Abstract
Insulin resistance is at the core of the metabolic syndrome, and men exhibit a higher incidence of metabolic syndrome than women in early adult life, but this sex advantage diminishes sharply when women reach the postmenopausal state. Because 17β-estradiol (E2) augments the excitability of the anorexigenic proopiomelanocortin (POMC) neurons, we investigated the neuroprotective effects of E2 against insulin resistance in POMC neurons from diet-induced obese (DIO) female and male mice. The efficacy of insulin to activate canonical transient receptor potential 5 (TRPC5) channels and depolarize POMC neurons was significantly reduced in DIO male mice but not in DIO female mice. However, the insulin response in POMC neurons was abrogated in ovariectomized DIO females but restored with E2 replacement. E2 increased T-type calcium channel Cav3.1 messenger RNA (mRNA) expression and whole-cell currents but downregulated stromal-interaction molecule 1 mRNA, which rendered POMC neurons more excitable and responsive to insulin-mediated TRPC5 channel activation. Moreover, E2 prevented the increase in suppressor of cytokine signaling-3 mRNA expression with DIO as seen in DIO males. As proof of principle, insulin [intracerebroventricular injection into the third ventricle (ICV)] decreased food intake and increased metabolism in female but not male guinea pigs fed a high-fat diet. The uncoupling of the insulin receptor from its downstream effector system was corroborated by the reduced expression of phosphorylated protein kinase B in the arcuate nucleus of male but not female guinea pigs following insulin. Therefore, E2 protects female POMC neurons from insulin resistance by enhancing POMC neuronal excitability and the coupling of insulin receptor to TRPC5 channel activation.
There is a sex difference in the development of central insulin resistance with obesity, and neuroprotection in females is provided, at least in part, by circulating estrogens.
Insulin resistance is at the core of the metabolic syndrome and causes abnormal insulin signaling in cells throughout the body. Neurons, similar to fat and muscle cells, can develop hyperinsulinemia-induced insulin resistance, which results in severe injury to the nervous system as seen in diabetic neuropathies (1). Moreover, males exhibit a higher incidence of metabolic syndrome than women in early adult life, but this sex difference diminishes sharply in hypoestrogenic states (2, 3). At the center of the regulation of energy homeostasis, and hence the central feedback of insulin (and leptin), are the anorexigenic proopiomelanocortin (POMC) and orexigenic neuropeptide Y(NPY)/agouti-related peptide (AgRP) neurons in the hypothalamic arcuate nucleus (ARH). These neuronal populations are integral components of the hypothalamic energy balance circuitry. Optogenetic and pharmacogenetic stimulation of NPY/AgRP neurons rapidly increases food consumption (4, 5), whereas prolonged stimulation of POMC neurons attenuates food intake (4).
The pleiotropic effects of leptin and insulin in POMC neurons are vital for both the short-term (excitability) and long-term (transcriptional) modulation of POMC neuronal activity and the control of food intake and energy homeostasis. POMC and NPY/AgRP neurons are major central nervous system (CNS) targets of insulin and leptin actions (6–9). Insulin delivered directly into the third ventricle decreases food intake in guinea pigs (9), mice (10, 11), and rats (12). Insulin depolarizes POMC neurons in both males and females via activation of canonical transient receptor potential 5 (TRPC5) channels and hyperpolarize NPY/AgRP neurons via activation of ATP-sensitive potassium channels (9), activity that is congruent with the anorexigenic effects of insulin. Moreover, deletion of TRPC5 channels specifically in POMC neurons results in a decrease in energy expenditure and increase in food intake and weight gain in mice (13). The increase in POMC cell excitability induced by insulin translates into heightened transcriptional activity [i.e., an increase in Fos expression in the arcuate nucleus and specifically in POMC neurons following an intracerebroventricular injection into the third ventricle (ICV) of insulin] (9). In the guinea pig, the insulin-induced decrease in food intake is correlated with alterations in energy expenditure as manifested by increases in O2 consumption, CO2 production, and metabolic heat production (9). The catabolic effects of insulin are blocked by melanocortin receptor 3, 4 antagonists (10), which argues for the critical central actions of insulin directly on POMC neurons to regulate energy homeostasis.
In POMC neurons the insulin receptor (InsR) couples to phosphatidylinositol 3-kinase (PI3K) p110β activation (14, 15), and the InsR-mediated excitation of POMC neurons is abrogated by inhibition of PI3K activity (9, 15–17). Activation of PI3K generates phosphatidylinositol-3,4,5-triphosphate, which stimulates phospholipase C and protein kinase B (Akt) (9, 18–20). Phospholipase C also hydrolyzes phosphatidylinositol 4,5-bisphosphate, which modulates TRPC4-5 channel activity (9, 21, 22). In addition, PI3K increases the insertion of TRPC5 channels into the plasma membrane from intracellular vesicular pools to further boost depolarization and Ca2+ entry into POMC neurons (23). Collectively, all of these PI3K-mediated effects are involved in the actions of insulin in POMC neurons. Therefore, we hypothesized that the TRPC5 channel represents a potential target for the critical uncoupling events leading to insulin resistance in obesity and type II diabetes and that 17β-estradiol (E2) may play a neuroprotective role in the female to prevent insulin resistance because E2 augments the excitability of POMC neurons through increasing ion channel expression and function and ultimately POMC protein and messenger RNA (mRNA) expression (24–27).
Materials and Methods
Animals and treatments
All animal procedures described in this study were performed in accordance with institutional guidelines based on National Institutes of Health standards and approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University or Western University of Health Sciences.
Mice
For the electrophysiology and single-cell reverse transcription (RT) polymerase chain reaction (PCR) experiments, transgenic mice expressing enhanced green fluorescent protein (EGFP) under the control of the Pomc promoter (PomcEGFP; from Dr. Malcolm Low, University of Michigan) were used in these studies. All animals were maintained under controlled temperature and photoperiod (lights on at 6 am and off at 6 pm) and given free access to food and water. We used a well-established, diet-induced obese mouse model to do cellular studies on POMC neurons (28, 29). PomcEGFP mice were put on a high-fat diet (HFD; 45% kcal from fat; Research Diets, New Brunswick, NJ; D12451) starting at 3 weeks of age for 12 weeks to induce diet-induced obesity (DIO). A control group of mice received normal grain-based chow (5L0D; Laboratory Diets, St. Louis, MO). After 12 weeks, we prepared coronal slices from both groups of mice and did whole-cell voltage-clamp recording from POMCEGFP (POMC) neurons. In females, the estrous cycle was monitored daily based on vaginal cell cytology for at least 2 weeks before using the animals for electrophysiological experiments on proestrus. Other animals were ovariectomized (OVX) and treated with estradiol benzoate and used for mRNA determinations as previously described (30). High circulating (proestrous) levels of E2 were verified by the uterine weights (>90 mg) at the time of hypothalamic slice preparation (31).
Body composition and food intake
Mice were housed individually, and body weight and food intake measurements were determined every 2 weeks. The evening prior to each glucose tolerance test (GTT), all mice were assessed for body composition (fat and lean mass) using a portable digital balance (Ohaus Scout Pro, Bradford, MA) and an EchoMRI 4-in-1-500 Body Composition Analyzer (Houston, TX). Food intake was determined as grams of diet consumed per day.
GTT
All mice were weaned at 3 weeks of age and given either a regular chow diet or a 45% fat diet. Age-matched control and DIO mice were fasted for 15 hours, and baseline glucose levels were measured with the aid of an Accu-Check Advantage blood glucose meter (Roche, Basel, Switzerland) using blood collected from the tail vein. Mice were injected intraperitoneally with glucose (1 mg/g lean mass as determined by magnetic resonance imaging) (32) in sterile phosphate-buffered saline, and blood glucose levels were measured 15, 30, 60, 90, and 120 minutes after injection. Glucose clearance (area under the curve) was calculated based on the glucose baseline levels at 0 minutes (33).
Visualized whole-cell patch recording
Whole-cell current clamp and voltage clamp recordings were made from POMCEGFP neurons as previously described (9, 17). Coronal arcuate slices (250 μm) were prepared from intact males and proestrous females, 10 weeks and older as previously described (9, 17). Whole-cell patch recordings were made from POMCEGFP neurons using an Olympus BX51 W1 fixed-stage scope outfitted with epifluorescence and infrared differential interference contrast video microscopy. Patch pipettes (A-M Systems; 1.5-mm outer diameter borosilicate glass; World Precision Instruments, Sarasota, FL) were pulled on a Brown/Flaming puller (P-97; Sutter Instrument, Novato, CA) and filled with the following solution: 128 mM potassium gluconate, 10 mM NaCl, 1 mM MgCl2, 11 mM EGTA, 10 mM HEPES, 2 mM adenosine triphosphate, and 0.25 mM GTP adjusted to pH 7.3 with KOH (295 mOsm). Pipette resistances ranged from 3.5 to 4 MΩ. In whole-cell configuration, access resistance was less than 30 MΩ; the access resistance was 80% compensated. The input resistance was calculated by measuring the slope of the current-voltage (I-V) relationship curve between –70 and –50 mV. Standard whole-cell patch recording procedures and pharmacological testing were performed as previously described (17). Electrophysiological signals were digitized with a Digidata 1322A (Axon Instruments), and the data were analyzed using p-Clamp software (Molecular Devices, Foster City, CA). The liquid junction potential was corrected for all data analysis. I-V relationships of the ligand-sensitive (i.e., insulin) currents were constructed by voltage ramps from –100 to +10 mV from a holding potential of –60 mV.
Electrophysiological solutions/drugs
A standard artificial cerebrospinal fluid was used (17). All drugs were purchased from Calbiochem (San Diego, CA) unless otherwise specified. Purified guinea pig insulin was purchased from Dr. Al Parlow (Harbor-UCLA Medical Center, Torrance, CA) through the National Hormone and Peptide Program. The depolarizing (inward current) response to purified guinea pig insulin was exactly the same as what we have published for bovine (Sigma-Aldrich I-1882) and human recombinant (Sigma-Aldrich I-9278) insulin (9). 2-Aminoethyl diphenylborinate (2-APB; 100 mM; Sigma-Aldrich, St. Louis, MO), rosiglitazone (100 mM; Cayman Chemical, Ann Arbor, MI), and GSK-7975A (10 mM; AOBIOUS Inc., Gloucester, MA) were dissolved in dimethyl sulfoxide.
ARH dissection for quantitative real-time PCR
The ARH was microdissected from grain-fed OVX-oil– and OVX-E2–treated female mice as described previously (34). Total RNA was extracted and treated with DNAse before reverse transcription and quantitative real-time PCR (qPCR) as described (34).
Cell harvesting of dispersed POMCEGFP neurons and qPCR
Cell harvesting and qPCR were conducted as previously described (30). Besides the original documentation that 99% of the Low mice POMCEGFP neurons immunostain for β-endorphin (35), we have shown that >95% of POMCEGFP neurons express Pomc mRNA and no Agrp mRNA in our single-cell profiling (9, 17). The ARH was microdissected from basal hypothalamic coronal slices obtained from control and DIO OVX-E2-treated females and intact male PomcEGFP mice (n = 4 to 5 animals/group). The POMCEGFP dispersed cells were visualized, patched, and then harvested (1 to 10 cells/tube) as described previously (30). Briefly, the tissue was incubated in protease [1 mg/mL in oxygenated artificial cerebrospinal fluid (aCSF)] for ∼15 minutes at 37°C and washed 4 times in low Ca2+ aCSF (and 2 times in aCSF). Gentle trituration with Pasteur pipettes of decreasing size were used to disperse the neurons onto a glass-bottom dish. We designed a glass-bottom 60mm dish to increase the surface area of the plate to allow greater segregation among cells. The healthy cells settled on the glass-bottom dish after approximately 15 to 20 minutes, at which time the aCSF was removed and fresh aCSF was added to the plate. Throughout the dispersion and harvesting procedure, a constant flow (2 mL/min) of oxygenated aCSF circulated into the plate while the effluent circulated out using a peristaltic pump. The aCSF flow helped ensure fresh, oxygenated media was reaching the cells and assisted in clearing out unhealthy cells and debris from the trituration. The cells harvested were those observed to be fully intact, with one to three processes and a smooth cell membrane. The cells were harvested using the XenoWorks Microinjector System (Sutter Instrument, Novato, CA). This microinjector system provides negative pressure in the pipette, which, together with the fine and gentle control of the suction, allows each cell to be drawn into the pipette essentially without fluid. Cells were harvested as single cells or as pools of 5 or 10 individual cells/tube. In view of publications that subpopulations of POMCEGFP neurons may coexpress Agrp (36), we tested individual POMCEGFP neurons (158 neurons from nine females) for Pomc mRNA, some of which were also reacted for Agrp mRNA. These data revealed that 98% of POMCEGFP neurons express Pomc, confirming our previous findings and those of others (9, 17, 37), and none of the POMCEGFP neurons express Agrp, although they are derived from the same progenitor cells (38).
Primers for the genes that encode for TRPC5, stromal-interaction molecule 1 (STIM1), p110β, β-actin, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were designed using Clone Manager software (Sci Ed Software, Denver, CO) to cross at least one intron-exon boundary and optimized as previously described using the Power SYBR Green method (30, 39), whereas the T-type calcium channel Cav3.1 (Cav3.1) mRNA was quantified using Taqman Gene Expression Assays (CaV3.1 assay ID Mm00486549_m1; β-actin assay ID mM01205647_g1). Primer sequences were as follows: suppressor of cytokine signaling-3 (Socs3; accession number NM_007707), forward primer 1913 to 1932 nt, reverse primer 2012 to 2031 nt, 119-bp product; Pik3cb (p110β) (accession number NM_029094), forward primer 1083 to 1101 nt, reverse primer 1149 to 1167 nt, 85-bp product; Stim1 (accession number NM_009287, qPCR: forward primer 987 to 1006 nt, reverse primer 1094 to 1111 nt, 125-bp product; single-cell PCR: forward primer 816 to 834 nt, reverse primer 932 to 950 nt; we have published the primers for Gapdh, Trpc5 (30), and Agrp (39). Given that the Socs3 gene only expresses one intron, which made it difficult to design efficient primers for PCR quantification, we designed a primer pair that did not cross exon-intron boundaries. Therefore, to avoid amplifying genomic DNA, the harvested neuronal pools used to measure Socs3 were treated with DNase I (1 U/μg DNA; Invitrogen, Grand Island, NY) at 10–3 U for 10 minutes and heat denatured (65°C) for 5 minutes. For CaV3.1, qPCR was performed using the Taqman Universal PCR Master Mix (Applied Biosystems, Foster City, CA) with predesigned Taqman Gene Expression Assays that include both primer and probe for the target and reference gene. Controls included neuronal pools reacted without RT, hypothalamic RNA reacted with RT and without RT, and water blanks. Primers for qPCR were further tested for efficiency (E = 10(–1/m) – 1) (30, 40, 41), which varied from 96% to 100%. The results were as follows: Pik3cb (p110β), m = –3.481, r2 = 0.95, efficiency = 96%; Gapdh, m = –3.35, r2 = 0.99, efficiency = 98.7%; Socs3, m = –3.294, r2 = 0.90, efficiency = 100%; Trpc5, m = –3.161, r2 = 0.95, efficiency = 100%; Stim1, m = –3.407, r2 = 0.98, efficiency = 97%.
qPCR was performed on a Quantstudio 7 Flex Real-Time PCR System (Life Technologies, Grand Island, NY) using Power SYBR Green Master Mix (Life Technologies) according to established protocols (30). The comparative cycle threshold (ΔΔCT) method (40, 41) was used to determine values from duplicate (or triplicate; Stim1) samples of 4 µL for the target genes Pik3cb, Socs3, Trpc5, Stim1, and CaV3.1 and 2 µL for the reference genes Gapdh and β-actin. The relative linear quantity was determined using the 2-ΔΔCT equation (30). To determine the relative expression levels of target genes in POMCEGFP neurons obtained from control grain-fed animals compared with HFD animals or OVX-oil–treated animals compared with OVX-E2–treated animals, the mean Δ CT for the target genes from the grain-fed (or OVX-oil–treated) samples were used as the calibrator. The data were expressed as n-fold change in gene expression normalized to the reference gene Gapdh (or β-actin) and relative to the calibrator, and the mean and standard error of the mean were calculated and used for statistical analysis.
Guinea pigs
Male and female Topeka guinea pigs (350 to 400 g, 35 to 40 days of age) were either purchased from Elm Hill Breeding Laboratories (Chelmsford, MA) or bred in the animal facilities at Western University of Health Sciences. Ovarian cyclicity was assessed through daily visual inspection of the vaginal aperture. Animals were randomly split into two groups (chow and HFD) either at the time of weaning (if bred in-house) or at the time of arrival (if purchased from Elm Hill) and exposed for at least 5 weeks to their assigned diet.
Surgical procedures
Stereotaxic implantation of guide cannulas into the third ventricle, assessment of energy intake and expenditure, and western blot analysis were performed as described previously (9, 42, 43). Briefly, anesthesia was induced by the subcutaneous injection of a ketamine/xylazine mixture (33 mg/kg and 6 mg/kg, respectively) and was maintained using isoflurane (1.5% to 2%) throughout the procedure. The anesthetized animal was secured in a stereotaxic frame (Stoelting, Wood Dale, IL), and a midline incision was made through the scalp. A hole was then drilled in the skull, through which a 22-gauge guide cannula (Plastics One, Roanoke, VA) was lowered 1 mm above the third ventricle at a 4° angle from the vertical plane using the following coordinates: anteroposterior: –2.1 mm, mediolateral: 0.7 mm, dorsoventral: –9.8 mm, tooth bar: –5.5 mm. Three additional holes were drilled that we used to insert anchor screws into the skull. The guide cannula was fastened in place with dental acrylic applied to the surgical field. Finally, a stylet was inserted into the guide cannula to keep the lumen patent. The animals were allowed to recover for 7 days prior to the start of experimentation. Only animals with verified third cerebroventricle cannula placement were included in the data analysis.
Assessment of energy intake and expenditure
We used a Comprehensive Laboratory Animal Monitoring System (Columbus Instruments, Columbus, OH) to evaluate energy balance as previously described and validated (9, 42). Briefly, animals were divided into two groups. One of the groups was started on a “westernized” HFD, from which 46% of the calories were fat derived, whereas the other group remained on a standard, grain-based diet, from which 11% of the calories were fat derived. At 6 weeks (1 week after the guide cannula implantations) in the males and 6 to 7 weeks (1 to 2 weeks after implantation and during the late follicular phase as noted by 2 consecutive days of vulvar swelling immediately preceding vaginal opening) in the females, the guinea pigs were allowed to acclimate to their Comprehensive Laboratory Animal Monitoring System chambers for 3 days, after which energy intake [mean hourly food intake over 5 days], O2 consumption, CO2 production, metabolic heat production, and the respiratory exchange ratio (RER) were experimentally measured for 5 days. During this time, the animals were injected either with purified guinea pig insulin (4 mU; ICV) or filtered 0.9% saline (2 μL; ICV) every morning at 8 am and then monitored for each of the aforementioned indices of energy balance.
Western blot analysis
Western blotting was carried out as previously reported (43). Briefly, at the end of the 5-day experimental period, we set out to determine whether diet-induced insulin resistance involved changes in the activation of the PI3K/Akt pathway in the ARH. Thus, animals fed either standard chow and an HFD were treated once again with insulin (4 mU; ICV) or its filtered 0.9% saline vehicle (2 µL, ICV), anesthetized 2 hours later with 32% isoflurane, and rapidly decapitated. Dorsal abdominal fat pads were removed and weighed to note any differences in adiposity. The brain was removed and coronal slices (1 mm in thickness) were prepared using a brain matrix (Ted Pella, Inc., Redding, CA). The ARH then was microdissected from the slices. Microdissected tissue was homogenized in cold lysis buffer [50 mM tris(hydroxymethyl)aminomethane (Tris)-HCl, pH 7.4, 0.5 M EDTA, 0.5 M EGTA] containing protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO). Protein levels were quantified using a Bradford assay (BioRad Laboratories, Hercules, CA) to establish equal loading into the gel. Proteins were separated by electrophoresis on a 10% sodium dodecyl sulfate polyacrylamide gel and transferred to a nitrocellulose membrane. Membranes were blocked for 1 hour with Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) and incubated overnight with primary antibodies directed against either total Akt (1:1000; Cell Signaling, Danvers, MA) or against phosphorylated Akt (pAkt; 1:500; Cell Signaling) at 4°C. All membranes were probed with anti-GAPDH (1:10,000; Millipore, Billerica, MA) as a loading control. They then were washed with Tris-buffered saline with Tween, followed by incubation with Odyssey infrared-conjugated secondary antibodies (LI-COR Biosciences) diluted 1:10,000 in Odyssey blocking buffer for 2 hours at room temperature. After washes with Tris-buffered saline with Tween, followed by Tris-buffered saline, membranes were scanned using an Odyssey infrared imager (LI-COR Biosciences). Data were analyzed by dividing the pAkt/GAPDH ratio by the total Akt/GAPDH ratio and then normalizing the resultant pAkt/total Akt ratio to the values observed in the appropriate control groups for sex and diet. Total Akt, pAkt, and GAPDH optical densities were quantified using Image Studio Software (LI-COR Biosciences).
Experimental design and statistical analysis
In visualized whole-cell patch recording experiments, only one recording was made per slice, and a maximum of three recordings were made from each PomcEGFP mouse. For cell harvesting of dispersed POMCEGFP neurons and qPCR measurements, 5 to 10 cells per pool and three pools from each animal were used. For the mouse body composition, food intake and GTT experiments, and assessment of guinea pig energy intake and expenditure followed by western blot analysis, all animal numbers are indicated in the figure legends. Statistical comparisons between two groups were performed using either an unpaired two-tailed Student t test or a Mann-Whitney U test. Comparisons between more than two groups were performed using the repeated-measures, multifactorial analysis of variance (ANOVA). If a significant interaction was encountered, we then moved to the one-way ANOVA, followed by the multiple range tests as specified in the appropriate figure legends. All data were analyzed using GraphPad Prism version 6 (La Jolla, CA). All data are presented as mean ± standard error of the mean (SEM). Differences were considered statistically significant if the probability of error was less than 5%.
Results
HFD increases body weight, fat deposition, and glucose intolerance in male and female mice
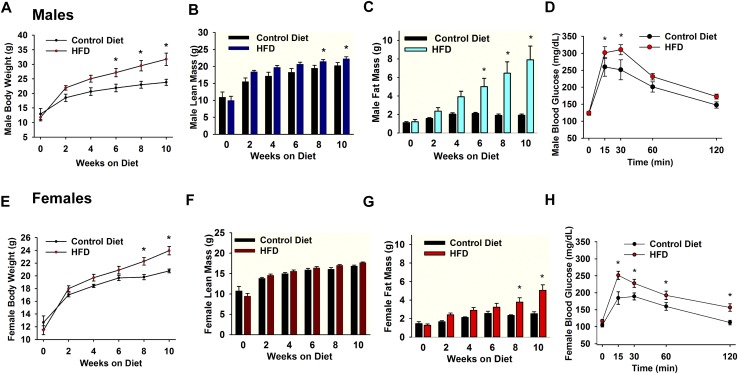
PomcEGFP mice were put on an HFD starting at 3 weeks of age for 10 weeks to induce DIO, and a control group of mice received normal grain-based chow (Fig. 1). The average food intake between male and female PomcEGFP mice within the control diet groups (males: 4.8 ± 0.2 g/d; females: 4.9 ± 0.2 g/d) or within the DIO groups (males: 3.4 ± 0.1 g/d; females: 3.1 ± 0.1 g/d) was not significantly different. However, the total body weight of DIO male and female PomcEGFP mice was significantly greater than control diet mice by week 6 or 8, respectively (Fig. 1A and 1E). Moreover, the average lean mass of DIO male PomcEGFP mice was significantly greater than mice fed a control diet by week 8 (DIO males: 21.4 ± 0.5 g; control males: 19.5 ± 0.8 g) (Fig. 1B), whereas the average lean mass of DIO female PomcEGFP mice was not significantly different than control diet mice (DIO females: 17.7 ± 0.2 g; control females: 16.8 ± 0.2 g) (Fig. 1F). More importantly, the average fat mass of DIO male PomcEGFP mice was significantly greater than control diet mice by week 6 (DIO males: 5.0 ± 0.9 g; control males: 1.9 ± 0.2 g) (Fig. 1C), whereas the average fat mass of DIO female PomcEGFP mice was significantly greater than control diet mice by week 8 (DIO females: 3.8 ± 0.5 g; control females: 2.3 ± 0.1 g) (Fig. 1G). After 10 weeks on their respective diets, all PomcEGFP mice were assessed for glucose tolerance using an intraperitoneal (i.p.) GTT. Both males and females started at relatively the same blood glucose levels after an overnight fast (Fig. 1D and 1H, time 0), suggesting similar homeostatic conditions within the body after fasting. However, DIO male (Fig. 1D) and female (Fig. 1H) PomcEGFP mice had higher glucose levels after i.p. glucose compared with control diet mice, indicating that DIO mice were glucose intolerant compared with control diet mice. Moreover, both DIO males and females had a significantly different glucose clearance rate than their age-matched controls (area under the curve: DIO females: 23,132 ± 1046 mg/dL × min, n = 7 vs control females: 18,356 ± 773 mg/dL × min, n = 5, t(10) = 3.389, P = 0.0069; DIO males: 28,030 ± 1067 mg/dL × min, n = 12 vs control males: 23,773 ± 1783 mg/dL × min, n = 8, t(18) = 2.183, P = 0.0425).
Figure 1.
Male and female mice on HFD become obese and glucose intolerant. (A–C) Male PomcEGFP mice were maintained on a HFD (45% fat) or a control grain-based diet for 10 weeks. (A) The HFD caused a significant weight gain and DIO over the 10-week period, which was significantly different from controls by week 6 [two-way ANOVA: main effect of treatment (F(1,54) = 23.743, P < 0.001), main effect of time (F(5,54) = 25.419, P < 0.001) and interaction (F(5,54) = 2.031, P = 0.089); control, n = 4, DIO mice, n = 7; post hoc Bonferroni test, *P < 0.05]. (B and C) Lean mass does not include bone and fluids within organs. Data are expressed in total grams. Total body fat was measured by nuclear magnetic resonance. The (B) lean and (C) fat content between groups became significant different at 8 and 6 weeks, respectively [two-way ANOVA for B: main effect of treatment (F(1,52) = 13.496, P < 0.001), main effect of time (F(5,52) = 39.497, P < 0.001) and interaction (F(5,52) = 1.252, P = 0.299); control, n = 4, DIO mice, n = 7; post hoc Bonferroni test, *P < 0.05; two-way ANOVA for C: treatment (F(1,52) = 26.962, P < 0.001), time (F(5,52) = 4.617, P = 0.001) and interaction (F(5,52) = 3.081, P = 0.016); control, n = 4, DIO mice, n = 7; post hoc Bonferroni test, *P < 0.05]. Consistent with the EchoMRI data, the perigonadal fat pad in DIO males was significantly heavier as compared with the control diet males (1982 ± 120 mg, n = 9 vs 405 ± 65 mg, n = 13, unpaired two-tailed t test, t(20) = 12.42, P < 0.0001; data not shown). (E–G) Female PomcEGFP mice were maintained on an HFD (45% fat) or a control grain-based diet. (E) The HFD caused a significant weight gain and DIO over the 10-week period, which was significantly different than controls by week 8 [two-way ANOVA: main effect of treatment (F(1,101) = 14.849, P < 0.001), main effect of time (F(5,101) = 76.846, P < 0.001) and interaction (F(5,101) = 3.091, P = 0.012); control, n = 9, DIO mice, n = 11; post hoc Bonferroni test, *P < 0.05]. (F and G) The body fat content between groups became significant different at 8 weeks. Two-way ANOVA for the (F) lean measurements: main effect of treatment (F(1,96) = 2.322, P = 0.131), main effect of time (F(5,96) = 65.715, P < 0.001) and interaction (F(5,96) = 1.814, P = 0.117); control, n = 9, HFD, n = 11. Two-way ANOVA for G: main effect of treatment (F(1,96) = 30.513, P < 0.001), main effect of time (F(5,96) = 12.571, P < 0.001) and interaction (F(5,96) = 3.327, P = 0.008); control, n = 9, HFD, n = 11; post hoc Bonferroni test, *P < 0.05. Consistent with the EchoMRI data, the perigonadal fat pad was significantly heavier in the DIO females compared with controls (1087 ± 133 mg, n = 9 vs 254 ± 20 mg, n = 11, unpaired two-tailed t test, t(18) = 6.829, P < 0.0001; data not shown). Both (D) DIO male and (H) female PomcEGFP mice at 10 weeks exhibited glucose intolerance vs their age-matched controls (fed grain-based diet). The GTTs showed significantly higher peak blood glucose levels 15 minutes after i.p. glucose (see “Materials and Methods”) and a delayed clearance. Two-way ANOVA for D: main effect of treatment (F(1,90) = 8.024, P = 0.006), main effect of time (F(4,90) = 35.919, P < 0.001) and interaction (F(4,90) = 1.316, P = 0.270); control, n = 8, HFD, n = 12; post hoc Bonferroni test, *P < 0.05. Two-way ANOVA for H: main effect of treatment (F(1,50) = 29.791, P < 0.001), main effect of time (F(4,50) = 33.646, P < 0.001) and interaction (F(4,50) = 1.537, P = 0.206); control, n = 5, HFD, n = 7; post hoc Bonferroni test, *P < 0.05.
POMC neurons in obese males are insulin resistant
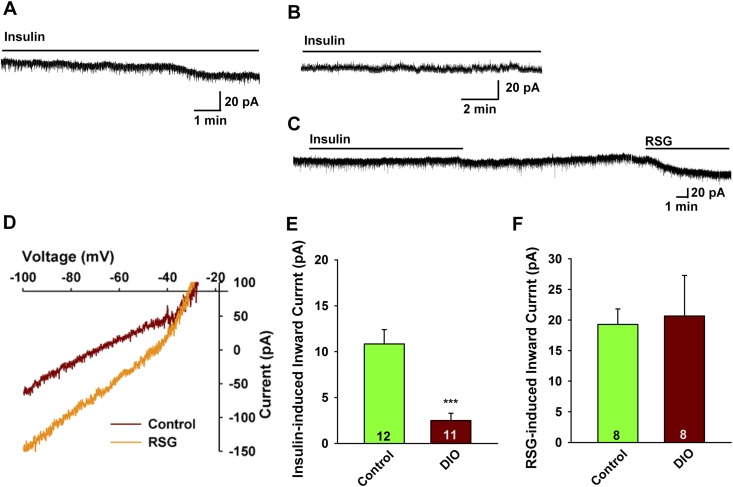
We prepared hypothalamic slices from the same cohort of DIO PomcEGFP mice after 10 weeks and did whole-cell current and voltage clamp recordings from POMC neurons in male mice using established protocols to determine the efficacy of insulin to depolarize POMC neurons (9). DIO male mice were ∼30% heavier at the time that they were euthanized for electrophysiology studies (38.0 ± 1.9 g, n = 9 vs 27.2 ± 0.8 g, n = 13, unpaired two-tailed t test, t(20) = 5.934, P < 0.0001) and had deposited ∼4 times as much total body fat (Fig. 1C). Consistent with the EchoMRI data, the perigonadal fat pad in DIO males was significantly heavier as compared with the control diet males (1982 ± 120 mg, n = 9 vs 405 ± 65 mg, n = 13, unpaired two-tailed t test, t(20) = 12.42, P < 0.0001). For the male POMC neurons, there was no difference in the resting membrane potential (DIO males: –73.1 ± 1.6 mV, n = 32 vs controls: –69.6 ± 1.6 mV, n = 35) or input resistance (DIO males: 1.1 ± 0.2 GΩ, n = 32 vs controls: 1.2 ± 0.1 GΩ, n = 35) between the two groups. Previously, we had established that POMC neurons are depolarized/excited by nanomolar concentrations of purified insulin through activation of nonselective cationic TRPC5 channels that generates an inward current with a reversal of ∼–25 mV and a double-rectifying current-voltage plot (I/V) (9). Although the insulin-induced current is relatively small (10 to 15 pA), it has pronounced depolarizing effects because of the high input resistance (>1 GΩ) of the POMC neurons. Therefore, we used voltage-clamp recordings in both control and DIO animals to assess the response to insulin. We also generated I/Vs before and after insulin to verify the activated conductance. As hypothesized, there was a significant difference in the steady-state response (inward current) to 20 nM insulin (DIO males: 2.5 ± 0.8 pA, n = 11 vs controls: 10.9 ± 1.6 pA, n = 12, unpaired two-tailed t test, t(21) = 4.646, P = 0.0001) (Fig. 2A, 2B, and 2E). Therefore, insulin appeared to be less efficacious in DIO males to activate TRPC5 channels.
Figure 2.
POMC neurons from DIO males are insulin resistant. In voltage clamp, insulin (20 nM) induced an inward current in POMC neurons from (A) male mice on a control diet but could not induce an inward current in POMC neurons from (B) DIO male mice. In this figure and Figs. 3 and 4, the line above the current trace designates the perfusion of insulin into the bath (at 1.25 mL/min), which took several minutes to reach a steady-state concentration. Vhold = –60 mV. (C) The TRPC5 channel opener rosiglitazone (RSG; 100 μM) induced an inward current in POMC neurons from DIO males similar to the effects of insulin in the control diet-fed males. Note: Rosiglitazone acts directly on the channel and therefore rapidly induces an inward current. Vhold = –60 mV. (D) The I-V relationship for the rosiglitazone-induced current from DIO male mice was obtained from C showing an inward current that reversed at –32 mV. (E and F) Bar graphs summarizing the responses to (E) insulin- and (F) rosiglitazone-induced inward currents in control diet and DIO male POMC mice. Data points represent the mean ± SEM. Unpaired two-tailed t test for E: t(21) = 4.646, ***P = 0.0001 vs control; unpaired two-tailed t test for F: t(14) = 0.1790, P = 0.8466. Cell numbers are indicated.
The thiazolidinedione drug rosiglitazone has been show to stimulate the opening of TRPC5 channels (44), and we have shown that it has similar effects as lanthanum to open TRPC5 channels in POMC neurons (9). Therefore, we tested rosiglitazone in POMC neurons from DIO males to access whether the number of active TPRC channels was diminished in the DIO state. Rosiglitazone (100 nM) was able to fully activate the channels (19.3 ± 2.5 pA, n = 8 in control males and 20.7 ± 6.6 pA, n = 8 in DIO males), and the I/V revealed that inward current generated by rosiglitazone tracked with insulin’s effects with a reversal potential of –29.6 ± 2.6 mV (n = 5), indicative of activation of a nonselective cationic (i.e., TRPC) channel (Fig. 2C, 2D, and 2F). Therefore, it appeared that the TRPC5 channels were not downregulated in DIO males, but instead, there was an uncoupling of InsRs from channel activation. Indeed, there was no difference in Trpc5 mRNA expression in POMC neurons from DIO vs control males based on our qPCR of POMC neurons (see later section).
POMC neurons in obese proestrous females maintain insulin sensitivity
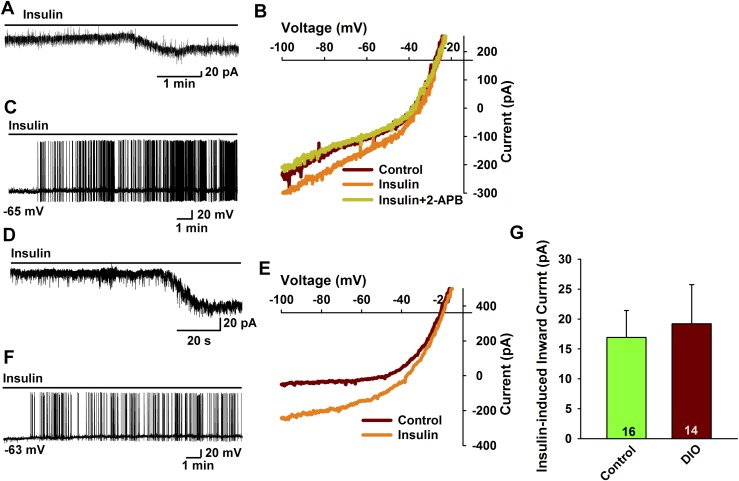
To test for a potential sex difference in the response to insulin with DIO, we did whole-cell current and voltage clamp recordings from female POMC neurons from DIO female PomcEGFP mice (9). The DIO female mice were ∼20% heavier at the time that they were euthanized for electrophysiology studies (27.2 ± 1.1 g, n = 9, vs 22.1 ± 0.4 g, n = 10, unpaired two-tailed t test, t(17) = 0.0003, P < 0.0001) and deposited ∼2 times as much total body fat (Fig. 1G). Consistent with the EchoMRI data, the perigonadal fat pad was significantly heavier in the DIO females compared with controls (1087 ± 133 mg, n = 9 vs 254 ± 20 mg, n = 11, unpaired two-tailed t test, t(18) = 6.829, P < 0.0001). For the female POMC neurons, there was no difference in the resting membrane potential (DIO females: –70.9 ± 1.7 mV, n = 44 vs controls: –69.2 ± 2.2 mV, n = 54) or input resistance (DIO females: 1.3 ± 0.1 GΩ, n = 44 vs controls: 1.1 ± 0.1 GΩ, n = 54) between the two groups. Again, we used sensitive voltage-clamp recordings to access the effects of insulin. In contrast to the males, the steady-state response (inward current) to 20 nM insulin was not attenuated with DIO (DIO females: –19.2 ± 6.5 pA, n = 14 vs controls: –16.9 ± 4.5 pA, n = 16) (Fig. 3A and 3D, summarized in Fig. 3G). The insulin-induced inward current reversed at –27.8 ± 4.4 mV (n = 6) in the control-fed females and –27.6 ± 7.0 mV (n = 5) in DIO females (Fig. 3B and 3E) and was antagonized by the TRPC3-6 channel blocker 2-aminoethyl diphenylborinate (45) (Fig. 3B). Therefore, we are confident that the insulin response is mediated by the opening of TPRC5 channels. Moreover in current clamp recordings in which we could monitor the firing activity of the POMC neurons, insulin robustly depolarized and increased firing of POMC neurons in both groups of animals (Fig. 3C and 3F). Hence, there is no attenuation of the insulin response (i.e., TRPC5 channel activation) in POMC neurons from DIO proestrous females, which indicates that there is a sex difference in the development of insulin resistance by POMC neurons in obesity.
Figure 3.
POMC neurons from DIO, proestrous females are protected against insulin resistance. (A–F) In voltage clamp, (A) insulin (20 nM) induced an inward current in POMC neurons from proestrous female mice on a control diet. Vhold = –60 mV. (B) The I-V relationship for the insulin-induced current was obtained from A, which showed an inward current that reversed at –25 mV. The inward current was blocked by TRPC channel antagonist 2-aminoethyl diphenylborinate (2-APB; 100 μM). (C) Insulin depolarized POMC neurons and induced firing from control proestrous females. Note: Due to the high-input resistance of POMC neurons (>1 GΩ), a small inward current is able to depolarize the cell and cause excitation (action potential firing) within a short time period. (D) In voltage clamp, insulin induced an inward current in POMC neurons from DIO proestrous females. Vhold = –60 mV. (E) The I-V relationship for the insulin-induced current was obtained from D, which showed the reversal potential of –20 mV. (F) Insulin depolarized POMC neurons and induced firing in DIO proestrous females. (G) Bar graph summarizing the responses of insulin-induced inward currents in control diet and DIO proestrous female PomcEGFP mice. Data points represent the mean ± SEM. Unpaired two-tailed t test, t(28) = 0.2924, P = 0.7722. Cell numbers are indicated.
E2 protects POMC neurons against insulin resistance
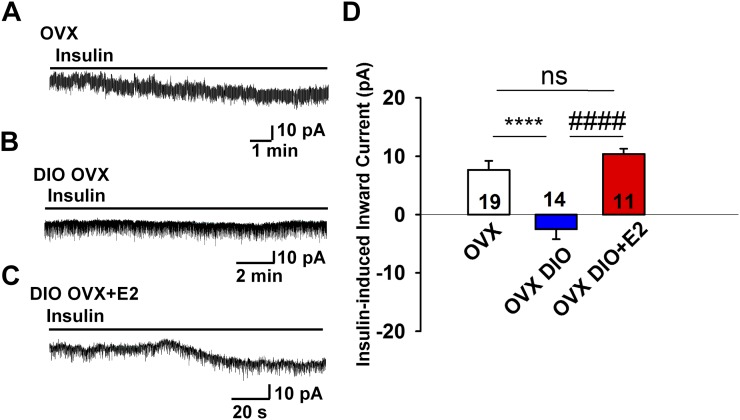
To explore the role of the gonadal steroids in preserving the insulin response in DIO females, we ovariectomized another cohort of female PomcEGFP mice that had been maintained on an HFD for 10 weeks. One week following ovariectomy, we gave one group (n = 3) an estradiol benzoate treatment (subcutaneous injection regimen that yielded proestrous serum levels of E2) (30) and treated the other group with the oil vehicle (n = 5). In contrast to insulin response in OVX females (n= 10) fed a control diet in which there was a significant insulin-mediated current (7.7 ± 1.6 pA, n = 19 neurons) (Fig. 4A and 4D), POMC neurons from OVX DIO females were completely refractory to the effects of insulin [i.e., there was no inward current (n = 14)] (Fig. 4B and 4D). Importantly, the TRPC5 channel opener rosiglitazone was able to generate a robust inward current in POMC neurons from OVX DIO females, indicating that TRPC5 channels were expressed and functional (data not shown). In contrast, POMC neurons from E2-treated OVX DIO females maintained their sensitivity to insulin [i.e., insulin induced a robust inward current and depolarized POMC neurons (10.4 ± 0.9 pA, n = 11)] (Fig. 4C and 4D). Therefore, in the absence of E2, there appears to be an uncoupling of InsR from activating TRPC5 channels in obese females, but the insulin response is rescued with E2 replacement.
Figure 4.
E2 replacement rescues insulin response in POMC neurons from OVX females. (A) In voltage clamp, insulin (20 nM) induced an inward current in POMC neurons from control diet-fed OVX female mice. Vhold = –60 mV. (B) In DIO OVX females, the insulin response was abrogated in POMC neurons. (C) However, the insulin response was rescued in DIO OVX females with E2 treatment (see “Materials and Methods”). (D) Bar graph summarizing the responses of insulin-induced currents in normal diet and OVX DIO and E2-treated female Pomc-EGFP mice. Data points represent the mean ± SEM. One-way ANOVA: effect of treatment, F(2, 41) = 17.74, P < 0.0001; Newman-Keuls multiple-comparison post hoc test. **** and #### indicate P < 0.001. Cell numbers are indicated. ns, no significant difference.
SOCS-3 is differentially regulated in obesity
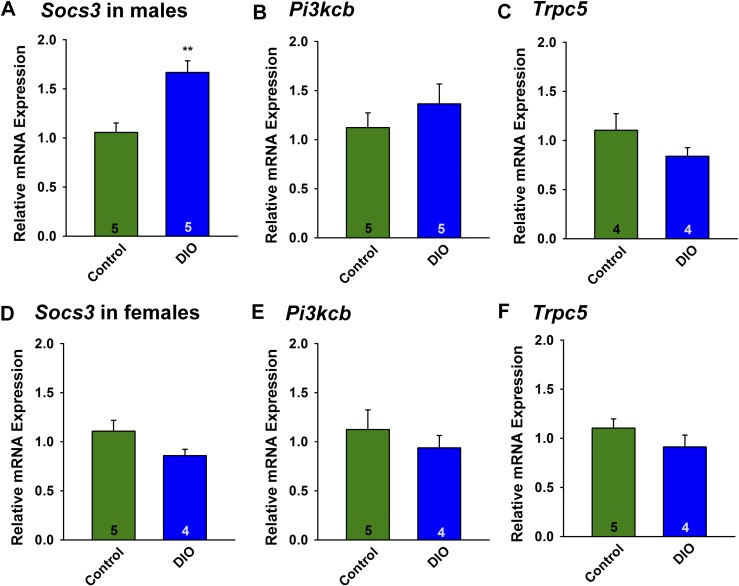
Leptin upregulates the expression of SOCS-3 in numerous cell types, including POMC neurons. SOCS-3 negatively regulates leptin signaling by inhibiting the coupling of the long form of the leptin receptor (LRb) to Janus kinase 2 and may also negatively regulate insulin signaling (46). Indeed, mice fed an HFD develop glucose intolerance, which is markedly improved by inactivating the Socs3 gene in POMC neurons (47). Therefore, we hypothesized that Socs3 mRNA expression may be upregulated in POMC neurons from the insulin-resistant DIO males but not in the insulin-responsive DIO females. Male and female mice were fed control grain-based diet or HFD as described previously, and after 10 weeks, PomcEGFP neurons were dispersed and harvested as previously described (30) and Socs3 and Pik3cb mRNA (encodes PI3K-p110β) were measured using qPCR. Socs3 mRNA, but not Pik3cb or Trpc5 mRNA, was increased in POMC neurons from DIO males as compared with the control diet–fed males (P < 0.01) (Fig. 5A–5C). In contrast, neither Socs3, Pik3cb, nor Trpc5 mRNA expression was altered in POMC neurons from E2-treated OVX DIO females as compared with E2-treated control females (Fig. 5D–5F), indicating that E2 prevented the increase in Socs3 mRNA expression with DIO, thereby maintaining insulin signaling in female POMC neurons.
Figure 5.
Socs3 mRNA expression is upregulated in POMC neurons from male but not female DIO mice. (A and D) HFD upregulated the Socs3 mRNA expression in ARH POMC neurons in males but not in females. Composite bar graphs illustrating the Socs3 mRNA levels determined in POMC neurons from (A) males or (D) females fed either an HFD or control diet. Data points represent the mean ± SEM. Unpaired two-tailed t test for A: t(8) = 4.006, P = 0.0039; unpaired two-tailed t test for D: t(7) = 1.826, P = 0.1122. Number of animals are indicated. **P < 0.005, values from DIO males that are significantly different from chow-fed controls. There were no differences in (B, E) Pik3cb mRNA or (C and F) Trpc5 mRNA expression in males or females fed either a control diet or HFD. Composite bar graphs illustrating the Pik3cb mRNA (p110 β) and Tripc5 mRNA determined in ARH POMC neurons from (B and C) males or (E and F) females fed either an HFD or control diet. Data points represent the mean ± SEM. Unpaired two-tailed t test for B: t(8) = 0.9674, P = 0.3617; unpaired two-tailed t test for E: t(7) = 0.7401, P = 0.4832; unpaired two-tailed t test for C: t(6) = 1.171, P = 0.2859; unpaired two-tailed t test for F: t(6) = 0.9513, P = 0.3782. Animal numbers are indicated with three pools of 10 cells from each animal.
E2 upregulates Cav3.1 and downregulates Stim1 mRNA expression
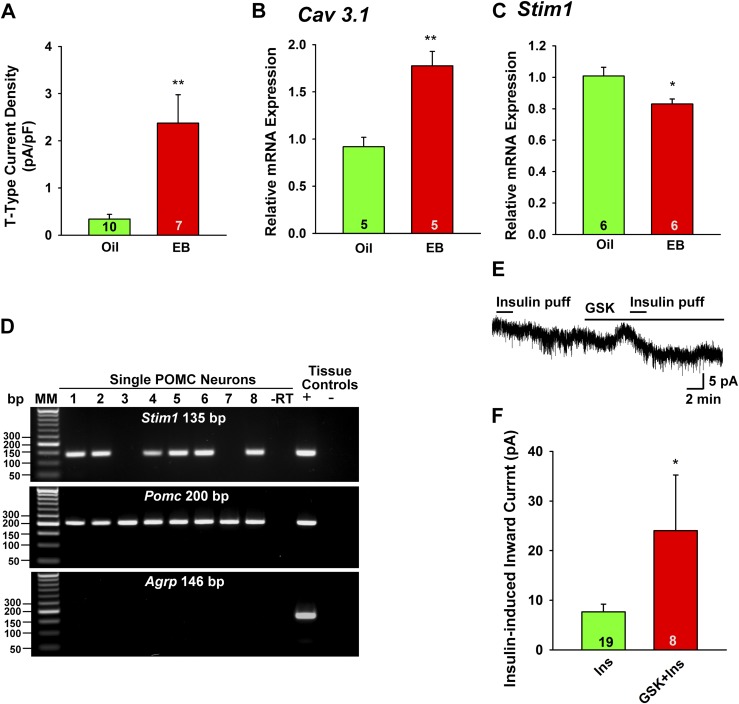
We hypothesized that the protective effects of E2 were mediated, at least in part, by upregulation of Ca2+ channel activity, which is known to enhance the overall excitability of POMC neurons (9, 17, 25). Because Trpc5 mRNA was not upregulated (Fig. 5C), we sought to measure voltage-gated calcium channels that are part of the TRPC channel signaling complexes in neurons (48, 49). Previously, we had found that T-type calcium channels facilitate TRPC4 channel activation in gonadotropin-releasing hormone (GnRH) neurons (22, 50). Therefore, we measured T-type calcium channel expression and function in POMC neurons. Adult female PomcEGFP mice (n = 10) were OVX and after 1 week were treated (subcutaneously) with oil vehicle (n = 5) or E2 (n = 5) to mimic proestrous levels of E2 (30). PomcEGFP neurons were dispersed and harvested from the oil-treated and E2-treated animals, and Cav3.1 transcripts were measured using qPCR (30). Both Cav3.1 mRNA and whole-cell T-type calcium currents were significantly upregulated in POMC neurons by E2 treatment (Fig. 6A and 6B). TRPC channels form either store-operated calcium channels (activated by depletion of calcium stores) or receptor-operated calcium channels (activated by membrane delimited receptors), which is dependent on their association with either the endoplasmic reticulum (ER) protein STIM1 for store-operated calcium channels or plasma membrane calcium channels for receptor-operated TRPC channels (51, 52). Therefore, we first measured Stim1 mRNA expression in another cohort of mice (n = 10) that were similarly treated with oil vehicle or E2 (n = 5/group). For the qPCR, we microdissected the arcuate nucleus rather than disperse the individual POMC neurons [see Bosch et al. (34) for details]. As we had hypothesized, E2 treatment significantly downregulated the expression of Stim1 (Fig. 6C), which would shift TRPC5 channels from being store-operated to receptor-operated (i.e., InsR) channels (51, 52). Also, Stim1 mRNA was highly expressed in POMC neurons (Fig. 6D), and the insulin-induced TRPC5 current in POMC neurons in OVX females was enhanced by approximately threefold (from 7.7 ± 1.6 pA, n = 19 to 24.0 ±11.2 pA, n = 8) in the presence of the store-operated Ca2+ channel inhibitor GSK 7975A (53) (Fig. 6E and 6F).
Figure 6.
E2 increases the whole-cell T-type calcium current and the mRNA expression of Cav3.1, but decreases the function of Stim1 in POMC neurons. (A) The current amplitude was normalized to the cell capacitance to calculate current density. Bar graphs summarize the density of T-type calcium current in POMC neurons from oil- and E2-treated animals. (B) Cav 3.1 mRNA expression was determined using the Taqman real-time PCR method in POMC neuronal pools (five cells in each pool). (C) Stim1 was measured in the arcuate nucleus in oil- and E2-treated females using SYBR Green real-time PCR (see “Materials and Methods”). (D) Based on single-cell RT-PCR analysis (65 cells from three female mice) >75% of POMC neurons express Stim1 mRNA. A representative gel illustrating that single POMC-EGFP neurons express mRNA for Pomc and Stim1, but not AgRP. –RT indicates that single cell reacted without RT; + indicates positive tissue control (with RT); – indicates negative tissue control (without RT). (E) In OVX females, insulin (Ins; 20 µM/4 µL “puff” application into bath) generated an inward current (4 pA) that washed out after several minutes, during which time the store-operated Ca2+ channel inhibitor GSK 7975A (10 µM) was applied. A second application of insulin (puff application) generated 8 pA current. Vhold = –60 mV. (F) GSK augmented the insulin-induced inward current by 2.9 ± 0.9-fold (n = 8). Data points represent the mean ± SEM. Unpaired two-tailed Student t test: t(15) = 3.986, P = 0.0012 (for T-type current); t(8) = 4.818, P = 0.0013 (for Cav3.1 mRNA); t(10) = 2.764, P = 0.020 (for Stim1 mRNA); t(25) = 2.184, P = 0.0386 (for GSK augmentation of insulin-induced inward current). *P < 0.05; **P < 0.01. EB, estradiol benzoate; MM, molecular marker.
Male but not female guinea pigs develop central insulin resistance
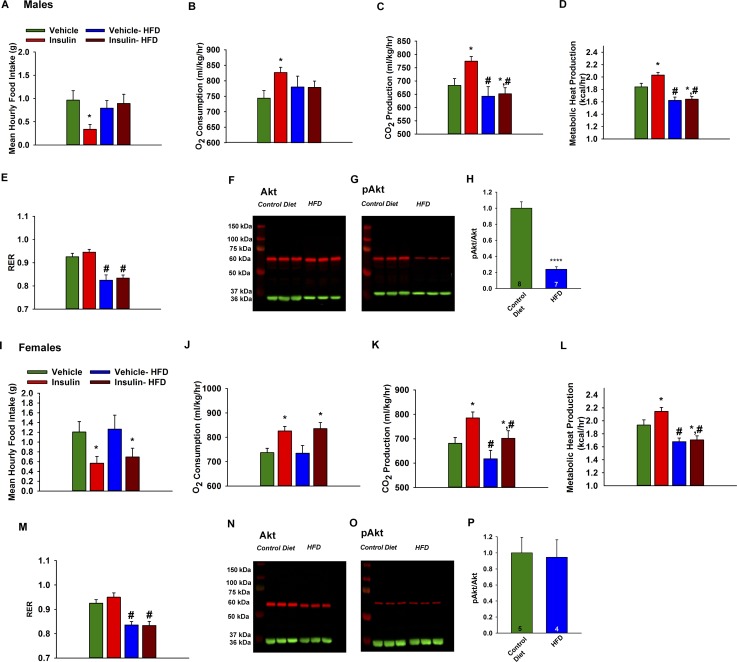
As proof of principle, we tested the effects of ICV insulin on a number of metabolic parameters in guinea pigs. We used the female guinea pig as a model because it has a long reproductive cycle (16 to 18 days) with clearly demarcated follicular (estrogen-driven) and luteal (progesterone-driven) phases similar to primates (54). Animals were randomly assigned to experimental groups and fed either standard chow or HFD (46% kcal from fat) for 6 weeks. After 6 weeks, there was not a significant increase in body weight or adiposity (dorsal abdominal fat pad weight), but the HFD did result in overt hypercholesterolemia in males (2.28 ± 0.50 mmol/L, n = 9 vs 0.80 ± 0.10 mmol/L, n = 10) and females (3.56 ± 0.54 mmol/L, n = 6 vs 1.05 ± 0.12 mmol/L, n = 6). In addition, HFD male guinea pigs exhibited decreased metabolic heat production and RER (Fig. 7A–7E). Moreover, the efficacy of ICV insulin to decrease food intake and increase O2 consumption and CO2 production was significantly attenuated. In periovulatory females, HFD significantly reduced CO2 production, metabolic heat production, and RER (Fig. 7I–7M). However, in contrast to the males, insulin decreased energy intake and increased O2 consumption and CO2 production in HFD females (Fig. 7I–7K). Moreover, western blot analysis of the microdissected ARH revealed that the expression of pAkt, a downstream target of PI3K activity, was reduced by approximately fivefold in males but was unchanged in female HFD guinea pigs (Fig. 7F–7H and 7N–7P). Therefore, it appears that the heightened levels of gonadal steroids (i.e., E2) in the periovulatory period protected females from developing CNS insulin resistance with the HFD.
Figure 7.
DIO male guinea pigs are insulin resistant whereas DIO females are protected against insulin resistance, and pAkt is reduced in ARH of DIO male but not in DIO female guinea pigs. In males, purified guinea pig insulin decreases (A) energy intake concomitant with increases in (B) O2 consumption, (C) CO2 production, and (D) metabolic heat production. The effects of insulin were markedly diminished by DIO. Data points represent the mean ± SEM of the mean hourly food intake, O2 consumption, CO2 production, metabolic heat production, and (E) RER seen in guinea pigs treated with either insulin (4 mU; ICV) or its filtered 0.9% saline vehicle (2 μL; ICV). Two-way ANOVA for A: main effect of diet (F(1,75) = 0.01, P = 0.9036), main effect of insulin (F(1,75) = 4.63, P = 0.0346) and interaction (F(1,75) = 4.54, P = 0.0364); two-way ANOVA for B: main effect of diet (F(1,74) = 1.66, P = 0.2019), main effect of insulin (F(1,74) = 1.14, P = 0.2888) and interaction (F(1,74) = 9.59, P = 0.0028); two-way ANOVA for C: main effect of diet (F(1,74) = 11.78, P = 0.0010), main effect of insulin (F(1,74) = 3.67, P = 0.0591) and interaction (F(1,74) = 6.83, P = 0.0109); two-way ANOVA for D: main effect of diet (F(1,74) = 17.11, P = 0.0001), main effect of insulin (F(1,74) = 21.06, P = 0.0000) and interaction (F(1,74) = 0.26, P = 0.6146); two-way ANOVA for E: main effect of diet (F(1,74) = 32.04, P = 0.0000), main effect of insulin (F(1,74) = 3.42, P = 0.068) and interaction (F(1,74) = 0.08, P = 0.7753); post hoc least-significant difference test, *P < 0.05, values from insulin-treated animals that are significantly different than those from vehicle-treated controls (n = 4). #P < 0.05, values from DIO animals that are significantly different than those fed standard chow (n = 4). Representative western blots illustrating the levels of (F) Akt, (G) pAkt, and their corresponding loading control GAPDH in the ARH microdissected from HFD and control diet-fed male guinea pigs. (H) Composite bar graphs illustrating the pAkt/Akt ratio, after normalizing to their respective loading control, determined in ARH microdissections from animals fed either HFD or control diet. Data points represent the mean ± SEM, respectively. Mann-Whitney U test: U = 1.0, P = 1.2081E-8. Animal numbers are indicated. **** indicates values from DIO animals that are significantly different from control diet-fed controls. In females, the insulin-induced decrease in (I) food intake and the increases in (J) O2 consumption and (K) CO2 production are preserved in DIO periovulatory females. Data points represent the mean ± SEM of the mean hourly food intake, O2 consumption, CO2 production, (L) metabolic heat production, and (M) the RER seen in guinea pigs treated with either insulin (4 mU; ICV) or its filtered 0.9% saline vehicle (2 μL; ICV). Two-way ANOVA for I: main effect of diet (F(1,75) = 0.18, P = 0.6688), main effect of insulin (F(1,75) = 8.08, P = 0.0058) and interaction (F(1,75) = 0.03, P = 0.8704); two-way ANOVA for J: main effect of diet (F(1,75) = 0.02, P = 0.8889), main effect of insulin (F(1,75) = 15.58, P = 0.0002) and interaction (F(1,75) = 0.06, P = 0.8028); two-way ANOVA for K: main effect of diet (F(1,75) = 6.40, P = 0.013), main effect of insulin (F(1,75) = 10.43, P = 0.0018) and interaction (F(1,75) = 0.12, P = 0.7321); two-way ANOVA for L: main effect of diet (F(1,75) = 26.23, P = 0.0000), main effect of insulin (F(1,75) = 4.74, P = 0.0325) and interaction (F(1,75) = 1.24, P = 0.2689); two-way ANOVA for M: main effect of diet (F(1,75) = 39.78, P = 0.0000), main effect of insulin (F(1,75) = 0.72, P = 0.3978) and interaction (F(1,75) = 0.50, P = 0.4821); post hoc least-significant difference test, *P < 0.05, values from insulin-treated animals that are significantly different than those from vehicle-treated controls (n = 4). #P < 0.05, values from DIO animals that are significantly different than those fed standard chow (n = 4). Representative western blots depicting the levels of (N) Akt, (O) pAkt, and their corresponding loading control GAPDH in the ARH microdissected from HFD or control diet-fed female guinea pigs. (P) Composite bar graphs showing the pAkt/Akt ratio determined in ARH microdissections from animals fed either HFD or control diet. Data points represent the mean ± SEM, respectively. Mann-Whitney U test: U = 101.0, P = 0.6084. Animal numbers are indicated. All of the triplicate bands for Akt and pAkt from control diet- and HFD-fed male and female guinea pig ARH seen in F to G, N, and O were obtained from the same gel. There was no difference in total Akt expression between the control and HFD female guinea pigs (Mann-Whitney U test: U = 73.0, P = 0.42), nor was there any difference between male and female control animals (Mann-Whitney U test: U = 233.0, P = 0.13).
Discussion
Although obesity produces dramatic alterations in metabolic phenotype in both males and females, E2 was able to protect females from the development of CNS (hypothalamic) insulin resistance. Insulin was fully efficacious to activate TRPC5 channels and depolarize POMC neurons in DIO proestrous and E2-treated OVX females but not in OVX female or male DIO mice. Treating OVX females with an estradiol regimen that mimicked proestrous serum levels of E2 restored the insulin response in POMC neurons. E2 upregulated Cav3.1 mRNA expression and T-type calcium channel currents but downregulated Stim1 mRNA, which rendered POMC neurons more excitable and responsive to insulin-mediated TRPC5 channel activation. Moreover, E2 prevented the increase in Socs3 expression with DIO, which is known to inhibit the coupling of the InsR with its downstream signaling cascade. Also, ICV insulin was fully efficacious in female but not male guinea pigs fed an HFD to reduce food intake and increase energy metabolism. Therefore, the present studies have identified a sex difference in the development of insulin resistance in hypothalamic (POMC) neurons. Moreover, we have discovered critical cellular signaling pathways in POMC neurons that are augmented by E2 to help protect females against insulin resistance.
The InsR in POMC neurons is coupled to TRPC5 channel activation through a PI3K signaling cascade in both mice and guinea pigs (9, 17). It has long been known that ovariectomy leads to hyperphagia, weight gain, and decreased metabolism (55–57). Deletion of estrogen receptor α (ERα) in POMC neurons recapitulates the hyperphagia (58), and E2 via ERα signaling increases PI3K activity and subsequently pAKT in female mouse POMC neurons (33). Presently, we found that pAkt activity in the arcuate nucleus was significantly reduced with HFD in male but not in female guinea pigs; and in preliminary electrophysiology experiments, the insulin-activated TRPC5 current was reduced in male but not female POMC neurons from guinea pigs on an HFD (unpublished data). Interestingly, there were no changes in Pik3cb (PI3K p110β) mRNA expression in POMC neurons in DIO mice of either sex, indicative of a posttranslational deficit in PI3K signaling. In addition, rosiglitazone, a TPRC5 channel opener (44), was able to fully activate the TRPC5 channels in POMC neurons from DIO males, indicating that the “uncoupling” of the InsR signaling pathway was upstream of TRPC5 channel activation.
We hypothesized that E2 could regulate the expression of voltage-gated Ca2+ channels and scaffolding proteins as we have demonstrated in other hypothalamic neurons (30, 31). Indeed, Cav3.1 mRNA and the associated T-type calcium current were upregulated in POMC neurons by E2 treatment. The T-type calcium channel Cav3.1 underlies burst firing in hypothalamic kisspeptin neurons (31), and it is tightly coupled to TRPC4 channel activation in GnRH neurons (22). In POMC neurons, we believe that the T-type calcium channel is also coupled to TRPC5 channel activation as has been shown for TRPC1 and l-type calcium (Cav1.3) channels in substantia nigra dopamine neurons (49) and TRPC3 and P/Q-type calcium (Cav2.1) channels in cerebellar Purkinje neurons (48). Also, we measured a decrease in Stim1 mRNA expression in the ARH with E2 treatment, and Stim1 mRNA is highly expressed in POMC neurons (Fig. 6D). STIM1 is localized to the endoplasmic membrane of cells, and its N-terminal domain contains an EF-hand that protrudes into the lumen of the ER to sense changes in ER Ca2+ concentrations (52). Upon depletion of ER Ca2+, STIM1 undergoes a conformational change, oligomerizes, and then interacts with plasma membrane TRPC channels (52, 59). Phosphorylation of STIM1 is required for oligomerization, and E2 is known to inhibit the phosphorylation of STIM1 and consequently its interaction with plasma membrane and hence store-operated Ca2+ entry (60). Therefore, in the absence of estrogens, TRPC channels are more associated with STIM1 and are store-operated Ca2+ channels (52, 59, 60). Indeed, we found that the insulin-induced TRPC5 current in POMC neurons in OVX females was enhanced in the presence of a store-operated Ca2+ channel inhibitor GSK 7975A. We have also observed that longer-term E2 treatment downregulated Stim1 mRNA expression by approximately twofold in the arcuate nucleus of female guinea pigs (unpublished data), which indicates that this E2-mediated mechanism for increasing TRPC5 channel coupling to InsRs may be conserved among mammals. The critical role of E2-mediated regulation of TRPC coupling may also explain our finding that the ability of insulin to activate TRPC5 channels was abrogated in OVX DIO females. In gonadal-intact DIO males, there was still a small response to insulin, which is indicative that circulating testosterone may be converted to E2 in the hypothalamus (61) and thereby help facilitate insulin signaling. Hence under normal physiological conditions, TRPC5 channels are associated with other plasma membrane channels (T-type calcium channels) in POMC neurons and coupled to plasma membrane receptors (InsR, LRb, serotonin 5HT2C) (9, 13, 17), but in cellular stressed states such as with obesity, TRPC5 channels may associate with STIM1 and are coupled to Ca2+ store depletion from the ER. Therefore, E2 appears to have a protective role by downregulating Stim1 expression.
In the periphery, Stim1 mRNA is upregulated in human glomerular mesangial cells with chronic high glucose exposure (diabetic model) (62) and in smooth muscle cells of hypertensive mice (angiotensin II induced) that exhibit vascular dysfunction and high (systolic) blood pressure (63). The vascular dysfunction/increased blood pressure is rescued in Stim1 knockout mice. In addition, E2 prevents the phosphorylation of STIM1 and its oligomerization and association with plasma membrane Ca2+ channels in transfected HEK293 cells (60). Therefore, STIM1 plays an important role peripherally and most likely centrally to regulate Ca2+ channels, and our findings of decreased Stim1 expression by E2 would indicate that E2 regulates InsR signaling by shifting TRPC5 channels from store-operated to receptor-operated channels (9).
Using the same PomcEGFP mouse line and same HFD feeding paradigm, Paeger et al. (29) recently found that the excitability of POMC neurons was markedly reduced in DIO males, and in congruence with our findings in males, the intracellular Ca2+ buffering capacity of POMC neurons was significantly attenuated. As a result of increased resting Ca2+ levels, the authors noted that the activity of small-conductance, calcium-activated potassium channels was significantly elevated, which contributed to the reduced excitability of POMC neurons in males. Given that the authors did not study females, we do not know if POMC small-conductance, calcium-activated potassium channel expression would be reduced by E2 treatment, as we have found in the GnRH neurons (30), and therefore help maintain POMC neuronal activity in females. Certainly, the control of intracellular calcium levels through calcium channel activity and ER buffering is most critical for not only the resting excitability of POMC neurons, but also the response to insulin.
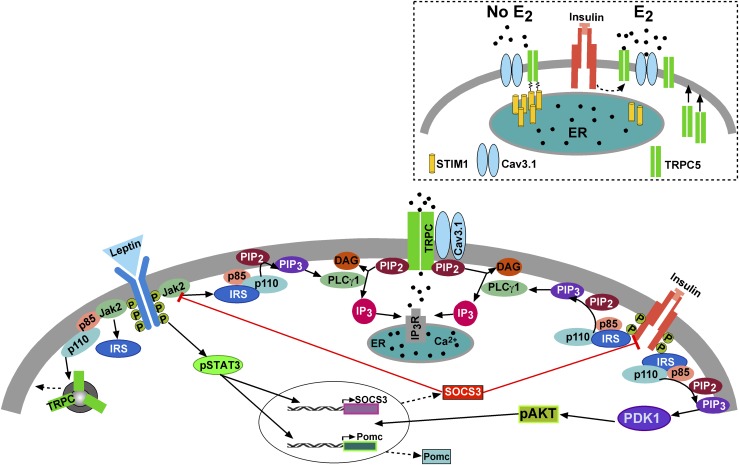
In males but not in females, Socs3 mRNA expression in POMC neurons was ∼1.6-fold higher with high-fat feeding. SOCS-3 is known to inhibit the tyrosine kinase activity of InsR and the interaction of insulin receptor substrate (IRS) proteins with InsR and also to direct IRS proteins toward degradation in fat and liver cells (64). Therefore, inhibition of InsR interaction with IRS proteins by SOCS-3 would effectively decrease all downstream signaling, including PI3K activity and TRPC5 channel activation (Fig. 8). The sex-specific increase in Socs3 expression with DIO may also explain the selective insulin resistance in male vs female POMC neurons. By limiting the Socs3 expression in DIO females, E2 preserved InsR signaling to depolarize POMC neurons via TRPC5 channel activation. Moreover, by upregulating Cav3.1 channel expression and shifting TRPC5 channels from store-operated to receptor-operated calcium channels in POMC neurons, E2 maintains the efficacy of insulin to depolarize and excite POMC neurons in DIO females, which is vital for the control of energy homeostasis (9, 13).
Figure 8.
A cellular model of insulin (and leptin) signaling via TRPC5 channel activation in POMC neurons. Based on the current findings and other published data, we propose that insulin signals via IRS-PI3K to activate TRPC5 channels in POMC neurons, which generates a robust inward cationic current to depolarize POMC neurons and increase their excitability. Similarly, leptin binding to its receptor (LRb) triggers the recruitment of the tyrosine kinase Janus kinase (JAK) 2, leading to the activation of the JAK/signal transducer and activator of transcription (STAT) signaling pathway and simultaneously activation of PI3K, which also activates TRPC5 channels (9, 17). PI3K (p85/p110) will also accelerate the rapid insertion of TPRC5 channels into the plasma membrane (23). With DIO (hyperleptinemia), there is an increase in Socs3 mRNA expression in POMC neurons in males but not in females, which induces insulin resistance by inhibiting the tyrosine kinase activity of InsR and the interaction of IRS proteins with InsR. However, under physiological stress and in the absence of E2, STIM1 interacts with TPRC5/Cav3.1 channels, thereby engaging these Ca2+ channels as store-operated channels (inset), which are activated with ER depletion of Ca2+ (52). However, under physiological conditions in cycling females, E2 downregulates the expression of STIM1 (see Fig. 6) and prevents the phosphorylation of STIM1, thereby converting the TRPC5/Cav3.1 signaling complex to receptor-operated (i.e., InsR) channels in POMC neurons (inset). PDK1, 3-phosphoinositide–dependent protein kinase-1.
Previous studies would indicate that signaling via ERα plays a critical role in mediating at least some of these transcriptional effects of E2 in POMC neurons (58). A recent publication has even implicated membrane ERα signaling because the ERα-selective agonist propyl pyrazole triol rapidly depolarized POMC neurons and induced firing, suggesting that a membrane-initiated signaling cascade, which excites POMC neurons and initiates new transcription, is involved (33). Also, the selective Gq-coupled membrane ER ligand STX, a novel diphenylacrylamide compound similar to E2, excites POMC neurons, initiates gene transcription, and inhibits food intake in both mice and guinea pigs (65). Collectively, these findings suggest that E2 can excite POMC neurons using multiple signaling pathways to preserve insulin signaling.
Although the developmental deletion of the InsR in either NPY/AgRP or POMC neurons does not alter control of energy homeostasis in the adult (66), deletion of both insulin and leptin receptors in POMC neurons causes overt systemic insulin resistance in both male and female mice (67). If left untreated insulin deficiency leads to hyperglycemia, polyuria, ketoacidosis, and death as seen in type 1 diabetes. Interestingly, insulin-deficient rodents are viable with leptin monotherapy and this life-saving therapy is effective in mice expressing leptin receptors only in hypothalamic POMC and GABAergic neurons (68). Fujikawa et al. (68) concluded that leptin and insulin must engage the same hypothalamic circuitry to maintain glucose homeostasis and hepatic function, and indeed we have shown at the cellular level that insulin and leptin engage a common signaling pathway to activate TRPC5 channels and depolarize POMC neurons (9). Consistent with these previous findings, preliminary studies in DIO males indicate that POMC neurons are also resistant to the depolarizing effects of leptin (reduced by 87%; unpublished data).
In contrast to POMC neurons, insulin and leptin inhibit the activity of NPY/AgRP neurons via a PI3K signaling pathway that activates ATP-sensitive potassium channels and hyperpolarizes these orexigenic neurons (9, 66, 69, 70). Also, in steroidogenic factor 1 (SF-1)–expressing neurons of the ventromedial nucleus of the hypothalamus, insulin inhibits action potential firing (71). SF-1 neurons provide glutamatergic input to POMC neurons (72, 73), and DIO impairs their activity and subsequently their excitatory input to POMC neurons (71). Again, these studies did not discriminate between the sexes, so it is not known if there are any sex differences in the activity of NPY/AgRP or SF-1 neurons as we have documented for POMC neurons in the current study. Regardless, our findings that circulating estrogens maintain insulin sensitivity in POMC neurons in obese females may help explain why premenopausal women are protected against development of insulin resistance in type II diabetes (2, 74).
Acknowledgments
We thank J. G. Bradner for excellent technical support.
Financial Support: This work was supported by US National Institutes of Health Grants NS038809 (to M.J.K.), NS043330 (to O.K.R.), DK068098 (to M.J.K. and O.K.R.), DA024314 (to E.J.W.), and P51 OD011092.
Author Contributions: J.Q. performed and analyzed the electrophysiology experiments. M.A.B. did the single-cell harvesting and quantitative PCR analysis of the harvested cells. C.M. performed and analyzed western blot experiments. U.-V.N. and C.CN. did the mouse in vivo analysis. C.M. did the guinea pig in vivo analysis. M.J.K., O.K.R., J.Q., and E.J.W. designed the experiments, analyzed the data, and wrote the manuscript.
Current Affiliation: C. Nestor’s current affiliation is the Department of Animal Science, North Carolina State University, Raleigh, North Carolina 27695.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- artificial cerebrospinal fluid
- AgRP
- agouti-related peptide
- Akt
- protein kinase B
- ANOVA
- analysis of variance
- ARH
- hypothalamic arcuate nucleus
- Cav3.1
- T-type calcium channel Cav3.1
- CNS
- central nervous system
- DIO
- diet-induced obesity
- E2
- 17β-estradiol
- EGFP
- enhanced green fluorescent protein
- ER
- endoplasmic reticulum
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GnRH
- gonadotropin-releasing hormone
- GTT
- glucose tolerance test
- HFD
- high-fat diet
- InsR
- insulin receptor
- ICV
- intracerebroventricular injection into the third ventricle
- i.p.
- intraperitoneal
- IRS
- insulin receptor substrate
- I-V
- current-voltage
- i.v.
- intravenous(ly)
- LRb
- the long form of the leptin receptor
- mRNA
- messenger RNA
- NPY
- neuropeptide Y
- OVX
- ovariectomized
- pAkt
- phosphorylated protein kinase B
- PCR
- polymerase chain reaction
- PI3K
- phosphatidylinositol 3-kinase
- POMC
- proopiomelanocortin
- qPCR
- quantitative real-time polymerase chain reaction
- RER
- respiratory exchange ratio
- RT
- reverse transcription
- SEM
- standard error of the mean
- SF-1
- steroidogenic factor 1
- Socs3
- suppressor of cytokine signaling-3
- STIM1
- stromal-interaction molecule 1
- Tris
- tris(hydroxymethyl)aminomethane
- TRPC5
- canonical transient receptor potential 5
- ΔΔCT
- comparative cycle threshold.
References
- 1.Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab. 2012;23(3):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: The study of women’s health across the nation. Arch Intern Med. 2008;168(14):1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gustafsson PE, Persson M, Hammarström A. Life course origins of the metabolic syndrome in middle-aged women and men: the role of socioeconomic status and metabolic risk factors in adolescence and early adulthood. Ann Epidemiol. 2011;21(2):103–110. [DOI] [PubMed] [Google Scholar]
- 4.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. [DOI] [PubMed] [Google Scholar]
- 7.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. [DOI] [PubMed] [Google Scholar]
- 8.Belgardt BF, Brüning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci. 2010;1212(1):97–113. [DOI] [PubMed] [Google Scholar]
- 9.Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, Rønnekleiv OK, Kelly MJ. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 2014;19(4):682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22(20):9048–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89(5):687–691. [DOI] [PubMed] [Google Scholar]
- 12.Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D’Alessio D, Tso P, Seeley RJ, Woods SC. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103(1):10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y, Yao T, Deng Z, Sohn J-W, Sun J, Huang Y, Kong X, Yu K-J, Wang R-T, Chen H, Guo H, Yan J, Cunningham KA, Chang Y, Liu T, Williams KW. TrpC5 mediates acute leptin and serotonin effects via pomc neurons. Cell Reports. 2017;18(3):583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115(4):951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, Chandarana K, Bell JD, Barsh GS, Smith AJH, Batterham RL, Ashford MLJ, Vanhaesebroeck B, Withers DJ. Dominant role of the p110β isoform of PI3K over p110α in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10(5):343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118(5):1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30(4):1560–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rameh LE, Rhee SG, Spokes K, Kazlauskas A, Cantley LC, Cantley LG. Phosphoinositide 3-kinase regulates phospholipase Cgamma-mediated calcium signaling. J Biol Chem. 1998;273(37):23750–23757. [DOI] [PubMed] [Google Scholar]
- 19.Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase C γ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17(2):414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae YS, Cantley LG, Chen C-S, Kim S-R, Kwon K-S, Rhee SG. Activation of phospholipase C-γ by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273(8):4465–4469. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Menchaca AA, Adney SK, Zhou L, Logothetis DE. Dual regulation of voltage-sensitive ion channels by PIP2. Front Pharmacol. 2012;3:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. Kisspeptin activation of TRPC4 channels in female GnRH neurons requires PIP2 depletion and cSrc kinase activation. Endocrinology. 2013;154(8):2772–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6(8):709–720. [DOI] [PubMed] [Google Scholar]
- 24.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23(29):9529–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu J, Bosch MA, Jamali K, Xue C, Kelly MJ, Rønnekleiv OK. Estrogen upregulates T-type calcium channels in the hypothalamus and pituitary. J Neurosci. 2006;26(43):11072–11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149(12):6113–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly MJ, Rønnekleiv OK. Minireview: neural signaling of estradiol in the hypothalamus. Mol Endocrinol. 2015;29(5):645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang C-Y, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449(7159):228–232. [DOI] [PubMed] [Google Scholar]
- 29.Paeger L, Pippow A, Hess S, Paehler M, Klein AC, Husch A, Pouzat C, Brüning JC, Kloppenburg P. Energy imbalance alters Ca2+ handling and excitability of POMC neurons. eLife. 2017;6:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosch MA, Tonsfeldt KJ, Rønnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17β-estradiol. Mol Cell Endocrinol. 2013;367(1-2):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Tonsfeldt KJ, Qiu J, Bosch MA, Kobayashi K, Steiner RA, Kelly MJ, Rønnekleiv OK. Molecular mechanisms that drive estradiol-dependent burst firing of Kiss1 neurons in the rostral periventricular preoptic area. Am J Physiol Endocrinol Metab. 2013;305(11):E1384–E1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP; NIH Mouse Metabolic Phenotyping Center Consortium . Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3(9-10):525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu L, Xu P, Cao X, Yang Y, Hinton AO Jr, Xia Y, Saito K, Yan X, Zou F, Ding H, Wang C, Yan C, Saha P, Khan SA, Zhao J, Fukuda M, Tong Q, Clegg DJ, Chan L, Xu Y. The ERα-PI3K cascade in proopiomelanocortin progenitor neurons regulates feeding and glucose balance in female mice. Endocrinology. 2015;156(12):4474–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosch MA, Hou J, Fang Y, Kelly MJ, Rønnekleiv OK. 17β-estradiol regulation of the mRNA expression of T-type calcium channel subunits: role of estrogen receptor α and estrogen receptor β. J Comp Neurol. 2009;512(3):347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. [DOI] [PubMed] [Google Scholar]
- 36.Lam BYH, Cimino I, Polex-Wolf J, Nicole Kohnke S, Rimmington D, Iyemere V, Heeley N, Cossetti C, Schulte R, Saraiva LR, Logan DW, Blouet C, O’Rahilly S, Coll AP, Yeo GSH. Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Mol Metab. 2017;6(5):383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padilla SL, Reef D, Zeltser LM. Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology. 2012;153(3):1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanz E, Quintana A, Deem JD, Steiner RA, Palmiter RD, McKnight GS. Fertility-regulating Kiss1 neurons arise from hypothalamic POMC-expressing progenitors. J Neurosci. 2015;35(14):5549–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nestor CC, Qiu J, Padilla SL, Zhang C, Bosch MA, Fan W, Aicher SA, Palmiter RD, Rønnekleiv OK, Kelly MJ. Optogenetic stimulation of arcuate nucleus Kiss1 neurons reveals a steroid-dependent glutamatergic input to POMC and AgRP neurons in male mice. Mol Endocrinol. 2016;30(6):630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 41.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farhang B, Pietruszewski L, Lutfy K, Wagner EJ. The role of the NOP receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology. 2010;59(3):190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borgquist A, Meza C, Wagner EJ. The role of AMP-activated protein kinase in the androgenic potentiation of cannabinoid-induced changes in energy homeostasis. Am J Physiol Endocrinol Metab. 2015;308(6):E482–E495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bon RS, Beech DJ. In pursuit of small molecule chemistry for calcium-permeable non-selective TRPC channels -- mirage or pot of gold? Br J Pharmacol. 2013;170(3):459–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57(4):427–450. [DOI] [PubMed] [Google Scholar]
- 46.Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006;17(9):365–371. [DOI] [PubMed] [Google Scholar]
- 47.Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4(2):123–132. [DOI] [PubMed] [Google Scholar]
- 48.Hartmann J, Karl RM, Alexander RP, Adelsberger H, Brill MS, Rühlmann C, Ansel A, Sakimura K, Baba Y, Kurosaki T, Misgeld T, Konnerth A. STIM1 controls neuronal Ca2+ signaling, mGluR1-dependent synaptic transmission, and cerebellar motor behavior. Neuron. 2014;82(3):635–644. [DOI] [PubMed] [Google Scholar]
- 49.Sun Y, Zhang H, Selvaraj S, Sukumaran P, Lei S, Birnbaumer L, Singh BB. Inhibition of L-Type Ca2+ channels by TRPC1-STIM1 complex is essential for the protection of dopaminergic neurons. J Neurosci. 2017;37(12):3364–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28(17):4423–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu Rev Pharmacol Toxicol. 2009;49(1):395–426. [DOI] [PubMed] [Google Scholar]
- 52.Salido GM, Jardín I, Rosado JA. The TRPC ion channels: association with Orai1 and STIM1 proteins and participation in capacitative and non-capacitative calcium entry. Adv Exp Med Biol. 2011;704:413–433. [DOI] [PubMed] [Google Scholar]
- 53.Gerasimenko JV, Gryshchenko O, Ferdek PE, Stapleton E, Hébert TOG, Bychkova S, Peng S, Begg M, Gerasimenko OV, Petersen OH. Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proc Natl Acad Sci USA. 2013;110(32):13186–13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav. 1975;6(4):329–349. [DOI] [PubMed] [Google Scholar]
- 55.Ahdieh HB, Wade GN. Effects of hysterectomy on sexual receptivity, food intake, running wheel activity, and hypothalamic estrogen and progestin receptors in rats. J Comp Physiol Psychol. 1982;96(6):886–892. [PubMed] [Google Scholar]
- 56.Geary N. Estradiol and the control of eating. Appetite. 1997;29(3):386. [PubMed] [Google Scholar]
- 57.Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav. 2004;82(1):35–41. [DOI] [PubMed] [Google Scholar]
- 58.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9(6):636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheridan JT, Gilmore RC, Watson MJ, Archer CB, Tarran R. 17β-Estradiol inhibits phosphorylation of stromal interaction molecule 1 (STIM1) protein: implication for store-operated calcium entry and chronic lung diseases. J Biol Chem. 2013;288(47):33509–33518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roselli CE, Resko JA. The distribution and regulation of aromatase activity in the central nervous system. Steroids. 1987;50(4-6):495–508. [DOI] [PubMed] [Google Scholar]
- 62.Chaudhari S, Wu P, Wang Y, Ding Y, Yuan J, Begg M, Ma R. High glucose and diabetes enhanced store-operated Ca2+ entry and increased expression of its signaling proteins in mesangial cells. Am J Physiol Renal Physiol. 2014;306(9):F1069–F1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kassan M, Ait-Aissa K, Radwan E, Mali V, Haddox S, Gabani M, Zhang W, Belmadani S, Irani K, Trebak M, Matrougui K. Essential role of smooth muscle STIM1 in hypertension and cardiovascular dysfunction. Arterioscler Thromb Vasc Biol. 2016;36(9):1900–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6(1):a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJA. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26(21):5649–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5(6):438–449. [DOI] [PubMed] [Google Scholar]
- 67.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho Y-R, Chuang J-C, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Brüning JC, Elmquist JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11(4):286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fujikawa T, Berglund ED, Patel VR, Ramadori G, Vianna CR, Vong L, Thorel F, Chera S, Herrera PL, Lowell BB, Elmquist JK, Baldi P, Coppari R. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab. 2013;18(3):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8(12):1376–1382. [DOI] [PubMed] [Google Scholar]
- 70.Plum L, Rother E, Münzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Könner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Brüning JC. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6(6):431–445. [DOI] [PubMed] [Google Scholar]
- 71.Klöckener T, Hess S, Belgardt BF, Paeger L, Verhagen LAW, Husch A, Sohn J-W, Hampel B, Dhillon H, Zigman JM, Lowell BB, Williams KW, Elmquist JK, Horvath TL, Kloppenburg P, Brüning JC. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci. 2011;14(7):911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sternson SM, Shepherd GMG, Friedman JM. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8(10):1356–1363. [DOI] [PubMed] [Google Scholar]
- 73.Conde K, Fabelo C, Krause WC, Propst R, Goethel J, Fischer D, Hur J, Meza C, Ingraham HA, Wagner EJ. Testosterone rapidly augments retrograde endocannabinoid signaling in proopiomelanocortin neurons to suppress glutamatergic input from steroidogenic factor-1 neurons via upregulation of diacylglycerol lipase-α. Neuroendocrinology. 2017;105(4):341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV; Women’s Health Initiative Investigators . Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–1187. [DOI] [PubMed] [Google Scholar]