Abstract
Purpose
Our recent studies raise the possibility of using sodium hydroxymethylglycinate (SMG), for pharmacologic therapeutic tissue cross-linking (TXL) of the cornea. The present study was performed to evaluate the antimicrobial effects of SMG for potential use in treating infectious keratitis.
Methods
In initial (group 1) experiments, methicillin-sensitive Staphylococcus aureus (MSSA), methicillin-resistant Staphylococcus aureus (MRSA), and Pseudomonas aeruginosa (PA) were treated with SMG (10–40 mM) for 10 to 120 minutes. In group 2 experiments, MRSA, PA, Candida albicans (CA), and vancomycin-resistant Enterococcus (VRE) were treated with SMG (20–200 mM) for 30 minutes. In group 2 experiments, BSA and neutralizing buffer were added to provide a proteinaceous medium, and to ensure precise control of SMG exposure times, respectively. SMG effectiveness was quantitated based on pathogen growth following a 24- to 48-hour incubation period.
Results
In group 1 experiments, as expected, time- and concentration-dependent bactericidal effects were noted using MSSA. In addition, the effect of SMG (40 mM) was greatest against MSSA (99.3%), MRSA (96.0%), and PA (97.4%) following a 2-hour exposure with lesser effects following 30- and 10-minute exposures. In group 2 experiments, concentration-dependent bactericidal effects were confirmed for MRSA (91%), PA (99%), and VRE (55%) for 200-mM SMG with 30-minute treatment. SMG was not as effective against CA, with a maximum kill rate of 37% at 80 mM SMG.
Conclusions
SMG solution exhibits a dose-dependent bactericidal effect on MSSA, MRSA, and PA, with milder effects on VRE and CA. These studies raise the possibility of using SMG TXL for the treatment of infectious keratitis.
Keywords: sodium hydroxymethylglycinate, tissue cross-linking/bonding, infectious keratitis, methicillin-resistant Staphylococcus aureus (MRSA)
As we continue to move forward in an age of emerging antimicrobial resistance, finding new approaches to treating soft tissue infections is critical. There is an urgent need to develop new antimicrobial agents, as the development of multidrug-resistant organisms increases. The World Health Organization (WHO) has recently published a list of antibiotic-resistant pathogens that have been prioritized as posing great risk to human health.1 Among those 12 bacteria listed (tuberculosis not included) include Staphylococcus aureus, methicillin-resistant, vancomycin-intermediate and resistant (MRSA); Pseudomonas aeruginosa (PA), carbapenem-resistant; and Enterococcus faecium, vancomycin-resistant (VRE). The list was intended to spur governments to incentivize basic science and advance research and development by both public and private sectors to invest in new antibiotic discovery.
Riboflavin/UVA corneal collagen cross-linking (CXL) is now approved by the Food and Drug Administration and a new effective treatment for keratoconus. The successful use of CXL in the treatment of corneal thinning diseases (i.e., keratoconus, post-LASIK keratectasias) has proven that induced tissue cross-linking can have beneficial therapeutic effects. The use of the CXL technique has now been extended to the treatment of infectious keratitis, because the riboflavin photochemical treatment is known to both kill bacteria and stabilize the extracellular matrix, where induced cross-linking imparts an increased resistance to enzymatic degradation, effectively inhibiting a major pathway used by invading (i.e., exogenous) microorganisms (i.e., bacteria, fungus, and so forth), and host leukocytes (i.e., endogenous) to degrade tissue, proteolytic (also known as enzymatic) collagen digestion. CXL is being used to treat the most difficult corneal infections and melts, where there exists an unmet clinical need and cross-linking therapy offers hope for a better treatment paradigm, particularly for difficult-to-treat infections and infections due to antibiotic-resistant organisms.
Although CXL is actively being used for these purposes, limitations regarding the photochemical technique remain, and include the need for epithelial removal and the intentional application of UVA light into a person's eye, exposing the lens and retina to potentially harmful irradiation. Several advantages could be gained by using a topical therapeutic tissue cross-linking (TXL) approach, including the omission of UVA light and epithelial removal, the ability to modulate the degree of cross-linking (by repeated treatments over time), and a homogeneous cross-linking effect (i.e., zone of effect should be guided by reactant diffusivity), and the possibility of self-administration. Furthermore, numerous delivery methods are available for corneal TXL, including simple viscous eye drops, hydrogel contact lenses (CLs), and corneal reservoirs.
Sodium hydroxymethylglycinate (SMG) is a small water-soluble compound (molecular weight = 127) and is one of a group of formaldehyde releasers (FARs) recently introduced for therapeutic TXL of the cornea. FARs are used as preservatives in a wide array of popular cosmetic and personal care products, such as skin care products, body wash, fingernail polish, and shampoo, where they are effectively used to prevent product spoilage from bacterial and fungal overgrowth.2 Although they have known antimicrobial effects, their specific use as a therapeutic agent in the treatment of infectious tissue processes, such as keratitis, has not been considered. Our recent studies raise the possibility of using SMG, an FAR, for pharmacologic TXL.3 The present study was performed to evaluate the in vitro antimicrobial effects of SMG, a candidate FAR compound for use as a TXL agent.4–12
Methods and Materials
Chemicals
SMG and sodium bicarbonate were obtained from Tyger Chemicals Scientific, Inc. (Ewing, NJ, USA) and Sigma-Aldrich Corp. (St. Louis, MO, USA), respectively. Fifty percent SMG solution (Suttocide) was purchased from Ashland (Columbus, OH, USA). BBL Trypticase Soy Broth (TSB), BBL Trypticase Soy Agar, Difco Sabouraud Dextrose Broth, and Difco D/E Neutralizing Broth, were purchased from Fisher Scientific (Waltham, MA, USA). Adult BSA was purchased from Sigma-Aldrich Corp. Balanced salt solution (BSS), BSS Plus (Alcon Laboratory, Inc., Fort Worth, TX, USA), was used to prepare all chemical solutions and buffers on the day within 60 minutes of experiment/application (details follow below).
Bacteria Strains
The following microorganisms were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA): Staphylococcus aureus (ATCC 6538), MRSA (ATCC 33592), PA (ATCC 27853), and Candida albicans (CA) (ATTC 11651). VRE was kindly provided by Dr. Shanta Modak of the Department of Surgery, Columbia University.
Group 1 Experiments (Using Methicillin-Sensitive S. aureus [MSSA], MRSA, and PA)
Antimicrobial Broth Experiments
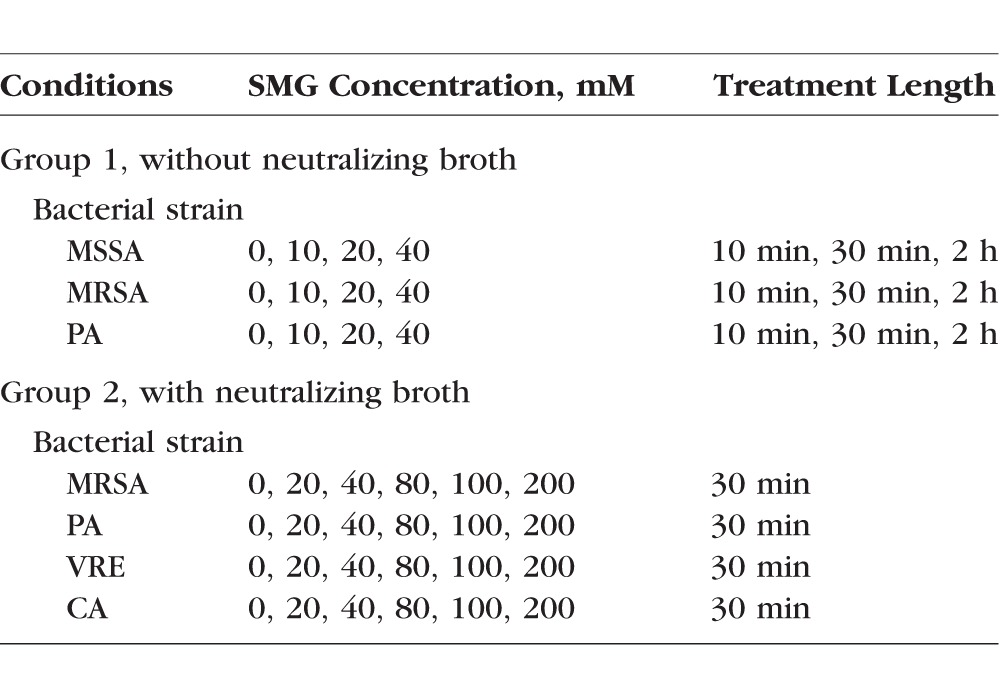
Treatment conditions (SMG concentration and incubation period) are summarized in Table 1. MSSA and MRSA were cultured in BBL TSB. The same initial culture and dilution was used for treatment and control plates for each experiment. From exponential growth phase, these bacteria were diluted to 3.3 × 103 colony-forming units (CFUs) per milliliter, then 50 μL of this culture was added to wells in a 96-well plate (6.86-mm diameter wells). Subsequently, 50 μL BSS (pH 7.5) with SMG was added to each well to a final SMG concentration of 10 mM, 20 mM, or 40 mM, and incubated for 10 minutes, 30 minutes, or 2 hours. After incubation, the samples were diluted in a 96-well plate with 200 μL TSB, and the 300-μL of diluted sample was added to and spread uniformly on a BBL Trypticase Soy Agar plate and incubated for 24 to 48 hours at 37°C. CFU counts were recorded when colonies were grown sufficiently to be counted by the naked eye. The bottom of each plate was divided into a minimum of four sections using a marking pen, and each section was counted separately. This was carried out to ensure accurate colony counting.
Table 1.
Summary of Experimental Conditions
Statistical Analysis
We have used both linear regression model and median regression model to evaluate the significance of the observed differences in colony counts between the control and treated groups. When there are only two groups to compare, we have used the Wilcoxon rank sum test. Kill rate for each plate was calculated using the number of colonies grown on a plate and the mean value of colonies grown on control plates for that experiment. Significance of statistical tests was based on an alpha value of 0.05 (P ≤ 0.05). Colony counts are reported as a mean bactericidal effect followed by the SD.
Group 2 Experiments (Using MRSA, PA, CA, and VRE, and Including Proteinaceous Media and Neutralizing Buffer)
Antimicrobial Broth Experiments
Treatment conditions (SMG concentration, incubation period, and use of neutralizing buffer) are summarized in Table 1. Pathogens were grown from a slant in either a TSB with 10% albumin for MRSA, PA, and VRE, or a Difco Sabouraud Dextrose Broth with 10% BSA for CA. The optical density of each pathogen was determined using a spectrophotometer set at 600 nm and zeroed using respective broths containing 10% BSA protein. From the exponential growth phase, 50 μL of 5 × 103 to 105 CFUs per milliliter were added to a 96-well flat bottom assay plate. Each well was treated with a final concentration of 20 mM, 40 mM, 80 mM, 100 mM, or 200 mM of SMG by pipetting 50 μL SMG dissolved in a BSS into each well. Control wells were treated with 50 μL BSS. Following addition of SMG, the lid was placed on the assay plate, and it was gently rocked back and forth five times to mix the SMG and pathogen. After a 30-minute treatment period, 200 μL Difco D/E Neutralizing Broth (39 mg/mL) was pipetted into each well to neutralize the SMG, and mixed by pipetting up and down five times. The resulting mixture of the pathogen, SMG, and neutralizing agent was pipetted onto a BBL trypticase soy agar plate and evenly spread with an L-spreader. Plates were incubated upside down in a Forma-Steri-Cycle CO2 incubator for 20 to 28 hours, except CA, which was incubated for a longer time period (48–58 hours) due to its slower growth rate. Plates were then manually counted by the naked eye and recorded. During the counting process, each colony was marked on the bottom of the culture plate with a fine point marking pen in order to assure accurate counting.
Statistical Analysis
We have performed three separate experiments on each pathogen collected from different sources. For each experiment, three to six plates were prepared to test each concentration (0 [control], 20, 40, 80, 100, and 200 mM). Kill rate for each plate was calculated using the number of colonies grown on a plate and the mean value of colonies grown on control plates for that experiment. To analyze the data, we fit linear regression models to compare differences in mean kill rates between doses adjusted for day (2-way ANOVA). For graphs, mean kill rates and SEs were calculated based on these regression models, and statistical analysis was conducted using STATA 13.1 software (StataCorp, College Station, TX, USA).
Results
Group 1 Experiments
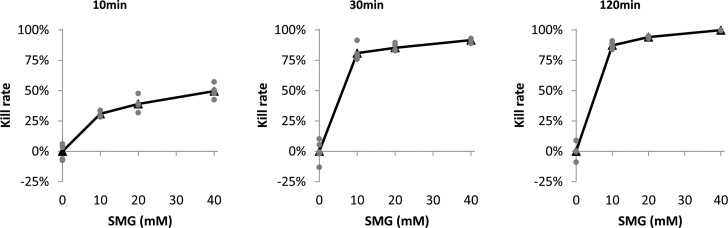
Bactericidal activity of an SMG solution against MSSA, expressed in kill rates, in the 10-minute incubation group was modest for 10 mM (30.9%, SD 2.5%), 20 mM (39.1%, SD 5.6%), and 40 mM (49.6%, SD 5.2%). These values increased in the 30-minute incubation group for 10 mM (80.9%, SD 6.0%), 20 mM (85.3%, SD 2.9%), and 40 mM (91.6%, SD 1.6%). The most robust bactericidal effect against MSSA was seen in the 2-hour incubation for 10 mM (87.3%, SD 2.7%), 20 mM (94.1%, SD 0.7%), and 40 mM (99.8%, SD 0.2%). These data are shown in Figure 1. In all experiments, control plates had colony counts ranging from 191 to 408 colonies per plate (for 103 seeding density).
Figure 1.
Bactericidal activity of SMG on MSSA. The percentage of MSSA bacteria killed, calculated by the number of colonies grown on the treated plate and the average of the number of colonies grown on control plates, were compared in relation to concentrations and incubation durations. Kill rate for each plate was calculated by using the number of colonies grown on that plate and the average of the number of colonies grown on control plates (gray dots). Mean kill rates (average of kill rates on treated plates) are expressed in black triangles. The method for counting was as described in Methods and Materials. The greatest effect was seen in the 2-hour incubation with 10 mM, 20 mM, and 40 mM.
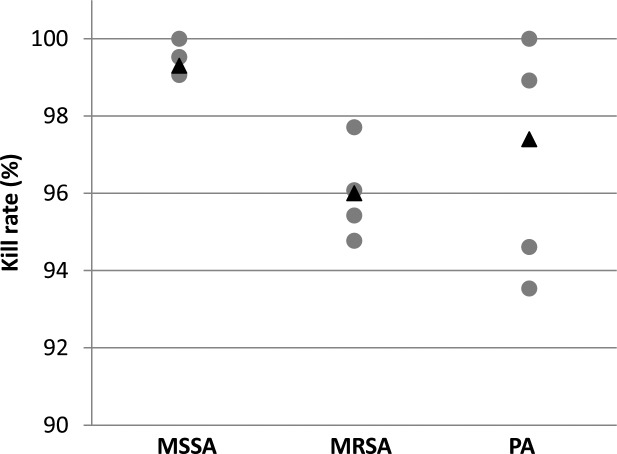
In additional rounds of experiments with 40 mM SMG and a 2-hour incubation, the bactericidal effect was equally robust against MSSA (99.3%, SD 0.4%), MRSA (96.0%, SD 1.1%), and PA (97.4, SD 3.1%). Control plates had colony counts ranging from 191 to 408 colonies per plate for MSSA, 98 to 347 for MRSA, and 86 to 103 for PA (Fig. 2).
Figure 2.
Bactericidal activity of 40 mM SMG for 120 minutes on MSSA, MRSA, and PA. With 40 mM SMG incubated over 120 minutes, the percentage of MSSA, MRSA, and PA bacteria killed were compared. Ten plates were prepared for each bacterium. Five plates were treated with 40 mM and five plates were treated with blank solution (control). Kill rate for each plate was calculated by using the number of colonies grown on that plate and the average of the number of colonies grown on control plates (gray dots). Mean kill rates (average of kill rates on treated plates) are expressed in black triangles. Methods for measurement, as described previously, via colonies per plate. The bactericidal effect was robust in each of the three samples.
Group 2 Experiments
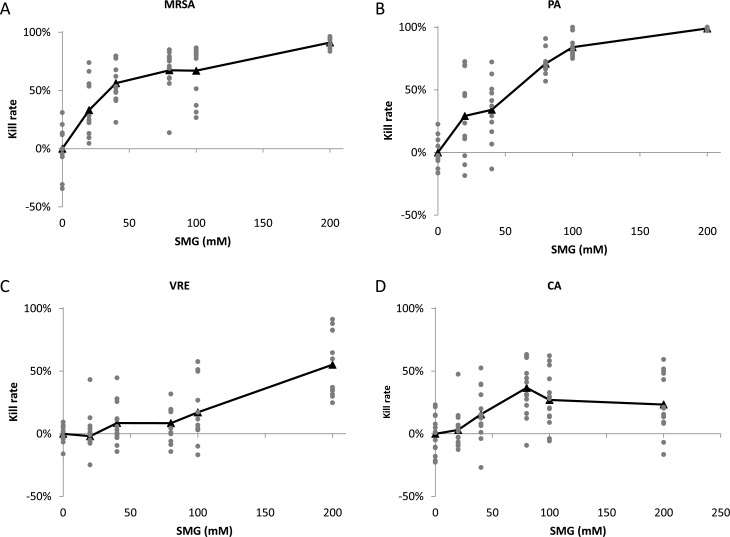
As shown in Figure 3 and Table 2, and consistent with the group 1 experiment, SMG exhibited the most effective bactericidal activity against MRSA (Fig. 3A) and PA (Fig. 3B), both in a dose-dependent manner. Thirty-minute incubation of SMG with MRSA showed the dosage-dependent bactericidal activity, and kill rates were as follows: 33% (SD 25%, vs. control P < 0.001) at 20 mM, 56% (SD 17%, vs. control P < 0.001) at 40 mM, 67% (SD 19%, vs. control P < 0.001) at 80 mM, and 67% (SD 23%, vs. control P < 0.001) at 100 mM. At 200 mM, the kill rate reached 91% (SD 4%, vs. control P < 0.001), which was equivalent to the bactericidal activity resulting from a 2-hour incubation at 40 mM from group 1 experiments. The most profound bactericidal activity was observed against PA at the highest concentration, 200 mM, resulting in 99% (SD 1%, vs. control P < 0.001) kill rate. The dose-dependent effect is noted with each increasing interval of SMG: 29% (SD 33%, vs. control P < 0.001) at 20 mM, 34% (SD 25%, vs. control P < 0.001) at 40 mM, 71% (SD 10%, vs. control P < 0.001) at 80 mM, and 84% (SD 8%, vs. control P < 0.001) at 100 mM. The numbers of colonies ranged from 17 to 4000 for MRSA and 0 to 2074 for PA, with the maximum number of colonies exhibited in the control plates and varied depending on the seeding density.
Figure 3.
Concentration-dependent bactericidal activity against (A) MRSA, (B) PA, (C) VRE, and (D) CA. In all of these experiments, BSA (10 mg/mL) and neutralizing buffer were added to the incubation mixtures to simulate a proteinaceous environment and to stop the reaction precisely, respectively. Pathogens were treated with 0 (control), 20, 40, 80, 100, and 200 mM SMG for 30 minutes, and kill rates were determined for each plate and plotted with gray dots. The average % kill rate was determined by the difference in the number of colony counts in treated versus control (untreated) samples and is expressed in black triangles. Each value represents the average of multiple (3–6) independent determinations carried out on a minimum of 3 separate days. SMG displayed greatest effects against MRSA and PA with marginal effects against VRE and was ineffective against CA. P value was calculated using regression models comparing differences in mean kill rates between doses adjusted for day (2-way ANOVA).
Table 2.
Group 2 Experiment: Mean Kill Rates ± SD of SMG on Four Different Bacterial Strains and P Values (Comparison Against 0 mM SMG)
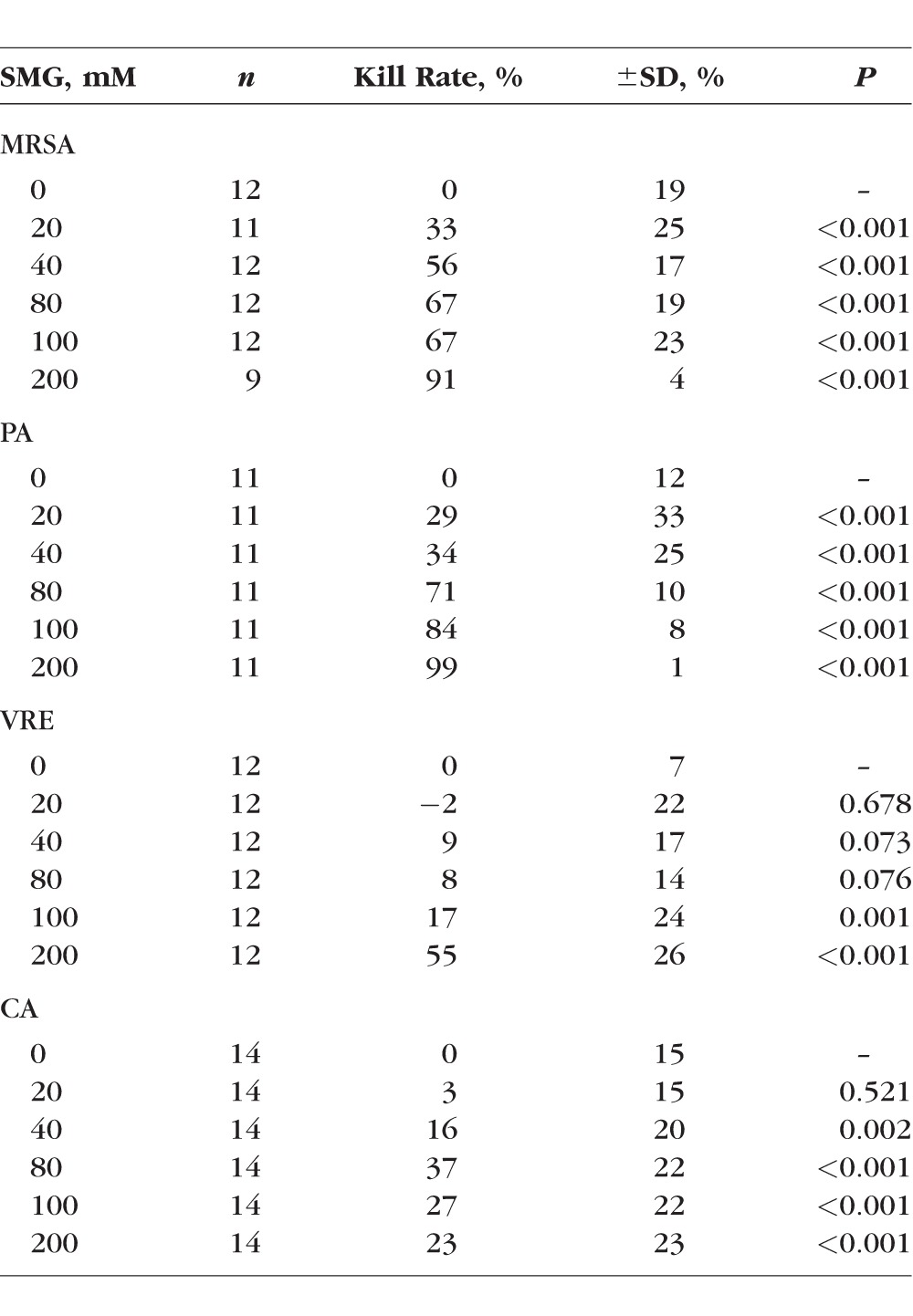
VRE is another bacterium that has a potential risk to human health due to lack of effective antibiotics. As shown in Figure 3C, SMG exhibited mild antimicrobial activity against VRE, with kill rates of −2% (SD 22%, vs. control P = 0.678) at 20 mM, 9% (SD 17%, vs. control P = 0.073) at 40 mM, and 8% (SD 14%, vs. control P = 0.076) at 80 mM. At 100 mM, the kill rate was 17% (SD 24%, vs. control P = 0.001), and at 200 mM, SMG was shown to be moderately effective with a kill rate of 55% (SD 26%, vs. control < 0.001). The numbers of colonies for VRE ranged from 63 to 1238, with the maximum number of colonies exhibited in the control plates.
A low level of bactericidal activity was observed against CA. As shown in Figure 3D, the kill rate plateaued at 40% with 80 mM SMG or higher, and there were no significant differences in kill rates at dosages between 80 and 200 mM (vs. control P < 0.001). Kill rates at 20 mM were 3% (SD 15%, vs. control P = 0.521) and 16% (SD 20%, vs. control P = 0.002) at 40 mM. Even at high concentrations of 200 mM SMG, the number of Candida colonies remained significantly unchanged. The numbers of colonies on Candida ranged from 73 to 330.
Discussion
Infectious keratitis is a major source of blindness worldwide and a significant inconvenience for millions of contact lens wearers.13,14 Infectious keratitis affects millions of people worldwide and is responsible for more than 1.5 million new cases of monocular blindness each year, many of which occur in the developing world.15 It is estimated that the rate of corneal ulceration may be 10 times greater in areas of the developing world than in the United States.13 An easy, inexpensive, yet effective topical cross-linking solution could expand the reach of therapy for infectious keratitis to areas of the world without access to antibiotics or antifungals.13
There are many reasons to consider new approaches to treating infectious corneal disease, including the emergence of antibiotic resistance, duration of treatment necessary, degree of residual scarring, and toxic effects of traditional antibiotics.16–18 TXL has the potential to simplify the treatment paradigm and improve patient outcomes with regard to these limitations of standard-of-care antibiotic therapy. CXL has shown equal efficacy against antibiotic-sensitive and antibiotic-resistant strains of bacteria.19 CXL has been shown in vitro to have a strong bactericidal effect in a single treatment, and our experiments with TXL show robust bactericidal activity with a one-time treatment.20 In a live rabbit model, CXL has shown to decrease the size of corneal scarring and shorten healing time, as this one-time intervention replaces weeks of frequent administration of toxic antimicrobials to the ocular surface.21
Traditional approaches to treating tissue infections rely primarily on direct microbial killing. On the other hand, cross-linking therapy (whether photochemical or topical) offers a dual approach to treating these infections. In addition to direct microbial killing, CXL induces cross-linking in the extracellular matrix and enhances corneal stromal resistance.22,23 The resistance alters the chemical accessibility to enzyme digestion via collagenases and, thus, slows tissue destruction, be it from endogenous (matrix metalloproteinases) or exogenous (i.e., bacterial collagenase) sources.24,25 This will help to control the infection as pathogens spread via enzymatic tissue destruction. The increased resistance to enzymatic degradation may additionally contribute to the reduction in size of corneal scars and negative outcomes of the most severe corneal ulcers, particularly corneal melts, seen in patients treated with CXL.22,24
Battling infectious keratitis, particularly the worst cases, has been problematic, and CXL and TXL offer hope for a better treatment option.18,24 FAR compounds like SMG are known to have antimicrobial properties and have been shown to have the capability to induce tissue cross-linking effects similar to the photochemical method.3 A significant amount of literature has accrued in recent years regarding the use of CXL in infectious keratitis (also known as “photoactivated chromophore for infectious keratitis–corneal collagen cross-linking” or “PACK-CXL”), and several recent reviews18,26,27 and even meta-analyses23,28 are now available. An overwhelming number of reports have shown that CXL is effective as an adjunct to standard antibiotic agents24,29 for bacterial keratitis. However, CXL also has been used with success as a primary therapy for infectious keratitis due to bacterial causes.30 The literature, however, is less convincing for fungal keratitis31 and acanthamoeba26 and should be avoided in viral keratitis.18 Importantly, PACK-CXL has been shown to have equal or improved bactericidal efficacy against antibiotic-resistant strains of Pseudomonas, Enterococcus, and S. aureus.19 Thus, TXL with SMG may be able to affect infectious corneal processes in a manner similar to the riboflavin photochemical (CXL) method, but without the need for de-epithelialization, intentional exposure to UV light, or costly CXL UV light source.
SMG solution exhibits a dose-dependent bactericidal effect on MSSA. This solution is also an effective bactericidal agent against MRSA, PA, and VRE. Although prior studies have shown the efficacy of SMG against various strains of bacteria and fungus, this is the first report showing efficacy against MRSA and VRE, which are increasingly troublesome bacteria with widespread effects. Furthermore, prior studies have focused on the antimicrobial activity of SMG as a preservative in personal care products, and, thus, used significantly different conditions than those used in the present study.2 That being said, the authors do report antimicrobial activity against S. aureus, PA, and CA, among several other pathogens. The concentrations used were significantly lower than those used in our study. As well, the incubation times were significantly longer than those used in our study (up to 28 days). To reiterate, the objectives in the study by Ghelardi et al.2 were to understand shelf-life properties and, thus, experiments lasting up to 28 days were reasonable. We, on the other hand, are interested in treating tissue infections and, thus, 10-minute to 2-hour incubations using higher concentrations were carried out. In any event, the current report in combination with prior studies does raise the possibility of using SMG for treating corneal tissue infections, an application that has not been previously suggested.
Regarding possible mechanisms for antimicrobial killing of FARs, in general, antiseptic agents (also known as biocides) have a broader spectrum of activity and multiple targets, as opposed to “traditional” antibiotics and antifungal agents that tend to have specific intracellular targets. Although the exact mechanism of microbial killing is not known completely either for free formaldehyde or for FARs, it is generally accepted that the killing process involves cross-linking reactions to various moieties of pathogen proteins and nucleic acids following penetration through the outer membrane or cell wall.32
These studies underscore an exciting new possibility for the use of this agent in clinical therapeutics, including infectious keratitis, because these concentrations are well-tolerated in live rabbits (Zyablitskaya M, et al. IOVS 2017;58:ARVO E-Abstract 4344). We have recently reported preliminary results from live rabbit studies that indicate the ocular tolerability of topical application of SMG at concentrations comparable to those used in the present in vitro study. In that report, we used up to 250 mM SMG applied via a corneal reservoir as well as topical eyedrops of 40 and 80 mM. An additional delivery method that has been used in our unpublished studies is via hydrogel CLs at concentrations up to 40 mM (Zyablitskaya M, et al. IOVS 2017;58:ARVO E-Abstract 4344).
The results of this study could open up an entirely new class of agents for treating tissue infections. Because they are broad-spectrum agents, these agents could have unique efficacy against emerging pathogens, such as MRSA, VRE, and extended-spectrum β-lactamases-resistant strains of Pseudomonas. As mentioned previously, WHO has recently published a list of antibiotic-resistant pathogens that have been prioritized as posing great risk to human health.1 Among those 12 bacteria listed include MRSA; PA, carbapenem-resistant; and VRE. These three species of bacteria were tested in the present study and found to have bactericidal activity, offering hope for developing agents, such as SMG, in the fight against antibiotic-resistant microorganisms.
Acknowledgments
The authors thank Shanta Modak, PhD, of Columbia University for her guidance and for providing the materials, and Jimmy Duong from the Design and Biostatistics Resource and the Biostatistical core facility of the Irving Institute at Columbia University Medical Center for biostatistical consultation.
Supported in part by Research to Prevent Blindness and by National Institutes of Health Grants NCRR UL1RR024156, NEI P30 EY019007, and NEI R01EY020495 (DCP). Patent pending through Columbia University (DCP).
Disclosure: P.B. Rapuano, None; A.H. Scanameo, None; D.E. Amponin, None; S.A. Paulose, None; M. Zyablitskaya, None; A. Takaoka, None; L.H. Suh, None; T. Nagasaki, None; S.L. Trokel, None; D.C. Paik, P
References
- 1. World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available at: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1.
- 2. Ghelardi E, Celandroni F, Gueye SA, Salvetti S, Campa M, Senesi S. . Antimicrobial activity of a new preservative for multiuse ophthalmic solutions. J Ocul Pharmacol Ther. 2013; 29: 586– 590. [DOI] [PubMed] [Google Scholar]
- 3. Babar N, Kim M, Cao K,et al. Cosmetic preservatives as therapeutic corneal and scleral tissue cross-linking agents. Invest Ophthalmol Vis Sci. 2015; 56: 1274– 1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chai D, Gaster RN, Roizenblatt R, Juhasz T, Brown DJ, Jester JV. . Quantitative assessment of UVA-riboflavin corneal cross-linking using nonlinear optical microscopy. Invest Ophthalmol Vis Sci. 2011; 52: 4231– 4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Groot A, White IR, Flyvholm MA, Lensen G, Coenraads PJ. . Formaldehyde-releasers in cosmetics: relationship to formaldehyde contact allergy. Part 2. Patch test relationship to formaldehyde contact allergy, experimental provocation tests, amount of formaldehyde released, and assessment of risk to consumers allergic to formaldehyde. Contact Dermatitis. 2010; 62: 18– 31. [DOI] [PubMed] [Google Scholar]
- 6. Kim SY, Babar N, Munteanu EL,et al. Evaluating the toxicity/fixation balance for corneal cross-linking with sodium hydroxymethylglycinate (SMG) and riboflavin-UVA (CXL) in an Ex vivo rabbit model using confocal laser scanning fluorescence microscopy. Cornea. 2016; 35: 550– 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laggner M, Pollreisz A, Schmidinger G,et al. Correlation between multimodal microscopy, tissue morphology, and enzymatic resistance in riboflavin-UVA cross-linked human corneas. Invest Ophthalmol Vis Sci. 2015; 56: 3584– 3592. [DOI] [PubMed] [Google Scholar]
- 8. Meek KM, Hayes S. . Corneal cross-linking—a review. Ophthalmic Physiol Opt. 2013; 33: 78– 93. [DOI] [PubMed] [Google Scholar]
- 9. Ni N, Nam EM, Hammersmith KM,et al. Seasonal, geographic, and antimicrobial resistance patterns in microbial keratitis: 4-year experience in eastern Pennsylvania. Cornea. 2015; 34: 296– 302. [DOI] [PubMed] [Google Scholar]
- 10. Paik DC, Solomon MR, Wen Q, Turro NJ, Trokel SL. . Aliphatic beta-nitroalcohols for therapeutic corneoscleral cross-linking: chemical mechanisms and higher order nitroalcohols. Invest Ophthalmol Vis Sci. 2010; 51: 836– 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paik DC, Wen Q, Airiani S, Braunstein RE, Trokel SL. . Aliphatic beta-nitro alcohols for non-enzymatic collagen cross-linking of scleral tissue. Exp Eye Res. 2008; 87: 279– 285. [DOI] [PubMed] [Google Scholar]
- 12. Pereira FQ, Bercht BS, Soares MG, da Mota MG, Pigatto JA. . Comparison of a rebound and an applanation tonometer for measuring intraocular pressure in normal rabbits. Vet Ophthalmol. 2011; 14: 321– 326. [DOI] [PubMed] [Google Scholar]
- 13. Whitcher JP, Srinivasan M, Upadhyay MP. . Corneal blindness: a global perspective. Bull World Health Organ. 2001; 79: 214– 221. [PMC free article] [PubMed] [Google Scholar]
- 14. Collier SA, Gronostaj MP, MacGurn AK,et al. Estimated burden of keratitis—United States, 2010. MMWR Morb Mortal Wkly Rep. 2014; 63: 1027– 1030. [PMC free article] [PubMed] [Google Scholar]
- 15. Ong HS, Corbett MC. . Corneal infections in the 21st century. Postgrad Med J. 2015; 91: 565– 571. [DOI] [PubMed] [Google Scholar]
- 16. Cheung N, Nagra P, Hammersmith K. . Emerging trends in contact lens-related infections. Curr Opin Ophthalmol. 2016; 27: 327– 332. [DOI] [PubMed] [Google Scholar]
- 17. Martins SA, Combs JC, Noguera G,et al. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci. 2008; 49: 3402– 3408. [DOI] [PubMed] [Google Scholar]
- 18. Price MO, Price FW Jr.. Corneal cross-linking in the treatment of corneal ulcers. Curr Opin Ophthalmol. 2016; 27: 250– 255. [DOI] [PubMed] [Google Scholar]
- 19. Makdoumi K, Backman A. . Photodynamic UVA-riboflavin bacterial elimination in antibiotic-resistant bacteria. Clin Exp Ophthalmol. 2016; 44: 582– 586. [DOI] [PubMed] [Google Scholar]
- 20. Schrier A, Greebel G, Attia H, Trokel S, Smith EF. . In vitro antimicrobial efficacy of riboflavin and ultraviolet light on Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, and Pseudomonas aeruginosa. J Refract Surg. 2009; 25: S799– S802. [DOI] [PubMed] [Google Scholar]
- 21. Tal K, Gal-Or O, Pillar S, Zahavi A, Rock O, Bahar I. . Efficacy of primary collagen cross-linking with photoactivated chromophore (PACK-CXL) for the treatment of Staphylococcus aureus-induced corneal ulcers. Cornea. 2015; 34: 1281– 1286. [DOI] [PubMed] [Google Scholar]
- 22. Garg P, Das S, Roy A. . Collagen cross-linking for microbial keratitis. Middle East Afr J Ophthalmol. 2017; 24: 18– 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Papaioannou L, Miligkos M, Papathanassiou M. . Corneal collagen cross-linking for infectious keratitis: a systematic review and meta-analysis. Cornea. 2016; 35: 62– 71. [DOI] [PubMed] [Google Scholar]
- 24. Said DG, Elalfy MS, Gatzioufas Z,et al. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014; 121: 1377– 1382. [DOI] [PubMed] [Google Scholar]
- 25. Spoerl E, Wollensak G, Seiler T. . Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004; 29: 35– 40. [DOI] [PubMed] [Google Scholar]
- 26. Tabibian D, Mazzotta C, Hafezi F. . PACK-CXL: corneal cross-linking in infectious keratitis. Eye Vis (Lond). 2016; 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan TC, Lau TW, Lee JW, Wong IY, Jhanji V, Wong RL. . Corneal collagen cross-linking for infectious keratitis: an update of clinical studies. Acta Ophthalmol. 2015; 93: 689– 696. [DOI] [PubMed] [Google Scholar]
- 28. Alio JL, Abbouda A, Valle DD, Del Castillo JM, Fernandez JA. . Corneal cross linking and infectious keratitis: a systematic review with a meta-analysis of reported cases. J Ophthalmic Inflamm Infect. 2013; 3: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Price MO, Tenkman LR, Schrier A, Fairchild KM, Trokel SL, Price FW Jr.. Photoactivated riboflavin treatment of infectious keratitis using collagen cross-linking technology. J Refract Surg. 2012; 28: 706– 713. [DOI] [PubMed] [Google Scholar]
- 30. Makdoumi K, Mortensen J, Sorkhabi O, Malmvall BE, Crafoord S. . UVA-riboflavin photochemical therapy of bacterial keratitis: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2012; 250: 95– 102. [DOI] [PubMed] [Google Scholar]
- 31. Uddaraju M, Mascarenhas J, Das MR,et al. Corneal cross-linking as an adjuvant therapy in the management of recalcitrant deep stromal fungal keratitis: a randomized trial. Am J Ophthalmol. 2015; 160: 131– 134.e5. [DOI] [PubMed] [Google Scholar]
- 32. McDonnell G, Russell AD. . Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999; 12: 147– 179. [DOI] [PMC free article] [PubMed] [Google Scholar]