quantification via quantitative reverse transcription PCR analysis. Our findings support that additional molecular binding studies would be helpful in fully defining the effects of these statins on ERRα signaling.
In conclusion, we identified ERRα agonists using ERR and PGC/ERR reporter cell lines in this study. The ERR reporter assay had an 82% confirmation rate between the primary and follow-up assay, whereas the PGC/ERR assay had an 85% confirmation rate. It was beneficial to screen two assays to identify ERR modulators, due to the possible dependence of ERR on different coactivators. The statin cluster was identified as a potential novel ERR agonist group. We cannot yet conclude definitively that ERR agonism will lead to measurable toxicity in humans. However, given the importance of this signaling pathway in maintaining metabolic homeostasis and the association of aberrant ERR signaling with enhanced aggressiveness of specific cancers, additional studies on the impact of ERR signaling disruption on human health are warranted. This study represents the first step in discovering potential ERR agonists within a large group of environmental compounds and pharmaceuticals.
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the statements, opinions, views, conclusions, or policies of the National Institute of Environmental Health Sciences (NIEHS), the National Center for Advancing Translational Sciences, the National Institutes of Health, or the US Government. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Financial Support: This work was supported by Interagency Agreement IAA No. NTR12003 from the National Institute of Environmental Health Sciences/Division of the National Toxicology Program to the National Center for Advancing Translational Sciences, National Institutes of Health. The statin-ERRα interaction study was supported in part by NIEHS/National Cancer Institute Grant U01ES026137 (City of Hope).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- cAMP
- cyclic adenosine monophosphate
- cDNA
- complementary DNA
- DMSO
- dimethyl sulfoxide
- ERα
- estrogen receptor α
- ERRα
- estrogen-related receptor α
- mRNA
- messenger RNA
- NR
- nuclear receptor
- PCR
- polymerase chain reaction
- PGC-1α
- peroxisome proliferator–activated receptor γ coactivator 1α
- qHTS
- quantitative high-throughput screening
- SAHA
- suberoylanilide hydroxamic acid
- siRNA
- small interfering RNA.
References
- 1.Giguère V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature. 1988;331(6151):91–94. [DOI] [PubMed] [Google Scholar]
- 2.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang N, Shigeta H, Shi H, Teng CT. Estrogen-related receptor, hERR1, modulates estrogen receptor-mediated response of human lactoferrin gene promoter. J Biol Chem. 1996;271(10):5795–5804. [DOI] [PubMed] [Google Scholar]
- 4.Deblois G, Hall JA, Perry M-C, Laganière J, Ghahremani M, Park M, Hallett M, Giguère V. Genome-wide identification of direct target genes implicates estrogen-related receptor α as a determinant of breast cancer heterogeneity. Cancer Res. 2009;69(15):6149–6157. [DOI] [PubMed] [Google Scholar]
- 5.Stein RA, Chang CY, Kazmin DA, Way J, Schroeder T, Wergin M, Dewhirst MW, McDonnell DP. Estrogen-related receptor α is critical for the growth of estrogen receptor–negative breast cancer. Cancer Res. 2008;68(21):8805–8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giguére V. To ERR in the estrogen pathway. Trends Endocrinol Metab. 2002;13(5):220–225. [DOI] [PubMed] [Google Scholar]
- 7.Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM. Erralpha and Gabpa/b specify PGC-1α-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA. 2004;101(17):6570–6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CY, McDonnell DP. Molecular pathways: the metabolic regulator estrogen-related receptor α as a therapeutic target in cancer. Clin Cancer Res. 2012;18(22):6089–6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villena JA, Kralli A. ERRalpha: a metabolic function for the oldest orphan. Trends Endocrinol Metab. 2008;19(8):269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor γ coactivator 1α expression in muscle. Proc Natl Acad Sci USA. 2003;100(12):7111–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo J, Sladek R, Carrier J, Bader J-A, Richard D, Giguère V. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor α. Mol Cell Biol. 2003;23(22):7947–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1α deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3(4):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin J, Wu P-H, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell. 2004;119(1):121–135. [DOI] [PubMed] [Google Scholar]
- 14.Park S, Chang C-Y, Safi R, Liu X, Baldi R, Jasper JS, Anderson GR, Liu T, Rathmell JC, Dewhirst MW, Wood KC, Locasale JW, McDonnell DP. ERRα-regulated lactate metabolism contributes to resistance to targeted therapies in breast cancer. Cell Reports. 2016;15(2):323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fradet A, Bouchet M, Delliaux C, Gervais M, Kan C, Benetollo C, Pantano F, Vargas G, Bouazza L, Croset M, Bala Y, Leroy X, Rosol TJ, Rieusset J, Bellahcène A, Castronovo V, Aubin JE, Clézardin P, Duterque-Coquillaud M, Bonnelye E. Estrogen related receptor alpha in castration-resistant prostate cancer cells promotes tumor progression in bone. Oncotarget. 2016;7(47):77071–77086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushima H, Mori T, Ito F, Yamamoto T, Akiyama M, Kokabu T, Yoriki K, Umemura S, Akashi K, Kitawaki J. Anti-tumor effect of estrogen-related receptor alpha knockdown on uterine endometrial cancer. Oncotarget. 2016;7(23):34131–34148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busch BB, Stevens WC Jr, Martin R, Ordentlich P, Zhou S, Sapp DW, Horlick RA, Mohan R. Identification of a selective inverse agonist for the orphan nuclear receptor estrogen-related receptor α. J Med Chem. 2004;47(23):5593–5596. [DOI] [PubMed] [Google Scholar]
- 18.Chisamore MJ, Cunningham ME, Flores O, Wilkinson HA, Chen JD. Characterization of a novel small molecule subtype specific estrogen-related receptor α antagonist in MCF-7 breast cancer cells. PLoS One. 2009;4(5):e5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willy PJ, Murray IR, Qian J, Busch BB, Stevens WC Jr, Martin R, Mohan R, Zhou S, Ordentlich P, Wei P, Sapp DW, Horlick RA, Heyman RA, Schulman IG. Regulation of PPARgamma coactivator 1α (PGC-1α) signaling by an estrogen-related receptor α (ERRalpha) ligand. Proc Natl Acad Sci USA. 2004;101(24):8912–8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng CT, Beames B, Alex Merrick B, Martin N, Romeo C, Jetten AM. Development of a stable cell line with an intact PGC-1α/ERRα axis for screening environmental chemicals. Biochem Biophys Res Commun. 2014;444(2):177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei W, Schwaid AG, Wang X, Wang X, Chen S, Chu Q, Saghatelian A, Wan Y. Ligand activation of ERRα by cholesterol mediates statin and bisphosphonate effects. Cell Metab. 2016;23(3):479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng CT, Hsieh J-H, Zhao J, Huang R, Xia M, Martin N, Gao X, Dixon D, Auerbach SS, Witt KL, Merrick BA. Development of novel cell lines for high-throughput screening to detect estrogen-related receptor alpha modulators. SLAS Discov. 2017;22(6):720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang R, Sakamuru S, Martin MT, Reif DM, Judson RS, Houck KA, Casey W, Hsieh J-H, Shockley KR, Ceger P, Fostel J, Witt KL, Tong W, Rotroff DM, Zhao T, Shinn P, Simeonov A, Dix DJ, Austin CP, Kavlock RJ, Tice RR, Xia M. Profiling of the Tox21 10K compound library for agonists and antagonists of the estrogen receptor alpha signaling pathway. Sci Rep. 2014;4(1):5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu C-W, Zhao J, Huang R, Hsieh J-H, Hamm J, Chang X, Houck K, Xia M. Quantitative high-throughput profiling of environmental chemicals and drugs that modulate farnesoid X receptor. Sci Rep. 2014;4(1):6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch C, Zhao J, Huang R, Xiao J, Li L, Heyward S, Xia M, Wang H. Quantitative high-throughput identification of drugs as modulators of human constitutive androstane receptor. Sci Rep. 2015;5(1):10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Huang R. Correction of microplate data from high-throughput screening In: Zhu H, Xia M, eds. High-Throughput Screening Assays in Toxicology. New York, NY: Springer New York; 2016:123–134. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Jadhav A, Southal N, Huang R, Nguyen D-T. A grid algorithm for high throughput fitting of dose-response curve data. Curr Chem Genomics. 2010;4:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci USA. 2006;103(31):11473–11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang R, Xia M, Cho M-H, Sakamuru S, Shinn P, Houck KA, Dix DJ, Judson RS, Witt KL, Kavlock RJ, Tice RR, Austin CP. Chemical genomics profiling of environmental chemical modulation of human nuclear receptors. Environ Health Perspect. 2011;119(8):1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohonen T. Self-organizing neural projections. Neural Netw. 2006;19(6-7):723–733. [DOI] [PubMed] [Google Scholar]
- 31.Audet-Walsh É, Giguére V. The multiple universes of estrogen-related receptor α and γ in metabolic control and related diseases. Acta Pharmacol Sin. 2014;36(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tam IS, Giguère V. There and back again: the journey of the estrogen-related receptors in the cancer realm. J Steroid Biochem Mol Biol. 2016;157:13–19. [DOI] [PubMed] [Google Scholar]
- 33.May FEB. Novel drugs that target the estrogen-related receptor alpha: their therapeutic potential in breast cancer. Cancer Manag Res. 2014;6:225–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fradet A, Bouchet M, Delliaux C, Gervais M, Kan C, Benetollo C, Pantano F, Vargas G, Bouazza L, Croset M, Bala Y, Leroy X, Rosol TJ, Rieusset J, Bellahcène A, Castronovo V, Aubin JE, Clézardin P, Duterque-Coquillaud M, Bonnelye E. Estrogen related receptor alpha in castration-resistant prostate cancer cells promotes tumor progression in bone. Oncotarget. 2016;7(47):77071–77086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui H, Moore J, Ashimi SS, Mason BL, Drawbridge JN, Han S, Hing B, Matthews A, McAdams CJ, Darbro BW, Pieper AA, Waller DA, Xing C, Lutter M. Eating disorder predisposition is associated with ESRRA and HDAC4 mutations. J Clin Invest. 2013;123(11):4706–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Jesús-Cortés H, Lu Y, Anderson RM, Khan MZ, Nath V, McDaniel L, Lutter M, Radley JJ, Pieper AA, Cui H. Loss of estrogen-related receptor alpha disrupts ventral-striatal synaptic function in female mice. Neuroscience. 2016;329:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suetsugi M, Su L, Karlsberg K, Yuan Y-C, Chen S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol Cancer Res. 2003;1(13):981–991. [PubMed] [Google Scholar]
- 38.Ambra R, Rimbach G, de Pascual Teresa S, Fuchs D, Wenzel U, Daniel H, Virgili F. Genistein affects the expression of genes involved in blood pressure regulation and angiogenesis in primary human endothelial cells. Nutr Metab Cardiovasc Dis. 2006;16(1):35–43. [DOI] [PubMed] [Google Scholar]
- 39.Huang Z, Fang F, Wang J, Wong C-W. Structural activity relationship of flavonoids with estrogen-related receptor gamma. FEBS Lett. 2009;584(1):22–26. [DOI] [PubMed] [Google Scholar]
- 40.Karamitri A, Shore AM, Docherty K, Speakman JR, Lomax MA. Combinatorial transcription factor regulation of the cyclic AMP-response element on the Pgc-1α promoter in white 3T3-L1 and brown HIB-1B preadipocytes. J Biol Chem. 2009;284(31):20738–20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richon VM, Emiliani S, Verdin E, Webb Y, Breslow R, Rifkind RA, Marks PA. A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci USA. 1998;95(6):3003–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin D, Ong JM, Hu J, Desmond JC, Kawamata N, Konda BM, Black KL, Koeffler HP. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res. 2007;13(3):1045–1052. [DOI] [PubMed] [Google Scholar]
- 43.Lu Q, Hutchins AE, Doyle CM, Lundblad JR, Kwok RPS. Acetylation of cAMP-responsive element-binding protein (CREB) by CREB-binding protein enhances CREB-dependent transcription. J Biol Chem. 2003;278(18):15727–15734. [DOI] [PubMed] [Google Scholar]
- 44.Ariazi EA, Kraus RJ, Farrell ML, Jordan VC, Mertz JE. Estrogen-related receptor α1 transcriptional activities are regulated in part via the ErbB2/HER2 signaling pathway. Mol Cancer Res. 2007;5(1):71–85. [DOI] [PubMed] [Google Scholar]
- 45.Lu N, Wang W, Liu J, Wong C-W. Protein kinase C epsilon affects mitochondrial function through estrogen-related receptor alpha. Cell Signal. 2011;23(9):1473–1478. [DOI] [PubMed] [Google Scholar]
- 46.Yan M, Audet-Walsh É, Manteghi S, Dufour CR, Walker B, Baba M, St-Pierre J, Giguère V, Pause A. Chronic AMPK activation via loss of FLCN induces functional beige adipose tissue through PGC-1α/ERRα. Genes Dev. 2016;30(9):1034–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craige SM, Kröller-Schön S, Li C, Kant S, Cai S, Chen K, Contractor MM, Pei Y, Schulz E, Keaney JF Jr. PGC-1α dictates endothelial function through regulation of eNOS expression. Sci Rep. 2016;6(1):38210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi YK, Park JH, Baek Y-Y, Won M-H, Jeoung D, Lee H, Ha K-S, Kwon Y-G, Kim Y-M. Carbon monoxide stimulates astrocytic mitochondrial biogenesis via L-type Ca2+ channel-mediated PGC-1α/ERRα activation. Biochem Biophys Res Commun. 2016;479(2):297–304. [DOI] [PubMed] [Google Scholar]
- 49.Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, Frankel SR. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007;109(1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu W, Yi S, Qiu L, Sun J, Tu P, Wang Y. BCL11B-mediated epigenetic repression is a crucial target for histone deacetylase inhibitors in cutaneous T-cell lymphoma. J Invest Dermatol. 2017;137(7):1523–1532. [DOI] [PubMed] [Google Scholar]
- 51.Saito K, Funayama T, Yokota Y, Murakami T, Kobayashi Y. Histone deacetylase inhibitors sensitize murine B16F10 melanoma cells to carbon ion irradiation by inducing G1 phase arrest. Biol Pharm Bull. 2017;40(6):844–851. [DOI] [PubMed] [Google Scholar]