Abstract
Purpose
To evaluate the relationship between the IOP-lowering effect of trabodenoson and the associated structural and functional changes in the trabecular meshwork (TM).
Methods
Six independent cohorts of young and aged mice were exposed to three different topical once-a-day formulations of trabodenoson and eyes were compared to those treated with placebo drops. IOP was measured daily just before drug administration using rebound tonometry. Outflow facility was measured in enucleated eyes. Flow patterns and morphology of conventional outflow tissues were monitored using tracer beads and standard histology, respectively. In parallel, three-dimensional human TM tissue constructs (3D-HTM) were grown and used in experiments to test effect of trabodenoson on the expression of collagen IV, fibronectin, matrix metalloproteinase (MMP)-2 and MMP-14 plus MMP-2 activity.
Results
Topical administration of trabodenoson significantly lowered IOP on every day tested, up to 7 days. After 2 days of treatment, outflow facility increased by 26% in aged mice and 30% overall (young and aged mice), which was significantly different from vehicle (P < 0.05). Outflow facility was 15% higher than controls after 7 days of treatment (P = 0.07). While gross morphology was not affected by treatment, the intensity of tracer bead distribution increased by day 7 (P = 0.05). Parallel experiments in 3D-HTM showed that trabodenoson treatment significantly increased MMP-2 activity and MMP-14 abundance, while decreasing fibronectin and collagen IV expression.
Conclusions
Trabodenoson alters ECM turnover by TM cells and increases conventional outflow facility, which accounts for its ability to lower IOP in young and aged mice.
Keywords: adenosine, conventional outflow, extracellular matrix, trabecular meshwork
Glaucoma is an optic neuropathy characterized by the progressive loss of retinal ganglion cells, causing irreversible visual field defects. In fact, vision loss due to glaucoma is the leading cause of irreversible blindness worldwide.1 Current treatments work exclusively to lower IOP, a primary risk factor for development of glaucoma. These topical medications lower IOP principally by either interfering with aqueous humor production or by increasing its drainage through the unconventional outflow pathway. Unfortunately, no available medications yet in the United States directly target the diseased tissue responsible for glaucomatous elevation in IOP, the conventional outflow pathway.
Mimetics of the naturally occurring purine nucleoside adenosine are under development to treat glaucoma because of their activity in the conventional outflow pathway. Adenosine exerts its biological effects by binding to four distinct, yet well-conserved G-protein coupled receptors (A1R, A2AR, A2BR, A3R) that are expressed ubiquitously throughout the body, including the eye.2–5 Pharmacologic studies using well-established agonists and antagonists to adenosine receptors have demonstrated previously effects on ocular blood flow as well as on IOP, depending on the receptor subtype activated.6–8 Avila et al.9 showed that only agonists to the A1R lower IOP, while agonists to the A2AR and A3R do the opposite, and elevate IOP. Adenosine A1 receptor activation by agonists such as N6-cyclohexyladenosine (CHA) lower IOP by increasing conventional outflow facility.2,10,11 Increased outflow facility is associated with elevated release of the protease matrix metalloproteinase 2 (MMP-2), which participates in the remodeling of the trabecular meshwork (TM) by digesting extracellular matrix proteins.2 Unfortunately, low corneal penetration by CHA in topical preparations has limited its clinical usefulness.12
In response, trabodenoson was synthesized as a novel adenosine mimetic with high specificity for the A1 receptor with good corneal penetration (Kim N, et al. IOVS 2009;50:ARVO E-Abstract 4061). Like CHA, trabodenoson effectively lowers IOP in several animal species, including humans.13 We designed and executed experiments to determine the mechanism of action responsible for the IOP-lowering effects of trabodenoson. Specifically, we examined the impact of trabodenoson treatment on the structure and function of the murine conventional outflow pathway, having a conventional outflow system similar to humans. Moreover, we examined the effects of trabodenoson on extracellular matrix homeostasis of human TM cells in a three-dimensional (3D) culture system.
Methods
Animals
Mice were handled in accordance with animal care and use guidelines of Duke University in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6 (C57) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA), bred, and housed in clear cages and kept in vivarium at 21°C with a 12-hour:12-hour light-dark cycle. Mice were treated and examined at ages 3 to 12 months.
IOP Measurements
Experiments were conducted to test effects of animal age, drug preparation/concentration and treatment duration on IOP. Two cohorts of C57 mice per experiment were tested, being age- and sex-matched. One cohort of sedated mice was administered 10 μL trabodenoson (either 3.0% or 6.0% [w/o preservative]) topically onto right eyes and 10 μL of placebo onto left eyes. Trabodenoson was prepared as an ophthalmic suspension of micronized powder in a stable, clinical-ready formulation (provided by Inotek13). Drops were given once/day for two or seven consecutive days. For comparisons, a second cohort was administered 10 μL of placebo topically to both eyes. In all cases, IOPs of both eyes were measured before eye drop administration using rebound tonometry (TonoLab) between 11 AM and 1 PM daily by the same person (GL).14 On four occasions during the course of the study IOP was measured by a second person (IN). Briefly, mice were anesthetized with ketamine (60 mg/kg) and xylazine (6 mg/kg) and IOP was measured immediately just as the mice stopped moving, while drifting into light sleep. Each IOP recorded was taken as the average of six independent measurements, giving a total of 36 measurements from the same eye. To prevent bias and maintain consistent measurements, we measured IOP in both eyes by taking turns between the right and left eyes (altered from right to left or from left to right eyes). For example, we would take two measurements for the right eye, two for the left eye; two for the left eye and then two measurements for the right eye, giving a total of six measurements for each IOP level.
Outflow Facility Measurements Using iPerfusion System
Due to the lipophilic nature of trabodenoson, a traditional perfusion experiment was not possible because we observed drug precipitation in infusion tubing/cannulation needles during the perfusion. To circumvent this issue, we conducted three independent experiments, testing either 2 or 7 days of topical trabodenoson treatment of mouse eyes. Mice were given 10 μL trabodenoson to one eye and placebo to the contralateral eye once/day for 2 or 7 days. At the end of the treatment period, animals were euthanized and eyes enucleated. The identity of treatment for each eye was masked before initiation of the perfusion protocol. Each freshly enucleated mouse eye was mounted on a stabilization platform in a perfusion chamber of the iPerfusion system using a small amount of cyanoacrylate glue (Loctite, Westlake, OH, USA).15,16 The perfusion chamber was filled (and eye submerged) with pre-warmed D-glucose in PBS (DBG, 5.5 mM) and temperature clamped at 35°C. A glass microneedle, back-filled with DBG was connected to the system and the microneedle was inserted into the anterior chamber of enucleated mouse eyes using a micromanipulator and stereo microscope.
Both eyes were perfused at a constant pressure of 9 mm Hg for 30 minutes to allow acclimatization, followed by nine sequential pressure steps of 4.5, 6, 7.5, 9, 10.5, 12, 15, 18 and 21 mm Hg. Flow during pressure steps was measured and recorded. Stable flow at each pressure was used for data analysis, which was performed as described previously.16 Briefly, a nonlinear flow-pressure model was used to account for the pressure dependence of outflow facility in mice, and the reference facility was analyzed at a reference pressure of 8 mm Hg (approximates the physiologic pressure drop across the conventional outflow pathway in living mice).
Microbead Tracer Infusion
Two pulled glass microneedles secured onto two micro-manipulators were filled with green fluorescent beads (100 nm, carboxylate-modified FluoSpheres; 1:750 dilutions; Molecular Probes, Eugene, OR, USA) in 1 × DBG (Dulbecco's PBS, pH 7.3 containing 5.5 mM D-glucose; Gibco Laboratories, Gaithersburg, MD, USA). The two needles were connected to two 1 mL syringes via tubing that was locked into a single syringe pump. Both eyes were perfused simultaneously at a rate of 0.167 μL/min for 1 hour, resulting in a total of 10 μL liquid containing bead suspension infused into each anterior chamber. After infusion, needles were withdrawn and mice were maintained for approximately 1 hour before euthanizing. Both eyes were collected and bisected at the equator. Lens, iris, and ciliary body were removed and anterior segments were flat-mounted with corneal epithelium side facing up. The fluorescence images were captured using a ×2.5 lens on a fluorescence microscope (Axioplan2; Carl Zeiss MicroImaging, Thornwood, NY, USA) using identical settings for both eyes. The automatic computation of the width and intensity of fluorescence in the TM region was done by a number of image processing operations as described previously.16
Histologic Analysis of Conventional Outflow Pathway
C57 mice were each given one 10 μL drop of 6% trabodenoson to their right eye and one 10 μL drop of placebo to their left eye, once/day for seven consecutive days. At the end of 7 days of treatment, both eyes were collected, immersed into 4% paraformaldehyde, and kept at 4°C overnight. The eyes were bisected and the posterior segments and lenses were removed. The anterior segments were cut into four quadrants and each quadrant was embedded into Epon (Electron Microscopy Sciences, ON, Canada). The blocks were cut into 0.5 μm semithin sections and stained with 1% methylene blue. Images of the conventional outflow pathway in cross-section were captured digitally using light microscopy.
Human TM (HTM) Cell Culture From Donor Eyes
Primary cultures of HTM cells were isolated from discarded (after keratoplasty) donor tissue rings and characterized by their expression of myocillin, αB-crystalline, and α-smooth muscle actin as described previously.17 HTM cells were plated in 75 cm2 cell culture flasks with 10% fetal bovine serum (FBS; Atlas Biologicals, Fort Collins, CO, USA) in improved minimal essential medium (IMEM; Corning Cellgro, Manassas, VA) with 0.1 mg/mL gentamicin. Fresh medium was supplied every 48 hours and the cells were maintained at 37°C in a humidified atmosphere with 5% carbon dioxide until 90% confluence. At this point the cells were trypsinized using 0.25% trypsin/0.5 mM EDTA (Gibco Laboratories) and subcultured. Cell cultures from four different human donor eyes (surgical discard anterior segment rings) were used during experiments. All studies were conducted using cells before the fifth passage.
Scaffold Preparation
SU-8 2010 (MicroChem Corp., Westborough, MA, USA) was used as free-standing biomimetic porous microstructures, serving as scaffolds onto which HTM cells were seeded. Scaffolds were fabricated using standard photolithographic techniques. The fabrication process begins with the spin-coating of a release (sacrificial) layer onto a silicon wafer followed by baking at 150°C. Next, SU-8 2010 was applied by spin-coating to a final thickness of 8 to 10 μm, followed by baking at 95°C. The resulting wafer was exposed to ultraviolet (UV) light through a mask containing the desired pattern, followed by baking at 95°C. The resulting wafer was developed using SU-8 developer (MicroChem Corp.) and the scaffolds containing the desired features were released from the substrate, washed with acetone and sterilized using 70% ethanol. Before cell seeding, scaffolds were coated with appropriate coating factors (1% gelatin coating and hyaluronic acid).
3D Culturing of HTM Cells on Scaffolds and Treatment
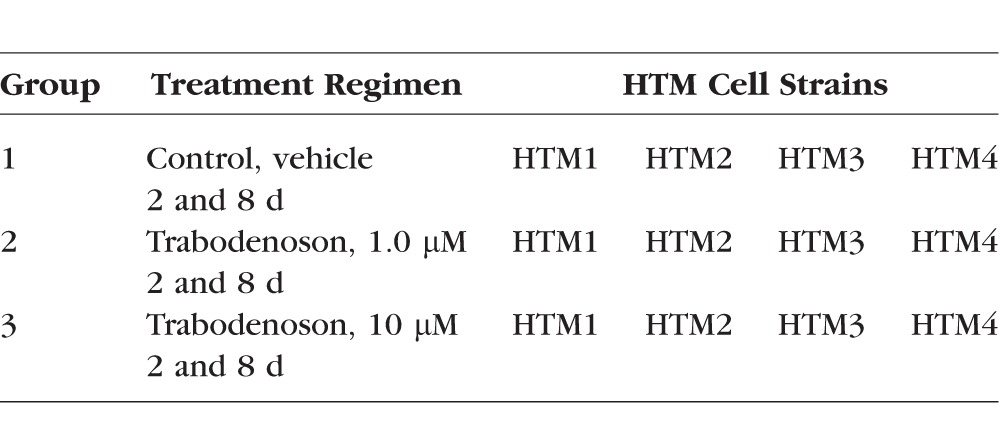
To create 3D-HTM constructs, the individual micro-fabricated SU-8 scaffolds were attached to aluminum rings (15 mm diameter) and placed in a 24-well plate followed by seeding with 40,000 to 50,000 HTM cells/scaffold. The cells were cultured in 10% FBS-IMEM for 14 days and the media was changed every other day. On day 14, the 3D-HTM constructs reached confluency and were exposed to a differentiation step (1% FBS-IMEM) for 6 days (with media changes every 3 days) before treatment was commenced. For each experimental group, the same number of cells was seeded onto each scaffold and all were treated identically before treatment. On day 6, the 3D-HTM constructs were subjected to the treatment groups as described in the Table. The 1 and 10 μM concentrations of trabodenoson selected for the in vitro studies were consistent with measured levels in the TM from unpublished PK studies performed by Inotek in multiple species following topical ocular administration.
Table.
Treatment Groups for 3D-HTM Constructs in Culture
On days 2 and 8 (after initiation of treatment with vehicle or trabodenoson), the supernatant and cell lysates from each group were collected and analyzed by Western blot, zymography, and electrochemiluminescence. Media was supplemented only for the day 8 samples; with media changes (1% FBS-IMEM supplemented with vehicle or trabodenoson) taking place on days 3 and 6 of the study.
Protein Extraction and Western Blot Analysis
After treatments, cellular proteins were extracted with ice-cold radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCL, pH 7.5, 150 mM sodium chloride, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 25 mM NaF, 0.1 mM sodium orthovanadate, 10 mM NaP4O7, 1 nM phenylmethyl sulfonyl fluoride) containing protease inhibitors (Complete Protease Inhibitor; Roche, Manheim, Germany) on ice. Total protein concentrations were quantified by a bicinchoninic acid assay (Thermo Fischer Scientific, Waltham, MA, USA). A total of 10 μg of proteins from each sample were separated by SDS polyacrylamide gel electrophoresis on a 4% to 12% gel in MOPS running buffer (Life Technologies, Carlsbad, CA, USA), transferred onto a polyvinylidene fluoride (PVDF) membrane and probed with the following primary antibodies: rabbit anti-MMP14, mouse anti-fibronectin, rabbit anti-collagen IV, and mouse anti-GAPDH (Abcam, Boston, MA, USA). HRP-conjugated goat anti-mouse or anti-rabbit secondary antibodies (Invitrogen, Carlsbad, CA, USA) were used. Bound antibody complexes were detected using FluorChem E (ProteinSimple, San Jose, CA, USA). Protein expression was analyzed by densitometry using ImageJ (National Institutes of Health [NIH], Bethesda, MD, USA), and normalized to the housekeeping protein, GAPDH. All experiments were performed in triplicate for each of four donor cells.
Gelatin Zymography
The culture supernatants were collected from vehicle, 1.0 μM trabodenoson and 10 μM trabodenoson treatments at the 2- and 8-day time points. Samples were mixed with tris-glycine SDS-PAGE sample buffer (1:1; Life Technologies, Carlsbad, CA, USA) without a reducing agent, and were subjected to electrophoretic analysis using tris-glycine-buffered SDS-polyacrylamide gel containing 0.1% gelatin (Thermo Fischer Scientific). The gel was developed according to manufacturer's instructions and stained with SimplyBlue Safestain (Life Technologies) before imaging. Active MMP-2 (Abcam) was loaded on the gel to help identify the MMP-2 bands for each sample. Densitometry was performed (using ImageJ) subtracting background and normalizing to vehicle-treated samples.
Secreted Protein Analysis by Electrochemiluminescence
A validated electrochemiluminescence assay for determining MMP-2 and TIMP-2 concentrations in culture supernatants collected from the vehicle, and 1.0 and 10 μM trabodenoson treatments at the 2- and 8-day time points was purchased from MSD (Meso Scale Discovery, Rockville, MD, USA). The assay was performed as per the manufacturer's instructions and plates were read using an MSD Sector 2400 Imager. Data were analyzed using MSD's Discovery Workbench software. Measured levels of MMP-2 and TIMP-2 from each sample (pg/mL) were normalized to the total protein contained within that sample. The total protein in each sample was quantified using the bicinchoninic acid assay (Thermo Fisher Scientific).
Immunocytochemistry and Confocal Microscopy
Samples treated with vehicle, 10 μM trabodenoson, and 1 and 3 μg/mL MMP-2, were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and blocked using 5% goat serum. To examine ECM proteins, samples were incubated with antibodies that specifically recognize collagen IV, fibronectin, and laminin (Abcam), as described previously.18 Samples were costained with 4′,6-diamidino-2-phenylendole (DAPI) to locate cell nuclei, followed by confocal imaging. Laser scanning confocal microscopy was performed using a Leica SP5 confocal microscope, and images were acquired at ×63 magnification using an oil-immersion objective. Confocal images were processed using Leica LasAF software, and all confocal images within a given experiment were imaged and captured using the same laser intensity and gain settings to compare intensities across samples (z-stacks of 20 optical sections for each image).
Statistical Analysis
Statistical significance between groups was assessed by a 2-tailed Student's t-test. A 2-tailed paired weighted t-test was applied to the log transformed outflow facility data to determine whether any observed difference in reference facility between contralateral eyes was statistically significant. For the analysis related to the 3D HTM cell culture, data are expressed as mean ± SD. The difference among vehicle-treated (controls), and 1 and 10 μM trabodenoson-treated 3D HTM samples was analyzed using 2-way ANOVA followed by Bonferroni post tests (GraphPad Prism 6.02; GraphPad Software, Inc., La Jolla, CA, USA). A value of P < 0.05 was considered statistically significant.
Results
Topical Trabodenoson Decreases IOP in Mice
We tested the IOP-lowering effects of two different concentrations of trabodenoson (3.0% and 6.0%) in young (3–4 months old) and aged (12 months old) mice over 7 days of QD treatment. Compared to vehicle-treated eyes, we observed an average IOP drop with 3.0% trabodenoson of 2.44 ± 0.22 mm Hg in young mice (Fig. 1). The IOP-lowering effect was immediate, resulting in an approximately 3.3 mm Hg drop one day after the first treatment and sustained over the treatment period; with the IOP significantly different than vehicle-treated eyes at every time point. Similar IOP-lowering efficacy and pattern was observed using the higher concentration of trabodenoson (6.0%), demonstrating an average IOP drop of 2.45 ± 0.38 mm Hg in young mice over 7 days (Supplementary Fig. S1). In this set of experiments, we also tested a preservative-free trabodenoson solution finding that the profile and average IOP decrease over 7 days was similar to solution containing preservative (2.45 ± 0.38 vs. 2.12 ± 0.27 mm Hg), significantly different from vehicle-treated eyes on days 3, 6, and 7 but not significantly different between the two preparations.
Figure 1.
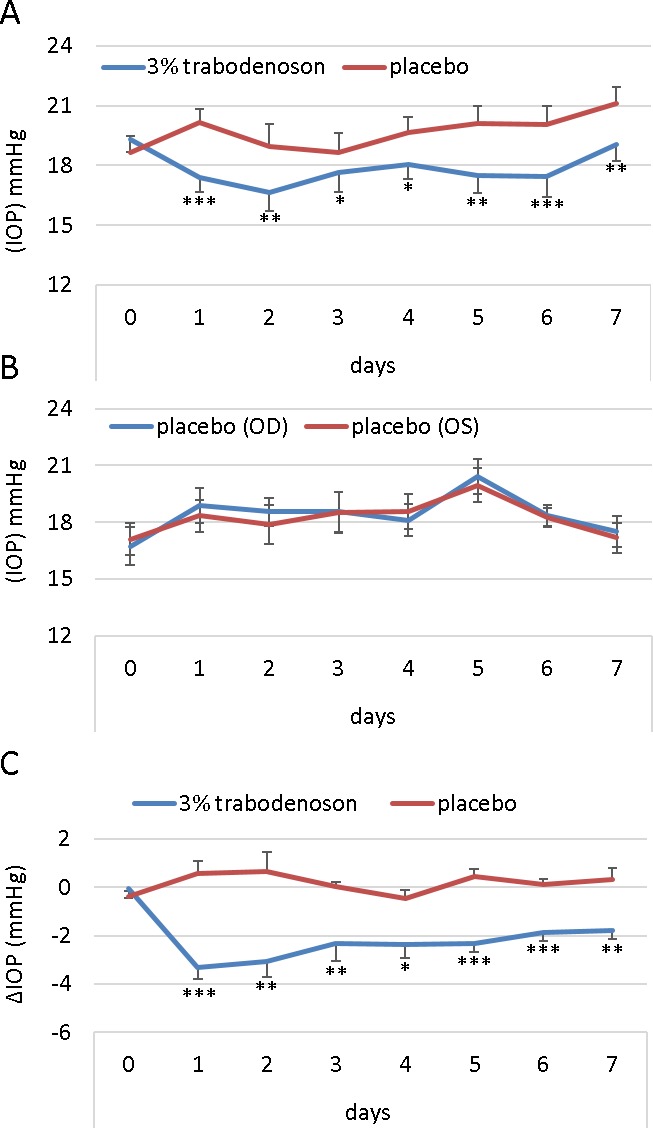
Trabodenoson decreases IOP in young mice. (A) IOP data (mean ± SEM, n = 10) from young C57 mice obtained using rebound tonometry. Right eyes were treated topically with 3% trabodenoson, while placebo was applied topically on the contralateral left eyes. (B) These data are compared to the second cohort, which was treated with placebo in both eyes (n = 8). All mice were treated once/day for seven consecutive days. (C) The difference in IOP (delta) between the trabodenoson and placebo groups (*P < 0.05; **P < 0.01; ***P < 0.001).
We next tested the effects of trabodenoson on a cohort of 12-month-old mice. We found that trabodenoson was equally effective at lowering IOP (2.31 ± 0.19 mm Hg) in aged mice treated with 6.0% trabodenoson (Fig. 2) as young mice. IOP in treated eyes of aged mice was significantly different than that in contralateral control eyes at every time tested except day 7.
Figure 2.
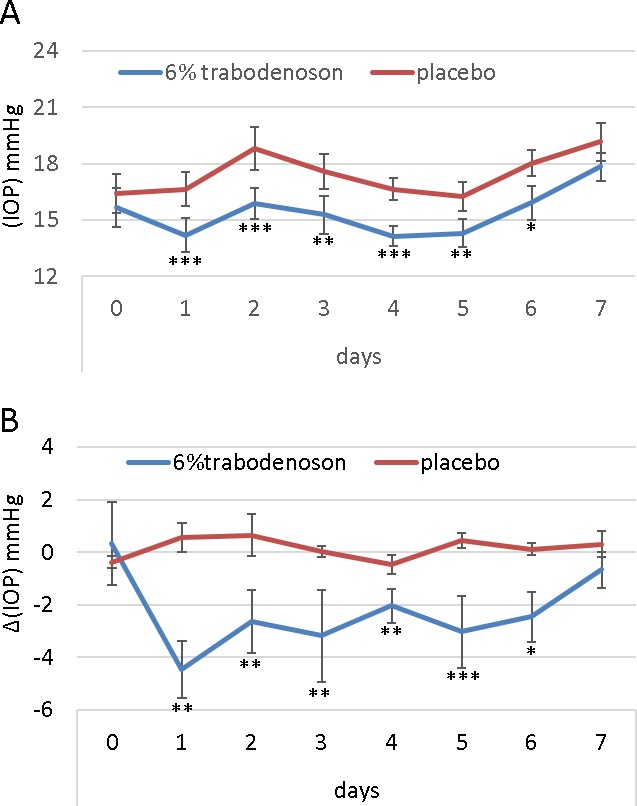
Trabodenoson decreases IOP in aged mice. Right eyes were treated with trabodenoson and the contralateral left eye of aged C57 mice with placebo. IOP was measured by rebound tonometry and mice received topical treatments once/day for seven consecutive days. (A) Raw IOP data (mean ± SEM, n = 18). (B) Change (delta) in IOP between trabodenoson and placebo groups (*P < 0.05; **P < 0.01; ***P < 0.001).
Trabodenoson Increases Conventional Outflow Facility
We next tested whether the IOP-lowering effects of trabodenoson were due to an increase in outflow facility via alterations in the conventional outflow pathway. In preliminary experiments, we were unable to maintain trabodenoson in solution during a standard perfusion protocol of enucleated mouse eyes. As an alternative, we decided to treat living mice (young and old), monitor IOP, euthanize mice at two time points, enucleate eyes, and then measure outflow facility of eye pairs. Based upon effect of trabodenoson in IOP studies, we chose to treat mice with 6% trabodenoson for 7 days and euthanize two cohorts at days 2 and 7 for perfusion studies. Outflow facility of treated eyes was measured using the iPerfusion system with DBG in the perfusion tubing (no drug). A representative flow trace at each of the pressure steps in the presence and absence of trabodenoson is shown in Figure 3A. These data were used to establish a flow-pressure relationship for each eye (Fig. 3C), from which outflow facility was estimated. As shown in Figure 3, outflow facility significantly improved after 2 days of treatment; increasing by 26% in old mice (3.25 ± 0.34 vs. 4.10 ± 0.52 nL/min/mm Hg, P = 0.048) and by 30% when all mice were grouped together (3.91 ± 0.39 vs. 5.09 ± 0.63 nL/min/mm Hg, P = 0.037). When young mice were analyzed separately, we observed an average 33% increase in facility; however, due to variability in data, this was not significantly different than control (4.49 ± 0.49 vs. 5.98 ± 0.85 nL/min/mm Hg, P = 0.12). Similarly, at 7 days of treatment we only saw a 15% increase in facility when all eyes were examined, which did not reach statistical significance (3.37 ± 0.23 vs. 3.85 ± 0.27 nL/min/mm Hg, P = 0.07). Interestingly, this downward trend matched the IOP profile over 7 days of treatment.
Figure 3.
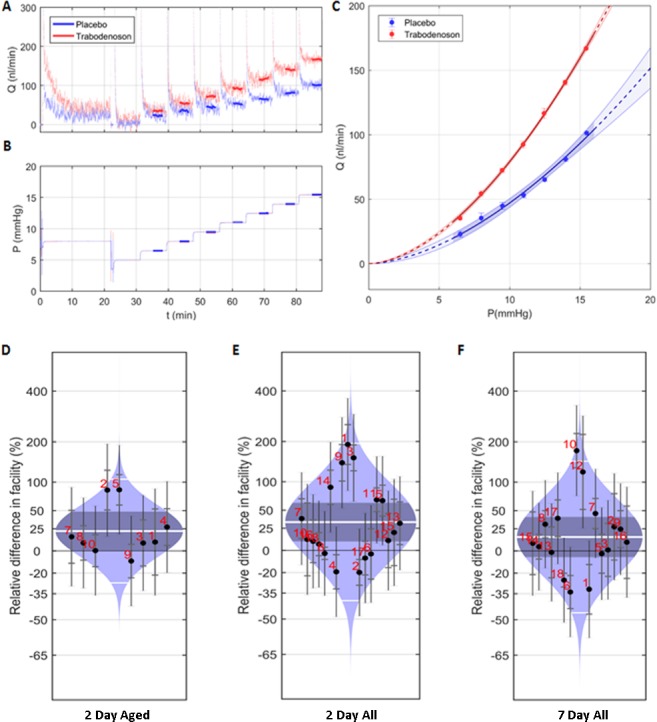
Trabodenoson increased outflow facility in mouse eyes. Living mice were treated with 6% trabodenoson daily topically in one eye and placebo in the contralateral eye for two or seven consecutive days, with IOP measured daily. Immediately before perfusion experiments were initiated, mice were euthanized, eyes enucleated, and outflow facility measurements performed using an iPerfusion system. (A) Representative flow trace, comparing measurements in paired eyes in the presence or absence of trabodenoson at each pressure step (B). These data are plotted in (C), showing a flow-pressure relationship for this single experiment. Outflow facility was derived from each flow-pressure relationship and combined to determine the magnitude of trabodenoson effects. Outflow facility measurements for each experiment (indicated by number) and mean ± SD outflow facility (white line and gray shaded area, respectively) are displayed in “cello plots” as percentage change in trabodenoson treated compared to control for 2-day treatment of aged mouse eyes (D), 2-day treatment of all eyes (E), and 7-day treatment of all eyes (F). The data showed mean ± SEM, n = 9 (2 days aged), n = 17 (2 days young + aged) and n = 15 (7 days).
Trabodenoson Increases Fluorescent Tracer Deposition in Outflow Tissues
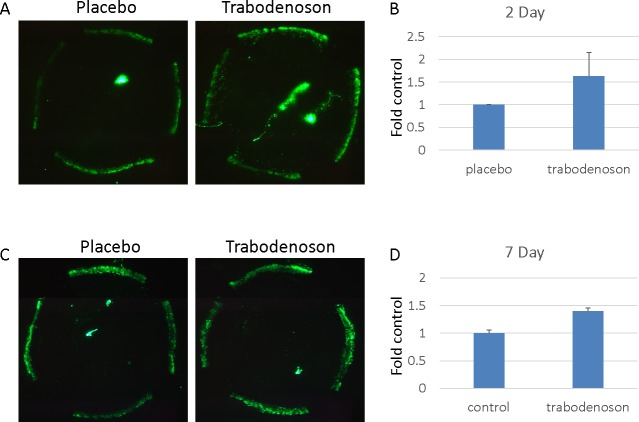
To test whether trabodenoson effects on outflow in living mouse eyes involves modifications in flow patterns in outflow tissues, fluorescent tracer was perfused simultaneously under constant flow conditions into paired eyes after 2 or 7 consecutive days of daily trabodenoson treatment. Anterior segments were flat-mounted, imaged, and fluorescence quantified. Compared to placebo control eyes perfused in parallel, trabodenoson-treated eyes at 2 days was on average approximately 60% more intense; however, due to variability this was not significant (Fig. 4, 1.6 ± 0.5, P = 0.24). The results in eyes treated with trabodenoson for 7 days were more consistent, increasing fluorescence intensity by 40% (1.4 ± 0.05, P = 0.05). Trabodenoson treatment resulted in no significant change in tracer decoration of outflow tissue area (2.8% decrease at 2 days and 18.6% increase at 7 days, P > 0.2, data not shown).
Figure 4.
Trabodenoson effects on tracer deposition in conventional outflow tissues. The experiments were conducted in two groups of young mice. One group of mice was treated daily with trabodenoson topically on one eye and placebo on the contralateral eye for two consecutive days. The second group was treated topically daily for 7 days. At end of the study, the mice were anesthetized and a fixed amount of fluorescent beads diluted in DBG were perfused simultaneously into paired eyes at a constant flow rate. Fluorescence intensity of tracer deposited in outflow tissues was visualized and captured on flat-mounted anterior segments using an epifluorescence microscope. (A, C) Representative flat-mounts from eyes treated for 2 and 7 days, respectively. (B, D) Combined data (mean ± SE). n = 8 for 2-day treatment group (B) and n = 6 for 7-day treatment group (D).
Trabodenoson Effects on Outflow Tissue Morphology
To visualize whether lowering IOP effects of trabodenoson is through gross modification of outflow tissue morphology, young C57 mice were treated with either 6% trabodenoson or placebo once/day for seven consecutive days. The eyes were collected, fixed, and anterior segments were Epon-embedded for histology. As shown in Figure 5, gross outflow tissue morphology did not show any obvious modifications by trabodenoson, being indistinguishable from vehicle-treated eyes.
Figure 5.
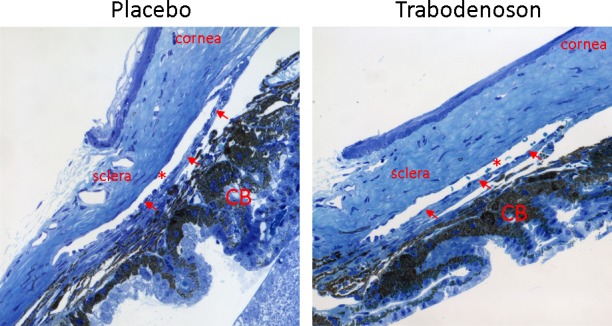
Trabodenoson effects on conventional outflow tissue morphology. Mouse eyes were treated with 6% trabodenoson topically in one eye and placebo in the contralateral eye once/day for seven consecutive days. At end of the study, all eyes were collected and fixed in 4% paraformaldehyde (PFA). The anterior segments were embedded in Epon and blocks were cut into 0.5 μm semithin sections and stained with 1% methylene blue. The images were captured digitally using light microscopy. Shown are representative images of sections from each quadrant of three pairs of mouse eyes that were examined. *Schlemm's canal; arrows show the trabecular meshwork (TM). CB, ciliary body.
Trabodenoson Effects on MMP-2 and TIMP-2 Abundance and Activity
Next, we examined the cellular changes responsible for alterations in IOP, outflow facility, and flow patterns. Cultures of human TM cells were grown on scaffolds and supernatant and cell lysates were collected after 2 or 8 days of treatment. Using an immunoassay, total MMP-2 and TIMP-2 levels in supernatant were quantified from four different TM cell strains and found not to be different in trabodenoson treatment groups versus vehicle control (Fig. 6A). In contrast, when supernatants were analyzed by zymography, a dose-related increase in MMP-2 activity compared to vehicle-treated controls, at both time points, was observed (Day 2, 1 ± 0.04 for control, 1.78 ± 0.41 for 1 μM trabodenoson and 2.16 ± 0.87 for 10 μM trabodenoson; Day 8, 1 ± 0.04 for control, 1.61 ± 0.58 for 1 μM trabodenoson, and 1.99 ± 0.47 for 10 μM trabodenoson; n = 12 for all conditions; Figs. 6B, 6C).
Figure 6.

Trabodenoson increases MMP-2 activity from TM cells. Three-dimensional human TM tissue constructs (3D-HTM) were treated with and 10 μM trabodenoson and the culture supernatants were analyzed at the 2- and 8-day time points. Secreted MMP-2 and TIMP-2 concentrations were measured using an electrochemiluminescence assay (A) and the activity of secreted MMP-2 from TM cells isolated from four donor on 3D-HTM constructs (TM1, TM2, TM3, TM4) was analyzed using gelatin zymography ([B, C]; n = 12, *P < 0.05, **P < 0.01).
Trabodenoson Effects on MMP-14, Fibronectin, and Collagen IV
The impact to structural components due to trabodenoson-mediated changes in MMP activity was assessed. Representative confocal micrographs of TM cells on 3D constructs following 48 hours of treatment with vehicle, trabodenoson (10 μM), and exogenous active MMP-2 (1 or 3 μg/mL) as positive controls are shown in Figure 7. Results show an obvious reduction in staining of fibronectin and collagen IV by trabodenoson treatment, comparable to exogenous MMP-2 in reducing these ECM proteins (Fig. 7A).
Figure 7.
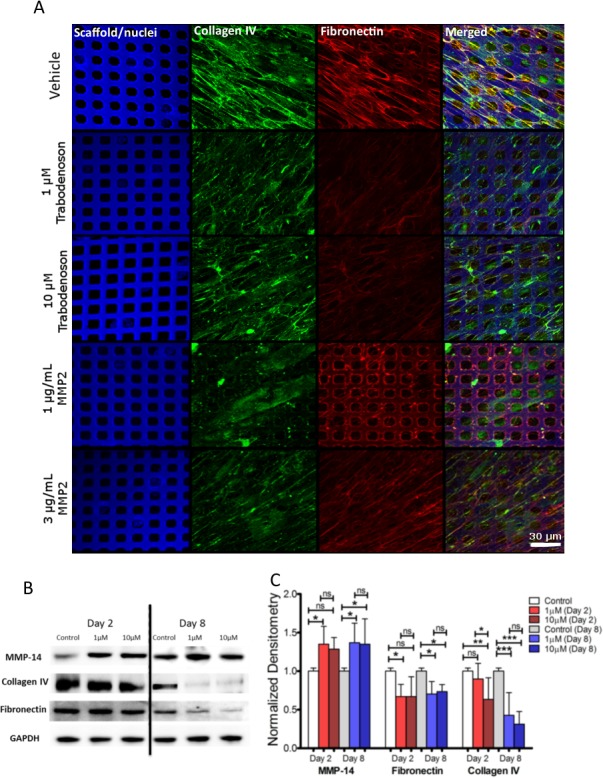
The effects of trabodenoson on MMP-14 and ECM proteins in TM cells. Three-dimensional human TM tissue constructs (3D-HTM) were treated with 1 and 10 μM trabodenoson and tissue constructs and their cell lysates were analyzed at the 2- and 8-day time points. After 2 days, treated 3D-HTM constructs were fixed and incubated with antibodies against collagen IV, and fibronectin and, in parallel, compared to 1 and 3 μg/mL MMP-2 treatments (A). Expression of active MMP-14, collagen IV, and fibronectin for 3D-HTM after 2- and 8-days of treatment was measured using Western blot. (B) Representative blots. (C) Densitometry analysis of all Western blot data (n = 12, *P < 0.05, **P < 0.01, ***P < 0.001).
The protein levels of fibronectin, collagen IV, and MMP-14 (an activator of MMP-2)19 were examined and quantified using Western blot analysis followed by densitometry (Figs. 7B, 7C). Significant increases in the levels of MMP-14 were detected in tissue lysates from cells treated with 1 μM (Day 2 and 8; n = 12, P ≤ 0.05) and 10 μM (Day 8; n = 12, P ≤ 0.05) trabodenoson compared to vehicle-treated controls. In the same samples, fibronectin levels decreased compared to vehicle-treated samples (n = 12, P ≤ 0.05) after 2 and 8 days of treatment with 1 and 10 μM trabodenoson. Similarly, collagen type IV also was affected by the treatments; 2-day treatment with 10 μM trabodenoson decreased collagen IV expression compared to vehicle-treated samples (n = 12, P ≤ 0.01). Treatment with trabodenoson for 8 days at 1 and 10 μM concentrations was effective at lowering collagen IV protein expression compared to vehicle-treated samples (n = 12, P ≤ 0.001 for both concentrations).
Discussion
In the present study, we investigated the IOP-lowering effects of trabodenoson, a highly selective adenosine A1 receptor agonist, on conventional outflow structure and function after topical delivery in living mice. Our results showed that trabodenoson lowered IOP in young and aged mice by increasing conventional outflow facility, without significant modifications of the gross outflow tissue structure or flow patterns. Instead, trabodenoson appears to increase outflow by impacting extracellular matrix (ECM) turnover related to fibronectin and collagen IV degradation due to increased MMP-2 activity via MMP-14 activation.
Elevated IOP is a major risk factor for glaucoma that is the result of aberrant homeostatic processes in the conventional pathway. Increases in IOP for a prolonged period of time in normal eyes lead to stretching of the extracellular matrix and cells within the conventional tract, resulting in a myriad of cellular activities.20 Typical compensatory responses to mechanical stretching initiate increased secretions of a variety of endogenous mediators, including adenosine and proteins involved in ECM turnover, such as MMP-2.21,22 Such activities work to remodel the conventional outflow pathway, modulating outflow resistance, and, thus, IOP. Our data here confirmed the role of adenosine A1 receptor signaling in the regulation of outflow facility. We showed for the first time to our knowledge the activity of trabodenoson in the conventional outflow pathway of mice, which have a lamellar TM and a true Schlemm's canal, like primates.
Similar to CHA, the IOP-lowering effects of trabodenoson appears to be related to increased conventional outflow facility via a MMP-2-dependent mechanism.10 Unlike CHA, our results showed that MMP-2 expression in the media did not change with treatment, but that its enzymatic activity increased in a dose-related manner. Coincident with such changes were elevated MMP-14 levels, an enzyme that activates pro-MMP-2 in the presence of TIMP-2,23 and decreases collagen IV and the collagen-binding protein, fibronectin. Bradley et al.24 first demonstrated that stretching of the TM leads to increases in enzymes to digest away ECM protein, decrease outflow resistance and lower IOP. Our results build on their initial observations and highlight the role of adenosine, and specifically A1 receptor downstream signaling in contributing to those enzymatic changes. These data and a recent study by Filla et al.25 emphasized the interrelationship between collagen IV and fibronectin deposition/degradation and importance in ECM turnover and outflow resistance generation. Such ECM changes were accompanied by subtle changes in flow patterns as measured by tracer bead deposition.
Due to the lipophilic nature of trabodenoson, early attempts to perfuse enucleated eyes failed because of precipitation of drug in the perfusion tubing/needle. This was despite numerous attempts to maintain trabodenoson in solution during dilution steps using different diluents that include DMSO, ethanol and β-hydroxylprople cyclodextrin. As an alternative, we treated living animals for up to 7 days with topical preparations, sacrificing cohorts of mice, enucleating eyes, and perfusing without drug in the perfusion media for facility measurements. Thus, any changes in outflow facility are the result of alterations in the conventional outflow tissues due to drug treatment in vivo. Using this method, we observed changes in outflow facility at 2 days of treatment, but not at 7 days. These data were consistent with IOP data that showed the greatest IOP decrease at 2 days.
Trabodenoson was equipotent in young and aged mice, with average IOP lowering of 2.45 versus 2.31 mm Hg over the 7 days of treatment. On day 2 of trabodenoson treatment, the mean IOP lowering for young mice was 2.3 mm Hg, compared to 2.9 mm Hg for old mice. Consistent with these data, the drug effect on outflow facility was similar. However, we were able to resolve significant changes in outflow facility only at 2 days in old mice but not young mice, due to more variability observed in the young mouse cohort.
Maximum IOP lowering effects of trabodenoson were noticed at days 1 and 2 in all groups studied, with IOP lowering less efficacious at later time points. While IOP was not statistically different between days 1 and 7 in mice treated with 3% trabodenoson, we observed a statistically significant difference in IOP between days 1 and 7 in mice treated daily with 6% trabodenoson. These data matched outflow facility measurements whereby we observed 33% increase in outflow at day 2 and only 15% at day 7. A possible explanation is adenosine A1 receptor desensitization/downregulation after repeated dosing. This hypothesis must be explored further in future studies by examining receptor densities in TM cells after different treatment durations.
In conclusion, trabodenoson is an IOP-lowering compound that targets and remodels ECM produced by conventional outflow cells, thereby increasing outflow facility. Future studies directed at examining the impact of trabodenoson on IOP in mouse models having increased ECM accumulation due to CTGF overexpression26 or prolonged corticosteroid treatment would be interesting.27
Supplementary Material
Acknowledgments
The authors thank Joseph Sherwood, PhD, for assistance in analyzing outflow facility data using iPerfusion system.
Supported by a P30 grant (EY005722) and an unrestricted departmental grant from Research to Prevent Blindness Foundation, which supports core services at Duke Eye Center. Glauconix, Inc., holds a patent for 3D-HTM, and Inotek Pharmaceuticals holds a patent for trabodenoson.
Disclosure: G. Li, None; K.Y. Torrejon, Glauconix (E); A.M. Unser, Glauconix (E); F. Ahmed, Glauconix (E); I.D. Navarro, None; R.A. Baumgartner, Inotek (E); D.S. Albers, Inotek (E); W.D. Stamer, Aerie (F, C, S), Allergan (F), Glauconix (C), Inotek (F), Ironwood (F)
References
- 1. World Health Organization (WHO). Prevention of Blindness and Visual Impairment. Available at: http://www.who.int/blindness/causes/en/. Accessed July 19, 2017.
- 2. Crosson CE, Sloan CF, Yates PW. . Modulation of conventional outflow facility by the adenosine A1 agonist N6-cyclohexyladenosine. Invest Ophthalmol Vis Sci. 2005; 46: 3795– 3799. [DOI] [PubMed] [Google Scholar]
- 3. Jacobson KA, Kim SK, Costanzi S, Gao ZG. . Purine receptors: GPCR structure and agonist design. Mol Interv. 2004; 4: 337– 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. . International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001; 53: 527– 552. [PMC free article] [PubMed] [Google Scholar]
- 5. Schlotzer-Schrehardt U, Zenkel M, Decking U,et al. . Selective upregulation of the A3 adenosine receptor in eyes with pseudoexfoliation syndrome and glaucoma. Invest Ophthalmol Vis Sci. 2005; 46: 2023– 2034. [DOI] [PubMed] [Google Scholar]
- 6. Jacobson KA, Civan MM. . Ocular purine receptors as drug targets in the eye. J Ocul Pharmacol Ther. 2016; 32: 534– 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmidl D, Weigert G, Dorner GT,et al. . Role of adenosine in the control of choroidal blood flow during changes in ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2011; 52: 6035– 6039. [DOI] [PubMed] [Google Scholar]
- 8. Polska E, Ehrlich P, Luksch A, Fuchsjager-Mayrl G, Schmetterer L. . Effects of adenosine on intraocular pressure, optic nerve head blood flow, and choroidal blood flow in healthy humans. Invest Ophthalmol Vis Sci. 2003; 44: 3110– 3114. [DOI] [PubMed] [Google Scholar]
- 9. Avila MY, Stone RA, Civan MM. . A(1)-, A(2A)- and A(3)-subtype adenosine receptors modulate intraocular pressure in the mouse. Br J Pharmacol. 2001; 134: 241– 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shearer TW, Crosson CE. . Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002; 43: 3016– 3020. [PubMed] [Google Scholar]
- 11. Dismuke WM, Overby DR, Civan MM, Stamer WD. . The value of mouse models for glaucoma drug discovery. J Ocul Pharmacol Ther. 2016; 32: 486– 487. [DOI] [PubMed] [Google Scholar]
- 12. Crosson CE. . Intraocular pressure responses to the adenosine agonist cyclohexyladenosine: evidence for a dual mechanism of action. Invest Ophthalmol Vis Sci. 2001; 42: 1837– 1840. [PubMed] [Google Scholar]
- 13. Myers JS, Sall KN, DuBiner H,et al. . A dose-escalation study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of 2 and 4 weeks of twice-daily ocular trabodenoson in adults with ocular hypertension or primary open-angle glaucoma. J Ocul Pharmacol Ther. 2016; 32: 555– 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li G, Farsiu S, Qiu J,et al. . Disease progression in iridocorneal angle tissues of BMP2-induced ocular hypertensive mice with optical coherence tomography. Mol Vis. 2014; 20: 1695– 1709. [PMC free article] [PubMed] [Google Scholar]
- 15. Sherwood JM, Reina-Torres E, Bertrand JA, Rowe B, Overby DR. . Measurement of Outflow Facility Using iPerfusion. PLoS One. 2016; 11: e0150694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li G, Mukherjee D, Navarro I,et al. . Visualization of conventional outflow tissue responses to netarsudil in living mouse eyes. Eur J Pharmacol. 2016; 787: 20– 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torrejon KY, Papke EL, Halman JR,et al. . TGFbeta2-induced outflow alterations in a bioengineered trabecular meshwork are offset by a rho-associated kinase inhibitor. Sci Rep. 2016; 6: 38319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torrejon KY, Papke EL, Halman JR,et al. . Bioengineered glaucomatous 3D human trabecular meshwork as an in vitro disease model. Biotechnol Bioeng. 2016; 113: 1357– 1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Visse R, Nagase H. . Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003; 92: 827– 839. [DOI] [PubMed] [Google Scholar]
- 20. Acott TS, Kelley MJ, Keller KE,et al. . Intraocular pressure homeostasis: maintaining balance in a high-pressure environment. J Ocul Pharmacol Ther. 2014; 30: 94– 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. WuDunn D. . Mechanobiology of trabecular meshwork cells. Exp Eye Res. 2009; 88: 718– 723. [DOI] [PubMed] [Google Scholar]
- 22. Stamer WD, Acott TS. . Current understanding of conventional outflow dysfunction in glaucoma. Curr Opin Ophthalmol. 2012; 23: 135– 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Butler GS, Butler MJ, Atkinson SJ,et al. . The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998; 273: 871– 880. [DOI] [PubMed] [Google Scholar]
- 24. Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. . Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001; 42: 1505– 1513. [PubMed] [Google Scholar]
- 25. Filla MS, Dimeo KD, Tong T, Peters DM. . Disruption of fibronectin matrix affects type IV collagen, fibrillin and laminin deposition into extracellular matrix of human trabecular meshwork (HTM) cells. Exp Eye Res. 2017; 165: 7– 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Junglas B, Kuespert S, Seleem AA,et al. . Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol. 2012; 180: 2386– 2403. [DOI] [PubMed] [Google Scholar]
- 27. Overby DR, Bertrand J, Tektas OY,et al. . Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Invest Ophthalmol Vis Sci. 2014; 55: 4922– 4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.