Abstract
The contributions of estradiol and testosterone to atherosclerotic lesion progression are not entirely understood. Cross-sex hormone therapy (XHT) for transgender individuals dramatically alters estrogen and testosterone levels and consequently could have widespread consequences for cardiovascular health. Yet, no preclinical research has assessed atherosclerosis risk after XHT. We examined the effects of testosterone XHT after ovariectomy on atherosclerosis plaque formation in female mice and evaluated whether adding low-dose estradiol to cross-sex testosterone treatments after ovariectomy reduced lesion formation. Six-week-old female ApoE−/− C57BL/6 mice underwent ovariectomy and began treatments with testosterone, estradiol, testosterone with low-dose estradiol, or vehicle alone until euthanized at 23 weeks of age. Atherosclerosis lesion progression was measured by Oil Red O stain and confirmed histologically. We found reduced atherosclerosis in the estradiol- and combined testosterone/estradiol–treated mice compared with those treated with testosterone or vehicle only in the whole aorta (−75%), aortic arch (−80%), and thoracic aorta (−80%). Plaque size was similarly reduced in the aortic sinus. These reductions in lesion size after combined testosterone/estradiol treatment were comparable to those obtained with estrogen alone. Testosterone/estradiol combined therapy resulted in less atherosclerosis plaque formation than either vehicle or testosterone alone after ovariectomy. Testosterone/estradiol therapy was comparable to estradiol replacement alone, whereas mice treated with testosterone only fared no better than untreated controls after ovariectomy. Adding low-dose estrogen to cross-sex testosterone therapy after oophorectomy could improve cardiovascular outcomes for transgender patients. Additionally, these results contribute to understanding of the effects of estrogen and testosterone on atherosclerosis progression.
Adding low-dose estradiol to cross-sex testosterone therapy in female mice after ovariectomy improved atherosclerosis plaque formation by 75% to 80%, comparable to effects of estradiol replacement alone.
The sex steroid hormones estradiol (E) and testosterone (T) are known to influence atherosclerosis formation and progression both in humans and experimental animals. The effect of these hormones is confounded by sex. Perhaps the most dramatic example allowing uncoupling of the effects of sex and sex steroid hormones is in the transgender population. Transgender individuals identify as a gender that differs from the one they were assigned at birth and make up an estimated 1,400,000 people in the United States, ∼0.6% of the national population (1). These individuals frequently undergo cross-sex hormone therapy (XHT) to alter secondary sex characteristics. For female-to-male (FtM) transgender patients, this involves lowering endogenous ovarian hormone levels (often by oophorectomy) and supplementing exogenous T to levels typical of nontransgender (cisgender) males. This can induce body and facial hair growth, voice deepening, and altered fat distribution (2). Although widespread statistics are not available, a recent study documented that 97% of transgender patients treated in a clinic were using XHT (3). XHT is generally used continuously and indefinitely, yet the long-term implications are unknown. Because gonadal steroids are involved in numerous physiological processes, XHT would be expected to cause changes that are not limited to the reproductive tract and secondary sex characteristics. Although limited research has been executed on long-term effects of XHT, observational studies have suggested various adverse health outcomes (4–7). Yet, those limited studies lacked both controls and long-term follow up (particularly for FtM individuals), suggesting the need for basic and translational research.
Atherosclerosis is the most common cause of death in Western societies. This cardiovascular disease is a chronic inflammatory process involving interactions among lipoproteins, monocyte-derived macrophages, T cells, and the arterial wall, which results in development of plaques or lesions that project out into the lumen of the vessel (8). Cardiovascular disease displays marked sex differences (9), with men twice as likely to die of cardiovascular disease as women (10, 11), and a growing body of research suggesting sex differences in the cells involved in human atherosclerosis (12). Because gonadal steroid hormones are implicated in cardiac health (11, 13), atherosclerosis risk is of particular concern for patients on XHT, although effects have not been previously studied.
Estrogens have been proposed to reduce atherosclerosis (11, 14–19), whereas T does not appear to have similar protective functions, yet data on this facet of endocrinology remain controversial. The 1998 Heart and Estrogen/Progestin Replacement Study (20) found no benefit to postmenopausal estrogen therapy in women with preexisting coronary artery disease, and the 2002 Women’s Health Initiative study (21) suggested potential adverse health outcomes, including increased coronary heart disease, following postmenopausal estrogen therapy. Yet, longer-term follow-up on the Women’s Health Initiative work has since revealed that estrogen therapy for younger participants improved atherosclerosis outcomes (22), which was confirmed in the Early Versus Late Intervention Trial With Estradiol study (23). In contrast, T therapy in males has been associated with increased cardiovascular disease (24). In postmenopausal women, serum T levels are directly associated with atherosclerosis progression, but estrogen levels are inversely correlated with lesion development (13, 18, 25, 26), Preclinical work supports these findings because female mice treated with high-dose T have decreased serum high-density lipoprotein (HDL) levels and increased atherosclerosis development, mimicking the outcomes seen in males (27), whereas estrogens can have cardioprotective effects in female rabbits and primates after ovariectomy (OVX) (28, 29). Taken together, this suggests that XHT T treatments after oophorectomy for FtM individuals could increase atherosclerosis risk. However, despite the prevalence of XHT use, no preclinical models or controlled studies have yet investigated the effects of the physiologically relevant high-dose T used in females as XHT.
Using an ApoE-null mouse model, we consider the effects of T-based XHT after OVX on atherosclerosis development in female mice. ApoE is a vital ligand for the uptake and clearance of atherogenic lipoproteins, including very low-density lipoproteins (LDLs) and chylomicrons (30). Mice do not develop atherosclerosis under normal conditions; however, targeted mutation of the ApoE gene results in severe hypercholesterolemia and early development of atherosclerotic lesions (30–34), making this murine model ideal for studying factors affecting cardiac disease progression. Because estrogen replacement therapy has been shown to reduce atherosclerosis progression in younger postmenopausal women (17), we investigate if adding low-dose estrogen to T XHT might reduce atherosclerosis risk after gonad removal in adolescent female mice.
Materials and Methods
Animals
This article was prepared according to Animal Research: Reporting of In Vivo Experiments guidelines (35). All animal experiments were conducted using female Apoetm1Unc mice (n = 24) obtained from the Jackson Laboratory (Bar Harbor, ME) in accordance with an approved Yale University Animal Care Committee protocol. All animals received a chow diet ad libitum. At 6 weeks of age, four groups of mice (n = 6 per group), randomly assigned, underwent OVX and began weekly injections of T (group T), E benzoate (group E), T and E mixed group (T+E), or vehicle control (group C), continuing through 22 weeks of age. Mice were weighed before undergoing OVX at 6 weeks and again before being euthanized at 23 weeks.
Bilateral OVX was performed with meloxicam analgesic and isoflurane anesthetic. Hormone treatments followed our previously published paradigm (36), with weekly subcutaneous 100 µL sesame oil injections. Mice in the E-treated group received a previously characterized dose of E benzoate used as a model for postmenopausal hormone replacement therapy in female mice after OVX, weight-adjusted to average adult C57Bl/6 bodyweight, which was 6.4 μg/wk E benzoate per mouse (37). T and T+E groups received T in the weight-adjusted amount as used for human XHT (31 µg/wk) (38–42); the T+E group received E benzoate at a weight-adjusted estrogen dose matching levels in cisgender males (0.8 µg/wk).
All mice were euthanized at 23 weeks of age after overnight fasting. Plasma was collected by cardiac puncture and stored overnight at 4°C before analysis. Whole aorta was dissected and stored in formalin at 4°C until being subjected to Oil Red O (ORO) staining. Whole hearts were extracted and cut in half, and the top half for each mouse was embedded in Optimal Cutting Temperature (Sakura Finetechnical Co, Ltd) compound after overnight fixation in 10% formalin, and frozen in −80°C until sections were cut for histology.
Plasma analysis
Standard commercial enzyme-linked immunosorbent assay kits were used according to the manufacturer’s protocol to determine E (Calbiotech, Spring Valley, CA) and T (Immuno-Biological Laboratories, Inc., Minneapolis, MN) levels. Commercial kits were used to measure plasma triglyceride (TG; Diagnostic Chemicals, Charlottetown, PEI, Canada), and total cholesterol (TC) and HDL (ThermoFisher Scientific Inc., Waltham, MA) concentrations. Subtracting HDL from TC gave LDL. Samples were run in duplicate. Standard commercial kits were similarly used to quantify plasma levels of alanine aminotransferase, blood urea nitrogen, creatinine, bilirubin, insulin, and glucose, and performed in the Yale core facility laboratories.
ORO staining
ORO staining solution was prepared by mixing 35 mL ORO solution (0.2%, weight-to-volume ratio) in methanol with 10 mL of 1M sodium hydroxide and filtered with filter paper (43). Whole aortas were washed in 1 mL 78% methanol for 5 minutes on a tilted roller, incubated in 1 mL ORO staining solution on tilted rollers for 50 minutes, destained in 1 mL 78% methanol for 5 minutes, and then transferred to phosphate-buffered saline until they were mounted and imaged.
Adventitial fat was removed under an Olympus SZX16 microscope with fine forceps. Then aortas were cut longitudinally using microdissecting spring scissors and pinned flat onto clear-bottom Sylgard-coated glass dissecting dishes, as described (44). Images were captured using an Olympus SZX16 microscope with an SDF PLAPO 0.5 XPF objective lens connected to an Olympus Model U-LH100HG camera (100 W 19 V) and analyzed using ImageJ software with a [0,131] red threshold, determined based on ability to visualize red stain in the E and T+E groups.
Histology
Sections of the aortic sinus were cut, mounted, and stained with hematoxylin-eosin (H&E) for histopathological analysis. Photographs were taken using a Nikon Eclipse 80i histology microscope, with a 4×/0.13 ± WD 17.1 objective lens. Lesion size was measured for all mice using ImageJ and is presented as average lesion area per animal for each treatment group, normalized to average lesion area for OVX/vehicle mice. Three sections were evaluated per mouse. No additional processing was done. Black arrows were added using GraphPad Prism for Mac.
Statistical analysis
All statistical analyses were performed on GraphPad Prism for Mac. The primary outcome for this study is aortic plaque formation, quantified by ORO staining. One-tailed t tests were used to compare plasma E and T levels. Analysis of variance with post hoc multiple comparisons tests using a Tukey correction were used to determine differences between groups for weight change, quantitative plaque analyses, and plasma lipids and cholesterol. No mice were excluded from the study. All quantitative data are presented with mean ± standard error of the mean (SEM).
Results
Hormone levels and body weight changes
To validate our model, we first checked plasma E and T levels in all groups, at 23 weeks (5 days postinjection). E levels were significantly higher in E-treated mice (mean E ± SEM, 77.2 ± 34.3 pg/mL) than in controls (3.9 ± 0.8 pg/mL; P = 0.05) or T-treated mice (3.0 ± 0.8 pg/mL; P = 0.03). Further, combined therapy mice had higher E levels (29.6 ± 13.3 pg/mL) than both control (P = 0.05) and T-treated mice (P = 0.04). T levels were higher in T-treated mice (22.3 ± 2.1 ng/dL) compared with controls (17.2 ± 1.2 ng/dL; P = 0.04) or E-treated mice (16.3 ± 1.2 ng/dL; P = 0.02). The increase in T levels was not substantial in the combined therapy group (18.0 ± 2.0 ng/dL).
We found a significant relationship between treatment and change in body weight from 6 (pre-OVX) to 23 weeks (F = 8.5, P = 0.001). The T group (mean weight gain ± SEM, +6.4 ± 0.74 g) did not differ from vehicle (+4.8 ± 0.2 g), E (+7.9 ± 0.5 g), or combined therapy (+8.0 ± 0.5g) mice, but E and T+E combined therapy groups had comparably greater weight gain than vehicle-treated controls (E, P = 0.003; T+E, P = 0.003).
These changes in hormone levels and body weight were not associated with important changes in renal or liver function, as measured by blood urea nitrogen, creatinine, alanine aminotransferase, and bilirubin levels (Supplemental Table 1). Random glucose and insulin levels were also within the normal range and not significantly different between groups (Supplemental Table 1).
Plaque formation in whole aorta and aortic root
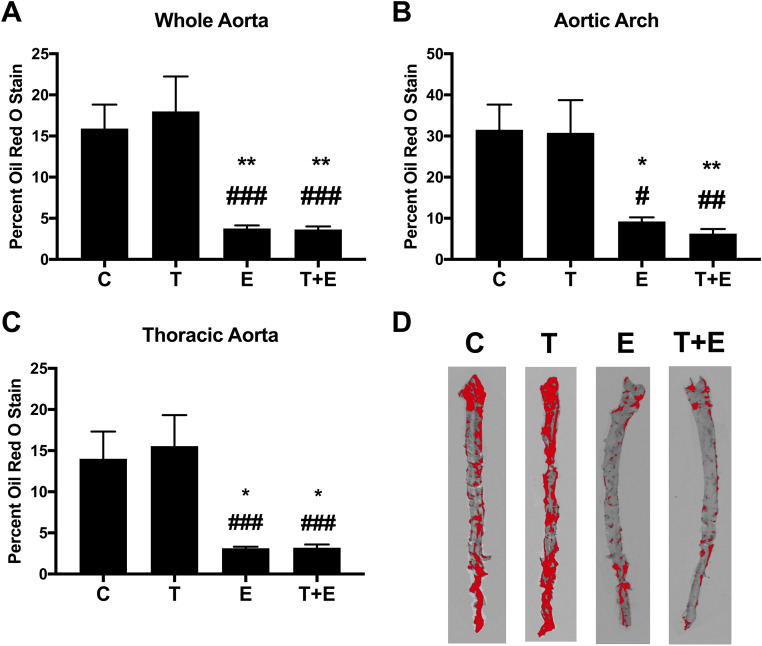
To investigate the effect of different treatments on atherosclerotic plaque formation, we used aortic ORO stain (relative staining with the quantification coloration threshold displayed in Fig. 1D). Treatment was related to percent ORO staining for whole aorta (F = 9.07, P = 0.006), aortic arch (F = 7.357, P = 0.002), and thoracic aorta (F = 7.659, P = 0.0015).
Figure 1.
Quantification of aorta ORO stain. Percent stained area is shown for (A) whole aorta, (B) aortic arch, and (C) thoracic aorta (vehicle control: n = 5; T, E, and T+E: n = 6 per group). Data are presented as mean ± SEM. There was a significant relationship between treatment and ORO staining for the whole aorta (F = 9.07, P = 0.006), aortic arch (F = 7.36, P = 0.002), and thoracic aorta (F = 7.67, P = 0.0015). Post hoc Tukey-corrected tests demonstrated that the E and T+E groups had significantly less lesion formation than mice treated with vehicle or with T alone, for whole aorta, aortic arch, and thoracic aorta. No other comparisons were significantly different. Significance compared with vehicle controls: *P = 0.03, **P = 0.02. Significance compared with T: #P = 0.03, ##P = 0.02, ###P < 0.01. (D) A representative aorta for each group with red quantification on ImageJ is shown.
Although T alone in the OVX group did not significantly alter lesion formation compared with vehicle, we found reduced stained lesion area in E only and combined therapy groups compared with mice treated with vehicle or T alone. This included 75% reduction in lipid plaque area in the whole aorta in E and T+E mice compared with vehicle controls (E, P = 0.02; T+E, P = 0.02; Fig. 1A) and 80% reduction compared with T-treated mice (E, P < 0.01; T+E, P < 0.01; Fig. 1A). Similar reductions were observed in the aortic arch, with E mice exhibiting 70% reduction in plaques compared with vehicle controls and T mice (vs. vehicle controls: P = 0.03, vs T: P = 0.03; Fig. 1B) and with combined therapy mice exhibiting 80% reduction in lesion formation compared with both C and T mice (vs. C: P = 0.02, vs T: P = 0.02; Fig. 1B). 80% reduction in plaques was apparent for both E and T/E groups in the thoracic aorta compared with both vehicle (E: P = 0.03, T+E: P = 0.04; Fig. 1C) and T (E, P < 0.01; T+E, P < 0.01; Fig. 1C) mice. The E and combination therapies yielded comparable results (P > 0.99).
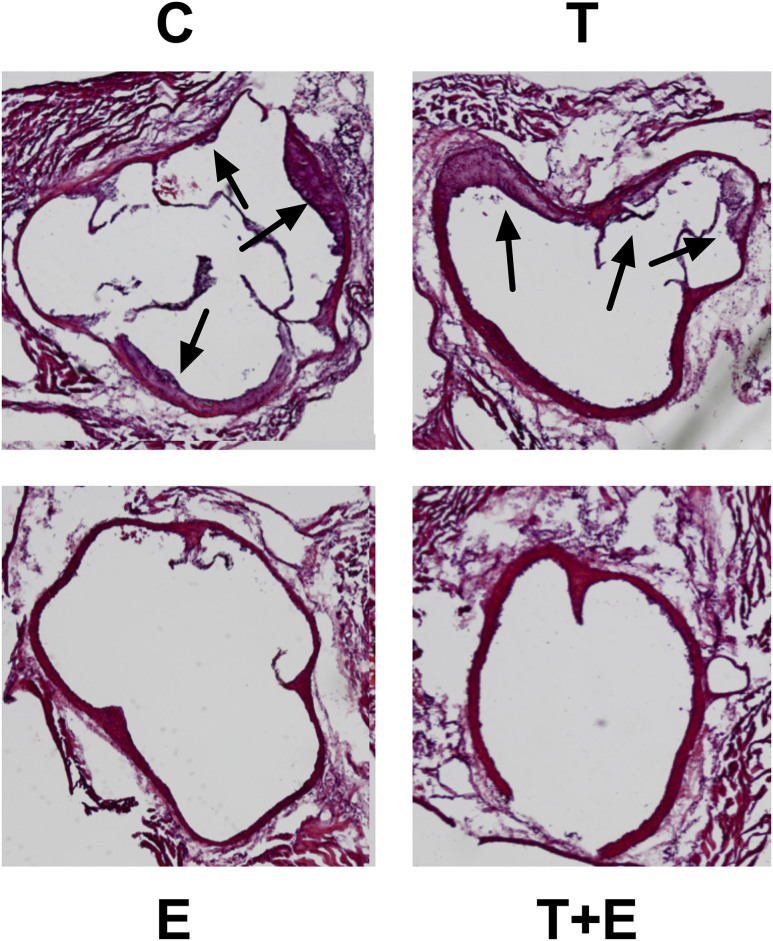
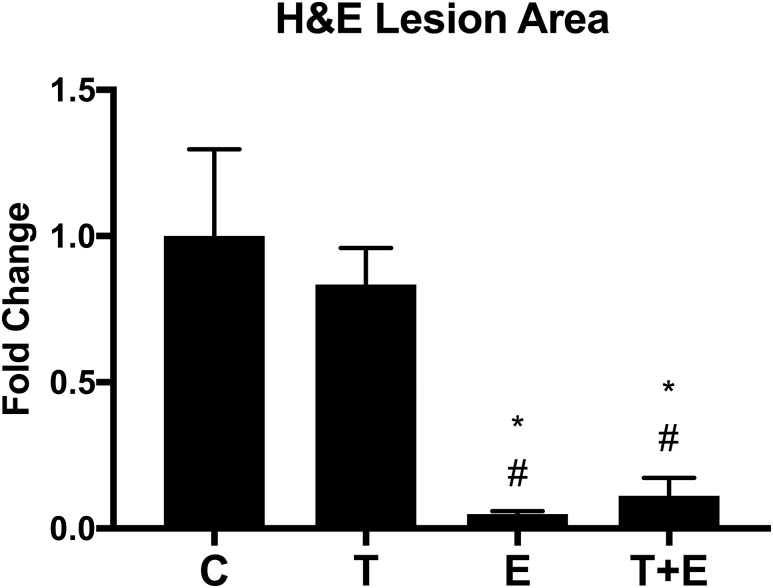
We confirmed these findings using H&E staining on aortic root sections, measuring lesion size. Vehicle- or T-treated OVX mice displayed noticeable large plaque formation (purple in contrast to the red of the endothelial wall, indicated with black arrows; Fig. 2), yet E and combination therapy-treated OVX groups did not (Fig. 2). This observation was supported quantitatively with a significant relationship between average lesion area per H&E-stained aortic root sample and treatment (F = 10.92, P = 0.0002). Measurement of average lesion area revealed that E-treated mice lesion size was 5% that of the control (P < 0.01; Fig. 3); combined therapy mice were 11% (P < 0.01; Fig. 3). E and T+E combined treatment mice both exhibited lower average lesion area compared with T mice (E, P < 0.01; T+E, P < 0.01; Fig. 3), which were 83% the area of controls (P = 0.87). E and combined therapy results were comparable (P > 0.99).
Figure 2.
H&E staining of the aortic root. Immunohistochemistry images are shown for representative mouse groups treated with vehicle control (C), T, E, and combined T+E after OVX. Photographs were taken at ×4 magnification; black arrows indicate large plaque formation.
Figure 3.
Average lesion area in H&E-stained aortic root. Average lesion area is presented as fold change compared with C, with bars representing SEM. We found a significant relationship between average lesion area per H&E-stained aortic root sample and treatment (F = 10.92, P = 0.0002). Post hoc Tukey-corrected tests demonstrated a reduction in average lesion area for E-treated and T+E-treated mice, compared with both C- and T-treated mice. Significance compared with C, *P < 0.01. Significance compared with T, #P < 0.01.
Plasma cholesterol and triglycerides
To determine if changes to circulating cholesterol and TG levels contributed to these observed differential outcomes, we measured the plasma concentration of TC, HDL, LDL, and TG in all mice from all groups. Treatment was related to TC (F = 5.587, P = 0.0064), HDL (F = 3.478, P = 0.0363), LDL (F = 5.459, P = 0.0071), and TG (F = 3.409, P = 0.0387). E therapy (E) resulted in significantly decreased TC (−35%, P < 0.01; Table 1), HDL (−45%, P = 0.03; Table 1), and LDL (−35%, P < 0.01; Table 1) compared with vehicle, and reduced TC (−30%, P = 0.03; Table 1), LDL (−30%, P = 0.03; Table 1), and TG (−30%, P = 0.04; Table 1) compared with T, consistent with previous characterizations of ovariectomized ApoE−/− mice treated with E (45). The reductions observed with combined therapy compared with vehicle and T-treated mice were not statistically significant (Table 1).
Table 1.
Plasma Lipoprotein and Lipid Concentrations
| Treatment | TC (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | TG (mg/dL) |
|---|---|---|---|---|
| C | 254.4 ± 25.51 | 6.69 ± 0.86 | 247.7 ± 24.77 | 56.01 ± 9.08 |
| T | 235.4 ± 20.16 | 5.46 ± 0.82 | 229.9 ± 19.83 | 74.88 ± 6.05 |
| E | 164.8 ± 9.17a,b | 3.66 ± 0.56c | 161.1 ± 9.09a,b | 50.84 ± 3.19d |
| T+E | 201.6 ± 8.26 | 5.0 ± 0.31 | 196.5 ± 8.36 | 64.69 ± 4.46 |
Data are presented as mean ± SEM (C: n = 5; T, E, T+E: n = 6 per group). There was a significant relationship between treatment and TC (F = 5.587, P = 0.0064), HDL (F = 3.478, P = 0.0363), LDL (F = 5.459, P = 0.0071), and TG (F = 3.409, P = 0.0387), according to analysis of variance. Post hoc Tukey-corrected tests demonstrated that the E therapy had significantly lower levels than mice treated with vehicle TC, HDL, and LDL, and compared with T mice for TC, LDL, and TG. No other comparisons were significantly different.
Abbreviation: C, vehicle control.
Significance compared with C: P < 0.01.
Significance compared with T: P = 0.03.
Significance compared with C: P = 0.03.
Significance compared with T: P = 0.04.
Discussion
In this study, we assessed the effects of sex steroid treatment following OVX in female ApoE knockout mice on atherosclerosis development and whether adding a low dose of estrogen to T treatment reduces plaque formation. The use of T in female mice uncouples the effects of sex from that of sex steroid hormones. T administration also models cross-sex T therapy after oophorectomy in transgender female-to-male sex-affirmative treatment in humans. Our findings suggest that T does not reduce atherosclerosis progression, but estrogen does; yet, adding a low dose of estrogen can mimic the reductions in atherosclerosis lesions observed with estrogen replacement alone.
Female ApoE null mice have been previously shown to exhibit accelerated atherosclerosis development after OVX; however, estrogen therapy reverses this effect (14). Here we found a substantial reduction in atherosclerosis lesion formation in both females treated with estrogen after OVX and females treated with a combined therapy of T and a low dose of estrogen, compared with those treated with T alone after OVX. However, this estrogen-dependent reduction in plaque formation did not appear to be dependent on improvements in plasma cholesterol and TG. Consistent with prior work (16), estrogen reduced atherosclerosis progression, independent of circulating cholesterol levels. Here we showed that reductions in both LDL and HDL cholesterol in E-treated mice, but no important alterations to the ratio, which is acknowledged to be the more meaningful cholesterol metric (46). The changes that we observed in lipid levels matched previously published results as well (45). These findings are consistent with clinical work suggesting that changes in plasma cholesterol are responsible for relatively little of estrogen-dependent protection against atherosclerosis (47); numerous other mechanisms might be responsible for the observed benefits (48). Such mechanisms include decreased recruitment of macrophages (central to all stages of atherosclerosis pathogenesis and progression) (49), increased nitric oxide production (50, 51) (which reduces atherosclerotic progression) (52–56), and reduced aortic endothelial vascular cell adhesion molecule-1 expression (57, 58) (which recruits immune cells to atherosclerotic lesions to bolster plaque formation) (59–61). In contrast, androgens cause opposite effects to estrogens on these three mechanisms (62–64).
Although some males have been shown to have reduced atherosclerosis formation with T treatments (65), that effect depends on T aromatization to E (66, 67). Preclinical models have demonstrated that estrogen receptor function mediates prevention of early atherosclerosis in both females and males (68–70). Clinically, this is supported by advanced atherosclerosis development in males with aromatase deficiency (71–74), which can be reduced by estrogen therapy both clinically (18) and in preclinical models (29, 66, 75–77). Cisgender men have higher estrogen levels than postmenopausal cisgender women because of aromatization of T (78, 79). Yet, aromatization has been shown to be sexually dimorphic, with cisgender men aromatizing T twice as much (0.4% vs. 0.2%) as cisgender women (80). If aromatization of T XHT in FtM transgender patients were not sufficient to increase E levels, supplementing the T therapy with a low dose of E could improve long-term outcomes.
One limitation of this study is the small extent of T increase with the combined therapy. Estrogen therapy lowered both endogenous T levels and the increase in T achieved with therapy. This needs to be further investigated to ensure that adding low-dose E does not interfere with developing the desired secondary sex characteristics.
Species differences are an additional limitation of this work. Mice lack sex hormone binding globulin (81), which may have influenced our findings. We followed the established rodent models of T and E injections that were dosed to mimic human hormone therapy (including as a replacement of endogenous hormones for males) (82–89). We selected this treatment paradigm to model human XHT as closely as possible. Our data are consistent with previous work demonstrating that high doses of T in female mice are also associated with atherosclerosis progression (27). Similarly, although mice have low endogenous estrogen levels, the dose used here is based on published postmenopausal estrogen replacement paradigms in female mice (37) and has been used previously to model XHT (36). Further work is needed to validate these doses and our treatment model. Finally, although aromatase in cardiovascular tissue can convert T to E, both our findings and prior research (90, 91) suggest that this aromatization is insufficient to sustain estrogen levels at the level required for reducing atherosclerosis after OVX.
With this study, we investigated relative contributions of E and T to atherosclerosis lesion progression in ovariectomized female mice, and found that a low dose of E is required for reducing atherosclerosis development after gonadectomy. This has implications for hormone action on atherosclerosis formation, but is perhaps most important for transgender individuals currently using T XHT after oophorectomy without estrogen supplementation. Our findings suggest a potentially improved treatment of reducing atherosclerosis risk of FtM transgender patients. If confirmed in humans, these results would offer a large contribution toward improving sex-affirming treatment of transgender individuals to reduce risk of cardiovascular disease.
Supplementary Material
Acknowledgments
Financial Support: This study was funded by National Institutes of Health Grant RO1 HD076422 and the Pierson College Mellon Senior Research Grant.
Current Affiliation: T.G. Goetz’s current affiliation is Columbia University, College of Physicians and Surgeons, New York, New York 10032.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- E
estradiol
- FtM
female-to-male
- HDL
high-density lipoprotein
- H&E
hematoxylin and eosin
- LDL
low-density lipoprotein
- ORO
Oil Red O
- OVX
ovariectomy
- SEM
standard error of the mean
- T
testosterone
- TC
total cholesterol
- T+E
testosterone and estradiol mixed group
- TG
triglyceride
- XHT
cross-sex hormone therapy.
References
- 1. Flores AR, Herman JL, Gates GJ, Brown TN. How Many Adults Identify as Transgender in the United States. Los Angeles, CA: The Williams Institute; 2016. [Google Scholar]
- 2. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ III, Spack NP, Tangpricha V, Montori VM; Endocrine Society . Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(9):3132–3154. [DOI] [PubMed] [Google Scholar]
- 3. Kailas M, Lu HMS, Rothman EF, Safer JD. Prevalence and types of gender-affirming surgery among a sample of transgender endocrinology patients prior to state expansion of insurance coverage. Endocr Pract. 2017;23(7):780–786. [DOI] [PubMed] [Google Scholar]
- 4. Wierckx K, Elaut E, Declercq E, Heylens G, De Cuypere G, Taes Y, Kaufman JM, T’Sjoen G. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur J Endocrinol. 2013;169(4):471–478. [DOI] [PubMed] [Google Scholar]
- 5. Wierckx K, Mueller S, Weyers S, Van Caenegem E, Roef G, Heylens G, T’Sjoen G. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med. 2012;9(10):2641–2651. [DOI] [PubMed] [Google Scholar]
- 6. Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol. 2014;170(6):809–819. [DOI] [PubMed] [Google Scholar]
- 7. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635–642. [DOI] [PubMed] [Google Scholar]
- 8. Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104(4):503–516. [DOI] [PubMed] [Google Scholar]
- 9. Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–1587. [DOI] [PubMed] [Google Scholar]
- 10. Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801–1811. [DOI] [PubMed] [Google Scholar]
- 11. Nathan L, Chaudhuri G. Estrogens and atherosclerosis. Annu Rev Pharmacol Toxicol. 1997;37(1):477–515. [DOI] [PubMed] [Google Scholar]
- 12. Franconi F, Rosano G, Basili S, Montella A, Campesi I. Human cells involved in atherosclerosis have a sex. Int J Cardiol. 2017;228:983–1001. [DOI] [PubMed] [Google Scholar]
- 13. Eckardstein A, Wu FC. Testosterone and atherosclerosis. Growth Horm IGF Res. 2003;13(Suppl A):S72–S84. [DOI] [PubMed] [Google Scholar]
- 14. Bourassa PA, Milos PM, Gaynor BJ, Breslow JL, Aiello RJ. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 1996;93(19):10022–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrington DM, Reboussin DM, Brosnihan KB, Sharp PC, Shumaker SA, Snyder TE, Furberg CD, Kowalchuk GJ, Stuckey TD, Rogers WJ, Givens DH, Waters D. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343(8):522–529. [DOI] [PubMed] [Google Scholar]
- 16. Marsh MM, Walker VR, Curtiss LK, Banka CL. Protection against atherosclerosis by estrogen is independent of plasma cholesterol levels in LDL receptor-deficient mice. J Lipid Res. 1999;40(5):893–900. [PubMed] [Google Scholar]
- 17. Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53(3):605–619. [DOI] [PubMed] [Google Scholar]
- 18. Rivin AU, Dimitroff SP. The incidence and severity of atherosclerosis in estrogen-treated males, and in females with a hypoestrogenic or a hyperestrogenic state. Circulation. 1954;9(4):533–539. [DOI] [PubMed] [Google Scholar]
- 19. Williams JK, Adams MR, Klopfenstein HS. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990;81(5):1680–1687. [DOI] [PubMed] [Google Scholar]
- 20. Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–613. [DOI] [PubMed] [Google Scholar]
- 21. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women’s Health Initiative I. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 22. Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP; ELITE Research Group . Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vigen R, O’Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–1836. [DOI] [PubMed] [Google Scholar]
- 25. Karim R, Hodis HN, Stanczyk FZ, Lobo RA, Mack WJ. Relationship between serum levels of sex hormones and progression of subclinical atherosclerosis in postmenopausal women. J Clin Endocrinol Metab. 2008;93(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, Szklo M. Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol. 2002;155(5):437–445. [DOI] [PubMed] [Google Scholar]
- 27. Paigen B, Holmes PA, Mitchell D, Albee D. Comparison of atherosclerotic lesions and HDL-lipid levels in male, female, and testosterone-treated female mice from strains C57BL/6, BALB/c, and C3H. Atherosclerosis. 1987;64(2-3):215–221. [DOI] [PubMed] [Google Scholar]
- 28. Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990;10(6):1051–1057. [DOI] [PubMed] [Google Scholar]
- 29. Bruck B, Brehme U, Gugel N, Hanke S, Finking G, Lutz C, Benda N, Schmahl FW, Haasis R, Hanke H. Gender-specific differences in the effects of testosterone and estrogen on the development of atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 1997;17(10):2192–2199. [DOI] [PubMed] [Google Scholar]
- 30. Curtiss LK, Boisvert WA. Apolipoprotein E and atherosclerosis. Curr Opin Lipidol. 2000;11(3):243–251. [DOI] [PubMed] [Google Scholar]
- 31. Breslow JL. Mouse models of atherosclerosis. Science. 1996;272(5262):685–688. [DOI] [PubMed] [Google Scholar]
- 32. Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14(1):133–140. [DOI] [PubMed] [Google Scholar]
- 33. Osada J, Joven J, Maeda N. The value of apolipoprotein E knockout mice for studying the effects of dietary fat and cholesterol on atherogenesis. Curr Opin Lipidol. 2000;11(1):25–29. [DOI] [PubMed] [Google Scholar]
- 34. van Dijk KW, Hofker MH, Havekes LM. Dissection of the complex role of apolipoprotein E in lipoprotein metabolism and atherosclerosis using mouse models. Curr Atheroscler Rep. 1999;1(2):101–107. [DOI] [PubMed] [Google Scholar]
- 35. Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG; NC3Rs Reporting Guidelines Working Group . Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160(7):1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goetz TG, Mamillapalli R, Devlin MJ, Robbins AE, Majidi-Zolbin M, Taylor HS. Cross-sex testosterone therapy in ovariectomized mice: addition of low-dose estrogen preserves bone architecture. Am J Physiol Endocrinol Metab. 2017;313(5):E540–E551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li J, McMurray RW. Effects of cyclic versus sustained estrogen administration on peripheral immune functions in ovariectomized mice. Am J Reprod Immunol. 2010;63(4):274–281. [DOI] [PubMed] [Google Scholar]
- 38. Center of Excellence for Transgender Health UCSF . Hormone Administration. Available at: http://transhealth.ucsf.edu/trans?page=protocol-hormones. Accessed 28 July 2017.
- 39. Dkhil MA, Al-Quraishy S, Abdel-Baki A-A, Ghanjati F, Arauzo-Bravo MJ, Delic D, Wunderlich F. Epigenetic modifications of gene promoter DNA in the liver of adult female mice masculinized by testosterone. J Steroid Biochem Mol Biol. 2015;145:121–130. [DOI] [PubMed] [Google Scholar]
- 40. Benten WP, Ulrich P, Kühn-Velten WN, Vohr HW, Wunderlich F. Testosterone-induced susceptibility to Plasmodium chabaudi malaria: persistence after withdrawal of testosterone. J Endocrinol. 1997;153(2):275–281. [DOI] [PubMed] [Google Scholar]
- 41. Wunderlich F, Mossmann H, Helwig M, Schillinger G. Resistance to Plasmodium chabaudi in B10 mice: influence of the H-2 complex and testosterone. Infect Immun. 1988;56(9):2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Delić D, Grosser C, Dkhil M, Al-Quraishy S, Wunderlich F. Testosterone-induced upregulation of miRNAs in the female mouse liver. Steroids. 2010;75(12):998–1004. [DOI] [PubMed] [Google Scholar]
- 43. Guevara NV, Kim H-S, Antonova EI, Chan L. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat Med. 1999;5(3):335–339. [DOI] [PubMed] [Google Scholar]
- 44. Andrés-Manzano MJ, Andrés V, Dorado B. Oil Red O and hematoxylin and eosin staining for quantification of atherosclerosis burden in mouse aorta and aortic root. Methods in Mouse Atherosclerosis. Springer: New York; 2015:85–99. [DOI] [PubMed] [Google Scholar]
- 45. Elhage R, Arnal J-F, Pieraggi M-T, Duverger N, Fiévet C, Faye JC, Bayard F. 17 β-estradiol prevents fatty streak formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1997;17(11):2679–2684. [DOI] [PubMed] [Google Scholar]
- 46. Fernandez ML, Webb D. The LDL to HDL cholesterol ratio as a valuable tool to evaluate coronary heart disease risk. J Am Coll Nutr. 2008;27(1):1–5. [DOI] [PubMed] [Google Scholar]
- 47. Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, Ernster VL, Cummings SR. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117(12):1016–1037. [DOI] [PubMed] [Google Scholar]
- 48. Gerhard M, Ganz P.. How do we explain the clinical benefits of estrogen? From Bedside to Bench. 1995;92(1):5–8. [DOI] [PubMed] [Google Scholar]
- 49. Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23(5):665–686. [DOI] [PubMed] [Google Scholar]
- 51. Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, Chambon P, Bayard F, Arnal JF. Estradiol alters nitric oxide production in the mouse aorta through the α-, but not β-, estrogen receptor. Circ Res. 2002;90(4):413–419. [DOI] [PubMed] [Google Scholar]
- 52. Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2(8567):1057–1058. [DOI] [PubMed] [Google Scholar]
- 53. Napoli C, de Nigris F, Williams-Ignarro S, Pignalosa O, Sica V, Ignarro LJ. Nitric oxide and atherosclerosis: an update. Nitric Oxide. 2006;15(4):265–279. [DOI] [PubMed] [Google Scholar]
- 54. Napoli C, Ignarro LJ. Nitric oxide and atherosclerosis. Nitric Oxide. 2001;5(2):88–97. [DOI] [PubMed] [Google Scholar]
- 55. Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104(4):448–454. [DOI] [PubMed] [Google Scholar]
- 56. Oemar BS, Tschudi MR, Godoy N, Brovkovich V, Malinski T, Lüscher TF. Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation. 1998;97(25):2494–2498. [DOI] [PubMed] [Google Scholar]
- 57. Simoncini T, Maffei S, Basta G, Barsacchi G, Genazzani AR, Liao JK, De Caterina R. Estrogens and glucocorticoids inhibit endothelial vascular cell adhesion molecule-1 expression by different transcriptional mechanisms. Circ Res. 2000;87(1):19–25. [DOI] [PubMed] [Google Scholar]
- 58. Caulin-Glaser T, Watson CA, Pardi R, Bender JR. Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest. 1996;98(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O’Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92(2):945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. O’Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996;93(4):672–682. [DOI] [PubMed] [Google Scholar]
- 61. Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85(2):199–207. [DOI] [PubMed] [Google Scholar]
- 62. Kimura M, Sudhir K, Jones M, Simpson E, Jefferis A-M, Chin-Dusting JP. Impaired acetylcholine-induced release of nitric oxide in the aorta of male aromatase-knockout mice: regulation of nitric oxide production by endogenous sex hormones in males. Circ Res. 2003;93(12):1267–1271. [DOI] [PubMed] [Google Scholar]
- 63. McCrohon JA, Jessup W, Handelsman DJ, Celermajer DS. Androgen exposure increases human monocyte adhesion to vascular endothelium and endothelial cell expression of vascular cell adhesion molecule-1. Circulation. 1999;99(17):2317–2322. [DOI] [PubMed] [Google Scholar]
- 64. Death AK, McGrath KC, Sader MA, Nakhla S, Jessup W, Handelsman DJ, Celermajer DS. Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-kappaB-dependent pathway. Endocrinology. 2004;145(4):1889–1897. [DOI] [PubMed] [Google Scholar]
- 65. Malkin CJ, Pugh PJ, Jones RD, Jones TH, Channer KS. Testosterone as a protective factor against atherosclerosis--immunomodulation and influence upon plaque development and stability. J Endocrinol. 2003;178(3):373–380. [DOI] [PubMed] [Google Scholar]
- 66. Nathan L, Shi W, Dinh H, Mukherjee TK, Wang X, Lusis AJ, Chaudhuri G. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci USA. 2001;98(6):3589–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mukherjee TK, Dinh H, Chaudhuri G, Nathan L. Testosterone attenuates expression of vascular cell adhesion molecule-1 by conversion to estradiol by aromatase in endothelial cells: implications in atherosclerosis. Proc Natl Acad Sci USA. 2002;99(6):4055–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Villablanca A, Lubahn D, Shelby L, Lloyd K, Barthold S. Susceptibility to early atherosclerosis in male mice is mediated by estrogen receptor α. Arterioscler Thromb Vasc Biol. 2004;24(6):1055–1061. [DOI] [PubMed] [Google Scholar]
- 69. Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest. 1997;99(10):2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Adams MR, Golden DL, Register TC, Anthony MS, Hodgin JB, Maeda N, Williams JK. The atheroprotective effect of dietary soy isoflavones in apolipoprotein E-/- mice requires the presence of estrogen receptor-α. Arterioscler Thromb Vasc Biol. 2002;22(11):1859–1864. [DOI] [PubMed] [Google Scholar]
- 71. Sudhir K, Chou TM, Chatterjee K, Smith EP, Williams TC, Kane JP, Malloy MJ, Korach KS, Rubanyi GM. Premature coronary artery disease associated with a disruptive mutation in the estrogen receptor gene in a man. Circulation. 1997;96(10):3774–3777. [DOI] [PubMed] [Google Scholar]
- 72. Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, Milliken EE, Issa JP. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res. 1999;43(4):985–991. [DOI] [PubMed] [Google Scholar]
- 73. Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994;89(4):1501–1510. [DOI] [PubMed] [Google Scholar]
- 74. Dong C, Yoon W, Goldschmidt-Clermont PJ. DNA methylation and atherosclerosis. J Nutr. 2002; 132(8, Suppl)2406S–2409S. [DOI] [PubMed] [Google Scholar]
- 75. Tse J, Martin-McNaulty B, Halks-Miller M, Kauser K, DelVecchio V, Vergona R, Sullivan ME, Rubanyi GM. Accelerated atherosclerosis and premature calcified cartilaginous metaplasia in the aorta of diabetic male Apo E knockout mice can be prevented by chronic treatment with 17 β-estradiol. Atherosclerosis. 1999;144(2):303–313. [DOI] [PubMed] [Google Scholar]
- 76. Buko VU, Lukivskaya O, Naruta E, et al. . Antiatherogenic effects of 17 beta-estradiol and 17 alpha-estradiol and its derivative J811 in cholesterol-fed rabbits with thyroid inhibition. Climacteric. 2001;4(1):49–57. [PubMed] [Google Scholar]
- 77. Hodgin JB, Maeda N. Minireview: estrogen and mouse models of atherosclerosis. Endocrinology. 2002;143(12):4495–4501. [DOI] [PubMed] [Google Scholar]
- 78. Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO III. Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994;90(2):786–791. [DOI] [PubMed] [Google Scholar]
- 79. Lew R, Komesaroff P, Williams M, Dawood T, Sudhir K. Endogenous estrogens influence endothelial function in young men. Circ Res. 2003;93(11):1127–1133. [DOI] [PubMed] [Google Scholar]
- 80. Longcope C, Kato T, Horton R. Conversion of blood androgens to estrogens in normal adult men and women. J Clin Invest. 1969;48(12):2191–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol (Oxf). 1974;3(1):69–96. [DOI] [PubMed] [Google Scholar]
- 82. Gardner WU. Ovarian and lymphoid tumors in female mice subsequent to roentgen-ray irradiation and hormone treatment. Proc Soc Exp Biol Med. 1950;75(2):434–436. [DOI] [PubMed] [Google Scholar]
- 83. Li MH, Gardner WU. Further studies on the pathogenesis of ovarian tumors in mice. Cancer Res. 1949;9(1):35–41. [PubMed] [Google Scholar]
- 84. Gardner W, Allen E, Smith G, Strong L. Carcinoma of the cervix of mice receiving estrogens. J Am Med Assoc. 1938;110(15):1182–1183. [Google Scholar]
- 85. Hooker CW, Pfeiffer CA. The morphology and development of testicular tumors in mice of the A strain receiving estrogens. Cancer Res. 1942;2(11):759–769. [Google Scholar]
- 86. Thompson JS, Crawford MK, Reilly RW, Severson CD. The effect of estrogenic hormones on immune responses in normal and irradiated mice. J Immunol. 1967;98(2):331–335. [PubMed] [Google Scholar]
- 87. Guo W, Bachman E, Vogel J, Li M, Peng L, Pencina K, Serra C, Sandor NL, Jasuja R, Montano M, Basaria S, Gassmann M, Bhasin S. The effects of short-term and long-term testosterone supplementation on blood viscosity and erythrocyte deformability in healthy adult mice. Endocrinology. 2015;156(5):1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guo W, Li M, Bhasin S. Testosterone supplementation improves anemia in aging male mice. J Gerontol A Biol Sci Med Sci. 2014;69(5):505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mariotti R, Fattoretti P, Malatesta M, Nicolato E, Sandri M, Zancanaro C. Forced mild physical training improves blood volume in the motor and hippocampal cortex of old mice. J Nutr Health Aging. 2014;18(2):178–183. [DOI] [PubMed] [Google Scholar]
- 90. van Kesteren P, Lips P, Gooren LJ, Asscheman H, Megens J. Long-term follow-up of bone mineral density and bone metabolism in transsexuals treated with cross-sex hormones. Clin Endocrinol (Oxf). 1998;48(3):347–354. [DOI] [PubMed] [Google Scholar]
- 91. Van Caenegem E, Wierckx K, Taes Y, Dedecker D, Van de Peer F, Toye K, Kaufman JM, T’Sjoen G. Bone mass, bone geometry, and body composition in female-to-male transsexual persons after long-term cross-sex hormonal therapy. J Clin Endocrinol Metab. 2012;97(7):2503–2511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.